Abstract
A series of iridium(III) triimine complexes incorporating 2,2′:6′,2″-terpyridine (terpy) and 2,6-bis(thiazol-2-yl)pyridine (dtpy) derivatives were successfully designed and synthesized to investigate the impact of the peripheral rings (pyridine, thiazole) and substituents (thiophene, bithiophene, EDOT) attached to the triimine skeleton on their photophysical properties. The Ir(III) complexes were fully characterized using IR, 1H, elemental analysis and single crystal X-ray analysis. Their thermal properties were evaluated using TGA measurements. Photoluminescence spectra of [IrCl3L1–6] were investigated in solution at 298 and 77 K. The experimental studies were accompanied by DFT/TDDFT calculations. The photophysical properties of the synthesized triimine ligands and Ir(III) complexes were studied in detail by electronic absorption and emission. In solution, they exhibited photoluminescence quantum yields ranging from 1.27% to 5.30% depending on the chemical structure. The experimental research included DFT/TDDFT calculations. The photophysical properties of the synthesized triimine ligands and Ir(III) complexes were conducted using electronic absorption and emission techniques. In solution, they displayed photoluminescence quantum yields ranging from 1.27% to 5.30% depending on the chemical structure.
1. Introduction
The 2,2′:6′,2″- terpyridine (terpy) as a family of N–heterocyclic ligands is one of most widely studied compounds as functional templates in coordination chemistry [1]. Terpyridines were first discovered by Morgan and Burstall in 1932 during solvatothermal reaction by heating the pyridine with anhydrous FeCl3 [2]. Since then, numerous functional groups have been introduced into the terpy skeleton in different positions. The terpy-based metal complexes have exhibited a great potential for application in molecular magnetism, molecular electronics, catalysis, and supramolecular chemistry. It has been proven that these compounds demonstrate activity as antitumor, antimicrobial and anti-HIV agents [3,4,5,6,7]. What is more, due to their photophysical and electrochemical properties, complexes based on terpy derivatives have been widely studied for potential applications covering light-to-electricity conversion, light-emitting electrochemical cells (LECs), (electro)luminescent systems, non-linear optical devices, and luminescent sensors [8]. Lately, particular attention has been devoted to terpy-like ligands, whose structural modification consists in replacing the perihedral pyridine rings with thiazole rings leading to 2,6-bis(thiazol-2-yl)pyridines (dtpy). Thiazole and pyridine rings exhibit different electronic characteristics, including σ-donor and π-acceptor properties. This difference enables the tuning of HOMO and LUMO energies, thereby facilitating the development of new, efficient phosphorescent materials. In contrast to the rich coordination chemistry of 2,2′:6′,2′′-terpyridine, the number of transition metal complexes based on 2,6–(bis(thiazol-2-yl)pyridines is limited. Among polypyridine complexes of d6 transition metals, great attention has been devoted to iridium(III) coordination compounds, which show structural diversity and are able to form mono-, bis-, and triscyclometallated compounds [9,10,11,12,13,14,15,16,17,18]. The modification of Ir(III) environment in those three types of compounds allows for tuning their emission properties that cover the full visible spectra up to the near infrared. The high structural variety allows better control of the excited state nature and emission properties of these complexes and Ir(III) species are known to exhibit higher triplet quantum yields due to the mixing of the singlet and triplet excited states through spin-orbital coupling, leading to high phosphorescence efficiencies [17,19].
The main aim of this work was to explore the impact of the triimine skeleton (terpy and dtpy) and substituents (thiophene, bithiophene, and EDOT) on the photophysical properties of Ir(III) complexes. Recently, some efforts have been made to understand the structure–property correlation in rhenium(I), platinum(I), gold(I), cobalt(II), and copper(II) [20,21,22,23,24,25,26] complexes by introducing the R substituent in the 4′-position of the terpy and/or dtpy rings. However, to the best of our knowledge, [IrCl3(L)] complexes incorporating terpy (ligands L1–L3) and dtpy derivatives (ligands L4–L6) are quite rare and will be reported here for the first time (see Table S15). Taking into account the ability to tune the luminescent properties of [IrCl3(4′-R-terpy)] and [IrCl3(4′-R-dtpy)], we chose Ir(III) complexes in order to investigate their photophysical properties. The used R substituents (Scheme 1) incorporated into the 4′-position of the terpy/dtpy skeleton have different electron donating or withdrawing properties as well as different steric effects. The luminescent properties were studied in solution at 298 K and 77 K. The density functional theory and time-dependent DFT calculations were performed to obtain insight into their electronic structure and spectroscopic properties.
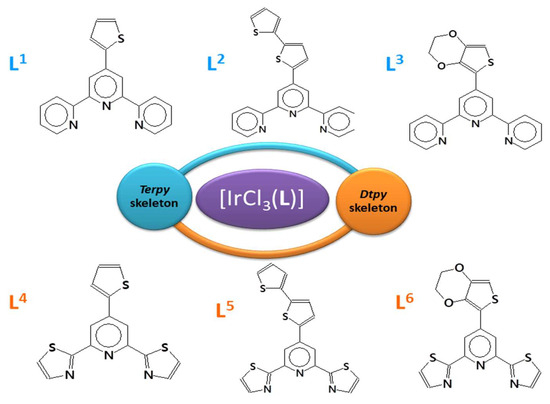
Scheme 1.
Ligands used in the synthesis of the complexes.
2. Results and Discussion
2.1. Synthesis and Structural Characterization
To synthesize iridium(III) complexes, the procedure based on the solvatothermal reaction of IrCl3 with an appropriate N-donor ligand in 2-methoxyethanol was employed. The successful coordination of the triimine ligands and formation of Ir(III) complexes have been confirmed by elemental analysis and spectroscopic tools (Figures S1 and S2) and X-ray diffraction studies. The last ones were performed for monocrystals of 1 and 6.
The crystallographic studies together with selected bond distances and angles are summarized in Tables S1 and S2 in the Supporting Information. The molecular structures of 1 and 6 as representative examples are depicted in Figure 1. The coordination of tridentate terpy/dtpy ligands results in significant distortions within the typically octahedral environment surrounding the iridium atom. Whereas the bond angles of Cl(1)–Ir(1)–Cl(3) (178.82(5)° in 1; 177.32(6)° in 6) and N(2)–Ir(1)–Cl(2) (177.30(16)° in 1; 177.75(14)° in 6) are both close to the ideal angle of 180°, that of N(1)–Ir(1)–N(3) is much smaller (161.00(17)° in 1; 160.4(2)° in 6) owing to the chelating coordination mode of modified ligand formation of two five-membered metallocycles. Notably, [IrCl3(L)] complexes (L = tridentate N-donor ligand) are relatively rare [27,28,29,30,31,32,33]. A search in the Cambridge Structure Database (Version 5.37) revealed only nine such systems (Table S10).
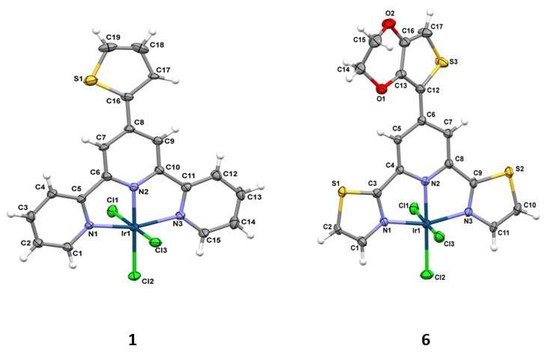
Figure 1.
Molecular structures of the compounds 1 and 6 together with the atom numbering. Displacement ellipsoids are drawn at the 50% probability level.
The central bond length Ir(1)–N(2) (1.942(4)Å in 1; 1.960(5)Å in 6) is significantly shorter than other two Ir(1)–N bond distances; this leads to a lengthening of the trans sited Ir(1)–Cl(2) bond distances. The crystal packing analysis (Mercury 3.10.2 program) [34] demonstrates that the molecules of 1 and 6 show weak C–H⋯S/π⋯π and C–H⋯O/C–H⋯S/C–H⋯Cl/π⋯π interactions, respectively. More structural details are given in Tables S3 and S4. The Hirshfeld surfaces were generated using Crystal Explorer 21.5 in order to quantify and visualize the intermolecular interactions in the crystal structures (Figure 2) [35,36].
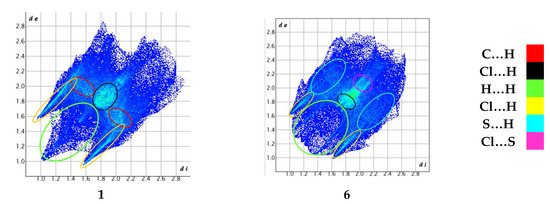
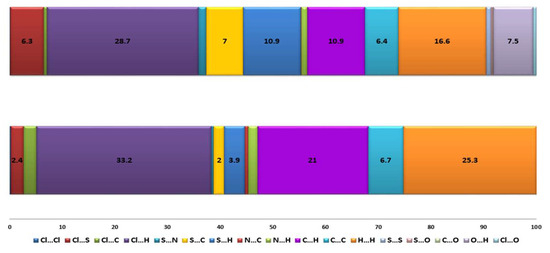
Figure 2.
Hirshfeld surface mapped with dnorm (top), graph showing a fraction of the individual close contacts on the Hirshfeld surfaces (down).
The Hirschfeld surfaces confirm that the C-H…Cl together with van der Waals interactions play a dominant role in the examined structures, the C…H, Cl…H, and H…H contributions are significant (21%, 33.2%, and 25.3% in 1 and 10.9%, 28.7%, and 16.6% in 6). In 6, the S…C (7%) and S…H (10.9%) are important in confirming the results of the intermolecular interactions analysis (see Tables S3 and S4 in ESI). In 6, the C(5)–H(5)…O(3) and C(11)–H(11)…O(2) interactions seems to be among the most important, and the O…H contribution (7.5%) confirms that the 3,4-ethylenedioxythiophene substituent in the dtpy skeleton actively participates in the creation of the interaction network.
2.2. Thermal Properties
In order to examine the thermal properties of [IrCl3(L1–L6)] complexes, thermogravimetric analysis (TGA) was applied. The results are collected in Table S5 and Figure S6. The TGA results show that the Ir(III) complexes based on the terpy skeleton are more stable than those based on dtpy derivatives; however, the effect of the substituent is also significant. The values of Tmax of the Ir(III) compounds EDOT-terpy (3) (423°) and EDOT-dtpy (6) (438°) are less stable than thiophene (508° in 1; 450° in 4) and bithiophene (493° in 2 and 466° in 5) derivatives. At these temperatures, the TG curves display one weight loss attributed to the release of the R substituent: 14.142% in 1 (calcd 13.54%); 22.218% in 3 (calcd 22.49%); 12.692% in 4 (calcd 13.26%); 23.30% in 5 (calcd 21.073%); 21.164% in 6 (calcd 20.61%). The compound 2 exhibits different thermal behavior than the other compounds due to the two-step decomposition of the bithiophene substituent. The first weight loss is 13.362% at 525° (calcd 23.71%), which can be assigned to the release of only one thiophene ring. Further bithiophene decomposition occurs at a higher temperature and seems to be accompanied by the decomposition of the terpy skeleton.
2.3. Electronic Absorption Spectra and TD-DFT Calculations
The electronic absorption spectra of 1–6 registered in DMSO are presented in Figure 3, and the relevant spectroscopic data are summarized in ESI. Comparing the spectra of the free ligands L1–L6, the high energy absorptions of 1–6 can be assigned to the π → π* transitions localized on the triimine ligand coordinated to the metal center in a tridentate mode (see Figure S4). The absorptions of 1–6 in the low-energy region with maxima in the range 498–563 nm and much lower molar extinction coefficients possess a charge transfer character. The maxima of the complexes 4–6 based on the dtpy skeleton are significantly red-shifted (550 nm in 4, 563 nm in 5, 535 in 6) in comparison to the compounds 1–3 incorporating ligands with the terpy core (508 nm in 1, 530 nm in 2, 498 nm in 3). Considering the influence of the R substituents, it can be noticed that the bithiophene Ir(III) derivatives (2 and 5) are the most red-shifted in comparison to the thiophene (1 and 4) and EDOT (3 and 6) Ir(III) systems (see Table S6).
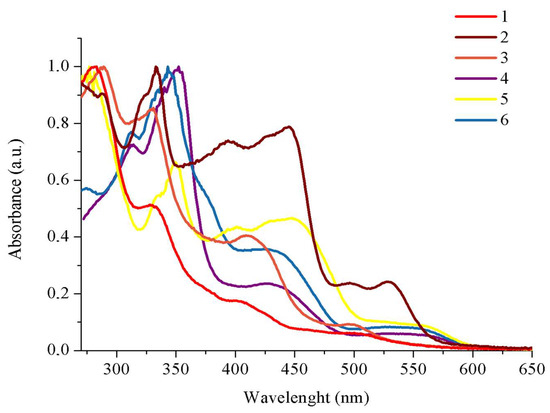
Figure 3.
UV–Vis profiles of 1–6 recorded in DMSO.
To deepen our understanding of the nature of the excited states involved in absorption processes, DFT/TD-DFT calculations were conducted for 1–6. The optimized structures of 1 and 6 agreed with the X-ray data (Table S2 in the ESI). An analysis of the molecular orbital energy levels graph for 1–6 reveals that energy gaps persist within the range of 2.69–3.33 eV. (Figure 4) The HOMO–LUMO gap is significantly lower in 2 (2.93 eV) and 5 (2.69 eV), which confirmed that the optical profile of [IrCl3(4′-bithiophene-terpy/dtpy)] differs from the other studied compounds. Moreover, the LUMO levels of 4–6 are destabilized in comparison with those for 1–3, which indicates significant differences between the terpy and dtpy core of the tridentate ligand. The HOMO orbitals for the pairs of the Ir(III) complexes incorporating thiophene and EDOT as R substituents lie in similar energy levels: –6.49 eV; –6.57 for 1/4 and –6.34 eV; –6.42eV for 3/6, respectively.
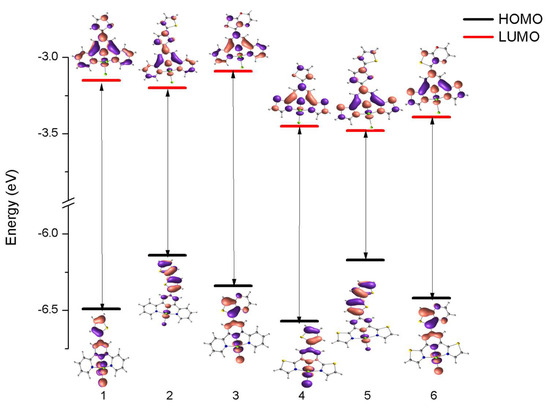
Figure 4.
Molecular orbital energy level graph of 1–6 at the DFT/B3LYP/LAN2DZ/6-31G* level.
The experimental and calculated electronic absorption spectra of 1–6 are compared in Figure 5. According to the theoretical calculations, the low-energy absorption band is composed of several transitions of different origins (see Tables S7–S12). Predominant contributions can be assigned to the excitations H-1→LUMO; HOMO→LUMO in 1, 4 and 6; and HOMO→LUMO in 2, 3, and 5. Based on the MO composition (Figure S5, Table S13) in 1, 3, and 6, the transitions H–1→LUMO can be assigned to MLCT [dπ(Ir)→π*(triimine)], while HOMO→LUMO possess an admixture of ILCT transition (charge transfer with electronic density originating from the R substituent unit to the π-conjugated triimine acceptor moiety. As previously described, the complexes 2 and 5 differ from the other complexes and their HOMO→LUMO transitions are dominated by intense ILCT transition (πR/→ π*(terpy) in 2; πR/→ π*(dtpy) in 5).
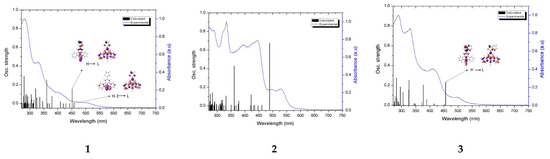
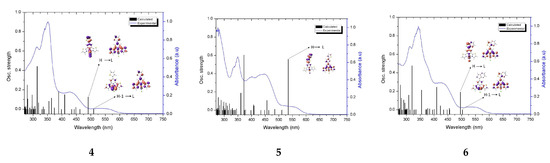
Figure 5.
Experimental (blue line) absorption spectra of 1–6 in DMSO alongside transitions (black lines) computed at the DFT/B3LYP/LAN2DZ/6-31G* level.
2.4. Emission Properties
The photoluminescence properties of [IrCl3(L1–L6)] (1–6) were investigated in deaerated DMSO solution at room temperature and in the EtOH–MeOH (4:1 v/v) rigid matrix at 77 K. All the Ir(III) complexes were found to be emissive under these experimental conditions and the summary of the photophysical data is presented in Table 1 and Figure 6.

Table 1.
Photoluminescent data (excitation λex and emission λem wavelength, quantum yield Φ, lifetime τ, and goodness-of-fit-χ2) of 1–6 in DMSO solution (5 × 10−5M), as well as in solid state and 77 K glass (EtOH-MeOH,4:1, v/v).
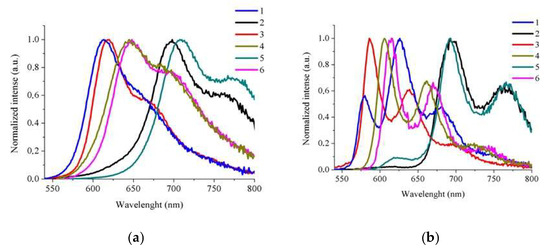
Figure 6.
The normalized emission spectra of 1–6 in DMSO at room temperature (a) and in a low-temperature matrix (77 K in EtOH–MeOH (4:1 v/v)) (b).
The excitation of iridium(III) complexes at the lowest absorption band gave rise to a room-temperature structured emission band with a maximum located in the orange-red spectral region (612–709 nm). The emission behavior of 1–6 is sensitive to the donor–acceptor properties of substituents incorporated into terpy (1–3) and dtpy skeletons (4–6). The attachment of thiazole and EDOT groups to the terpy ligand in the complexes 1 and 3 provides a broad band with weak shoulder with maxima at 612 nm, 660 nm (sh) nm in 1; and 620 nm, 677 nm (sh) nm in 3. The same effect is observed for the pair of compounds based on the dtpy skeleton. The bands of 4 and 6 showed a more distinct shoulder with red-shifted maxima by ca. 30 nm. The compounds 2 and 5 containing bithiophene as a substituent are the most shifted with two well-separated bands towards red wavelengths with the maxima 698 nm, 767 nm for 2 and 709 nm, 771 nm for 5. The emission lifetimes fall in the microsecond time regime in the case of the complexes 1, 2, and 5, while the compounds 3, 4, and 6 have nanosecond lifetimes. The decay curves for all of the Ir(III) complexes described by a biexponential fit indicate emission from more than one excited state (3MLCT mixed with 3IL/3ILCT). The luminescence quantum yields for Ir(III) based on the terpy skeleton are higher (4.42–5.30%) in comparison to dtpy–Ir(III) derivatives.
At low temperature (77 K), strong vibronic coupling features emerge, clearly indicating that the ligand affects the lowest-energy excited state (3IL/3ILCT).
The emission slightly shifts to higher energies and shows significant increases in the excited state lifetimes relative to room temperature.
2.5. Electrochemistry
The electrochemical properties of the Ir(III) complexes were studied at room temperature in DMF. The obtained data are gathered in Table S14 in ESI. No oxidation processes were observed for 1–6 up to +2.00 V. In general, it is expected that metal-based oxidation is shifted towards more positive potentials; so in terpy–like Ir(III) systems, IrIV/IrIII redox couple cannot be observed in the conventional potential window [37]. For 1–6, one reversible one-electron reduction process can be observed from −1.63 to −1.43V. By comparison to similar Ir(III) complexes, this process can be attributed to the reduction of the terpyridine/2,6-bis(thiazol-2-yl)pyridine ligands, and the LUMO may be located on the π* orbital of the polypyridyl ligand.
3. Materials and Methods
As a starting materials, IrCl3 was employed, while solvents of reagent grade were utilized for ligand synthesis. The solvents (HPLC grade) were used for spectroscopic studies. All materials were commercially available and used without additional purification. The N–heterocyclic ligands (L1–L5) were synthesized following literature procedures involving the condensation of 2-acetylpyridine or 2-acetylpyridine with appropriate commercially available benzaldehyde [38,39,40]. The 3,4-(Ethylenedioxy)thiophene-2-carboxaldehyde for ligand L6 was prepared according to the literature method [41] (Scheme 2).
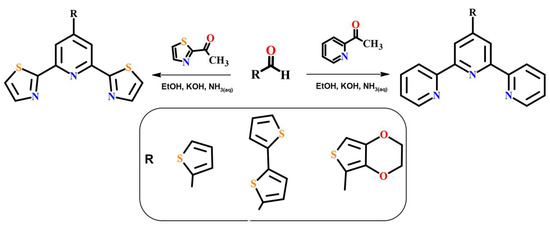
Scheme 2.
Ligands L1–L6: Synthetic route.
Data were processed with the aid of CrysAlis Pro software [42], Olex2 software [43], SHELXT, and SHELXL-2018/3 package [44]. Single crystals of 1 and 6 were grown from acetonitrile by slow evaporation of the solvent at room temperature. X-ray diffraction was performed using an Oxford Diffraction four-circle diffractometer Gemini A Ultra model, equipped with an Atlas CCD detector. Graphite-monochromated MoK radiation with a wavelength (λ) of 0.71073 Å was utilized for data collection, conducted at room temperature. CrysAlis Pro software [42], Olex2 software [43], SHELXT, and the SHELXL-2018/3 package [44] were used for data processing. All the non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated positions and refined with riding constraints: d(C–H) = 0.93 Å, Uiso(H) = 1.2 Ueq(C) (for aromatic) and d(C–H) = 0.96 Å, Uiso(H) = 1.5 Ueq(C) (for methyl). Crystallographic data for 1 and 6 were deposited with the Cambridge Crystallographic Data Center, CCDC 2352214-2352215.
IR spectra were obtained using a Nicolet iS5 FT-IR spectrophotometer (ThermoScientific, Waltham, MA, USA) within the range of 4000–400 cm−1. Electronic absorption spectra were recorded in DMSO with a concentration of c = 2.5 × 10−5 mol dm−3 using a Nicolet Evolution UV-VIS-NIR spectrophotometer (range: 650–250 nm). The 1H NMR spectra for 1–6 were produced at room temperature in DMSO-d6 using a Bruker 400 MHz spectrometer, while elemental analyses (C, H, N) were carried out on a Perkin–Elmer CHN-2400 analyzer (Perkin–Elmer, Waltham, MA, USA). The FLS-980 spectrofluorimeter (Edinburgh Instruments Ltd., Livingston, Scotland) were used for recording photoluminescence spectra. The room-temperature spectra were prepared in DMSO (c = 2.5 × 10−5 mol dm−3). The low-temperature emission spectra were measured in an ethanol:methanol mixture (4:1) frozen-glass matrix at the temperature of liquid nitrogen with a Dewar assembly. The time-resolved measurements were conducted at optically diluted solutions with an optical density ranging from 0.05 to 0.1 at room temperature. The experiments utilized time-correlated single photon counting (TCSPC) methods on the FLS-980 spectrofluorimeter. The excitation wavelength was obtained using a set of picosecond pulsed diodes, specifically EPLED–340 nm and EPLED–375 nm, serving as the light source. A PMT (Hamamatsu, R928P) housed in a cooled environment was employed as the detector, with the system alignment performed at the emission wavelength. Additionally, to analyze fluorescence decay, an instrument response function (IRF) was acquired. This IRF provides details regarding the time response of the entire optical and electronic system. For its determination, a ludox solution was utilized as a standard at the excitation wavelengths. Quantum yields were measured using the integrating sphere absolute method at room temperature and using the solvent (DMSO) as a blank. The samples were optically diluted to avoid the inner filter effect and re-absorption.
Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) measurements were performed using the Autolab potentiostat (Eco Chemie) under ambient conditions. A three-electrode, one-compartment cell was utilized to contain the deaerated solution of complexes and supporting electrolyte in DMF. The complexes and supporting electrolyte (n-Bu4NPF6) concentrations were equal to 10−6 mol dm−3 and 0.01 mol dm−3, respectively. The scan rate was equal to 0.1 V s−1. A glassy carbon disk working electrode (3 mm diam.) and a Ag/Ag+ reference electrode were used. TGA for 1–6 was carried out on a Perkin Elmer Pyris1 instrument (Perkin Elmer, Waltham, MA, USA).
Preparation of Iridium(III) Complexes
IrCl3 (1 mmol) and appropriate ligands L1–L6 (1 mmol) were dissolved in 2-methoxyethanol (25 mL). The resulting solution was heated in an autoclave for 20 h to 150 °C, maintained at this temperature for 30 h, and then cooled down to room temperature for another 30 h. X-ray-quality brown (1, 6) crystals were obtained by filtration and were washed with diethyl ether.
1: Yield: 60%. Elemental analysis calcd: C 37.17%, H 2.13%, N 6.84% found: C 37.22%, H 2.18%, N 7.00%; 1H NMR (500 MHz, DMSO) δ 9.22 (ddd, J = 5.6, 1.4, 0.5 Hz, 2H), 9.00 (s, 2H), 8.92 (d, J = 8.0 Hz, 2H), 8.31 (td, J = 7.8, 1.5 Hz, 2H), 8.26 (dd, J = 3.7, 1.1 Hz, 1H), 8.00–7.96 (m, 3H), 7.42 (dd, J = 5.0, 3.7 Hz, 1H) Ir (KBR; cm–1): 1604 (vs), 1424 (vs), 1254 (m), 786 (vs), 724 (s).
2: Yield: 65%. Elemental analysis calcd: C 39.69%, H 2.17%, N 6.04% found: C 39.58%, H 2.27% N, 6.37%; 1H NMR (500 MHz, DMSO) δ 9.22 (d, J = 7.1 Hz, 2H), 9.00 (s, 1H), 8.93 (d, J = 7.6 Hz, 2H), 8.31 (dd, J = 16.9, 2.8 Hz, 3H), 8.26 (d, J = 4.0 Hz, 1H), 8.02–7.97 (m, 2H), 7.69–7.66 (m, 2H), 7.54 (d, J = 3.6 Hz, 1H), 7.22 (dd, J = 5.1, 3.6 Hz, 1H). Ir (KBR; cm–1): 1606 (vs), 1464 (m) 1445 (vs), 1242 (m), 788 (s).
3: Yield: 70%. Elemental analysis calcd: C 37.42%, H 2.54%, N 6.23%; found C 37.67%, H 2.45%, N 6.56%; 1H NMR (500 MHz, DMSO) δ 9.22 (d, 2H), 8.81–8.74 (m, 4H), 8.27 (t, 2H), 7.98 (t, 2H), 7.09 (s, 1H), 4.54 (s, 2H), 4.38 (s, 2H). Ir (KBR; cm–1): 1604 (s), 1495 (vs), 1474 (s), 1420 (m), 1358 (m), 1060 (m), 790 (m)
4: Yield: 60%. Elemental analysis calcd: C 28.78%, H 1.45%, N 6.71%; found: C 28.46%, H 1.43%, N 6.77%; 1H NMR (500 MHz, DMSO) δ 8.94 (s, 2H), 8.59 (d, J = 3.4 Hz, 2H), 8.27 (d, J = 3.4 Hz, 2H), 8.23 (d, J = 3.7 Hz, 1H), 7.95 (d, J = 6.0 Hz, 1H), 7.41–7.38 (m, 1H). Ir (KBR; cm–1): 1606 (vs), 1482 (m), 1247 (m), 740 (m), 726 (w).
5: Yield: 60%. Elemental analysis calcd: C 32.23%, H 1.57%, N 5.93%; found: C 31.96%, H 1.84%, N 6.01%; 1H NMR (500 MHz, DMSO) δ 8.96 (s, 2H), 8.59 (d, J = 3.3 Hz, 2H), 8.27 (d, J = 3.4 Hz, 2H), 8.23 (d, J = 4.0 Hz, 1H), 7.68 (dd, J = 5.1, 1.1 Hz, 1H), 7.63 (d, J = 3.9 Hz, 1H), 7.55 (dd, J = 3.6, 1.1 Hz, 1H), 7.22–7.21 (m, 1H). Ir (KBR; cm–1): 1601 (vs), 1464 (vs), 1246 (m), 732(m).
6: Yield: 70%. Elemental analysis calcd: C 29.68%, H 1.77% N 6.13%; found: C 30.68%, H 1.85% N 6.45%; 1H NMR (500 MHz, DMSO) δ 8.68 (s, 2H), 8.59 (d, J = 3.4 Hz, 2H), 8.27 (d, J = 3.4 Hz, 2H), 7.09 (s, 1H), 4.55 (s, 4H), 4.38 (s, 4H). Ir (KBR; cm–1): 1599 (s), 1495 (vs), 1436 (v), 1358 (w), 1177 (m), 1059 (s), 763 (m).
4. Conclusions
Concluding, we present the synthesis and structural, thermal, electrochemical, and photophysical characterization of iridium(III) complexes of the general formula [IrCl3(L1−6)]. The adaptation of synthesis method to solvatothermal conditions gave rise to synthesized complexes with good yields (60–70%) and of the high purity, which was confirmed via FT-IR, NMR, and elemental analysis. Additionally, for the complexes 1 and 6, the X-ray analysis has been performed, confirming a pseudooctahedral structure around iridium(III) ions. Crystal packing analysis together with Hirshfeld surface analysis indicates 3D supramolecular assembly in both complexes. The compounds 1–6 display good thermal stability with Tmax values in the range 450–508 °C depending on the triimine skeleton and substituent attached. CVs and DPVs show only ligand-based reduction without an oxidation wave, indicating that the LUMOs have a π*(terpy/dtpy) character. The HOMOs, estimated via DFT, are in the majority localized on substituents. The lowest-energy absorption band is sensitive to both the type of triimine skeleton as well as the kind of substituent attached. Accordingly to TD-DFT calculations, this band can be attributed to MLCT/IL/ILCT transitions for 1, 3 and 4, 6, while it became predominately ILCT in the case of 2 and 5. The complexes display room-temperature red-orange phosphorescence in solution with quantum yields in the range 1.27–5.30% and lifetimes of hundreds of nanoseconds to a few microseconds. The structured shape of the emission band at both room and low temperatures indicates a substantial share of the ligand in the emission process. The emission bands of bithiophene-substituted compounds (shifted to the red) may be associated with 3ILCT contribution, while in the case of thiophene- and EDOT-appended based complexes the emitting state is more mixed between 3MLCT and 3IL/3ILCT. Our studies clearly evidence that the substituent attached to the triimine skeleton governs the character of LEES, while the triimine skeleton plays a vital role in the overall ratio between the radiative and non-radiative processes influencing the quantum yield and lifetime of the excited state. We also plan to examine early photophysical processes for [Ir(L)(terpy)]/[Ir(L)(dtpy)], where L represents N-donor polypyridine and/or cyclometalating ligands. Among the six Ir(III) compounds, the compounds 2 and 5 seem to be the most interesting and will be used for the preparation of bis(terpy)/bis(dtpy) complexes of iridium(III). The compounds 2 and 5 definitely differ from the other Ir(III) complexes, taking into account the nature of their HOMO→LUMO transitions as well as their photophysical profiles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29112496/s1. Refs [45,46] are cited in the Supplementary Materials. Figure S1. FT-IR spectra of 1–6; Figure S2. 1H NMR spectra of 1–6 (a) and 1H NMR and 13C NMR spectra of L6 (b); Figure S3. TGA of 1–6 under dry N2 atmosphere; Figure S4. UV-Vis spectra of complexes 1–6 together with ligand L1–L6; Figure S5. Composition of frontier molecular orbitals of complexes 1–6 (blue – Ir, red – 3Cl green – R’ substituent, violet – terpy/dtpy skeleton). Table S1. Crystal data and structure refinement; Table S2. Selected bond lengths (Å) and angles (deg) for 1 and 6; Table S3. Short intra–and intermolecular contacts; Table S4. Short π•••π stacking interactions; Table S5. TGA data for 1–6; Table S6. The absorption maxima for complexes [IrCl3(L1–L6)] (1–6); Table S7. The energies and characters of the selected spin-allowed electronic transitions for 1 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S8. The energies and characters of the selected spin-allowed electronic transitions for 2 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S9. The energies and characters of the selected spin-allowed electronic transitions for 3 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S10. The energies and characters of the selected spin-allowed electronic transitions for 4 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S11. The energies and characters of the selected spin-allowed electronic transitions for 5 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S12. The energies and characters of the selected spin-allowed electronic transitions for 6 calculated with the TDDFT/PBE1PBE method, together with assignment to the experimental absorption bands; Table S13. Frontier molecular orbitals of complexes 1–6; Table S14. Electrochemical properties of the Ir(III) complexes in DMF; Table S15. Structural and photophysical comparison of related Ir(III) complexes.
Author Contributions
Conceptualization, A.M.; Methodology, B.Z. and A.Ś.; Software, B.Z.; Investigation, B.Z. and M.S.; Writing—original draft, A.Ś.; Visualization, A.Ś. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre of Poland, SONATA grant no. 2020/39/D/ST4/00286. The research activities were co-financed by the funds granted under the Research Excellence Initiative of the University of Silesia in Katowice (POB).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hofmeier, H.; Schuber, U.S. Recent developments in the supramolecular chemistry of terpyridine–metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.T.; Burstall, F.H. Dehydrogenation of pyridine by anhydrous ferric chloride. J. Chem. Soc. 1932, 20–30. [Google Scholar] [CrossRef]
- Cummings, S.D. P latinum complexes of terpyridine: Interaction and reactivity with biomolecules. Coord. Chem. Rev. 2009, 253, 1495–1516. [Google Scholar] [CrossRef]
- de Paula, Q.A.; Mangrum, J.B.; Farrell, N.P. Zinc finger proteins as templates for metal ion exchange: Substitution effects on the C-finger of HIV nucleocapsid NCp7 using M(chelate) species (M = Pt, Pd, Au). J. Inorg. Biochem. 2009, 103, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Manikandamathavan, V.M.; Nair, B.U. Novel mononuclear Cu (II) terpyridine complexes: Impact of fused ring thiophene and thiazole head groups towards DNA/BSA interaction, cleavage and antiproliferative activity on HepG2 and triple negative CAL-51 cell line. Eur. J. Med. Chem. 2013, 68, 244–446. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Macedo, T.S.; Moreira, D.R.M.; Soares, M.B.P.; Leite, A.C.L.; Neves, J.K.; Pereira, V.R.A.; Avecilla, F.; Azam, A. Synthesis of 4′-(2-ferrocenyl)-2,2′:6′2′′-terpyridine: Characterization and antiprotozoal activity of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes. Eur. J. Med. Chem. 2014, 75, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-W.; Wang, Y.; Du, K.-J.; Li, G.-Y.; Guan, R.-L.; Ji, L.-N.; Chao, H. Synthesis, DNA interaction and anticancer activity of copper(II) complexes with 4′-phenyl-2,2′:6′,2″-terpyridine derivatives. J. Inorg. Biochem. 2014, 141, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Winter, A.; Schlutter, F.; Schubert, U.S. Advances in the field of π-conjugated 2,2′:6′,2″-terpyridine. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Sanguantrakun, N.; Schulze, B.; Schubert, U.S.; Berlin-Guette, C.P. Bis(tridentate) Ruthenium–Terpyridine Complexes Featuring Microsecond Excited-State Lifetimes. J. Am. Chem. Soc. 2012, 134, 12354–12357. [Google Scholar] [CrossRef]
- Presselt, M.; Dietzek, B.; Schmitt, M.; Rau, S.; Winter, A.; Jäger, M.; Schubert, U.S.; Popp, J. A Concept to Tailor Electron Delocalization: Applying QTAIM Analysis to Phenyl−Terpyridine Compounds. J. Phys. Chem. A 2010, 114, 13163–13174. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Lahtinen, M.; Kalenius, E.; Mal, P.; Rissanen, K. 2,2′:6′,2″-Terpyridine Trimethylplatinum(IV) Iodide Complexes as Bifunctional Halogen Bond Acceptors. Cryst. Growth Des. 2016, 16, 2527–2534. [Google Scholar] [CrossRef]
- Ghosh, B.N.; Topić, F.; Sahoo, P.K.; Mal, P.; Linnera, J.; Kalenius, E.; Tuononen, H.M.; Rissanen, K. Synthesis, structure and photophysical properties of a highly luminescent terpyridine diphenylacetylene hybrid fluorophore and its metal complexes. Dalton Trans. 2015, 44, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.W.; Dimitrov, V.S.; Long, N.J.; Orrell, K.G.; Osborne, A.G.; Sik, V.; Hursthouse, M.B.; Mazid, M.A. 2,2′:6′,2″-Terpyridine (terpy) acting as a fluxional bidentate ligand. Part 1. Trimethylplatinum(IV) halide complexes [PtXMe3(terpy)](X = Cl, Br or I): Nuclear magnetic resonance studies of their solution dynamics and crystal structure of [PtIMe3(terpy)]. J. Chem. Soc. Dalton Trans. 1993, 291–298. [Google Scholar] [CrossRef]
- Abhijnakrishna, R.; Magesh, K.; Ayushi, A.; Velmathi, S. Advances in the Biological Studies of Metal-Terpyridine Complexes: An Overview From 2012 to 2022. Coord. Chem. Rev. 2023, 496, 215380. [Google Scholar] [CrossRef]
- Lo, K.K.-W.; Louie, M.-W.; Zhang, K.Y. Design of luminescent iridium(III) and rhenium(I) polypyridine complexes as in vitro and in vivo ion, molecular and biological probes. Coord. Chem. Rev. 2010, 254, 2603–2622. [Google Scholar] [CrossRef]
- Lu, C.; Xu, W.; Shah, H.; Liu, B.; Xu, W.; Sun, L.; Qian, S.Y.; Sun, W. In Vitro Photodynamic Therapy of Mononuclear and Dinuclear Iridium(III) Bis(terpyridine) Complexes. Appl. Bio Mater. 2020, 3, 6865–6875. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W.; Chung, C.-K.; Nga, D.C.-M.; Zhu, N. Syntheses, characterisation and photophysical studies of novel biological labelling reagents derived from luminescent iridium(III) terpyridine complexes. New J. Chem. 2002, 26, 81–88. [Google Scholar]
- Maekawa, M.; Teradab, K.; Odab, S.; Sugimotoc, K.; Okubob, T.; Kuroda-Sowa, T. Syntheses and structural characterizations of mononuclear Ir(III) hydride complexes with 2,2′:6′,2″-terpyridine in the κ2N,N’ and κ3N,N’,N″ coordination modes. Inorg. Chim. Acta 2021, 514, 119962. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Yamabe, S.; Kanehisa, N.; Kai, Y.; Takashima, H.; Tsukahara, K. Detailed Description of the Metal-to-Ligand Charge-Transfer State in Monoterpyridine IrIII Complexes. Eur. J. Inorg. Chem. 2007, 1911–1919. [Google Scholar] [CrossRef]
- Choroba, K.; Penkala, M.; Palion-Gazda, J.; Malicka, E.; Machura, B. Pyrenyl-Substituted Imidazo [4,5-f][1,10]phenanthroline Rhenium(I) Complexes with Record-High Triplet Excited-State Lifetimes at Room Temperature: Steric Control of Photoinduced Processes in Bichromophoric Systems. Inorg. Chem. 2023, 62, 19256–19269. [Google Scholar] [CrossRef]
- Palion-Gazda, J.; Machura, B.; Szłapa-Kula, A.; Maroń, A.M.; Nycz, J.E.; Ledwon, P.; Schab-Balcerzak, E.; Siwy, M.; Grzelak, J.; Maćkowski, S. Effect of carbazole and pyrrolidine functionalization of phenanthroline ligand on ground- and excited-state properties of rhenium(I) complexes. Interplay between 3MLCT and 3IL/3ILCT. Dye. Pigment. 2022, 200, 110113. [Google Scholar] [CrossRef]
- Małecka, M.; Szlapa-Kula, A.; Maroń, A.M.; Ledwon, P.; Siwy, M.; Schab-Balcerzak, E.; Sulowska, K.; Maćkowski, S.; Erfurt, K.; Machura, B. Impact of the Anthryl Linking Mode on the Photophysics and Excited-State Dynamics of Re(I) Complexes [ReCl(CO)3(4′-An-terpy-κ2N)]. Inorg. Chem. 2022, 61, 15070–15084. [Google Scholar] [CrossRef]
- Maron, A.M.; Choroba, K.; Pedzinski, T.; Machura, B. Towards better understanding of photophysics of platinum(II) coordination compounds with anthracene and pyrene substituted 2,6-bis(thiazol-2-yl)pyridines. Dalton Trans. 2020, 49, 13440–13448. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Szlapa-Kula, A.; Malecki, J.G.; Raposo, L.; Roma-Rodrigues, C.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Square planar Au(III), Pt(II) and Cu(II) complexes with quinoline-substituted 2,2′:6′,2″-terpyridine ligands: From in vitro to in vivo biological properties. Eur. J. Med. Chem. 2021, 218, 113404. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Palion-Gazda, J.; Machura, B.; Bieńko, A.; Wojtala, D.; Bieńko, D.; Rajnák, C.; Boča, R.; Ozarowski, A.; Ozerov, M. Large Magnetic Anisotropy in Mono- and Binuclear cobalt(II) Complexes: The Role of the Distortion of the Coordination Sphere in Validity of the Spin-Hamiltonian Formalism. Inorg. Chem. 2024, 63, 1068–1082. [Google Scholar] [CrossRef] [PubMed]
- Choroba, K.; Machura, B.; Erfurt, K.; Casimiro, A.R.; Cordeiro, S.; Baptista, P.V.; Fernandes, A.R. Copper(II) Complexes with 2,2′:6′,2″-Terpyridine Derivatives Displaying Dimeric Dichloro-μ-Bridged Crystal Structure: Biological Activities from 2D and 3D Tumor Spheroids to In Vivo Models. J. Med. Chem. 2024, 67, 5813–5836. [Google Scholar] [CrossRef] [PubMed]
- Vogler, L.M.; Scott, B.; Brewer, K.J. Investigation of the Photochemical, Electrochemical, and Spectroelectrochemical Properties of an Iridium(III)/Ruthenium(II) Mixed-Metal Complex Bridged by 2,3,5,6-Tetrakis(2-pyridyl)pyrazine. Inorg. Chem. 1993, 32, 898–903. [Google Scholar] [CrossRef]
- Qin, Q.P.; Meng, T.; Tan, M.X.; Liu, Y.C.; Luo, X.J.; Zou, B.Q.; Liang, H. Synthesis and in vitro biological evaluation of three 40 -(4- methoxyphenyl)-2,2’:6’,2”-terpyridine iridium(III) complexes as new telomerase inhibitors. Eur. J. Med. Chem. 2018, 143, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, L.M.; Young, V.G., Jr.; Mann, K.R. Mono- and bis-tolylterpyridine iridium(III) complexes. Acta Cryst. Sect. C Cryst. Struct. Commun. 2010, 66, m62. [Google Scholar] [CrossRef]
- Dobroschke, M.; Geldmacher, Y.; Ott, I.; Harlos, M.; Kater, L.; Wagner, L.; Gust, R.; Sheldrick, W.S.; Prokop, A. Cytotoxic Rhodium(III) and Iridium(III) Polypyridyl Complexes: Structure–Activity Relationships, Antileukemic Activity, and Apoptosis Induction. ChemMedChem 2009, 4, 177–187. [Google Scholar] [CrossRef]
- Toda, T.; Saitoh, K.; Yoshinari, A.; Ikariya, T.; Kuwata, S. Synthesis and Structures of NCN Pincer-Type Ruthenium and Iridium Complexes Bearing Protic Pyrazole Arms. Organometallics 2017, 36, 1188–1195. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, Z.-L.; Ho, C.-L.; Yiu, S.-M.; Lee, C.-S.; Suramitr, S.; Hannongbua, S.; Chi, Y. Stepwise Access of Emissive Ir(III) Complexes Bearing a Multi Dentate Heteroaromatic Chelate: Fundamentals and Applications. Inorg. Chem. 2022, 61, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Davaasuren, B.; Padhy, H.; Rothenberger, A. Crystal structure of trichlorido(4’-ferrocenyl-2,2’:6’,2”-terpyridine-κ3N,N’,N”)iridium(III) acetonitrile disolvate. Acta Cryst. Sec. E Cryst. Commun. 2015, 71, m69. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edfinfton, P.R.; McCabe, P.; Pidcock, E.; Rodríguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Perth, WA, Australia, 2017. [Google Scholar]
- Mamo, A.; Stefio, I.; Parisi, M.F.; Credi, A.; Venturi, M.; Di Pietro, C.; Campagna, S. Luminescent and Redox-Active Iridium(III)-Cyclometalated Compounds with Terdentate Ligands. Inorg. Chem. 1997, 36, 5947–5950. [Google Scholar] [CrossRef] [PubMed]
- Klemens, T.; Świtlicka-Olszewska, A.; Machura, B.; Grucela, M.; Schab-Balcerzak, E.; Smolarek, K.; Mackowski, S.; Szlapa, A.; Kula, A.; Krompiec, S.; et al. Rhenium(I) terpyridine complexes– synthesis, photophysical properties and application in organic light emitting devices. Dalton Trans. 2016, 45, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Maroń, A.; Szlapa, A.; Klemens, T.; Kula, S.; Machura, B.; Krompiec, S.; Małecki, J.G.; Świtlicka-Olszewska, A.; Erfurt, K.; Chrobok, A. Tuning the photophysical properties of 4’-substituted terpyridines–an experimental and theoretical study. Org. Biomol. Chem. 2016, 14, 3793–3808. [Google Scholar] [CrossRef] [PubMed]
- Klemens, T.; Czerwińska, K.; Szlapa-Kula, A.; Kula, S.; Świtlicka, A.; Kotowicz, S.; Siwy, M.; Bednarczyk, K.; Krompiec, S.; Smolarek, K.; et al. Synthesis, spectroscopic, electrochemical and computational studies of rhenium(I) tricarbonyl complexes based on bidentate-coordinated 2,6-di(thiazol-2-yl)pyridine derivatives. Dalton Trans. 2017, 46, 9605–9620. [Google Scholar] [CrossRef] [PubMed]
- Grucela-Zajac, M.; Bijak, K.; Kula, S.; Filapek, M.; Wiacek, M.; Janeczek, H.; Skorka, L.; Gasiorowski, J.; Hingerl, K.; Sariciftci, N.S.; et al. (Photo)physical Properties of New Molecular Glasses End-Cappedwith Thiophene Rings Composed of Diimide and Imine Units. J. Phys. Chem. C 2014, 118, 13070–13086. [Google Scholar] [CrossRef]
- CrysAlis PRO; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2011.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.a.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Yamabe, S.; Kanehisa, N.; Inoue, T.; Takashima, H.; Tsukahara, K. Detailed Description of the Metal-to-Ligand Charge-Transfer State in Monoterpyridine IrIII Complexes. Eur. J. Inorg. Chem. 2009, 2067–2073. [Google Scholar] [CrossRef]
- Tessore, F.; Roberto, D.; Ugo, R.; Pizzotti, M. Terpyridine Zn(II), Ru(III), and Ir(III) Complexes: The Relevant Role of the Nature of the Metal Ion and of the Ancillary Ligands on the Second-Order Nonlinear Response of Terpyridines Carrying Electron Donor or Electron Acceptor Groups. Inorg. Chem. 2005, 44, 8967–8978. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).