Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug
Abstract
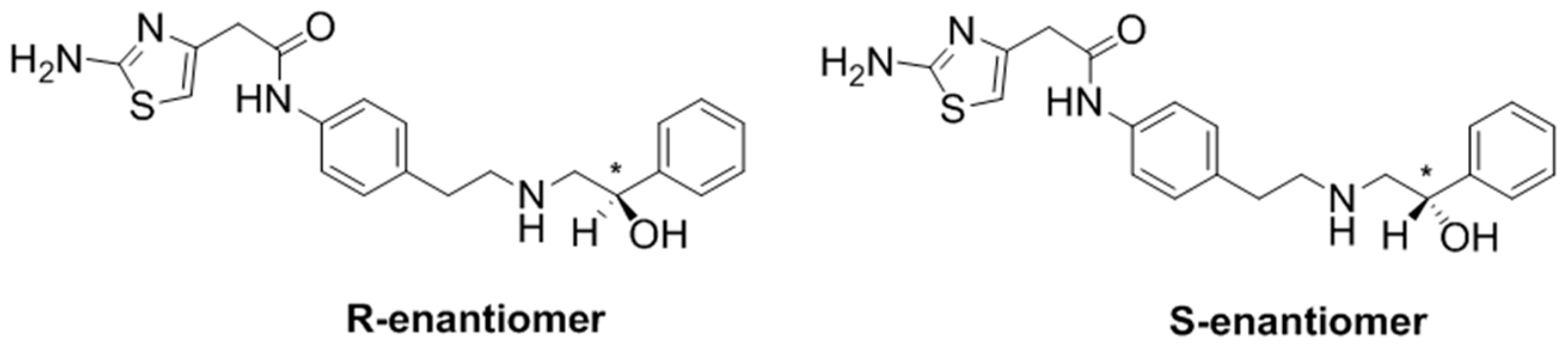
1. Introduction
2. Results and Discussion
2.1. Pre-Steady-State Hydrolysis of Mirabegron
2.2. Initial and Steady-State Hydrolysis of Mirabegron
3. Materials and Methods
3.1. Chemicals and Enzymes
3.2. Enzyme Titration
3.3. Steady-State Hydrolysis of Mirabegron
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lockridge, O. Review of Human Butyrylcholinesterase Structure, Function, Genetic Variants, History of Use in the Clinic, and Potential Therapeutic Uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Silman, I. The Multiple Biological Roles of the Cholinesterases. Prog. Biophys. Mol. Biol. 2021, 162, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Shaihutdinova, Z.; Lockridge, O. Drug and Pro-Drug Substrates and Pseudo-Substrates of Human Butyrylcholinesterase. Biochem. Pharmacol. 2023, 218, 115910. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.M. Acetylcholinesterase: Enzyme Structure, Reaction Dynamics, and Virtual Transition States. Chem. Rev. 1987, 87, 955–979. [Google Scholar] [CrossRef]
- Masson, P.; Froment, M.-T.; Gillon, E.; Nachon, F.; Darvesh, S.; Schopfer, L.M. Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Acetanilides. Biochim. Biophys. Acta 2007, 1774, 1139–1147. [Google Scholar] [CrossRef]
- Iitsuka, H.; Van Gelderen, M.; Katashima, M.; Takusagawa, S.; Sawamoto, T. Pharmacokinetics of Mirabegron, a Β3-Adrenoceptor Agonist for Treatment of Overactive Bladder, in Healthy East Asian Subjects. Clin. Ther. 2015, 37, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A.M.; Dudka, J. Mirabegron, a Selective Β3-Adrenergic Receptor Agonist, as a Potential Anti-Obesity Drug. J. Clin. Med. 2023, 12, 6897. [Google Scholar] [CrossRef]
- Sun, X.; Sui, W.; Mu, Z.; Xie, S.; Deng, J.; Li, S.; Seki, T.; Wu, J.; Jing, X.; He, X.; et al. Mirabegron Displays Anticancer Effects by Globally Browning Adipose Tissues. Nat. Commun. 2023, 14, 7610. [Google Scholar] [CrossRef]
- Takusagawa, S.; Yajima, K.; Miyashita, A.; Uehara, S.; Iwatsubo, T.; Usui, T. Identification of Human Cytochrome P450 Isoforms and Esterases Involved in the Metabolism of Mirabegron, a Potent and Selective Β3—Adrenoceptor Agonist. Xenobiotica 2012, 42, 957–967. [Google Scholar] [CrossRef]
- Konishi, K.; Minematsu, T.; Nagasaka, Y.; Tabata, K. Physiologically-Based Pharmacokinetic Modeling for Mirabegron: A Multi-Elimination Pathway Mediated by Cytochrome P450 3A4, Uridine 5′-Diphosphate-Glucuronosyltransferase 2B7, and Butyrylcholinesterase. Xenobiotica 2019, 49, 912–921. [Google Scholar] [CrossRef]
- Masson, P. Time-Dependent Kinetic Complexities in Cholinesterase-Catalyzed Reactions. Biochem. Mosc. 2012, 77, 1147–1161. [Google Scholar] [CrossRef]
- Frieden, C. Slow Transitions and Hysteretic Behavior in Enzymes. Annu. Rev. Biochem. 1979, 48, 471–489. [Google Scholar] [CrossRef] [PubMed]
- Kurganov, B.I.; Dorozhko, A.I.; Kagan, Z.S.; Yakovlev, V.A. The Theoretical Analysis of Kinetic Behaviour of “Hysteretic” Allosteric Enzymes. I. The Kinetic Manifestations of Slow Conformational Change of an Oligomeric Enyzme in the Monod, Wyman and Changeux Model. J. Theor. Biol. 1976, 60, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Neet, K.E.; Robert Ainslie, G. Hysteretic Enzymes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1980; Volume 64, pp. 192–226. ISBN 978-0-12-181964-4. [Google Scholar]
- Lushchekina, S.V.; Nemukhin, A.V.; Varfolomeev, S.D.; Masson, P. Molecular Modeling Evidence for His438 Flip in the Mechanism of Butyrylcholinesterase Hysteretic Behavior. J. Mol. Neurosci. 2014, 52, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Froment, M.-T.; Fort, S.; Ribes, F.; Bec, N.; Balny, C.; Schopfer, L.M. Butyrylcholinesterase-Catalyzed Hydrolysis of N-Methylindoxyl Acetate: Analysis of Volume Changes upon Reaction and Hysteretic Behavior. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 2002, 1597, 229–243. [Google Scholar] [CrossRef]
- Masson, P.; Schopfer, L.M.; Froment, M.-T.; Debouzy, J.-C.; Nachon, F.; Gillon, E.; Lockridge, O.; Hrabovska, A.; Goldstein, B.N. Hysteresis of Butyrylcholinesterase in the Approach to Steady-State Kinetics. Chem.-Biol. Interact. 2005, 157–158, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Froede, H.C.; Wilson, I.B. Direct Determination of Acetyl-Enzyme Intermediate in the Acetylcholinesterase-Catalyzed Hydrolysis of Acetylcholine and Acetylthiocholine. J. Biol. Chem. 1984, 259, 11010–11013. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, L.M.; David, E.; Hinrichs, S.H.; Lockridge, O. Human Butyrylcholinesterase in Cohn Fraction IV-4 Purified in a Single Chromatography Step on Hupresin. PLoS ONE 2023, 18, e0280380. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, W. The Number of Catalytic Sites in Acetylcholinesterase. Biochem. J. 1971, 123, 139–141. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Muhammad Ghali, U.; Teralı, K.; Dalmızrak, Ö.; Özer, N. Rethinking Common Solvents in Butyrylcholinesterase Activity Assays. Inorg. Chem. Commun. 2022, 143, 109796. [Google Scholar] [CrossRef]
- Sands, D.; Davis, A.; Banfield, S.; Pottie, I.R.; Darvesh, S. Solvents and Detergents Compatible with Enzyme Kinetic Studies of Cholinesterases. Chem.-Biol. Interact. 2023, 383, 110667. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Masson, P. Kinetic Evidence for Thermally Induced Conformational Change of Butyrylcholinesterase. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1987, 916, 193–199. [Google Scholar] [CrossRef]
- Lushchekina, S.V.; Masson, P. Slow-Binding Inhibitors of Acetylcholinesterase of Medical Interest. Neuropharmacology 2020, 177, 108236. [Google Scholar] [CrossRef]
- Vinces, T.C.; De Souza, A.S.; Carvalho, C.F.; Visnardi, A.B.; Teixeira, R.D.; Llontop, E.E.; Bismara, B.A.P.; Vicente, E.J.; Pereira, J.O.; De Souza, R.F.; et al. Monomeric Esterase: Insights into Cooperative Behavior, Hysteresis/Allokairy. Biochemistry 2024, 63, 1178–1193. [Google Scholar] [CrossRef]






| BChE form E | BChE form E′ | |
|---|---|---|
| Km | 3.9 ± 0.5 μM | 23.5 ± 3.9 μM |
| kcat | 7.32 ± 0.21 min−1 | 1.63 ± 0.08 min−1 |
| kcat/Km | 1.8 × 106 ± 0.28 × 106 M−1min−1 | 7.1 × 104 ± 1.4 × 104 M−1min−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaihutdinova, Z.; Masson, P. Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug. Molecules 2024, 29, 2356. https://doi.org/10.3390/molecules29102356
Shaihutdinova Z, Masson P. Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug. Molecules. 2024; 29(10):2356. https://doi.org/10.3390/molecules29102356
Chicago/Turabian StyleShaihutdinova, Zukhra, and Patrick Masson. 2024. "Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug" Molecules 29, no. 10: 2356. https://doi.org/10.3390/molecules29102356
APA StyleShaihutdinova, Z., & Masson, P. (2024). Pre-Steady-State and Steady-State Kinetic Analysis of Butyrylcholinesterase-Catalyzed Hydrolysis of Mirabegron, an Arylacylamide Drug. Molecules, 29(10), 2356. https://doi.org/10.3390/molecules29102356








