Abstract
The synthesis and design of low-cost visible-light-active catalysts for the photodegradation of organic dyes have been regarded as an efficient way to use solar energy in addressing environmental issues. We report the fabrication of MoS2/CdS nanoparticles functionalized with Bi2S3 nanoflakes. The ternary composites of “MoS2/CdS/Bi2S3” were synthesized in situ by a hydrothermal method at different temperatures. The changes in structural, optical, and morphological properties of the synthesized CdS/MoS2/Bi2S3 were explored. The effects of Bi2S3 on CdS/MoS2 were thoroughly studied by performing an X-ray diffractometer (XRD), a scanning electron microscope (SEM), an ultra-violet–visible spectrometer (Uv–vis), and Fourier transform infrared spectroscopic (FT-IR) studies of the nanoparticles. XRD confirms the cubical crystal structure of the nanoparticles. SEM studies possess the modulation in the surface morphology with the tenability in volume ratios of “MoS2/CdS/Bi2S3” composites. It was observed that the bandgaps calculated using absorption measurements could be manipulated from 2.40 eV to 0.97 eV with varying Bi2S3 in the MoS2/CdS nanostructures. FT-IR confirmed the synthesis of “MoS2/CdS/Bi2S3” nanoparticles. On allowing the visible light to fall for 120 min, it was observed that “MoS2/CdS/Bi2S3” degrades the methylene blue up to 90%. The calculated results of “MoS2/CdS/Bi2S3” suggest that the synthesized material could be a strong candidate for photodegradation applications. This research work explains the synthesis of MoS2/CdS/Bi2S3-based nanocomposites for the degradation of dye using a photocatalytic process. The final results show that this catalyst effectively degrades the dye.
1. Introduction
Water is necessary for many aspects of life, including growth and development. Safer and healthier drinking water is essential for maintaining public health [1]. Water is frequently used as one of the vital sources of life [2]. Water is a precious resource for many purposes, including domestic, industrial, and agricultural ones. It is also crucial for metabolic processes and cell function [3].
However, as the environment continues to deteriorate, there are various problems related to water pollution that also contaminate other parts of the ecosystem [4]. Furthermore, once polluted, the treatment of water is challenging and expensive, making it almost always impossible [5]. Water and water resources are essential for ensuring that all living species have access to enough food and a fertile habitat [6]. As the human population and economic activity have increased, so has the global need for freshwater [7]. Critical water resources are being placed under increasing pressure due to the adverse impacts of a growing global population, a changing climate, and different consumption habits, leading to widespread water stress in many countries [8]. Due to this, people are becoming more conscious of the urgent need to reduce their water use [9]. While developing nations face severe water pollution problems, industrialized nations still face pollution problems today [10]. Currently, 1.1 billion people are in danger because of a shortage of clean water, and 35% of fatal illnesses associated with water use occur in developing countries [11].
The development of technology has led to the introduction of several physical, chemical, and biological pollutants into the water supply. Wastewater includes liquid waste from houses, institutions, and industrial organizations. The pollution of waterways by industrial effluent is a global problem. There are high levels of several organic contaminants, heavy metals, and non-degradable compounds. Effectively eliminating these contaminants from industrial wastewater is of critical importance at present. Successful cleansing processes are required to eliminate these contaminants before they may be thrown away. Treatment methods for industrial wastewater may be divided into three broad categories: chemical, physical, and biological. The most often used technologies may be categorized as pre-treatments, primary treatments, secondary treatments, and tertiary treatments. Primary treatments, regularly used for essential cleaning, are size-based separations utilizing physical procedures similar to sedimentation and filtration. Secondary treatment can include physical, chemical, or biological methods to remove 80–90% of BOD, COD, and TSS from the wastewater. Tertiary treatment is used to further clean the effluent after secondary treatment by removing any harmful or dangerously recalcitrant pollutants (in some instances, this may be as high as 99%).
One of the methods available for efficiently treating wastewater is the use of nanomaterials. Water purification utilizing inexpensive nanoadsorbents and nanofiltration is an innovative use for nanomaterials. In this overview, we look at how nanoparticles are used to clean up industrial wastewater, emphasizing MB removal.
Industrial wastewater must be cleaned of organic dyes using practical, affordable methods that should be sustainable for the environment. The use of nanocomposite photocatalysts has been a very efficient way to treat industrial waste waters.
The use of photocatalysts is the most effective strategy for reducing organic dyes in manufacturing waste. The photocatalytic technique may be used to lessen environmental damage caused by industrial waste. CdS is one of the most promising photocatalysts for its nontoxicity, low cost, and photostability [9,10]. However, because of its large bandgap (2.42 eV), this frequently used CdS has poor photocatalytic effectiveness and is only photocatalytically active when exposed to UV light. Due to the smaller bandgap, visible-light photocatalysis was made possible [11]. However, several investigations have shown that CdS has weak photocatalytic activity. Metal-nonmetal doping is often used to create a defect level in the CdS band that is outlawed to optimize sunlight use [12,13].
The recombination impact of carriers further reduces the photocatalytic efficiency, yet a modest number of flaws are still virtually inadequate to improve the absorption of visible light radiation [14,15]. The reduced CdS (Cds-x) shows excellent visible light absorption and possesses a Cd2+ or sulfur vacancy defect. Under xenon-light illumination, it was found that the rate of methylene blue (MB) deterioration was 2.7 times greater than that of pure CdS. CdS-x offers active sites in the prohibited bandgap and maintains the natural structure, in contrast to impurity incorporation.
Although the decreased CdS (CdS-x) had more flaws, substantial photoelectron and hole recombination continued to occur in single-phase CdS, resulting in poor photocatalytic efficiency and hampering its use [16]. Heterostructures have been used to reduce carrier recombination by using semiconductor compounds, the deposition of noble metals, and the modification of carbon materials [17,18].
Nanoparticles are the most common sort of nanostructured material. According to the nanoscale model, nanoparticles are self-existent material units that are entirely isolated from one another by neighboring particles of a uniform size. They may also have a wide range of morphologies [17]. The creation of customized nanoparticles and their optimization for applications in various technological fields, including electronics, biomedicine, and catalysis, have received much attention during the last 20 years.
Nanoparticles fabricated from inorganic phases (metals, alloys, oxides, and composites) are typically preferred because of their enhanced electrical, optical, magnetic, and mechanical capabilities and the increased opportunities afforded by the smaller-than-nanometer-scale coupling between them [18]. Most industrial, environmental, and biological activities are based on aqueous chemistry, which is especially suitable for inorganic nanoparticles.
Furthermore, it has been suggested that nanoparticles could be used to filter out organic dyes from drinking water. Numerous early studies back up the idea that some particles may irreversibly remove one or more organic dyes and metals from industrial wastewater.
The thermal instability of these modified materials is causing a significant decline in photocatalytic activity [19,20]. Due to its thermal stability and capacity to offer catalytic activity, MoS2/Bi2S3 is a favored material. The photocatalytic characteristics of all the produced CdS/MoS2/Bi2S3 heterostructure photocatalysts are superior to those of pure CdS due to improved charge carrier separation efficiency [21,22].
Though, the synthesis technique of CdS/MoS2 composites is laborious, and the recycling process is challenging;, still the material becomes more efficient by intimate contact of MoS2 on the surface of CdS The photocatalytically active, micron-sized CdS/MoS2 composite photocatalysts are currently made using a three-step external source [23,24]. We proposed the loading of Bi2S3 on the surface of CdS/MoS to synthesize a new composite with further enhanced photocatalytic activity. Through a unique hydrothermal process under embedded sintering conditions has been used in this study to manufacture an in-situ grey CdS/MoS2/Bi2S3 hetero-structure photocatalyst in a single step. Earlier research has revealed its ability to treat high, medium, and low turbidity water. It can also be used as a softening or dewatering agent, so its importance in wastewater treatment cannot be overstated.
When “CdS/MoS2/Bi2S3” is compared with conventional chemical coagulants, it has the following advantages: cost effectiveness, availability, biodegradable sludge, eco-friendliness, low sludge volume, it does not produce harmful by-products, it is easily handled as it is not corrosive, and it does not affect the pH of water. In the light of the above advantages, “CdS/MoS2/Bi2S3” is environmentally friendly and available at a low cost, which can be a good alternative to chemical photocatalysis with a potential application in wastewater treatment in industry. Therefore, using “CdS/MoS2/Bi2S3” as a photocatalyst in the industrial waste strategy to improve water quality will provide the best results.
2. Results and Discussion
It was possible to identify the crystal structure using an X-ray diffractometer. A scanning electron microscope (SEM) was used to analyze surface morphology. FT-IR confirms the synthesis of MoS2/CdS/Bi2S3 nanoparticles.
2.1. Morphological Studies
The nanoscale MoS2 particles are visible and successfully prepared composites of MoS2 nanostructure. The morphology of MoS2/CdS was characterized using surface morphology as it was created. Figure 1a,b, respectively, show SEM images of CdS on MOS2. The CdS, which resembled an olive, was shaped like a nanoflake’s surface [25,26].

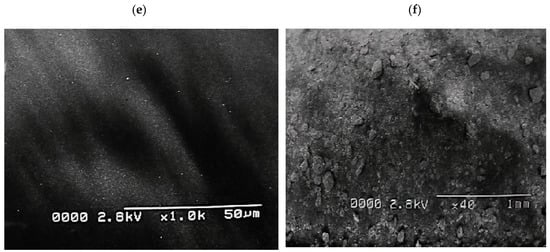
Figure 1.
(a) CdS, (b) MoS2, (c) CdS/MoS2/Bi2S3 composites (1%/1%/1%), (d) CdS/MoS2/Bi2S3 composites (1%/5%/5%), (e) CdS/MoS2/Bi2S3 Composites (2%/5%/5%), and (f) CdS/MoS2/Bi2S3 composites (0.1%/1%/1%).
MoS2 was connected irregularly to the edge of the CdS in the MoS2/CdS/Bi2S3 composite, increasing the catalytic edge sites [27,28].
The brilliance of the orange colour in the MoS2/CdS composite decreased when the ratio of MoS2/Bi2S3 increased. The surface-to-volume ratio of nanostructured materials makes assessing their surface highlights a must for several essential applications. Figure 1c shows the CdS/MoS2/Bi2S3 composites (1%/1%/1%), the Figure 1d CdS/MoS2/Bi2S3 composites (1%/0.5%/0.5%), the Figure 1e CdS/MoS2/Bi2S3 composites (2%/5%/5%), and the Figure 1f CdS/MoS2/Bi2S3 composites (0.1%/1%/1%). The particles of nanosized CdS, MoS2, and Bi2S3 are obviously clear, proposing a successful composite of CdS, MoS2, and Bi2S3 nanostructures. SEM images of complete CdS/MoS2/Bi2S3 nanoparticles demonstrate CdS/MoS2/Bi2S3 nanoparticles.
2.2. Structural Studies
The overlapped FTIR spectra of the synthesized materials have been shown in Figure 2. The FTIR spectra cover an array of 4000 cm−1 to 400 cm−1. The major peaks are at 3399 cm−1, 1624 cm−1, 1112 cm−1, and 418 cm−1. The extending style of the OH group, which is specified by water contents, is designated by an eclectic band at 3399 cm−1. The H-O-H bending vibrational mode is responsible for the band about 1624 cm−1, which is absorbed by water in the air. The FT-IR spectra of the precursor and product MoS2/CdS/Bi2S3 nanocomposites are also shown in Figure 2. The I.R. bands at 428 cm−1 and 508 cm−1 are symptomatic of the stretching vibrations of the Mo-S and (S-S)2 bonds [25,29].
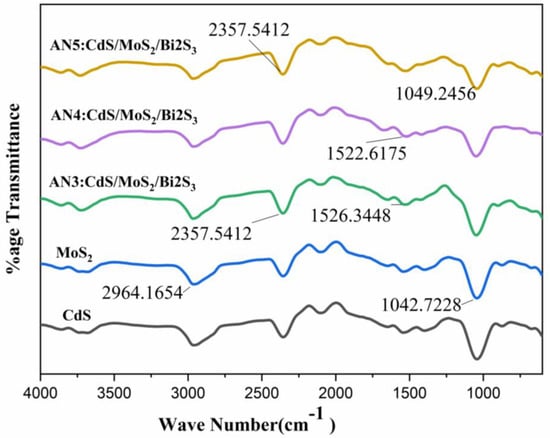
Figure 2.
FTIR spectra of CdS, MoS2, (AN3)CdS/MoS2/Bi2S3 composite (1%/1%/1%), (AN4)CdS/MoS2/Bi2S3 composite (1%/5%/5%), and (AN5)CdS/MoS2/Bi2S3 composite (2%/5%/5%).
Both the stretching vibration of the CdS link at 630 cm−1 and the bending vibration of water at 1560 cm−1 were both identified. Specifically, the prominent peak between 3200 and 3500 cm−1 was associated with the O-H bond vibration [26]. This suggested that photoreduction, rather than adsorption, was the primary interaction mechanism on the MoS2/CdS composite, as no new bands appeared following the reaction [30,31].
The absorptions at 1627, 1427, 1112, 875, and 607 cm−1 are all ascribed to MoS2, although the bands at 3000–2800 cm−1 are produced by the stretching of the C-H alkyl stretching band in polyethylene glycol. Finally, FTIR concerns have long established the favorable implementation of MoS2 nanocomposites [32].
We examined the FTIR range of the contrived MoS2 nanoparticles and shown in Figure 2. Wide-ranging absorption bands were found at 639 cm−1, 893 cm−1, 1402 cm−1, and 1622 cm−1 which are accredited to MoS2. The band at approximately 483 cm-1 is due to the S-S bond. The 931 cm−1 band is owed to the S-S bond. The peaks of about 3182 cm−1 are distinguishing features of the O-H group [33,34].
Strong hydrogen bonding between the O-H bands at 3170 cm−1 causes them to commonly overlap the C–H absorbance. CdS particles showed two C-O stretching bands at 997 cm−1 and 653 cm−1, and CH3 (acetone) bending, whether acute or wide, was papered at 1484 cm–1. Impurities such as SO4 can be detected as a small absorption peak at 853 cm−1. The absorption at 2002–2926 cm−1 is recognized as a stretching vibration of carbon and hydrogen.
Along with the peaks at 400–1000 cm−1, which are linked with Bi-S stretching vibration, an alternative peak seems to be at 1110 cm−1, which is accompanied by C–O, C=O, and C–S bending vibration.
2.3. XRD Analysis
Since atoms in different crystals release X-rays in different directions. Using an X-ray diffractometer (XRD JDX-3532 JEOL, Tokyo Japan), the crystallinity of as-prepared MoS2/CdS/Bi2S3 was determined. A three-dimensional arrangement of electron density may be indomitable by evaluating the angles and scattering intensities of these beams. This technique may be used to determine crystal structure, chemical bonding, and other similar phenomena. Porous molybdenum disulfide’s XRD pattern can be seen in Figure 3. Porous MoS2 was effectively synthesized, as evidenced by peaks at 14.3° and weak peaks at 24.3° and 29.2°. (JCPDS card No. 37-1492).
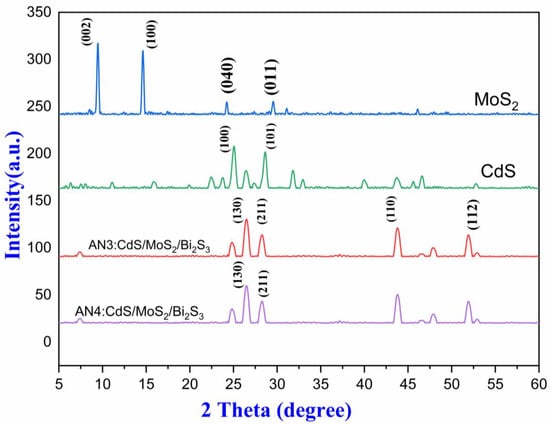
Figure 3.
XRD spectra (CdS, AN3, AN4, and MoS2).
Furthermore, the XRD spectrum of CdS nanoparticles is presented in Figure 3; peaks at 25.8, 43.2, and 51.3 °C agree with JCPDS 42-1411, representing the triumph for the production. Figure 3 also pertains to AN3 (CdS/MoS2/Bi2S3) 1%1%/1% nanocomposites at 26.9°, 44.7°, and 52.5° [35]. Figure 3 also has to do with AN4 (CdS/MoS2/Bi2S3, 1%:5%:5%) at 25.1°, 27.3°, and 46.1° to sanction the manifestation of the components Cd, S, and Mo in the manufactured nanoparticles and nanocomposites. The crystal configuration of MoS2/CdS was deliberated using XRD analysis [36]. The intense diffraction peak of the (101) plane shows that CdS crystals grew in that orientation. The diffraction peak intensity of MoS2/CdS was big and crisp, indicating excellent crystallization. The lack of a noticeable shift in the diffraction peak of MoS2/CdS composites when compared to CdS revealed that the loading of MoS2 rather than doping was the cause. The lack of a diffraction peak in MoS2 could be due to its low content [37]. After the photocatalytic reaction, the diffraction peaks did not significantly shift, indicating that the MoS2/Cd/Bi2S3 composite was stable. It is acknowledged that high crystallinity is related to a reduction in crystal defects, which prevents the recombination of photogenerated electrons and holes and improves photocatalytic activity.
2.4. UV–Vis Absorbance Spectra
The optical traits of MoS2/CdS nanocomposites were studied using UV-Vis and photocatalytic studies. UV-Vis spectra (for MoS2, CdS, and the MoS2/CdS nanocomposite) are presented in Figure 4. The UV-Vis absorption of MoS2 is very weak, but when CdS is incorporated into MoS2 to produce a MoS2/CdS nanocomposite, the absorption is enhanced and pushed down to shorter wavelengths. Studies in the UV-Vis range have been used to learn about the optical properties of MoS2/CdS nanocomposites. It is important to note that MoS2 has weak UV-Vis absorption; however, that UV-Vis absorption is enhanced by a MoS2/CdS nanocomposite.
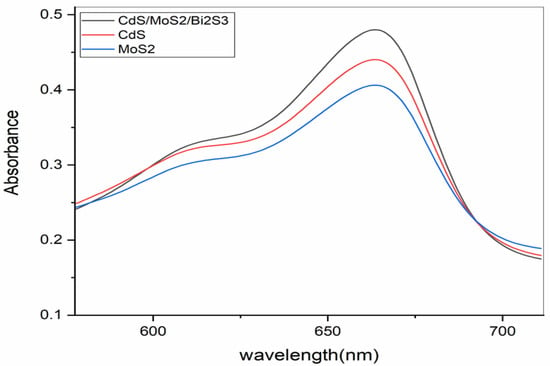
Figure 4.
UV-Vis spectrum (CdS/MoS2/Bi2S3, CdS, and MoS2) before degradation.
CdS nanoparticles, as shown in Figure 5a, feature a 3.4 eV bandgap and a maximum wavelength of 454 nm. The absorption spectra in Figure 4 show MoS2’s 1.6 eV bandgap [38]. Nanocomposites MoS2/CdS have a 2.8 eV bandgap, and their absorption spectra are shown in Figure 6 [36]. Due to the incorporation of MoS2, the bandgap energy was raised. The MoS2/CdS nanocomposite also exhibited an enhanced absorption curve in the visible spectrum.
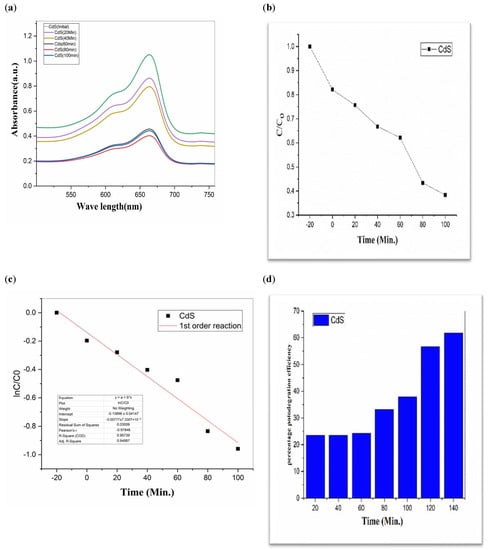
Figure 5.
CdS (a) graphical representation of degradation of methylene blue; (b) C/C0; (c) lnC/C0; (d) %age efficiency.
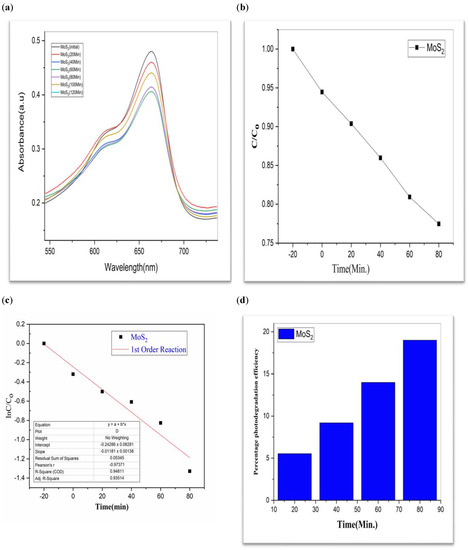
Figure 6.
MoS2 (a) graphical representation of degradation of methylene blue; (b) C/C0; (c) lnC/C0; (d) %age efficiency.
The bandgap was found by using the following formula:
2.5. Photocatalytic Activity of CdS/MoS2/Bi2S3
When exposed to ultraviolet (UV) or visible light, the catalyst becomes active, producing free electrons and holes on its surface in addition to hydroxyl radicals. These free radicals oxidize and break down or eliminate organic pollutants. Reacting with the surface, oxygen is found naturally in the air, and water frees electrons trapped in the CdS. Due to its interaction with unstable oxygen free radicals, the O2- anion is formed, further oxidizing the organic molecules (superoxide).
CdS/MoS2 is a useful photocatalyst because its surface undergoes many processes necessary for the degradation of organic dyes. The following formula was used to determine the effective photocatalytic degradation of organic dyes.
where C0 is the dye solution concertation before photoirradiation, and C is the concertation of solutions in suspension after photoirradiation for a given time t [39,40].
photocatalytic degradation efficiency = (C0 − C/C0) × 100
To investigate the photocatalytic degradation of organic dyes like methylene blue, nanoparticles of CdS, MoS2, and Bi2S3 were utilized. Figure 5 shows the typical degradation of methylene blue dye in the manifestation of CdS, MoS2, and Bi2S3, as well as the chemical and structural characteristics of the organic dyes used in this work [41]. Using lamps with wavelengths of 254 nm and 765 nm, ultraviolet light was used to expose the dyes. The Figure 7, Figure 8 and Figure 9 show a typical measurement of organic dye absorbance vs. reaction time after exposure to a UV-Vis lamp with a longer wavelength of 670 nm. In methylene blue, the hetero-poly aromatic bond between the S and N atoms accounts for the high absorbance at 664 nm [42,43].
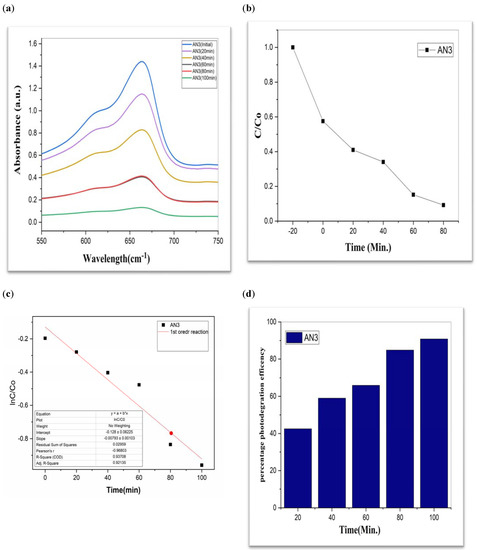
Figure 7.
AN3 (a) graphical representation of degradation of methylene blue; (b) C/C0; (c) lnC/C0; (d) %age efficiency.
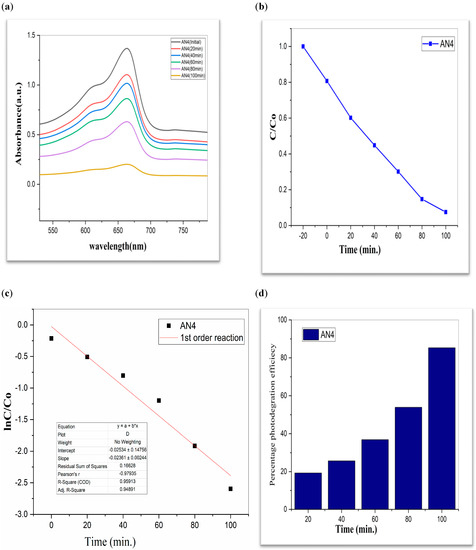
Figure 8.
AN4 (a) graphical representation of degradation of methylene blue; (b) C/C0; (c) lnC/C0; (d) %age efficiency.
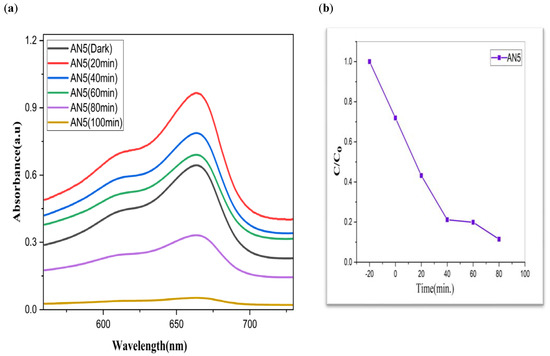
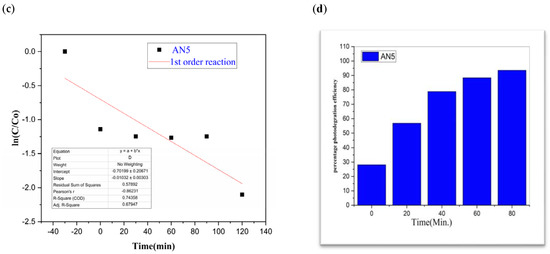
Figure 9.
AN5 (a) graphical representation of degradation of methylene blue; (b) C/C0; (c) lnC/C0; (d) %age efficiency.
Due to n to π* (the nitrogen atom has an unpaired electron pair) and to π* (a system of double bonds that are conjugated in aromatic rings), absorbance peaks at 664 and 615 nm indicated that an electronic transition was occurring in the MB solution [21,22].
The value of MB solution absorbance in the absence of UV light and the CdS/MoS2/Bi2S3 catalyst both dropped after 30 min, indicating that the CdS/MoS2/Bi2S3 catalyst has a strong degrading capability. When CdS/MoS2/Bi2S3 was utilized, the MB was completely damaged after 120 min of irradiation, but only 92% degraded after 100 min. Other researchers have reported comparable results using CdS/MoS2/Bi2S3 for photocatalytic MB breakdown (Figure 7, Figure 8 and Figure 9).
Although CdS/MoS2/Bi2S3 particles had higher photocatalytic activity, the composite has nearly two times more dye absorption under similar conditions, indicating that the CdS/MoS2/Bi2S3 phase had lower catalytic performance (Figure 7, Figure 8 and Figure 9).
Improved photocatalytic activity is anticipated from the anatase phase CdS/optimal MoS2’s size quantization effect, which is the consequence of a larger surface area and a larger band gap as a result of smaller particles. Though MB degradation effectiveness was discovered to be 90% in the contemporary analysis, which might be connected to the presence of more oxygen, as mentioned above, as well as smaller particle size obtained during CdS/MoS2/Bi2S3 production.
It has been advocated that the low electron hole pair recombination rate of semiconductor nanoparticles is the most important factor in their efficient photocatalytic activity. Due to electronic excitation inside “CdS/MoS2/Bi2S3”, the photocatalytic process is kicked off when nanocomposite “CdS/MoS2/Bi2S3” is exposed to UV light.
Direct activation of the nanoparticles is possible with energy superior to the bandgap of CdS, which is 3.2 eV (385 nm) [44,45]. According to numerous studies in the literature, the holes and electrons fashioned during photolysis can oxidize organic molecules, resulting in all of them being oxidized to •OH radicals when they interact with water or hydroxides. Photogenerated electrons may also enter the dye or combine with absorbed O2 on nanoparticle planes to form the superoxide radical anion O2• [46].
The succeeding Equations (1)–(8) recapitulate the overall deterioration process as demonstrated graphically in Figure 10 [15].
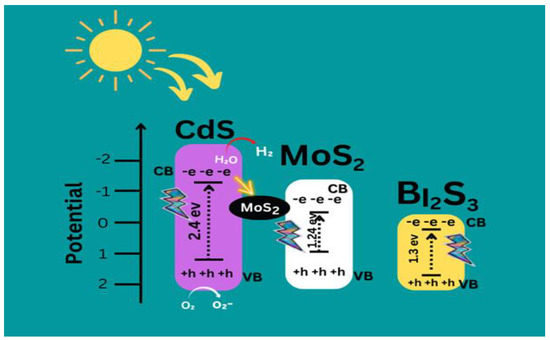
Figure 10.
Proposed Mechanism of CdS/MoS2/Bi2S3.
So,
examining methylene blue’s photocatalytic activity in its breakdown.
hv = photons,
h = holes and e = electrons
CdS/MoS2/Bi2S3 + hv (UV) → CdS/MoS2/Bi2S3 (eCB− + hVB+)
CdS/MoS2/Bi2S3 (hVB+) + H2O → CdS/MoS2/Bi2S3 + H+ + •OH
CdS/MoS2/Bi2S3(hVB+) + OH− →CdS/MoS2/Bi2S3 + •OH
CdS/MoS2/Bi2S3 (eCB−) + O2 → CdS/MoS2/Bi2S3 + O2− •
O2− • + H • → HO2•
methylene blue dye + OH• →degradation yields
methylene blue dye + hVB+ → chemical by- yields of oxidation
methylene blue dye + eCB− → reducing substances
The photocatalytic activity of CdS/MoS2/Bi2S3 nanoparticles for dye degradation was tested utilizing extended UV irradiation. The photocatalytic activity of “CdS/MoS2/Bi2S3” against MB is shown in the figure. The activity was set up to be appropriate in both conditions, though the degradation efficiency was found to be lower when associated with short UV irradiation. Other dyes were also studied to investigate the ability of composites to degrade them when exposed to UV light over longer periods of time the results of which will be published in our future research.
The graphs demonstrate the degradation of MB dye in the presence of CdS/MoS2/Bi2S3. Photocatalytic experiments with various compositions of nanocomposites demonstrated that the highest photocatalytic efficacy of about 90% was found to be for MB dye. Especially the photocatalytic degradation efficiency of “CdS/MoS2/Bi2S3” was found to be maximum i.e., 91% at ration of 2%:5%:5% (Table 1).

Table 1.
Degradation %age efficiency of synthesized materials.
While comparing, CdS has a 60% efficiency in the photodegradation of MB, whereas MoS2 only manages 19% (Table 1). But when we switched from Bi2S3 to CdS/MoS2, we saw a 90% boost in efficiency. The optimal outcome was obtained using a composition of CdS, MoS2, and Bi2S3. The “CdS/MoS2/Bi2S3” performed the best i.e., 91% (Table 1).
We looked at the photocatalytic deterioration of MB in the presence of visible light. Different molar ratios in “CdS/MoS2/Bi2S3” samples showed more significant photocatalytic activity when compared to dark blue “CdS/MoS2/Bi2S3”. The enhanced photocatalytic performance of “CdS/MoS2/Bi2S3” composite photocatalysts was also found. The “CdS/MoS2/Bi2S3” samples with microstructure also have a lot of potential applications in water treatment.
3. Materials and Methods
3.1. Synthesis of MoS2 Photocatalyst
The graphical representation of the synthesis of photocatalyst composites has been shown in Figure 11. A 0.92 g sample of ammonium heptamolybdate tetrahydrate was diluted in 50 mL of ethylene glycol. Then, it was continuously mixed with 5 mL of hydrazine for 30 min. The mixture was then heated to 200 °C for 24 h while enclosed in a hydrothermal reactor coated with Teflon. After the reaction was complete, the container was allowed to cool at room temperature, and distilled water was used to wash the product. After multiple washes in ethanol, the resulting material was placed into a heated oven at 60 °C for 24 h in order to dehydrate. The product was allowed to cool at room temperature until further characterization and photocatalytic investigation.
Figure 11.
Product of CdS/MoS2/BiS3.
3.2. Synthesis of CdS Photocatalyst
In the first step, 3.085 g of “Cd(NO3)2•4H2O” cadmium nitrate tetrahydrate was dissolved in 25 mL of distilled water. After that, 1.565 g of “Na2S” and 0.316 g of “Na2SO3” were added to the aqueous solution of cadmium nitrate tetrahydrate. A 5 mL solution of acetic acid, 20 mL of ethylene glycol, and 1 g of “Na2EDTA” were then added with constant stirring for 30 min. Furthermore, the solutions were mixed well in a stainless-steel autoclave lined with Teflon, which was then sonicated for an hour. After that, the autoclave was cooked in an air-blowing thermostatic oven at 180 °C for six hours. The residue was dried in a vacuum chamber at room temperature overnight, followed by washing with ethanol and water.
3.3. Synthesis of CdS/MoS2/Bi2S3 Composites
To obtain the wanted solution, we took 0.5 g of ascorbic acid dissolved in distillation water, added 0.5 g of MoS2, 0.5 g of CdS, 0.485 g of Bi(NO3)3.5H2O as a source of Bi, and 0.1126 g of thioacetamide as a source of sulfur, followed by 1 mL of ethylene diamine and 30 min of stirring (Figure 12). This solution was sonicated for 1 h after being well mixed in a Teflon-lined stainless steel autoclave. The autoclave, formed of stainless steel and layered with Teflon material, was placed in a thermostatic oven that heated the air to 200 °C for 24 h. After being washed in ethanol and water, the residue was dried in a vacuum compartment at room temperature for 12 h. We synthesized three different composites by varying the ratios of CdS, MoS2, and Bi2S3. The synthesized composites with specific ratios were as follows: AN3 with a ratio of 1:1:1; AN4 with a ratio of 1%/5%/5%; and AN5 with a ratio of 2%/5%/5% of CdS, MoS2, and Bi2S3, respectively.
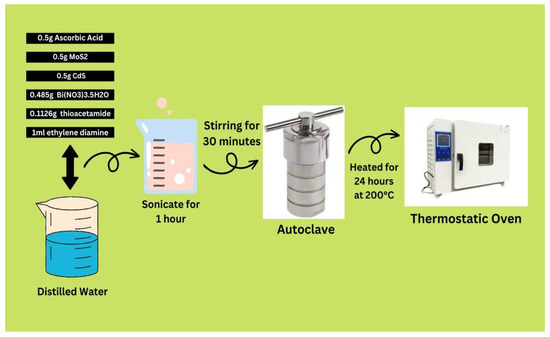
Figure 12.
Synthesis of CdS/MoS2/Bi2S3 composites.
4. Conclusions
We have successfully made “CdS/MoS2/Bi2S3” nanohybrids with different structures and looked at their photodegradation processes. The 3D designs of “CdS/MoS2/Bi2S3” nanohybrids displayed unusual sunlight-induced photocatalytic activities for decomposing three organic compounds. In sunlight irradiation, the “CdS/MoS2/Bi2S3” nano heterojunctions found extraordinarily high photodegradation efficiencies, resulting in the breakdown of 90.3% of the MB dye within 120 min. Superior photodegradation activity has been attained because of the synergistic effect’s high density between “CdS/MoS2” and Bi2S3. This effectively reduces the recombination rate and increases the charge separation process. Significant data demonstrate how varied “CdS/MoS2/Bi2S3” nanohybrid morphologies impact the heterojunction density. This work emphasizes the straightforward method for creating “CdS/MoS2/Bi2S3” heterojunctions for the outstanding photodegradation of organic azo dyes and pharmaceutical waste driven by sunlight. It can be shown that the cost-effective “CdS/MoS2/Bi2S3” nanohybrids are particularly useful for several industrial applications, including H2 production, solar cells, and water splitting.
Author Contributions
Conceptualization, M.S.T.; methodology, G.M.K.; software, M.B.T.; validation, M.S.; formal analysis, X.Z.; funding, B.J.; data curation, A.N.; writing—original draft preparation, A.N.; visualization, M.S.T.; supervision, G.M.K.; project administration, M.S.T.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China at the Chinese Academy of Science, and the APC was funded by Grant numbers 21974149 and 22174152.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Hollaway, M.J.; Beven, K.J.; Benskin, C.M.H.; Collins, A.; Evans, R.; Falloon, P.; Forber, K.J.; Hiscock, K.M.; Kahana, R.; Macleod, C.J.A. The challenges of modelling phosphorus in a headwater catchment: Applying a ‘limits of acceptability’uncertainty framework to a water quality model. J. Hydrol. 2018, 558, 607–624. [Google Scholar] [CrossRef]
- Shah, V.D. Fluid Thoughts-Water: Structure and Mysticism; Notion Press: Chennai, India, 2017. [Google Scholar]
- Ashfaq, A.; Clochard, M.-C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M.J.P. Polymerization reactions and modifications of polymers by ionizing radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as source of bioactive substances and skin care therapy—cosmeceuticals, algotheraphy, and thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Hirschon, R. Essential Objects and the Sacred: Interior and Exterior Space in an Urban Greek Locality 1. In Women and Space; Routledge: Oxford, UK, 2021; pp. 70–86. [Google Scholar]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Water chemistry in the biological studies by using nuclear analytical techniques. In Water Engineering Modeling and Mathematic Tools; Elsevier: Amsterdam, The Netherlands, 2021; pp. 133–156. [Google Scholar]
- Tan, L.; Dong, W.; Liu, K.; Luo, T.; Gu, X. Thermal decomposition in-situ preparation of gray rutile TiO2-x/Al2O3 composite and its enhanced visible-light-driven photocatalytic properties. Opt. Mater. 2021, 111, 110716. [Google Scholar] [CrossRef]
- Lewis, N.S. Research opportunities to advance solar energy utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.; Huang, W.; Taghipour, F. Recent progress and perspectives in the photocatalytic CO2 reduction of Ti-oxide-based nanomaterials. Appl. Surf. Sci. 2017, 396, 1696–1711. [Google Scholar] [CrossRef]
- Dong, W.; Liu, H.; Bao, Q.; Gu, X. Facile synthesis of metastable CaTi2O5 nanostructure and its photocatalytic properties. Opt. Mater. 2020, 105, 109921. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Matos, J.; Ocares-Riquelme, J.; Poon, P.S.; Montaña, R.; García, X.; Campos, K.; Hernández-Garrido, J.C.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid Interface Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- Qi, S.; Zhang, K.; Zhang, Y.; Zhang, R.; Xu, H. TiO2/Zn0.5Cd0.5S heterojunction for efficient photocatalytic degradation of methylene blue and its photocatalytic mechanism. Chem. Phys. Lett. 2022, 798, 139614. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Chandraprabha, M.; Krishna, R.H.; Samrat, K.; Sakunthala, A.; Sasikumar, M. Optical and antibacterial activity of biogenic core-shell ZnO@ TiO2 nanoparticles. J. Indian Chem. Soc. 2022, 99, 100361. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, H.; Wu, Y.; Ma, Z.; Zhang, R.; Tu, H.; Jiang, L. A new strategy to construct cellulose-chitosan films supporting Ag/Ag2O/ZnO heterostructures for high photocatalytic and antibacterial performance. J. Colloid Interface Sci. 2022, 609, 188–199. [Google Scholar] [CrossRef]
- Tahir, M.B.; Nabi, G.; Khalid, N.R. Enhanced photocatalytic performance of visible-light active graphene-WO3 nanostructures for hydrogen production. Mater. Sci. Semicond. Process. 2018, 84, 36–41. [Google Scholar] [CrossRef]
- Bilal Tahir, M.; Nabi, G.; Rafique, M.; Khalid, N.R. Role of fullerene to improve the WO3 performance for photocatalytic applications and hydrogen evolution. Int. J. Energy Res. 2018, 42, 4783–4789. [Google Scholar] [CrossRef]
- Luo, J.; Li, R.; Chen, Y.; Zhou, X.; Ning, X.; Zhan, L.; Ma, L.; Xu, X.; Xu, L.; Zhang, L.J.S.; et al. Rational design of Z-scheme LaFeO3/SnS2 hybrid with boosted visible light photocatalytic activity towards tetracycline degradation. Sep. Purif. Technol. 2019, 210, 417–430. [Google Scholar] [CrossRef]
- Adeleke, J.; Theivasanthi, T.; Thiruppathi, M.; Swaminathan, M.; Akomolafe, T.; Alabi, A.B. Photocatalytic degradation of methylene blue by ZnO/NiFe2O4 nanoparticles. Appl. Surf. Sci. 2018, 455, 195–200. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Xu, J.; Cao, X. Characterization and mechanism of MoS2/CdS composite photocatalyst used for hydrogen production from water splitting under visible light. Chem. Eng. J. 2015, 260, 642–648. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, J.; Huang, X.; Mei, P.; Wang, H.; Research, P. Boosting photocatalytic efficiency of MoS2/CdS by modulating morphology. Environ. Sci. Pollut. Res. 2022, 29, 73282–73291. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, L.; Zhang, J.; Wang, Z.; Zhu, W.; Wang, Y.; Zou, Z. Dual MOF-Derived MoS2/CdS Photocatalysts with Rich Sulfur Vacancies for Efficient Hydrogen Evolution Reaction. Chem. A Eur. J. 2022, 28, e202202019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. MoS2/CdS rod-like nanocomposites as high-performance visible light photocatalyst for water splitting photocatalytic hydrogen production. Int. J. Hydrogen Energy 2022, 47, 8247–8260. [Google Scholar] [CrossRef]
- Liu, X.; Wang, B.; Heng, Q.; Chen, W.; Li, X.; Mao, L.; Shangguan, W. Promoted charge separation on 3D interconnected Ti3C2/MoS2/CdS composite for enhanced photocatalytic H2 production. Int. J. Hydrogen Energy 2022, 47, 8284–8293. [Google Scholar] [CrossRef]
- Zheng, X.; Han, H.; Liu, J.; Yang, Y.; Pan, L.; Zhang, S.; Meng, S.; Chen, S. Sulfur Vacancy-Mediated Electron–Hole Separation at MoS2/CdS Heterojunctions for Boosting Photocatalytic N2 Reduction. ACS Appl. Energy Mater. 2022, 5, 4475–4485. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, S.; Cheng, W.; Han, G.; Wu, Q.; Jiang, J.J.C.; Physicochemical, S.A.; Aspects, E. Construction of mechanically robust and recyclable photocatalytic hydrogel based on nanocellulose-supported CdS/MoS2/Montmorillonite hybrid for antibiotic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128035. [Google Scholar] [CrossRef]
- Allahyar, S.; Taheri, M.; Allahyar, S. Facile synthesis of few-Layered MoS2 Nanoroses Covering TiO2 Nanowires as improved bacterial inactivation and photodegradation devices. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, L.; Zhang, Y.; Wang, P.; Wang, G.; Bai, F.; Zhao, Z.; Gong, F.; Liu, J. Metal Sulfides Yolk–Shell Nanoreactors with Dual Component for Enhanced Acidic Electrochemical Hydrogen Production. Small Struct. 2022, 4, 2200247. [Google Scholar] [CrossRef]
- Qin, Y.; Xiao, K.; Sun, S.; Wang, Y.; Kang, C. Fabrication of a novel pyramidal 3D MoS2/2D PbTiO3 nanocomposites and the efficient photocatalytic removal of organic pollutants: Effects of the PbTiO3 internal electric field and S-scheme heterojunction formation. Appl. Surf. Sci. 2023, 615, 156431. [Google Scholar] [CrossRef]
- Han, C.; Cheng, C.; Liu, F.; Li, X.; Wang, G.; Li, J. Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity. Nanotechnol. Rev. 2023, 12, 20220503. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, D.; Hiragond, C.B.; Lee, J.; Jung, J.-W.; Cho, C.-H.; In, I.; In, S.-I. Phase-controlled 1T/2H-MoS2 interaction with reduced TiO2 for highly stable photocatalytic CO2 reduction into CO. J. CO2 Util. 2023, 67, 102324. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y. Engineering. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Tien, T.-M.; Chen, E.L. S-Scheme System of MoS2/Co3O4 Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methyl Violet Dye Removal under Visible Light Irradiation. Coating 2023, 13, 80. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Fang, Y.; Sun, S.; Yu, Z. Compounds. Construction of MoS2/CdS/TiO2 ternary composites with enhanced photocatalytic activity and stability. J. Alloys Compd. 2016, 684, 335–341. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Chen, X.; Feng, H.; Shen, D.; Huang, B.; Jia, Y.; Zhou, Y.; Liang, Y. Effect of sulfur source on photocatalytic degradation performance of CdS/MoS2 prepared with one-step hydrothermal synthesis. J. Environ. Sci. 2018, 65, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, M.; Fang, Y.; Sun, S.; He, J. Ag2S and MoS2 as dual, co-catalysts for enhanced photocatalytic degradation of organic pollutions over CdS. J. Mater. Sci. 2016, 51, 779–787. [Google Scholar] [CrossRef]
- Ghasemipour, P.; Fattahi, M.; Rasekh, B.; Yazdian, F. Developing the ternary ZnO doped MoS2 nanostructures grafted on CNT and reduced graphene oxide (RGO) for photocatalytic degradation of aniline. Sci. Rep. 2020, 10, 4414. [Google Scholar] [CrossRef] [PubMed]
- Ritika, M.K.; Umar, A.; Mehta, S.K.; Singh, S.; Kansal, S.K.; Fouad, H.; Alothman, O.Y. Rapid solar-light driven superior photocatalytic degradation of methylene blue using MoS2-ZnO heterostructure nanorods photocatalyst. Materials 2018, 11, 2254. [Google Scholar] [CrossRef]
- El Malti, W.; Hijazi, A.; Abou Khalil, Z.; Yaghi, Z.; Medlej, M.K.; Reda, M. Comparative study of the elimination of copper, cadmium, and methylene blue from water by adsorption on the citrus Sinensis peel and its activated carbon. RSC Adv. 2022, 12, 10186–10197. [Google Scholar] [CrossRef]
- Mirsalari, S.A.; Nezamzadeh-Ejhieh, A.; Massah, A.R.; Research, P. A designed experiment for CdS-AgBr photocatalyst toward methylene blue. Env. Sci. Pollut. Res. Int. 2022, 29, 33013–33032. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, C.; Yin, J.; Chen, S.; Zhao, W.; Zhao, C. Clearance of methylene blue by CdS enhanced composite hydrogel materials. Environ. Technol. 2022, 43, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Zhang, Y.; Zheng, J.; Ge, S.; Lin, J.; Pan, D.; Naik, N.; Guo, Z. In-situ constructing visible light CdS/Cd-MOF photocatalyst with enhanced photodegradation of methylene blue. Particuology 2022, 69, 111–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).