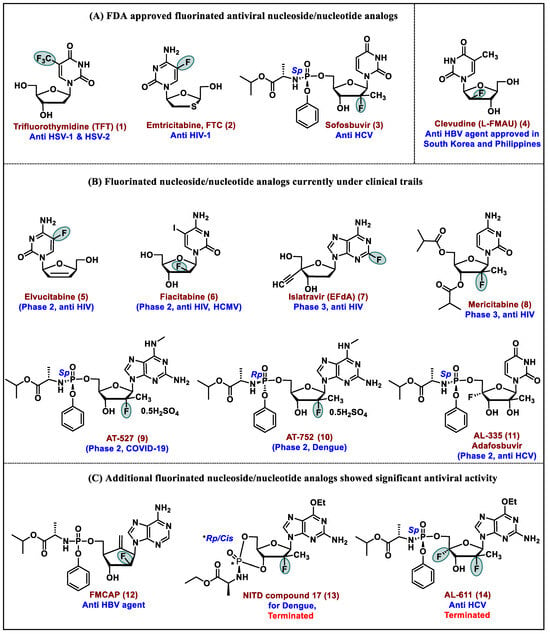
Abstract
The FDA has approved several drugs based on the fluorinated nucleoside pharmacophore, and numerous drugs are currently in clinical trials. Fluorine-containing nucleos(t)ides offer significant antiviral and anticancer activity. The insertion of a fluorine atom, either in the base or sugar of nucleos(t)ides, alters its electronic and steric parameters and transforms the lipophilicity, pharmacodynamic, and pharmacokinetic properties of these moieties. The fluorine atom restricts the oxidative metabolism of drugs and provides enzymatic metabolic stability towards the glycosidic bond of the nucleos(t)ide. The incorporation of fluorine also demonstrates additional hydrogen bonding interactions in receptors with enhanced biological profiles. The present article discusses the synthetic methodology and antiviral activities of FDA-approved drugs and ongoing fluoro-containing nucleos(t)ide drug candidates in clinical trials.
1. Introduction
In the past several decades, many fluoro-containing nucleos(t)ide drugs have been approved both in antiviral and anticancer therapies [1]. Nucleos(t)ides are fundamental nucleic acid fragments and are essential molecules for all living systems, including for the synthesis of DNA and RNA [2]. These molecules also play a significant role as chemotherapeutic agents in treating cancer and viral infections via selectively targeting certain enzymes of cancer and enzymes necessary for viral replication [3,4]. In the late 1980s, the unprecedented condition of acquired immunodeficiency syndrome (AIDS) caused by the human immunodeficiency virus (HIV) accelerated the discovery and development of nucleoside molecules as antivirals and since then, several FDA-approved nucleoside drugs have emerged in clinical practice [5]. Recently, it has been made evident that nucleos(t)ide-based drugs have played a prominent role in the cure and eradication of the COVID-19 infection, and these molecules have contributed significant aid in helping to overcome the pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [6].
Medicinal chemists engage in the design and synthesis of modified nucleos(t)ides with an altered nucleobase or sugar to discover new nucleoside-based therapeutic agents. The altered nucleobase and sugar techniques increase the selectivity and efficacy of nucleos(t)ides against specific viral enzymes without causing toxicity to the host [3,7]. In the past two decades, much advancement has been made in the design and synthesis of modified nucleos(t)ide analogs, and these endeavors invented various new classes of nucleos(t)ide-based drugs [8]. The selective insertion of fluorine atoms in a naturally or biologically active moiety often reveals an enhanced biological profile of interest. The electronegativity of fluorine and its capacity to add an additional hydrogen bond in receptors has gained much attention in drug design. In the area of nucleos(t)ide drugs, the incorporation of a fluorine atom either in the base or sugar also demonstrates better antiviral and anticancer activities and several fluoro-nucleos(t)ide drugs are in clinics. It has been estimated that more than 20% of drug candidates and 50% of agrochemicals contain one or more F atoms [9,10].
Fluorine is a magical atom in chemistry, which displays diverse pharmacological effects in biologically active molecules. The unique property of fluorine may substantially change the chemical, physical, and biological properties of active molecules [11]. It modulates and influences the pharmacokinetic and pharmacodynamic properties of drugs [12]. Incorporating a fluorine atom in organic moiety is beneficial due to its high electronegativity (3.98 on the Pauling electronegativity scale) [13]. The electronegativity of fluorine favors a firm and highly polarized C-F bond that improves the stability of small organic molecules. It also affects the acidic and basic properties of adjacent functional groups by impacting the overall pKa of the molecule [14]. The greater stability of the C-F bond compared to the C-H bond can prevent oxidative metabolism, thus making the C-F bond more resistant to oxidation and degradation than the C-H bond and restricting the formation of undesired metabolites [15]. The relatively small size of fluorine (van der Waals radius of 1.47 Å) closely mimics hydrogen without changing the geometry of molecules and demonstrates additional hydrogen bonding to a receptor or enzyme with minimal steric effects [16]. Fluorine is a bioisostere of hydrogen that expresses better lipophilicity than hydrogen with a reduced basicity of organic molecules, which enhances the cell penetration of molecules and drives them for easy delivery to the active site [17]. It also generates a significant role in the oral bioavailability of drugs via better absorption. Furthermore, the mentioned rationales underscore the significance of fluorine insertion, elucidating its pivotal role in drug design and the discovery of novel clinical drug candidates [18].
The presence of a fluorine atom in the ribose or carbocyclic ring of nucleos(t)ide impacts the glycosidic bond strength and increases enzymatic and metabolic stability [19]. It also affects the dipole–dipole, gauche, and F-base interactions [15]. The potential activity and stability of fluorinated nucleos(t)ides varies according to the substitution of fluorine atom(s) in different positions on nucleoside [20]. It is predicated but not proven that fluorine at the 2′-β-position of the ribose favors a south conformation of the molecule and is linked to DNA virus activity. In contrast, the 2′-α-position favors a north conformation and demonstrates activity against RNA viruses [21]. The dynamic equilibrium between two furanose puckering forms is the characteristic 3′-endo/2′-exo or ‘North’ (N) and 2′-endo/3′-exo or ‘South’ (S) ring conformations [22].
The development of the fluorinated drug 5-fluorouracil (5-FU) as an anticancer agent prompted extensive interest in developing fluorinated nucleoside/nucleotide analogs (NA) as therapeutic agents [23]. This has further been accelerated by the rapid development of synthetic methodologies in organofluorine chemistry. The substitution of the hydroxy or hydrogen in the sugar part or base of nucleosides by the fluorine atom leads to minimal steric effects and enhances the metabolic stability of nucleos(t)ides [18]. The present review article sheds light on the synthesis and antiviral activity of fluorinated nucleos(t)ides. In this article, we emphasize the synthesis and therapeutic importance of fluorinated nucleos(t)ides as antiviral drugs, along with candidates that are currently under clinical trials.
2. Synthesis of Fluorinated-Nucleos(t)ides
Two main approaches have been implemented in the synthesis of fluorinated nucleos(t)ides: one is the direct fluorination of the nucleoside moiety (divergent approach), and the second is a coupling of a fluorinated sugar or nucleobase with each other (convergent approach) [21]. Direct fluorination on the nucleoside is a linear strategy that retains the original configuration of the nucleoside. However, the coupling of nucleobases or heterocycles with a ribose sugar or carbocyclic ring limits the construction of the selective desired β-conformation of the nucleoside and often deals with a poor stereoselective N-glycosylation or coupling [19].
3. General Synthetic Approaches for The Fluoro-containing Nucleosides
The N-glycosylation of the fluoro-containing sugar or carbocyclic ring system with nucleobases is a common strategy. The coupling of the fluorinated sugar with base or heterocycles is a preferable method, where the direct fluorination on nucleoside is either not feasible or retains low yields with fluorinating agents. There are several known modifications on ribose and carbocyclic rings at 1′,2′,3′,4′ and 5′ that have been reported [3,7]. Among these, 2-deoxy-2′-F-ribo nucleos(t)ides demonstrated potential antiviral activities. It is well known that the substitution of 2′-OH by fluorine slows or even abolishes the enzymatic catalysis of the glycosidic bond without changing the nucleoside’s original confirmation [24,25].
The biological profile of C-2′-transformed nucleosides demonstrates enhanced antiviral properties of this class of molecules. Initially, Fox and co-workers introduced a series of 2′-deoxy-2′-fluoro analogs of uridine, 5-fluorouridine, and cytidine by treatment of 2,2′-anhydro nucleosides with hydrogen fluoride [26]. Since then, many strategies have been invented for the synthesis of 2′-fluoro nucleoside analogs, and these molecules have been examined thoroughly for biological properties (especially as an antiviral and anticancer agent). A general strategy for synthesizing fluoro-containing nucleos(t)ide is depicted in Figure 1.
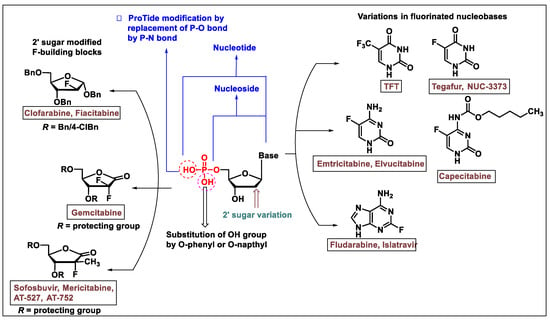
Figure 1.
A representation of the general modifications at base, sugar, and prodrug forms of the approved nucleosides or nucleotide molecules, which are either approved drug or in various stages of clinical development.
The synthesis of the 2′-deoxy-2′-β-fluoro ribanofuranose (ara-F nucleosides) and 2-deoxy-2′-α-fluoro of nucleos(t)ide analogs are well-explored in the antiviral drug discovery. The insertion of fluorine at the 2′-position of ribose or carbocyclic rings enhances the antiviral activity. The convergent approach is more frequently used in the synthesis of 2′-α/β-fluoro nucleosides. Convergent approaches allow nucleoside chemists to perform variations in the sugar or carbocyclic moieties in diverse ways. For specific nucleos(t)ides where a fluorine atom is strategically placed at either purine or pyrimidine bases (as shown in Figure 1) the desired fluorinated bases are employed in N-glycosylation reactions to synthesize the required fluorinated nucleosides. Various drugs have been introduced that contain fluorine atoms on the nucleobase and are synthesized via a convergent approach [1,3,19].
4. Biological Importance of Fluorinated Nucleos(t)ide
In the past 50 years, based on fluorinated nucleos(t)ide molecules, several clinical candidates have emerged for the treatment of viral infections and cancers [1,27]. Majorly, fluoro-nucleos(t)ides and fluoro heterocyclic bases target thymidylate synthase (TS), ribonucleotide diphosphate reductase (RDPR) [28], and viral polymerases by which these molecules express anticancer and antiviral potency. Installing fluorine in the nucleos(t)ides also enhances the selectivity and specificity of these moieties towards the viral DNA and RNA polymerase [29].
6. Conclusions
Nucleos(t)ides (NAs) are widely known for their antiviral and anticancer potency; among them, fluoro-containing nucleos(t)ides exert more prominent clinical candidates than their non-fluoro parent counterparts. Several fluoro-containing nuclosi(t)des have emerged as FDA-approved drugs for the treatment of viral infections. The insertion of fluorine/CF3 at the sugar or bases moiety of a nucleoside and the installation of 5-F/CF3, especially at the pyrimidine ring of nucleosides, have resulted in the invention of numerous antiviral drug candidates. The therapeutic molecules of this class selectively and specifically target the viral DNA/RNA polymerase and inhibit viral growth. This review article elucidates the synthesis and antiviral activity of FDA-approved fluro-nucleos(t)ide drugs and covers various fluoro nucleosides, which are at the various stages of clinical development as antiviral agents.
Author Contributions
Conceptualization, Y.K., R.A.J., C.K.C. and U.S.S.; writing—original draft preparation: Y.K. and R.A.J.; writing—review, and editing: C.K.C. and U.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are thankful to Emily Elizabeth Chanceller and Harishchandra Prasad Thoomu for proofreading.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Clercq, E.; Li, G.D. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [PubMed]
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Zenchenko, A.A.; Drenichev, M.S.; Il’icheva, I.A.; Mikhailov, S.N. Antiviral and Antimicrobial Nucleoside Derivatives: Structural Features and Mechanisms of Action. Mol. Biol. 2021, 55, 786–812. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Overbaugh, J.; Phillips, A.; Buchbinder, S. HIV infection. Nat. Rev. Dis. Primers 2015, 1, 15035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zheng, H.; Xiao, G.; Xie, X.; Rang, J.; Peng, D. Effectiveness and safety of azvudine in older adults with mild and moderate COVID-19: A retrospective observational study. BMC Infect. Dis. 2024, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Al Awadh, A.A. Nucleotide and nucleoside-based drugs: Past, present, and future. Saudi. J. Biol. Sci. 2022, 29, 103481. [Google Scholar]
- Inoue, M.; Sumii, Y.; Shibata, N. Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 2020, 5, 10633–10640. [Google Scholar] [CrossRef]
- Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23, 101467. [Google Scholar] [CrossRef]
- Buer, B.C.; Marsh, E.N. Fluorine: A new element in protein design. Protein Sci. 2012, 21, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Kitteringham, N.R.; Neill, P.M. Metabolism of Fluorine-Containing Drugs. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 443–470. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Taguchi, T.; Ojima, I. Unique Properties of Fluorine and their Relevance to Medicinal Chemistry and Chemical Biology. In Fluorine in Medicinal Chemistry and Chemical Biology; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–46. [Google Scholar]
- Shah, P.; Westwell, A.D. The role of fluorine in medicinal chemistry. J. Enzym. Inhib. Med. Chem. 2007, 22, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L. The C-F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem. 2010, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Faeh, C.; Diederich, F. Fluorine in pharmaceuticals: Looking beyond intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Meanwell, N.A. The Influence of Bioisosteres in Drug Design: Tactical Applications to Address Developability Problems. Tactics Contemp. Drug Des. 2015, 9, 283–381. [Google Scholar]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Sharon, A.; Chu, C.K. Fluorinated nucleosides: Synthesis and biological implication. J. Fluor. Chem. 2008, 129, 743–766. [Google Scholar] [CrossRef] [PubMed]
- Evich, M.; Spring-Connell, A.M.; Germann, M.W. Impact of modified ribose sugars on nucleic acid conformation and function. Heterocycl. Commun. 2017, 23, 155–165. [Google Scholar] [CrossRef]
- Cavaliere, A.; Probst, K.C.; Westwell, A.D.; Slusarczyk, M. Fluorinated nucleosides as an important class of anticancer and antiviral agents. Future Med. Chem. 2017, 9, 1809–1833. [Google Scholar] [CrossRef]
- Maderia, M.; Shenoy, S.; Van, Q.N.; Marquez, V.E.; Barchi, J.J., Jr. Biophysical studies of DNA modified with conformationally constrained nucleotides: Comparison of 2′-exo (north) and 3′-exo (south) ‘locked’ templates. Nucleic Acids Res. 2007, 35, 1978–1991. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.I.; Houlihan, G.; Holliger, P. Beyond DNA and RNA: The Expanding Toolbox of Synthetic Genetics. Cold Spring Harb. Perspect. Biol. 2019, 11, 6. [Google Scholar] [CrossRef]
- Schaerer, O.D.; Verdine, G.L. A Designed Inhibitor of Base-Excision DNA Repair. J. Am. Chem. Soc. 1995, 117, 10781–10782. [Google Scholar] [CrossRef]
- Lee, S.; Bowman, B.R.; Ueno, Y.; Wang, S.; Verdine, G.L. Synthesis and structure of duplex DNA containing the genotoxic nucleobase lesion N7-methylguanine. J. Am. Chem. Soc. 2008, 130, 11570–11571. [Google Scholar] [CrossRef]
- Codington, J.F.; Doerr, I.L.; Fox, J.J. Nucleosides. XVIII. Synthesis of 2′-Fluorothymidine, 2′-Fluorodeoxyuridine, and Other 2′-Halogeno-2′-Deoxy Nucleosides1,2. J. Org. Chem. 1964, 29, 558–564. [Google Scholar] [CrossRef]
- Hevey, R. The Role of Fluorine in Glycomimetic Drug Design. Chem. Eur. J. 2021, 27, 2240–2253. [Google Scholar] [CrossRef]
- Fukushima, M.; Fujioka, A.; Uchida, J.; Nakagawa, F.; Takechi, T. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur. J. Can. 2001, 37, 1681–1687. [Google Scholar] [CrossRef]
- Shet, H.; Sahu, R.; Sanghvi, Y.S.; Kapdi, A.R. Strategies for the Synthesis of Fluorinated Nucleosides, Nucleotides and Oligonucleotides. Chem. Rec. 2022, 22, e202200066. [Google Scholar] [CrossRef]
- Pal, S.; Chandra, G.; Patel, S.; Singh, S. Fluorinated Nucleosides: Synthesis, Modulation in Conformation and Therapeutic Application. Chem. Rec. 2022, 22, e202100335. [Google Scholar] [CrossRef]
- Choi, Y.K. Emerging and re-emerging fatal viral diseases. Exp. Mol. Med. 2021, 53, 711–712. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Dis. 2013, 12, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics. Curr. Hiv. Res. 2017, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.F.; Ayoup, M.S. Review on fluorinated nucleoside/non-nucleoside FDA-approved antiviral drugs. Rsc. Adv. 2022, 12, 31032–31045. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Agrofoglio, L.A. Nucleosides and emerging viruses: A new story. Drug Discov. Today 2022, 27, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
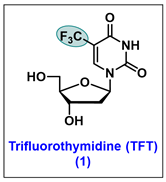
- Carmine, A.A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Trifluridine: A review of its antiviral activity and therapeutic use in the topical treatment of viral eye infections. Drugs 1982, 23, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Heidelberger, C. Therapeutic Antiviral Action of 5-Trifluoromethyl-2′-Deoxyuridine in Herpes Simplex Keratitis. Science 1964, 145, 585–586. [Google Scholar] [CrossRef]
- Umeda, M.; Heidelberger, C. Comparative studies of fluorinated pyrimidines with various cell lines. Cancer Res. 1968, 28, 2529–2538. [Google Scholar]
- Yamashita, J.; Takeda, S.; Matsumoto, H.; Unemi, N.; Yasumoto, M. Studies on antitumor agents. 8. Antitumor activities of O-alkyl derivatives of 2′-deoxy-5-(trifluoromethyl)uridine and 2′-deoxy-5-fluorouridine. J. Med. Chem. 1989, 32, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Prusoff, W.H.; Zucker, M.; Mancini, W.R.; Otto, M.J.; Lin, T.S.; Lee, J.J. Basic biochemical and pharmacological aspects of antiviral agents. Antivir. Res. 1985, 5, 1–10. [Google Scholar] [CrossRef]
- Wilhelmus, K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015, 1, CD002898. [Google Scholar] [CrossRef]
- Matsuoka, K.; Nakagawa, F.; Kobunai, T.; Takechi, T. Trifluridine/tipiracil overcomes the resistance of human gastric 5-fluorouracil-refractory cells with high thymidylate synthase expression. Oncotarget 2018, 9, 13438–13450. [Google Scholar] [CrossRef]
- Burness, C.B.; Duggan, S.T. Trifluridine/Tipiracil: A Review in Metastatic Colorectal Cancer. Drugs 2016, 76, 1393–1402. [Google Scholar] [CrossRef]
- Andersen, S.E.; Andersen, I.B.; Jensen, B.V.; Pfeiffer, P.; Ota, T.; Larsen, J.S. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta. Oncol. 2019, 58, 1149–1157. [Google Scholar] [CrossRef]
- Heidelberger, C.; Parsons, D.; Remy, D.C. Syntheses of 5-trifluoromethyluracil and 5-trifluoromethyl-2 ″-deoxyuridine. J. Am. Chem. Soc. 1962, 84, 3597–3598. [Google Scholar] [CrossRef]
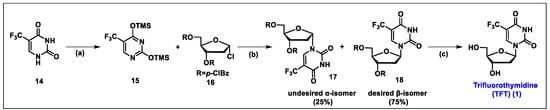
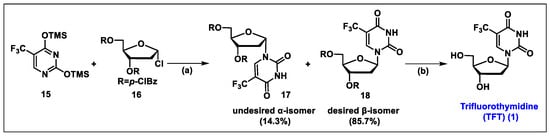
- Kobayashi, Y.; Yamamoto, K.; Asai, T.; Nakano, M.; Kumadaki, I. Studies on organic fluorine compounds. Part 35. Trifluoromethylation of pyrimidine- and purine-nucleosides with trifluoromethyl–copper complex. J. Chem. Soc. Perkin Trans. 1980, 1, 2755–2761. [Google Scholar] [CrossRef]
- Kawakami, H.; Takashi, E.; Koshi, K.; Hajime, M.; Yoshitake, N.; Kazuo, I.; Nobuhiro, M. The Synthesis of 2′-Deoxy-5-trifluoromethyluridine Utilizing a Coupling Reaction. Heterocycles 1990, 31, 569–574. [Google Scholar] [CrossRef]
- Komatsu, H.; Umetani, H. Synthesis of Trifluorothymidine: Green Glycosylation Condition Using Neither Chloroform nor Transition Metals. Org. Process Res. Dev. 2002, 6, 847–850. [Google Scholar] [CrossRef]
- Salvetti, R.; Pregnolato, M.; Verri, A.; Focher, F.; Spadari, S.; Marchand, A.; Mathe, C.; Gosselin, G. Synthesis and In Vitro Activity of D- and L-Enantiomers of 5-(Trifluoromethyl)Uracil Nucleoside Derivatives. Nucleosides Nucleotides Nucleic Acids 2001, 20, 1123–1125. [Google Scholar] [CrossRef]
- Kataoka, Y.; Iimori, M.; Niimi, S.; Tsukihara, H.; Wakasa, T.; Saeki, H.; Oki, E.; Maehara, Y.; Kitao, H. Cytotoxicity of trifluridine correlates with the thymidine kinase 1 expression level. Sci. Rep. 2019, 9, 7964. [Google Scholar] [CrossRef]
- Safrin, S. Treatment of acyclovir-resistant herpes simplex and varicella zoster virus infections. Antiv. Chemother. 4 1996, 394, 59–66. [Google Scholar]
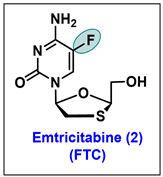
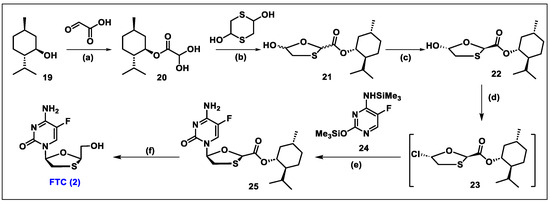
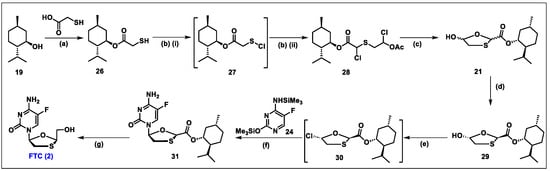
- Frampton, J.E.; Perry, C.M. Emtricitabine—A review of its use in the management of HIV infection. Drugs 2005, 65, 1427–1448. [Google Scholar] [CrossRef]
- Liotta, D.C.; Painter, G.R. Discovery and Development of the Anti-Human Immunodeficiency Virus Drug, Emtricitabine (Emtriva, FTC). Acc. Chem. Res. 2016, 49, 2091–2098. [Google Scholar] [CrossRef]
- Beach, J.W.; Jeong, L.S.; Alves, A.J.; Pohl, D.; Kim, H.O.; Chang, C.N.; Doong, S.L.; Schinazi, R.F.; Cheng, Y.C.; Chu, C.K. Synthesis of enantiomerically pure (2′R,5′S)-(-)-1-(2-hydroxymethyloxathiolan-5-yl)cytosine as a potent antiviral agent against hepatitis B virus (HBV) and human immunodeficiency virus (HIV). J. Org. Chem. 1992, 57, 2217–2219. [Google Scholar] [CrossRef]
- Schinazi, R.F.; McMillan, A.; Cannon, D.; Mathis, R.; Lloyd, R.M.; Peck, A.; Sommadossi, J.P.; St Clair, M.; Wilson, J.; Furman, P.A.; et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob. Agents Chemother. 1992, 36, 2423–2431. [Google Scholar] [CrossRef]
- Schinazi, R.F.; Boudinot, F.D.; Ibrahim, S.S.; Manning, C.; McClure, H.M.; Liotta, D.C. Pharmacokinetics and metabolism of racemic 2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rhesus monkeys. Antimicrob. Agents Chemother. 1992, 36, 2432–2438. [Google Scholar] [CrossRef]
- Mandala, D.; Thompson, W.A.; Watts, P. Synthesis routes to anti-HIV drugs. Tetrahedron 2016, 72, 3389–3420. [Google Scholar] [CrossRef]
- Gumina, G.; Song, G.Y.; Chu, C.K. L-Nucleosides as chemotherapeutic agents. FEMS Microbiol. Lett. 2001, 202, 9–15. [Google Scholar]
- Kraus, J.L.; Attardo, G. Synthesis of New 2,5-Substituted 1,3-Oxathiolanes. Intermediates in Nucleoside Chemistry. Synthesis 1991, 1991, 1046–1048. [Google Scholar] [CrossRef]
- Jin, H.; Siddiqui, M.A.; Evans, C.A.; Tse, H.A.; Mansour, T.S.; Goodyear, M.D.; Ravenscroft, P.; Beels, C.D. Diastereoselective synthesis of the potent antiviral agent (-)-2′-deoxy-3′-thiacytidine and its enantiomer. J. Org. Chem. 1995, 60, 2621–2623. [Google Scholar] [CrossRef]
- Cousins, R.P.C.; Mahmoudian, M.; Youds, P.M. Enzymic resolution of oxathiolane intermediates—An alternative approach to the anti-viral agent lamivudine (3TC™). Tetrahedron Asymmetry 1995, 6, 393–396. [Google Scholar] [CrossRef]
- Kraus, J.L. New Phosphonate Analogues of 3′-Thia-2′,3′-dideoxycytidine(BCH-189) Synthesis and Anti-HIV Evaluation. Nucleosides Nucleotides 1993, 12, 157–162. [Google Scholar] [CrossRef]
- Snead, D.R.; McQuade, D.T.; Ahmad, S.; Krack, R.; Stringham, R.W.; Burns, J.M.; Abdiaj, I.; Gopalsamuthiram, V.; Nelson, R.C.; Gupton, B.F. An Economical Route to Lamivudine Featuring a Novel Strategy for Stereospecific Assembly. Org. Process Rese. Dev. 2020, 24, 1194–1198. [Google Scholar] [CrossRef]
- Goodyear, M.D.; Hill, M.L.; West, J.P.; Whitehead, A.J. Practical enantioselective synthesis of lamivudine (3TC (TM)) via a dynamic kinetic resolution. Tetrahedron Lett. 2005, 46, 8535–8538. [Google Scholar] [CrossRef]
- Mandala, D.; Watts, P. An Improved Synthesis of Lamivudine and Emtricitabine. Chemistryselect 2017, 2, 1102–1105. [Google Scholar] [CrossRef]
- Kashinath, K.; Snead, D.R.; Burns, J.M.; Stringham, R.W.; Gupton, B.F.; McQuade, D.T. Synthesis of an Oxathiolane Drug Substance Intermediate Guided by Constraint-Driven Innovation. Org. Process Res. Dev. 2020, 24, 2266–2270. [Google Scholar] [CrossRef]
- Emtriva (Emtricitabine) Capsule Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021500s019lbl.pdf (accessed on 5 December 2023).
- Ng, H.H.; Stock, H.; Rausch, L.; Bunin, D.; Wang, A.; Brill, S.; Gow, J.; Mirsalis, J.C. Tenofovir disoproxil fumarate: Toxicity, toxicokinetics, and toxicogenomics analysis after 13 weeks of oral administration in mice. Int. J. Toxicol. 2015, 34, 4–10. [Google Scholar] [CrossRef]
- Richman, D.D. Antiretroviral activity of emtricitabine, a potent nucleoside reverse transcriptase inhibitor. Antivir. Ther. 2001, 6, 83–88. [Google Scholar] [CrossRef]
- Perry, C.M. Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Disoproxil Fumarate Single-Tablet Regimen (Stribild®): A Review of Its Use in the Management of HIV-1 Infection in Adults. Drugs 2014, 74, 75–97. [Google Scholar] [CrossRef]
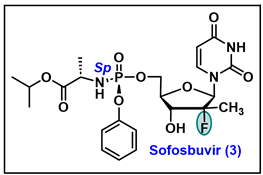
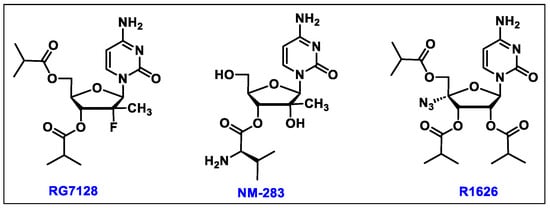
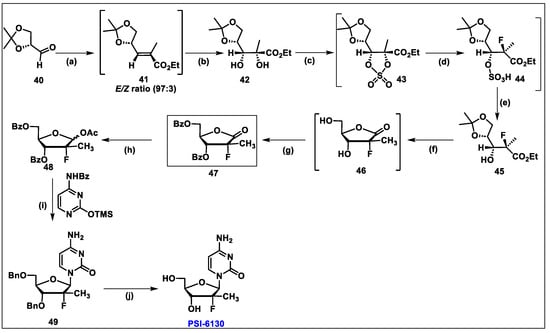
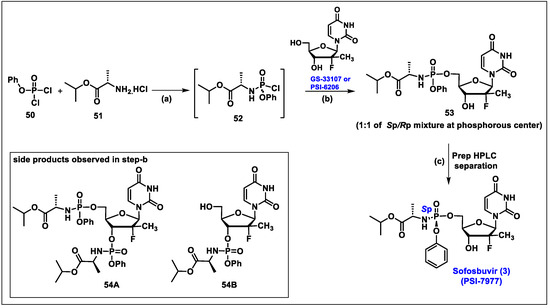
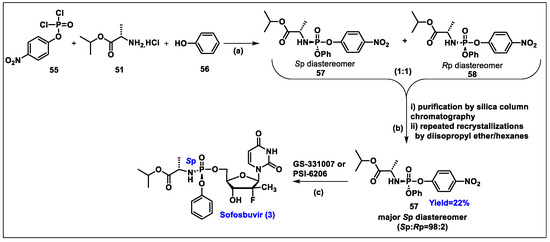
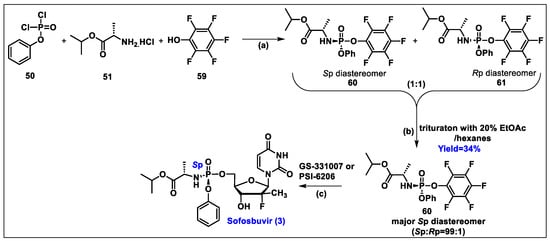
- Sofia, M.J.; Bao, D.; Chang, W.; Du, J.; Nagarathnam, D.; Rachakonda, S.; Reddy, P.G.; Ross, B.S.; Wang, P.; Zhang, H.R.; et al. Discovery of a beta-d-2′-deoxy-2′-alpha-fluoro-2′-beta-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. [Google Scholar] [CrossRef]
- Khalil, A.; El-Khouly, A.S.; Elkaeed, E.B.; Eissa, I.H. The Inhibitory Potential of 2′-dihalo Ribonucleotides against HCV: Molecular Docking, Molecular Simulations, MM-BPSA, and DFT Studies. Molecules 2022, 27, 4530. [Google Scholar] [CrossRef]
- Appleby, T.C.; Perry, J.K.; Murakami, E.; Barauskas, O.; Feng, J.; Cho, A.; Fox, D.; Wetmore, D.R.; McGrath, M.E.; Ray, A.S.; et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 2015, 347, 771–775. [Google Scholar] [CrossRef]
- de Albuquerque, P.; Santos, L.H.S.; Antunes, D.; Caffarena, E.R.; Figueiredo, A.S. Structural insights into NS5B protein of novel equine hepaciviruses and pegiviruses complexed with polymerase inhibitors. Virus Res. 2020, 278, 197867. [Google Scholar] [CrossRef]
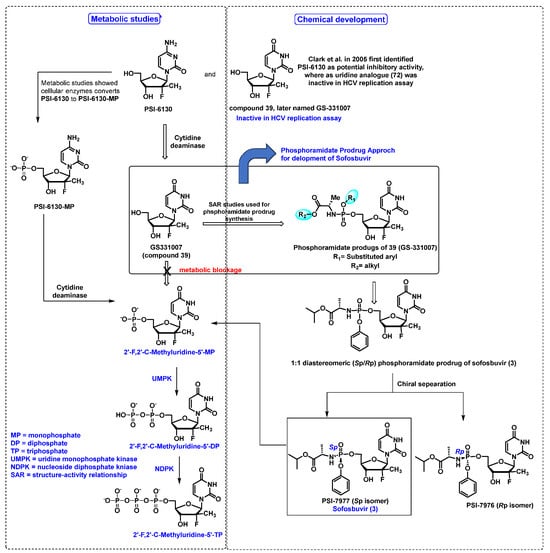
- Murakami, E.; Niu, C.; Bao, H.; Micolochick Steuer, H.M.; Whitaker, T.; Nachman, T.; Sofia, M.A.; Wang, P.; Otto, M.J.; Furman, P.A. The mechanism of action of beta-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine involves a second metabolic pathway leading to beta-D-2′-deoxy-2′-fluoro-2′-C-methyluridine 5′-triphosphate, a potent inhibitor of the hepatitis C virus RNA-dependent RNA polymerase. Antimicrob. Agents Chemother. 2008, 52, 458–464. [Google Scholar] [CrossRef]
- FDA Approves New Treatment for Pediatric Patients with Any Strain of Hepatitis C. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-pediatric-patients-any-strain-hepatitis-c (accessed on 21 February 2024).
- Xiao, F.; Fofana, I.; Thumann, C.; Mailly, L.; Alles, R.; Robinet, E.; Meyer, N.; Schaeffer, M.; Habersetzer, F.; Doffoel, M.; et al. Synergy of entry inhibitors with direct-acting antivirals uncovers novel combinations for prevention and treatment of hepatitis C. Gut 2015, 64, 483–494. [Google Scholar] [CrossRef]
- Clark, J.L.; Hollecker, L.; Mason, J.C.; Stuyver, L.J.; Tharnish, P.M.; Lostia, S.; McBrayer, T.R.; Schinazi, R.F.; Watanabe, K.A.; Otto, M.J.; et al. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J. Med. Chem. 2005, 48, 5504–5508. [Google Scholar] [CrossRef]
- Pockros, P.J. Emerging therapies for chronic hepatitis C virus. Gastroenterol. Hepatol. 2008, 4, 729–734. [Google Scholar]
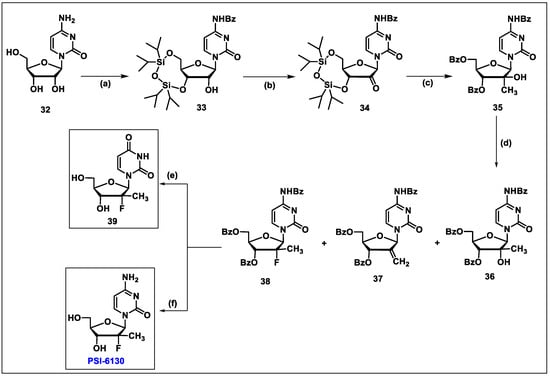
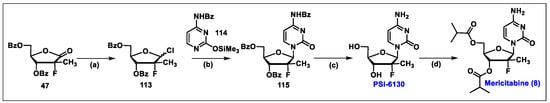
- Wang, P.; Chun, B.K.; Rachakonda, S.; Du, J.; Khan, N.; Shi, J.; Stec, W.; Cleary, D.; Ross, B.S.; Sofia, M.J. An efficient and diastereoselective synthesis of PSI-6130: A clinically efficacious inhibitor of HCV NS5B polymerase. J. Org. Chem. 2009, 74, 6819–6824. [Google Scholar] [CrossRef]
- Gao, Y.; Sharpless, K.B. Vicinal diol cyclic sulfates. Like epoxides only more reactive. J. Am. Chem. Soc. 1988, 110, 7538–7539. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, W.R.; Robledo, N.; Leveque, V.; Ali, S.; Lara-Jaime, T.; Masjedizadeh, M.; Smith, D.B.; Cammack, N.; Klumpp, K.; et al. Characterization of the metabolic activation of hepatitis C virus nucleoside inhibitor beta-D-2′-Deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130) and identification of a novel active 5′-triphosphate species. J. Biol. Chem. 2007, 282, 29812–29820. [Google Scholar] [CrossRef]
- Murakami, E.; Bao, H.; Ramesh, M.; McBrayer, T.R.; Whitaker, T.; Micolochick Steuer, H.M.; Schinazi, R.F.; Stuyver, L.J.; Obikhod, A.; Otto, M.J.; et al. Mechanism of activation of beta-D-2′-deoxy-2′-fluoro-2′-c-methylcytidine and inhibition of hepatitis C virus NS5B RNA polymerase. Antimicrob. Agents Chemother. 2007, 51, 503–509. [Google Scholar] [CrossRef]
- Thornton, P.J.; Kadri, H.; Miccoli, A.; Mehellou, Y. Nucleoside Phosphate and Phosphonate Prodrug Clinical Candidates. J. Med. Chem. 2016, 59, 10400–10410. [Google Scholar] [CrossRef]
- Slusarczyk, M.; Serpi, M.; Pertusati, F. Phosphoramidates and phosphonamidates (ProTides) with antiviral activity. Antivir. Chem. Chemother. 2018, 26, 2040206618775243. [Google Scholar] [CrossRef]
- Ross, B.S.; Reddy, P.G.; Zhang, H.R.; Rachakonda, S.; Sofia, M.J. Synthesis of Diastereomerically Pure Nucleotide Phosphoramidates. J. Org. Chem. 2011, 76, 8311–8319. [Google Scholar] [CrossRef]
- EPCLUSA® (Sofosbuvir and Velpatasvir) Tablets, for Oral Use Initial U.S. Approval: 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208341s009lbl.pdf (accessed on 16 December 2023).
- Sulkowski, M.S. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N. Engl. J. Med. 2014, 370, 1469. [Google Scholar] [CrossRef]
- Mumtaz, N.; Jimmerson, L.C.; Bushman, L.R.; Kiser, J.J.; Aron, G.; Reusken, C.; Koopmans, M.P.G.; van Kampen, J.J.A. Cell-line dependent antiviral activity of sofosbuvir against Zika virus. Antivir. Res. 2017, 146, 161–163. [Google Scholar] [CrossRef]
- Dragoni, F.; Boccuto, A.; Picarazzi, F.; Giannini, A.; Giammarino, F.; Saladini, F.; Mori, M.; Mastrangelo, E.; Zazzi, M.; Vicenti, I. Evaluation of sofosbuvir activity and resistance profile against West Nile virus in vitro. Antivir. Res. 2020, 175, 104708. [Google Scholar] [CrossRef]
- Xu, H.T.; Colby-Germinario, S.P.; Hassounah, S.A.; Fogarty, C.; Osman, N.; Palanisamy, N.; Han, Y.S.; Oliveira, M.; Quan, Y.D.; Wainberg, M.A. Evaluation of Sofosbuvir (beta-D-2′-deoxy-2′-alpha-fluoro-2′-beta-C-methyluridine) as an inhibitor of Dengue virus replication. Sci. Rep. 2017, 7, 6345. [Google Scholar] [CrossRef]
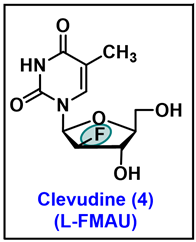
- Chu, C.K.; Ma, T.; Shanmuganathan, K.; Wang, C.; Xiang, Y.; Pai, S.B.; Yao, G.Q.; Sommadossi, J.P.; Cheng, Y.C. Use of 2′-fluoro-5-methyl-beta-L-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob. Agents Chemother. 1995, 39, 979–981. [Google Scholar] [CrossRef]
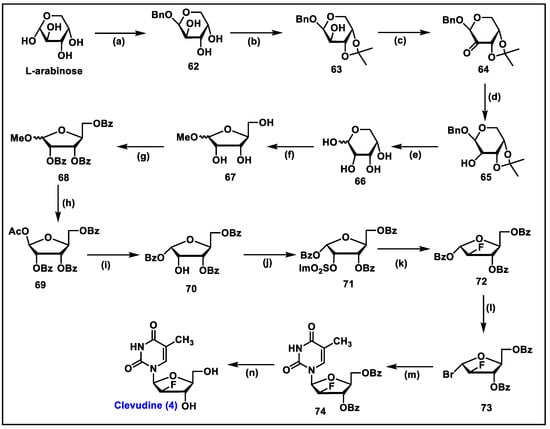
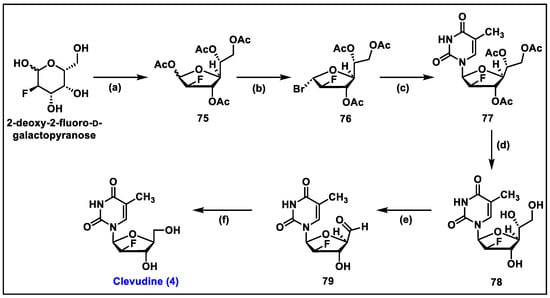
- Sharon, A.; Jha, A.K.; Chu, C.K. Clevudine, to Treat Hepatitis B Viral Infection. In Analogue-Based Drug Discovery II; edn.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 383–408. [Google Scholar]
- Du, J.; Choi, Y.; Lee, K.; Chun, B.K.; Hong, J.H.; Chu, C.K. A practical synthesis of L-FMAU from L-arabinose. Nucleosides Nucleotides 1999, 18, 187–195. [Google Scholar] [CrossRef]
- Sznaidman, M.L.; Almond, M.R.; Pesyan, A. New Synthesis of L-Fmau from L-Arabinose. Nucleosides Nucleotides Nucleic Acids 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Tremblay, T.; Alcée, J.B.; Giguère, D. Protecting-group-free synthesis of clevudine (l-FMAU), a treatment of the hepatitis B virus. Org. Biomol. Chem. 2022, 20, 8859–8863. [Google Scholar] [CrossRef]
- Yao, G.Q.; Liu, S.H.; Chou, E.; Kukhanova, M.; Chu, C.K.; Cheng, Y.C. Inhibition of Epstein-Barr virus replication by a novel L-nucleoside, 2′-fluoro-5-methyl-beta-L-arabinofuranosyluracil. Biochem. Pharmacol. 1996, 51, 941–947. [Google Scholar] [CrossRef]
- Niu, C.R.; Bao, H.Y.; Tolstykh, T.; Steuer, H.M.M.; Murakami, E.; Korba, B.; Furman, P.A. Evaluation of the in vitro anti-HBV activity of clevudine in combination with other nucleoside/nucleotide inhibitors. Antivir. Ther. 2010, 15, 401–412. [Google Scholar] [CrossRef]
- Lee, H.S.; Chung, Y.H.; Lee, K.; Byun, K.S.; Paik, S.W.; Han, J.Y.; Yoo, K.; Yoo, H.W.; Lee, J.H.; Yoo, B.C. A 12-week clevudine therapy showed potent and durable antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology 2006, 43, 982–988. [Google Scholar] [CrossRef]
- Korba, B.E.; Furman, P.A.; Otto, M.J. Clevudine: A potent inhibitor of hepatitis B virus in vitro and in vivo. Expert Rev. Anti. Infect. Ther. 2006, 4, 549–561. [Google Scholar] [CrossRef]
- Peek, S.F.; Cote, P.J.; Jacob, J.R.; Toshkov, I.A.; Hornbuckle, W.E.; Baldwin, B.H.; Wells, F.V.; Chu, C.K.; Gerin, J.L.; Tennant, B.C.; et al. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 2001, 33, 254–266. [Google Scholar] [CrossRef]
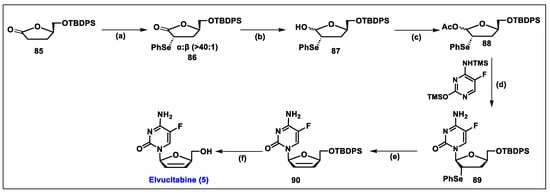
- An Open-Label Treatment Protocol to Provide Continued Elvucitabine Treatment. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT00675844 (accessed on 12 March 2024).
- Esposito, F.; Corona, A.; Tramontano, E. HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions. Mol. Biol. Int. 2012, 2012, 586401. [Google Scholar] [CrossRef]
- Lin, T.S.; Luo, M.Z.; Liu, M.C.; Zhu, Y.L.; Gullen, E.; Dutschman, G.E.; Cheng, Y.C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-beta-L-cytidine (beta-L-d4C) and 2′,3′-dideoxy 2′,3′-didehydro-beta-L-5-fluorocytidine (beta-L-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J. Med. Chem. 1996, 39, 1757–1759. [Google Scholar]
- Chen, S.H.; Li, X.Y.; Li, J.; Niu, C.S.; Carmichael, E.; Doyle, T.W. Stereoselective syntheses of beta-L-FD4C and beta-L-FddC. J. Org. Chem. 1997, 62, 3449–3452. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, N.D.; Seong, B.L. Discovery and Development of Anti-HBV Agents and Their Resistance. Molecules 2010, 15, 5878–5908. [Google Scholar] [CrossRef]
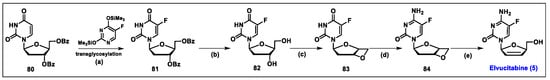
- Colucci, P.; Pottage, J.C.; Robison, H.; Turgeon, J.; Schurmann, D.; Hoepelman, I.M.; Ducharme, M.P. Multiple-Dose Pharmacokinetic Behavior of Elvucitabine, a Nucleoside Reverse Transcriptase Inhibitor, Administered over 21 Days with Lopinavir-Ritonavir in Human Immunodeficiency Virus Type 1-Infected Subjects. Antimicrob. Agents Chemother. 2009, 53, 662–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, S.H.; Wang, Q.; Mao, J.; King, I.; Dutschman, G.E.; Gullen, E.A.; Cheng, Y.C.; Doyle, T.W. Synthesis and biological evaluation of a series of 2′-fluorinated-2′,3′-dideoxy-2′,3′-didehydro-(l)-nucleosides. Bioorg. Med. Chem. Lett. 1998, 8, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; McAtee, J.J.; Schlueter Wirtz, S.; Tharnish, P.; Juodawlkis, A.; Liotta, D.C.; Schinazi, R.F. Synthesis and Biological Evaluation of 2‘,3‘-Didehydro-2‘,3‘-dideoxy-5- fluorocytidine (D4FC) Analogues: Discovery of Carbocyclic Nucleoside Triphosphates with Potent Inhibitory Activity against HIV-1 Reverse Transcriptase. J. Med. Chem. 1999, 42, 859–867. [Google Scholar] [CrossRef] [PubMed]
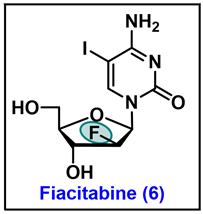
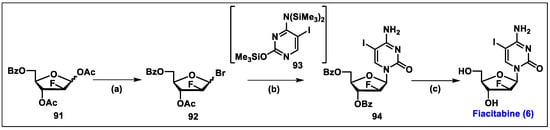
- The Safety and Effectiveness of FIAC in the Treatment of Cytomegalovirus (CMV) in Patients with AIDS. Available online: https://clinicaltrials.gov/ct2/show/NCT00000981?term=Fiacitabine&draw=2&rank=1 (accessed on 2 December 2023).
- Watanabe, K.A.; Su, T.L.; Klein, R.S.; Chu, C.K.; Matsuda, A.; Chun, M.W.; Lopez, C.; Fox, J.J. Nucleosides. 123. Synthesis of antiviral nucleosides: 5-substituted 1-(2-deoxy-2-halogeno-beta-D-arabinofuranosyl)cytosines and -uracils. Some structure-activity relationships. J. Med. Chem. 1983, 26, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Rollinson, E.A. Comparative efficacy of three 2′-fluoropyrimidine nucleosides and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (BW B759U) against pseudorabies and equine rhinopneumonitis virus infection in vitro and in laboratory animals. Antivir. Res. 1987, 7, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Coen, N.; Duraffour, S.; Topalis, D.; Snoeck, R.; Andrei, G. Spectrum of activity and mechanisms of resistance of various nucleoside derivatives against gammaherpesviruses. Antimicrob. Agents Chemother. 2014, 58, 7312–7323. [Google Scholar] [CrossRef]
- Colacino, J.M.; Lopez, C. Efficacy and Selectivity of Some Nucleoside Analogs as Anti-Human Cytomegalovirus Agents. Antimicrob. Agents Chemother. 1983, 24, 505–508. [Google Scholar] [CrossRef]
- Nikkels, A.F.; Pierard, G.E. Recognition and treatment of shingles. Drugs 1994, 48, 528–548. [Google Scholar] [CrossRef]
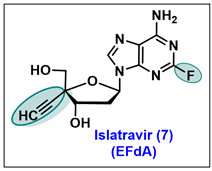
- Molina, J.M.; Yazdanpanah, Y.; Afani Saud, A.; Bettacchi, C.; Chahin Anania, C.; Klopfer, S.O.; Grandhi, A.; Eves, K.; Hepler, D.; Robertson, M.N.; et al. Brief Report: Efficacy and Safety of Oral Islatravir Once Daily in Combination with Doravirine through 96 Weeks for Treatment-Naive Adults with HIV-1 Infection Receiving Initial Treatment with Islatravir, Doravirine, and Lamivudine. J. Acquir. Immune. Defic. Syndr. 2022, 91, 68–72. [Google Scholar] [CrossRef]
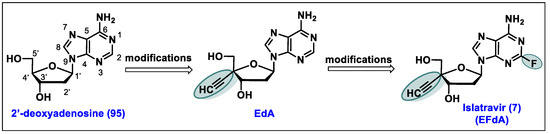
- Markowitz, M.; Sarafianos, S.G. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591: A novel HIV-1 reverse transcriptase translocation inhibitor. Curr. Opin. HIV AIDS 2018, 13, 294–299. [Google Scholar] [CrossRef]
- MK-8591. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=mk-8591&cntry=&state=&city=&dist= (accessed on 12 January 2024).
- Kohgo, S.; Yamada, K.; Kitano, K.; Iwai, Y.; Sakata, S.; Ashida, N.; Hayakawa, H.; Nameki, D.; Kodama, E.; Matsuoka, M.; et al. Design, efficient synthesis, and anti-HIV activity of 4′-C-cyano- and 4′-C-ethynyl-2′-deoxy purine nucleosides. Nucleosides Nucleotides Nucleic Acids 2004, 23, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Ohrui, H.; Kohgo, S.; Hayakawa, H.; Kodama, E.; Matsuoka, M.; Nakata, T.; Mitsuya, H. 2′-Deoxy-4′-C-ethynyl-2-fluoroadenosine: A nucleoside reverse transcriptase inhibitor with highly potent activity against all HIV-1 strains, favorable toxic profiles and stability in plasma. Nucleic Acids Symp. Ser. 2006, 50, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Schurmann, D.; Rudd, D.J.; Zhang, S.; De Lepeleire, I.; Robberechts, M.; Friedman, E.; Keicher, C.; Huser, A.; Hofmann, J.; Grobler, J.A.; et al. Safety, pharmacokinetics, and antiretroviral activity of islatravir (ISL, MK-8591), a novel nucleoside reverse transcriptase translocation inhibitor, following single-dose administration to treatment-naive adults infected with HIV-1: An open-label, phase 1b, consecutive-panel trial. Lancet HIV 2020, 7, e164–e172. [Google Scholar] [PubMed]
- Salie, Z.L.; Kirby, K.A.; Michailidis, E.; Marchand, B.; Singh, K.; Rohan, L.C.; Kodama, E.N.; Mitsuya, H.; Parniak, M.A.; Sarafianos, S.G. Structural basis of HIV inhibition by translocation-defective RT inhibitor 4′-ethynyl-2-fluoro-2′-deoxyadenosine (EFdA). Proc. Natl. Acad. Sci. USA 2016, 113, 9274–9279. [Google Scholar] [CrossRef]
- Ohrui, H. 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine, a nucleoside reverse transcriptase inhibitor, is highly potent against all human immunodeficiency viruses type 1 and has low toxicity. Chem. Rec. 2006, 6, 133–143. [Google Scholar] [CrossRef] [PubMed]
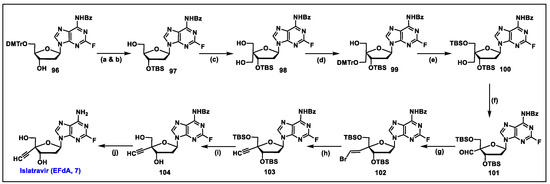
- Kageyama, M.; Miyagi, T.; Yoshida, M.; Nagasawa, T.; Ohrui, H.; Kuwahara, S. Concise synthesis of the anti-HIV nucleoside EFdA. Biosci. Biotechnol. Biochem. 2012, 76, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Ohrui, H.; Kuwahara, S. Synthesis of EFdA via a diastereoselective aldol reaction of a protected 3-keto furanose. Org. Lett. 2015, 17, 828–831. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Kong, J.; Belyk, K.M.; Chen, B.; Gibson, A.W.; Keen, S.P.; Lieberman, D.R.; Milczek, E.M.; Moore, J.C.; Murray, D.; et al. Enantioselective Synthesis of 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) via Enzymatic Desymmetrization. Org. Lett. 2017, 19, 926–929. [Google Scholar] [CrossRef] [PubMed]
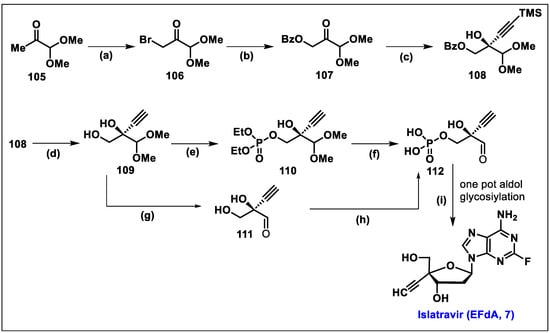
- Patel, N.R.; Nawrat, C.C.; McLaughlin, M.; Xu, Y.; Huffman, M.A.; Yang, H.; Li, H.; Whittaker, A.M.; Andreani, T.; Lévesque, F.; et al. Synthesis of Islatravir Enabled by a Catalytic, Enantioselective Alkynylation of a Ketone. Org. Lett. 2020, 22, 4659–4664. [Google Scholar] [CrossRef]
- Huffman, M.A.; Fryszkowska, A.; Alvizo, O.; Borra-Garske, M.; Campos, K.R.; Canada, K.A.; Devine, P.N.; Duan, D.; Forstater, J.H.; Grosser, S.T.; et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 2019, 366, 1255–1259. [Google Scholar] [CrossRef]
- Stoddart, C.A.; Galkina, S.A.; Joshi, P.; Kosikova, G.; Moreno, M.E.; Rivera, J.M.; Sloan, B.; Reeve, A.B.; Sarafianos, S.G.; Murphey-Corb, M.; et al. Oral Administration of the Nucleoside EFdA (4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine) Provides Rapid Suppression of HIV Viremia in Humanized Mice and Favorable Pharmacokinetic Properties in Mice and the Rhesus Macaque. Antimicrob. Agents Chemother. 2015, 59, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
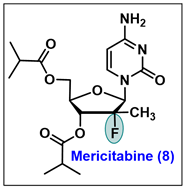
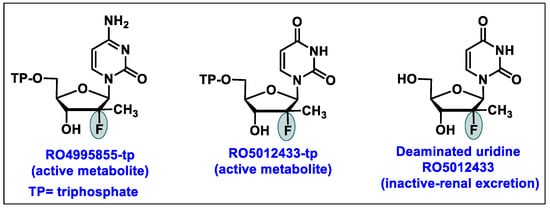
- Chen, Y.C.; Haznedar, J.; Kulkarni, R.; Vistuer, C.; Washington, C.; Liu, M.; Smith, P. Evaluation of the effect of mericitabine at projected therapeutic and supratherapeutic doses on cardiac repolarization in healthy subjects: A thorough QT/QTc study. Clin. Pharmacol. Drug Dev. 2014, 3, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Stuyver, L.J.; McBrayer, T.R.; Tharnish, P.M.; Clark, J.; Hollecker, L.; Lostia, S.; Nachman, T.; Grier, J.; Bennett, M.A.; Xie, M.Y. Inhibition of hepatitis C replicon RNA synthesis by β-D-2′-deoxy-2′-fluoro-2′-C-methylcytidine: A specific inhibitor of hepatitis C virus replication. Antivir. Chem. Chemother. 2006, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Washington, C.; Moreira, S.; Haznedar, J.; Goelzer, P.; Chen, Y.C. Single-dose Pharmacokinetics of the HCV Polymerase Inhibitor Mericitabine in Healthy Caucasian and Japanese Subjects. Drug Metab. Pharmacokinet. 2014, 29, 141–147. [Google Scholar] [CrossRef]
- Mericitabine. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=Mericitabine&cntry=&state=&city=&dist= (accessed on 1 February 2024).
- Carr, R.; Hildbrand, S.; Hodges, M.L.; Kammrer, M.; Lang, J.F.; Lawrimore, W.J., III; Nguyen, D. Process for the Preparation of 2-Deoxy-2-fluoro-2-methyl-D-dibofuranosyl Nucleoside Compounds. U.S. Patent 13/903,726, 12 2013. [Google Scholar]
- Pawlotsky, J.M.; Najera, I.; Jacobson, I. Resistance to mericitabine, a nucleoside analogue inhibitor of HCV RNA-dependent RNA polymerase. Antivir. Ther. 2012, 17, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Leveque, V.; Le Pogam, S.; Ma, H.; Philipp, F.; Inocencio, N.; Smith, M.; Alker, A.; Kang, H.; Najera, I.; et al. Selected Replicon Variants with Low-Level In Vitro Resistance to the Hepatitis C Virus NS5B Polymerase Inhibitor PSI-6130 Lack Cross-Resistance with R1479. Antimicrob. Agents Chemother. 2008, 52, 4356–4369. [Google Scholar] [CrossRef]
- Reddy, P.G.; Bao, D.; Chang, W.; Chun, B.K.; Du, J.; Nagarathnam, D.; Rachakonda, S.; Ross, B.S.; Zhang, H.R.; Bansal, S.; et al. 2′-deoxy-2′-alpha-fluoro-2′-beta-C-methyl 3′,5′-cyclic phosphate nucleotide prodrug analogs as inhibitors of HCV NS5B polymerase: Discovery of PSI-352938. Bioorg. Med. Chem. Lett. 2010, 20, 7376–7380. [Google Scholar] [CrossRef]
- Pockros, P.J.; Jensen, D.; Tsai, N.; Taylor, R.; Ramji, A.; Cooper, C.; Dickson, R.; Tice, A.; Kulkarni, R.; Vierling, J.M.; et al. A randomized trial of mericitabine plus pegylated interferon alpha-2a/ribavirin for 24 weeks in treatment-naive HCV genotype 1/4 patients. Hepatology 2013, 58, 514–523. [Google Scholar] [CrossRef]
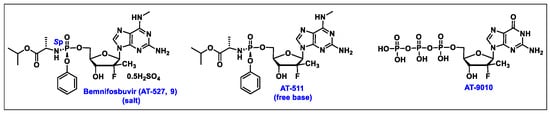
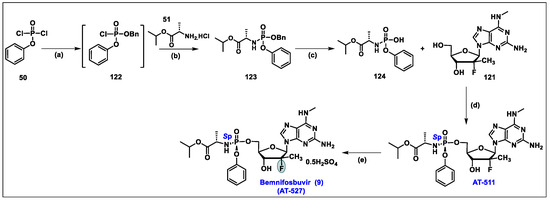
- Good, S.S.; Moussa, A.; Zhou, X.J.; Pietropaolo, K.; Sommadossi, J.P. Preclinical evaluation of AT-527, a novel guanosine nucleotide prodrug with potent, pan-genotypic activity against hepatitis C virus. PLoS ONE 2020, 15, 1. [Google Scholar] [CrossRef]
- Good, S.S.; Westover, J.; Jung, K.H.; Zhou, X.J.; Moussa, A.; La Colla, P.; Collu, G.; Canard, B.; Sommadossi, J.P. AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19. Antimicrob. Agents Chemother. 2021, 65, 4. [Google Scholar] [CrossRef]
- Berliba, E.; Bogus, M.; Vanhoutte, F.; Berghmans, P.J.; Good, S.S.; Moussa, A.; Pietropaolo, K.; Murphy, R.L.; Zhou, X.J.; Sommadossi, J.P. Safety, pharmacokinetics and antiviral activity of AT-527, a novel purine nucleotide prodrug, in HCV-infected subjects with and without cirrhosis. Antimicrob. Agents Chemother. 2019, 63, 12. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/study/NCT05904470 (accessed on 10 February 2024).
- Moussa, A. Stereoselective Manufacture of Selected Purine Phosphoramidates. U.S. Patent Application No. WO 2022/040473 A1, 24 February 2022. [Google Scholar]
- Study to Evaluate the Effects of AT-527 in Non-Hospitalized Adult Patients with Mild or Moderate COVID-19. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04709835 (accessed on 10 February 2024).
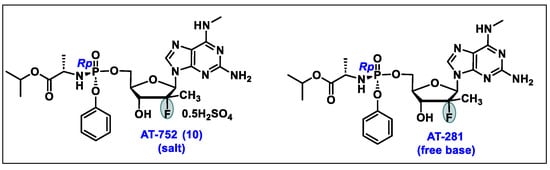
- AT-752. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=AT-752&cntry=&state=&city=&dist= (accessed on 10 February 2024).
- Good, S.S.; Shannon, A.; Lin, K.; Moussa, A.; Julander, J.G.; La Colla, P.; Collu, G.; Canard, B.; Sommadossi, J.P. Evaluation of AT-752, a Double Prodrug of a Guanosine Nucleotide Analog with In Vitro and In Vivo Activity against Dengue and Other Flaviviruses. Antimicrob. Agents Chemother. 2021, 65, e0098821. [Google Scholar] [CrossRef] [PubMed]
- Study of AT-752 in Healthy Subjects. Available online: https://clinicaltrials.gov/study/NCT04722627?cond=AT-752&rank=1 (accessed on 16 February 2024).
- Lin, K.; Good, S.S.; Julander, J.G.; Weight, A.E.; Moussa, A.; Sommadossi, J.P. AT-752, a double prodrug of a guanosine nucleotide analog, inhibits yellow fever virus in a hamster model. PLoS Negl. Trop. Dis. 2022, 16, e0009937. [Google Scholar] [CrossRef] [PubMed]
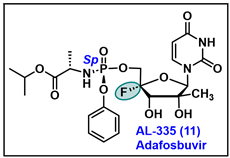
- Available online: https://clinicaltrials.gov/study/NCT02339207?cond=HCV%20Infection&term=AL-335&rank=2 (accessed on 6 March 2024).
- Available online: https://clinicaltrials.gov/study/NCT02569710?cond=HCV%20Infection&term=AL-335&rank=3&tab=results (accessed on 6 March 2024).
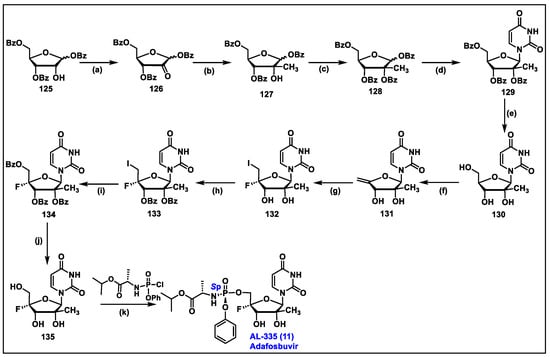
- Wang, G.; Dyatkina, N.; Prhavc, M.; Williams, C.; Serebryany, V.; Hu, Y.; Huang, Y.; Wan, J.; Wu, X.; Deval, J.; et al. Synthesis and Anti-HCV Activities of 4′-Fluoro-2′-Substituted Uridine Triphosphates and Nucleotide Prodrugs: Discovery of 4′-Fluoro-2′-C-methyluridine 5′-Phosphoramidate Prodrug (AL-335) for the Treatment of Hepatitis C Infection. J. Med. Chem. 2019, 62, 4555–4570. [Google Scholar] [CrossRef] [PubMed]
- McClure, M.W.; Berliba, E.; Tsertsvadze, T.; Streinu-Cercel, A.; Vijgen, L.; Astruc, B.; Patat, A.; Westland, C.; Chanda, S.; Zhang, Q.; et al. Safety, tolerability, and pharmacokinetics of AL-335 in healthy volunteers and hepatitis C virus-infected subjects. PLoS ONE 2018, 13, e0204974. [Google Scholar] [CrossRef] [PubMed]
- Leonid, B.; Guangyi, W.; David, B.S. Substituted Nucleosides, Nucleotdes and Analogs Thereof. U.S. Patent 9,243,022, 26 January 2016. [Google Scholar]
- Wang, G.; Lim, S.P.; Chen, Y.L.; Hunziker, J.; Rao, R.; Gu, F.; She, C.C.; Ghafar, N.A.; Xu, H.Y.; Chan, K.; et al. Structure-activity relationship of uridine-based nucleoside phosphoramidate prodrugs for inhibition of dengue virus RNA-dependent RNA polymerase. Bioorg. Med. Chem. Lett. 2018, 28, 2324–2327. [Google Scholar] [CrossRef] [PubMed]
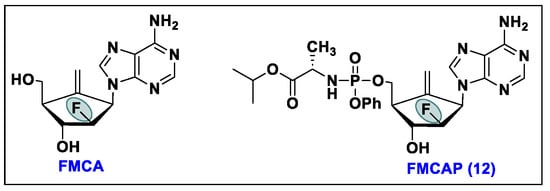
- Singh, U.S.; Mulamoottil, V.A.; Chu, C.K. 2′-Fluoro-6′-methylene carbocyclic adenosine and its phosphoramidate prodrug: A novel anti-HBV agent, active against drug-resistant HBV mutants. Med. Res. Rev. 2018, 38, 977–1002. [Google Scholar] [CrossRef] [PubMed]
- Rawal, R.K.; Singh, U.S.; Chavre, S.N.; Wang, J.N.; Sugiyama, M.; Hung, W.; Govindarajan, R.; Korba, B.; Tanaka, Y.; Chu, C.K. 2 ‘-Fluoro-6 ‘-methylene-carbocyclic adenosine phosphoramidate (FMCAP) prodrug: In vitro anti-HBV activity against the lamivudine-entecavir resistant triple mutant and its mechanism of action. Bioorg. Med. Chem. Lett. 2013, 23, 503–506. [Google Scholar] [CrossRef]
- Gadthula, S.; Rawal, R.K.; Sharon, A.; Wu, D.; Korba, B.; Chu, C.K. Synthesis and antiviral activity of cyclopropyl-spirocarbocyclic adenosine, (4R,5S,6R,7R)-4-(6-amino-9H-purin-9-yl)-7-(hydroxymethyl)spiro[2.4]heptane-5,6-diol against hepatitis C virus. Bioorg. Med. Chem. Lett. 2011, 21, 3982–3985. [Google Scholar] [CrossRef]
- Jin, Y.H.; Liu, P.; Wang, J.; Baker, R.; Huggins, J.; Chu, C.K. Practical Synthesis of d- and l-2-Cyclopentenone and Their Utility for the Synthesis of Carbocyclic Antiviral Nucleosides against Orthopox Viruses (Smallpox, Monkeypox, and Cowpox Virus). J. Org. Chem. 2003, 68, 9012–9018. [Google Scholar] [CrossRef]
- Gemal, A.L.; Luche, J.L. Lanthanoids in organic synthesis. 6. Reduction of. alpha.-enones by sodium borohydride in the presence of lanthanoid chlorides: Synthetic and mechanistic aspects. J. Am. Chem. Soc. 1981, 103, 5454–5459. [Google Scholar] [CrossRef]
- Wang, J.N.; Singh, U.S.; Rawal, R.K.; Sugiyama, M.; Yoo, J.; Jha, A.K.; Scroggin, M.; Huang, Z.H.; Murray, M.G.; Govindarajan, R.; et al. Antiviral activity of novel 2′-fluoro-6′-methylene-carbocyclic adenosine against wild-type and drug-resistant hepatitis B virus mutants. Bioorg. Med. Chem. Lett. 2011, 21, 6328–6331. [Google Scholar] [CrossRef] [PubMed]
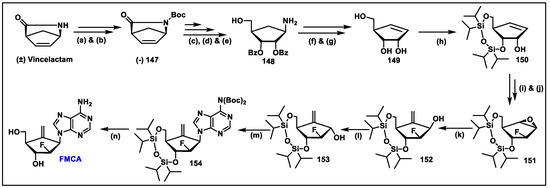
- Singh, U.S.; Mishra, R.C.; Shankar, R.; Chu, C.K. Stereoselective Synthesis of 2′-Fluoro-6′-methylene Carbocyclic Adenosine via Vince Lactam. J. Org. Chem. 2014, 79, 3917–3923. [Google Scholar] [CrossRef] [PubMed]
- Luche, J.L. Lanthanides in Organic-Chemistry. 1. Selective 1,2 Reductions of Conjugated Ketones. J. Am. Chem. Soc. 1978, 100, 2226–2227. [Google Scholar] [CrossRef]
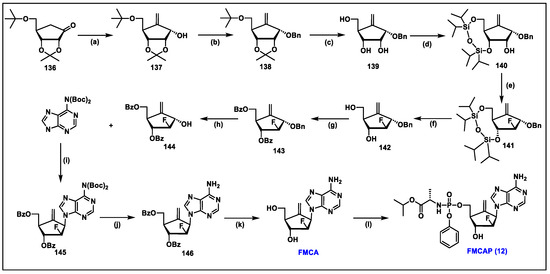
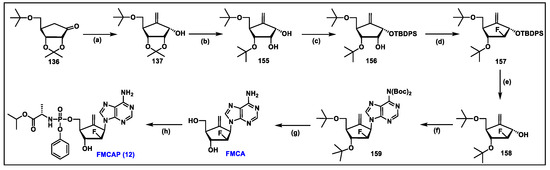
- Singh, U.S.; Mulamoottil, V.A.; Chu, C.K. Synthesis of an Anti-hepatitis B Agent, 2′-Fluoro-6′-methylene-carbocyclic Adenosine (FMCA) and Its Phosphoramidate (FMCAP). J. Org. Chem. 2019, 84, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Villet, S.; Ollivet, A.; Pichoud, C.; Barraud, L.; Villeneuve, J.P.; Trepo, C.; Zoulim, F. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J. Hepatol. 2007, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.W.; Langley, D.R.; Colonno, R.J.; Tenney, D.J. Mechanistic Characterization and Molecular Modeling of Hepatitis B Virus Polymerase Resistance to Entecavir. PLoS ONE 2010, 5, 9195. [Google Scholar] [CrossRef] [PubMed]
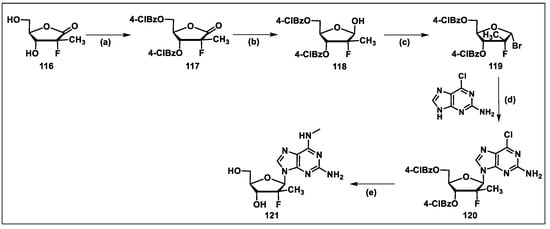
- Chang, W.; Bao, D.H.; Chun, B.K.; Naduthambi, D.; Nagarathnam, D.; Rachakonda, S.; Reddy, P.G.; Ross, B.S.; Zhang, H.R.; Bansal, S.; et al. Discovery of PSI-353661, a Novel Purine Nucleotide Prodrug for the Treatment of HCV Infection. ACS Med. Chem. Lett. 2011, 2, 130–135. [Google Scholar] [CrossRef] [PubMed]
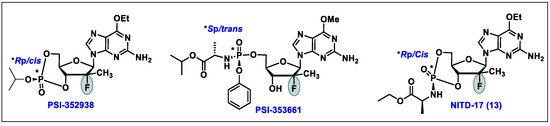
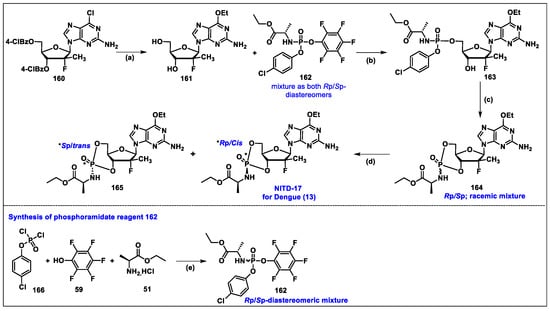
- Karuna, R.; Yokokawa, F.; Wang, K.; Zhang, J.; Xu, H.; Wang, G.; Ding, M.; Chan, W.L.; Abdul Ghafar, N.; Leonardi, A.; et al. A Cyclic Phosphoramidate Prodrug of 2′-Deoxy-2′-Fluoro-2′-C-Methylguanosine for the Treatment of Dengue Virus Infection. Antimicrob. Agents Chemother. 2020, 64, 12. [Google Scholar] [CrossRef]
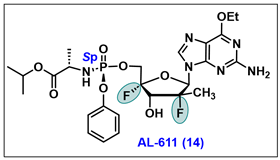
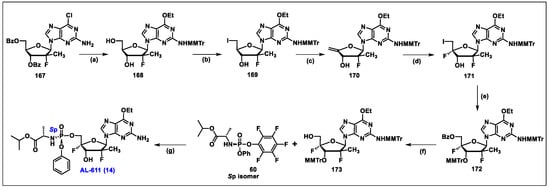
- Wang, G.; Dyatkina, N.; Prhavc, M.; Williams, C.; Serebryany, V.; Hu, Y.; Huang, Y.; Wu, X.; Chen, T.; Huang, W.; et al. Synthesis and Anti-HCV Activity of Sugar-Modified Guanosine Analogues: Discovery of AL-611 as an HCV NS5B Polymerase Inhibitor for the Treatment of Chronic Hepatitis C. J. Med. Chem. 2020, 63, 10380–10395. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).