Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities
Abstract
1. Introduction
2. Results
2.1. Ultrasound–Microwave Combined Extraction of Lycium barbarum Leaf Polysaccharides (LLP)
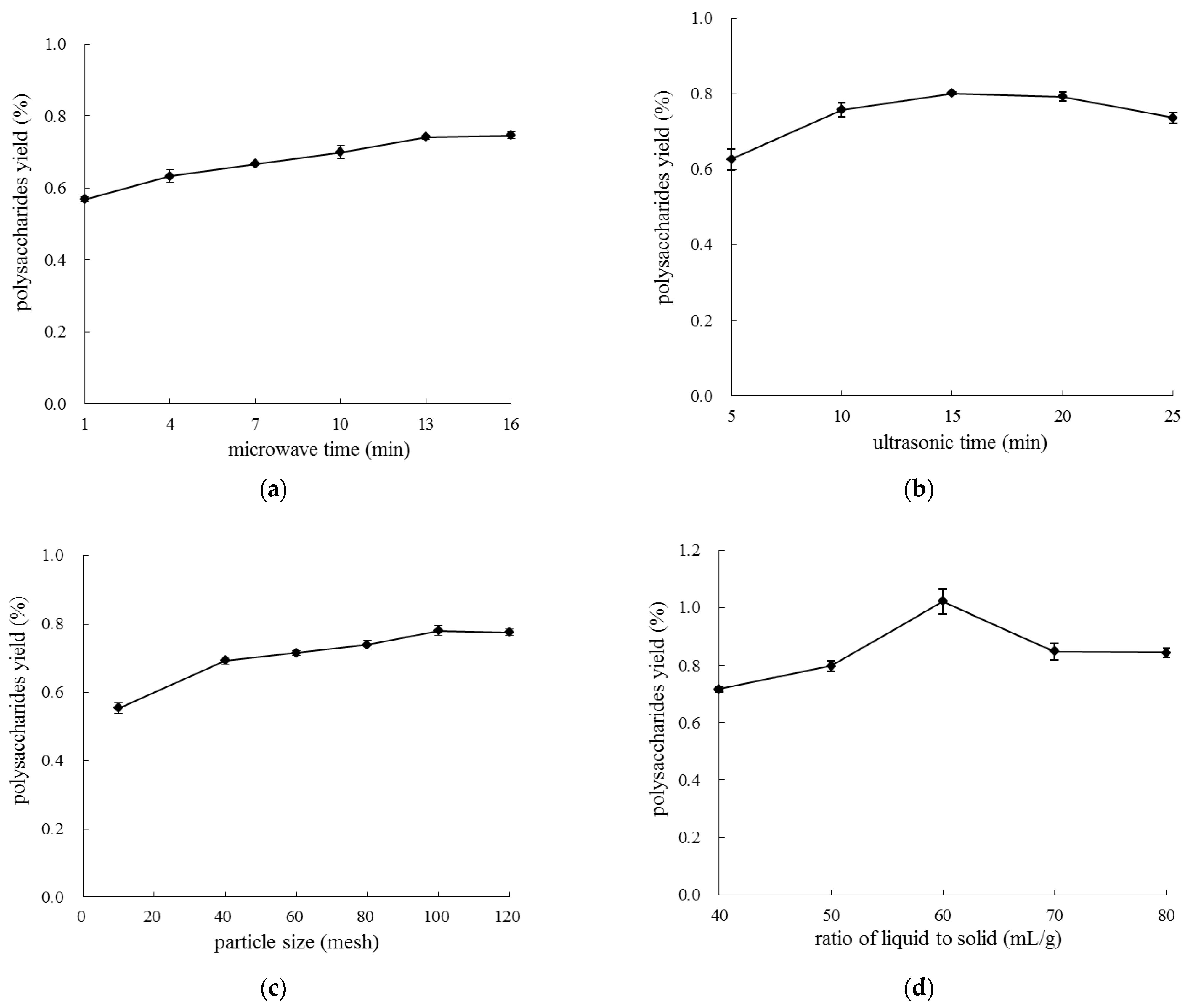
2.1.1. Effects of Extraction Variables on Extraction Yield of LLP
2.1.2. Optimization of LLP Extraction by Response Surface Method (RSM)
Model Fitting and Statistical Analysis
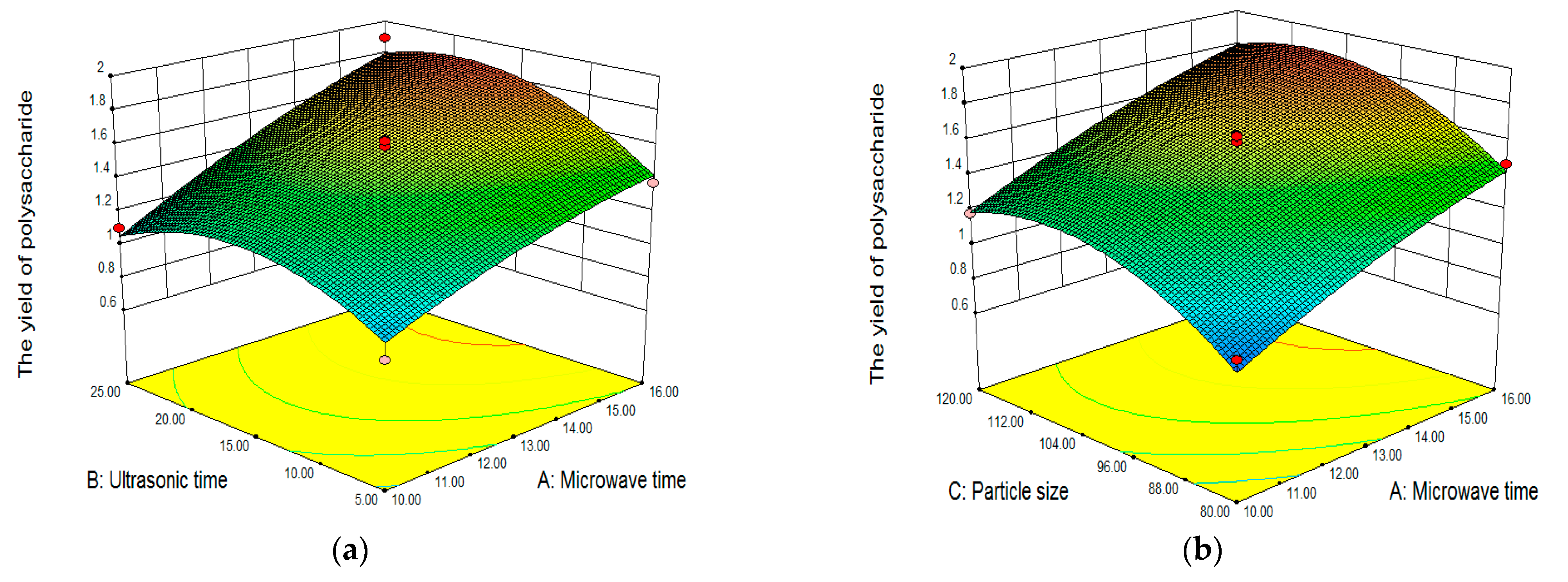
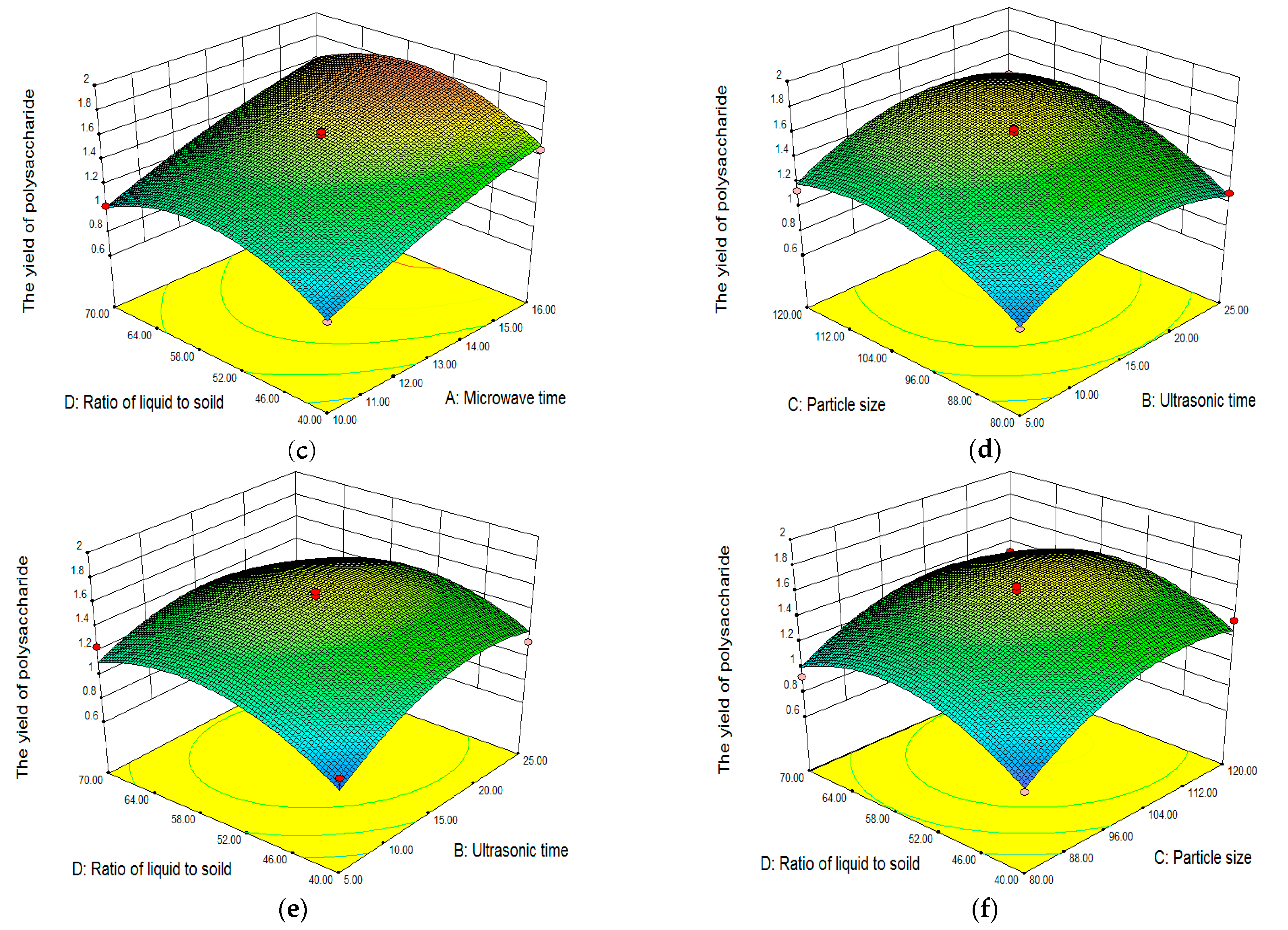
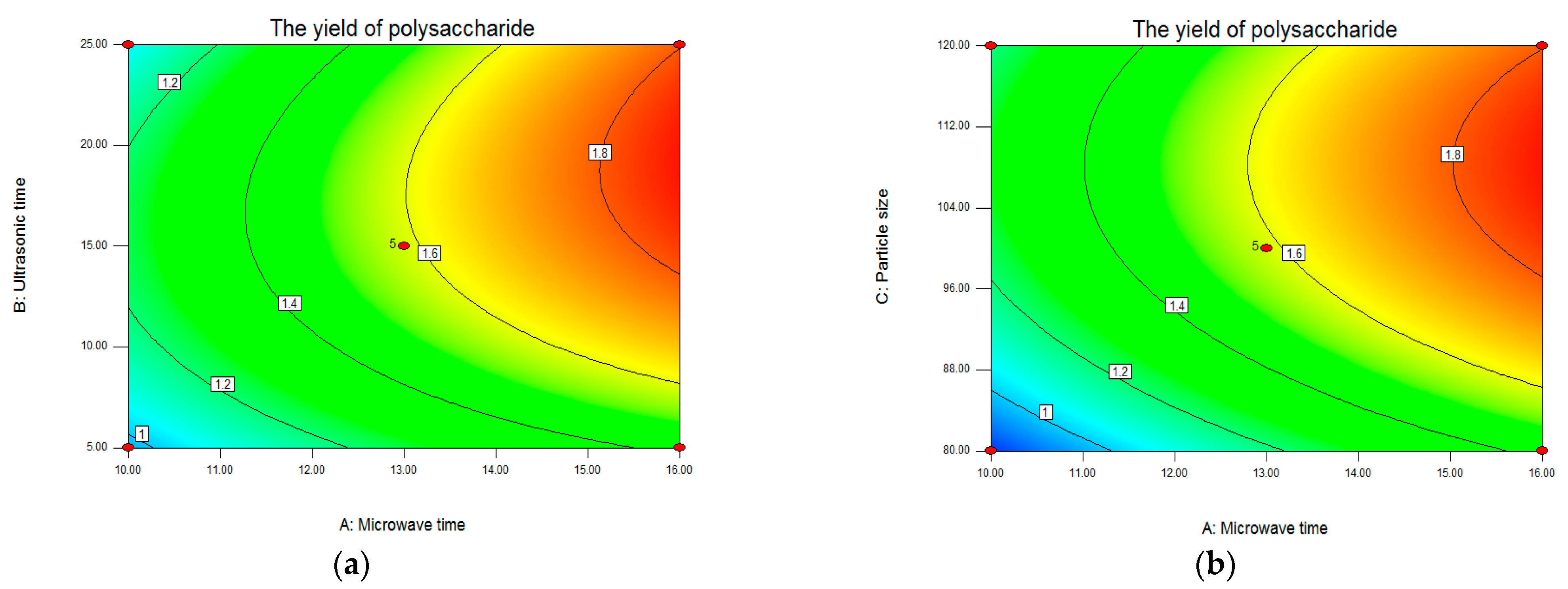
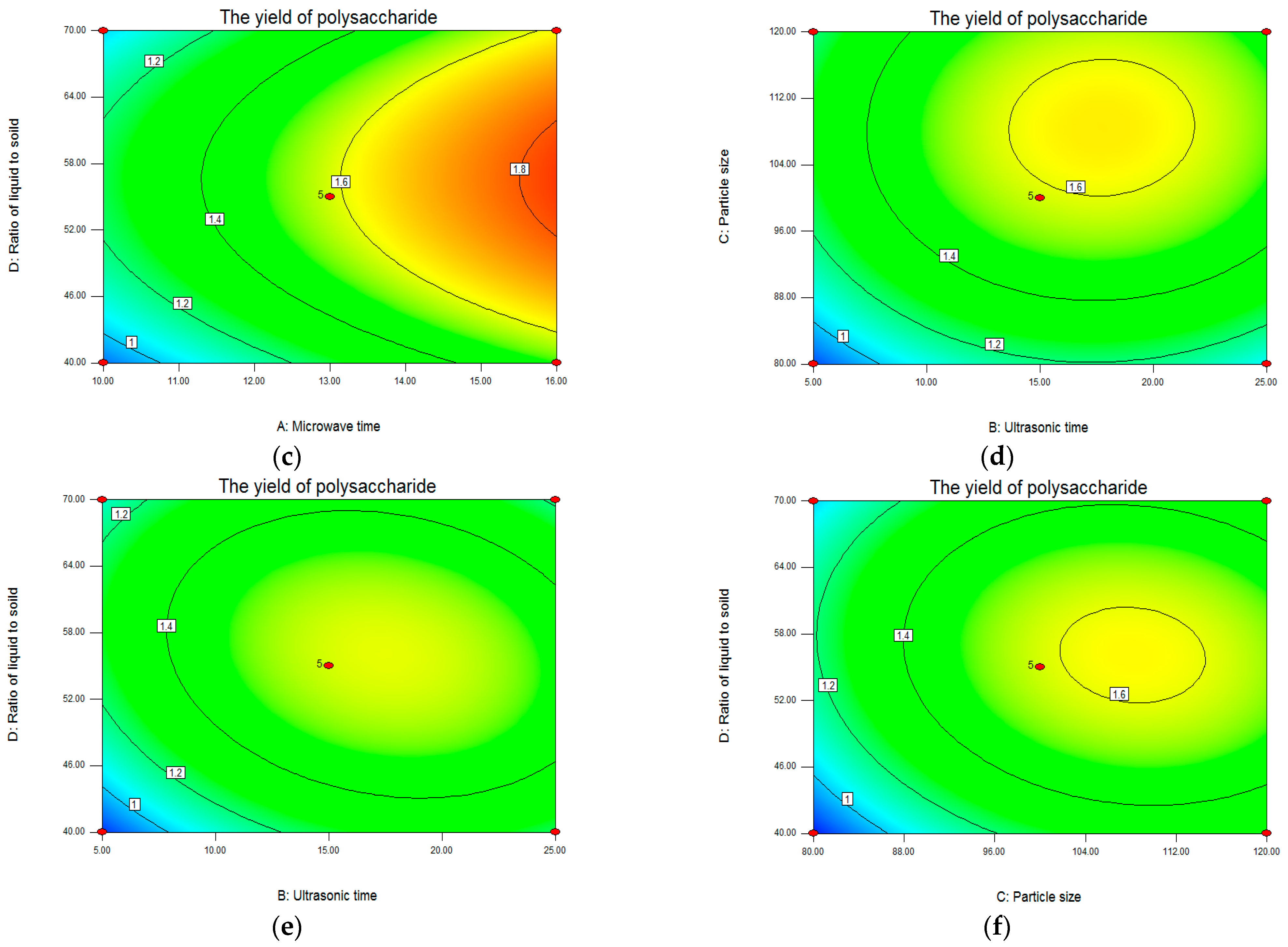
Analysis of Response Surface Plots and Contour Plots
2.1.3. Verification of Ultrasound–Microwave Combined Extraction
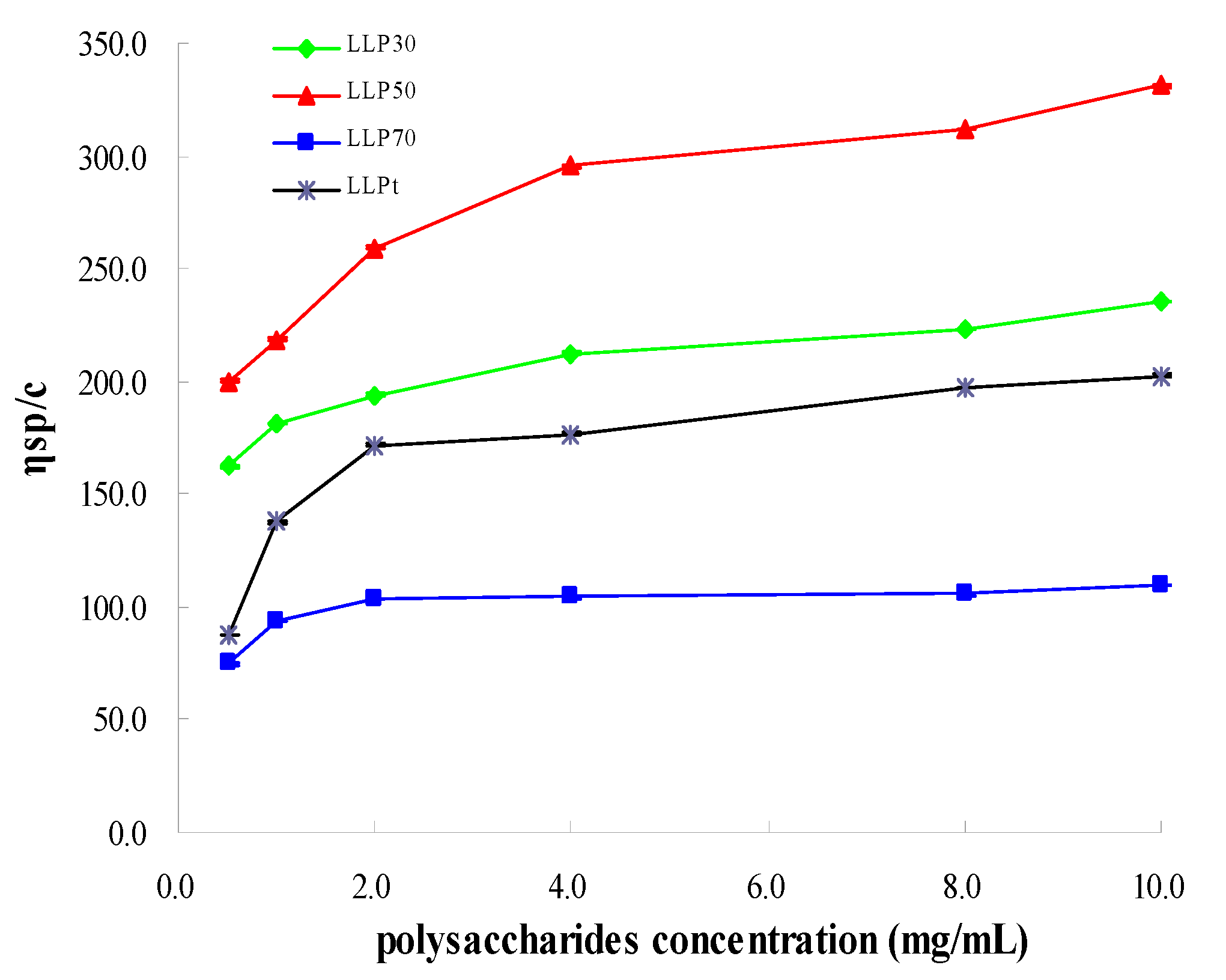
2.2. Chemical Characterization of Polysaccharides and Their Fractions
2.3. In Vitro Hypoglycemic and Antioxidant Activities of LLP
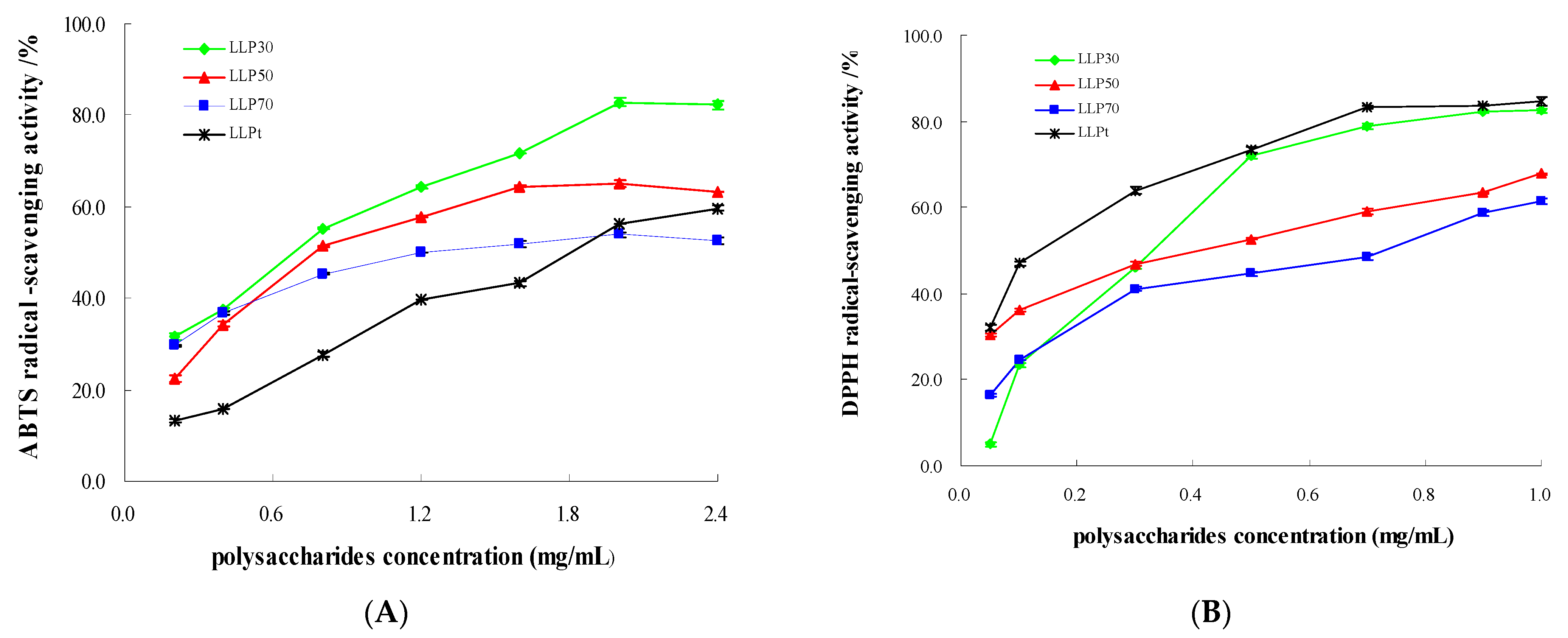
2.3.1. Antioxidant Activity
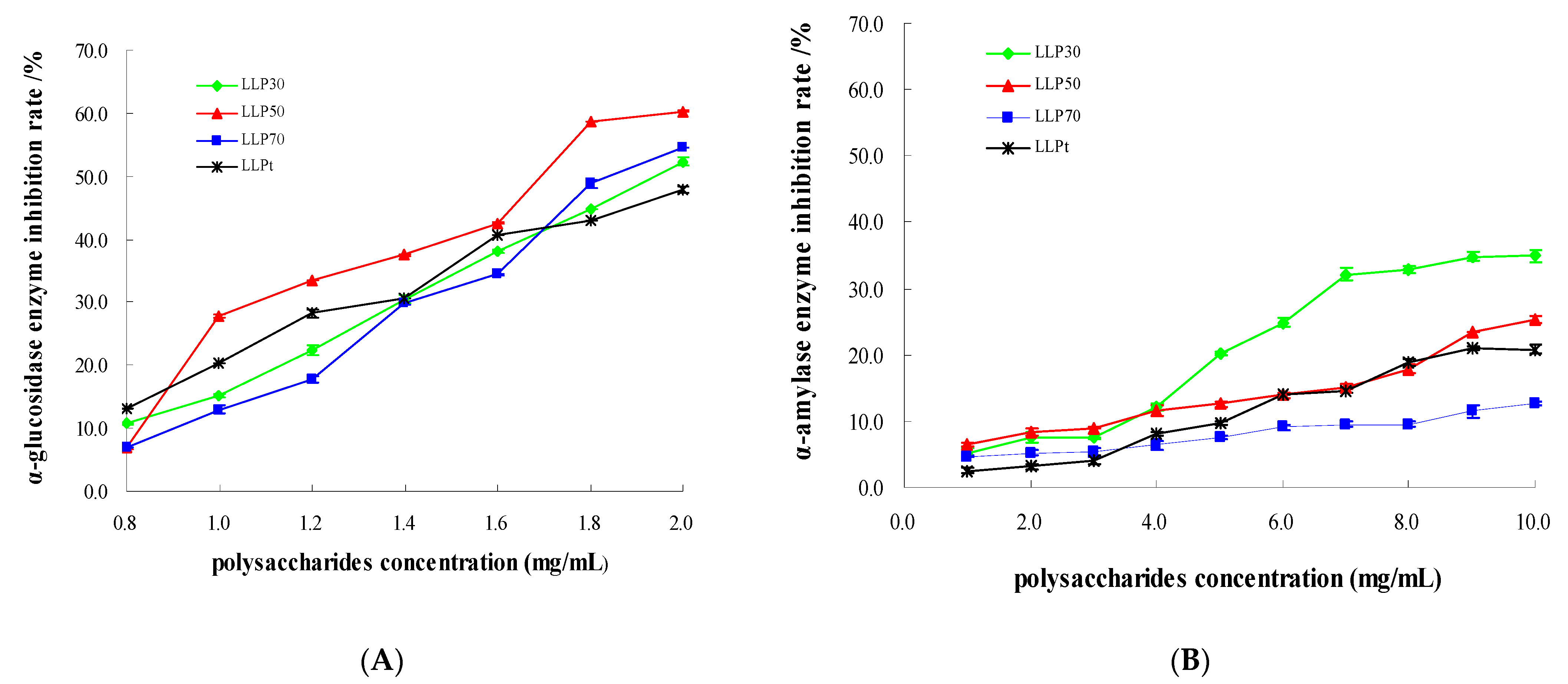
2.3.2. Inhibitory Effect on α-Glucosidase
2.3.3. Inhibitory Effect on α-Amylase
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Reagents
4.2. Polysaccharides Extraction
4.2.1. UMCE Method
4.2.2. Conventional Extraction Methods
4.2.3. Determination of Extraction Yield of Polysaccharides
4.3. Purification and Fractionation of Polysaccharides
4.4. Qualitative Analysis of Polysaccharides
4.4.1. Molish Assay
4.4.2. Fehling’s Reaction
4.4.3. Iodine–Potassium Iodide Method
4.5. Measurement of Viscosity-Average Molecular Weights
4.6. Antioxidant Activities of LLP In Vitro
4.6.1. Scavenging Ability of LLP on ABTS Radicals
4.6.2. Scavenging Ability of LLP on DPPH Radicals
4.7. Hypoglycemic Activities of LLP In Vitro
4.7.1. Inhibitory Effects of LLP on α-Glucosidase
4.7.2. Inhibitory Effects of LLP on α-Amylase
4.8. Experimental Design and Statistical Analysis
4.8.1. BBD
4.8.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, W.X.; Yang, X.H.; Zhang, H.F.; Feng, J.; Zhang, M.Y. Chemical structure, hypoglycemic activity and mechanism of action of selenium-polysaccharides. Biol. Trace Elem. Res. 2022, 200, 4404–4418. [Google Scholar] [CrossRef]
- Xu, X.; Shan, B.; Liao, C.H.; Xie, J.H.; Wen, P.W.; Shid, J.Y. Anti-diabetic properties of Momordica charantia L. polysaccharide inalloxan-induced diabetic mice. Int. J. Biol. Macromol. 2015, 81, 538–543. [Google Scholar] [CrossRef]
- Zaghloul, N.; Awaisu, A.; Mahfouz, A.; Alyafei, S.; Elewa, H. A 5-year trend in the use of sodium-glucose co-transporter 2 inhibitors and other oral antidiabetic drugs in a Middle Eastern country. Int. J. Clin. Pharm. 2022, 44, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.L.; Hui, J.M.H.; Lee, Y.H.A.; Satti, D.I.; Shum, Y.K.L.; Kiu, P.T.H.; Wai, A.K.C.; Liu, T.; Wong, W.T.; Chan, J.S.K.; et al. Sulfonylurea is associated with higher risks of ventricular arrhythmia or sudden cardiac death compared with metformin: A population-based Cohort study. J. Am. Heart Assoc. 2022, 11, e026289. [Google Scholar] [CrossRef]
- Zhang, L.; Hogan, S.; Li, J.R.; Sun, S.; Canning, C.; Zheng, S.J.; Zhou, K.Q. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozonic-treated mice. Food Chem. 2011, 126, 466–471. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, X.H. Bioactive substances in Lycium leaves and their application to food industry. Sci. Technol. Food Ind. 2010, 31, 369–373. [Google Scholar]
- Tang, H.L.; Chen, C.; Wang, S.K.; Sun, G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Duan, G.; Fan, G.; Peng, N. Effect of Lycium barbarum polysaccharides on cell signal transduction pathways. Biomed. Pharmacother. 2022, 147, 112620. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Lu, D.Y.; Wang, Y. Analysis of flavonoids from leaves of cultivated Lycium barbarum L. Plant Foods Hum. Nutr. 2009, 64, 199–204. [Google Scholar] [CrossRef]
- Quan, N.; Wang, Q.; Li, X.Y.; Yang, X.H.; Zhu, C.Y.; Zhang, H.F. Antioxidant capacity and inhibitory activity against DNA damage of Lycium barbarum leaves and fruits. Nat. Prod. Res. Dev. 2018, 30, 134–140. [Google Scholar]
- Jiang, L.; Mei, L.J.; Liu, Z.G.; Li, J.Q.; Wang, Q.L.; Shao, Y.; Tao, Y.D. Extraction and hypoglycemic effect of crude polysaccharides from Chinese wolfberry (Lycium chinense) leaves. Food Sci. 2013, 34, 42–46. [Google Scholar]
- Zhang, H.F.; Yang, X.H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Khedmat, L.; Izadi, A.; Mofid, V.; Mojtahedi, S.Y. Recent advances in extracting pectin by single and combined ultrasound techniques: A review of techno-functional and bioactive health-promoting aspects. Carbohydr. Polym. 2020, 229, 115474. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, B.; Lv, W.; Wu, Y. Effects of microwave power and hot air temperature on the physicochemical properties of dried ginger (Zingiber officinale) using microwave hot-air rolling drying. Food Chem. 2023, 404, 134741. [Google Scholar] [CrossRef]
- Zeng, H.L.; Zhang, Y.; Lin, S.; Jian, Y.Y.; Miao, S.; Zheng, B.D. Ultrasonic-microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Michalaki, A.; Karantonis, H.C.; Kritikou, A.S.; Thomaidis, N.S.; Dasenaki, M.E. Ultrasound-assisted extraction of total phenolic compounds and antioxidant activity evaluation from Oregano (Origanum vulgare ssp. hirtum) using response surface methodology and identification of specific phenolic compounds with HPLC-PDA and Q-TOF-MS/MS. Molecules 2023, 28, 2033. [Google Scholar] [PubMed]
- Prabhu, A.A.; Mandal, B.; Dasu, V.V. Medium optimization for high yield production of extracellular human interferon-γ from Pichia pastoris: A statistical optimizationand neural network-based approach. Korean J. Chem. Eng. 2017, 34, 1109–1121. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.Q. Box-Behnken response surface design for the optimization of electrochemical detection of cadmium by square wave anodic stripping voltammetry on bismuth film/glassy carbon electrode. Sens. Actuators B Chem. 2016, 235, 67–73. [Google Scholar] [CrossRef]
- Liu, Y.; Qiang, M.L.; Sun, Z.G.; Du, Y.Q. Optimization of ultrasonic extraction of polysaccharides from Hovenia dulcis peduncles and their antioxidant potential. Int. J. Biol. Macromol. 2015, 80, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shu, L.; Liang, T.; Li, Y.; Liu, Y.; Zhong, X.; Xing, L.; Zeng, W.; Zhao, R.; Wang, X. Extraction optimization, physicochemical property, antioxidant activity, and α-glucosidase inhibitory effect of polysaccharides from lotus seedpods. J. Sci. Food Agric. 2022, 102, 4065–4078. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Qi, S.; Fan, M.; Zheng, S.; Huang, Q.; Lu, X. Ultrasonic-microwave-assisted extraction for enhancing antioxidant activity of Dictyophora indusiata polysaccharides: The difference mechanisms between single and combined assisted extraction. Ultrason. Sonochem. 2023, 95, 106356. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, H.F.; Cao, X.Y.; Yang, X.H.; Wang, F.Z.; Guo, Q.; Sun, C.Q. Enzymatic water extraction of polysaccharides from Epimedium brevicornu and their antioxidant activity and protective effect against DNA damage. J. Food Biochem. 2017, 41, e12298. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Sintsova, O.; Gladkikh, I.; Kalinovskii, A.; Zelepuga, E.; Monastyrnaya, M.; Kim, N.; Shevchenko, L.; Peigneur, S.; Tytgat, J.; Kozlovskaya, E.; et al. Magnificamide, a β-defensin-like peptide from the mucus of the sea anemone Heteractis magnifica, is a strong inhibitor of mammalian α-amylases. Mar. Drugs 2019, 17, 542. [Google Scholar] [CrossRef]
- Zhang, H.F.; Niu, L.L.; Yang, X.H.; Li, L. Analysis of water-soluble polysaccharides in an edible medicinal plant Epimedium: Method development, validation, and application. J. AOAC Int. 2014, 97, 784–790. [Google Scholar] [CrossRef]
- Wei, C.; He, P.; He, L.; Ye, X.; Cheng, J.; Wang, Y.; Li, W.; Liu, Y. Structure characterization and biological activities of a pectic polysaccharide from cupule of Castanea henryi. Int. J. Biol. Macromol. 2018, 109, 65–75. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Yang, X.H.; Duan, M.Y.; Zhang, H.F. Structural characterization of novel fraction EP80 of Epimedium sagittatum polysaccharides and its effects on enzymes related to alcohol metabolism. Nat. Prod. Res. Dev. 2022, 34, 1539–1547. [Google Scholar]
- Ma, C.; Bai, J.; Shao, C.; Liu, J.; Zhang, Y.; Li, X.; Yang, Y.; Xu, Y.; Wang, L. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Res. Int. 2021, 143, 110281. [Google Scholar] [CrossRef]
- Zhang, H.F.; Zhang, X.; Yang, X.F.; Qiu, N.X.; Wang, Y.; Wang, Z.Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crops Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- An, Y.J.; Zhou, X.X.; Wang, Y.; Yang, M.; Zhang, H.F. Microwave-ultrasound assisted extraction of epimedin F from Epimedii Folium and its effects on α-glucosidase and α-amylase. J. Shaanxi Norm. Univ. 2020, 48, 26–33. [Google Scholar]
| Variables | Symbol Codes | Levels | ||
|---|---|---|---|---|
| 1 | 0 | −1 | ||
| Microwave time (min) | A | 10 | 13 | 16 |
| Ultrasonic time (min) | B | 5 | 15 | 25 |
| Particle size (mesh) | C | 80 | 100 | 120 |
| Ratio of liquid to solid (mL/g) | D | 40 | 55 | 70 |
| Runs | Microwave Time A (min) | Ultrasonic Time B (min) | Particle Size C (mesh) | Ratio of Liquid to Solid D (mL/g) | Polysaccharide Yield Y (%) |
|---|---|---|---|---|---|
| 1 | −1 (10) | 0 (15) | 1 (120) | 0 (55) | 1.184 ± 0.171 |
| 2 | 0 (13) | 0 (15) | 0 (100) | 0 (55) | 1.589 ± 0.090 |
| 3 | −1 (10) | 0 (15) | −1 (80) | 0 (55) | 0.902 ± 0.132 |
| 4 | −1 (10) | 0 (15) | 0 (100) | 1 (70) | 1.022 ± 0.240 |
| 5 | 0 (13) | 0 (15) | 0 (100) | 0 (55) | 1.557 ± 0.046 |
| 6 | 0 (13) | −1 (5) | 0 (100) | −1 (40) | 0.930 ± 0.223 |
| 7 | 1 (16) | 0 (15) | 1 (120) | 0 (55) | 1.763 ± 0.139 |
| 8 | 0 (13) | −1 (5) | 0 (100) | 1 (70) | 1.242 ± 0.070 |
| 9 | −1 (10) | −1 (5) | 0 (100) | 0 (55) | 0.869 ± 0.051 |
| 10 | 0 (13) | 1 (25) | 1 (120) | 0 (55) | 1.439 ± 0.126 |
| 11 | 1 (16) | 0 (15) | 0 (100) | −1 (40) | 1.456 ± 0.274 |
| 12 | 0 (13) | 0 (15) | −1 (80) | 1 (70) | 0.931 ± 0.089 |
| 13 | 0 (13) | 1 (25) | −1 (80) | 0 (55) | 1.082 ± 0.210 |
| 14 | 0 (13) | 0 (15) | 0 (100) | 0 (55) | 1.521 ± 0.032 |
| 15 | −1 (10) | 0 (15) | 0 (100) | −1 (40) | 0.889 ± 0.110 |
| 16 | 0 (13) | 1 (25) | 0 (100) | −1 (40) | 1.131 ± 0.135 |
| 17 | 0 (13) | 0 (15) | 0 (100) | 0 (55) | 1.627 ± 0.239 |
| 18 | 0 (13) | 0 (15) | −1 (80) | −1 (40) | 0.775 ± 0.101 |
| 19 | 0 (13) | 0 (15) | 1 (120) | 1 (70) | 1.317 ± 0.183 |
| 20 | 1 (16) | −1 (5) | 0 (100) | 0 (55) | 1.372 ± 0.063 |
| 21 | −1 (10) | 1 (25) | 0 (100) | 0 (55) | 1.103 ± 0.211 |
| 22 | 0 (13) | 1 (25) | 0 (100) | 1 (70) | 1.114 ± 0.088 |
| 23 | 0 (13) | −1 (5) | −1 (80) | 0 (55) | 0.839 ± 0.197 |
| 24 | 0 (13) | 0 (15) | 1 (120) | −1 (40) | 1.338 ± 0.142 |
| 25 | 1 (16) | 0 (15) | 0 (100) | 1 (70) | 1.596 ± 0.111 |
| 26 | 0 (13) | 0 (15) | 0 (100) | 0 (55) | 1.621 ± 0.229 |
| 27 | 1 (16) | 0 (15) | −1 (80) | 0 (55) | 1.468 ± 0.243 |
| 28 | 1 (16) | 1 (25) | 0 (100) | 0 (55) | 1.898 ± 0.215 |
| 29 | 0 (13) | −1 (5) | 1 (120) | 0 (55) | 1.142 ± 0.153 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2.555 | 14 | 0.183 | 25.344 | <0.0001 | ** |
| A | 1.070 | 1 | 1.070 | 148.624 | <0.0001 | ** |
| B | 0.157 | 1 | 0.157 | 21.812 | 0.0004 | ** |
| C | 0.398 | 1 | 0.398 | 55.291 | <0.0001 | ** |
| D | 0.041 | 1 | 0.041 | 5.718 | 0.0314 | * |
| AB | 0.021 | 1 | 0.021 | 2.960 | 0.1074 | |
| AC | 0.000 | 1 | 0.000 | 0.006 | 0.9400 | |
| AD | 0.000 | 1 | 0.000 | 0.002 | 0.9677 | |
| BC | 0.001 | 1 | 0.001 | 0.101 | 0.7551 | |
| BD | 0.027 | 1 | 0.027 | 3.757 | 0.0730 | |
| CD | 0.008 | 1 | 0.008 | 1.088 | 0.3147 | |
| A2 | 0.017 | 1 | 0.017 | 2.373 | 0.1457 | |
| B2 | 0.318 | 1 | 0.318 | 44.170 | <0.0001 | ** |
| C2 | 0.311 | 1 | 0.311 | 43.228 | <0.0001 | ** |
| D2 | 0.487 | 1 | 0.487 | 67.594 | <0.0001 | ** |
| Residual | 0.101 | 14 | 0.007 | |||
| Lack of fit | 0.093 | 10 | 0.009 | 4.682 | 0.0751 | |
| Pure Error | 0.008 | 4 | 0.002 | |||
| Total | 2.656 | 28 |
| Samples | ABTS | DPPH | ||||
|---|---|---|---|---|---|---|
| Fitting Equations | R2 | EC50 (mg/mL) | Fitting Equations | R2 | EC50 (mg/mL) | |
| VC | Y = −949942X2 + 18852X + 7.6294 | 0.9916 | 0.003 | Y = 27.688 ln(X) + 231.41 | 0.9625 | 0.002 |
| LLPt | Y = 22.243X + 9.2361 | 0.9809 | 1.833 | Y = 17.423 ln(X) + 85.569 | 0.9877 | 0.129 |
| LLP30 | Y = −9.399X2 + 48.731X + 20.263 | 0.9870 | 0.706 | Y = 16.775 ln(X) + 80.264 | 0.9784 | 0.165 |
| LLP50 | Y = −13.29X2 + 50.981X + 16.908 | 0.9899 | 0.828 | Y = −21.895X2 + 59.008X + 29.253 | 0.9889 | 0.416 |
| LLP70 | Y = −6.6817X2 + 26.332X + 27.829 | 0.9920 | 1.219 | Y = 14.259 ln(X) + 57.793 | 0.9697 | 0.579 |
| Samples | α-Glucosidase | α-Amylase | ||||
|---|---|---|---|---|---|---|
| Fitting Equations | R2 | IC50 (mg/mL) | Fitting Equations | R2 | IC50 (mg/mL) | |
| Acarbose | Y = 14.131 ln(X) + 70.565 | 0.9534 | 0.0002 | Y = 3.046X2 − 7.8893X + 8.9804 | 0.9960 | 5.187 |
| LLPt | Y = −7.225X2 + 49.279X − 21.649 | 0.9871 | 2.101 | Y = 2.3427X − 1.2074 | 0.9744 | 21.858 |
| LLP30 | Y = 35.678X − 19.384 | 0.9962 | 1.945 | Y = −0.1034X2 + 5.076X − 2.7315 | 0.9469 | 14.928 |
| LLP50 | Y = 54.854 ln(X) + 22.13 | 0.9507 | 1.659 | Y = 0.1567X2 + 0.2861X + 6.7586 | 0.9800 | 15.721 |
| LLP70 | Y = 41.227X − 28.403 | 0.9813 | 1.902 | Y = 0.0343X2 + 0.5153X + 3.9659 | 0.9760 | 29.885 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, N.; Wang, Y.-D.; Li, G.-R.; Liu, Z.-Q.; Feng, J.; Qiao, C.-L.; Zhang, H.-F. Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules 2023, 28, 3880. https://doi.org/10.3390/molecules28093880
Quan N, Wang Y-D, Li G-R, Liu Z-Q, Feng J, Qiao C-L, Zhang H-F. Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules. 2023; 28(9):3880. https://doi.org/10.3390/molecules28093880
Chicago/Turabian StyleQuan, Na, Yi-Dan Wang, Guo-Rong Li, Zi-Qi Liu, Jing Feng, Chun-Lei Qiao, and Hua-Feng Zhang. 2023. "Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities" Molecules 28, no. 9: 3880. https://doi.org/10.3390/molecules28093880
APA StyleQuan, N., Wang, Y.-D., Li, G.-R., Liu, Z.-Q., Feng, J., Qiao, C.-L., & Zhang, H.-F. (2023). Ultrasound–Microwave Combined Extraction of Novel Polysaccharide Fractions from Lycium barbarum Leaves and Their In Vitro Hypoglycemic and Antioxidant Activities. Molecules, 28(9), 3880. https://doi.org/10.3390/molecules28093880





