[FeIIICl(TMPPH2)][FeIIICl4]2: A Stand-Alone Molecular Nanomedicine That Induces High Cytotoxicity by Ferroptosis
Abstract
1. Introduction
2. Results and Discussion
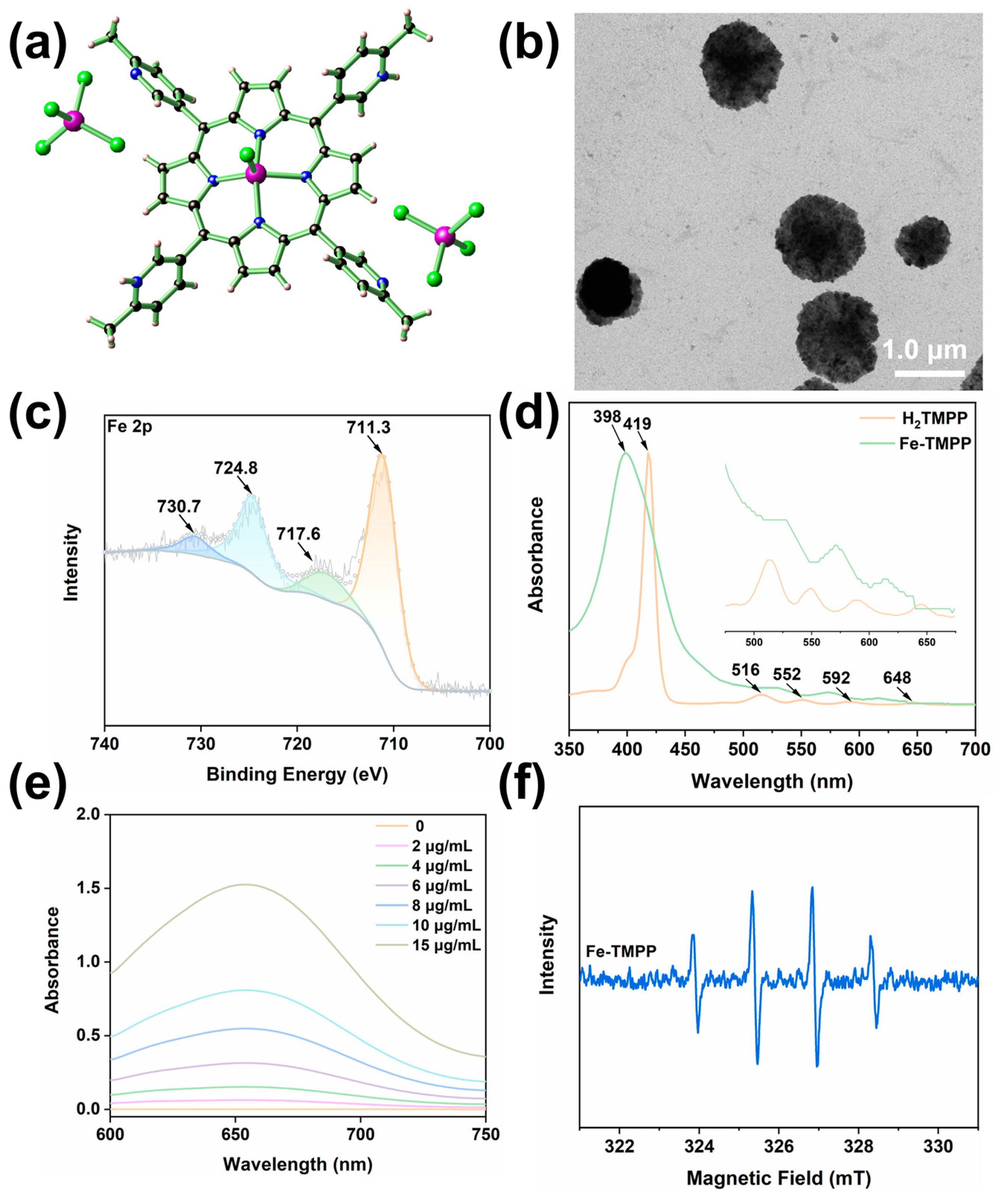
2.1. Material Synthesis and Structure Descriptions
2.2. Spectroscopic Characterizations of Fe-TMPP
2.3. Characterizations of Fe-TMPP Nanoparticles
2.4. Detection of Fe-TMPP-induced Generation of ROS in Solution
2.5. In Vitro ROS Detection and Cytotoxicity Assay
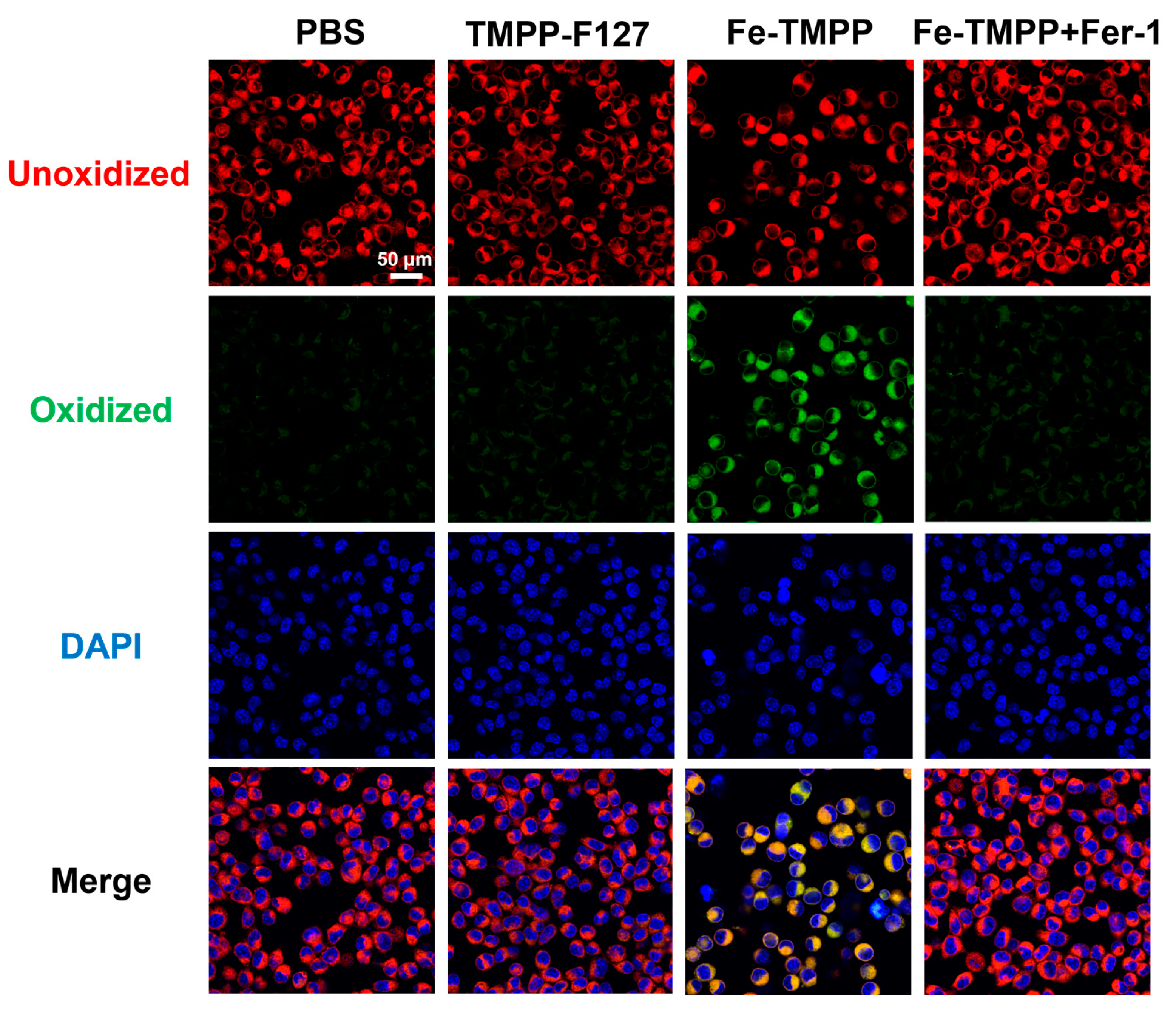
2.6. Ferroptosis Assay
3. Materials and Methods
3.1. General
3.2. Synthesis and Characterization of TMPP-F127
3.3. Synthesis and Characterization of Fe-TMPP
3.4. Single Crystal X-ray Crystallography
3.5. •OH Detection
3.6. 1O2 Detection
3.7. MTT or CCK-8 Cytotoxicity Assay
3.8. Intracellular ROS Detection
3.9. Flow Cytometric Apoptosis Assay
3.10. Ferroptosis Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, Z.; Yang, J.; Zheng, W.; Sun, J.; Wang, W.; Qian, H. Recent advances on modulation of H2O2 in tumor microenvironment for enhanced cancer therapeutic efficacy. Coord. Chem. Rev. 2023, 481, 215049. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, F.; Li, X.; Niu, G.; Yang, Y.; Li, H.; Jiang, Y. Tumor microenvironment-responsive Fenton nanocatalysts for intensified anticancer treatment. J. Nanobiotechnology 2022, 20, 69. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-M.; Li, R.-T.; Yu, L.; Huang, T.; Peng, J.; Meng, W.; Sun, B.; Zhang, W.-H.; Jiang, Z.-H.; Chen, J.; et al. Reprogramming of the tumor microenvironment using a PCN-224@IrNCs/d-Arg nanoplatform for the synergistic PDT, NO, and radiosensitization therapy of breast cancer and improving anti-tumor immunity. Nanoscale 2023, 15, 10715–10729. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, R.; Ye, Q.; Zou, Y.; Lu, X.; Zhang, W.; Chen, J.; Zhao, Y. Mn3O4 nanoshell coated metal–organic frameworks with microenvironment-driven O2 production and GSH exhaustion ability for enhanced chemodynamic and photodynamic cancer therapies. Adv. Healthc. Mater. 2023, 12, 2202280. [Google Scholar] [CrossRef]
- Huang, N.; Tang, X.-Y.; Meng, W.; Lai, Y.-H.; Zhou, X.; Yu, X.-Z.; Zhang, W.-H.; Chen, J.-X. Immunogenic radiation therapy for enhanced antitumor immunity via a core–shell nanosensitizer-mediated immunosuppressive tumor microenvironment modulation. ACS Nano 2023, 17, 19853–19864. [Google Scholar] [CrossRef]
- Hou, Y.-K.; Zhang, Z.-J.; Li, R.-T.; Peng, J.; Chen, S.-Y.; Yue, Y.-R.; Zhang, W.-H.; Sun, B.; Chen, J.-X.; Zhou, Q. Remodeling the tumor microenvironment with core–shell nanosensitizer featuring dual-modal imaging and multimodal therapy for breast cancer. ACS Appl. Mater. Interfaces 2023, 15, 2602–2616. [Google Scholar] [CrossRef]
- Pan, W.-L.; Tan, Y.; Meng, W.; Huang, N.-H.; Zhao, Y.-B.; Yu, Z.-Q.; Huang, Z.; Zhang, W.-H.; Sun, B.; Chen, J.-X. Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials 2022, 283, 121449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, W.; Chen, M.; Zhang, B.; Zhang, L.; Li, P. The applications of nanozymes in cancer therapy: Based on regulating pyroptosis, ferroptosis and autophagy of tumor cells. Nanoscale 2023, 15, 12137–12156. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Qin, X.; Liu, J. Photothermal-chemotherapy integrated nanoparticles with tumor microenvironment response enhanced the induction of immunogenic cell death for colorectal cancer efficient treatment. ACS Appl. Mater. Interfaces 2019, 11, 43393–43408. [Google Scholar] [CrossRef]
- Huang, N.; Qian, A.; Zou, Y.; Lin, M.; Pan, W.; Chen, M.; Meng, W.; Zhang, W.; Chen, J. Immunogenic radiation therapy for enhanced anti-tumor immunity via core-shell nanocomposite-mediated multiple strategies. Theranostics 2023, 13, 4121–4137. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H. Oxaliplatin induces ferroptosis and oxidative stress in HT29 colorectal cancer cells by inhibiting the Nrf2 signaling pathway. Exp. Ther. Med. 2022, 23, 394. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Yang, X.; Wu, Q.; An, P.; Jin, X.; Liu, W.; Huang, X.; Li, Y.; Yan, S.; et al. Auranofin mitigates systemic iron overload and induces ferroptosis via distinct mechanisms. Signal Transduct. Target. Ther. 2020, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, S.; Wang, T.; Li, S.; Wang, L.; Li, D.; Tian, Y.; Zhang, Q. Four-photon absorption iron complex for magnetic resonance/photoacoustic dual-model imaging and an enhanced ferroptosis process. Anal. Chem. 2023, 95, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Deng, Z.; Gao, J.; Liang, C.; Xia, H.; Zhang, P. An osmium-peroxo complex for photoactive therapy of hypoxic tumors. Nat. Commun. 2022, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ouyang, A.; He, J.; Xie, J.; Banerjee, S.; Zhang, Q.; Zhang, P. An ultrasound activated cyanine-rhenium(i) complex for sonodynamic and gas synergistic therapy. Chem. Commun. 2022, 58, 3314–3317. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yuan, H.; Chen, Y.; Peng, X.-X.; Wu, Y.; He, W.; Guo, Z. Type I photoreaction and photoinduced ferroptosis by a Ru(II) complex to overcome tumor hypoxia in photodynamic therapy. CCS Chem. 2022, 5, 1583–1591. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Zhang, J.; Sun, J.; Zhao, X.; Zhu, Y.; Wei, W.; Zhao, J.; Guo, Z. A ferroptosis-inducing iridium(III) complex. Sci. China Chem. 2020, 63, 65–72. [Google Scholar] [CrossRef]
- Liu, J.; Kang, D.W.; Fan, Y.; Nash, G.T.; Jiang, X.; Weichselbaum, R.R.; Lin, W. Nanoscale covalent organic framework with staggered stacking of phthalocyanines for mitochondria-targeted photodynamic therapy. J. Am. Chem. Soc. 2024, 146, 849–857. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, J.; Shen, W.; Lu, H.; Hou, X.; Liu, Y.; Xu, Y.; Wu, Q.; Xuan, Z.; Yang, Y.; et al. Engineering mitochondrial uncoupler synergistic photodynamic nanoplatform to harness immunostimulatory pro-death autophagy/mitophagy. Biomaterials 2022, 289, 121796. [Google Scholar] [CrossRef]
- Ke, L.; Wei, F.; Xie, L.; Karges, J.; Chen, Y.; Ji, L.; Chao, H. A biodegradable iridium(III) coordination polymer for enhanced two-photon photodynamic therapy using an apoptosis–ferroptosis hybrid pathway. Angew. Chem. Int. Ed. 2022, 61, e202205429. [Google Scholar] [CrossRef]
- Yuan, H.; Han, Z.; Chen, Y.; Qi, F.; Fang, H.; Guo, Z.; Zhang, S.; He, W. Ferroptosis photoinduced by new cyclometalated iridium(III) complexes and its synergism with apoptosis in tumor cell inhibition. Angew. Chem. Int. Ed. 2021, 60, 8174–8181. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Chu, X.; Dong, H.-L.; Hou, H.-Y.; Liu, Y. Recent advances in augmenting Fenton chemistry of nanoplatforms for enhanced chemodynamic therapy. Coord. Chem. Rev. 2023, 479, 215004. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine meets Fenton chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, S.; Liu, B.; Bian, Y.; Yuan, M.; Yang, C.; Meng, Q.; Chen, C.; Ma, P.a.; Lin, J. Fe(III)-naphthazarin metal–phenolic networks for glutathione-depleting enhanced ferroptosis–apoptosis combined cancer therapy. Small 2023, 19, 2207825. [Google Scholar] [CrossRef] [PubMed]
- Meda, L.; Ranghino, G.; Moretti, G.; Cerofolini, G.F. XPS detection of some redox phenomena in Cu-zeolites. Surf. Interface Anal. 2002, 33, 516–521. [Google Scholar] [CrossRef]
- Li, Q.; Xu, B.-W.; Zou, Y.-M.; Niu, R.-J.; Chen, J.-X.; Zhang, W.-H.; Young, D.J. Nanoscale two-dimensional FeII- and CoII-based metal−organic frameworks of porphyrin ligand for the photodynamic therapy of breast cancer. Molecules 2023, 28, 2125. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.-J.; Zhou, W.-F.; Liu, Y.; Yang, J.-Y.; Zhang, W.-H.; Lang, J.-P.; Young, D.J. Morphology-dependent third-order optical nonlinearity of a 2D Co-based metal–organic framework with a porphyrinic skeleton. Chem. Commun. 2019, 55, 4873–4876. [Google Scholar] [CrossRef] [PubMed]
- Valicsek, Z.; Horváth, O. Application of the electronic spectra of porphyrins for analytical purposes: The effects of metal ions and structural distortions. Microchem. J. 2013, 107, 47–62. [Google Scholar] [CrossRef]
- Basalla, A.J.; Kendrick, B.S. Correcting ultraviolet-visible spectra for baseline artifacts. J. Pharm. Sci. 2023, 112, 3240–3247. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, X.; Nie, Y.; Zheng, Q.; Shangguan, J.; Zhu, C.; Bustillo, K.C.; Ercius, P.; Wang, L.; Limmer, D.T.; et al. Defect-mediated ripening of core-shell nanostructures. Nat. Commun. 2022, 13, 2211. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef]
- Howard, M.D.; Lu, X.; Jay, M.; Dziubla, T.D. Optimization of the lyophilization process for long-term stability of solid–lipid nanoparticles. Drug Dev. Ind. Pharm. 2012, 38, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Rahman, S. Storage stabilisation of albumin-loaded chitosan nanoparticles by lyoprotectants. Trop. J. Pharm. Res. 2013, 2, 135–142. [Google Scholar]
- Mahesh, K.V.; Singh, S.K.; Gulati, M. A comparative study of top-down and bottom-up approaches for the preparation of nanosuspensions of glipizide. Powder Technol. 2014, 256, 436–449. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Zu, Y.; Zhang, Y.; Li, Y.; Sun, W.; Shan, C.; Ge, Y. Preparation and characterization of betulin nanoparticles for oral hypoglycemic drug by antisolvent precipitation. Drug Deliv. 2014, 21, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Chou, H.-L.; Peng, Y.-K. Disclosing the origin of transition metal oxides as peroxidase (and catalase) mimetics. ACS Appl. Mater. Interfaces 2022, 14, 22728–22736. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-T.; Zhu, Y.-D.; Li, W.-Y.; Hou, Y.-K.; Zou, Y.-M.; Zhao, Y.-H.; Zou, Q.; Zhang, W.-H.; Chen, J.-X. Synergistic photothermal-photodynamic-chemotherapy toward breast cancer based on a liposome-coated core–shell AuNS@NMOFs nanocomposite encapsulated with gambogic acid. J. Nanobiotechnology 2022, 20, 212. [Google Scholar] [CrossRef] [PubMed]
- Cacaccio, J.; Durrani, F.; Cheruku, R.R.; Borah, B.; Ethirajan, M.; Tabaczynski, W.; Pera, P.; Missert, J.R.; Pandey, R.K. Pluronic F-127: An efficient delivery vehicle for 3-(1′-hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH or Photochlor). Photochem. Photobiol. 2020, 96, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Entradas, T.; Waldron, S.; Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Çol, S.; Emirik, M.; Alım, Z.; Baran, A. Physical–chemical studies of new, versatile carbazole derivatives and zinc complexes: Their synthesis, investigation of in vitro inhibitory effects on α-glucosidase and human erythrocyte carbonic anhydrase I and II isoenzymes. Appl. Organomet. Chem. 2022, 36, e6799. [Google Scholar] [CrossRef]
- Rozenberga, L.; Skinner, W.; Lancaster, D.G.; Bloch, W.M.; Blencowe, A.; Krasowska, M.; Beattie, D.A. A europium metal–organic framework for dual Fe3+ ion and pH sensing. Sci. Rep. 2022, 12, 11982. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-L.; Yuan, Y.-Q.; Chao, M.-Y.; Young, D.J.; Zhang, W.-H.; Lang, J.-P. Deciphering the structural relationships of five Cd-based metal–organic frameworks. Inorg. Chem. 2017, 56, 6522–6531. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Shi, B.; Ge, F.; Liu, Y.; Mao, Z.; Zhu, H.; Wang, S.; Yu, G.; Huang, F.; et al. Dual-emissive platinum(II) metallacage with a sensitive oxygen response for imaging of hypoxia and imaging-guided chemotherapy. Angew. Chem. Int. Ed. 2020, 59, 20208–20214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, J.; Huang, W.; Zhao, Y.; Yang, X. Thermosensitive nanogels with cross-linked Pd(II) ions for improving therapeutic effects on platinum-resistant cancers via intratumoral formation of hydrogels. Chem. Mater. 2019, 31, 5089–5103. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, H.; Li, Y.; Qin, Y.; Xu, L.; Bowers, D.J.; Gangoda, M.; Li, X.; Yang, H.-B.; Zheng, Y.-R. Coordination-driven self-assembly of a Pt(IV) prodrug-conjugated supramolecular hexagon. Chem. Commun. 2018, 54, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wu, X.; Zhao, G.; Feng, K.; Ni, K.; Sun, X. Renal clearable ultrasmall single-crystal Fe nanoparticles for highly selective and effective ferroptosis therapy and immunotherapy. J. Am. Chem. Soc. 2021, 143, 15812–15823. [Google Scholar] [CrossRef]
- Wu, M.; Ling, W.; Wei, J.; Liao, R.; Sun, H.; Li, D.; Zhao, Y.; Zhao, L. Biomimetic photosensitizer nanocrystals trigger enhanced ferroptosis for improving cancer treatment. J. Control. Release 2022, 352, 1116–1133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, W.; Abdul Razak, S.R.; Han, T.; Ahmad, N.H.; Li, X. Ferroptosis as a potential target for cancer therapy. Cell Death Dis. 2023, 14, 460. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.-l.; Huang, Z.-j.; Lin, Z.-t.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, M.; Bhattarai, P.; Hameed, S.; Tang, Y.; Dai, Z. Complementing cancer photodynamic therapy with ferroptosis through iron oxide loaded porphyrin-grafted lipid nanoparticles. ACS Nano 2021, 15, 20164–20180. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Song, L.; Pan, W.; Zhong, H.; Li, N.; Tang, B. Tumor-targeted cascade nanoreactor based on metal–organic frameworks for synergistic ferroptosis–starvation anticancer therapy. ACS Nano 2020, 14, 11017–11028. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS (Version 2.03): Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]
- Conrad, M.; Proneth, B. Broken hearts: Iron overload, ferroptosis and cardiomyopathy. Cell Res. 2019, 29, 263–264. [Google Scholar] [CrossRef]
- Fang, X.; Cai, Z.; Wang, H.; Min, J.; Wang, F. Role of iron overload and ferroptosis in heart disease. Chin. Sci. Bull. 2019, 64, 2974–2987. [Google Scholar]


| Fe-TMPP | |
|---|---|
| CCDC number | 2330690 |
| Formula | C44H34Cl9Fe3N8 |
| Formula weight | 1161.39 |
| Crystal system | Triclinic |
| Space group | P − 1 |
| T (K) | 213(2) |
| a/Å | 9.5980(6) |
| b/Å | 11.0408(7) |
| c/Å | 14.4682(10) |
| α/° | 70.702(3) |
| β/° | 86.792(3) |
| γ/° | 75.171(2) |
| V/Å3 | 1398.16(16) |
| Z | 1 |
| Dc/(g cm−3) | 1.379 |
| F(000) | 585 |
| μ (Mo–Kα)/mm−1 | 1.234 |
| Total reflections | 29,635 |
| Unique reflections | 4006 |
| Observed reflections | 2844 |
| No parameters | 339 |
| Rint | 0.0778 |
| R a | 0.1007 |
| wR b | 0.2811 |
| GOF c | 1.055 |
| Drug Formulation | Cell Line | IC50 | Reference |
|---|---|---|---|
| Cisplatin | 4T1 | 7.43 μM | [43] |
| Cisplatin | MCF7 | 43.0 μM | [44] |
| Cisplatin | A549 | 16.4 μM | [45] |
| Cisplatin | HT29 | 24.8 μM | [45] |
| Fe-TBP | CT26 | 3.10 μM | [46] |
| bcc-USINPs | Hep-G2 | 15.7 μg mL−1 | [47] |
| AE@RBC/Fe NCs | HSC-3 | 7.80 μM | [48] |
| Fe-TMPP | HCT-116 | 3.97 μM | This work † |
| Fe-TMPP | 4T1 | 2.00 μM | This work † |
| Fe-TMPP | HuH-7 | 3.68 μM | This work † |
| Fe-TMPP | BXPC3 | 3.11 μM | This work † |
| Fe-TMPP | DLD-1 | 0.231 μM | This work † |
| Fe-TMPP | PC3 | 0.213 μM | This work † |
| Fe-TMPP | AGS | 0.0975 μM | This work † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Feng, J.-H.; Zeng, C.-M.; Zhang, Z.-S.; Cao, F.-L.; Zhang, W.-H.; Chen, J.-X.; Young, D.J. [FeIIICl(TMPPH2)][FeIIICl4]2: A Stand-Alone Molecular Nanomedicine That Induces High Cytotoxicity by Ferroptosis. Molecules 2024, 29, 2495. https://doi.org/10.3390/molecules29112495
Wang X, Feng J-H, Zeng C-M, Zhang Z-S, Cao F-L, Zhang W-H, Chen J-X, Young DJ. [FeIIICl(TMPPH2)][FeIIICl4]2: A Stand-Alone Molecular Nanomedicine That Induces High Cytotoxicity by Ferroptosis. Molecules. 2024; 29(11):2495. https://doi.org/10.3390/molecules29112495
Chicago/Turabian StyleWang, Xiao, Jia-Hao Feng, Chun-Mei Zeng, Ze-Sheng Zhang, Feng-Lin Cao, Wen-Hua Zhang, Jin-Xiang Chen, and David J. Young. 2024. "[FeIIICl(TMPPH2)][FeIIICl4]2: A Stand-Alone Molecular Nanomedicine That Induces High Cytotoxicity by Ferroptosis" Molecules 29, no. 11: 2495. https://doi.org/10.3390/molecules29112495
APA StyleWang, X., Feng, J.-H., Zeng, C.-M., Zhang, Z.-S., Cao, F.-L., Zhang, W.-H., Chen, J.-X., & Young, D. J. (2024). [FeIIICl(TMPPH2)][FeIIICl4]2: A Stand-Alone Molecular Nanomedicine That Induces High Cytotoxicity by Ferroptosis. Molecules, 29(11), 2495. https://doi.org/10.3390/molecules29112495







