Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae)
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of Essential Oils
2.1.1. Chemical Constituents of Citrus Oil
2.1.2. Chemical Constituents of Chuan-Shan Pepper Oil
2.1.3. Chemical Constituents of Zanthoxylum Oil
2.1.4. Chemical Constituents of Pomelo Peel Oil
2.1.5. Chemical Constituents of Orange Leaf Oil
2.2. Laboratory Bioassay
2.3. Pot Experiments
2.3.1. Control Efficacy of Citrus Oil against T. flavus
2.3.2. Control Efficacy of Chuan-Shan Pepper Oil against T. flavus
2.3.3. Control Efficacy of Zanthoxylum Oil against T. flavus
2.3.4. Control Efficacy of Pomelo Peel Oil against T. flavus
2.3.5. Control Efficacy of Orange Leaf Oil against T. flavus
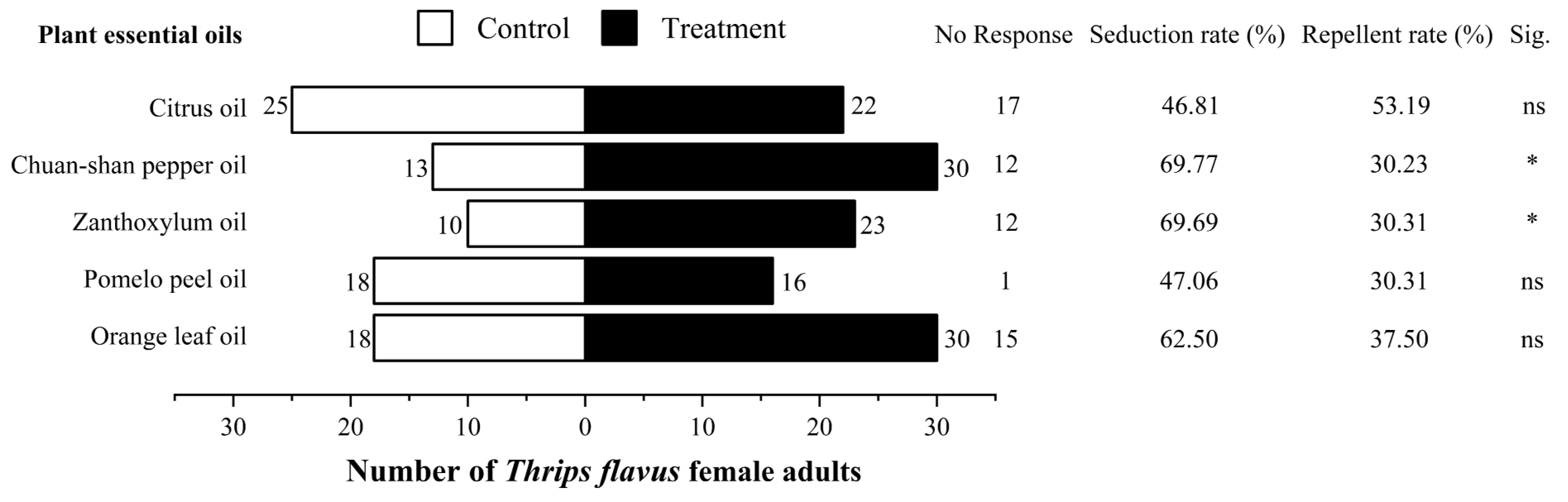
2.4. Olfactometer Test
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Plant Essential Oils
4.3. GC–MS Analysis
4.4. Laboratory Bioassay
4.5. Pot Experiments
4.6. Olfactory Test
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nickle, D.A. Commonly intercepted thrips at US ports of entry from Africa, Europe, and the Mediterranean. III. The genus Thrips Linnaeus, 1758 (Thysanoptera: Thripidae). Proc. Entomol. Soc. Wash. 2008, 110, 165–185. [Google Scholar] [CrossRef]
- Minaei, K. Thrips (Insecta, Thysanoptera) of Iran: A revised and updated checklist. ZooKeys 2013, 330, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Mound, L.; Collins, D.; Hastings, A. Thysanoptera Britannica et Hibernica–Thrips of the British Isles; Identic Pty Ltd.: Coolangatta, QLD, Australia, 2018; Available online: https://keys.lucidcentral.org/keys/v3/british_thrips/ (accessed on 21 May 2021).
- Boonham, N.; Smith, P.; Walsh, K.; Tame, J.; Morris, J.; Spence, N.; Bennison, J.; Barker, I. The detection of tomato spotted wilt virus (TSWV) in individual thrips using real time fluorescent RT-PCR (TaqMan). J. Virol. Methods 2002, 101, 37–48. [Google Scholar] [CrossRef]
- Singh, S.J.; Krishanareddy, M. Watermelon bud necrosis: A new tospovirus disease. Acta Hortic. 1996, 431, 68–77. [Google Scholar] [CrossRef]
- Zvaríková, M.; Masarovič, R.; Prokop, P.; Fedor, P. An updated checklist of thrips from Slovakia with emphasis on economic species. Plant Prot. Sci. 2020, 56, 292–304. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.J.; Wang, D.; Yang, J.; Ding, N.; Shi, S.S. Effect of different plants on the growth and reproduction of Thrips flavus (Thysanoptera: Thripidae). Insects 2021, 12, 502. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, S.S.; Xu, M.L.; Cui, J. Current research on soybean pest management in China. Oil Crop Sci. 2018, 3, 215–227. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.J.; Ding, N.; Gao, B.S.; Gao, Y.; Shi, S.S. Biological activity tests and field trials of eight kinds of insecticides to Thrips flavus. Agrochemicals 2021, 60, 220–222. (In Chinese) [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.L.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.; Funderburk, J.E. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef]
- Gholami, Z.; Sadeghi, A. Management strategies for western flower thrips in vegetable greenhouses in Iran: A review. Plant Protect. Sci. 2016, 52, 87–98. [Google Scholar] [CrossRef]
- Gao, Y.L.; Lei, Z.R.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kalscheur, N.A.; Kemp, K.E. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 2009, 102, 1567–1579. [Google Scholar] [CrossRef]
- Chang-Geun, Y.; Byeoung-Ryeol, C.; Hyung-Man, P.; Chang-Gyu, P.; Young-Joon, A. Fumigant toxicity of plant essential oils to Thrips palmi (Thysanoptera: Thripidae) and Orius strigicollis (Heteroptera: Anthocoridae). J. Econ. Entomol. 2006, 99, 1733–1738. [Google Scholar] [CrossRef]
- Koundal, R.; Dolma, S.K.; Chand, G.; Agnihotri, V.K.; Reddy, S. Chemical composition and insecticidal properties of essential oils against diamondback moth (Plutella xylostella L.). Toxin Rev. 2018, 39, 371–381. [Google Scholar] [CrossRef]
- Koschier, E.H. Essential oil compounds for thrips control—A review. Nat. Prod. Commun. 2008, 3, 1171–1182. [Google Scholar] [CrossRef]
- Saroukolai, A.T.; Nouri-Ganbalani, G.; Hadian, J.; Rafiee-Dastjerdi, H. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Protect. Sci. 2014, 50, 207–216. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Inhibition of reproduction of Acanthoscelides obtectus Say (Coleoptera), a kidney bean (Phaseolus vulgaris) bruchid, by aromatic essential oils. Crop Prot. 1994, 13, 624–628. [Google Scholar] [CrossRef]
- Wang, Y.; An, Z.; Zhen, C.; Liu, Q.Z.; Shi, W.P. Composition of the essential oil of Cynanchum mongolicum (Asclepiadaceae) and insecticidal activities against Aphis glycines (Hemiptera: Aphidiae). Pharmacogn. Mag. 2014, 10, S130–S134. [Google Scholar] [CrossRef]
- Wagan, T.A.; Nawaz, M.; Khan, M.M.; Hua, H.X.; Zhao, J. Bioactivity of three plants’ essential oils against Aphis glycines. J. Kans. Entomol. Soc. 2018, 91, 71–84. [Google Scholar] [CrossRef]
- Sanini, C.; Massarolli, A.; Krinski, D.; Butnariu, A.R. Essential oil of spiked pepper, Piper aduncum L. (Piperaceae), for the control of caterpillar soybean looper, Chrysodeixis includens Walker (Lepidoptera: Noctuidae). Braz. J. Bot. 2017, 40, 399–404. [Google Scholar] [CrossRef]
- Santos, N.C.; De Silva, J.E.; Santos, A.C.C.; Dantas, J.O.; Tavares, S.R.S.A.; Andrade, V.S.; Oliveira, S.D.S.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Bioactivity of essential oils from Croton grewioides and its major compounds: Toxicity to soybean looper Chrysodeixis includens and selectivity to the predatory stink bug Podisus nigrispinus. Environ. Sci. Pollut. Res. 2022, 30, 18798–18809. [Google Scholar] [CrossRef] [PubMed]
- González, J.O.W.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Biological activity of essential oils from Aloysia polystachya and Aloysia citriodora (Verbenaceae) against the soybean pest Nezara viridula (Hemiptera: Pentatomidae). Nat. Prod. Commun. 2010, 5, 301–306. [Google Scholar] [CrossRef]
- Werdin, G.J.O.; Gutiérrez, M.M.; Murray, A.P.; Ferrero, A.A. Composition and biological activity of essential oils from Labiatae against Nezara viridula (Hemiptera: Pentatomidae) soybean pest. Pest. Manag. Sci. 2011, 67, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Cossolin, J.F.S.; Pereira, M.J.B.; Martínez, L.C.; Turchen, L.M.; Fiaz, M.; Bozdoğan, H.; Serrão, J.E. Cytotoxicity of Piper aduncum (Piperaceae) essential oil in brown stink bug Euschistus heros (Heteroptera: Pentatomidae). Ecotoxicology 2019, 28, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.C.; Zanuncio, T.V.; Ramalho, F.S.; Da Silva, C.A.D.; Serrão, J.E.; Zanuncio, J.C. Feeding and oviposition of Anticarsia gemmatalis (Lepidoptera: Noctuidae) with sublethal concentrations of ten condiments essential oils. Ind. Crop. Prod. 2015, 74, 139–143. [Google Scholar] [CrossRef]
- Myrtsi, E.D.; Koulocheri, S.D.; Evergetis, E.; Haroutounian, S.A. Agro-industrial co-products upcycling: Recovery of carotenoids and fine chemicals from Citrus sp. juice industry co-products. Ind. Crop. Prod. 2022, 186, 115190. [Google Scholar] [CrossRef]
- Nahar, L.; El-Seedi, H.R.; Khalifa, S.A.M.; Mohammadhosseini, M.; Sarker, S.D. Ruta essential oils: Composition and bioactivities. Molecules 2021, 26, 4766. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of Citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus by-products into value added products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Atiq, H.T.; Koraqi, H.; Javaid, Z.; Alhag, S.K.; Al-Shuraym, L.A.; Bader, D.M.D.; Almarzuq, M.; Afifi, M.; et al. Citrus waste as source of bioactive compounds: Extraction and utilization in health and food industry. Molecules 2023, 28, 1636. [Google Scholar] [CrossRef]
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Liu, Y.B. Anisole is an environmentally friendly fumigant for postharvest pest control. J. Stored Prod. Res. 2021, 93, 101842. [Google Scholar] [CrossRef]
- Bissim, S.; Kenmogne, S.B.; Tcho, A.T.; Lateef, M.; Ahmed, A.; Happi, E.N.; Wansi, J.D.; Ali, M.S.; Waffo, A.F.K. Bioactive acridone alkaloids and their derivatives from Citrus aurantium (Rutaceae). Phytochem. Lett. 2019, 29, 148–153. [Google Scholar] [CrossRef]
- Jian, R.C.; Lin, Y.; Li, Y.L.; Wu, W.F.; Ren, X.F.; Liang, Z.Y.; Kong, L.J.; Cai, J.L.; Lao, C.Y.; Wu, M.; et al. Larvicidal activity of two Rutaceae plant essential oils and their constituents against Aedes albopictus (Diptera: Culicidae) in multiple formulations. J. Med. Entomol. 2022, 59, 1669–1677. [Google Scholar] [CrossRef]
- Mayura, S.; Siriporn, P. Adulticidal, larvicidal, pupicidal and oviposition deterrent activities of essential oil from Zanthoxylum limonella Alston (Rutaceae) against Aedes aegypti (L.) and Culex quinquefasciatus (Say). Asian Pac. J. Trop. Biomed. 2017, 7, 967–978. [Google Scholar] [CrossRef]
- Pereira, K.D.C.; Quintela, E.D.; Do Nascimento, V.A.; Da Silva, D.J.; Rocha, D.V.M.; Silva, J.F.A.; Arthurs, S.P.; Forim, M.R.; Silva, F.G.; Cazal, C.d.M. Characterization of Zanthoxylum rhoifolium (Sapindales: Rutaceae) essential oil nanospheres and insecticidal effects to Bemisia tabaci (Sternorrhyncha: Aleyrodidae). Plants 2022, 11, 1135. [Google Scholar] [CrossRef]
- Goudoum, A.; Ngamo, L.S.; Ngassoum, M.B.; Tatsadjieu, L.N.; Mbofung, C.M. Tribolium castaneum (Coleoptera: Curculionidae) sensitivity to repetitive applications of lethal doses of imidacloprid and extracts of Clausena anisata (Rutaceae) and Plectranthus glandulosus (Lamiaceae). Int. J. Biol. Chem. Sci. 2010, 4, 1242–1250. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Do Nascimento, A.F.; Monteiro, V.B.; De Moraes, M.M. Larvicidal, ovicidal and antifeedant activities of essential oils and constituents against Spodoptera frugiperda. Arch. Phytopathol. Plant Prot. 2022, 55, 851–873. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; Awad, M.; Amer, A.; Hassan, N.N.; Ibrahim, E.-D.S.; Ali, H.M.; Akrami, M.; Salem, M.Z.M. Insecticidal activity of lemongrass essential oil as an eco-friendly agent against the black cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). Insects 2021, 12, 737. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Okunowo, W.O.; Osuntoki, A.A.; Olabode, T.B.; Ayo-Folorunso, F. Insecticidal and biochemical activity of essential oil from Citrus sinensis peel and constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. [Google Scholar] [CrossRef]
- Liu, Z.L. Chemical composition and toxicity of essential oil of Boenninghausenia sessilicarpa (Rutaceae) against two grain storage insects. J. Med. Plants Res. 2012, 6, 2920–2924. [Google Scholar] [CrossRef]
- Moshrefi, Z.Z.; Soltaninezhad, B.; Hashemi, M.; Noori, S.M.A. A review of effective essential oils and their biologically active compounds to protect the safety of food stored against insect pests. J. Essent. Oil Res. 2022, 34, 111–122. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito Machado, D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and chemodiversity approaches: New insights on modern study of plant secondary metabolite diversity at different spatiotemporal and organizational scales. Rev. Bras. Farmacogn. 2003, 33, 49–72. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tomou, E.M.; Goula, K.; Dimakopoulou, K.; Tzortzakis, N.; Skaltsa, H.; Sideritis, L. essential oils: A systematic review. Phytochemistry 2023, 4, 113607. [Google Scholar] [CrossRef]
- Chi, P.T.L.; Van Hung, P.; Le Thanh, H.; Phi, N.T.L. Valorization of citrus leaves: Chemical composition, antioxidant and antibacterial activities of essential oils. Waste Biomass Valorization 2020, 11, 4849–4857. [Google Scholar] [CrossRef]
- Meryem, S.; Mohamed, D.; Nour-eddine, C.; Faouzi, E. Chemical composition, antibacterial and antioxidant properties of three Moroccan citrus peel essential oils. Sci. Afr. 2023, 20, e01592. [Google Scholar] [CrossRef]
- Manzur, M.; Luciardi, M.C.; Blázquez, M.A.; Alberto, M.R.; Cartagena, E.; Arena, M.E. Citrus sinensis essential oils an innovative antioxidant and antipathogenic dual strategy in food preservation against spoliage bacteria. Antioxidants 2023, 12, 246. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, B.N.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Liang, S.; Hu, W.; Cheng, W.; Zhang, S.; Zou, R. Zanthoxylum bungeanum essential oil: Extraction and component analysis for α-glucosidase inhibitory activity and the underlying mechanism based on molecular docking. Appl. Sci. 2023, 13, 2627. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Labarrere, B.; Prinzing, A.; Dorey, T.; Chesneau, E.; Hennion, F. Variations of secondary metabolites among natural populations of sub-Antarctic ranunculus species suggest functional redundancy and versatility. Plants 2019, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Devi, M.L.; Biona, T.; Sharma, N.; Das, S.; Chakravorty, J.; Mukherjee, P.K.; Rajashekar, Y. Phytochemical composition and antimicrobial activity of essential oil from the leaves of Artemisia vulgaris L. Molecules 2023, 28, 2279. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Martínez, R.M.; Cases, M.A.; Sotomayor, J.A. Watering level effect on Thymus hyemalis Lange essential oil yield and composition. J. Agric. Food Chem. 2003, 51, 5420–5427. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Firoznezhad, M.; Craker, L.; Akbarzadeh, M. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia, at two phenological stages. Rev. Bras. Farmacogn. 2013, 23, 861–869. [Google Scholar] [CrossRef]
- de Andrade Rodrigues, R.M.B.; da Silva Fontes, L.; de Carvalho Brito, R.; Barbosa, D.R.S.; das Graças Lopes Citó, A.M.; do Carmo, I.S.; de Jesus Sousa, E.M.; Silva, G.N. A sustainable approach in the management of Callosobruchus maculatus: Essential oil of Protium heptaphyllum and its major compound D-limonene as biopesticides. J. Plant Dis. Prot. 2022, 129, 831–841. [Google Scholar] [CrossRef]
- Qi, X.J.; Feng, Y.X.; Pang, X.; Du, S.S. Insecticidal and repellent activities of essential oils from seed and root of celery (Apium graveolens L.) against three stored product insects. J. Essent. Oil Bear. Plants 2021, 24, 1169–1179. [Google Scholar] [CrossRef]
- Wang, D.C.; Qiu, D.R.; Shi, L.N.; Pan, H.Y.; Li, Y.W.; Sun, J.Z.; Xue, Y.J.; Wei, D.S.; Li, X.; Zhang, Y.M.; et al. Identification of insecticidal constituents of the essential oils of Dahlia pinnata Cav. against Sitophilus zeamais and Sitophilus oryzae. Nat. Prod. Res. 2015, 29, 1748–1751. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, N.; Chen, H.L.; Zhong, B.L.; Yang, A.X.; Kuang, F.; Ouyang, Z.G.; Chun, J. Fumigant activity of sweet orange essential oil fractions against red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2017, 110, 1556–1562. [Google Scholar] [CrossRef]
- Liang, J.-Y.; An, Y.; Hou, Z.-B.; Wang, X.-D.; Zhou, F.; Zhang, J.; Wang, J.-L. Acute toxicity of Zanthoxylum bungeanum against two stored product insects and synergistic interactions between two major compounds limonene and linalool. J. Environ. Sci. Health B 2022, 57, 739–744. [Google Scholar] [CrossRef]
- Masakazu, M.; Isamu, N.; Hirosuke, Y. Insecticidal joint action of pipercide and co-occurring compounds isolated from Piper nigrum L. Agric. Biolog. Chem. 1980, 44, 1701–1703. [Google Scholar] [CrossRef]
- Bekele, J.; Hassanali, A. Blend effects in the toxicity of the essential oil constituents of Ocimum kilimandscharicum and Ocimum kenyense (Labiateae) on two postharvest insect pests. Phytochemistry 2001, 57, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Scott-Brown, A.S.; Arnold, S.E.J.; Kite, G.C.; Farrell, I.W.; Farman, D.I.; Collins, D.W.; Stevenson, P.C. Mechanisms in mutualisms: A chemically mediated thrips pollination strategy in common elder. Planta 2019, 250, 367–379. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Mitchell, V.J.; McLaren, G.F.; Manning, L.M.; Bunn, B.; Suckling, D.M. Attraction of the New Zealand flower thrips, Thrips obscuratus, to cis-jasmone, a volatile identified from Japanese honeysuckle flowers. J. Chem. Ecol. 2009, 35, 656–663. [Google Scholar] [CrossRef]
- Koschier, E.H.; De Kogel, W.J.; Visser, J.H. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2000, 26, 2643–2655. [Google Scholar] [CrossRef]
- Katerinopoulos, H.E.; Pagona, G.; Afratis, A.; Stratigakis, N.; Roditakis, N. Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J. Chem. Ecol. 2005, 31, 111–122. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, J.J.; Qin, Y.C.; Pan, P.L.; Tu, H.T.; Du, W.X.; Zhou, W.F.; Baxendale, F.P. Reducing whiteflies on cucumber using intercropping with less preferred vegetables. Entomol. Exp. Appl. 2013, 150, 19–27. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Akhtar, Y.; Isman, M.B.; Seffrin, R.C.; Born, F.S. Repellent activity of essential oils from two species of Citrus against Tetranychus urticae in the laboratory and greenhouse. Crop. Prot. 2015, 74, 110–115. [Google Scholar] [CrossRef]
- Sun, X.L.; Wang, G.C.; Cai, X.M.; Jin, S.; Gao, Y.; Chen, Z.M. The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics. J. Chem. Ecol. 2010, 36, 388–395. [Google Scholar] [CrossRef]
- Rachid, B.; Abdelhalim, M.; Karim, E.-F.; Ali, O.; Abdelhadi, S.; Abderrahim, A.; Mustapha, E.-B. Chemical composition, and insecticidal activities of four plant essential oils from Morocco against larvae of Helicoverpa armigera (Hub.) under field and laboratory conditions. Crop Prot. 2021, 144, 105607. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. α-humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, C.; El Fakhouri, K.; Sbaghi, M.; Bouharroud, R.; Boulamtat, R.; Aasfar, A.; Mesfioui, A.; El Bouhssini, M. Chemical composition and insecticidal potential of six essential oils from morocco against Dactylopius opuntiae (Cockerell) under Field and laboratory conditions. Insects 2021, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Silva, G.T.; Figueiredo, K.G.; Alves, D.S.; de Oliveira, D.F.; Silva, G.H.; de Souza e Silva, G.T.; de Oliveira, M.S.; Biondi, A.; Carvalho, G.A. Survival and demography of the tomato borer (Tuta absoluta) exposed to citrus essential oils and major compounds. Agriculture 2023, 13, 538. [Google Scholar] [CrossRef]
- Gao, Y.; Ding, N.; Wang, D.; Zhao, Y.J.; Cui, J.; Li, W.B.; Pei, T.H.; Shi, S.S. Effect of temperature on the development and reproduction of Thrips flavus (Thysanoptera: Thripidae). Agric. Forest Entomol. 2022, 24, 279–288. [Google Scholar] [CrossRef]
- Huang, X.; Ge, S.Y.; Liu, J.H.; Wang, Y.; Liang, X.Y.; Yuan, H.B. Chemical composition and bioactivity of the essential oil from Artemisia lavandulaefolia (Asteraceae) on Plutella xylostella (Lepidoptera: Plutellidae). Fla. Entomol. 2018, 101, 44–48. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Sun, X.L.; Xin, Z.J.; Luo, Z.X.; Gao, Y.; Bian, L.; Chen, Z.M. Identification and field evaluation of non-host volatiles disturbing host location by the tea geometrid, Ectropis obliqua. J. Chem. Ecol. 2013, 39, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-Y.; Zhang, C.-X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
| No. | Compounds 1 | Relative Percentage (%) | ||||
|---|---|---|---|---|---|---|
| Citrus Oil (Citrus reticulata Blanco) | Chuan-Shan Pepper Oil (Zanthoxylum piasezkii Maxim.) | Zanthoxylum Oil (Zanthoxylum bungeanum Maxim.) | Pomelo Peel Oil (Citrus maxima (Burm.) Merr.) | Orange Leaf Oil (Citrus sinensis (L.) Osbeck) | ||
| 1 | Alpha-pinene | 0.899 | 0.772 | 1.100 | ||
| 2 | Sabenene | 0.573 | 8.291 | 17.131 | 0.507 | |
| 3 | (1S)-(1)-Beta-pinene | 0.336 | 1.421 | |||
| 4 | Beta-myrcene | 2.912 | 1.874 | 3.698 | 2.811 | |
| 5 | 1,1′-Oxydi-2-propanol | 0.853 | ||||
| 6 | 2,2′-Oxydipropanol | 0.750 | ||||
| 7 | p-Cymene | 0.289 | ||||
| 8 | 2-(2-Hydroxypropoxy)-1-propanol | 1.045 | ||||
| 9 | d-limonene | 95.617 | 11.832 | 22.145 | 78.236 | |
| 10 | Gamma-terpinene | 1.853 | ||||
| 11 | Linalool | 64.101 | 52.690 | 8.018 | ||
| 12 | Styralyl acetate | 1.760 | ||||
| 13 | Alpha-terpineol | 0.545 | ||||
| 14 | Linalyl anthranilate | 1.073 | 1.145 | 5.468 | 0.441 | |
| 15 | (–)-Isocaryophyllene | 0.658 | ||||
| 16 | 2-Methyl-4-phenyl-2-butanol | 3.461 | ||||
| 17 | Beta-caryophyllene | 0.864 | ||||
| 18 | Jasmone | 0.367 | ||||
| 19 | Allyl cyclohexylpropionate | 0.660 | ||||
| 20 | Butylated hydroxytoluene | 5.348 | ||||
| 21 | 2-(Oct-2-enyl) cyclopentan-1-one | 0.861 | ||||
| 22 | Methyl jasmonate | 51.502 | ||||
| 23 | Methyl dihydrojasmonate | 14.477 | ||||
| 24 | Alpha-hexyl cinnamaldehyde | 13.328 | ||||
| 25 | Phenethyl phenylacetate | 4.897 | ||||
| 26 | Germacrene D | 1.834 | 1.424 | |||
| 27 | Methyl (3Z,7E,10E)-3,7,10,12-tridecatetraenoate | 5.686 | ||||
| 28 | 1,5-Cyclooctadiene, 3-(1-Methyl-2-propen-1-yl)- | 4.446 | ||||
| Essential Oils | LC50 (g/L) | 95% Confidence Interval | Regression Equation | Related Coefficient | χ2 | p-Value |
|---|---|---|---|---|---|---|
| Orange leaf oil | 0.26 | 0.11~0.37 | y = 5.9807 + 1.6867x | 0.8381 | 4.7591 | 0.1903 |
| Zanthoxylum oil | 0.27 | 0.16~0.36 | y = 6.3029 + 2.3067x | 0.8581 | 5.768 | 0.1235 |
| Pomelo peel oil | 0.44 | 0.35~0.51 | y = 6.2064 + 3.3408x | 0.9353 | 5.4535 | 0.1414 |
| Chuan-shan pepper oil | 0.58 | 0.51~0.69 | y = 5.9531 + 4.0840x | 0.9426 | 7.4438 | 0.0590 |
| Citrus oil | 2.73 | 1.98~3.90 | y = 4.2585 + 1.7001x | 0.7511 | 10.7618 | 0.0131 |
| Essential Oils | Concentration Gradients (g a.i.·hm−2) | Control Efficacy (%) | ||
|---|---|---|---|---|
| After 1 Day | After 3 Days | After 7 Days | ||
| Orange leaf oil | 180.00 | 16.05 ± 1.24 b | 24.95 ± 5.40 c | 69.44 ± 7.35 c |
| 360.00 | 38.27 ± 7.51 ab | 52.22 ± 5.88 b | 77.78 ± 3.68 bc | |
| 540.00 | 41.98 ± 6.88 ab | 56.67 ± 6.94 b | 93.06 ± 3.67 ab | |
| 720.00 | 64.20 ± 8.64 a | 67.78 ± 4.01 b | 97.22 ± 1.39 a | |
| 900.00 | 65.43 ± 2.47 a | 90.00 ± 3.85 a | 100.00 a | |
| Pomelo peel oil | 180.00 | 19.75 ± 7.51 d | 27.40 ± 5.97 c | 18.37 ± 5.40 d |
| 360.00 | 28.40 ± 4.45 cd | 54.79 ± 2.37 bc | 44.89 ± 1.94 cd | |
| 540.00 | 45.68 ± 4.94 bc | 76.71 ± 8.98 ab | 48.31 ± 2.18 c | |
| 720.00 | 58.02 ± 3.27 b | 82.19 ± 3.62 ab | 83.68 ± 4.08 b | |
| 900.00 | 90.13 ± 3.27 a | 95.89 ± 4.11 a | 100.00 a | |
| Zanthoxylum oil | 180.00 | 15.54 ± 6.24 c | 34.94 ± 4.17 b | 48.10 ± 2.53 c |
| 360.00 | 32.14 ± 2.06 bc | 28.92 ± 2.41 b | 46.84 ± 2.19 c | |
| 540.00 | 39.29 ± 9.45 bc | 54.22 ± 3.19 ab | 69.62 ± 4.38 bc | |
| 720.00 | 60.71 ± 2.06 ab | 69.88 ± 6.38 a | 87.34 ± 4.56 b | |
| 900.00 | 71.43 ± 5.46 a | 81.93 ± 9.56 a | 98.73 ± 1.27 a | |
| Chuan-shan pepper oil | 180.00 | 16.67 ± 2.38 b | 21.69 ± 2.41 c | 34.18 ± 3.35 c |
| 360.00 | 34.52 ± 3.15 ab | 43.37 ± 2.41 b | 51.9 ± 3.35 b | |
| 540.00 | 35.71 ± 4.12 a | 54.22 ± 4.34 ab | 62.02 ± 4.38 b | |
| 720.00 | 32.14 ± 3.57 ab | 48.19 ± 2.41 ab | 62.03 ± 2.19 b | |
| 900.00 | 34.52 ± 6.63 ab | 59.04 ± 3.19 a | 82.28 ± 3.35 a | |
| Citrus oil | 900.00 | 5.95 ± 3.15 b | 59.04 ± 8.69 a | 53.16 ± 10.13 a |
| 1800.00 | 26.19 ± 3.15 ab | 48.19 ± 6.71 a | 54.43 ± 5.80 a | |
| 2700.00 | 44.05 ± 6.30 a | 56.63 ± 4.17 a | 59.49 ± 5.06 a | |
| 3600.00 | 48.81 ± 7.24 a | 54.22 ± 6.38 a | 50.63 ± 9.56 a | |
| 4500.00 | 46.43 ± 9.45 a | 54.22 ± 9.64 a | 62.03 ± 4.38 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, T.-H.; Zhao, Y.-J.; Wang, S.-Y.; Li, X.-F.; Sun, C.-Q.; Shi, S.-S.; Xu, M.-L.; Gao, Y. Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae). Molecules 2023, 28, 2998. https://doi.org/10.3390/molecules28072998
Pei T-H, Zhao Y-J, Wang S-Y, Li X-F, Sun C-Q, Shi S-S, Xu M-L, Gao Y. Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae). Molecules. 2023; 28(7):2998. https://doi.org/10.3390/molecules28072998
Chicago/Turabian StylePei, Tian-Hao, Yi-Jin Zhao, Sheng-Yuan Wang, Xiao-Feng Li, Chen-Qi Sun, Shu-Sen Shi, Meng-Lei Xu, and Yu Gao. 2023. "Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae)" Molecules 28, no. 7: 2998. https://doi.org/10.3390/molecules28072998
APA StylePei, T.-H., Zhao, Y.-J., Wang, S.-Y., Li, X.-F., Sun, C.-Q., Shi, S.-S., Xu, M.-L., & Gao, Y. (2023). Preliminary Study on Insecticidal Potential and Chemical Composition of Five Rutaceae Essential Oils against Thrips flavus (Thysanoptera: Thripidae). Molecules, 28(7), 2998. https://doi.org/10.3390/molecules28072998