Abstract
Two series of novel [1,2,4]triazolo[4,3-c]- and [1,2,4]triazolo[1,5-c]quinazoline fluorophores with 4′-amino[1,1′]-biphenyl residue at position 5 have been prepared via Pd-catalyzed cross-coupling Suzuki–Miyaura reactions. The treatment of 2-(4-bromophenyl)-4-hydrazinoquinazoline with orthoesters in solvent-free conditions or in absolute ethanol leads to the formation of [4,3-c]-annulated triazoloquinazolines, whereas [1,5-c] isomers are formed in acidic media as a result of Dimroth rearrangement. A 1D-NMR and 2D-NMR spectroscopy, as well as a single-crystal X-ray diffraction analysis, unambiguously confirmed the annelation type and determined the molecular structure of p-bromophenyl intermediates and target products. Photophysical properties of the target compounds were investigated in two solvents and in the solid state and compared with those of related 3-aryl-substituted [1,2,4]triazolo[4,3-c]quinazolines. The exclusion of the aryl fragment from the triazole ring has been revealed to improve fluorescence quantum yield in solution. Most of the synthesized structures show moderate to high quantum yields in solution. Additionally, the effect of solvent polarity on the absorption and emission spectra of fluorophores has been studied, and considerable fluorosolvatochromism has been stated. Moreover, electrochemical investigation and DFT calculations have been performed; their results are consistent with the experimental observation.
1. Introduction
[1,2,4]Triazoloquinazoline represents polyazaheterocycle, which consists of triazolo moiety fused to a quinazoline ring. Its derivatives are widely known as an important class of heterocyclic aromatic compounds for various pharmaceutical applications [1,2]. Moreover, triazoloquinazolines provide a promising molecular platform for materials sciences. Each of the fragments is of great interest owing to their electron withdrawing properties and use in the design of donor–acceptor small molecules displaying characteristics preferable for optical materials. The quinazoline component has been explored in the context of fundamental research [3,4,5], with some quinazoline derivatives revealed to have potential application in optoelectronics [6,7,8,9], detection of analytes [10,11], bioimaging [12], etc. [1,2,4]Triazole derivatives, in turn, are considered as blue phosphorescent, TADF emitters or host materials for OLED devices [13,14,15]. Other D-π-A-π-D structures with a triazole ring as an acceptor part show strong emission in solution and potential for optoelectronic purposes [16,17]. Due to high photoluminescence (PL) efficiency, as well as good affinity to analytes, triazole-based materials have great potential to be used as sensitive and selective fluorescence probes [18].
The advantages of fused triazoles, such as the extended π-conjugation system, rigid planar stricture, tuning of the molecular energy levels, and good thermal and morphological stabilities were taken into account when creating the [1,2,4]triazolo[4,3-a]pyridine and [1,2,4]triazolo[1,5-a]pyridine luminophores [19,20,21]. Moreover, [1,2,4]triazolopyridine derivatives are suitable scaffolds for the designing of highly twisted π-conjugation structures, maintaining high triplet energies. This strategy was realized in the synthesis of 9,9′-(2-([1,2,4]triazolo[1,5-a]pyridin-2-yl)-1,3-phenylene)bis(9H-carbazole) host materials [22]; devices based on this heterocycle demonstrate remarkable electroluminescence performance comparable to that for reported Ir-based PhOLEDs.
Notably, the data concerning photophysical properties of [1,2,4]triazoloquinazoline derivatives are scarce. Some related compounds have been shown to be effective luminescent components for light-emitting devices [23,24,25,26,27,28]. Recently the [1,2,4]triazolo[5,1-b]quinazoline probe toward Fe3+ ions has been reported [29]. However, to date, there are no systematic structure–property relationship studies.
In this context, design, synthesis, and investigation of photophysical properties of triazolo-annulated quinazolines are highly important and interesting for field of both fundamental and applied chemistry. Previously, we have reported synthesis and photophysical properties of 3-aryl-substituted 5-(4′-amino[1,1′]-biphenyl)[1,2,4]triazolo[4,3-c]quinazolines A, Figure 1 [30]. Some of the obtained compounds were shown to exhibit strong fluorescence, both in solution and in solid state, as well as emission solvatochromism and sensory ability toward water and acid. It was revealed that the annulation of [1,2,4]triazole ring to the quinazoline core had a considerable impact on emission behavior and solvatochromic properties compared to 4-morpholinylquinazolines, 4-cyanoquinazolines, or quinazolin-4-one counterparts B, Figure 1 [31,32].
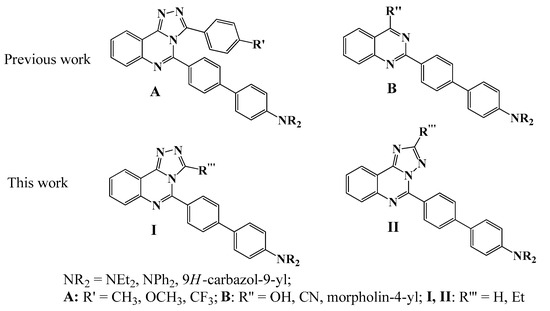
Figure 1.
Previously reported 3,5-diaryl[1,2,4]triazolo[4,3-c]quinazolines (A), 5-(4′-amino[1,1′]-biphenyl)quinazolines (B), and target triazoloquinazolines (I,II).
Herein, we aim to modify the triazole fragment and design of 5-(4′-amino[1,1′]-biphenyl)[1,2,4]triazolo[4,3-c]quinazolines I unsubstituted at position 3 and their 3-ethyl analogues. We suppose that excluding the aryl fragment from the triazole ring might have considerable influence on photophysical properties. Moreover, we are interested in whether the isomeric arrangement of the triazoloquinazoline ring will have a significant impact on photophysical characteristics; for this purpose, we developed [1,2,4]triazolo[1,5-c]quinazoline derivatives II. We used 2-(4-Bromophenyl)- 4-hydrazinoquinazoline and orthoester as starting materials for the construction of polycyclic [1,2,4]triazolo[4,3-c]quinazoline core of compounds I. Their [1,5-c] isomers were obtained in acidic media as a result of Dimroth rearrangement of [1,2,4]triazolo[4,3-c]quinazolines. The cross-coupling of bromophenyl derivatives with boronic acids under the typical conditions was applied for the synthesis of target fluorophores. Photophysical and electrochemical properties for compounds I and II were carefully studied experimentally and theoretically using DFT calculations. Additionally, characteristics of target fluorophores I and II and their 3-aryltriazolo[4,3-c]quinazoline counterparts A were compared.
2. Results
2.1. Synthesis
The synthetic approach (Scheme 1) is based on the use of previously described 2-(4-bromophenyl)-4-hydrazino-quinazoline 1 [33] as starting material. [1,2,4]Triazolo[4,3-c]quinazolines 2a,b were prepared by solvent-free cyclocondensation of 1 with triethyl orthoformate or triethyl orthopropionate under reflux for 4 h in good yields of 83 and 84%, respectively. A similar procedure was described previously for related compounds [34]. It was shown that the refluxing of staring hydrazine 1 with orthoesters in anhydrous ethanol gives triazolo[4,3-c]quinazolines 2a,b, with comparable yields. However, using 95% ethanol as a solvent resulted in the mixture of isomers 2 and 3 and the ring-opening product 3b′. The compounds 2a,b were successfully converted into triazolo[1,5-c]quinazoline isomers 3a,b by refluxing in glacial acetic acid for 4 h. The reaction progress can be easily monitored by TLC analysis. The Rf values of isomers are significantly different (for example, 0.16 for 2a and 0.71 for 3a in a 1/1 mixture of hexane/EtOAc).
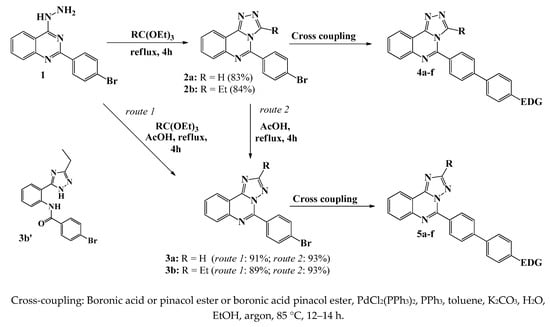
Scheme 1.
Synthesis of 5-(4′-amino[1,1′]-biphenyl)[1,2,4]triazolo[4,3-c]quinazolines 4a–f and 5-(4′-amino[1,1′]-biphenyl)[1,2,4]triazolo[1,5-c]quinazolines 5a–f.
The transformation, probably, is based on an H+-catalyzed Dimroth rearrangement (Scheme S1) proposed for a similar [1,2,4]triazolopyrimidine heterocycle; the mechanism of this process generally involves the addition of an electrophile, a ring opening, and a ring closure [35]. Each isomer 2a and 3a or 2b and 3b was distinguished by their 1H NMR spectra (Figures S1–S4). For example, the most prominent peak in the spectrum of compound 2a was observed at 9.32 ppm as a singlet attributed to the triazole proton, while a similar singlet in the spectrum of isomer 3a was observed upfield at 8.63 ppm, Figure 2a. In the case of the ethyl-substituted derivatives 2b and 3b, there is considerable difference in the position of signals attributed to ethyl group, Figure 2b. Moreover, the signals of phenylene protons (H-2 and H-6) of [1,5-c] isomers 3a,b shifted downfield compared to [4,3-c] ones 2a,b, which indicates an increase in the electron-withdrawing effect of the annulated triazole cycle. The NMR correlations are in good agreement with the literature data for triazolo-annelated azacycles [36,37]. Additionally, we performed a nuclear Overhauser effect spectroscopy (NOESY) and heteronuclear multiple bond correlation experiment (HMBC) for compounds 2a and 3b (Figures S1 and S3). In the 1H-1H NOESY spectra of compound 2a we observed a cross-peak between H-3 and H-2′ proton signals, whereas correlations with triazole proton in spectrum of compound 3a did not appear. 1H-13C HMBC spectrum of 2a contains a cross-peak of the C(5) atom with an H-3 proton of the triazole cycle; in the case of its isomer 3a, the corresponding cross-peak is absent. These findings are consistent with proposed structures and confirm spatial arrangement of molecules. Notably, the melting point of [4,3-c] isomers 2a,b is higher than that of [1,5-c] counterparts 3a,b (for example, 250–252 °C for 2a and 186–188° for 3a, respectively).
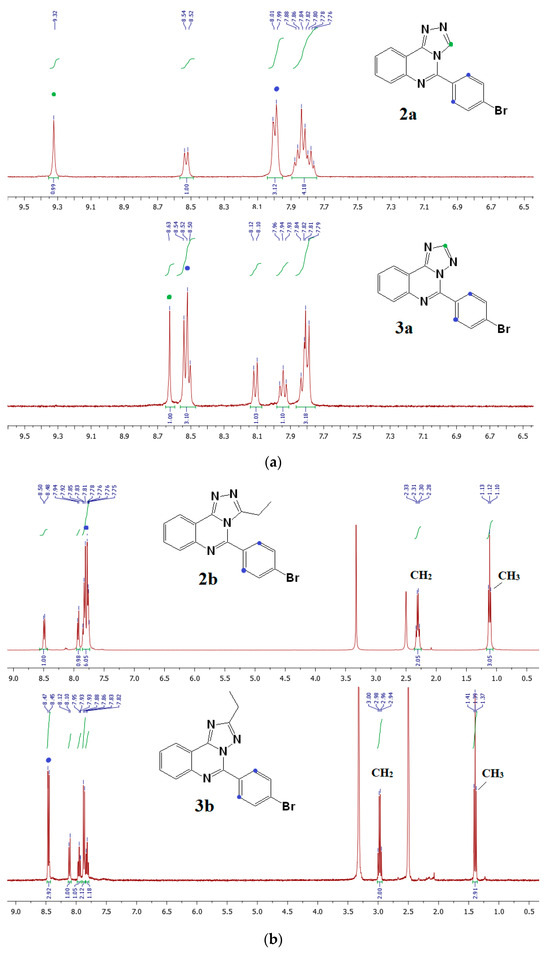
Figure 2.
(a) 1H NMR spectra of 2a and 3a in DMSO-d6. (b) 1H NMR spectra of 2b and 3b in DMSO-d6. Hydrogen chemical shifts (δ) in ppm.
Furthermore, it was established that both compounds 3a and 3b can be obtained directly from 2-(4-bromophenyl)-4-hydrazinoquinazoline 1 by treatment with orthoester in acidic media; the refluxing of the reaction mixture for 16 h generated the desired products in 91% and 89% yields, respectively, after recrystallization from DMSO. The reaction progress was monitored by NMR spectroscopy after 4, 8, and 16 h, Figure S5. Each time after cooling the reaction mixture to room temperature, water was added and the precipitate that formed was filtered off, dried, and analyzed. The spectroscopic data shows that no signals of the starting compound that has been observed at 4 h in both cases, whereas signals of both isomers, as well as ring-opening product 3b′, appear in the first case (Figure S5a). Probably, the formation of triazolo[1,5-c]quinazoline proceeds through [4,3-c] isomers with subsequent rearrangement. After 16 h the ring-opening products were fully converted into corresponding triazolo[1,5-c]quinazolines. It is worth noting, we succeeded in isolating the ring-opening product 3b′ (Scheme 1), which seems to be the intermediate during 5-(4-bromophenyl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline formation, by column chromatography. The amide 3b′ probably formed as a result of the hydrolysis; the structure of proposed compound 3b′ is consistent with the 1H NMR spectroscopy and the mass spectrometry data (Figure S6).
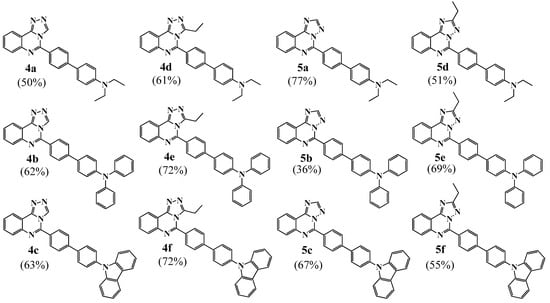
Both isomers participate in cross-coupling reactions under typical conditions described elsewhere [31,38,39] and form products 4a–f or 5a–f in moderate to good yields (from 36 to 77% after purification by column chromatography on silica gel or recrystallisation from DMSO). Their structure was confirmed by spectroscopic and analytical data. Notably, the NMR spectra of products 4 and 5 are significantly different depending on annelation type, similar to their parent bromo derivatives 2 and 3, (Figures S1–S4). To obtain an unambiguous structural assignment of each isomer, we grew single crystals of 4a and 4e, as well as 5d and 5e, for X-ray diffraction analysis (Figure 3, Tables S1–S8). Single crystals were obtained by the slow evaporation technique from an n-hexane/CH2Cl2 mixture for 4a, 4e, and 5d or an n-hexane/EtOAc mixture for 5e.
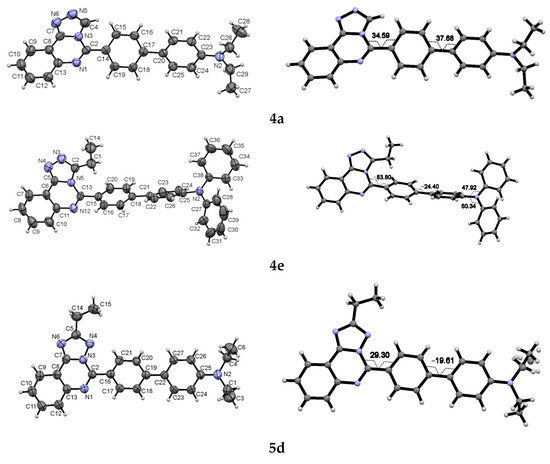
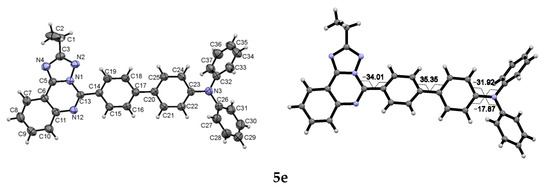
Figure 3.
Molecular structure and selected torsion angles of the compounds 4a, 4e, 5d, and 5e in the thermal ellipsoids of 50% probability.
According to XRD data, the compounds are crystallized in the centrosymmetric space groups of the monoclinic or triclinic systems. The general geometry, bond distances, and angles of the compound are near to expectations. In particular, the nitrogen atom of the diethylaminophenyl or diphenylaminophenyl substituents has a planar configuration with neighboring carbon atoms. The triphenylamino group of compounds 4e and 5e is twisted and has a propeller-like shape. The compounds 4a and 5e demonstrate the disordering of the ethyl groups. All the compounds are characterized by twisted conformation of the biphenylene moiety around a heteroaromatic core with the highest torsion angle in 4e N(5)C(13)C(15)C(20) = 83.8°. For other studied compounds, the torsion angle between the heterocycle and phenylene substituents is significantly lesser due to the effect of the π-π conjugation. In the crystals the shortened intermolecular π-π contacts are observed. For compound 5d, the contact C(9)…C(20) [x − 1, y, z] 3.253 Å between π-accepted heterocyclic and π-donated biphenylene moieties was noticed. The compound 5e forms the π-interacted centrosymmetric dimers with distance C(14)…C(16) [1 − x, 1 − y, 1 − z] 3.329 Å. For compound 4e, π-π interaction is observed between heterocyclic moieties [2 − x, −y, 1 − z] with a distance of 3.24 Å between the least-squared planes. For compound 4a most principal intermolecular contacts are weak H-bonds C(4)H…N(5) [1 − x, −y, 1 − z] contributing to the formation of H-bonded dimers. However, the π-π interactions for this compound are insignificant.
2.2. UV/Vis and Fluorescence Spectroscopy
The UV/Vis absorption and photoluminescence (PL) spectroscopic data of [1,2,4]triazolo[4,3-c]quinazolines 4a–f and [1,2,4]triazolo[1,5-c]quinazolines 5a–f were studied for toluene and MeCN solutions at c ~10−5 M and presented in Table 1; the corresponding spectra are shown in Figure S19.

Table 1.
Photophysical properties of [1,2,4]triazoloquinazolines 4a–f and 5a–f (~10−5 M) in toluene and MeCN solutions and solid state (powder) under normal conditions.
Normalized absorption spectra of compounds 4a–f and 5a–f in MeCN are combined in Figure 4. As can be seen, the lowest energy absorption maxima are affected by the nature of the aminoaryl fragment. 9H-Carbazol-9-yl-containing triazoloquinazolines 4c, 4f, 5c, and 5f are characterized by similar absorption features and display maxima in the range of 312–328 nm, whereas the absorption band of their Et2N or Ph2N counterparts is red-shifted, and the maxima are located in the range of 340–375 nm. On the other hand, the presence of the Et group at the triazole ring of [1,2,4]triazolo[4,3-c]quinazolines results in hypsochromically-shifted absorption (compounds 4d, 4e, and 4f in contrast to 4a, 4b and 4c) that can be associated with considerable twisting of biphenyl moiety influenced by the ethyl group. In the case of [1,2,4]triazolo[1,5-c]quinazolines 5a–f the presence of ethyl substituent at triazole skeleton has little effect on the absorption wavelength (pairs 5d–5a, 5e–5b, and 5f–5c in Table 1 and Figure 4b). The [1,5-c] annelation type, in general, leads to a shift of the absorption maxima in the red region compared to the [4,3-c] one.
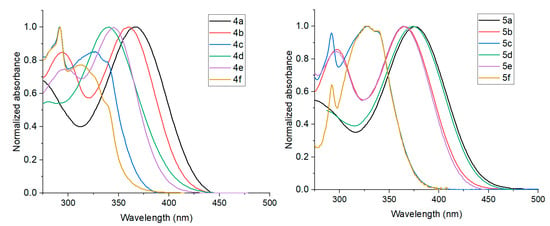
Figure 4.
Normalized absorption spectra of compounds 4a–f (left) and 5a–f (right) in MeCN.
Compared to the MeCN solution, the absorption band in toluene is slightly shifted to the red region, Table 1, but, in general, the influence of solvent polarity on the absorption band is minor.
All the compounds of 4 and 5 are emissive in both solvents with different fluorescence intensity and quantum yield. The emission maxima of Et2N- and Ph2N-substituted triazoloquinazolines are in the range of 465–486 nm in toluene, whereas carbazolyl-containing triazoloquinazolines emit in the blue–purple region with maxima at 420–441 nm. The influence of the arrangement of the triazole core, as well as the presence of the Et-group, is negligible, Figure S20. However, all fluorophores are found to be sensitive in response to the polarity of the solvent. When going from toluene to MeCN, the emission band shifts to the red region and the maxima appear at 530–548 nm in the case of carbazolyl-derivatives 4c, 4f, 5c, and 5f, and in the range of 593–609 nm for other counterparts, 4a,b,d,e and 5a,b,d,e. According to the obtained quantum yields in the two solvents, compounds can be divided into several groups, namely: compounds 5d and 5f with ΦF ≥ 90% in both solvents; compounds 4a, 4b, 5a, 5b, and 5e with ΦF ≥ 90% in toluene (non-polar media); compounds 4c, 4d, 4e, and 5c with moderate ΦF (11–75%, depending on solvent); and compound 4f with low emission, less 3%. Moreover, the compounds 4a, 4b, and 5a–f demonstrate a decrease in quantum yield when going from non-polar toluene to polar MeCN whereas the compounds 4c, 4d, 4e, and 4f show enhancement emission in polar media compared to a non-polar one.
For detailed investigation of photophysical properties we measured the fluorescence lifetime (τ) of chromophores 4a–d and 5a–f in toluene (Figure S21), and also calculated radiative decay rate constant (kr) and non-radiative decay rate constant (knr) (Table 2). Emission spectra for fluorophores 4a,b,d and 5a–f fit the single exponential function, whereas decay trace is bi-exponential in the case of compounds 4c and 4e (Table S9); this can probably be attributed to the solvent effect or existence of several emitting states [41,42,43]; lifetimes are on a nanosecond timescale. In each series of compounds 4a–c, 5a–c, and 5d–f, diphenylamino-derivatives 4b, 5b, and 5e are characterized by the highest values of singlet excited-state lifetimes in the range of 1.66–1.85 ns. The values obtained for triazoloquinazolines 4a–d and 5a–f are similar to the lifetimes reported for 4-morpholin-4-yl- and 4-oxoquinazoline systems [31,32]. According to the calculations (Table 2) the relevant radiative decay constants (kr) of 4a, 4b, and 5a–f are similar and ranges from 52.02 × 107 s−1 to 61.25 × 107 s−1. In general, energy dissipation in compounds 4a,b and 5a–f mainly occurred through radiative channels due to the high π-conjugation length of molecules (kr > knr), while knr exceeds kr for derivatives 4c,d,e, probably due to considerable twisting of the structure.

Table 2.
Fluorescence lifetime (τ), radiative decay rate constant (kr) and non-radiative decay rate constant (knr) of chromophores 4a–e and 5a–f in toluene at r. t.
We compared the photophysical properties of unsubstituted [1,2,4]triazolo[4,3-c]quinazoline fluorophores 4a–c with those of 3-aryl[1,2,4]triazolo[4,3-c]quinazolines A (Scheme 1) reported previously [30]. The region of absorption and the emission band for unsubstituted-at-C(3) position compounds 4a–c is rather similar to their aryl-substituted counterparts A; the identical correlation in the influence of aryl fragment nature on photophysical properties is observed. However, removal of the aryl fragment, in general, leads to an increase in the quantum yield in solutions, probably due to the reduction in non-radiative energy losses.
The solid-state luminescent properties of compounds 4a–f and 5a–f were also investigated at room temperature. Triazoloquinazolines show luminescence in the yellow, green, cyan, and blue regions, Figure 5, under irradiation by a hand-held UV lamp.

Figure 5.
Photographs of 4a–f and 5a–f taken in the dark upon irradiation with a hand-held UV lamp (λem = 366 nm).
The measured spectra correlated with visual results, Table 1. The introduction of ethyl group into triazole ring of [1,2,4]triazolo[4,3-c]quinazolines 4a–f causes a hypsochromic shift by ~50 nm (for example, compound 4a regarding 4d), whereas the emission of 5a–f is not influenced by ethyl substituent. The proximity of the ethyl group to biphenyl moiety, probably, results in twisted conformation of molecule and reduced conjugation length. Some of compounds are characterized by good quantum yields of up to 42%; the values are comparable to 2-(amino[1,1′-biphenyl]-4-yl)-4-(morpholin-4-yl)quinazolines and their 4-oxo counterparts [31,32].
2.3. Effects of Solvent Polarity for Compounds 4 and 5
As long as synthesized triazoloquinazolines 4 and 5 represent push–pull systems with electron-withdrawing triazoloquinazoline core and electron-donating arylamino moiety, separated by a π-system, they are promising fluorosolvatochromic candidates. We studied the absorption and emission properties for some new compounds 4a, 4d, 5a, 5d, 5e, and 5f in the solvents of different polarity (Figure 6 and Figure S22, and Tables S10–S15). The shape and energy of the absorption bands were revealed to be weakly dependent on the solvent polarity, whereas the fluorescence spectra show a strong dependence on the solvent polarity and a remarkable positive solvatochromism (142–193 nm) when going from non-polar cyclohexane to polar MeCN or MeOH. The photograph (Figure 6c) of fluorophore 4a solutions, as an example, taken under a UV light, exhibited a wide range of colors, from deep blue to orange. The results indicate a low molecular dipole moment in the ground state and the large dipole moment in the excited state. The fluorosolvatochromism suggests a potential intramolecular charge transfer between the donor and acceptor units upon photoexitation. Notably, all compounds show a structured emission in non-polar cyclohexane, and a broad and structureless emission in other solvents of moderate and high polarity suggesting ICT states [44].
Figure 6.
Absorption (a) and emission (b) spectra of compounds 4a in different solvents: cyclohexane, toluene, THF, DCM, DMSO, MeCN and MeOH; (c) photograph of solutions of 4a taken in the dark upon irradiation with a hand-held UV lamp (λem = 366 nm).
To further analyze solvatochromic properties the Lippert–Mataga equation [44,45,46] was employed in which the Stokes shift (Δν) was plotted as a function of the orientation polarizability (Δf) of the solvents, Figure S23. The clear linear trend (R2 > 0.92, Table 3) indicates the increase in dipole moment in the excited state compared to the ground state and supports the ICT nature of the excited state. A higher slope for 4d, 5e, and 5f than for other fluorophores suggests that they exhibit a more pronounced charge transfer process.

Table 3.
Data from Lippert–Mataga plot and DFT calculations for quinazolines 4a, 4d, 5a and 5d–f.
Onsager radii of the molecules, calculated from the Van der Waals volume [47,48] or by the DFT method, were employed to determine the change in dipole moments Δµ1 and Δµ2, respectively, Table 3. The obtained values of the difference Δµ1 between the dipole moments of the ground and excited states were calculated to be in the range of 11.05–14.14 D or 33.08–42.81 D. We also calculated Δµ using DFT theory and obtained results ranging from 15.59 to 30.69 D. The underestimation of Δµ1 could be attributed to the assuming a spherical model for molecule. Summary of all data suggests remarkable polar structure in excited state. [1,2,4]Triazolo[4,3-c]quinazolines 5e and 5f exhibit the highest Δµ value in the considered series.
2.4. Electrochemical Studies of [1,2,4]Triazoloquinazolines
The electrochemical behavior of the compounds 4a–f and 5a–f was studied using cyclic voltammetry in CH2Cl2 (Figures S24 and S25, Table 4). As can be seen from Figure S24, compounds 4a–f and 5a–f are characterized by quasi-reversible peaks of oxidation, while in the range of the electrochemical stability window of the supporting electrolyte peaks of reduction was not observed. In general, the electrochemical behavior of compounds in the anodic region remains almost unchanged when going from [4,3-c] to [1,5-c] isomers or from H-substituted to ethyl-substituted derivatives and is determined exclusively by the donor fragment. Based on the obtained oxidation onset potentials, we calculated the HOMO energy for the presented compounds (Table 4). The energies of the HOMO, determined by electrochemistry, match very well with those calculated by DFT.

Table 4.
Electrochemical properties of [1,2,4]triazoloquinazolines 4a–f, 5a–f in CH2Cl2 solutions and energy of FMOs calculated by DFT.
2.5. Quantum-Chemical Calculations
The distribution plots of the HOMOs and LUMOs, as well as energy levels and energy gaps in the gas phase are presented in Table S16 and Table 4. For all the compounds, the HOMO electrons are mainly distributed on the electron-donating aminoaryl unit and phenylene moiety; however, the participation of phenylene spacer is less in carbazol-9-yl-derivatives 4c,f and 5c,f than in its Et2N- (4a,d, 5a,d) and Ph2N-containing (4b,e, 5b,e) counterparts that confirm shorten π-conjugation of the former molecules, due to the twisting of the rigid carbazol-9-yl fragment, and corresponds with the experimental data. The LUMOs plots are similar for the compounds 4a–f and 5a–f; electrons are located in the [1,2,4]triazoloquinazoline framework and the biphenylene part, with partial involvement of the nitrogen atom of donor group in the case of Et2N- and Ph2N-sabstituted triazoloquinazolines. The value of the energy gap is slightly lower in [1,2,4]triazolo[4,3-c]quinazolines 5a–f (Eg = 3.47–3.78 eV) than in [4,3-c] isomers 4a–f (Eg = 3.58–3.95 eV).
Supplementary Table S17 shows the optimized geometries calculated for the electronic ground state (S0) of all molecules in gas phase. The selected dihedral angles α1–α4, which account for the internal twisting of molecule fragments, are collected in Table S18. The angles α1 present value of 6–7° in triazoloquinazolines 4a–c and value of 10–14° for their Et-substituted counterparts 4d–f, whereas the same angles are close to 1–2° in the case of [1,5-c] isomers 5a–f. Moreover, [1,2,4]triazolo[4,3-c]quinazolines 4a–f are characterized by highly twisted phenylene residues (angles α2 more than 36°) relative to heterocycle core, while α2 is around 20° for compounds 5a–f. This difference is most probably the result of the steric hindrance introduced by a hydrogen atom or the Et group of [4,3-c]-arranged structure. The angle α4 define the deviation from planarity of aminoaryl donor part, the value, predictable, increases when going from Et2N- to Ph2N- and to carbazol-9-yl derivatives in each of the sets of fluorophores that ascribe to the steric hindrance caused by the phenyl groups of Ph2N moiety or the rigid planar structure of the carbazole unit. Overall, triazolo[4,3-c]quinazolines are more twisted than their [1,5-c] isomers and tend to absorb at higher energetic wavelength displaying a hipsochromically shifted absorption band, that is consistent with the experimental results.
After geometry optimizations, the electronic transition properties (excitation energy (eV), absorption wavelength (nm), oscillator strength (fosc), nature of the transition, and major contributions of molecular orbitals) were calculated, Table S19. The predicted UV/Vis absorption spectra are presented in Figure S26. According to calculations, the lowest excited singlet state (S1) for compounds 4, 5 origins from HOMO/LUMO transitions with contributions >93%, with the energy of Franck–Condon states in the range of 3.13−3.49 eV for compounds of series 4 and 3.09−3.26 eV for compounds of series 5. The HOMO–LUMO transitions demonstrate a pronounced charge transfer from electron-donating aminoaryl part to [1,2,4]triazoloquinazoline fragment, which is responsible for the underestimation of the energy of the S1 transition calculated using TD-DFT by up to 0.3 eV in comparison with the experimental one. In order to further characterize the electronic transitions in the compounds under study, hole–electron analysis for S0–S1 transitions was carried out (the results are presented in the Table 5) [50].

Table 5.
Hole–electron interaction analysis result for compounds 4, 5.
Calculated parameters indicate that the transitions exhibit notable overlap in the spatial distributions of electrons and holes (Sr~0.4–0.5), but also significant delocalization, as indicated by high D-indices (more than one bond length) and positive t-indices, meaning that there is a substantial separation of the hole and electron distributions. Based on the results obtained, we can conclude that the S0-S1 transitions in these compounds have a pronounced charge transfer character, most pronounced for compounds 4c, 4f, 5c, and 5f.
To gain insights into the fluorescence properties of the compounds 4a–f and 5a–f, the optimized geometries for the electronic excited state (S1) were calculated in toluene and MeCN, Table S18, Figure 7. As can be seen from Table S19, the deviation of biphenyl residue from plane of triazolo[4,3-c]quinazoline core (α1) in compounds 4a–f increases more than twice in the excited state compared to the ground state. However, biphenyl moiety tends to shorten angles α2 and α3. Therefore, triazolo[4,3-c]quinazolines 4a–f, characterized by the simultaneous planarization of a biphenyl fragment and the twisting of a polycycle fragment, formed a pincer-like arrangement of the molecule. Contrary, all angles, α1–α3, in triazolo[1,5-c]quinazolines 5a–f decrease in excited state, forming highly planar 5-biphenyltriazolo[1,5-c]quinazoline fragments.
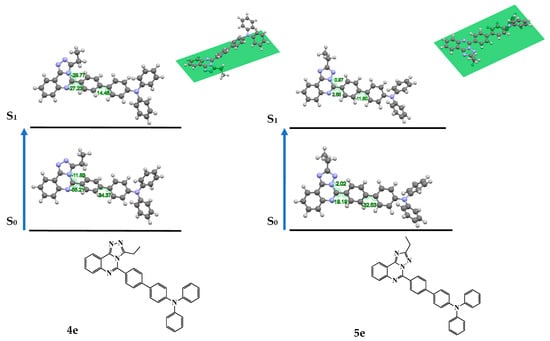
Figure 7.
Changes of selected dihedral angles of 4e and 5e in MeCN upon photoexcitation.
In addition, bond lengths L1 and L2 in 4a–f and 5a–f in the excited states were shortened (Table S18), indicating conjugation enhancement of structures in excited states conducting a probable ICT process.
3. Experimental Methods
3.1. General Information
Unless otherwise indicated, all common reagents and solvents were used from commercial suppliers without further purification. Reagent 1 was dried by azeotropic distillation using toluene. Melting points were determined on Boetius-combined heating stages. 1H NMR and 13C NMR spectra were recorded at room temperature, on a Bruker DRX-400 or Bruker DRX-600 spectrometer (Billerica, MA, USA). Hydrogen chemical shifts (δ in ppm) were referenced to the hydrogen resonance of the corresponding solvent (DMSO-d6, δ = 2.50 ppm or CDCl3, δ = 7.26 ppm). Carbon chemical shifts (δ in ppm) were referenced to the carbon resonances of the solvent (DMSO-d6, δ = 39.5 ppm CDCl3, δ = 77.2 ppm). Peaks are labeled as singlet (s), doublet (d), triplet (t), quartet (q), or multiplet (m). Mass spectra were recorded on the Shimadzu GCMS-QP2010 Ultra instrument (Kyoto, Japan) with electron ionization (EI) of the sample. The elemental analysis was carried out with the use of a Perkin Elmer 2400 Series II C,H,N-analyzer (Waltham, MA, USA).
3.2. Photophysical Characterization
UV/vis absorption spectra were recorded on the Shimadzu UV-1800 Spectrophotometer using quartz cells with 1 cm path length at room temperature. Emission spectra were measured on the Horiba FluoroMax-4 (Kyoto, Japan) at room temperature using quartz cells with 1 cm path length. Fluorescence quantum yield of the target compounds in solution and solid state were measured by using the Integrating Sphere Quanta-φ of the Horiba-Fluoromax-4 [41]. Time-resolved fluorescence measurements were carried out using time-correlated single-photon counting (TCSPC) with a nanosecond LED (λ = 370 nm).
3.3. Electrochemical Studies
Cyclic voltammetry was carried out on a Metrohm Autolab PGSTAT302N potentiostat (Herisau, Switzerland) with a standard three-electrode configuration. Typically, a three-electrode cell equipped with a platinum disk working electrode (3 mm), a glass carbon disk counter electrode (3 mm), and a Ag/AgNO3 (0.01 M) pseudo-reference electrode was used. Measurements were made in dry CH2Cl2 with tetrabutylammonium hexafluorophosphate (0.1 M) as the supporting electrolyte under an argon atmosphere at a scan rate of 100 mV/s. The potential of reference electrode was calibrated by using the ferrocene/ferrocenium redox couple (Fc+/Fc).
3.4. Quantum-Chemical Calculations
Conformational search was carried out before DFT calculations using the AQME program [51]. DFT calculations were performed using the Orca 5.0.3 program. The ground-state geometry optimizations were performed at the PBE0-D3BJ/def2-TZVP level of theory in the gas phase. Frequency analyses were carried out at the same theoretical level to ensure that the optimized geometries correspond to a local minimum on the potential energy surface; all compounds were characterized by only real vibrational frequencies. The absorption spectra and optimal geometry of S1-state were calculated by TDDFT at the same theoretical level. The Chemcraft program was used for the visualization [Chemcraft—graphical software for visualization of quantum chemistry computations, Version 3.8, https://www.chemcraftprog.com, accessed on 5 March 2024.
3.5. Crystallography
The single crystal of compound 4a (yellow block of 0.41 × 0.29 × 0.17), 4e (yellow irregular crystal of 0.44 × 0.26 × 0.15), 5d (yellow block of 0.48 × 0.39 × 0.27) and 5e (light yellow block of 0.46 × 0.35 × 0.28) was used for X-ray analysis. Structural studies of the compounds were performed using equipment available in the Collaborative Access Centre “Spectroscopy and Analysis of the Organic compound” at the Postovsky Institute of the Organic Synthesis, Ural Branch, Russian Academy of Sciences. The X-ray diffraction analysis was performed at room temperature on the Xcalibur 3 diffractometer (Oxford Diffraction, Abingdon, UK). Using Olex2 [52], the structure was solved with the ShelXT structure solution program using intrinsic phasing and refined with the ShelXL [53] refinement package using full-matrix least squares minimization. All non-hydrogen atoms were refined in an anisotropic approximation; the H atoms were placed in calculated positions and refined isotropically in the “rider” model.
Crystal data for 4a C25H23N5 includes the following: M = 393.48, monoclinic, a = 7.4970(4) Å, b = 12.6068(6) Å, c = 22.0871(14) Å, α = 90°, β = 96.507(5)°, γ = 90°, V = 2074.07(19) Å3, space group P21/n, Z = 4, and μ(Mo Kα) = 0.077 mm−1. On the angles 2.46 < 2Θ < 30.91°, 9749 reflections were measured and 2557 unique (Rint = 0.0739), which were used in all calculations. Goodness to fit was recorded at F2 0.996; the final R1 = 0.1548, wR2 = 0.1555 (all data) and R1 = 0.0601, wR2 = 0.1139 (I > 2σ(I)). The largest diff. peak and hole was 0.135 and –0.187 ēÅ−3.
Crystal data for 4e C35H27N5 includes the following: M = 517.61, monoclinic, a = 11.2955(12) Å, b = 13.9226(11) Å, c = 17.6642(17) Å, α = 90°, β = 103.110(10)°, γ = 90°, V = 2705.5(5) Å3, space group P21/n, Z = 4, and μ(Mo Kα) = 0.076 mm−1. On the angles 3.522 < 2Θ < 26.367°, 19,711 reflections measured, 2529 unique (Rint = 0.0847), which were used in all calculations. Goodness to fit was recorded at F2 0.985; the final R1 = 0.1503, wR2 = 0.2332 (all data) and R1 = 0.0672, wR2 = 0.1692 (I > 2σ(I)). The largest diff. peak and hole was 0.297 and –0.159 ēÅ−3.
Crystal data for 5d C27H27N5 includes the following: M = 421.53, triclinic, a = 8.7546(7) Å, b = 11.8118(11) Å, c = 12.9418(10) Å, α = 111.946(7)°, β = 100.965(7)°, γ = 106.434(7)°, V = 1122.79(17) Å3, space group P −1, Z = 2, and μ(Mo Kα) = 0.076 mm−1. On the angles 3.597 < 2Θ < 31.000°, 11,319 reflections measured, 3163 unique (Rint = 0.0487) which were used in all calculations. Goodness to fit was recorded at F2 1.022; the final R1 = 0.1244, wR2 = 0.2334 (all data) and R1 = 0.0672, wR2 = 0.1711 (I > 2σ(I)). The largest diff. peak and hole was 0.292 and –0.204 ēÅ−3.
Crystal data for 5e C35H27N5 includes the following: M = 517.61, monoclinic, a = 9.4980(7) Å, b = 29.6579(18) Å, c = 10.0462(10) Å, α = 90°, β = 107.435(7)°, γ = 90°, V = 2699.9(3) Å3, space group P21/n, Z = 4, and μ(Mo Kα) = 0.077 mm−1. On the angles 3.550 < 2Θ < 29.570°, 19,422 reflections measured, 3419 unique (Rint = 0.1029) which were used in all calculations. Goodness to fit was recorded at F2 0.957; the final R1 = 0.1511, wR2 = 0.2311 (all data) and R1 = 0.0780, wR2 = 0.1758 (I > 2σ(I)). The largest diff. peak and hole was 0.236 and –0.286 ēÅ−3.
The results of X-ray diffraction analysis for compounds 4a, 4e, 5d, and 5e were deposited in the Cambridge Crystallographic Data Centre (CCDC 2,336,242 for 4a, CCDC 2,336,250 for 4e, CCDC 2,336,251 for 5d, and CCDC 2,336,243 for 5e). The data are free and can be available at www.ccdc.cam.ac.uk.
3.6. Synthesis of Compounds 2a,b, 3a,b, 4a–f and 5a–f
3.6.1. General Procedure for the Synthesis of [1,2,4]Triazolo[4,3-c]quinazolines (2a,b)
Method 1. In a round-bottom flask equipped with a magnetic stirred bar, 2-(4-bromophenyl)-4-hydrazinoquinazoline 1 (0.23 g, 0.72 mmol) in absolute ethanol (17 mL) and corresponding ortho ester (4.30 mmol) were added. The mixture was refluxed for 4 h. Condenser was equipped with a calcium chloride drying tube. After cooling down and partial evaporation the solid was filtered off, washed with water, dried and used in the next step without further purification. A pure sample for analysis was obtained by crystallization from DMSO.
Method 2. In a round-bottom flask equipped with a magnetic stirred bar, dried 2-(4-bromophenyl)-4-hydrazinoquinazoline 1 (0.28 g, 0.89 mmol) and corresponding ortho ester (7.20 mmol) were added. The mixture was refluxed for 4 h. A condenser was equipped with a calcium chloride drying tube. After cooling down the solid was filtered off, washed with water and dried.
- 5-(4-Bromophenyl)-[1,2,4]triazolo[4,3-c]quinazoline (2a). The general procedure was applied using 1 and triethyl orthoformate: colorless powder, yield 83% (method 1), yield 86% (method 2); mp 250–252 °C; 1H NMR (DMSO-d6, 400 MHz) δ 7.78–7.86 (4H, m), 7.99–8.01 (3H, m), 8.52–8.54 (1H, m, H-7 or H-10), 9.32 (1H, s, H-3); 13C {1H} NMR (DMSO-d6, 100 MHz, 40 °C) δ 115.5, 122.7, 125.1, 128.2, 129.1, 130.9, 131.4, 131.8, 131.9, 137.0, 140.8, 144.2, 147.4; EIMS m/z 326 [M + 2]+ (96), 325 [M + 1]+ (54), 324 [M]+ (100), 298 [M − N2 + 2]+ (16), 217 [M − N2 − Br]+ (37); C15H9BrN4 (324.00).
- 5-(4-Bromophenyl)-3-ethyl-[1,2,4]triazolo[4,3-c]quinazoline (2b). The general procedure was applied using 1 and triethyl orthopropionate: beige powder, yield 83%; mp 189–191 °C; 1H NMR (DMSO-d6, 400 MHz) δ 1.12 (3H, t, 3J = 7.3 Hz, CH3), 2.30 (2H, q, 3J = 7.3 Hz, CH2), 7.75–7.85 (6H, m), 7.92–7.94 (1H, m), 8.48–8.50 (1H, m, H-7 or H-10); 13C {1H} NMR (DMSO-d6, 100 MHz) δ 11.2 (CH3), 21.1 (CH2), 116.3, 122.4, 124.1, 127.9, 129.2, 131.1, 131.2, 132.7, 140.2, 145.0, 148.5, 159.7; EIMS m/z 354 [M + 2]+ (97), 353 [M + 1]+ (83), 352 [M]+ (100), 337 [M − CH3]+ (19), 102 (74); C17H13BrN4 (352.03).
3.6.2. General Procedure for the Synthesis of [1,2,4]Triazolo[1,5-c]quinazolines (3a,b)
Method 1. In a round-bottom flask equipped with a magnetic stirred bar, corresponding [1,2,4]triazolo[4,3-c]quinazoline 2 (0.61 mmol) and glacial acetic acid (5 mL) were added together. The mixture was refluxed for 4 h. After cooling down the water was added until the formation of precipitate. The product was filtered off and washed with water, dried and used in the next step without further purification. A pure sample for analysis was obtained by crystallization from DMSO.
Method 2. In a round-bottom flask equipped with a magnetic stirred bar, 2-(4-bromophenyl)-4-hydrazino-quinazoline 1 (0.95 mmol) in glacial acetic acid (7 mL) and a corresponding orthoester (4.70 mmol) were added together. The mixture was refluxed for 16 h. After cooling down the water was added until the formation of precipitate. The product was filtered off and washed with water, dried and used in the next step without further purification. Pure sample for analysis was obtained by crystallization from DMSO.
- 5-(4-Bromophenyl)-[1,2,4]triazolo[1,5-c]quinazoline (3a). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2a as the starting material: beige powder, yield 93% (method 1), yield 91% (method 2); mp 186–188 °C; 1H NMR (DMSO-d6, 400 MHz) δ 7.79–7.84 (3H, m, H-3′, H-5′, H-8 or H-9), 7.93–7.96 (1H, m, H-8 or H-9), 8.10–8.12 (1H, m, H-7 or H-10), 8.50–8.54 (3H, m, H-2′, H-6′, H-7 or H-10), 8.63 (1H, s, H-2); 13C {1H} NMR (DMSO-d6, 100 MHz, 50 °C) δ 117.0, 123.0, 125.2, 128.2, 128.7, 130.4, 131.2, 132.0, 132.2, 141.9, 144.9, 151.2, 153.5; EIMS m/z 326 [M + 2]+ (100), 325 [M + 1]+ (48), 324 [M]+ (97), 298 [M − N2 + 2]+ (17), 217 [M − N2 − Br]+ (49); C15H9BrN4 (324.00).
- 5-(4-Bromophenyl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline (3b). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2b as the starting material: colorless powder, yield 93% (method 1), yield 89% (method 2); mp 132–134 °C; 1H NMR (DMSO-d6, 400 MHz) δ 1.39 (3H, t, 3J = 7.5 Hz, CH3), 2.97 (2H, q, 3J = 7.5 Hz, CH2), 7.80–7.83 (1H, m, H-8 or H-9), 7.86–7.88 (2H, m, H-3′, H-5′), 7.93–7.97 (1H, m, H-8 or H-9), 8.10–8.12 (1H, m, H-7 or H-10), 8.85–8.47 (3H, m, H-2′, H-6′, H-7 or H-10); 13C {1H} NMR (DMSO-d6, 100 MHz, 50 °C) δ 12.1 (CH3), 21.5 (CH2), 116.7, 123.1, 125.2, 128.3, 128.6, 130.7, 131.2, 132.0, 132.2, 142.0, 144.7, 151.7, 167.6; EIMS m/z 354 [M + 2]+ (68), 353 [M + 1]+ (47), 352 [M]+ (70), 339 [M − CH3 + 2]+ (14), 102 (100); C17H13BrN4 (352.03).
- 4-Bromo-N-(2-(3-ethyl-1H-[1,2,4]triazol-5-yl)phenyl)benzamide (3b′). The method 2 was applied. The reaction was stopped after 8 h. After cooling down the water was added until the formation of a precipitate. The product was filtered off, washed with water, and dried. Then it was purified by column chromatograpgy on silica gel, eluent EtOAc/hexane (3:7) to pure EtOAc. Pale orange powder, mp 220–222 °C; 1H NMR (DMSO-d6, 400 MHz) δ 1.33 (3H, t, 3J = 7.4 Hz, CH3), 2.88 (2H, q, 3J = 7.4 Hz, CH2), 7.20–7.24 (1H, m, benzo), 7.44–7.46 (1H, m, benzo), 7.80–7.82 (2H, m, 4-BrC6H4), 8.01–8.04 (2H, m, 4-BrC6H4), 8.17–8.19 (1H, m, benzo), 8.74–8.76 (1H, m, benzo), 12.8 (1H, s, NH), 14.2 (1H, s, NH); EIMS m/z 372 [M + 2]+ (75), 370 [M]+ (78), 185 [C7H4BrO + 2]+ (98), 183 [C7H4BrO]+ (100); C17H15BrN4O (370.04).
3.6.3. General Procedures for the Synthesis of Target Products 4a–f and 5a–f
The corresponding boronic acid or boronic acid pinacol ester (0.64 mmol), PdCl2(PPh3)2 (48 mg, 68 μmol), PPh3 (36 mg, 136 μmol), saturated solution of K2CO3 (3.7 mL) and EtOH (3.7 mL) were added to the suspension of the corresponding derivative 2a,b or 3a,b (0.60 mmol) in toluene (22 mL). The mixture was stirred at 85 °C for 12–14 h in argon atmosphere in round-bottom pressure flask equipped with magnetic stirred bar. The reaction mixture was cooled to room temperature, and EtOAc/H2O (10/10 mL) mixture was added. The organic layer was separated, additionally washed with water (10 mL), and evaporated at reduced pressure. The product was purified by column chromatography on silica gel, hexane/ethyl acetate mixture was used as an eluent.
- 5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[4,3-c]quinazoline (4a). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2a and 4-(diethylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (2/8) → EtOAc/hexane (1/1). Yellow powder, yield 50%; mp 155–157 °C; 1H NMR (CDCl3, 400 MHz) δ 1.22 (6H, t, 3J = 6.9 Hz, 2CH3), 3.43 (4H, q, 3J = 6.9 Hz, 2CH2), 6.78–8.80 (2H, m, 2CHphenylene), 7.58–7.60 (2H, m, 2CHphenylene), 7.71–7.73 (1H, m, H-8 or H-9), 7.78–7.82 (3H, m, 2CHphenylene, H-8 or H-9), 7.96–7.98 (2H, m, 2CHphenylene), 8.88–8.68 (1H, m, H-7 or H-10), 9.07 (1H, s, H-3); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.7 (2CH3), 44.5 (2CH2), 112.0, 115.9, 123.6, 125.8, 126.6, 128.2, 128.7, 129.0, 129.1, 132.0, 136.3, 141.7, 144.8, 145.0, 148.1, 148.7; EIMS m/z 394 [M + 1]+ (20), 393 [M]+ (67), 378 [M − CH3]+ (100); anal. calcd for C25H23N5 (393.20): C 76.31, H 5.89, N 17.80%. Found C 76.25, H 6.11, N 17.56%.
- 5-(4′-Diphenylamino-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[4,3-c]quinazoline (4b). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2a and 4-(diphenylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (1/3) → EtOAc. Additionally, the product was recrystallized from DMSO. Yellow powder, yield 62%; mp 110–112 °C; 1H NMR (CDCl3, 400 MHz) δ 7.06–7.10 (2H, m, 2CHphenyl), 7.16–7.19 (6H, m, 4CHphenyl, 2CHphenylene), 7.29–7.32 (4H, m, 4CHphenyl), 7.55–7.57 (2H, m, 2CHphenylene), 7.73–7.76 (1H, m, H-8 or H-9), 7.81–7.85 (3H, m, 2CHphenylene, H-8 or H-9), 8.00–8.07 (3H, m, 2CHphenylene, H-7 or H-10), 8.68–8.70 (1H, m, H-7 or H-10), 9.06 (1H, s, H-3); 13C {1H} NMR (CDCl3, 150 MHz) 115.9, 123.4, 123.6, 123.7, 125.0, 127.5, 128.0, 128.8, 129.1, 129.4, 129.6, 130.5, 132.2, 132.8, 136.2, 141.7, 144.5, 144.6, 147.5, 148.5, 148.8; EIMS m/z 490 [M + 1]+ (36), 489 [M]+ (100); anal. calcd for C33H23N5×DMSO×1/2H2O: C 72.24, H 4.78, N 12.76%. Found C 72.38, H 4.02, N 12.74%.
- 5-(4′-(9H-Carbazol-9-yl)-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[4,3-c]quinazoline (4c). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2a and 4-(9H-carbazol-9-yl)phenylboronic acid pinacol ester. Eluent for column chromatography: EtOAc/hexane (1/3) → EtOAc. Additionally, the product was recrystallized from DMSO. Beige powder, yield 63%; mp > 250 °C; 1H NMR (CDCl3, 400 MHz) δ 7.31–7.34 (2H, m, 2CHcarbaz.), 7.43–7.47 (2H, m, 2CHcarbaz.), 7.50–7.52 (2H, m, 2CHcarbaz.), 7.73–7.78 (3H, m), 7.83–7.87 (1H, m, H-8 or H-9), 8.92–8.94 (2H, m), 8.97–8.99 (2H, m), 8.08–8.12 (3H, m) 8.17–8.19 (2H, m), 8.70–8.72 (1H, m, H-7 or H-10), 9.09 (1H, s, H-2); 13C {1H} NMR (CDCl3, 150 MHz) δ 109.9, 116.0, 120.4, 120.6, 123.7, 123.8, 126.2, 127.7, 128.2, 128.9, 129.3, 129.5, 131.5, 132.3, 136.2, 138.2, 138.6, 140.8, 141.6, 144.1, 144.4, 148.8; EIMS m/z 488 [M + 1]+ (38), 487 [M]+ (100); anal. calcd for C33H21N5 (487.18): C 81.29, H 4.34, N 14.34%. Found C 81.35, H 4.18, N 14.67%.
- 5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[4,3-c]quinazoline (4d). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2b and 4-(diethylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (1/3) → EtOAc. Additionally, the product was recrystallized twice from the mixture of EtOAc/hexane. Pale yellow powder, yield 61%; mp 154–156 °C; 1H NMR (CDCl3, 400 MHz) δ 1.19–1.24 (9H, m, 3CH3), 2.57 (2H, q, 3J = 7.3 Hz, CH2), 3.43 (4H, q, 3J = 7.3 Hz, 2CH2), 6.78–6.80 (2H, m, 2CHphenylene), 7.59–7.64 (4H, m, 4CHphenylene), 7.64–7.69 (1H, m, H-8 or H-9), 7.75–7.77 (3H, m, 2CHphenylene, H-8 or H-9), 7.96–7.8 (1H, m, H-7 or H-10), 8.66–8.68 (1H, m, H-7 or H-10); 13C {1H} NMR (CDCl3, 100 MHz) δ 11.9 (CH3), 12.2 (2CH3), 21.9 (CH2), 44.5 (2CH2), 112.0, 116.6, 123.3, 125.8, 125.9, 128.0, 128.2, 128.9, 129.0, 130.4, 131.5, 140.8, 143.9, 145.9, 147.9, 149.6, 150.5; EIMS m/z 422 [M + 1]+ (25), 421 [M]+ (76), 406 [M − CH3]+ (100); anal. calcd for C27H27N5 (421.23): C 76.93, H 6.46, N 16.61%. Found C 76.73, H 6.24, N 16.31%.
- 5-(4′-Diphenylamino-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[4,3-c]quinazoline (4e). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2b and 4-(diphenylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (7/3) → EtOAc/hexane (1/1). Pale yellow powder, yield 72%; mp 154–156 °C; 1H NMR (CDCl3, 400 MHz) δ 1.22 (3H, t, 3J = 7.2 Hz, CH3), 2.55 (2H, q, 3J = 7.2 Hz, CH2), 7.06–7.09 (2H, m, 2CHphenyl), 7.15–7.20 (6H, m, 4CHphenyl, 2CHphenylene), 7.28–7.32 (4H, m, 4CHphenyl), 7.56–7.58 (2H, m, 2CHphenylene), 7.66–7.73 (3H, m, 2CHphenylene, H-8 or H-9), 7.76–7.80 (3H, m, 2CHphenylene, H-8 or H-9), 7.97–7.99 (1H, m, H-7 or H-10), 8.67–8.69 (1H, m, H-7 or H-10); 13C {1H} NMR (CDCl3, 150 MHz) δ 12.0 (CH3), 22.0 (CH2), 116.7, 123.4, 123.5, 123.6, 124.9, 126.8, 128.0, 128.3, 129.2, 129.3, 129.5, 131.6, 131.8, 133.1, 140.9, 143.4, 145.7, 147.6, 148.3, 149.6, 150.5; EIMS m/z 518 [M + 1]+ (44), 517 [M]+ (100); anal. calcd for C35H27N5 (517.23): C 81.21, H 5.26, N 13.53%. Found C 81.05, H 5.11, N 13.22%.
- 5-(4′-(9H-Carbazol-9-yl)-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[4,3-c]quinazoline (4f). The general procedure was applied using [1,2,4]triazolo[4,3-c]quinazoline 2b and 4-(9H-carbazol-9-yl)phenylboronic acid pinacol ester. Eluent for column chromatography: EtOAc/hexane (1/3) → EtOAc. Additionally, the product was washed with hexane. Pale beige powder, yield 72%; mp 255–257 °C; 1H NMR (DCCl3, 400 MHz) δ 1.26 (3H, t, 3J = 7.3 Hz, CH3), 2.59 (2H, q, 3J = 7.3 Hz, CH2), δ 7.31–7.34 (2H, m, 2CHcarbaz.), 7.43–7.47 (2H, m, 2CHcarbaz.), 7.50–7.52 (2H, m, 2CHcarbaz.), 7.71–7.81 (6H, m), 7.92–7.94 (4H, m), 7.99–8.01 (1H, m, H-7 or H-10), 8.17–8.19 (2H, m), 8.69–8.71 (1H, m, H-7 or H-10); 13C {1H} (CDCl3, 100 MHz) δ 12.0 (CH3), 22.1 (CH2), 109.9, 116.8, 120.3, 120.6, 123.5, 123.7, 126.2, 127.5, 127.7, 128.4, 128.8, 129.5, 131.8, 132.7, 138.8, 140.8, 140.9, 143.1, 145.4, 149.7, 150.4; EIMS m/z 516 [M + 1]+ (42), 515 [M]+ (100); anal. calcd for C35H25N5 (515.21): C 81.51, H 4.89, N 13.58%. Found C 80.43, H 5.20, N 13.26%.
- 5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[1,5-c]quinazoline (5a). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3a and 4-(diethylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (1/9). Yellow powder, yield 77%; mp 170–172 °C; 1H NMR (CDCl3, 400 MHz) δ 1.22 (6H, t, 3J = 7.0 Hz, 2CH3), 3.42 (4H, q, 3J = 7.0 Hz, 2CH2), 6.77–6.79 (2H, m, 2CHphenylene), 7.69–7.73 (1H, m, H-8 or H-9), 7.77–7.80 (2H, m, 2CHphenylene), 7.83–7.87 (1H, m, H-8 or H-9), 8.12–8.14 (1H, m, H-7 or H-10), 8.48 (1H, s, H-2), 8.55–8.61 (3H, m, 2CHphenylene, H-7 or H-10); 13C {1H} NMR (CDCl3, 100 MHz) δ 13.1 (2CH3), 44.9 (2CH2), 112.3, 117.8, 124.0, 126.1, 126.9, 128.5, 128.6, 129.1, 129.2, 131.2, 132.6, 143.5, 145.0, 147.0, 148.2, 152.5, 153.9; EIMS m/z 394 [M + 1]+ (21), 393 [M]+ (70), 378 [M − CH3]+ (100); anal. calcd for C25H23N5 (393.20): C 76.31, H 5.89, N 17.80%. Found C 76.55, H 6.26, N 18.21%.
- 5-(4′-Diphenylamino-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[1,5-c]quinazoline (5b). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3a and 4-(diphenylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (3/17). Yellow-green powder, yield 36%; mp 170–172 °C; 1H NMR (CDCl3, 400 MHz) δ 7.05–7.09 (2H, m, 2CHphenyl), 7.16–7.18 (6H, m, 4CHphenyl, 2CHphenylene), 7.26–7.32 (4H, m, 4CHphenyl), 7.57–7.59 (2H, m, 2CHphenylene), 7.72–7.75 (1H, m, H-8 or H-9), 7.72–7.75 (2H, m, 2CHphenylene), 7.85–7.89 (1H, m, H-8 or H-9), 8.14–8.16 (1H, m, H-7 or H-10), 8.49 (1H, s, H-2), 8.57–8.59 (1H, m, H-7 or H-10), 8.62–8.64 (2H, m, 2CHphenylene); 13C {1H} NMR (100 MHz, CDCl3) δ 117.6, 123.4, 123.6, 124.9, 126.6, 128.1, 128.5, 128.9, 129.5, 130.0, 131.0, 132.4, 133.7, 143.1, 144.0, 146.5, 147.6, 148.2, 152.2, 153.6; EIMS m/z 490 [M + 1]+ (40), 489 [M]+ (100); anal. calcd for C33H23N5 (489.20): C 80.96, H 4.74, N 14.31%. Found C 80.88, H 5.00, N 14.04%.
- 5-(4′-(9H-Carbazol-9-yl)-[1,1′]-biphenyl-4-yl)-[1,2,4]triazolo[1,5-c]quinazoline (5c). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3a and 4-(9H-carbazol-9-yl)phenylboronic acid pinacol ester. After cooling the reaction mixture product was filtered off, washed with hexane. Pale grey powder, yield 67%; mp 257–259 °C; 1H NMR (DMSO-d6, 600 MHz) δ 7.31–7.34 (2H, m, 2CHcarbaz.), 7.46–7.51 (4H, m, 4CHcarbaz.), 7.80–7.81 (2H, m), 7.85–7.87 (1H, m, H-8 or H-9), 7.99–8.01 (1H, m, H-8 or H-9), 8.10–8.11 (2H, m), 8.13–8.14 (2H, m), 8.18–8.19 (1H, m, H-7 or H-10), 8,27–8.28 (2H, m), 8.53–8.54 (1H, m, H-7 or H-10), 8.69–8.71 (2H, m, 2CHphenylene), 8.79 (1H, s, H-2); 13C {1H} NMR (150 MHz, DMSO-d6, 55 °C) δ 109.5, 117.1, 120.1, 120.5, 122.8, 123.2, 126.3, 126.5, 127.1, 128.4, 128.6, 128.7, 130.6, 131.0, 132.4, 136.9, 138.0, 140.0, 142.1, 142.2, 145.7, 151.4, 153.6; EIMS m/z 488 [M + 1]+ (37), 487 [M]+ (100); anal. calcd for C33H21N5 (487.20): C 81.29, H 4.34, N 14.34%. Found C 81.18, H 4.15, N 14.39%.
- 5-(4′-Diethylamino-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline (5d). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3b and 4-(diethylamino)phenylboronic acid. Eluent for column chromatography: EtOAc/hexane (1/2) → EtOAc/hexane (1/1). Additionally, the product was recrystallized from a mixture of CH2Cl2/hexane. Yellow powder, yield 51%; mp 116–118 °C; 1H NMR (CDCl3, 400 MHz) δ 1.22 (6H, t, 3J = 6.5 Hz, 2CH3), 1.51 (3H, t, 3J = 7.5 Hz, CH3), 3.08 (2H, q, 3J = 7.5 Hz, CH2), 3.43 (4H, q, 3J = 6.5 Hz, 2CH2), 6.78–6.80 (2H, m, 2CHphenylene), 7.60–7.62 (2H, m, 2CHphenylene), 7.66–7.70 (1H, m, H-8 or H-9), 7.77–7.85 (3H, m, 2CHphenylene, H-8 or H-9), 8.09–8.11 (1H, m, H-7 or H-10), 8.53–8.55 (1H, m, H-7 or H-10), 8.62–8.64 (2H, m, 2CHphenylene); 13C {1H} NMR (CDCl3, 100 MHz) δ 12.8 (2CH3), 12.9 (CH3), 22.6 (CH2), 44.6 (2CH2), 112.0, 117.2, 123.7, 125.8, 126.8, 128.0, 128.3, 128.8, 129.1, 130.9, 132.0, 143.2, 144.5, 146.6, 147.9, 152.7, 168.5; EIMS m/z 422 [M + 1]+ (25), 421 [M]+ (80), 406 [M − CH3]+ (100); anal. calcd for C27H27N5 (421.23): C 76.93, H 6.46, N 16.61%. Found C 76.72, H 6.22, N 16.42%.
- 5-(4′-Diphenylamino-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline (5e). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3b and 4-(diphenylamino)phenylboronic acid. Eluent for column chromatography: hexane → EtOAc/hexane (8/2). Additionally, the product was washed with hexane. Yellow–green powder, yield 69%; mp 185–187 °C; 1H NMR (CDCl3, 400 MHz) δ 1.51 (3H, t, 3J = 7.2 Hz, CH3), 3.08 (2H, q, 3J = 7.2 Hz, CH2), 7.05–7.08 (2H, m, 2CHphenyl), 7.16–7.18 (6H, m, 4CHphenyl, 2CHphenylene), 7.28–7.31 (4H, m, 4CHphenyl), 7.57–7.59 (2H, m, 2CHphenylene), 7.68–7.71 (1H, m, H-8 or H-9), 7.79–7.86 (3H, m, 2CHphenylene, H-8 or H-9), 8.10–8.12 (1H, m, H-7 or H-10), 8.54–8.56 (1H, m, H-7 or H-10), 8.65–8.67 (2H, m, 2CHphenylene); 13C {1H} NMR (100 MHz, CDCl3) δ 12.8 (CH3), 22.5 (CH2), 117.3, 123.4, 123.6, 123.7, 124.9, 126.5, 128.0, 128.2, 128.8, 129.5, 130.2, 131.0, 132.0, 133.8, 143.1, 143.8, 146.2, 147.6, 148.1, 152.7, 168.6; EIMS m/z 518 [M + 1]+ (41), 517 [M]+ (100); anal. calcd for C35H27N5 (517.23): C 81.21, H 5.26, N 13.53%. Found C 82.34, H 5.48, N 14.04%.
- 5-(4′-(9H-Carbazol-9-yl)-[1,1′]-biphenyl-4-yl)-2-ethyl-[1,2,4]triazolo[1,5-c]quinazoline (5f). The general procedure was applied using [1,2,4]triazolo[1,5-c]quinazoline 3b and 4-(9H-carbazol-9-yl)phenylboronic acid pinacol ester. After cooling the reaction mixture product was filtered off, washed with hexane and recrystallized from DMSO. Pale beige powder, yield 55%; mp 286–288 °C; 1H NMR (DMSO-d6, 400 MHz) δ 1.49 (3H, t, 3J = 7.5 Hz, CH3), 3.03 (2H, q, 3J = 7.5 Hz, CH2), δ 7.26–7.30 (2H, m, 2CHcarbaz.), 7.41–7.44 (2H, m, 2CHcarbaz.), 7.49–7.51 (2H, m, 2CHcarbaz.), 7.76–7.80 (3H, m), 7.90–7.94 (1H, m, H-8 or H-9), 8.00–8.02 (2H, m), 8.08–8.12 (3H, m), 8.18–8.19 (2H, m), 8.48–8.50 (1H, m, H-7 or H-10), 8.77–8.79 (2H, m, 2CHphenylene); 13C NMR was not recorded due to poor solubility of the sample. EIMS m/z 516 [M + 1]+ (40), 515 [M]+ (100); anal. calcd for C35H25N5 (515.21): C 81.51, H 4.89, N 13.58%. Found C 81.46, H 5.04, N 14.05%.
4. Conclusions
We have developed a synthetic approach to 5-(4-bromophenyl)-[1,2,4]triazolo[4,3-c]quinazolines and their [1,5-c]-isomers. The bromophenyl derivatives were successfully functionalized by introducing aminoaryl donor fragments via palladium-catalyzed cross-coupling reactions with boronic acids or their esters; two series of 5-(4′-EDG-[1,1′]-biphenyl-4-yl)[1,2,4]triazoloquinazoline fluorophores were prepared. The structures of the ring-opening product and target fluorophores were unambiguously confirmed by NMR spectroscopy, mass-spectrometry, and XRD data. The photophysical properties of [1,2,4]triazoloquinazolines have been studied in two solvents and in the solid state. 9H-Carbazol-9-yl-containing triazoloquinazolines are characterized by absorption maxima in the range of 312–328 nm in MeCN, whereas the band of their Et2N or Ph2N counterparts is red-shifted, and the maxima are located in the 340–375 nm range; the absorption band slightly shifts to red region in toluene compared to the MeCN solution. All the compounds are emissive in the blue–cyan region in toluene and in the yellow–orange region in MeCN, with different fluorescence intensities and quantum yields. Some of triazolo[4,3-c]quinazolines exhibited medium to high quantum yields, both in solution and in solid state. Triazoloquinazolines with a [1,5-c] annelation type turned out to be more effective fluorophores with quantum yields of over 75% in toluene solutions. The presence of the ethyl group has considerable impact on photophysical properties of triazolo[4,3-c]quinazolines due to steric hindrance between the close-located biphenyl substituent and ethyl group. The quantum yields of presented compounds are found to be higher than those of their 3-aryl-[1,2,4]triazolo[4,3-c]quinazoline counterparts. The experimental findings were conducted by theoretical calculations. Notably, synthesized push–pull organic systems exhibit strong fluorosolvatochromism as a consequence of the large dipole moment in the excited state, with the strong emission intensity in aprotic solvents, thus making them interesting candidates for practical application as fluorescence probes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29112497/s1, Figures S1–S18: NMR and mass spectra of [1,2,4]triazoloquinazolines 2a,b, 3a,b, 4a–f, and 5a–f and product 3b′; Scheme S1: The proposed mechanism of the Dimroth rearrangement [35]; Tables S1–S8: Selected bond lengths and angles of compounds 4a, 4e, 5d, and 5e; Figure S19: Absorption and emission spectra of compounds 4a–f and 5a–f in toluene and MeCN; Figure S20: Combined emission spectra of fluorophores 4a–f and 5a–f in MeCN; Table S9, Figure S21: Detailed data of the fluorescence lifetime measurements of 4a–f and 5a–f; Figure S22: Absorption and emission spectra of compounds 4a, 4d, 5a, and 5d–f in different solvents, Tables S10–S15: Orientation polarizability for solvents (Δf), absorption and emission maxima (λabs, λem, nm), and Stokes shift (nm, cm−1) of compounds 4a, 4d, 5a, and 5d–f in different solvents; Figure S23: Lippert–Mataga plot of fluorophores 4a, 4d, 5a, 5d, 5e and 5f; Figures S24 and S25: General view of voltammograms for 4a–f and 5a–f in CH2Cl2; Table S16: The electronic distribution in HOMO/LUMO of 4a–f and 5a–f; Table S17: The optimized geometries of 4a–f and 5a–f in gas phase, toluene, and MeCN; Table S18: Selected dihedral angles (α) and bond lengths (L) in the ground and the excited states of 4a–f and 5a–f in toluene and MeCN; Table S19: The calculated electronic transitions of compounds 4a–f and 5a–f; Figure S26: Computed absorption spectra for compounds 4a–f and 5a–f; Table S20: Hole–electron interaction analysis result for compounds 4a–f and 5a–f.
Author Contributions
Conceptualization, V.N.C.; methodology, A.E.K., M.A.I. and T.N.M.; investigation, A.E.K., E.S.S., D.A.G., O.S.E. and P.A.S.; writing—original draft preparation, T.N.M., E.V.N. and D.S.K.; supervision, V.N.C.; project administration, V.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (grant number No. 23-73-01147, https://rscf.ru/en/project/23-73-01147/, accessed on 23 March 2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on request from the corresponding authors.
Acknowledgments
D.A.G. is grateful for the financial support from the state assignment of IOS UB RAS (Theme No. 124020500039-0).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jabeen, T.; Aslam, S.; Ahmad, M.; ul Haq, A.; Al-Hussain, S.A.; Zaki, M.E.A. Triazoloquinazoline: Synthetic Strategies and Medicinal Importance. In Recent Advances on Quinazoline [Working Title]; IntechOpen: London, UK, 2023. [Google Scholar]
- Abuelizz, H.A.; Al-Salahi, R. An Overview of Triazoloquinazolines: Pharmacological Significance and Recent Developments. Bioorg. Chem. 2021, 115, 105263. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, M.; Doan, T.H.; Hibner-Kulicka, P.; Otake, R.; Lukarska, M.; Lohier, J.F.; Ozawa, K.; Nanbu, S.; Alayrac, C.; Suzuki, Y.; et al. Synthesis and Structure-Photophysics Evaluation of 2-N-Amino-Quinazolines: Small Molecule Fluorophores for Solution and Solid State. Chem. Asian J. 2021, 16, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Zhang, X.; Zhu, B.; Wang, J.; Wu, G.; Yin, Y.; Song, Q. Comparative Studies of Organic Dyes with a Quinazoline or Quinoline Chromophore as π-Conjugated Bridges for Dye-Sensitized Solar Cells. Dye. Pigment. 2016, 124, 72–81. [Google Scholar] [CrossRef]
- Bonnaud, T.; Scaviner, M.; Robin-le Guen, F.; Achelle, S. 4-substituted Push-pull Quinazoline Chromophores with Extended π-conjugated Linker. J. Heterocycl. Chem. 2024, 61, 358–364. [Google Scholar] [CrossRef]
- Lipunova, G.N.; Nosova, E.V.; Charushin, V.N.; Chupakhin, O.N. Functionalized Quinazolines and Pyrimidines for Optoelectronic Materials. Curr. Org. Synth. 2018, 15, 793–814. [Google Scholar] [CrossRef]
- Li, P.; Xiang, Y.; Gong, S.; Lee, W.K.; Huang, Y.H.; Wang, C.Y.; Yang, C.; Wu, C.C. Quinazoline-Based Thermally Activated Delayed Fluorescence Emitters for High-Performance Organic Light-Emitting Diodes with External Quantum Efficiencies about 28%. J. Mater. Chem. C 2021, 9, 12633–12641. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Su, S.; Guo, F.; Cao, Y.; Zhang, Y. Quinazoline-Based Thermally Activated Delayed Fluorecence for High-Performance OLEDs with External Quantum Efficiencies Exceeding 20%. Adv. Opt. Mater. 2019, 7, 1801496. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Wang, H.; Shen, B.; Zhang, J.; Hao, J.; Cao, J.; Wang, Z. Synthesis, Photophysical and Optoelectronic Properties of Quinazoline-Centered Dyes and Their Applications in Organic Light-Emitting Diodes. Dye. Pigment. 2016, 125, 299–308. [Google Scholar] [CrossRef]
- Plaza-Pedroche, R.; Georgiou, D.; Fakis, M.; Fihey, A.; Katan, C.; Guen, F.R.-l.; Achelle, S.; Rodríguez-López, J. Effect of Protonation on the Photophysical Properties of 4-Substituted and 4,7-Disubstituted Quinazoline Push-Pull Chromophores. Dye. Pigment. 2021, 185, 108948. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar Singh, N.; Mukhopadhyay, S.; Shankar Pandey, D. AIE Active Quinazoline Based Probes for Selective Detection of Fe3+ and Acidochromism. Inorganica Chim. Acta 2023, 546, 121294. [Google Scholar] [CrossRef]
- Dwivedi, B.K.; Singh, V.D.; Paitandi, R.P.; Pandey, D.S. Substituent-Directed ESIPT-Coupled Aggregation-Induced Emission in Near-Infrared-Emitting Quinazoline Derivatives. ChemPhysChem 2018, 19, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, G.; Wong, W.Y. Functionalization of Phosphorescent Emitters and Their Host Materials by Main-Group Elements for Phosphorescent Organic Light-Emitting Devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Wang, Q.; Ao, L.; Zhong, C.; Yang, C.; Qin, J.; Ma, D. Highly Efficient Phosphorescent Organic Light-Emitting Diodes Hosted by 1,2,4-Triazole-Cored Triphenylamine Derivatives: Relationship between Structure and Optoelectronic Properties. J. Phys. Chem. C 2010, 114, 601–609. [Google Scholar] [CrossRef]
- Lee, J.; Shizu, K.; Tanaka, H.; Nomura, H.; Yasuda, T.; Adachi, C. Oxadiazole- and Triazole-Based Highly-Efficient Thermally Activated Delayed Fluorescence Emitters for Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1, 4599–4604. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Światkowski, M. Highly Luminescent 4H-1,2,4-Triazole Derivatives: Synthesis, Molecular Structure and Photophysical Properties. Materials 2020, 13, 5627. [Google Scholar] [CrossRef] [PubMed]
- Olesiejuk, M.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Synthesis of 4-Alkyl-4H-1,2,4-Triazole Derivatives by Suzuki Cross-Coupling Reactions and Their Luminescence Properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef] [PubMed]
- Abdurahman, A.; Wang, L.; Zhang, Z.; Feng, Y.; Zhao, Y.; Zhang, M. Novel Triazole-Based AIE Materials: Dual-Functional, Highly Sensitive and Selective Fluorescence Probe. Dye. Pigment. 2020, 174, 108050. [Google Scholar] [CrossRef]
- Wu, J.; You, Q.; Lan, J.; Guo, Q.; Li, X.; Xue, Y.; You, J. Cu-Catalysed Direct C-H (Hetero)Arylation of [1,2,4]Triazolo[4,3-a]Pyridine to Construct Deep-Blue-Emitting Luminophores. Org. Biomol. Chem. 2015, 13, 5372–5375. [Google Scholar] [CrossRef] [PubMed]
- Vadagaonkar, K.S.; Yang, C.J.; Zeng, W.H.; Chen, J.H.; Patil, B.N.; Chetti, P.; Chen, L.Y.; Chaskar, A.C. Triazolopyridine Hybrids as Bipolar Host Materials for Green Phosphorescent Organic Light-Emitting Diodes (OLEDs). Dye. Pigment. 2019, 160, 301–314. [Google Scholar] [CrossRef]
- Cao, C.; Chen, W.C.; Tian, S.; Chen, J.X.; Wang, Z.Y.; Zheng, X.H.; Ding, C.W.; Li, J.H.; Zhu, J.J.; Zhu, Z.L.; et al. A Novel D-π-A Blue Fluorophore Based on [1,2,4]Triazolo[1,5-a] Pyridine as an Electron Acceptor and Its Application in Organic Light-Emitting Diodes. Mater. Chem. Front. 2019, 3, 1071–1079. [Google Scholar] [CrossRef]
- Song, W.; Shi, L.; Gao, L.; Hu, P.; Mu, H.; Xia, Z.; Huang, J.; Su, J. Triazolo[1,5-a]Pyridine as Building Blocks for Universal Host Materials for High-Performance Red, Green, Blue and White Phosphorescent Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 5714–5722. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Fang, R.; Liu, S. Organic Compound for Light-Emitting Device, and Organic Light-Emitting Device Comprising the Same. Patent WO2022078250, 21 April 2022. [Google Scholar]
- Sun, E.; Fang, R.; Liu, S. Organic Compound for Light-Emitting Device, Application of Organic Compound and Organic Light-Emitting Device. Patent CN112174968A, 5 January 2021. [Google Scholar]
- Sun, E.; Zeng, L.; Liu, S.; Fang, R.; Wu, J. Preparation of Spiro[Acridine-Fluorene]-Derivative Luminescent Material for Organic Electroluminescent Devices. Patent CN112442037, 5 March 2021. [Google Scholar]
- Sun, E.; Liu, S.; Li, Z.; Zhang, X. Compound and Organic Electroluminescent Device Using Same. Patent CN109824672, 31 May 2019. [Google Scholar]
- Sun, E.; Liu, S.; Wu, J.; Feng, J. Organic Electroluminescent Material and Device. Patent WO2019206242, 31 October 2019. [Google Scholar]
- Sun, E.; Liu, S.; Wu, J.; Shao, S. Organic Electroluminescent Material and Device. Patent CN110407838, 5 November 2019. [Google Scholar]
- Li, Z.-P.; Zhao, H.; Zhang, Z.-H.; Qiu, Z.-X.; Li, X.-F.; Huang, L.-J. A Novel [1, 2, 4]Triazolo[5,1-b]Quinazoline Derivative as a Fluorescent Probe for Highly Selective Detection of Fe3+ Ions. J. Asian Nat. Prod. Res. 2024, 26, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kopotilova, A.E.; Moshkina, T.N.; Nosova, E.V.; Lipunova, G.N.; Starnovskaya, E.S.; Kopchuk, D.S.; Kim, G.A.; Gaviko, V.S.; Slepukhin, P.A.; Charushin, V.N. 3-Aryl-5-Aminobiphenyl Substituted [1,2,4]Triazolo[4,3-c]Quinazolines: Synthesis and Photophysical Properties. Molecules 2023, 28, 1937. [Google Scholar] [CrossRef] [PubMed]
- Moshkina, T.N.; Nosova, E.V.; Permyakova, J.V.; Lipunova, G.N.; Valova, M.S.; Slepukhin, P.A.; Sadieva, L.K.; Charushin, V.N. Synthesis and Photophysical Properties of 2-Aryl-4-(Morpholin-4-yl)Quinazoline Chromophores: The Effect of π-Linker Moiety. Dye. Pigment. 2022, 206, 110592. [Google Scholar] [CrossRef]
- Moshkina, T.N.; Nosova, E.V.; Permyakova, J.V.; Lipunova, G.N.; Zhilina, E.F.; Kim, G.A.; Slepukhin, P.A.; Charushin, V.N. Push-Pull Structures Based on 2-Aryl/Thienyl Substituted Quinazolin-4(3H)-Ones and 4-Cyanoquinazolines. Molecules 2022, 27, 7156. [Google Scholar] [CrossRef] [PubMed]
- Nosova, E.V.; Kopotilova, A.E.; Ivan’kina, M.A.; Moshkina, T.N.; Kopchuk, D.S. Synthesis of 5-(4-Bromophenyl)- and 5-(5-Bromothiophen-2-yl)-Substituted 3-Aryl[1,2,4]Triazolo[4,3-c]Quinazolines. Russ. Chem. Bull. 2022, 71, 1483–1487. [Google Scholar] [CrossRef]
- Postovskii, I.Y.; Vereshchagina, N.N.; Mertsalov, S.L. Researches on benzodiazines VI. Synthesis of 2-R-4-hydrazinoquinazolines, 5-R-[8,4-c]-s-triazolo-and 5-R-[1,5-c]tetrazoloquinazolines. Chem. Heterocycl. Compd. 1966, 2, 94–97. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A.; Kadyrova, M.S. The Dimroth Rearrangement in the Synthesis of Condensed Pyrimidines—Structural Analogs of Antiviral Compounds. Chem. Heterocycl. Compd. 2021, 57, 342–368. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Geronikaki, A.; Spinelli, D.; Hovakimyan, A.A.; Noravyan, A.S. Synthesis and Structure of Condensed Triazolo- and Tetrazolopyrimidines. Tetrahedron 2013, 69, 10637–10643. [Google Scholar] [CrossRef]
- Vorob’ev, E.V.; Kletskii, M.E.; Krasnikov, V.V.; Mezheritskii, V.V.; Steglenko, D.V. Studies on mechanisms of the rearrangement of thieno[3,2-e][1,2,4]triazolo[4,3-c]pyrimidines into thieno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines. Russ Chem Bull. 2006, 55, 2247–2255. [Google Scholar] [CrossRef]
- Moshkina, T.N.; Le Poul, P.; Barsella, A.; Pytela, O.; Bureš, F.; Robin-Le Guen, F.; Achelle, S.; Nosova, E.V.; Lipunova, G.N.; Charushin, V.N. Electron-Withdrawing Substituted Quinazoline Push-Pull Chromophores: Synthesis, Electrochemical, Photophysical and Second-Order Nonlinear Optical Properties. European J. Org. Chem. 2020, 2020, 5445–5454. [Google Scholar] [CrossRef]
- Starnovskaya, E.S.; Valieva, M.I.; Aluru, R.; Kopchuk, D.S.; Khasanov, A.F.; Taniya, O.S.; Novikov, A.S.; Kalinichev, A.A.; Santra, S.; Zyryanov, G.V.; et al. Carbazole/Fluorene-Substituted 5-Phenyl-2,2′-Bipyridine D-π-A Fluorophores: Photophysical Data, Hyperpolarizability and CT-Indices. New J. Chem. 2023, 47, 12393–12402. [Google Scholar] [CrossRef]
- Porrès, L.; Holland, A.; Pålsson, L.O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, I.S.; Taniya, O.S.; Sadieva, L.K.; Volkova, N.N.; Minin, A.S.; Grzhegorzhevskii, K.V.; Gorbunov, E.B.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N.; et al. Bola-Type PAH-Based Fluorophores/Chemosensors: Synthesis via an Unusual Clemmensen Reduction and Photophysical Studies. J. Photochem. Photobiol. A Chem. 2021, 420, 113466. [Google Scholar] [CrossRef]
- Kournoutas, F.; Fihey, A.; Malval, J.P.; Spangenberg, A.; Fecková, M.; Le Poul, P.; Katan, C.; Robin-Le Guen, F.; Bureš, F.; Achelle, S.; et al. Branching Effect on the Linear and Nonlinear Optical Properties of Styrylpyrimidines. Phys. Chem. Chem. Phys. 2020, 22, 4165–4176. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Peng, Z.; Wang, Z.; Wang, Y.; Lu, P. Preparation and Photophysical Properties of Quinazoline-Based Fluorophores. RSC Adv. 2020, 10, 30297–30303. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Lippert, E. Spektroskopische Bestimmung Des Dipolmomentes Aromatischer Verbindungen Im Ersten Angeregten Singulettzustand. Z. Elektrochem. Berichte Bunsenges. Phys. Chem. 1957, 61, 962–975. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956, 29, 465–470. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Fast Calculation of van Der Waals Volume as a Sum of Atomic and Bond Contributions and Its Application to Drug Compounds. J. Org. Chem. 2003, 68, 7368–7373. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Maka, V.K.; Moorthy, J.N. Remarkable Influence of ‘Phane Effect’ on the Excited-State Properties of Cofacially Oriented Coumarins. Phys. Chem. Chem. Phys. 2017, 19, 4758–4767. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, T.; Chen, Q. An Sp-Hybridized All-Carboatomic Ring, Cyclo[18]Carbon: Electronic Structure, Electronic Spectrum, and Optical Nonlinearity. Carbon N. Y. 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Alegre-Requena, J.V.; Sowndarya, S.V.S.; Pérez-Soto, R.; Alturaifi, T.M.; Paton, R.S. AQME: Automated Quantum Mechanical Environments for Researchers and Educators. WIREs Comput. Mol. Sci. 2023, 13, e1663. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).