Determination and Characterization of Gold Nanoparticles in Liquor Using Asymmetric Flow Field-Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Investigation of the Effects of Ethanol on Au NPs
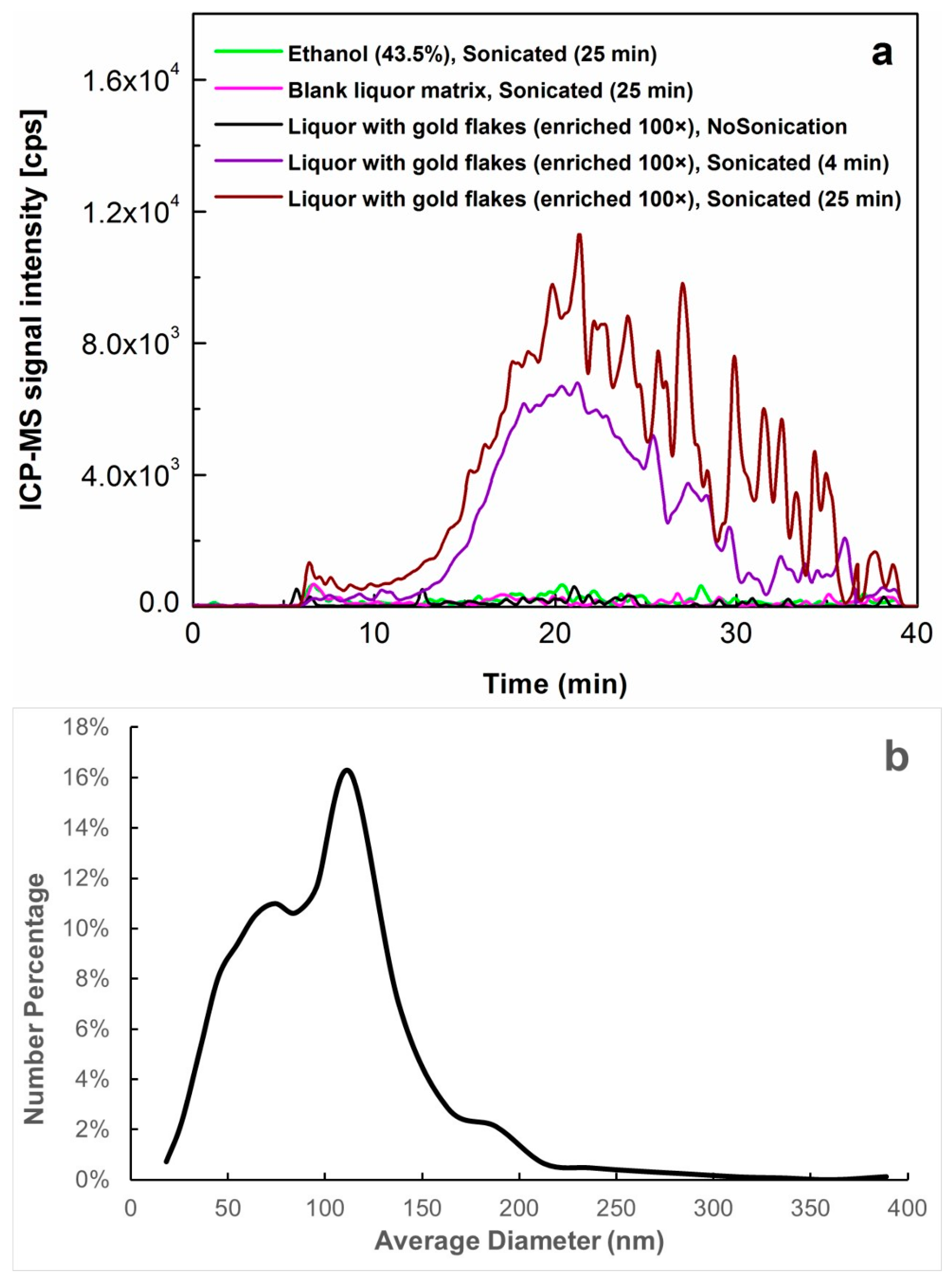
2.2. Determination of Au NPs Released after Ultrasound Treatment in Gold-Containing Liquor by AF4-ICP-MS
2.2.1. Protocol Optimization for the Sample Preparation
2.2.2. Characterization of Size and Number-Based Size Distribution of Au NPs
2.2.3. Mass Quantification of Au NPs with Pre-Channel Mass Calibration
2.3. Measurement of the Recovery in Au NPs Detection by AF4-ICP-MS with Pre-Channel Calibration
3. Materials and Methods
3.1. Instrumentation
3.2. Reagents and Materials
3.3. Sample Preparation for Release and Extraction of Au NPs
3.4. Size Characterization of Au NPs by AF4-ICP-MS
3.5. Quantification of Au NPs by Pre-Channel Mass Calibration
3.6. Measurement of Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.7. Evaluation of Recovery Rates in Au NPs Analysis by AF4-ICP-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA Panel on Food Additives and Nutrients Sources added to Food. Scientific opinion on the re-evaluation of gold (E 175) as a food additive. EFSA J. 2016, 14, 4362. [Google Scholar]
- Chirino, Y.I.; Pedraza-Chaverri, J. Food additives containing nanoparticles induce gastrotoxicity, hepatotoxicity and alterations in animal behavior: The unknown role of oxidative stress. Food Chem. Toxicol. 2020, 146, 111814. [Google Scholar]
- Medina-Reyes, E.I.; Rodríguez-Ibarra, C.; Déciga-Alcaraz, A.; Díaz-Urbina, D.; Imai, K. Concern of carcinogenic risk of eating gold leaf (gold foil)—In relation to asbestos carcinogenesis mechanism. Nanobiomedicine 2018, 10, 26–30. [Google Scholar]
- Russell, M.A.; King, L.E., Jr.; Boyd, A.S. Lichen planus after consumption of a gold containing liquor. N. Engl. J. Med. 1996, 334, 603. [Google Scholar] [CrossRef]
- Russell, M.A.; Langley, M.; Truett, A.P.; King, L.E., Jr.; Boyd, A.S. Lichenoid dermatitis after consumption of gold-containing liquor. J. Am. Acad. Dermatol. 1997, 36, 841–844. [Google Scholar] [CrossRef]
- Guenthner, T.; Stork, C.M.; Cantor, R.M. Goldschlager allergy in a gold allergic patient. Vet. Hum. Toxicol. 1999, 41, 246. [Google Scholar]
- Möller, H. Contact allergy to gold as a model for clinical-experimental research. Contact Dermat. 2010, 62, 193–200. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Poulsen, M.; Nielsen, E. Toxicological risk assessment of elemental gold following oral exposure to sheets and NPs—A review. Regul. Toxicol. Pharmacol. 2015, 72, 216–221. [Google Scholar] [CrossRef]
- Evariste, L.; Lamas, B.; Ellero-Simatos, S.; Khoury, L.; Cartier, C.; Gaultier, E.; Chassaing, B.; Feltin, N.; Devoille, L.; Favre, G.; et al. A 90-day oral exposure to food-grade gold at relevant human doses impacts the gut microbiota and the local immune system in a sex-dependent manner in mice. Part. Fibre Toxicol. 2023, 20, 27. [Google Scholar] [CrossRef]
- Leong, T.S.H.; Manickam, S.; Martin, G.J.; Li, W.; Ashokkumar, M. Ultrasonic Production of Nano-Emulsions for Bioactive Delivery in Drug and Food Applications; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Firouz, M.S.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of high-intensity ultrasound to improve food processing efficiency: A review. Foods 2022, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Manyatsi, T.S.; Morata, A.; Tiwari, B.K. Ultrasound-assisted production of alcoholic beverages: From fermentation and sterilization to extraction and aging. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5243–5271. [Google Scholar] [CrossRef] [PubMed]
- Lomthong, T.; Siripornvisal, S.; Khunnamwong, P. Ultrasound-assisted enzymatic hydrolysis of broken Riceberry rice for sugar syrup production as a substrate for bacterial cellulose facial mask development. J. Appl. Biol. Biotechnol. 2022, 10, 96–101. [Google Scholar]
- Estivi, L.; Brandolini, A.; Condezo-Hoyos, L.; Hidalgo, A. Impact of low-frequency ultrasound technology on physical, chemical and technological properties of cereals and pseudocereals. Ultrason. Sonochem. 2022, 86, 106044. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, D.A. Gold nanoparticles: Recent aspects for human toxicology. J. Occup. Med. Toxicol. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Enea, M.; Pereira, E.; Peixoto de Almeida, M.; Araújo, A.M.; Bastos, M.d.L.; Carmo, H. Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Wang, Q.; Li, M.; Dang, F.; Zhou, D.M. Nonselective uptake of silver and gold nanoparticles by wheat. Nanotoxicology 2019, 13, 1073–1086. [Google Scholar] [CrossRef]
- García, R.Á.F.; Fernández-Iglesias, N.; López-Chaves, C.; Sánchez-González, C.; Llopis, J.; Montes-Bayón, M.; Bettmer, J. Complementary techniques (spICP-MS, TEM, and HPLC-ICP-MS) reveal the degradation of 40 nm citrate-stabilized Au nanoparticles in rat liver after intraperitoneal injection. J. Trace Elem. Med. Biol. 2019, 55, 1–5. [Google Scholar] [CrossRef]
- Witzler, M.; Küllmer, F.; Hirtz, A.; Günther, K. Validation of gold and silver nanoparticle analysis in fruit juices by single-particle ICP-MS without sample pretreatment. J. Agric. Food Chem. 2016, 64, 4165–4170. [Google Scholar] [CrossRef]
- Donovan, A.R.; Adams, C.D.; Ma, Y.; Stephan, C.; Eichholz, T.; Shi, H. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere 2016, 144, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.C.; Stephan, C.; Lead, J.R. Single-particle inductively coupled plasma mass spectroscopy analysis of size and number concentration in mixtures of monometallic and bimetallic (core-shell) nanoparticles. Talanta 2017, 162, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Mehtala, J.G.; Wei, A. Nanometric resolution in the hydrodynamic size analysis of ligand-stabilized gold nanorods. Langmuir 2014, 30, 13737–13743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nie, X.; Ji, Y.; Liu, Y.; Wu, X.; Chen, C.; Fang, X. Quantitative biokinetics and systemic translocation of various gold nanostructures are highly dependent on their size and shape. J. Nanosci. Nanotechnol. 2014, 14, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Bi, X.; Reed, R.B.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ. Sci. Technol. 2014, 48, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Y.; Yu, W.; Xu, L.; Ge, C.; Liu, J.; Gu, N. Linear aggregation of gold nanoparticles in ethanol. Colloids Surf. A 2003, 223, 177–183. [Google Scholar] [CrossRef]
- Hussain, I.; Wang, Z.; Cooper, A.; Brust, M. Formation of spherical nanostructures by the controlled aggregation of gold colloids. Langmuir 2006, 22, 2938–2941. [Google Scholar] [CrossRef]
- López-Sanz, S.; Fariñas, N.R.; Martín-Doimeadios, R.d.C.R.; Ríos, Á. Analytical strategy based on asymmetric flow field flow fractionation hyphenated to ICP-MS and complementary techniques to study gold nanoparticles transformations in cell culture medium. Anal. Chim. Acta 2019, 1053, 178–185. [Google Scholar] [CrossRef]
- Mekprayoon, S.; Siripinyanond, A. Performance evaluation of flow field-flow fractionation and electrothermal atomic absorption spectrometry for size characterization of gold nanoparticles. J. Chromatogr. A 2019, 1604, 460493. [Google Scholar] [CrossRef]
- Bocca, B.; Battistini, B.; Petrucci, F. Silver and gold nanoparticles characterization by SP-ICP-MS and AF4-FFF-MALS-UV-ICP-MS in human samples used for biomonitoring. Talanta 2020, 220, 121404. [Google Scholar] [CrossRef]
- Techarang, T.; Siripinyanond, A. Use of electrical field-flow fractionation for gold nanoparticles after improving separation efficiency by carrier liquid optimization. Anal. Chim. Acta 2021, 1144, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Chan, W.C.W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Goebl, J.; Lu, Z.; Yin, Y. Role of salt in the spontaneous assembly of charged gold nanoparticles in ethanol. Langmuir 2011, 27, 5282–5289. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chua, S.L.; Ch’ng, A.L.; Yu, D.; Koh, S.P.; Phang, H.; Chiew, P. An effective approach for size characterization and mass quantification of silica nanoparticles in coffee creamer by AF4-ICP-MS. Anal. Bioanal. Chem. 2020, 412, 5499–5512. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A. Separation and size characterization of highly polydisperse titanium dioxide nanoparticles (E171) in powdered beverages by using asymmetric flow field-flow fractionation hyphenated with multi-angle light scattering and inductively coupled plasma mass spectrometry. J. Chromatogr. A 2021, 1643, 462059. [Google Scholar] [PubMed]
- Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A. Detection, identification and size distribution of silver nanoparticles (AgNPs) in milk and migration study for breast milk storage bags. Molecules 2022, 27, 2539. [Google Scholar] [CrossRef] [PubMed]
- Verleysen, E.; Waegeneers, N.; De Vos, S.; Brassinne, F.; Ledecq, M.; Van Steen, F.; Andjelkovic, M.; Janssens, R.; Mathioudaki, S.; Delfosse, L.; et al. Physicochemical characterization of nanoparticles in food additives in the context of risk identification. EFSA J. 2021, 18, 6678E. [Google Scholar] [CrossRef]
- Gaborskia, T.R.; Snyder, J.L.; Striemer, C.C.; Fang, D.Z.; Hoffman, M.; Fauchet, P.M.; McGrath, J.L. High performance separation of nanoparticles with ultrathin porous nanocrystalline silicon membranes. ACS Nano 2010, 4, 6973–6981. [Google Scholar] [CrossRef]
- Geiss, O.; Cascio, C.; Gilliland, D.; Franchini, F.; Barrero-Moreno, J. Size and mass determination of silver nanoparticles in an aqueous matrix using asymmetric field flow fractionation coupled to inductively coupled plasma mass spectrometer and ultraviolet–visible detectors. J. Chromatogr. A 2013, 1321, 100–108. [Google Scholar] [CrossRef]
- Gray, E.P.; Bruton, T.A.; Higgins, C.P.; Halden, R.U.; Westerhoff, P.; Ranville, J.F. Analysis of gold nanoparticle mixtures: A comparison of hydrodynamic chromatography (HDC) and asymmetrical flow field-flow fractionation (AF4) coupled to ICP-MS. J. Anal. At. Spectrom. 2012, 27, 1532–1539. [Google Scholar] [CrossRef]
- Mudalige, T.K.; Qu, H.; Sanchez-Pomales, G.; Sisco, P.N.; Linder, S.W. Simple functionalization strategies for enhancing nanoparticle separation and recovery with asymmetric flow field flow fractionation. Anal. Chem. 2015, 87, 1764–1772. [Google Scholar] [CrossRef] [PubMed]

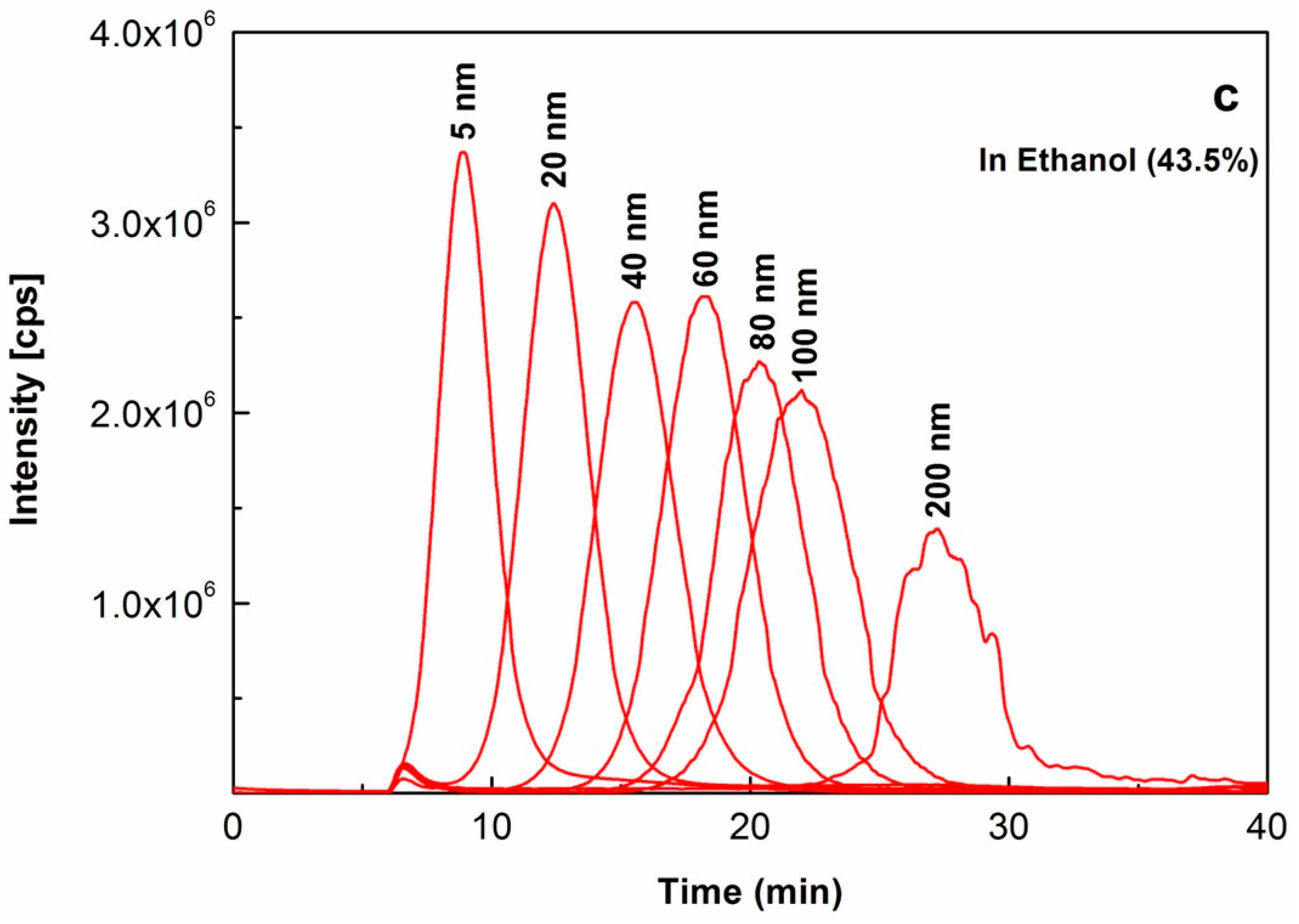
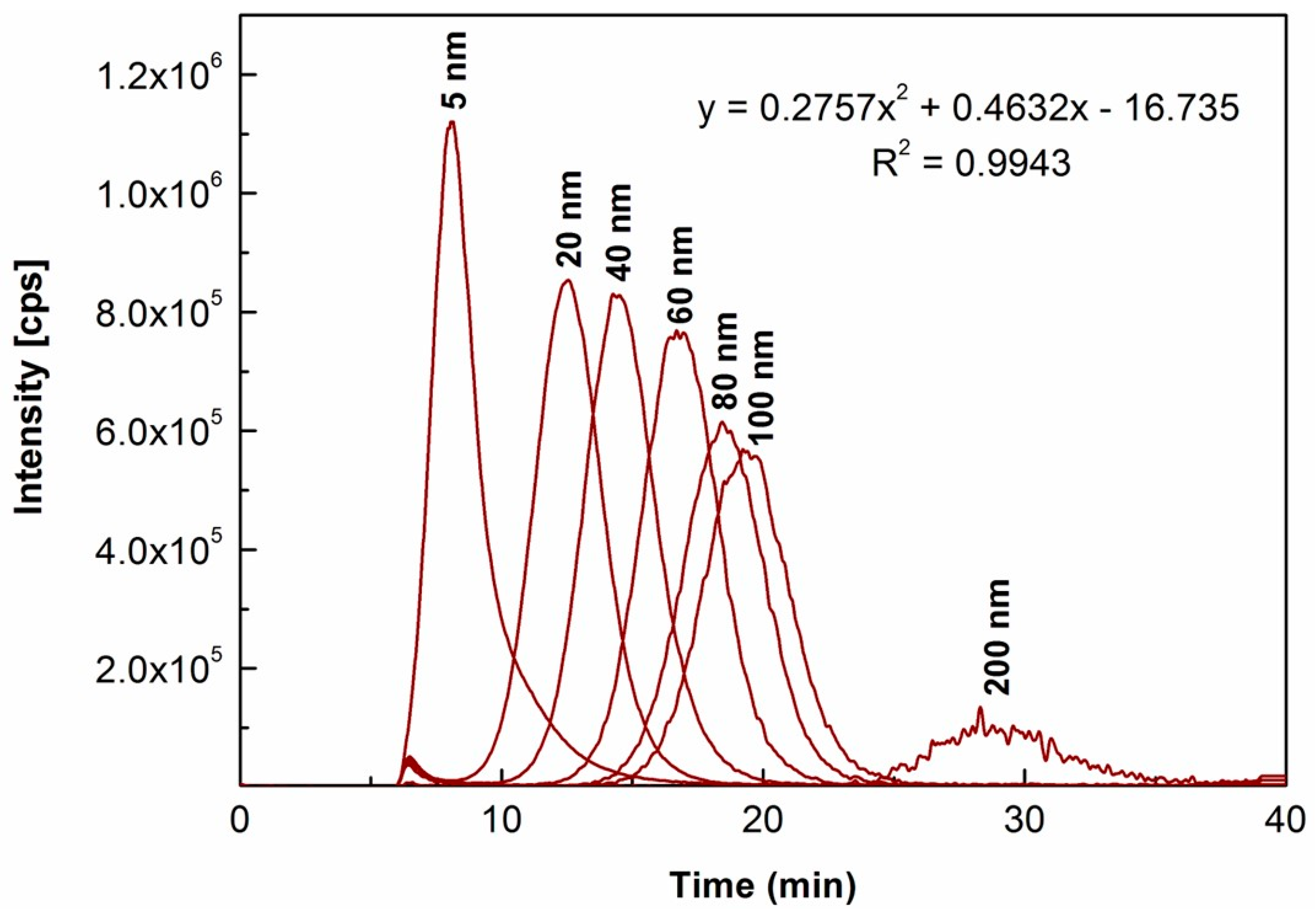
| Size Reported in Product Certificate a | DLS Analysis Value b (nm) | |||
|---|---|---|---|---|
| Normal Size (nm) | TEM Diameter (nm) | Hydrodynamic Diameter (DLS) (Dh) (nm) | Z-Average Diameter (nm) | PDI |
| 5 | 5.0 ± 0.6 | ─ c | 10.1 ± 0.5 | 0.311 ± 0.034 |
| 20 | 18.9 ± 1.5 | 24.0 | 22.4 ± 0.1 | 0.141 ± 0.002 |
| 40 | 40.0 ± 5.0 | 45.0 | 43.1 ± 0.3 | 0.113 ± 0.010 |
| 60 | 60.0 ± 6.0 | 68.0 | 70.2 ± 0.3 | 0.094 ± 0.013 |
| 80 | 77.0 ± 10.0 | 83.0 | 83.3 ± 0.3 | 0.158 ± 0.013 |
| 100 | 103.0 ± 10.0 | 105.0 | 110.5 ± 0.2 | 0.063 ± 0.007 |
| 200 | 200.0 | 213.0 | 212.9 ± 3.3 | 0.097 ± 0.019 |
| Product a | Retention Time Range (min) | Hydrodynamic Diameter (Dh) (nm) | |
|---|---|---|---|
| Major Diameter (nm) b | Size Range (nm) c | ||
| Gold-containing Liquor | (8.7 ± 0.2)~(38.0 ± 0.1) | 123.7 ± 5.5 | (8.3 ± 1.1)~(398.0 ± 2.7) |
| Edible Gold Flakes (EGF) | (11.4 ± 0.4)~(38.3 ± 0.4) | 126.6 ± 9.8 | (17.9 ± 1.4)~(454.8 ± 12.3) |
| Mass Concentration of Au NPs in Liquor (μg L−1) a | Limit of Detection (LOD) (µg L−1) d | Limit of Quantification (LOQ) (µg L−1) d | |
|---|---|---|---|
| No Sonication | Sonication b | ||
| Liquor with 100-Fold Enrichment | |||
| No Detection | 48.1 ± 0.6 c | 1.4 | 3.7 |
| Nominal Size (nm) | Recovery Rate (R)— Overall (%) | Recovery Rate (RA)— Matrix (%) | Recovery Rate (RB)— AF4 Channel (%) |
|---|---|---|---|
| 5 | 82 ± 2 | 89 ± 1 | 92 ± 2 |
| 20 | 93 ± 2 | 101 ± 3 | 93 ± 1 |
| 60 | 92 ± 7 | 94 ± 1 | 98 ± 7 |
| 100 | 95 ± 2 | 98 ± 1 | 97 ± 2 |
| 200 | 91 ± 2 | 97 ± 1 | 94 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Chua, S.L.; Yu, D.; Chan, S.H.; Li, A. Determination and Characterization of Gold Nanoparticles in Liquor Using Asymmetric Flow Field-Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry. Molecules 2024, 29, 248. https://doi.org/10.3390/molecules29010248
Li B, Chua SL, Yu D, Chan SH, Li A. Determination and Characterization of Gold Nanoparticles in Liquor Using Asymmetric Flow Field-Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry. Molecules. 2024; 29(1):248. https://doi.org/10.3390/molecules29010248
Chicago/Turabian StyleLi, Bin, Sew Lay Chua, Dingyi Yu, Sheot Harn Chan, and Angela Li. 2024. "Determination and Characterization of Gold Nanoparticles in Liquor Using Asymmetric Flow Field-Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry" Molecules 29, no. 1: 248. https://doi.org/10.3390/molecules29010248
APA StyleLi, B., Chua, S. L., Yu, D., Chan, S. H., & Li, A. (2024). Determination and Characterization of Gold Nanoparticles in Liquor Using Asymmetric Flow Field-Flow Fractionation Hyphenated with Inductively Coupled Plasma Mass Spectrometry. Molecules, 29(1), 248. https://doi.org/10.3390/molecules29010248






