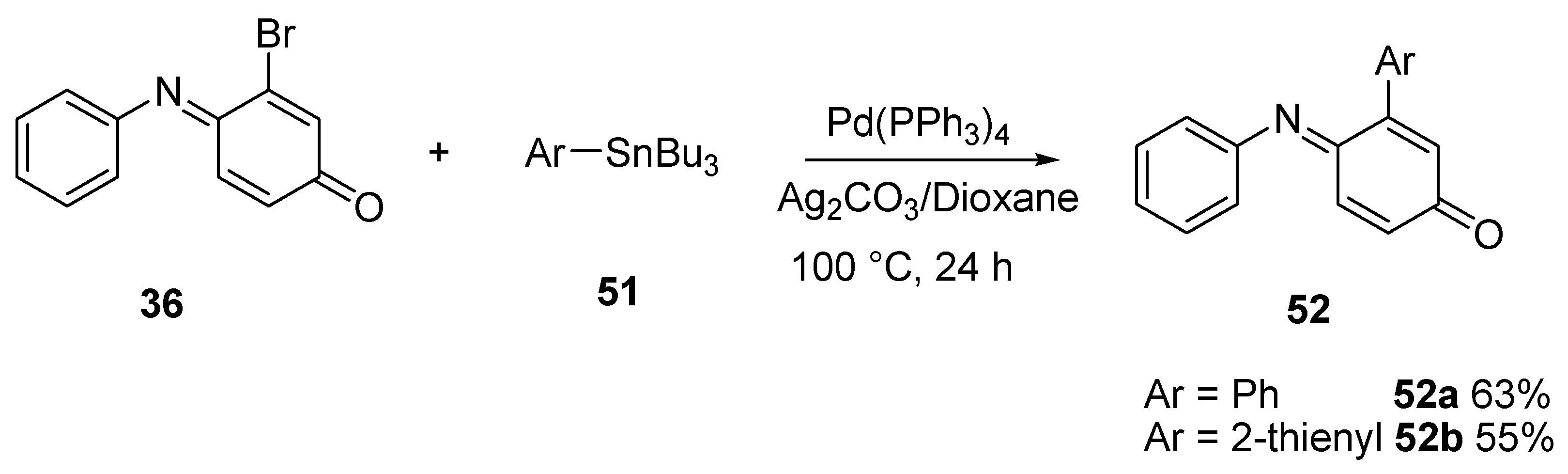Diversity-Orientated Synthesis and Biological Properties of Compounds Based on the N-Phenylquinoneimine Scaffold
Abstract
1. Introduction
1.1. N-Phenylquinoneimine and Its Pharmacological Significance
1.2. Quinoneimines in Natural Products and Dyes
1.3. Biological Activity of N-Phenylquinoneimine Scaffolds
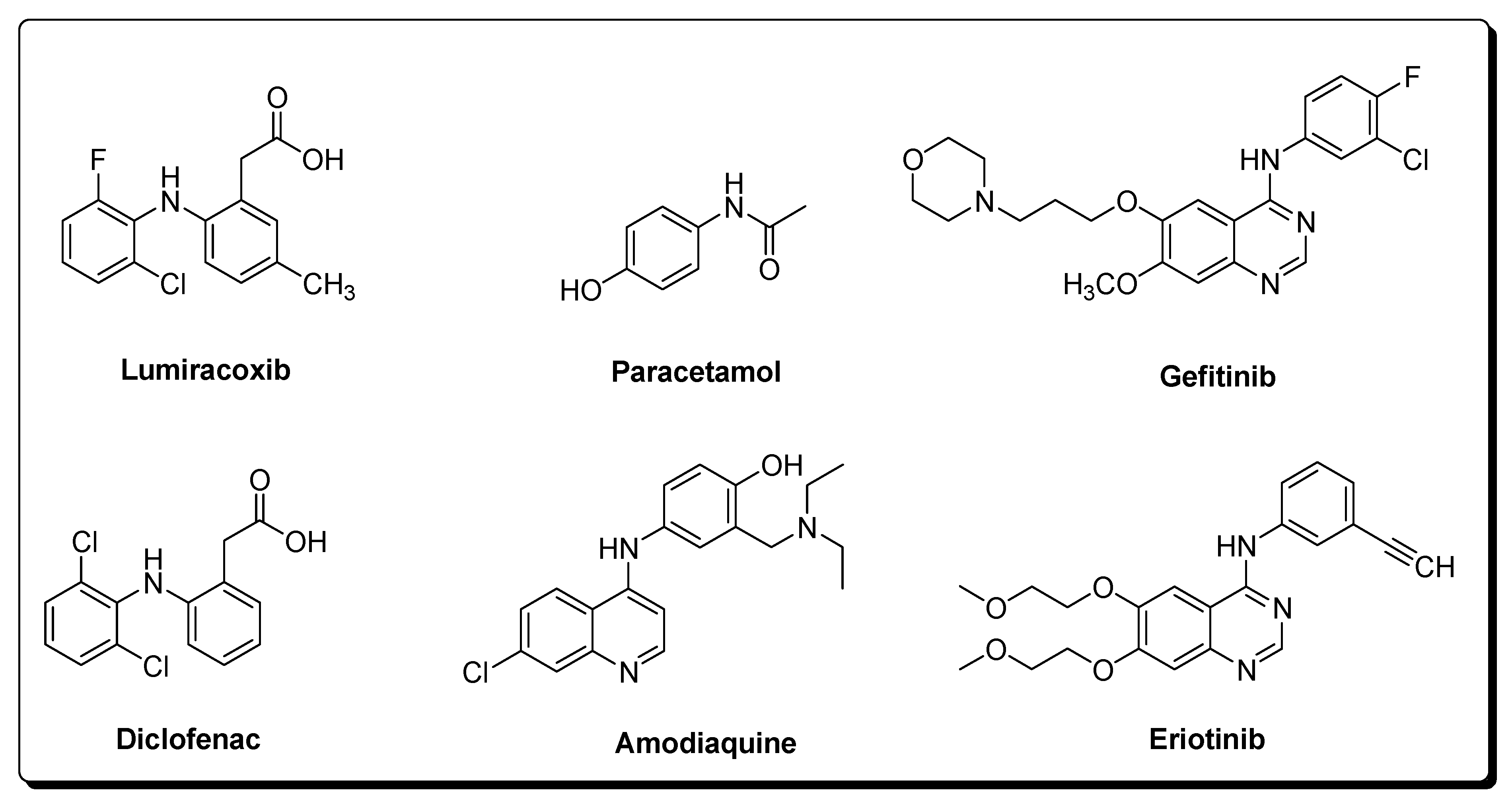
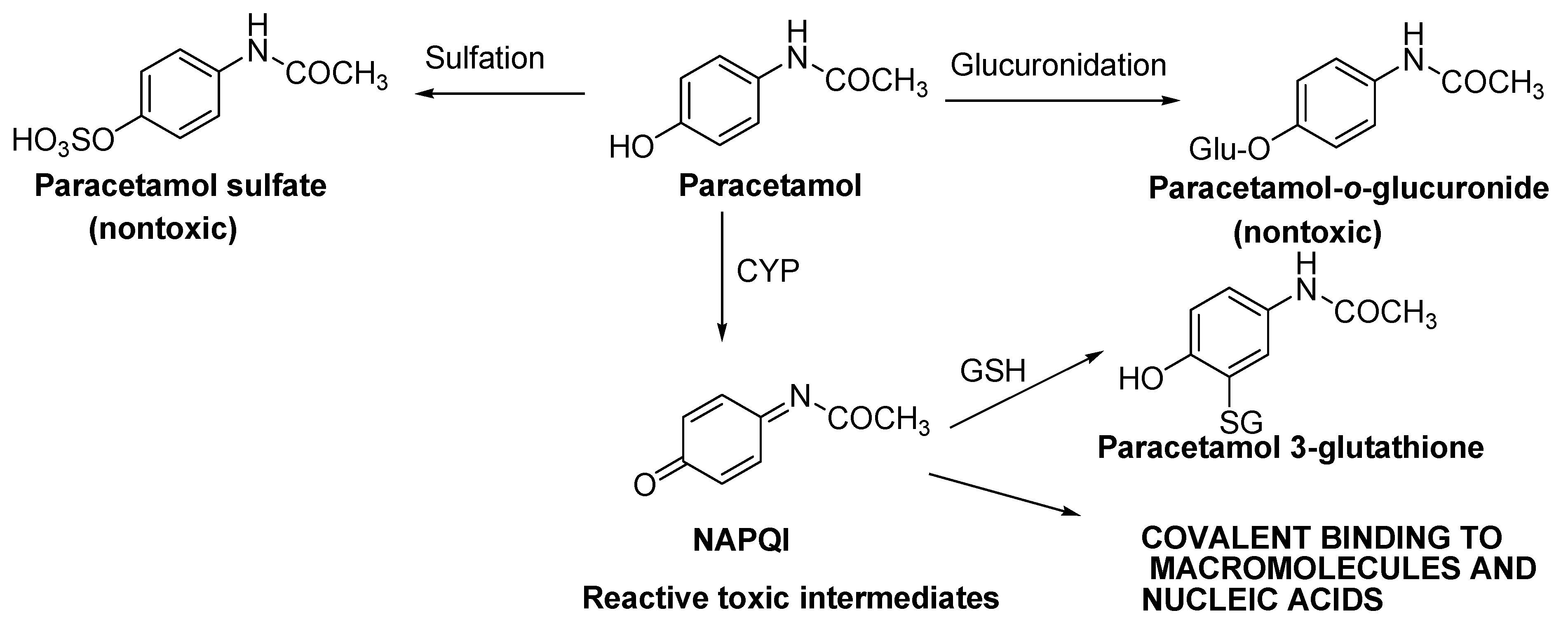
1.4. Significance of Quinoneimine-Based Drugs
1.5. Synthesis of Quinoneimines
2. Molecular Diversity from N-Phenylquinoneimine
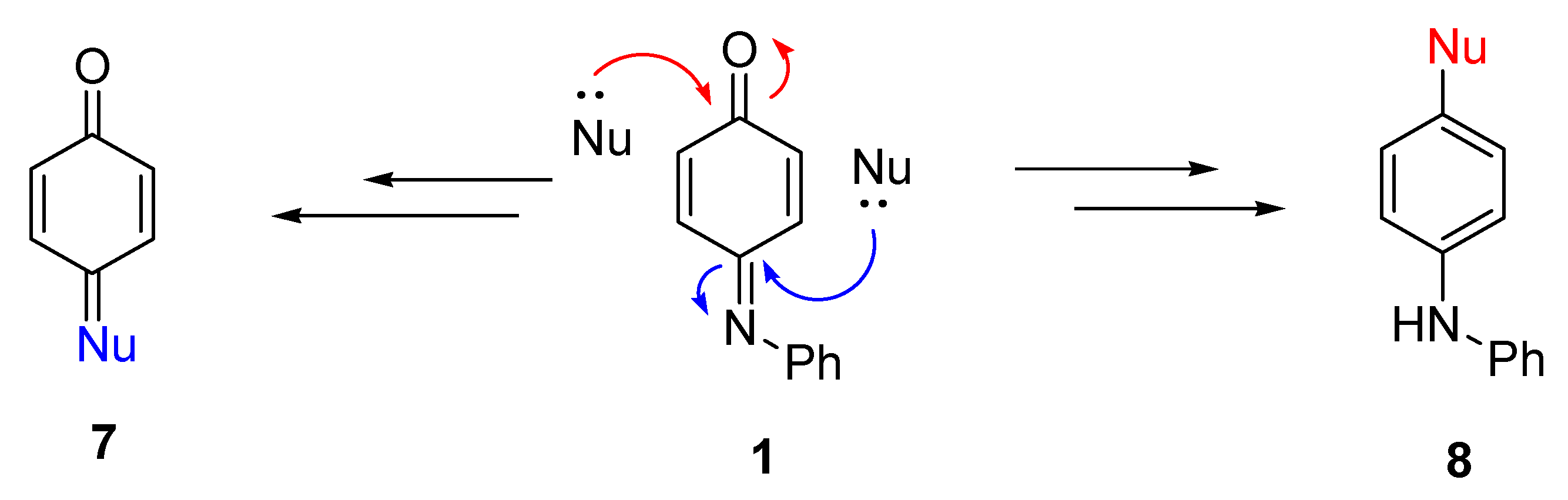
2.1. Nucleophilic Addition Reactions of N-Phenylquinoneimine
2.1.1. Direct Addition
2.1.2. Conjugate Addition
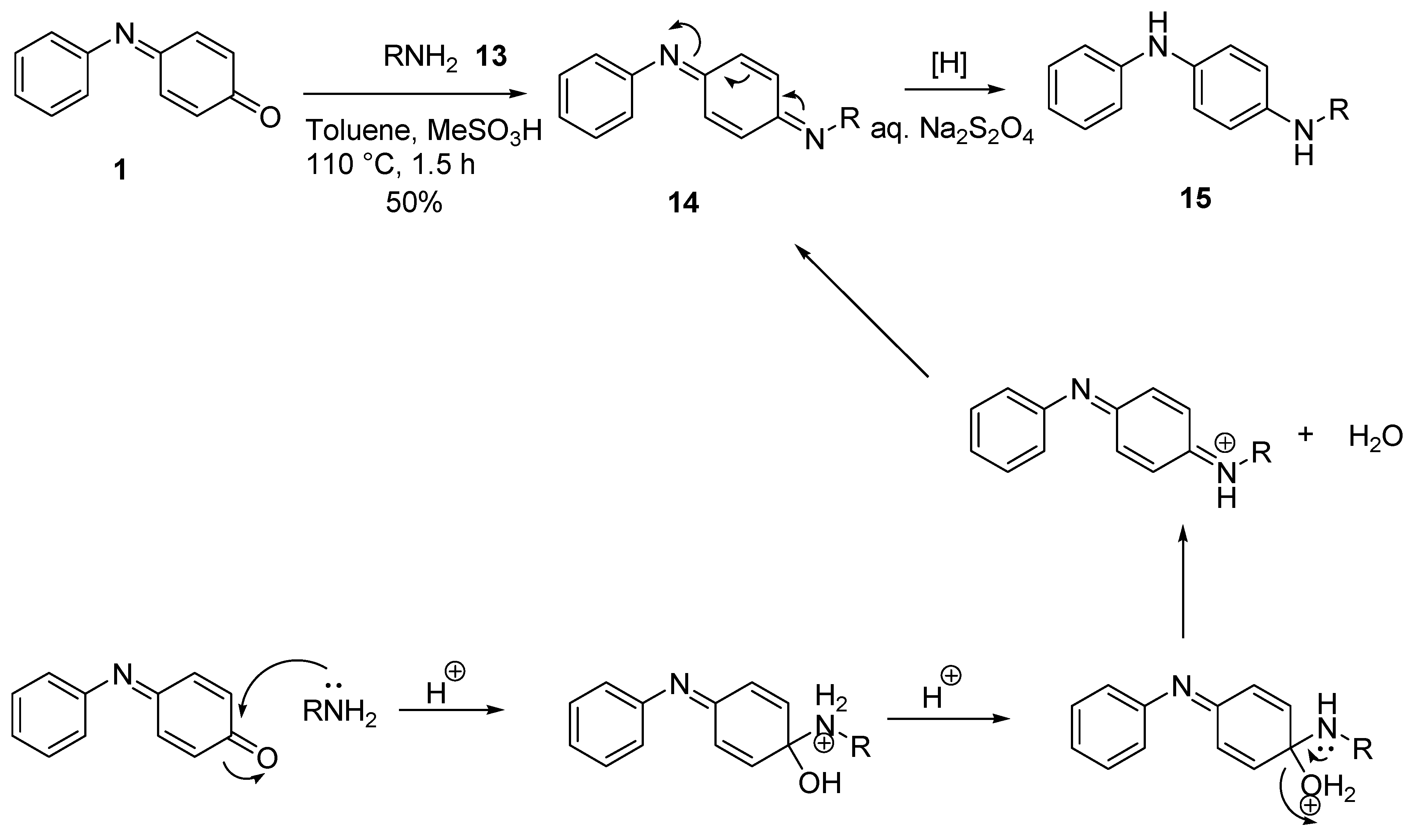
2.2. Reaction with Nitrogen Nucleophiles
2.2.1. Aliphatic Amines
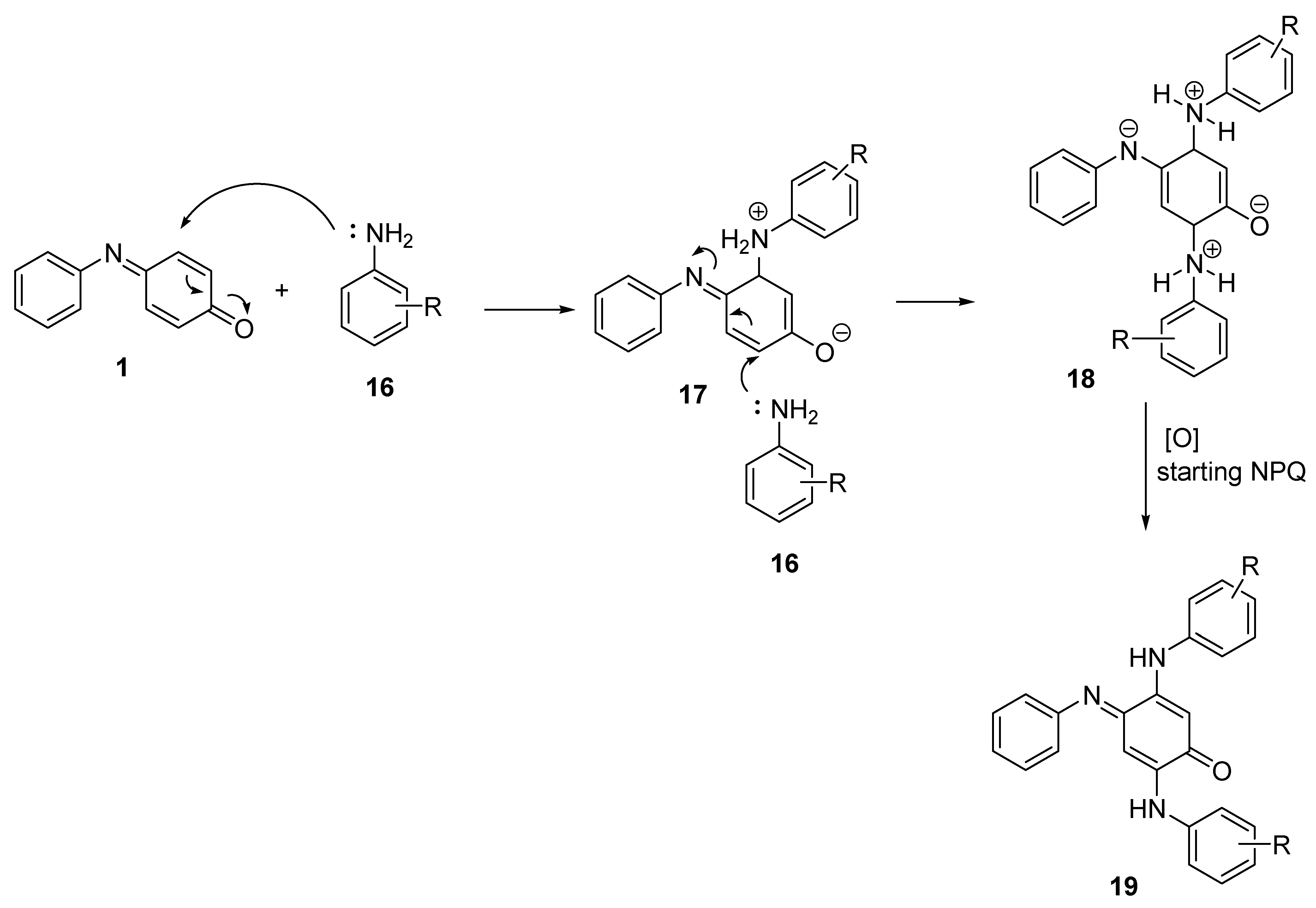
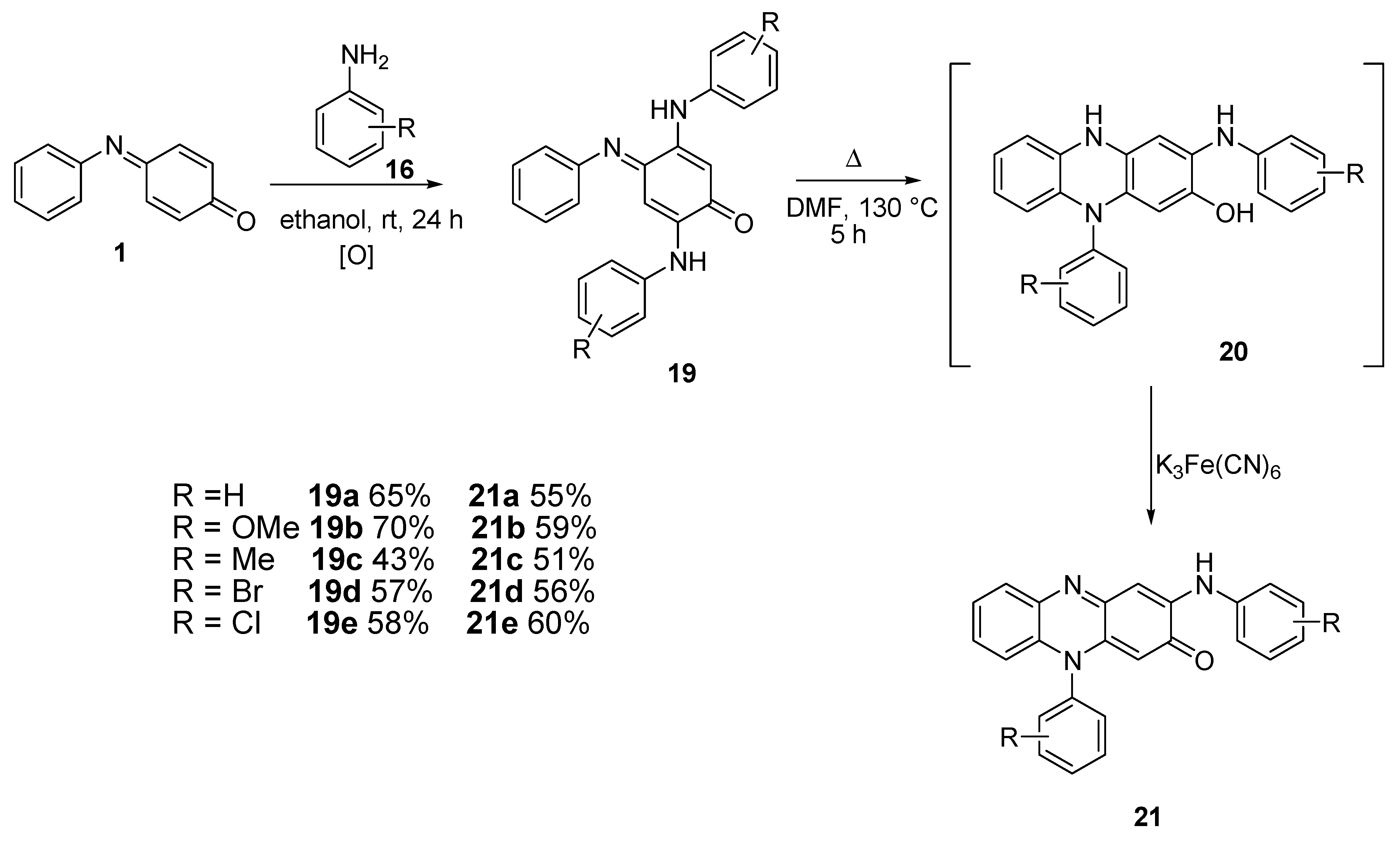
2.2.2. Aromatic Amines
2.3. Reaction with Sulfur Nucleophiles
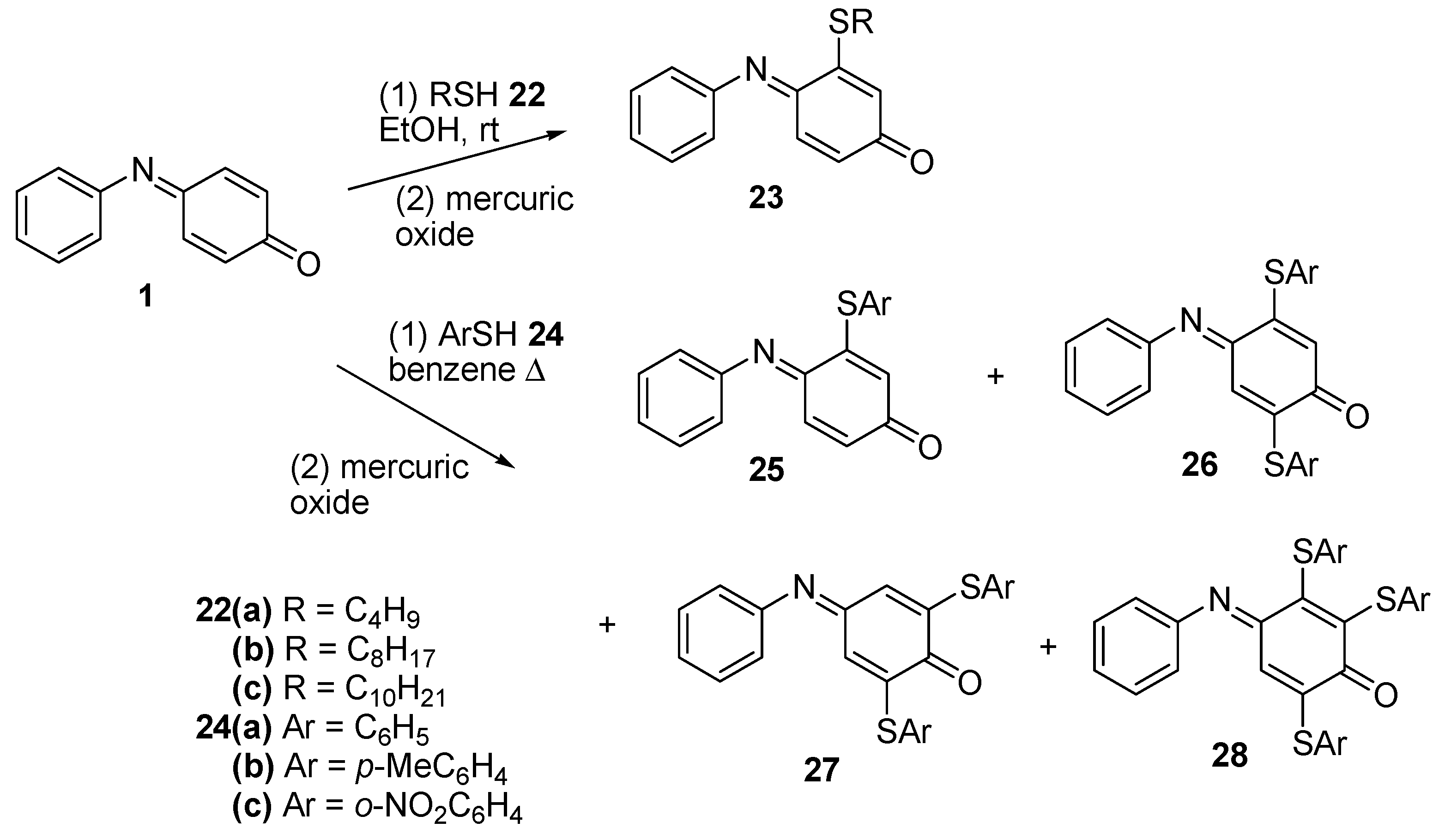
2.3.1. Thiols
2.3.2. Sulfinates
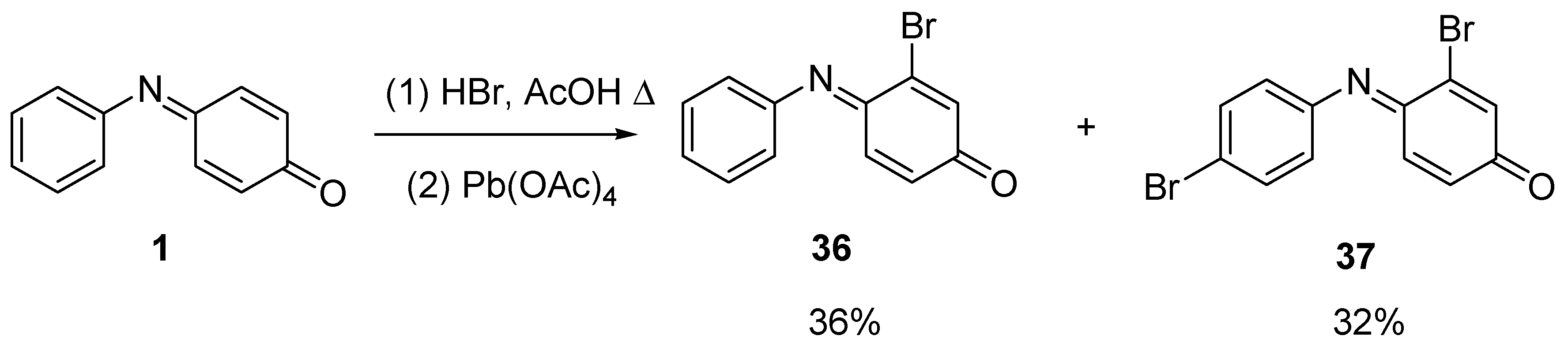
2.4. Reaction with Halogens
2.5. Reaction with Carbon Nucleophiles
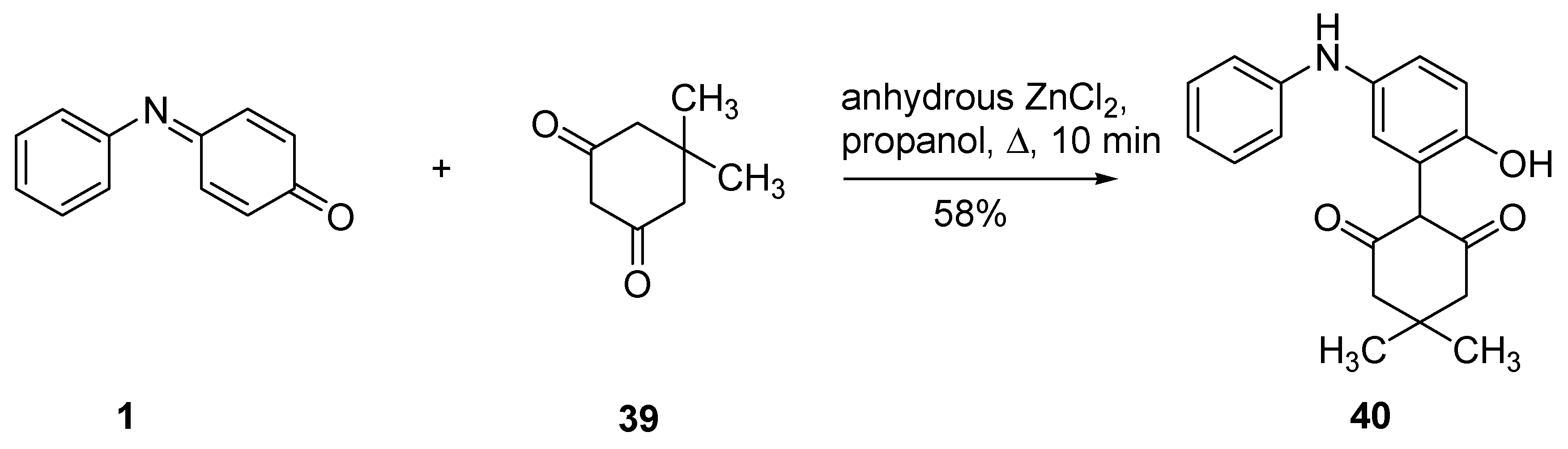
2.5.1. Enolates
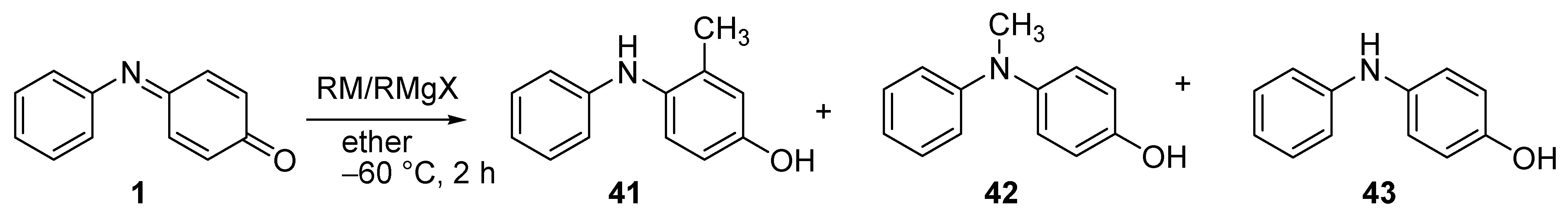
2.5.2. Organometallic Reagents
3. Oxidation of N-Phenylquinoneimine
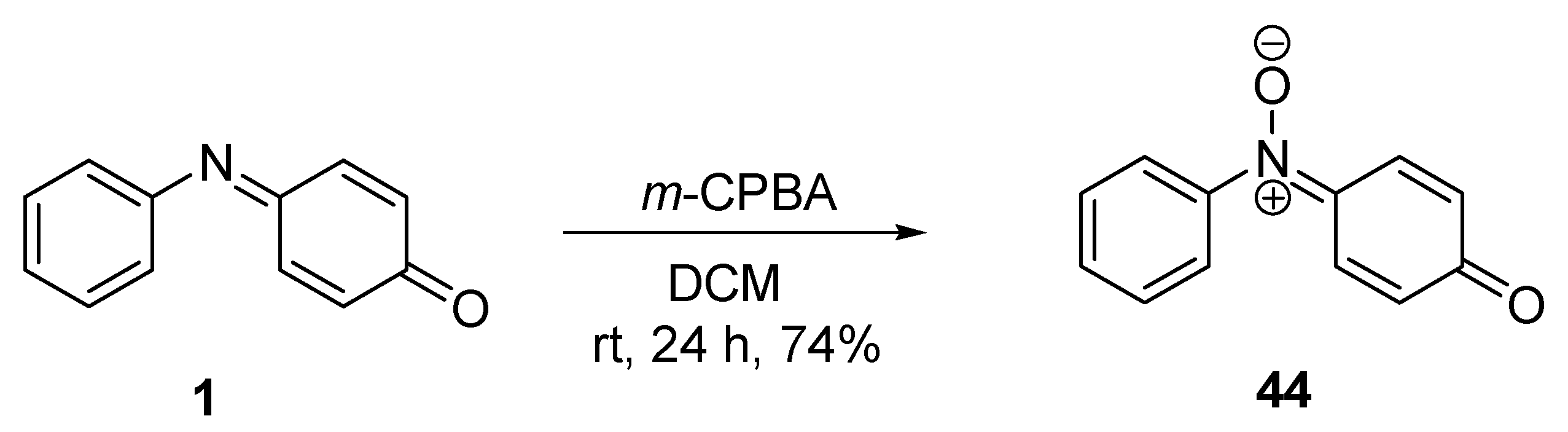
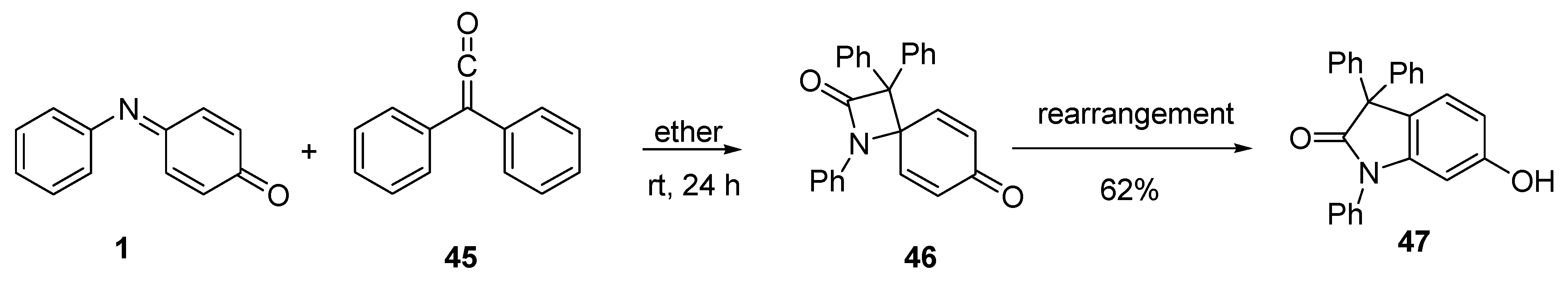
3.1. Cycloaddition Reactions
3.2. Coupling Reactions
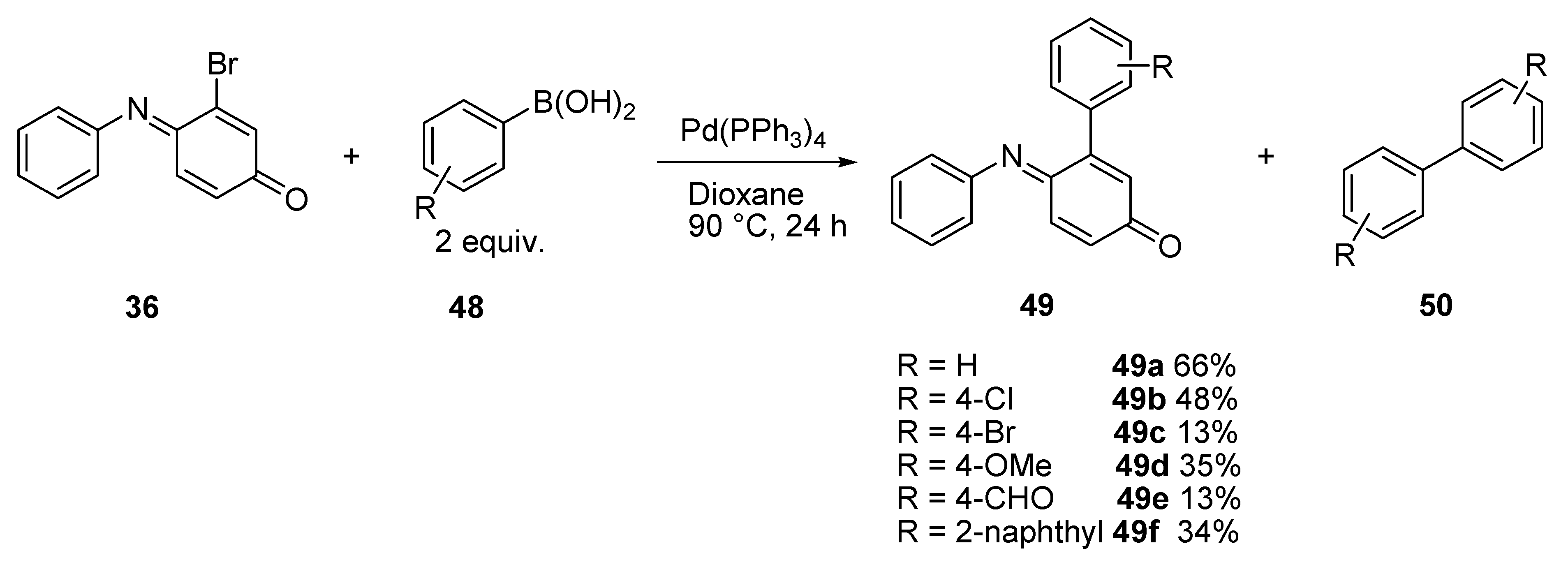
3.2.1. Suzuki Coupling
3.2.2. Stille Coupling
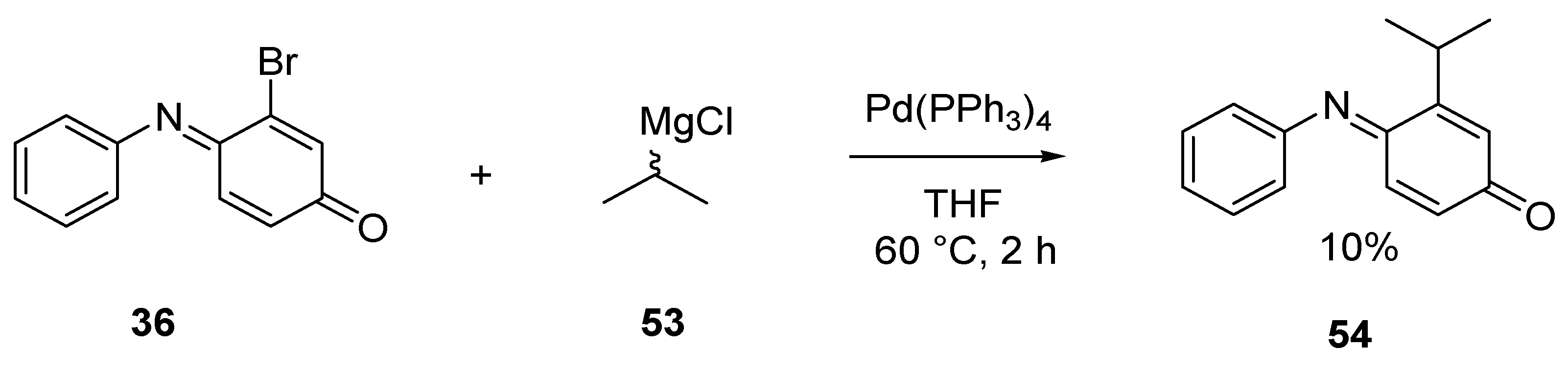
3.2.3. Kumada Coupling
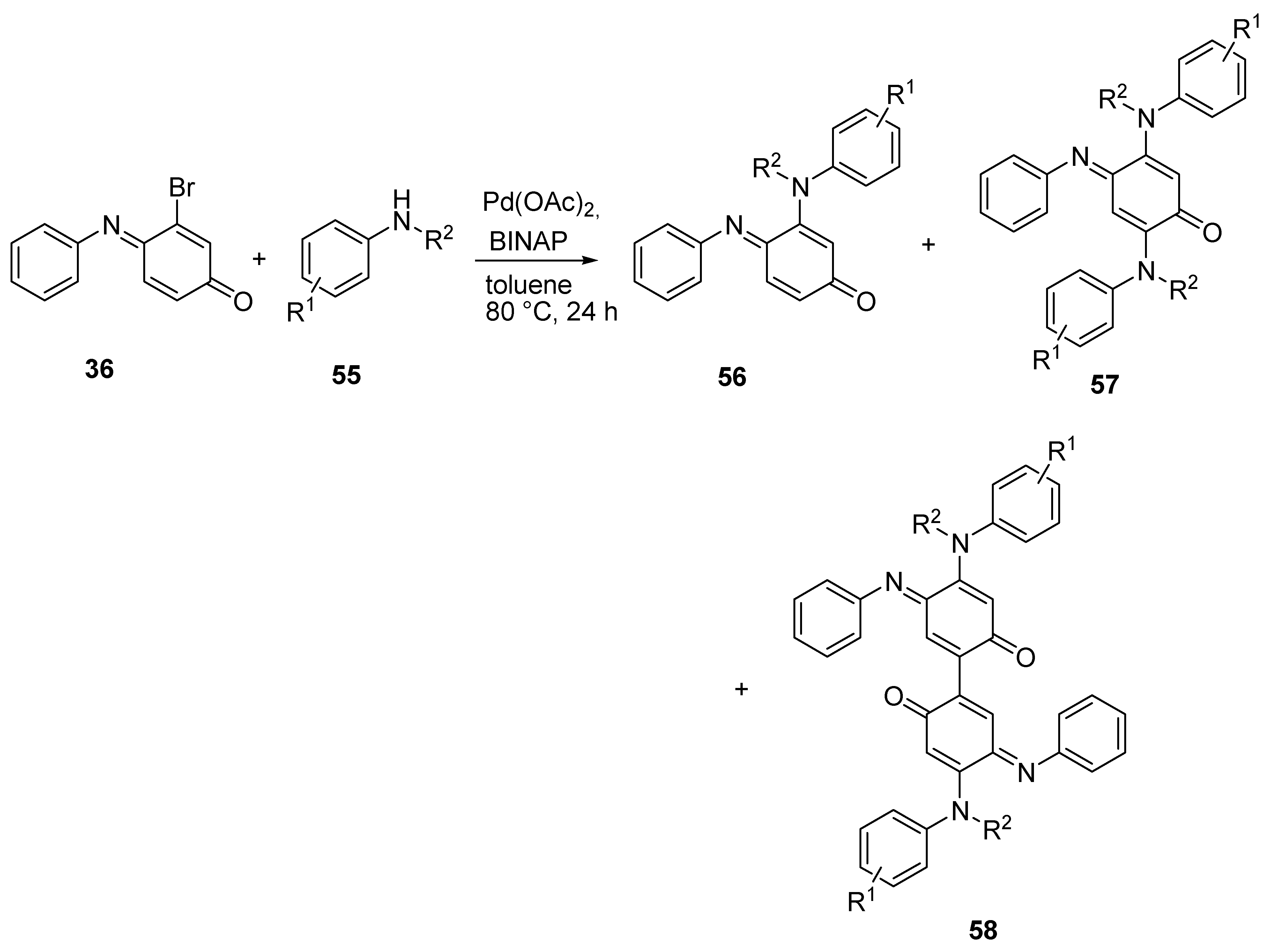
3.2.4. Buchwald–Hartwig Coupling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 2009, 15, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.D.; Schreiber, S.L. A Planning Strategy for Diversity-Oriented Synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Spring, D.R. Diversity-oriented synthesis; a challenge for synthetic chemists. Org. Biomol. Chem. 2003, 1, 3867–3870. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castro, M.; Zimmermann, S.; Sankar, M.G.; Kumar, K. Scaffold Diversity Synthesis and Its Application in Probe and Drug Discovery. Angew. Chem. Int. Ed. 2016, 55, 7586–7605. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Murata, Y.; Nakamura, S. The Relationship between the Structures and Absorption Spectra of Cyan Color Indoaniline Dyes. J. Org. Chem. 1993, 58, 5238–5244. [Google Scholar] [CrossRef]
- Tao, W.; Barra, M. Inhibition of quinone-imine dye deamination by complexation with para-sulfonated calixarenes. J. Org. Chem. 2001, 66, 2158–2160. [Google Scholar] [CrossRef]
- Barra, M.; Tan, A.; Wong, S. Hydroxide-catalysed decomposition of benzoquinone-imine dyes. Dye. Pigment. 2004, 61, 63–67. [Google Scholar] [CrossRef]
- Imai, S.; Noguchi, T.; Seto, H. Studies on cell growth stimulating substances of low molecular weight: Part 2. Exfoliazone and lavanducyanin, potent growth-promoting substances of rat liver cell line, RLN-8, produced by streptomyces exfoliatus and streptomyces aeriouvifer. J. Antibiot. 1993, 46, 1232–1238. [Google Scholar] [CrossRef]
- Imai, S.; Shimazu, A.; Furihata, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Isolation and Structure of a New Phenoxazine Antibiotic, Exfoliazone, Produced by Streptomyces Exfoliatus. J. Antibiot. 1990, 43, 1606–1607. [Google Scholar] [CrossRef]
- Bolognese, A.; Correale, G.; Manfra, M.; Lavecchia, A.; Mazzoni, O.; Novellino, E.; Barone, V.; Pani, A.; Tramontano, E.; La Colla, P.; et al. Antitumor agents. 1. Synthesis, biological evaluation, and molecular modeling of 5H-pyrido[3,2-a]phenoxazin-5-one, a compound with potent antiproliferative activity. J. Med. Chem. 2002, 45, 5205–5216. [Google Scholar] [CrossRef]
- Cortés-Salazar, F.; Beggah, S.; van der Meer, J.R.; Girault, H.H. Electrochemical As(III) whole-cell based biochip sensor. Biosens. Bioelectron. 2013, 47, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Boone, S.R.; Pierpont, C.G. Stereoselective oxidation of a coordinated phenoxazinylate radical with molecular oxygen. J. Am. Chem. Soc. 1990, 112, 4561–4562. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Pierpont, C.G. Semiquinone Imine Complexes of Ruthenium. Coordination and Oxidation of the 1-Hydroxy-2, 4, 6, 8-Tetra-tert-Butylphenoxazinyl Radical. Inorg. Chem. 1992, 31, 2020–2029. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Del. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Stephanopoulos, N.; Carrico, Z.M.; Francis, M.B. Nanoscale integration of sensitizing chromophores and porphyrins with bacteriophage MS2. Angew. Chem. Int. Ed. 2009, 48, 9498–9502. [Google Scholar] [CrossRef]
- Yadav, A.; Mathur, P. p-Quinoneimine as an intermediate in the oxidative coupling of 2-amino-5-methylphenol to 4a,7-dimethyldihydro-2-aminophenoxazinone catalyzed by a monomeric copper(II) complex. Catal. Commun. 2014, 55, 1–5. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Shkoulev, V.; Menashe, N.; Greenwald, M.; Aizikovich, A.; Ofer, D.; Byk, G.; et al. Approaches for introducing high molecular diversity in scaffolds: Fast parallel synthesis of highly substituted 1H-quinolin-4-one libraries. Mol. Divers. 2004, 8, 437–448. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Sekar, G. An efficient synthesis of iminoquinones by a chemoselective domino ortho-hydroxylation/oxidation/imidation sequence of 2-aminoaryl ketones. Org. Biomol. Chem. 2016, 14, 3053–3060. [Google Scholar] [CrossRef]
- Adesina, A.D. Molecular Diversity from N-phenylquinoneimine. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2020. Available online: https://theses.ncl.ac.uk/jspui/handle/10443/5040 (accessed on 17 December 2020).
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef] [PubMed]
- Tintore, M.; Vidal-Jordana, A.; Sastre-Garriga, J. Treatment of multiple sclerosis—Success from bench to bedside. Nat. Rev. Neurol. 2019, 15, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Vittum, P.W.; Brown, G.H. Indoaniline dyes. I. Some phenol blue derivatives with substituents in the phenol ring. J. Am. Chem. Soc. 1946, 68, 2235–2239. [Google Scholar] [CrossRef]
- Josephy, P.D.; Mason, R.P.; Eling, T. Chemical structure of the adducts formed by the oxidation of benzidine in the presence of phenols. Carcinogenesis 1982, 3, 1227–1230. [Google Scholar] [CrossRef]
- Ren, J.; Liu, D.; Tian, L.; Wei, Y.; Proksch, P.; Zeng, J.; Lin, W. Venezuelines A–G, new phenoxazine-based alkaloids and aminophenols from Streptomyces venezuelae and the regulation of gene target Nur77. Bioorg. Med. Chem. Lett. 2013, 23, 301–304. [Google Scholar] [CrossRef]
- Maskey, R.P.; Li, F.C.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins A-C: Production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. 2003, 56, 622–629. [Google Scholar] [CrossRef]
- Gomes, P.B.; Nett, M.; Dahse, H.M.; Sattler, I.; Martin, K.; Hertweck, C. Bezerramycins A–C, antiproliferative phenoxazinones from Streptomyces griseus featuring carboxy, carboxamide or nitrile substituents. Wiley Online Libr. 2010, 2010, 231–235. [Google Scholar] [CrossRef]
- Gomes, P.B.; Nett, M.; Dahse, H.-M.; Hertweck, C. Pitucamycin: Structural merger of a phenoxazinone with an epoxyquinone antibiotic. J. Nat. Prod. 2010, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Gripenberg, J. Fungus pigments VIII: The structure of cinnabarin and cinnabarinic acid. Acta Chem. Scand. 1958, 12, 603–610. [Google Scholar] [CrossRef]
- Stepanov, B.I. Vvedenie v khimiiu I tekhnologiiu organicheskikh krasitelei. In The Great Soviet Encyclopedia, 3rd ed.; Prokhorov, A.M., Ed.; Soviet Encyclopedia: Moscow, Russia, 1971. [Google Scholar]
- Supriatin, Y.; Sumirat, V.A.; Herdiani, M. Growth Analysis of Escherichia coli and Salmonella typhi on MacConkey Agar Modification. J. Phys. Conf. Ser. 2021, 1764, 012207. [Google Scholar] [CrossRef]
- Wichmann, C.F.; Liesch, J.M.; Schwartz, R.E. L-671,329, A New Antifungal Agent II. Structure Determination. J. Antibiot. 1989, 42, 168–173. [Google Scholar] [CrossRef]
- Jose, J.; Burgess, K. Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron 2006, 62, 11021–11037. [Google Scholar] [CrossRef]
- Kerman, K.; Oezkan, D.; Kara, P.; Karadeniz, H.; Oezkan, Z.; Erdem, A.; Jelen, F.; Oezsoez, M. Electrochemical detection of specific DNA sequences from PCR amplicons on carbon and mercury electrodes using Meldola’s blue as an intercalator. Turk. J. Chem. 2004, 28, 523–533. Available online: https://www.acarindex.com/turkish-journal-of-chemistry/electrochemical-detection-of-specific-dna-sequences-from-pcr-amplicons-on-carbon-and-mercury-electrodes-using-meldola39s-blue-as-an-intercalator-783586 (accessed on 25 January 2022).
- Reut, V.E.; Kozlov, S.O.; Kudryavtsev, I.V.; Grudinina, N.A.; Kostevich, V.A.; Gorbunov, N.P.; Grigorieva, D.V.; Kalvinkovskaya, J.A.; Bushuk, S.B.; Varfolomeeva, E.Y.; et al. New Application of the Commercially Available Dye Celestine Blue B as a Sensitive and Selective Fluorescent “Turn-On” Probe for Endogenous Detection of HOCl and Reactive Halogenated Species. Antioxidants 2022, 11, 1719–1733. [Google Scholar] [CrossRef]
- Swenton, J.S.; Shih, C.; Chen, C.P.; Chou, C.T. Preparation of quinone imine ketals via intramolecular condensation of amino-substituted quinone monoketals. Anodic oxidation chemistry of trifluoroacetamide derivatives of 1,4-dimethoxybenzenes and 4-methoxyphenols. J. Org. Chem. 1990, 55, 2019–2026. [Google Scholar] [CrossRef]
- Aubart, K.M.; Heathcock, C.H. A Biomimetic Approach to the Discorhabdin Alkaloids: Total Syntheses of Discorhabdins C and E and Dethiadiscorhabdin D. J. Org. Chem. 1999, 64, 16–22. [Google Scholar] [CrossRef]
- Koutentis, P.A.; Loizou, G.; Lo Re, D. Synthesis of Triazafluoranthenones via Silver(I)-Mediated Nonoxidative and Oxidative Intramolecular Palladium-Catalyzed Cyclizations. J. Org. Chem. 2011, 76, 5793–5802. [Google Scholar] [CrossRef]
- Inoue, K.; Ishikawa, Y.; Nishiyama, S. Synthesis of tetrahydropyrroloiminoquinone alkaloids based on electrochemically generated hypervalent iodine oxidative cyclization. Org. Lett. 2010, 12, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Rajesh, C.; Dhanya, R.; Rath, N.P. Lewis-acid promoted annulation of p-quinoneimines by allylsilanes: A facile entry into benzofused heterocycles. Org. Lett. 2002, 4, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Eggert, C. Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol. Res. 1997, 152, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, J.; Yokomizo, K.; Awata, Y.; Shibata, M.; Nohara, T.; Teramine, T.; Takahashi, K. Structures of phytotoxins, AV-toxins C, D and E, produced by zonate leaf spot fungus of mulberry. Tetrahedron Lett. 1987, 28, 3697–3698. [Google Scholar] [CrossRef]
- Marsh, J.P., Jr.; Goodman, L. Synthesis of possible actinomycin D precursors. Can. J. Chem. 1966, 44, 799–806. [Google Scholar] [CrossRef]
- Almeida, R.G.; Valença, W.O.; Rosa, L.G.; de Simone, C.A.; de Castro, S.L.; Barbosa, J.M.C.; Pinheiro, D.P.; Paier, C.R.K.; de Carvalho, G.G.C.; Pessoa, C.; et al. Synthesis of quinone imine and sulphur-containing compounds with antitumor and trypanocidal activities: Redox and biological implications. RSC Med. Chem. 2020, 11, 1145–1160. [Google Scholar] [CrossRef]
- Lackner, H.; Bahner, I.; Shigematsu, N.; Pannell, L.K.; Mauger, A.B. Structures of five components of the actinomycin Z complex from Streptomyces fradiae, two of which contain 4-chlorothreonine. J. Nat. Prod. 2000, 63, 352–356. [Google Scholar] [CrossRef]
- Westley, J.W.; Liu, C.M.; Blount, J.F.; Todaro, L.; Sello, L.H.; Troupe, N. Isolation and characterization of three novel polyether antibiotics and three novel actinomycins as cometabolites of the same Streptomyces sp. X-14873, ATCC 31679. J. Antibiot. 1986, 39, 1704–1711. [Google Scholar] [CrossRef]
- Green, D.M. Wilm’s tumour. Eur. J. Cancer 1997, 33, 409–418. [Google Scholar] [CrossRef]
- Womer, R.B. Soft tissue sarcomas. Eur. J. Cancer 1997, 33, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, T.; Bess, J.; Henderson, L.E.; Levin, J.G. Actinomycin D Inhibits Human Immunodeficiency Virus Type 1 Minus-Strand Transfer in In Vitro and Endogenous Reverse Transcriptase Assays. J. Virol. 1998, 72, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Dactinomycin. The American Society of Health-System Pharmacists. Available online: https://www.drugs.com/monograph/dactinomycin.html (accessed on 1 April 2007).
- Klopčič, I.; Dolenc, M.S. Chemicals and Drugs Forming Reactive Quinone and Quinone Imine Metabolites. Chem. Res. Toxicol. 2019, 32, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Porubek, D.; Rundgren, M.; Larsson, R.; Albano, E.; Ross, D.; Nelson, S.D.; Moldéus, P. Quinone Imines as Biological Reactive Intermediates. In Biological Reactive Intermediates III: Mechanisms of Action in Animal Models and Human Disease; Kocsis, J.J., Jollow, D.J., Witmer, C.M., Nelson, J.O., Snyder, R., Eds.; Springer: Boston, MA, USA, 1986; pp. 631–644. [Google Scholar] [CrossRef]
- Burmistrov, K.S.; Burmistrov, S.I. Redox potentials of N-arylquinonimines. Ukr. Khimicheskii Zhurnal 1978, 44, 832–835. [Google Scholar]
- Baragona, F.; Lomberget, T.; Duchamp, C.; Henriques, N.; Lo Piccolo, E.; Diana, P.; Montalbano, A.; Barret, R. Synthesis of 5-substituted 2,3-dihydrobenzofurans in a one-pot oxidation/cyclization reaction. Tetrahedron 2011, 67, 8731–8739. [Google Scholar] [CrossRef]
- Adams, R.; Looker, J.H. Quinone Imides. IV. p-Quinone Monosulfonimides. J. Am. Chem. Soc. 1951, 73, 1145–1149. [Google Scholar] [CrossRef]
- Fields, D.L., Jr.; Lodaya, J.S. Preparation of Quinoneimines from Hydroxyphenylamines Using Hypochlorite as Oxidizing Agent. WO9952860A1, 21 October 1999. [Google Scholar]
- Fields, D.L., Jr.; Lohr, R. Preparation of Quinoneimines from Hydroxyphenylamines Using Hydrogen Peroxide and a Catalyst. WO9952859A1, 21 October 1999. [Google Scholar]
- Fields, D.L., Jr.; Stern, M.K.; Lodaya, J.S. Preparation of N-Phenylbenzoquinoneimines via Oxidation of Hydroxydiphenylamines Using a Modified Activated Carbon Catalyst. WO9936395A2, 22 July 1999. [Google Scholar]
- Ma, H.; Wu, S.; Sun, Q.; Li, H.; Chen, Y.; Zhao, W.; Ma, B.; Guo, Q.; Lei, Z.; Yan, J. A new method for the synthesis of iminoquinones via DMP-mediated oxidative reaction. Synthesis 2010, 19, 3295–3300. [Google Scholar] [CrossRef]
- Cottman, K.S. Preparation of a N-Substituted Phenylenediamine. U.S. Patent 4968843A, 6 November 1990. [Google Scholar]
- Capehart, S.L.; ElSohly, A.M.; Obermeyer, A.C.; Francis, M.B. Bioconjugation of Gold Nanoparticles through the Oxidative Coupling of ortho-Aminophenols and Anilines. Bioconjugate Chem. 2014, 25, 1888–1892. [Google Scholar] [CrossRef]
- Barret, R.; Daudon, M. Synthesis of Quinone-Imines with Iodoxybenzene. Synth. Commun. 1990, 20, 1543–1549. [Google Scholar] [CrossRef]
- Barret, R.; Daudon, M. Preparation of quinone-imide ketals from amides with hypervalent organo-iodine compounds. Tetrahedron Lett. 1991, 32, 2133–2134. [Google Scholar] [CrossRef]
- Balogh, V.; Fetizon, M.; Golfier, M. Oxidations with Silver Carbonate/Celite. V. Oxidations of Phenols and Related Compounds. J. Org. Chem. 1971, 36, 1339–1341. [Google Scholar] [CrossRef]
- Mehrdadian, M.; Khazalpour, S.; Amani, A. Electrochemical synthesis of new quinone-imines with assisted of 4-ethynylaniline and para-toluidine as nucleophile. Electrochim. Acta 2022, 427, 140849. [Google Scholar] [CrossRef]
- Schreiber, S.L. Molecular diversity by design. Nature 2009, 457, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Cottman, K.S.; Kuczkowski, J.A. Preparation of N-alkyl-N’-phenyl-p-phenylenediamines. EP617004A1, 28 September 1994. [Google Scholar]
- Lewis, R.J. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2007; p. 59. [Google Scholar]
- Verschueren, K. Handbook of Environmental Data on Organic Chemicals. Volumes 1–2, 4th ed.; John Wiley & Sons: New York, NY, USA, 2001; p. 179. [Google Scholar]
- Tsoi, E.V.; Afanas’eva, G.B.; Chupakhin, O.N. Research in the chemistry of heterocyclic quinoneimines. 3. Oxidative cyclization of 2,5-diarylamino-substituted 1,4-benzoquinone-4-phenylimines—Simple method for the preparation of 2-arylamino-5-aryl-3-phenazinones. Chem. Heterocycl. Compd. 1984, 20, 263–267. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Druzhkov, N.O.; Kurskii, Y.A.; Abakumova, L.G.; Shavyrin, A.S.; Fukin, G.K.; Poddel’skii, A.I.; Cherkasov, V.K.; Okhlopkova, L.S. Quinone imines and aminophenols as precursors of new heterocycles. Russ. Chem. Bull. 2005, 54, 2571–2577. [Google Scholar] [CrossRef]
- Afanaseva, G.B.; Tsoi, E.V.; Chupakhin, O.N.; Sidorov, E.O.; Konovalov, S.V. Thiylation of 1,4-Benzoquinone-4-Phenylimine by Alkanethiol and Arenethiol. Zh. Org. Khim. 1985, 21, 1926–1932. [Google Scholar]
- Varlamov, V.T.; Gadomsky, S.Y. Kinetics and mechanism of the chain reaction between N-phenyl-1,4-benzoquinone monoimine and thiophenol. Russ. J. Phys. Chem. 2017, 91, 835–842. [Google Scholar] [CrossRef]
- Konovalova, S.A.; Avdeenko, A.P.; Santalova, A.A.; D’Yakonenko, V.V.; Palamarchuk, G.V.; Shishkin, O.V. Reaction of N-aryl-1,4-benzoquinone imines with sodium arenesulfinates. Russ. J. Org. Chem. 2014, 50, 1757–1762. [Google Scholar] [CrossRef]
- Tsoi, E.V.; Afanas’eva, G.B.; Chupakhin, O.N.; Sidorov, E.O. Reactions of N-Phenyl-1,4-benzoquinonemono imine benzoanalogs. Zh. Org. Khim. 1989, 25, 2409–2416. [Google Scholar]
- Fink, J.K. High Performance Polymers; William Andrew Inc.: Norwich, NY, USA, 2008. [Google Scholar]
- Drill, V.A.; DiPalma, J.R. Drill’s Pharmacology in Medicine; McGraw-Hill: New York, NY, USA, 1971. [Google Scholar]
- Burmistrov, K.S.; Toropin, N.V.; Burmistrov, S.I. Reaction of Hydrogen Bromide with N-Aryl-1,4-Benzoquinonemonoimines. Zh. Org. Khim. 1993, 29, 1170–1174. [Google Scholar]
- Burmistrov, K.S.; Yurchenko, A.G. Addition of Hydrogen-Chloride to N-Aryl-1,4-Benzoquinonmonoimines. Zh. Org. Khim. 1985, 21, 575–578. [Google Scholar]
- Novikov, V.P.; Bolibrukh, L.D.; Makovetsky, V.P.; Kolesnikov, V.T.; Pivovarova, N.S.; Pirozhenko, V.V. Reaction of N-phenyl-1,4-benzoquinone monoimine with dimedone. Dopov. Nats. Akad. Nauk Ukr. 1995, 6, 99–100. [Google Scholar]
- Tlustakova, M.; Honzl, J. Reaction of N-phenyl-p-quinoneimine with organometallic agents. Collect. Czechoslov. Chem. Commun. 1972, 37, 4031–4034. [Google Scholar] [CrossRef]
- Pedersen, C.J. Products of the photochemical decomposition of N,N′-disubstituted p-quinonediimine-N,N′-dioxides. J. Am. Chem. Soc. 1957, 79, 5014–5019. [Google Scholar] [CrossRef]
- Bird, C.W. The addition of diphenylketene to benzoquinone N-phenylimine. J. Chem. Soc. 1965, 3016. [Google Scholar] [CrossRef]
- Wang, S.; Yao, L.; Ying, J.; Wu, X.-F. Palladium-catalyzed carbonylation of iminoquinones and aryl iodides to access aryl p-amino benzoates. Org. Biomol. Chem. 2021, 19, 8246–8249. [Google Scholar] [CrossRef]
- Jillella, R.; Raju, S.; Hsiao, H.-C.; Hsu, D.-S.; Chuang, S.-C. Pd-Catalyzed Redox-Neutral C–N Coupling Reaction of Iminoquinones with Electron-Deficient Alkenes without External Oxidants: Access of Tertiary (E)-Enamines and Application to the Synthesis of Indoles and Quinolin-4-ones. Org. Lett. 2020, 22, 6252–6256. [Google Scholar] [CrossRef]
- Bailey, T.R. Unsymmetrical heterobiaryl synthesis. A highly efficient palladium-catalyzed cross-coupling reaction of heteroaryl trialkylstannanes with aryl halides. Tetrahedron Lett. 1986, 27, 4407–4410. [Google Scholar] [CrossRef]
- McGlacken, G.P.; Fairlamb, I.J.S. Palladium-Catalysed Cross-Coupling and Related Processes: Some Interesting Observations That Have Been Exploited in Synthetic Chemistry. Eur. J. Org. Chem. 2009, 2009, 4011–4029. [Google Scholar] [CrossRef]
- Iffland, L.; Petuker, A.; Van Gastel, M.; Apfel, U.P. Mechanistic Implications for the Ni(I)-Catalyzed Kumada Cross-Coupling Reaction. Inorganics 2017, 5, 78. [Google Scholar] [CrossRef]
- Takano, H.; Hiraishi, M.; Yaguchi, S.; Iwata, S.; Shoda, S.; Kobayashi, S. Alternating copolymerization of cyclic germylenes with N-phenyl-p-quinoneimine via oxidation-reduction process. Polym. J. 2016, 48, 969–972. [Google Scholar] [CrossRef]
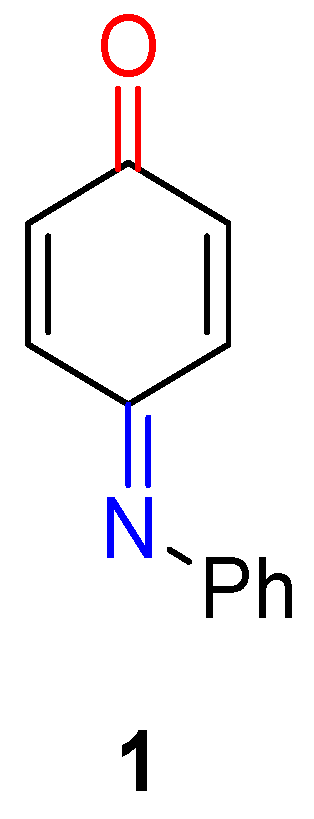
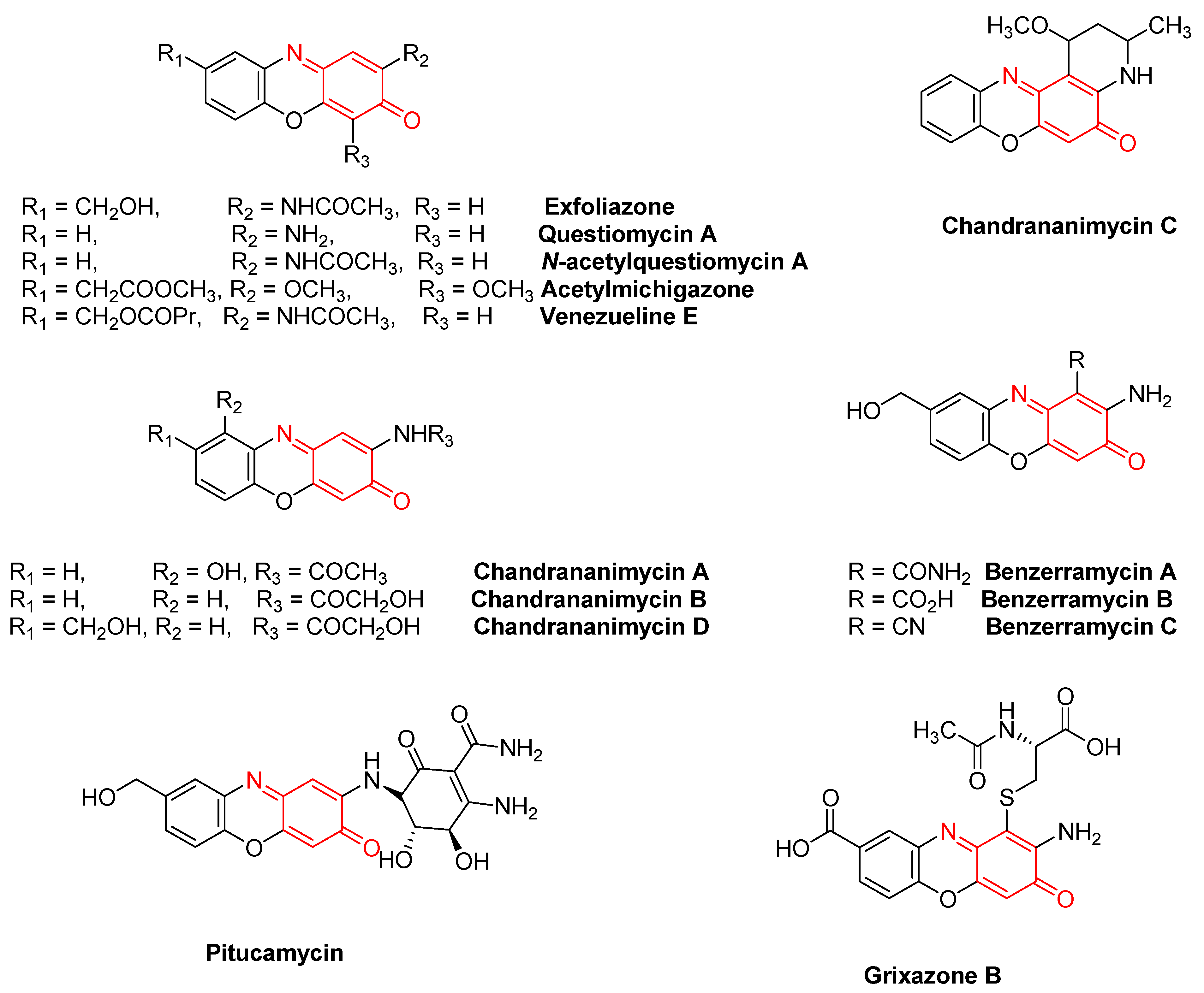
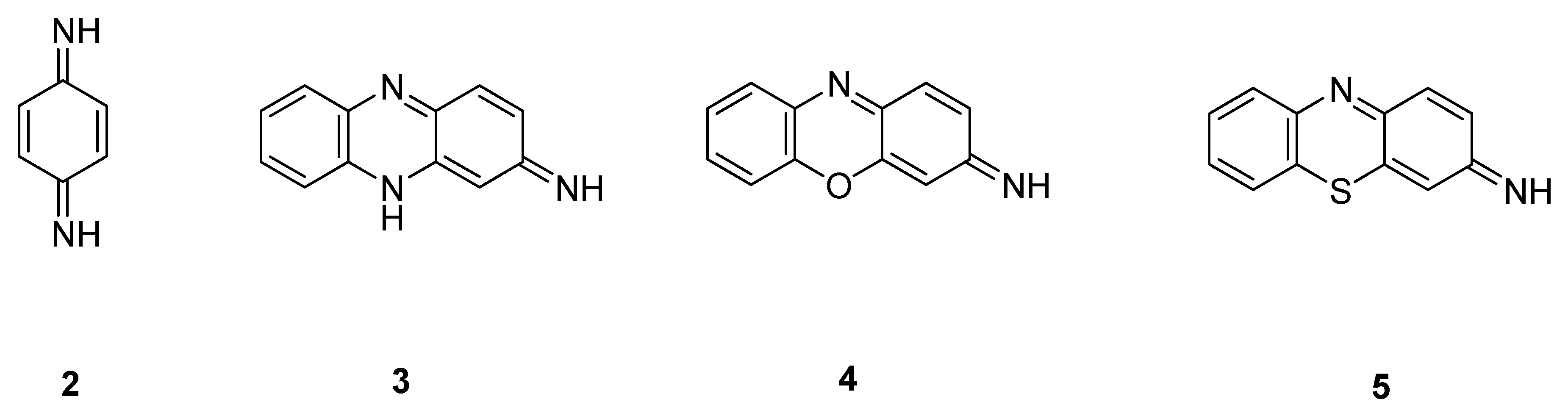
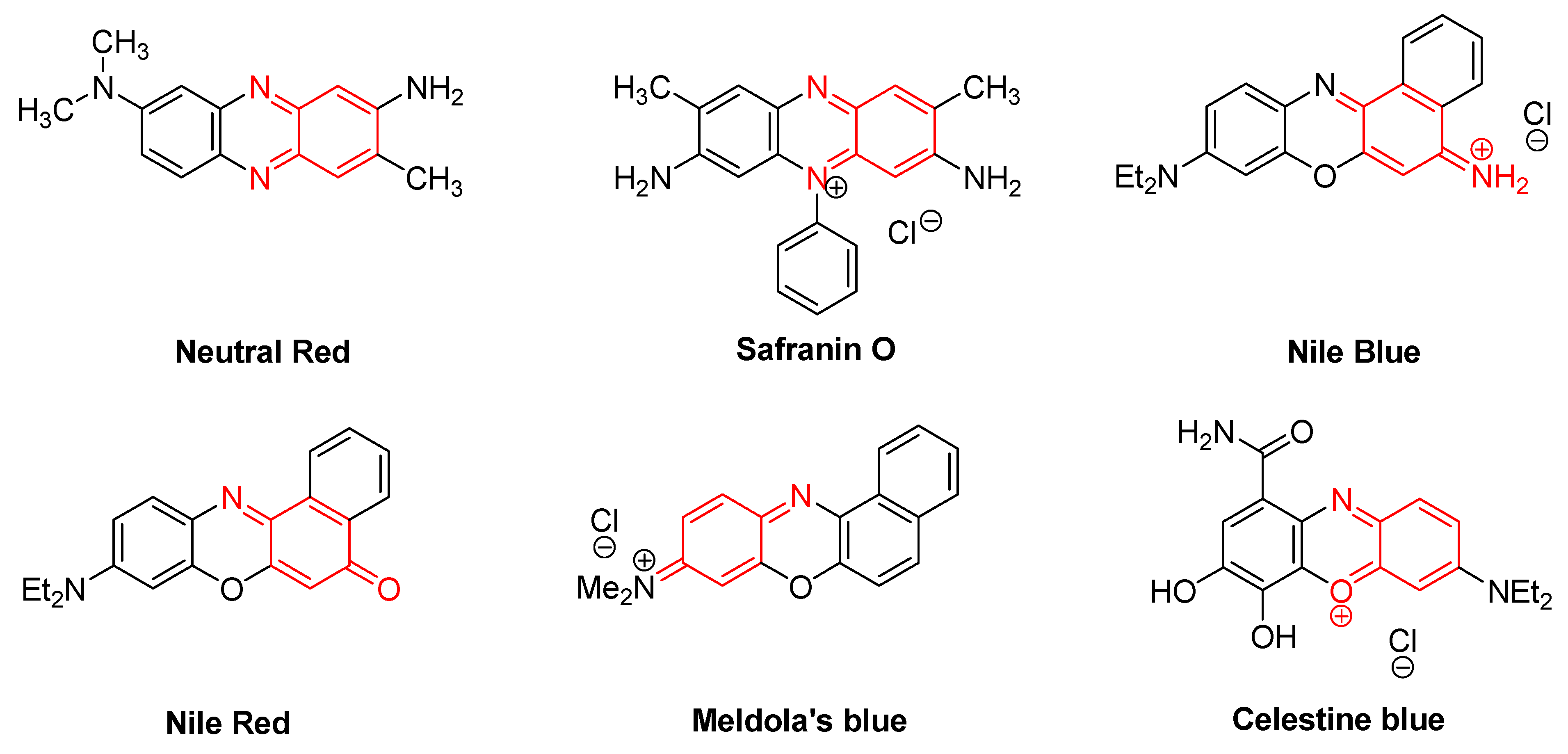
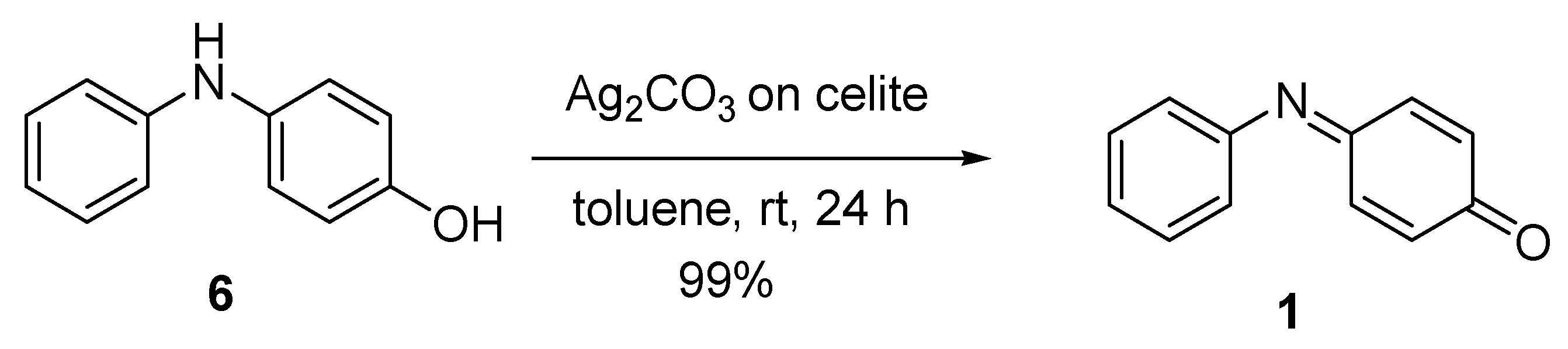






| Quineimine Derivatives as Natural Products | Biological Activities (References) |
|---|---|
| Exfoliazone | Antibiotic, antifungal, antitumor, growth-promoting activities [9,10] |
| Questiomycin A, N-acetylquestiomycin A, and Acetylmichigazone | Growth stimulatory and inhibitory effects [9] |
| Venezuelines A–G | Cytotoxic and antitumor activities [30] |
| Chandrananimycins A–D | Antibacterial, antifungal, antialgal, phytotoxic, and anticancer activities [31,33] |
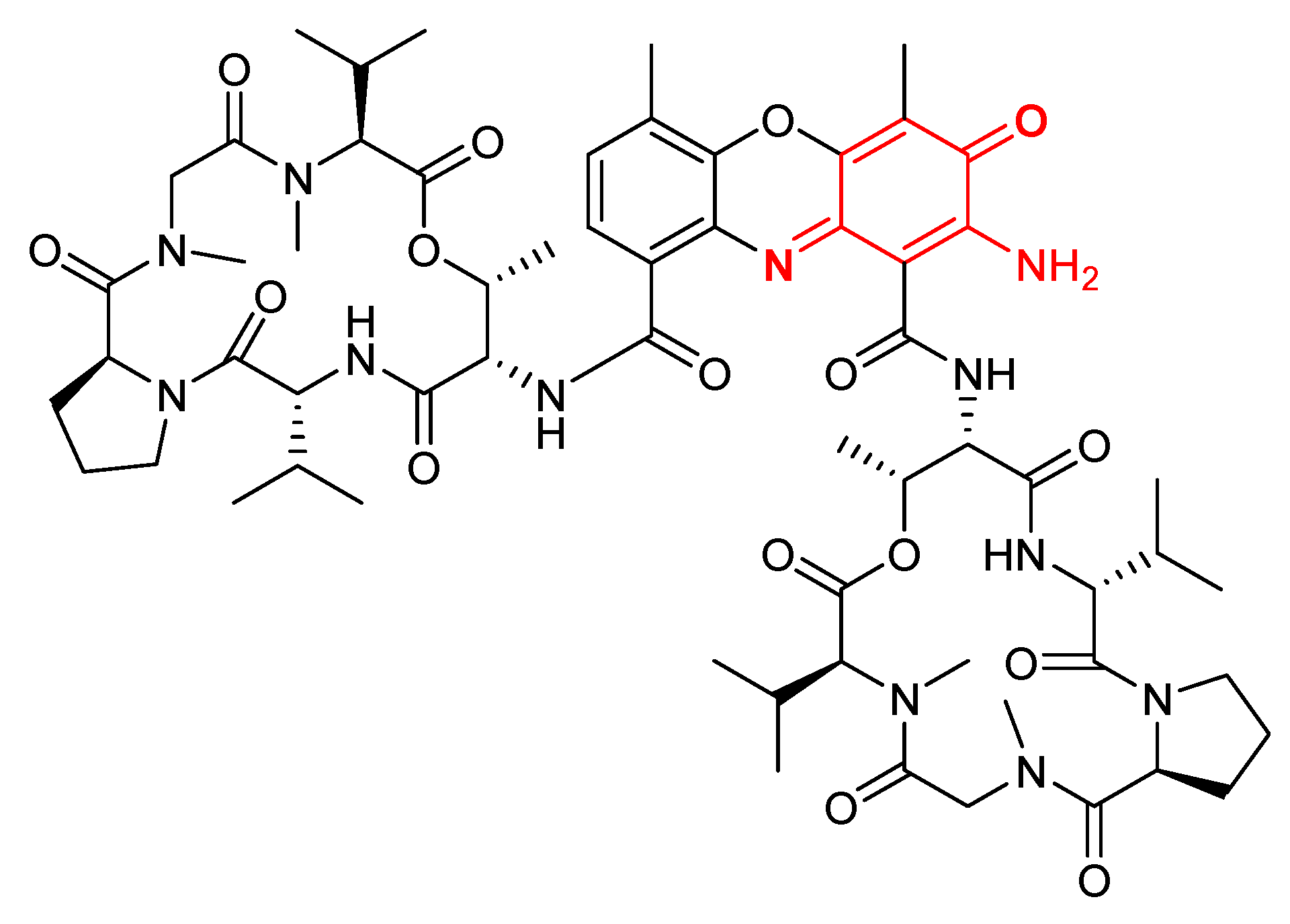
| Actinomycins | Antibacterial, antitumor, and anticancer activities [52,53,54,55] |
| Pitucamycin | Antiproliferative and cytotoxic activities [33] |
| Grixazone B | Antimicrobial [33] |
| Benzerramycin A–C | Antiproliferative [32] |
| Cinnabarin and Cinnabarinic acid | Antibacterial, antimicrobial [34] |
| Substrate | Oxidising Agent | Solvent | Temp (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 6 | HgO | Benzene | Reflux | 1 | 78 | [59] |
| 6 | Ag2CO3 on Celite | Toluene | rt | 30 min | 99 | [60] |
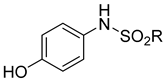 | Pb(OAc)4 | Acetic acid | rt | 1 | 58 | [61] |
| 6 | Hypochlorite | Heptane | rt | 1 | 99 | [62] |
| 6 | Hydrogen peroxide | Toluene | 35 | 25 min | 99 | [63] |
| 6 | Activated carbon catalyst | Methanol | 50 | 1 | 90 | [64] |
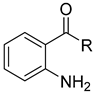 | 2-iodoxybenzoic acid | Dimethyl sulfoxide | rt | 35 min | 84–98 | [20] |
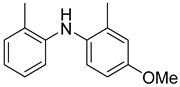 | Dess–Martin periodinane | Dichloromethane | rt | 8 | 56 | [65] |
| 6 | K2Cr2O7 | Acetone | 40 | 45 min | 97 | [66] |
 | K3[Fe(CN)6] | Phosphate buffer | rt | 30 min | n.r. | [67] |
| Reagent | Solvent | 41 (%) | 42 (%) | 43 (%) |
|---|---|---|---|---|
| CH3Li | THF a | 9.8 | 9.3 | 9.8 |
| CH3Li | Ether | 13.6 | 14.1 | 13.9 |
| CH3MgI | Ether | 15.6 | 14.0 | 14.8 |
| CH3CuLiI | Ether | 0 | 30.5 | 28.7 |
| CH3CuLiI | THF a | 0 | 28.1 | 57.7 |
| CH3Li + CuCl b | THF a | 0 | 44.0 | 7.8 |
| CH3MgI + CuI c | Ether | 23.4 | 17.8 | 18.4 |
| Entry | Aniline 55 | R1 | R2 | 56 (%) | 57 (%) | 58 (%) |
|---|---|---|---|---|---|---|
| 1 | a | H | H | 79 | 13 | nd |
| 2 | b | 4-OMe | H | 66 | 17 | nd |
| 3 | c | 4-Cl | H | 29 | 15 | nd |
| 4 | d | 4-CF3 | H | 29 | 3 | nd |
| 5 | e | 2-Me | H | 72 | t | 5 |
| 6 | f | H | Me | 59 | 8 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adesina, A.; Skouta, R. Diversity-Orientated Synthesis and Biological Properties of Compounds Based on the N-Phenylquinoneimine Scaffold. Molecules 2024, 29, 249. https://doi.org/10.3390/molecules29010249
Adesina A, Skouta R. Diversity-Orientated Synthesis and Biological Properties of Compounds Based on the N-Phenylquinoneimine Scaffold. Molecules. 2024; 29(1):249. https://doi.org/10.3390/molecules29010249
Chicago/Turabian StyleAdesina, Adebimpe, and Rachid Skouta. 2024. "Diversity-Orientated Synthesis and Biological Properties of Compounds Based on the N-Phenylquinoneimine Scaffold" Molecules 29, no. 1: 249. https://doi.org/10.3390/molecules29010249
APA StyleAdesina, A., & Skouta, R. (2024). Diversity-Orientated Synthesis and Biological Properties of Compounds Based on the N-Phenylquinoneimine Scaffold. Molecules, 29(1), 249. https://doi.org/10.3390/molecules29010249