Emerging Strategies for Enhancing Propionate Conversion in Anaerobic Digestion: A Review
Abstract
1. Introduction
2. Production and Metabolism of Propionate in Anaerobic Digesters
2.1. Production of Propionate
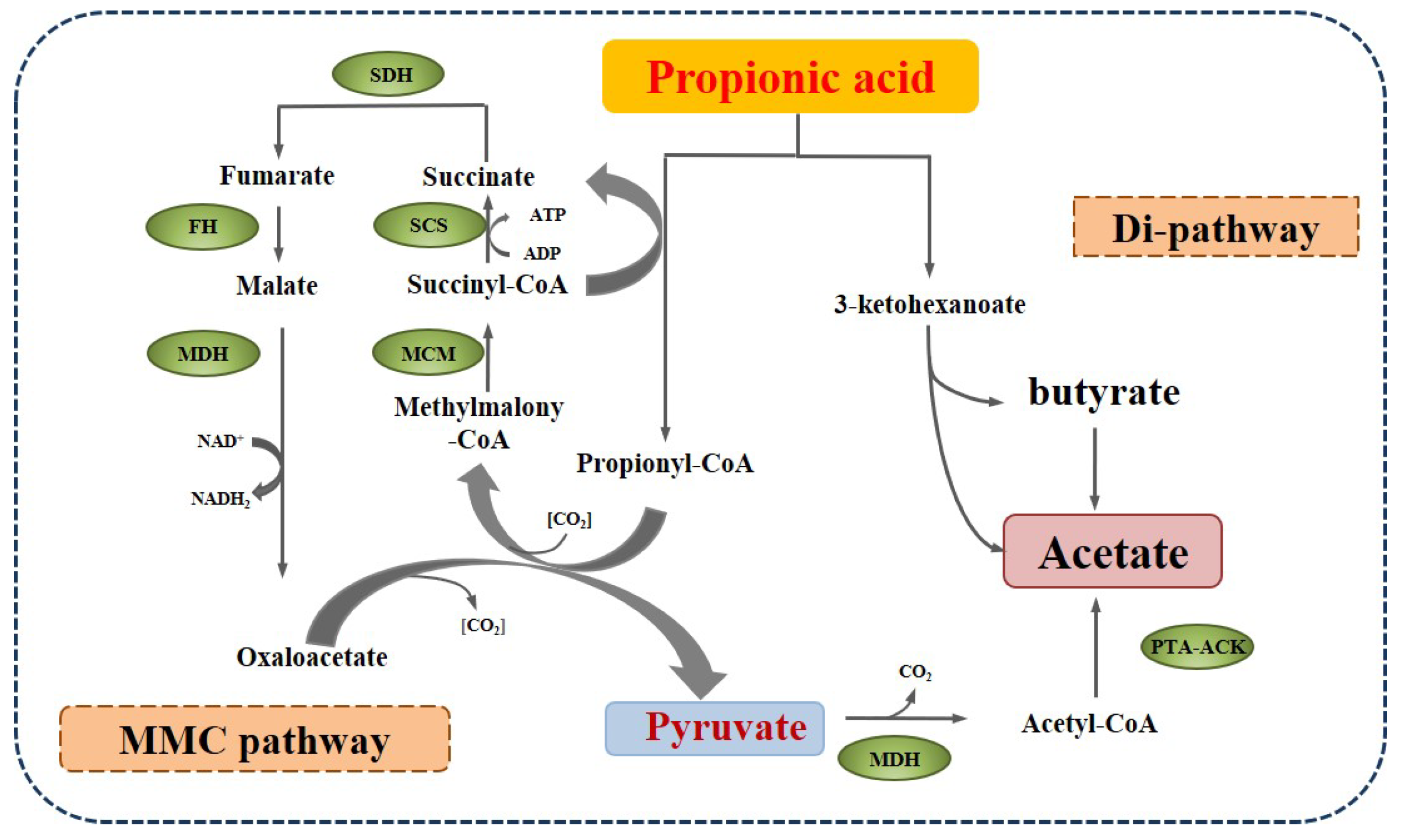
2.2. Metabolism of Propionate
3. Critical Parameters Influencing the Biodegradation of Propionate
4. Engineering Strategies for Enhancing the Biodegradation of Propionate
4.1. Buffering Addition
4.2. Bioaugmentation
4.3. Supplementary Trace Elements
4.4. Addition of Sulfate
4.5. Addition of Nitrogen-Containing Compound
4.6. Addition of Conductive Material
4.7. Nano-Sized Additives
4.8. Degasification of Dissolved Hydrogen
5. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mardani, A.; Zavadskas, E.K.; Khalifah, Z.; Zakuan, N.; Jusoh, A.; Nor, K.M.; Khoshnoudi, M. A review of multi-criteria decision-making applications to solve energy management problems: Two decades from 1995 to 2015. Renew. Sustain. Energy Rev. 2017, 71, 216–256. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, K.; Zhu, W.; Zhang, L.; Zhao, Z.; Xu, L.; Yang, J.; Shen, B. Effects of oxygen deficient and flue gas torrefaction on the surface property and structural feature of typical agriculture waste: Rice husk. Fuel 2022, 327, 125211. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Lee, C.T.; Klemeš, J.J.; Ho, C.S.; Ho, W.S. The characterisation and treatment of food waste for improvement of biogas production during anaerobic digestion—A review. J. Clean. Prod. 2018, 172, 1545–1558. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yi, X.; Yang, F.; Wang, D.; Han, H. Dissimilatory manganese reduction facilitates synergistic cooperation of hydrolysis, acidogenesis, acetogenesis and methanogenesis via promoting microbial interaction during anaerobic digestion of waste activated sludge. Environ. Res. 2023, 218, 114992. [Google Scholar] [CrossRef]
- Akman, H.E.; Perendeci, N.A.; Ertekin, C.; Yaldiz, O. Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules 2022, 27, 6891. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Y.; Xie, H.; Wang, H.; Liu, L.; Du, H.; Imanaka, T.; Igarashia, Y.; Luo, F. Performance on a novel rotating bioreactor for dry anaerobic digestion: Efficiency and biological mechanism compared with wet fermentation. Energy 2022, 254, 124404. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. A review of process parameters influence in solid-state anaerobic digestion: Focus on performance stability thresholds. Renew. Sustain. Energy Rev. 2022, 167, 112756. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Xie, H.; Cao, W.; Chen, R.; Kong, Z.; Zhang, Y. Insight into the effects and mechanism of cellulose and hemicellulose on anaerobic digestion in a CSTR-AnMBR system during swine wastewater treatment. Sci. Total Environ. 2023, 869, 161776. [Google Scholar] [CrossRef]
- Lima, V.D.O.; de Barros, V.G.; Duda, R.M.; de Oliveira, R.A. Anaerobic digestion of vinasse and water treatment plant sludge increases methane production and stability of UASB reactors. J. Environ. Manag. 2023, 327, 116451. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Ma, H. The key regulative parameters in pilot-scale IC reactor for effective incineration landfill leachate treatment: Focus on the process performance and microbial community. J. Water Process. Eng. 2023, 51, 103322. [Google Scholar] [CrossRef]
- Mortezaei, Y.; Amani, T.; Elyasi, S. Corrigendum to ’High-rate anaerobic digestion of yogurt wastewater in a hybrid EGSB and fixed-bed reactor: Optimizing through response surface methodology’ [Process 113 (2018) 255–263]. Process. Saf. Environ. Prot. 2023, 171, 895. [Google Scholar] [CrossRef]
- Yang, H.; Deng, L.; Wang, L.; Zheng, D.; Liu, Y.; Wang, S.; Huang, F. Comparison of three biomass-retaining reactors of the ASBR, the UBF and the USR treating swine wastewater for biogas production. Renew. Energy 2019, 138, 521–530. [Google Scholar] [CrossRef]
- Veiga, S.; Romero, M.; Faccio, R.; Segobia, D.; Apesteguía, C.; Pérez, A.L.; Brondino, C.D.; Bussi, J. Biogas dry reforming over Ni-La-Ti catalysts for synthesis gas production: Effects of preparation method and biogas composition. Fuel 2023, 346, 128300. [Google Scholar] [CrossRef]
- Tavera, C.G.; Raab, T.; Trujillo, L.H. Valorization of biogas digestate as organic fertilizer for closing the loop on the economic viability to develop biogas projects in Colombia. Clean. Circ. Bioecon. 2023, 4, 100035. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Nguyen, D.; Surendra, K.C.; Shrestha, S.; Rajendran, K.; Oechsner, H.; Xie, L.; Khanal, S.K. Anaerobic biorefinery: Current status, challenges, and opportunities. Bioresour. Technol. 2016, 215, 304–313. [Google Scholar] [CrossRef]
- Edwards, J.; Othman, M.; Burn, S. A review of policy drivers and barriers for the use of anaerobic digestion in Europe, the United States and Australia. Renew. Sustain. Energy Rev. 2015, 52, 815–828. [Google Scholar] [CrossRef]
- Bangalore, M.; Hochman, G.; Zilberman, D. Policy incentives and adoption of agricultural anaerobic digestion: A survey of Europe and the United States. Renew. Energy 2016, 97, 559–571. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.-Y.; Qiao, W.; Wang, X.; Takayanagi, K. Sulfate addition as an effective method to improve methane fermentation performance and propionate degradation in thermophilic anaerobic co-digestion of coffee grounds, milk and waste activated sludge with AnMBR. Bioresour. Technol. 2015, 185, 308–315. [Google Scholar] [CrossRef]
- Paranjpe, A.; Saxena, S.; Jain, P. A Review on Performance Improvement of Anaerobic Digestion Using Co-Digestion of Food Waste and Sewage Sludge. J. Environ. Manag. 2023, 338, 117733. [Google Scholar] [CrossRef]
- Perez-Esteban, N.; Vinardell, S.; Vidal-Antich, C.; Peña-Picola, S.; Chimenos, J.; Peces, M.; Dosta, J.; Astals, S. Potential of anaerobic co-fermentation in wastewater treatments plants: A review. Sci. Total. Environ. 2022, 813, 152498. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Liu, X.; Liu, X.; Liu, R.; Wang, Z.; Li, Y.; Sun, C.; Abd-Alla, M.H.; Rasmey, A.-H.M. Integration of two-stage anaerobic digestion process with in situ biogas upgrading. Bioresour. Technol. 2023, 369, 128475. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Green, H.; Tao, W. Reversibility of propionic acid inhibition to anaerobic digestion: Inhibition kinetics and microbial mechanism. Chemosphere 2020, 255, 126840. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Wei, Y.; Guan, R.; Li, X.; Lu, X.; Rong, S.; Zuo, X.; Yuan, H. High-solids anaerobic co-digestion performances and microbial community dynamics in co-digestion of different mixing ratios with food waste and highland barley straw. Energy 2023, 262, 125529. [Google Scholar] [CrossRef]
- Lu, X.; Ni, J.; Zhen, G.; Kubota, K.; Li, Y.-Y. Response of morphology and microbial community structure of granules to influent COD/SO42—Ratios in an upflow anaerobic sludge blanket (UASB) reactor treating starch wastewater. Bioresour. Technol. 2018, 256, 456–465. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, T.; Xing, W.; Li, R.; Yang, T.; Yao, N.; Lv, D. Links between carbon/nitrogen ratio, synergy and microbial characteristics of long-term semi-continuous anaerobic co-digestion of food waste, cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126094. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, P.; Wang, Y.; Wu, P.; Li, Y.; Ma, L. Enhancing methane production in anaerobic co-digestion of sewage sludge and food waste by regulating organic loading rate. Bioresour. Technol. 2022, 363, 127988. [Google Scholar] [CrossRef]
- Meyer, G.; Okudoh, V.; van Rensburg, E. A rumen based anaerobic digestion approach for lignocellulosic biomass using barley straw as feedstock. S. Afr. J. Chem. Eng. 2022, 41, 98–104. [Google Scholar] [CrossRef]
- Tenci, N.A.; Ammam, F.; Huang, W.E.; Thompson, I.P. Anaerobic co-digestion of Euphorbia tirucalli with pig blood for volatile fatty acid production. Bioresour. Technol. Rep. 2023, 21, 101333. [Google Scholar] [CrossRef]
- Stanley, J.T.; Thanarasu, A.; Kumar, P.S.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential pre-treatment of lignocellulosic biomass for the enhancement of biomethane production through anaerobic digestion—A review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production–A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Hanum, F.; Atsuta, Y.; Daimon, H. Methane Production Characteristics of an Anaerobic Co-Digestion of Pig Manure and Fermented Liquid Feed. Molecules 2022, 27, 6509. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Karrabi, M.; Ranjbar, F.M.; Shahnavaz, B.; Seyedi, S. A comprehensive review on biogas production from lignocellulosic wastes through anaerobic digestion: An insight into performance improvement strategies. Fuel 2023, 340, 127239. [Google Scholar] [CrossRef]
- Liu, L.; Yun, S.; Ke, T.; Wang, K.; An, J.; Liu, J. Dual utilization of aloe peel: Aloe peel-derived carbon quantum dots enhanced anaerobic co-digestion of aloe peel. Waste Manag. 2023, 159, 163–173. [Google Scholar] [CrossRef]
- González, J.F.; Parralejo, A.I.; González, J.; Álvarez, A.; Sabio, E. Optimization of the production and quality of biogas in the anaerobic digestion of different types of biomass in a batch laboratory biodigester and pilot plant: Numerical modeling, kinetic study and hydrogen potential. Int. J. Hydrogen Energy 2022, 47, 39386–39403. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yuan, Z.; Wang, R.; Angelidaki, I.; Zhu, G. Syntrophy mechanism, microbial population, and process optimization for volatile fatty acids metabolism in anaerobic digestion. Chem. Eng. J. 2023, 452, 139137. [Google Scholar] [CrossRef]
- Herrero, M.; Stuckey, D. Bioaugmentation and its application in wastewater treatment: A review. Chemosphere 2015, 140, 119–128. [Google Scholar] [CrossRef]
- Leng, L.; Yang, P.; Singh, S.; Zhuang, H.; Xu, L.; Chen, W.-H.; Dolfing, J.; Li, D.; Zhang, Y.; Zeng, H.; et al. A review on the bioenergetics of anaerobic microbial metabolism close to the thermodynamic limits and its implications for digestion applications. Bioresour. Technol. 2018, 247, 1095–1106. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, S.; Jing, Z.; Zhang, Y.; Wang, J.; Liu, J.; Wang, Y.; Guo, W.; Li, Y.; Feng, L.; et al. Understanding of the interrelationship between methane production and microorganisms in high-solid anaerobic co-digestion using microbial analysis and machine learning. J. Clean. Prod. 2022, 373, 133848. [Google Scholar] [CrossRef]
- Abu-Zeid, M.A.E.R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. pH and hydraulic retention time regulation for anaerobic fermentation: Focus on volatile fatty acids production/distribution, microbial community succession and interactive correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Wilkins, D.; Chen, J.; Ng, S.-K.; Lu, H.; Jia, Y.; Lee, P.K.H. Metagenomic Reconstruction of Key Anaerobic Digestion Pathways in Municipal Sludge and Industrial Wastewater Biogas-Producing Systems. Front. Microbiol. 2016, 7, 778. [Google Scholar] [CrossRef] [PubMed]
- Srirangan, K.; Bruder, M.; Akawi, L.; Miscevic, D.; Kilpatrick, S.; Moo-Young, M.; Chou, C.P. Recent advances in engineering propionyl-CoA metabolism for microbial production of value-added chemicals and biofuels. Crit. Rev. Biotechnol. 2017, 37, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, B.; Wong, J.W.; Zhang, Y. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: A mini-review focusing on acidogenic metabolic pathways. Bioresour. Technol. 2018, 248, 68–78. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Boone, D.R.; Bryant, M.P. propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl. Environ. Microbiol. 1980, 40, 626–632. [Google Scholar] [CrossRef]
- Liu, Y.; Balkwill, D.L.; Aldrich, H.C.; Drake, G.R.; Boone, D.R. Characterization of the anaerobic propionate-degradating synytophs Smithella propionica gen. nov., sp. nov. and Syntrophobacter wolonii. Int. J. Syst. Bacteriol. 1999, 49, 545–556. [Google Scholar] [CrossRef]
- de Bok, F.A.M.; Stams, A.J.M.; Dijkema, C.; Boone, D.R. Pathway of Propionate Oxidation by a Syntrophic Culture of Smithella propionica and Methanospirillum hungatei. Appl. Environ. Microbiol. 2001, 67, 1800–1804. [Google Scholar] [CrossRef]
- Sun, J.; Rene, E.R.; He, Y.; Ma, W.; Hu, Q.; Qiu, B. Carbon, iron, and polymer-based conductive materials for improving methane production in anaerobic wastewater treatment systems: A review on their direct interspecific electron transfer mechanism. Fuel 2023, 342, 127703. [Google Scholar] [CrossRef]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Usman, M.; et al. Biochar assisted anaerobic digestion for biomethane production: Microbial symbiosis and electron transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Sun, H.; Zhao, P.; Wang, Q.; Wu, C.; Gao, M. Response of semi-continuous anaerobic digestion of food waste to progressively increasing temperature: Methanogen community, correlation analysis, and energy balance. Ind. Crop. Prod. 2023, 192, 116066. [Google Scholar] [CrossRef]
- Jang, H.M.; Choi, Y.-K.; Kan, E. Effects of dairy manure-derived biochar on psychrophilic, mesophilic and thermophilic anaerobic digestions of dairy manure. Bioresour. Technol. 2018, 250, 927–931. [Google Scholar] [CrossRef]
- Ban, Q. Syntrophic Propionate Degradation Response to Temperature Decrease and Microbial Community Shift in an UASB Reactor. J. Microbiol. Biotechnol. 2013, 23, 382–389. [Google Scholar] [CrossRef]
- Jiang, M.; Qiao, W.; Wang, Y.; Zou, T.; Lin, M.; Dong, R. Balancing acidogenesis and methanogenesis metabolism in thermophilic anaerobic digestion of food waste under a high loading rate. Sci. Total. Environ. 2022, 824, 153867. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Guo, R.; Fan, X.; Zhao, X. Accelerated methanogenesis from effluents of hydrogen-producing stage in anaerobic digestion by mixed cultures enriched with acetate and nano-sized magnetite particles. Bioresour. Technol. 2015, 190, 132–139. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, W.; Wang, X.; Takayanagi, K.; Shofie, M.; Li, Y.-Y. Kinetic characterization of thermophilic and mesophilic anaerobic digestion for coffee grounds and waste activated sludge. Waste Manag. 2015, 36, 77–85. [Google Scholar] [CrossRef]
- Zhao, J.; Westerholm, M.; Qiao, W.; Yin, D.; Bi, S.; Jiang, M.; Dong, R. Impact of temperature and substrate concentration on degradation rates of acetate, propionate and hydrogen and their links to microbial community structure. Bioresour. Technol. 2018, 256, 44–52. [Google Scholar] [CrossRef]
- Gensollen, G.; Pourcher, A.-M.; Duedal, A.-L.; Picard, S.; Le Roux, S.; Peu, P. Impact of pH in the first-stage of a two-stage anaerobic digestion on metabolic pathways and methane production. Bioresour. Technol. Rep. 2022, 20, 101256. [Google Scholar] [CrossRef]
- Li, J.; Ban, Q.; Zhang, L.; Jha, A.K. Syntrophic propionate degradation in anaerobic digestion: A review. Int. J. Agric. Biol. 2012, 14, 843–850. [Google Scholar]
- Zhang, L.; Ban, Q.; Li, J.; Jha, A.K. Response of Syntrophic Propionate Degradation to pH Decrease and Microbial Community Shifts in an UASB Reactor. J. Microbiol. Biotechnol. 2016, 26, 1409–1419. [Google Scholar] [CrossRef]
- Du, C.; Yan, H.; Zhang, Y.; Li, Y.; Cao, Z. Use of oxidoreduction potential as an indicator to regulate 1,3-propanediol fermentation by Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2006, 69, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Lizama, A.C.; Figueiras, C.C.; Pedreguera, A.Z.; Espinoza, J.E.R. Enhancing the performance and stability of the anaerobic digestion of sewage sludge by zero valent iron nanoparticles dosage. Bioresour. Technol. 2018, 275, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, W.; Li, R. Real-time recovery strategies for volatile fatty acid-inhibited anaerobic digestion of food waste for methane production. Bioresour. Technol. 2018, 265, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, R.; Shi, X.; Wang, C.; Wang, L.; Dai, M. Magnetite nanoparticles enable a rapid conversion of volatile fatty acids to methane. RSC Adv. 2016, 6, 25662–25668. [Google Scholar] [CrossRef]
- Satoh, H.; Bandara, W.M.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Enhancement of organic matter degradation and methane gas production of anaerobic granular sludge by degasification of dissolved hydrogen gas. Bioresour. Technol. 2017, 244, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Cazier, E.; Trably, E.; Steyer, J.; Escudie, R. Biomass hydrolysis inhibition at high hydrogen partial pressure in solid-state anaerobic digestion. Bioresour. Technol. 2015, 190, 106–113. [Google Scholar] [CrossRef]
- Kim, M.; Ahn, Y.-H.; Speece, R. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Li, A. Semi-continuous anaerobic digestion of extruded OFMSW: Process performance and energetics evaluation. Bioresour. Technol. 2018, 247, 103–115. [Google Scholar] [CrossRef]
- Saidi, R.; Hamdi, M.; Bouallagui, H. Enhanced hydrogen and methane production from date fruit wastes using semi continuous two-stage anaerobic digestion process with increasing organic loading rates. Process Saf. Environ. Prot. 2023, 174, 267–275. [Google Scholar] [CrossRef]
- McCarty, P.L.; Smith, D.P. Anaerobic wastwater treatment. Environ. Sci. Technol. 1986, 20, 1200–1206. [Google Scholar] [CrossRef]
- Zheng, K.; Wang, Y.; Guo, H.; Zhu, T.; Zhao, Y.; Liu, Y. Potassium permanganate pretreatment effectively improves methane production from anaerobic digestion of waste activated sludge: Reaction kinetics and mechanisms. Sci. Total. Environ. 2022, 847, 157402. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef]
- Yu, L.; Wensel, P.C. Mathematical Modeling in Anaerobic Digestion (AD). J. Bioremed. Biodegrad. 2013, S4, 003. [Google Scholar] [CrossRef]
- Lima, D.R.S.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; de Aquino, S.F. Influence of different thermal pretreatments and inoculum selection on the biomethanation of sugarcane bagasse by solid-state anaerobic digestion: A kinetic analysis. Ind. Crop. Prod. 2018, 111, 684–693. [Google Scholar] [CrossRef]
- Zhang, W.; Lang, Q.; Wu, S.; Li, W.; Bah, H.; Dong, R. Anaerobic digestion characteristics of pig manures depending on various growth stages and initial substrate concentrations in a scaled pig farm in Southern China. Bioresour. Technol. 2014, 156, 63–69. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and ternary trace elements to enhance anaerobic digestion of cattle manure: Focusing on kinetic models for biogas production and digestate utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- Bala, R.; Gupta, G.K.; Dasgupta, B.V.; Mondal, M.K. Pretreatment optimisation and kinetics of batch anaerobic digestion of liquidised OFMSW treated with NaOH: Models verification with experimental data. J. Environ. Manag. 2019, 237, 313–321. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Panico, A.; D’Antonio, G. Mathematical modelling of disintegration-limited co-digestion of OFMSW and sewage sludge. Water Sci. Technol. 2008, 58, 1513–1519. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.-H.; Wu, G.; Zhan, X. Impact of total solids content on anaerobic co-digestion of pig manure and food waste: Insights into shifting of the methanogenic pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef]
- Adarme, O.F.H.; Baêta, B.E.L.; Filho, J.B.G.; Gurgel, L.V.A.; de Aquino, S.F. Use of anaerobic co-digestion as an alternative to add value to sugarcane biorefinery wastes. Bioresour. Technol. 2019, 287, 121443. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Tao, J.; Wang, Z.; Chen, C.; Mu, L.; Ruan, H.; Yon, Y.R.; Su, H.; Yan, B.; Chen, G. Modification of anaerobic digestion model No.1 with Machine learning models towards applicable and accurate simulation of biomass anaerobic digestion. Chem. Eng. J. 2023, 454, 140369. [Google Scholar] [CrossRef]
- Gao, S.; Huang, Y.; Yang, L.; Wang, H.; Zhao, M.; Xu, Z.; Huang, Z.; Ruan, W. Evaluation the anaerobic digestion performance of solid residual kitchen waste by NaHCO3 buffering. Energy Convers. Manag. 2015, 93, 166–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Yang, Y.; Li, D.; Wang, Y.; Che, H.; Deng, G. Contribution distinguish between emission reduction and meteorological conditions to “Blue Sky”. Atmos. Environ. 2018, 190, 209–217. [Google Scholar] [CrossRef]
- Lin, Y.; Lü, F.; Shao, L.; He, P. Influence of bicarbonate buffer on the methanogenetic pathway during thermophilic anaerobic digestion. Bioresour. Technol. 2013, 137, 245–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liu, F.; Yan, H.; Li, J. Mediative mechanism of bicarbonate on anaerobic propionate degradation revealed by microbial community and thermodynamics. Environ. Sci. Pollut. Res. 2018, 25, 12434–12443. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Zheng, P.; Wang, Y. Anaerobic digestion of food waste stabilized by lime mud from papermaking process. Bioresour. Technol. 2014, 170, 270–277. [Google Scholar] [CrossRef]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Lu, J.; Jia, Z.; Wang, P.; Yang, X.; Lin, P.; Ren, L.; Farghali, M. Restoration of acidified dry anaerobic digestion of food waste: Bioaugmentation of butyric acid-resistant microbes. J. Environ. Chem. Eng. 2021, 10, 106935. [Google Scholar] [CrossRef]
- Guo, Q.; Ji, J.; Ling, Z.; Zhang, K.; Xu, R.; Leng, X.; Mao, C.; Zhou, T.; Wang, H.; Liu, P.; et al. Bioaugmentation improves the anaerobic co-digestion of cadmium-containing plant residues and cow manure. Environ. Pollut. 2021, 289, 117885. [Google Scholar] [CrossRef]
- Jo, Y.; Rhee, C.; Choi, H.; Shin, J.; Shin, S.G.; Lee, C. Long-term effectiveness of bioaugmentation with rumen culture in continuous anaerobic digestion of food and vegetable wastes under feed composition fluctuations. Bioresour. Technol. 2021, 338, 125500. [Google Scholar] [CrossRef]
- Acharya, S.M.; Kundu, K.; Sreekrishnan, T.R. Improved Stability of Anaerobic Digestion through the Use of Selective Acidogenic Culture. J. Environ. Eng. 2015, 141, 04015001. [Google Scholar] [CrossRef]
- Tzirita, M.; Papanikolaou, S.; Quilty, B. Degradation of Fat by a Bioaugmentation Product Comprising of Bacillus spp. before and after the Addition of a Pseudomonas sp. Eur. J. Lipid Sci. Technol. 2018, 120, 1700264. [Google Scholar] [CrossRef]
- Lee, J.T.; Dutta, N.; Zhang, L.; Tsui, T.T.; Lim, S.; Tio, Z.K.; Lim, E.Y.; Sun, J.; Zhang, J.; Wang, C.-H.; et al. Bioaugmentation of Methanosarcina thermophila grown on biochar particles during semi-continuous thermophilic food waste anaerobic digestion under two different bioaugmentation regimes. Bioresour. Technol. 2022, 360, 127590. [Google Scholar] [CrossRef]
- Van Duc, L.; Nagao, S.; Mojarrad, M.; Miyagawa, Y.; Li, Z.-Y.; Inoue, D.; Tajima, T.; Ike, M. Bioaugmentation with marine sediment-derived microbial consortia in mesophilic anaerobic digestion for enhancing methane production under ammonium or salinity stress. Bioresour. Technol. 2023, 376, 128853. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Inoue, D.; Ike, M. Mitigating ammonia-inhibition in anaerobic digestion by bioaugmentation: A review. J. Water Process Eng. 2023, 52, 103506. [Google Scholar] [CrossRef]
- Yan, M.; Wang, C.; Li, Y.; Tian, H.; Sun, Y. Effect of bioaugmentation on psychrotrophic anaerobic digestion: Bioreactor performance, microbial community, and cellular metabolic response. Chem. Eng. J. 2023, 455, 140173. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef]
- Lee, J.T.; Wang, Q.; Lim, E.Y.; Liu, Z.; He, J.; Tong, Y.W. Optimization of bioaugmentation of the anaerobic digestion of Axonopus compressus cowgrass for the production of biomethane. J. Clean. Prod. 2020, 258, 120932. [Google Scholar] [CrossRef]
- Li, M.-T.; Rao, L.; Wang, L.; Gou, M.; Sun, Z.-Y.; Xia, Z.-Y.; Song, W.-F.; Tang, Y.-Q. Bioaugmentation with syntrophic volatile fatty acids-oxidizing consortia to alleviate the ammonia inhibition in continuously anaerobic digestion of municipal sludge. Chemosphere 2022, 288, 132389. [Google Scholar] [CrossRef]
- Tale, V.; Maki, J.; Struble, C.; Zitomer, D. Methanogen community structure-activity relationship and bioaugmentation of overloaded anaerobic digesters. Water Res. 2011, 45, 5249–5256. [Google Scholar] [CrossRef]
- Tale, V.; Maki, J.; Zitomer, D. Bioaugmentation of overloaded anaerobic digesters restores function and archaeal community. Water Res. 2015, 70, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Carballa, M.; Van De Caveye, P.; Verstraete, W. Enhanced propionic acid degradation (EPAD) system: Proof of principle and feasibility. Water Res. 2009, 43, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Usman, M.; Zheng, Z.; Zhao, X.; Meng, X.; Hu, K.; Shen, X.; Wang, X.; Cai, Y. Two microbial consortia obtained through purposive acclimatization as biological additives to relieve ammonia inhibition in anaerobic digestion. Water Res. 2023, 230, 119583. [Google Scholar] [CrossRef] [PubMed]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Cai, Y.; Meng, X.; Hu, K.; Zhao, X.; Usman, M.; Esposito, G.; Shen, X.; Chen, S. A novel strategy to reduce trace element supplementation in the semi-solid anaerobic digestion with gradient ammonia concentration: The role of biochar. Fuel 2023, 338, 127332. [Google Scholar] [CrossRef]
- Frunzo, L.; Fermoso, F.; Luongo, V.; Mattei, M.; Esposito, G. ADM1-based mechanistic model for the role of trace elements in anaerobic digestion processes. J. Environ. Manag. 2019, 241, 587–602. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Joyce, A.; Ijaz, U.Z.; Nzeteu, C.; Vaughan, A.; Shirran, S.; Botting, C.H.; Quince, C.; O’Flaherty, V.; Abram, F. Linking Microbial Community Structure and Function during the Acidified Anaerobic Digestion of Grass. Front. Microbiol. 2018, 9, 540. [Google Scholar] [CrossRef]
- Schmidt, T.; Nelles, M.; Scholwin, F.; Pröter, J. Trace element supplementation in the biogas production from wheat stillage—Optimization of metal dosing. Bioresour. Technol. 2014, 168, 80–85. [Google Scholar] [CrossRef]
- Osuna, M.B.; Zandvoort, M.H.; Iza, J.M.; Lettinga, G.; Lens, P. Effects of trace element addition on volatile fatty acid conversions in anaerobic granular sludge reactors. Environ. Technol. 2003, 24, 573–587. [Google Scholar] [CrossRef]
- Zitomer, D.H.; Johnson, C.C.; Speece, R.E. Metal Stimulation and Municipal Digester Thermophilic/Mesophilic Activity. J. Environ. Eng. 2008, 134, 42–47. [Google Scholar] [CrossRef]
- Ezebuiro, N.C.; Techamanoon, K.; Körner, I. Synergistic and antagonistic influences of trace elements on volatile fatty acids degradation and methane production during the methanization of a mixture of volatile fatty acids. J. Environ. Chem. Eng. 2018, 6, 1455–1467. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Bioresour. Technol. 2018, 275, 172–182. [Google Scholar] [CrossRef]
- Worm, P.; Fermoso, F.G.; Lens, P.N.; Plugge, C.M. Decreased activity of a propionate degrading community in a UASB reactor fed with synthetic medium without molybdenum, tungsten and selenium. Enzym. Microb. Technol. 2009, 45, 139–145. [Google Scholar] [CrossRef]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Banks, C.; Heaven, S.; Longhurst, P. Investigation of the impact of trace elements on anaerobic volatile fatty acid degradation using a fractional factorial experimental design. Water Res. 2017, 125, 458–465. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Ruiz, D.; Santa-Catalina, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Bernet, N.; Delgenès, J.-P.; et al. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018, 260, 157–168. [Google Scholar] [CrossRef]
- Thabet, O.B.D.; Bouallagui, H.; Cayol, J.-L.; Ollivier, B.; Fardeau, M.-L.; Hamdi, M. Anaerobic degradation of landfill leachate using an upflow anaerobic fixed-bed reactor with microbial sulfate reduction. J. Hazard. Mater. 2009, 167, 1133–1140. [Google Scholar] [CrossRef]
- St-Pierre, B.; Wright, A.-D.G. Implications from distinct sulfate-reducing bacteria populations between cattle manure and digestate in the elucidation of H2S production during anaerobic digestion of animal slurry. Appl. Microbiol. Biotechnol. 2017, 101, 5543–5556. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Takayanagi, K.; Li, Q.; Shofie, M.; Gao, F.; Dong, R.; Li, Y.-Y. Thermodynamically enhancing propionic acid degradation by using sulfate as an external electron acceptor in a thermophilic anaerobic membrane reactor. Water Res. 2016, 106, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Colleran, S.; Pender, S. Mesophilic and thermophilic anaerobic digestion of sulphate-containing wastewaters. Water Sci. Technol. 2002, 45, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Isa, Z.; Grusenmeyer, S.; Verstraete, W. Sulfate reduction relative to methane production in high-rate anaerobic digestion: Microbiological aspects. Appl. Environ. Microbiol. 1986, 51, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Rim, J.M. Competition and Inhibition of Sulfate Reducers and Methane Producers in Anaerobic Treatment. Water Sci. Technol. 1991, 23, 1259–1264. [Google Scholar] [CrossRef]
- Erdirencelebi, D.; Ozturk, I.; Cokgor, E.U. System Performance in UASB Reactors Receiving Increasing Levels of Sulfate. CLEAN-Soil Air Water 2007, 35, 275–281. [Google Scholar] [CrossRef]
- Harada, H.; Uemura, S.; Momonoi, K. Interaction between sulfate-reducing bacteria and methane-producing bacteria in UASB reactors fed with low strength wastes containing different levels of sulfate. Water Res. 1994, 28, 355–367. [Google Scholar] [CrossRef]
- Jiménez, J.; Barrera, E.L.; De Vrieze, J.; Boon, N.; Demeester, S.; Spanjers, H.; Romero, O.R.; Dewulf, J. Microbial community dynamics reflect reactor stability during the anaerobic digestion of a very high strength and sulfate-rich vinasse. J. Chem. Technol. Biotechnol. 2018, 93, 975–984. [Google Scholar] [CrossRef]
- Lu, X.; Zhen, G.; Ni, J.; Kubota, K.; Li, Y.-Y. Sulfidogenesis process to strengthen regranulation for biodegradation of methanolic wastewater and microorganisms evolution in an UASB reactor. Water Res. 2017, 108, 137–150. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Xu, Z.; Quan, X.; Chen, S. Enhancement of sludge granulation in anaerobic acetogenesis by addition of nitrate and microbial community analysis. Biochem. Eng. J. 2015, 95, 104–111. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Quan, X.; Zhang, J.; Chen, S.; Afzal, S. Enhanced anaerobic fermentation with azo dye as electron acceptor: Simultaneous acceleration of organics decomposition and azo decolorization. J. Environ. Sci. 2014, 26, 1970–1976. [Google Scholar] [CrossRef]
- Im, S.; Yun, Y.-M.; Song, Y.-C.; Kim, D.-H. Enhanced anaerobic digestion of glycerol by promoting DIET reaction. Biochem. Eng. J. 2019, 142, 18–26. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Huang, Z.; He, C.; Dong, F.; Su, K.; Yuan, S.; Hu, Z.; Wang, W. Granular activated carbon and exogenous hydrogen enhanced anaerobic digestion of hypersaline phenolic wastewater via syntrophic acetate oxidation and hydrogenotrophic methanogenesis. Bioresour. Technol. 2022, 365, 128155. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J. Direct interspecies electron transfer mechanism in enhanced methanogenesis: A mini-review. Bioresour. Technol. 2021, 330, 124980. [Google Scholar] [CrossRef]
- Guan, Q.; Qu, Y.; Zhai, Y.; Shi, W.; Zhao, M.; Huang, Z.; Ruan, W. Enhancement of methane production in anaerobic digestion of high salinity organic wastewater: The synergistic effect of nano-magnetite and potassium ions. Chemosphere 2023, 318, 137974. [Google Scholar] [CrossRef]
- Zhong, Y.; He, J.; Wu, F.; Zhang, P.; Zou, X.; Pan, X.; Zhang, J. Metagenomic analysis reveals the size effect of magnetite on anaerobic digestion of waste activated sludge after thermal hydrolysis pretreatment. Sci. Total. Environ. 2022, 851, 158133. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: Performance and associated mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Morita, M.; Malvankar, N.S.; Franks, A.E.; Summers, Z.M.; Giloteaux, L.; Rotaru, A.E.; Rotaru, C.; Lovley, D.R. Potential for direct interspecies eletron transfer in methanogenic wastwater digester aggregates. mBio 2011, 2, e00159-11. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Liu, F.; Philips, J.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014, 173, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Jiao, P.; Zhang, X.; Qiu, S.; Zhou, X.; Tian, Z.; Liang, Y.; Zhang, Y.; Ma, L. Pyrite-enhanced Sludge Digestion via Stimulation of Direct Interspecies Electron Transfer between Syntrophic Propionate- and Sulfur-oxidizing Bacteria and Methanogens: Genome-centric Metagenomics Evidence. Chem. Eng. J. 2023, 456, 141089. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, G.; Kuang, B.; Jia, J.; Liu, C.; Cai, G.; Li, C. Novel insights into the anaerobic digestion of propionate via Syntrophobacter fumaroxidans and Geobacter sulfurreducens: Process and mechanism. Water Res. 2021, 200, 117270. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Z.; Gu, M.; Yin, Q.; Wu, G. Coupled effects of ferroferric oxide supplement and ethanol co-metabolism on the methanogenic oxidation of propionate. Sci. Total. Environ. 2020, 723, 137992. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Yu, Q.; Zhang, Y. Enhancing methanogenesis from anaerobic digestion of propionate with addition of Fe oxides supported on conductive carbon cloth. Bioresour. Technol. 2020, 302, 122796. [Google Scholar] [CrossRef]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Yu, Q.; Dang, Y.; Li, Y.; Quan, X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016, 102, 475–484. [Google Scholar] [CrossRef]
- Viggi, C.C.; Rossetti, S.; Fazi, S.; Paiano, P.; Majone, M.; Aulenta, F. Magnetite Particles Triggering a Faster and More Robust Syntrophic Pathway of Methanogenic Propionate Degradation. Environ. Sci. Technol. 2014, 48, 7536–7543. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ji, D.; Li, X.; Zhang, J.; Zang, L. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion. Bioresour. Technol. 2019, 287, 121373. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Dhar, B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018, 266, 259–266. [Google Scholar] [CrossRef]
- Yamada, C.; Kato, S.; Ueno, Y.; Ishii, M.; Igarashi, Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015, 119, 678–682. [Google Scholar] [CrossRef]
- Walker, D.J.F.; Nevin, K.P.; Holmes, D.E.; Rotaru, A.-E.; Ward, J.E.; Woodard, T.L.; Zhu, J.; Ueki, T.; Nonnenmann, S.S.; McInerney, M.J.; et al. Syntrophus conductive pili demonstrate that common hydrogen-donating syntrophs can have a direct electron transfer option. ISME J. 2020, 14, 837–846. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Nevin, K.P.; Woodard, T.L.; Mu, B.-Z.; Lovley, D.R. Expanding the Diet for DIET: Electron Donors Supporting Direct Interspecies Electron Transfer (DIET) in Defined Co-Cultures. Front. Microbiol. 2016, 7, 236. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2018, 275, 200–206. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.; Stuckey, D.C. The effect of Fe2NiO4 and Fe4NiO4Zn magnetic nanoparticles on anaerobic digestion activity. Sci. Total. Environ. 2018, 642, 276–284. [Google Scholar] [CrossRef]
- Nguyen, D.; Visvanathan, C.; Jacob, P.; Jegatheesan, V. Effects of nano cerium (IV) oxide and zinc oxide particles on biogas production. Int. Biodeterior. Biodegrad. 2015, 102, 165–171. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Yu, C.; Zhou, J. Effects of nano-sized MnO2 on methanogenic propionate and butyrate degradation in anaerobic digestion. J. Hazard. Mater. 2019, 364, 11–18. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Yin, J.; Shen, D. Effect of nano-magnetite on the propionic acid degradation in anaerobic digestion system with acclimated sludge. Bioresour. Technol. 2021, 334, 125143. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Fang, Q.; Chen, Y.; Ding, W.; Xiao, Y.; Wang, Z.; Zhou, W. Effect of Fe3O4 on propionic acid production by anaerobic fermentation of waste cooking oil and aerobic sludge. J. Water Process Eng. 2022, 49, 102910. [Google Scholar] [CrossRef]
- Bandara, W.M.; Kindaichi, T.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Anaerobic treatment of municipal wastewater at ambient temperature: Analysis of archaeal community structure and recovery of dissolved methane. Water Res. 2012, 46, 5756–5764. [Google Scholar] [CrossRef] [PubMed]
- Fotidis, I.A.; Wang, H.; Fiedel, N.R.; Luo, G.; Karakashev, D.B.; Angelidaki, I. Bioaugmentation as a Solution to Increase Methane Production from an Ammonia-Rich Substrate. Environ. Sci. Technol. 2014, 48, 7669–7676. [Google Scholar] [CrossRef]
- Visnyei, M.; Bakonyi, P.; Bélafi-Bakó, K.; Nemestóthy, N. Integration of gas-liquid membrane contactors into anaerobic digestion as a promising route to reduce uncontrolled greenhouse gas (CH4/CO2) emissions. Bioresour. Technol. 2022, 364, 128072. [Google Scholar] [CrossRef]



| Reaction | ΔG (kJ/mol, 25 °C) |
|---|---|
| (i) CH3COO− + H2O → HCO3− + CH4 | −31.0 |
| (ii) CH3CH2COO− + 3 H2O → CH3COO− + HCO3− + H+ + 3 H2 | +76.1 |
| (iii) CH3CH2CH2COO− + 2H2O → 2CH3COO− + H+ + 2H2 | +48.4 |
| (iv) CH3CH2CH2CH2COO− + 2H2O → CH3COO− + CH3CH2COO− + H+ + 2H2 | +25.1 |
| (M) 4 H2 + HCO3− + H+ → CH4 + 3 H2O | −135.6 |
| (ii + M) 4 CH3CH2COO− + 3H2O → 4 CH3COO− + HCO3− + H+ + 3 CH4 | −102.4 |
| Possible Electrophilic Micro-Organism or Electron Acceptor | Possible Electron Donator | Conduit | Employed Concentration | References |
|---|---|---|---|---|
| Methanothrix | Syntrophobacteraceae Thiobacillaceae | Pyrite | 5–40 g/L | [144] |
| Syntrophobacter fumaroxidans | Geobacter sulfurreducens | electric wire | / | [145] |
| Geobacter, Syntrophobacter, Smithella, and Methanosaeta | Geobacter | coupled effects of ethanol and Fe3O4 | 500 mg COD/L ethanol + 10 g/L Fe3O4 | [146] |
| Methanothrix | Levilinea | Fe2O3-loaded carbon cloth | / | [147] |
| Methanospirillum, Methanosphaerula | Thauera sp. | Magnetite | 10–1000 mg/L | [148] |
| Methanosaeta sp. | Geobacter sp. | biochar | 5 g/L | [149] |
| Methanosaeta sp., Methanosarcina sp. | Geobacter sp. | Electronically conductive pili | [149] | |
| CO2-reducing methanogens | Propionate-oxidazing acetogens | Magnetite | [150] | |
| Methanobacterium | Thauera sp. | Magnetite | 20 mM | [64] |
| Methanosaeta and Methanosarcina sp. | Geobacter | Graphite felt | [151] | |
| Methanobacterium sp. | Anaerolineae and Clostridia | Granular activated carbon | 0∼5.0 g | [152] |
| Methanosaeta sp. | Propionate and Butyrate | carbon fibers | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; Wang, Y.; Xu, F.; Li, J.; Tao, J.; Sun, Y.; Song, Y.; Duan, Z.; Li, S.; Chen, G. Emerging Strategies for Enhancing Propionate Conversion in Anaerobic Digestion: A Review. Molecules 2023, 28, 3883. https://doi.org/10.3390/molecules28093883
Mu L, Wang Y, Xu F, Li J, Tao J, Sun Y, Song Y, Duan Z, Li S, Chen G. Emerging Strategies for Enhancing Propionate Conversion in Anaerobic Digestion: A Review. Molecules. 2023; 28(9):3883. https://doi.org/10.3390/molecules28093883
Chicago/Turabian StyleMu, Lan, Yifan Wang, Fenglian Xu, Jinhe Li, Junyu Tao, Yunan Sun, Yingjin Song, Zhaodan Duan, Siyi Li, and Guanyi Chen. 2023. "Emerging Strategies for Enhancing Propionate Conversion in Anaerobic Digestion: A Review" Molecules 28, no. 9: 3883. https://doi.org/10.3390/molecules28093883
APA StyleMu, L., Wang, Y., Xu, F., Li, J., Tao, J., Sun, Y., Song, Y., Duan, Z., Li, S., & Chen, G. (2023). Emerging Strategies for Enhancing Propionate Conversion in Anaerobic Digestion: A Review. Molecules, 28(9), 3883. https://doi.org/10.3390/molecules28093883




