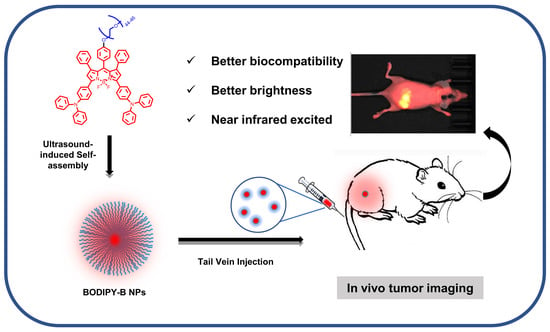
Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging
Abstract
1. Introduction
2. Results and Discussion
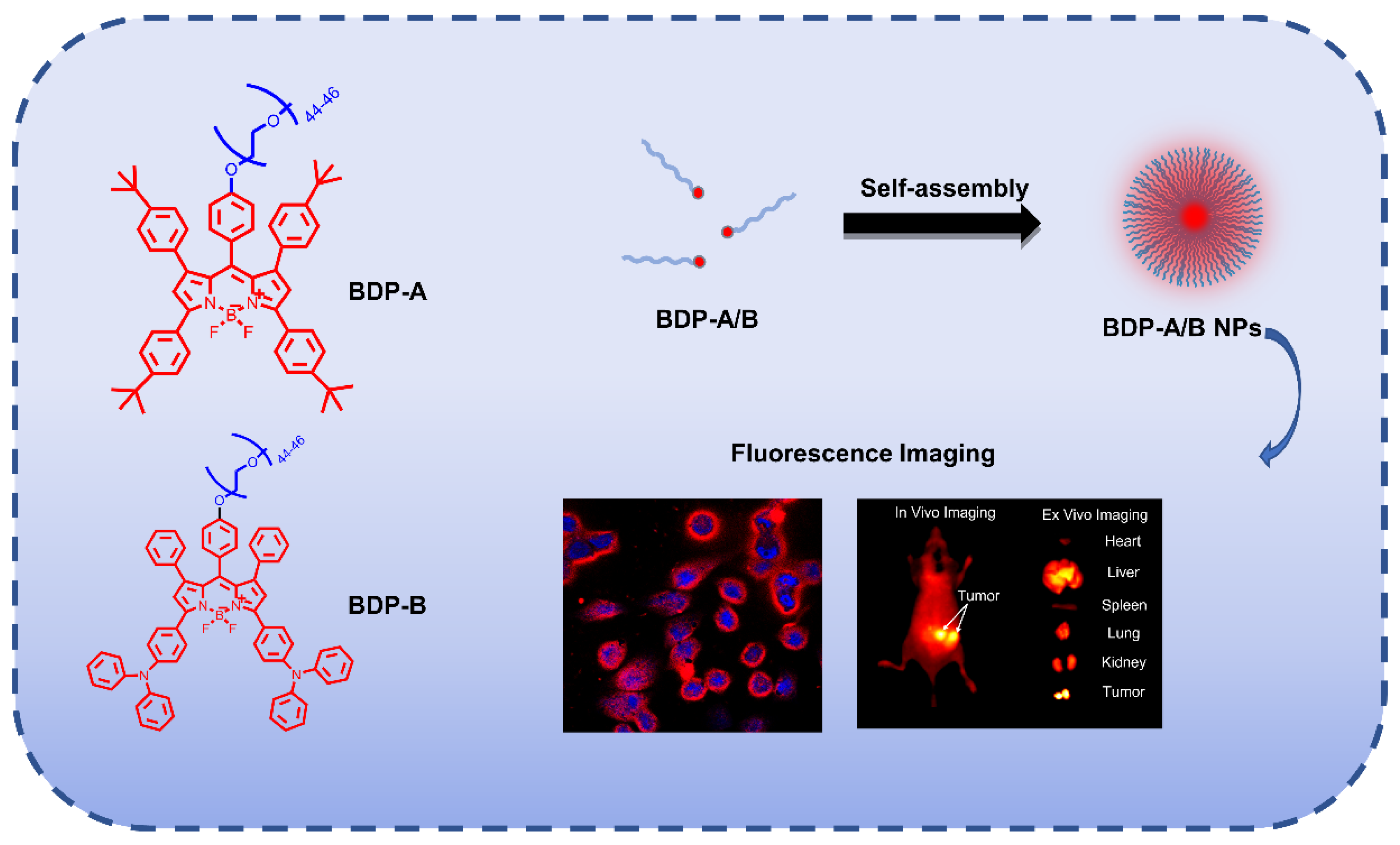
2.1. Synthesis and Characterization
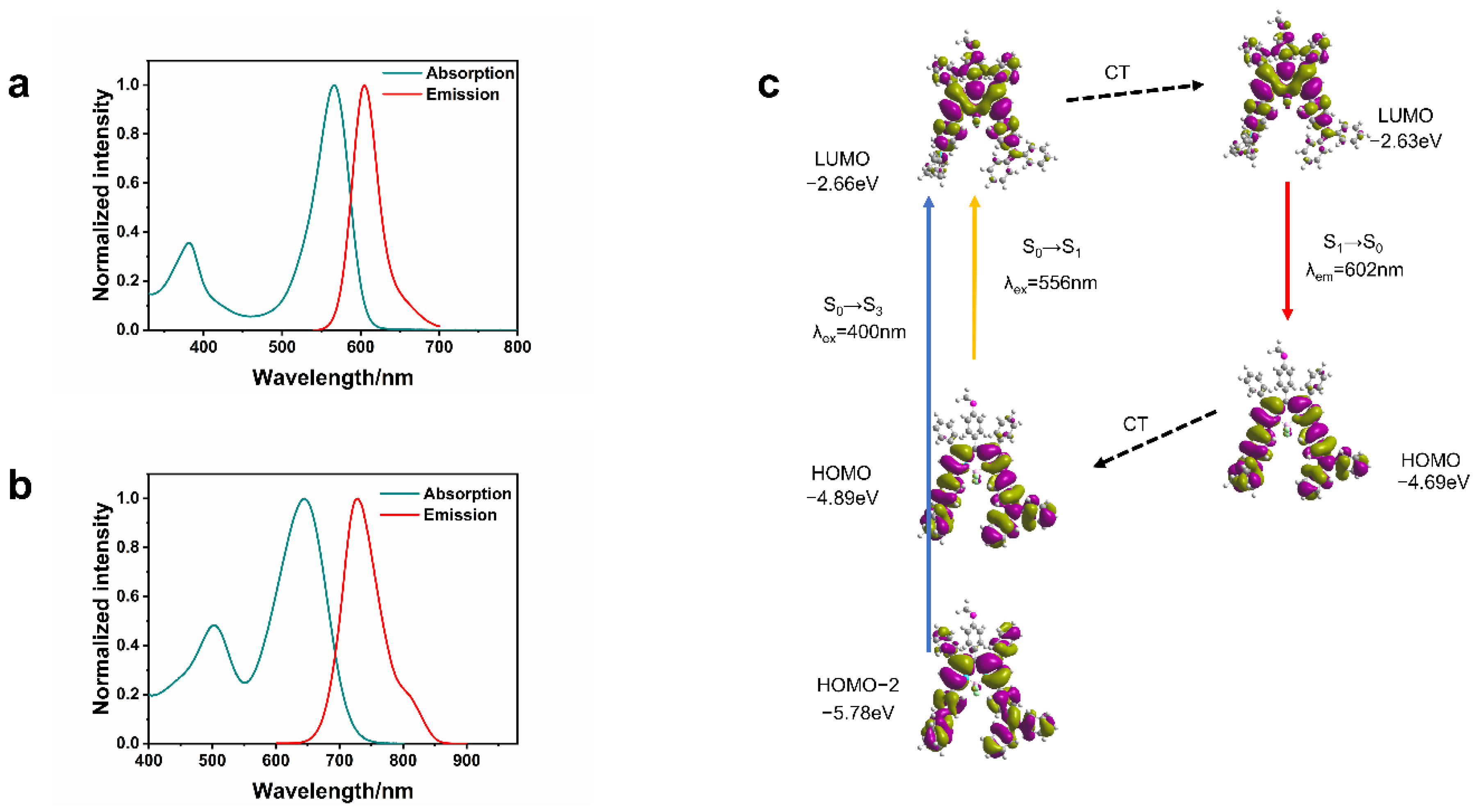
2.2. Absorption and Fluorescence
2.3. Theoretical Calculation
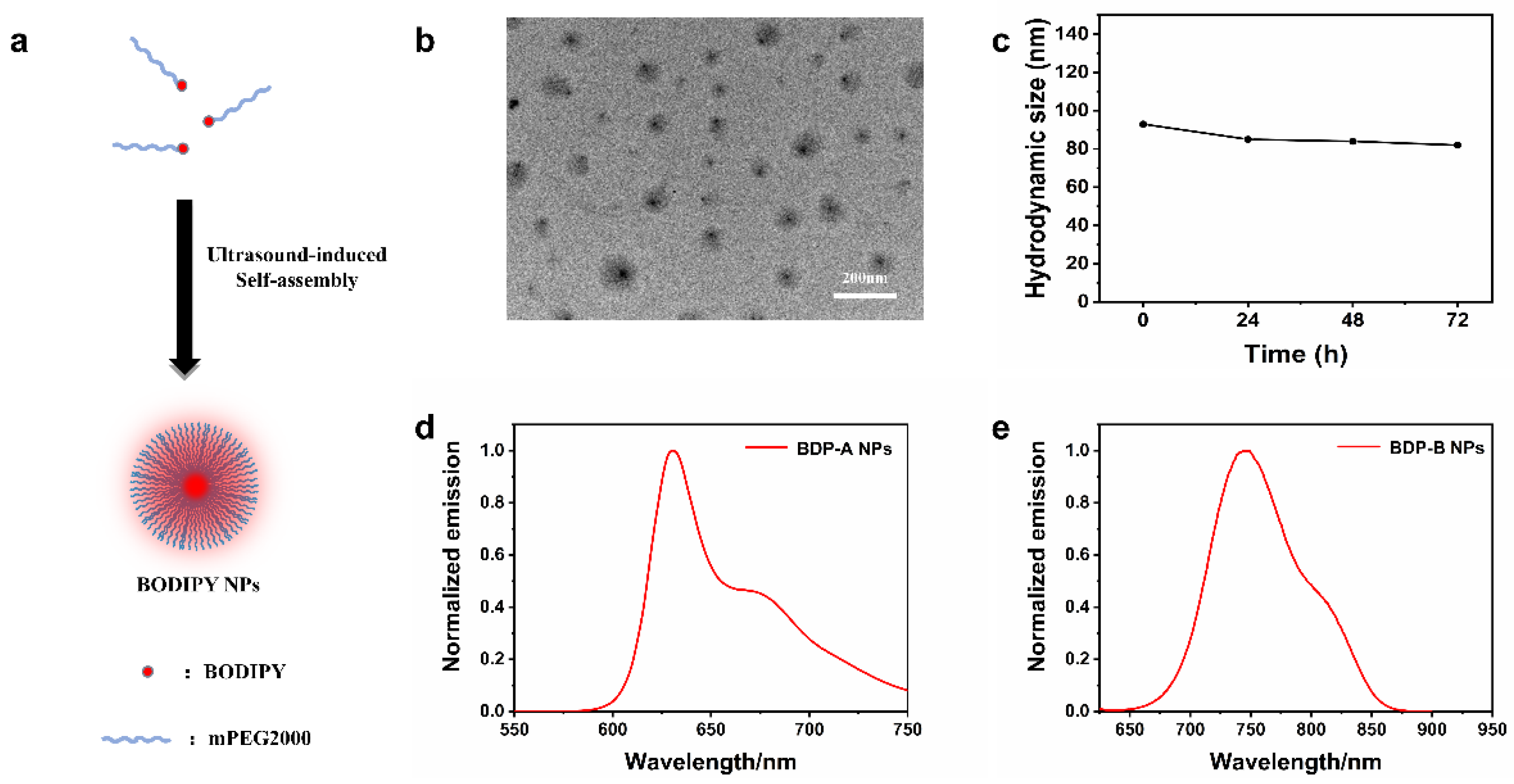
2.4. Self-Assembly and Characterization
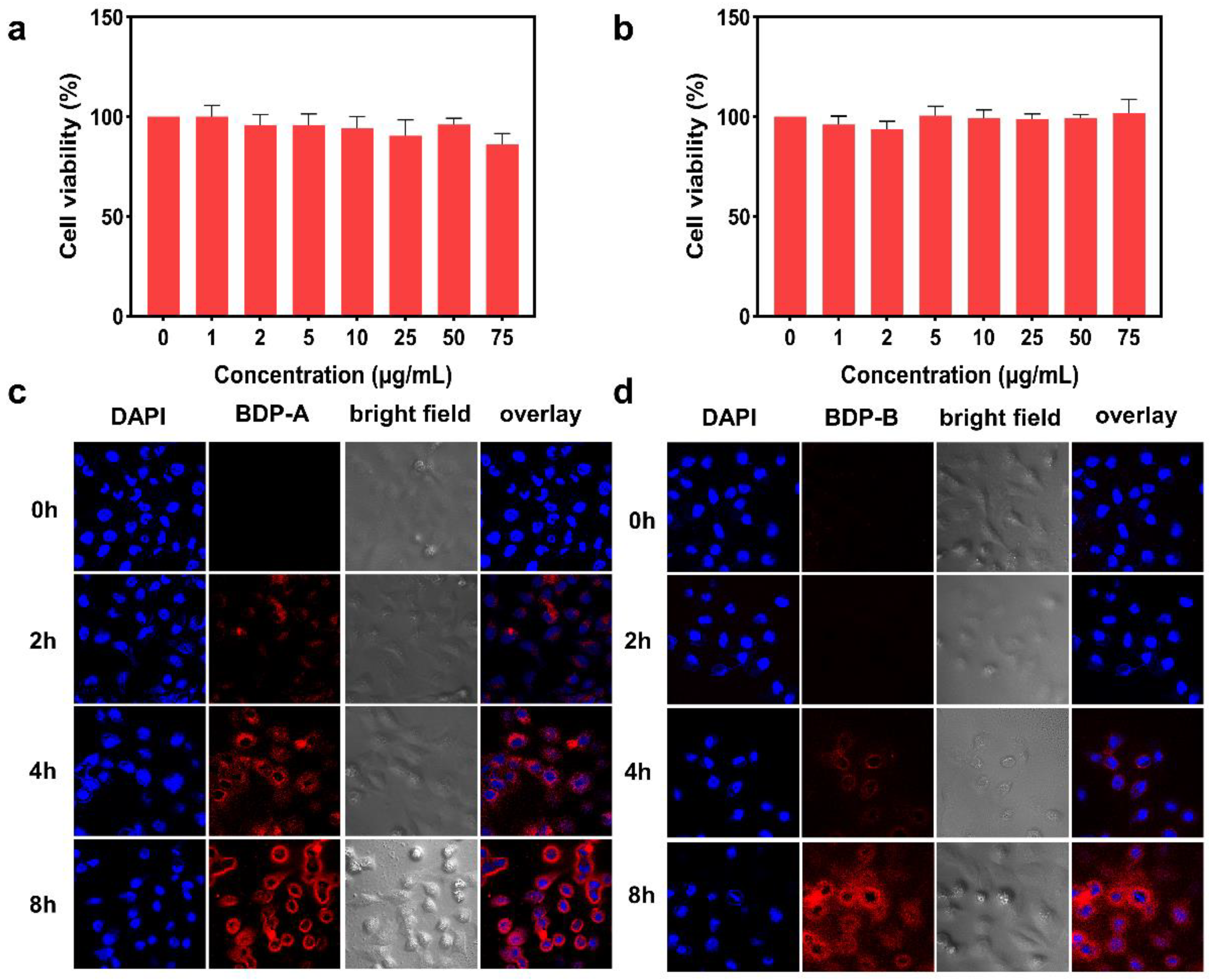
2.5. Biocompatibility Assessments of BDP-A/B NPs
2.6. Monitoring BDP-A/B NPs in Cells
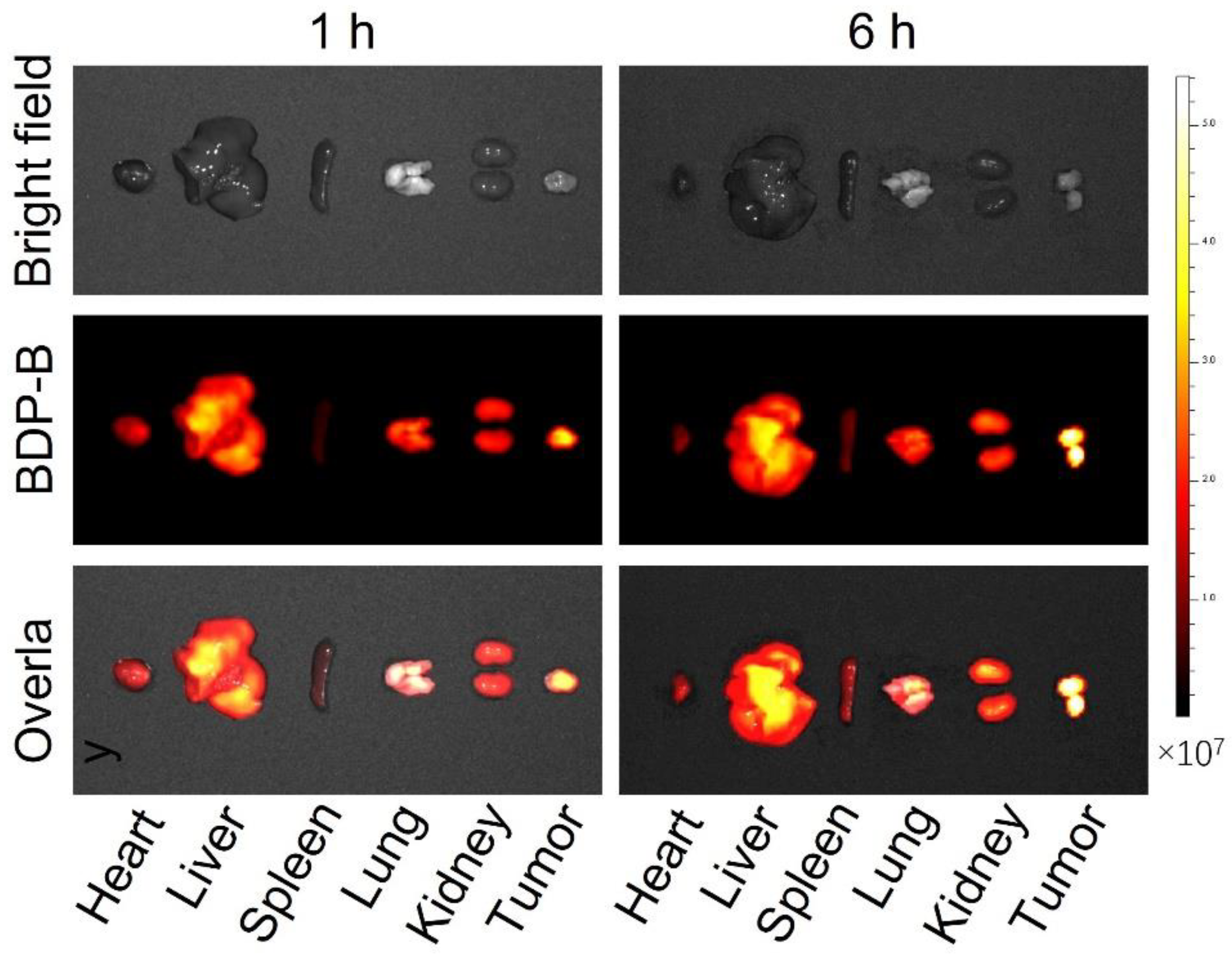
2.7. In Vivo Fluorescence Imaging of Mice Tumors
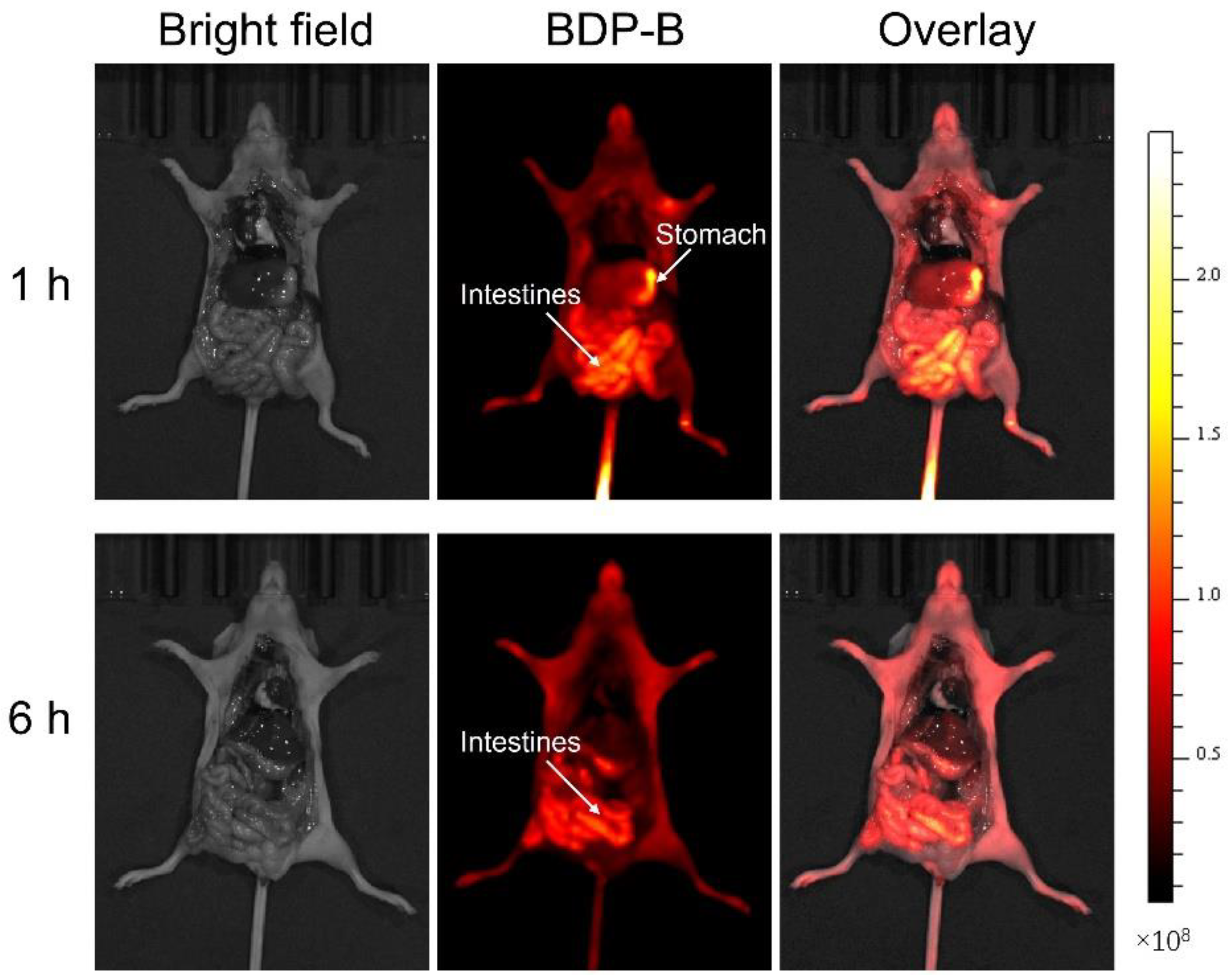
2.8. In Vivo Metabolism of BDP-B NPs
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, Y.; Shi, W.; Li, X.; Ma, H. Recent Advances in Fluorescent Probes for Lipid Droplets. Chem. Commun. 2022, 58, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Fam, T.K.; Klymchenko, A.; Collot, M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials 2018, 11, 1768. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, X.; Liu, Y.; Xu, Y.; Zhang, J.; Huang, F.; Li, B.; Miao, Y.; Sun, Y.; Li, Y. Dual-Light Triggered Metabolizable Nano-Micelles for Selective Tumor-Targeted Photodynamic/Hyperthermia Therapy. Acta Biomater. 2021, 119, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent Progress in the Development of near-Infrared Fluorescent Probes for Bioimaging Applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef]
- Hande, P.E.; Shelke, Y.; Datta, A.; Gharpure, S.J. Recent Advances in Small Molecule-Based Intracellular Ph Probes. Chembiochem 2022, 23, e202100448. [Google Scholar] [CrossRef]
- Dartar, S.; Ucuncu, M.; Karakus, E.; Hou, Y.; Zhao, J.; Emrullahoglu, M. Bodipy-Vinyl Dibromides as Triplet Sensitisers for Photodynamic Therapy and Triplet-Triplet Annihilation Upconversion. Chem. Commun. 2021, 57, 6039–6042. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators Based on Bodipy. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Z.; Song, C.; Tang, C.; Han, W.; Dong, X. Optical Nano-Agents in the Second near-Infrared Window for Biomedical Applications. Chem. Soc. Rev. 2019, 48, 22–37. [Google Scholar] [CrossRef]
- Lu, B.; Shuxian, M.; Feng, Y. Progress of Fluorescent Bio-Probe Based on Water-Soluble Boron-Dipyrromethene. Chin. J. Org. Chem. 2018, 38, 350. [Google Scholar] [CrossRef]
- Wang, L.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Recent Advances of Nir Dyes of Pyrrolopyrrole Cyanine and Pyrrolopyrrole Aza-Bodipy: Synthesis and Application. Dye. Pigment. 2022, 198, 110040. [Google Scholar] [CrossRef]
- Yin, J.F.; Hu, Y.; Wang, H.; Jin, Z.; Zhang, Y.; Kuang, G.C. Near-Infrared-Emissive Amphiphilic Bodipy Assemblies Manipulated by Charge-Transfer Interaction: From Nanofibers to Nanorods and Nanodisks. Chem. Asian J. 2017, 12, 3088–3095. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Lin, Y.; Yang, L.; Gao, F.; Zhao, Y.; Qiao, Z.; Zhao, Q.; Fan, Y.; Chen, Z.; Wang, H. Co-Self-Assembled Nanoaggregates of Bodipy Amphiphiles for Dual Colour Imaging of Live Cells. Chem. Commun. 2015, 51, 12447–12450. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Sun, Y.; Das, A.; Stang, P.; Lee, C.Y. Bodipy Based Metal-Organic Macrocycles and Frameworks: Recent Therapeutic Developments. Coord. Chem. Rev. 2022, 45, 124308. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Fan, Y.; Sun, Y.; Zhang, F. Beyond 1000 nm Emission Wavelength: Recent Advances in Organic and Inorganic Emitters for Deep-Tissue Molecular Imaging. Adv. Healthcare Mater. 2019, 8, e1900260. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, J.; Otsuka, Y.; Zhang, S.; Takahashi, M.; Yamada, K. A Bodipy-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials 2020, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Q.; Zou, X.; Chen, M.; Feng, W.; Shi, Y.; Li, F. Near-Infrared in Vivo Bioimaging Using a Molecular Upconversion Probe. Chem. Commun. 2016, 52, 7466–7469. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, H.; Guang, S.H.; Baev, A.; Xia, J.; Huang, W.; Prasad, P.N. Organic Nir-Ii Photoacoustic Agent Utilizing Combined Two-Photon and Excited State Absorption at 1064 Nm. ACS Photon. 2020, 7, 3161–3165. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; Yetisen, A.; et al. Near-Infrared-Ii Nanoparticles for Cancer Imaging of Immune Checkpoint Programmed Death-Ligand 1 and Photodynamic/Immune Therapy. ACS Nano 2021, 15, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.R.; Efres, B.-R.; Pina, J.; da Silva, M.C.; Pinto, S.C.S.; Gallo, J.; Costa, S.P.G.; Manuela, M.; Raposo, M. Bioimaging of Lysosomes with a Bodipy Ph-Dependent Fluorescent Probe. Molecules 2022, 27, 8065. [Google Scholar] [CrossRef]
- Gurubasavaraj, P.M.; Sajjan, V.; Munoz-Flores, B.; Perez, V.J.; Hosmane, N.S. Recent Advances in Bodipy Compounds: Synthetic Methods, Optical and Nonlinear Optical Properties, and Their Medical Applications. Molecules 2022, 27, 1877. [Google Scholar] [CrossRef]
- Abuduwaili, W.; Wang, X.; Huang, A.-T.; Sun, J.-L.; Xu, R.-C.; Zhang, G.-C.; Liu, Z.-Y.; Wang, F.; Zhu, C.-F.; Liu, T.-T.; et al. Iridium Complex-Loaded Sorafenib Nanocomposites for Synergistic Chemo-Photodynamic Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2022, 14, 37356–37368. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe Von Di- Und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Huang, F.; Li, Y.; Liu, J.; Zhang, J.; Wang, X.; Li, B.; Chang, H.; Miao, Y.; Sun, Y. Intraperitoneal Injection of Cyanine-Based Nanomicelles for Enhanced near-Infrared Fluorescence Imaging and Surgical Navigation in Abdominal Tumors. ACS Appl. Bio Mater. 2021, 4, 5695–5706. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, W.; Liu, X.; Wang, S.; Wang, Y. Bodipy-Based Fluorescent Surfactant for Cell Membrane Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2020, 3, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-F.; Watanabe, M.; Takagaki, A.; Song, J.T.; Ishihara, T. Pyridyl-Anchored Type Bodipy Sensitizer-Tio2 Photocatalyst for Enhanced Visible Light-Driven Photocatalytic Hydrogen Production. Catalysts 2020, 10, 535. [Google Scholar] [CrossRef]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of Small Molecule Aza-Bodipy: From Rational Structural Design to in Vivo Investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.H.; Widmaier, J.; Ziessel, R. Near-Infrared Fluorescent Nanoparticles Formed by Self-Assembly of Lipidic (Bodipy) Dyes. Chemistry 2011, 17, 11709–11714. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Lv, Y.; Dong, J.; Xuan, G.; Yang, J.; Wu, D.; Zhou, J.; Yu, G.; Tang, G.; et al. Nanomedicine Fabricated from a Boron-Dipyrromethene (Bodipy)-Embedded Amphiphilic Copolymer for Photothermal-Enhanced Chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 4463–4473. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Huang, F.; Sun, Y.; Li, Y.; Miao, Y. Near-Infrared Frequency Upconversion Luminescence Bioimaging Based on Cyanine Nanomicelles. ACS Appl. Polym. Mater. 2022, 4, 5566–5573. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, F. Molecular Fluorophores for Deep-Tissue Bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. Bodipy Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Duan, X.; Jiang, Z.; Ding, D.; Chen, Y.; Zhang, G.; Liu, Z. J-Aggregates of Meso-[2.2]Paracyclophanyl-Bodipy Dye for Nir-Ii Imaging. Nat. Commun. 2021, 12, 2376. [Google Scholar] [CrossRef] [PubMed]
- Kowada, T.; Maeda, H.; Kikuchi, K. Bodipy-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fang, Y.; Feng, W.; Xia, Q.; Feng, G. A Colorimetric and Ratiometric Fluorescent Probe with Enhanced near-Infrared Fluorescence for Selective Detection of Cysteine and Its Application in Living Cells. Dye. Pigment. 2017, 146, 103–111. [Google Scholar] [CrossRef]
- Hinton, D.A.; Ng, J.; Sun, J.; Lee, S.; Saikin, S.; Logsdon, J.; White, D.; Marquard, A.; Cavell, A.; Krasecki, V.; et al. Mapping Forbidden Emission to Structure in Self-Assembled Organic Nanoparticles. J. Am. Chem. Soc. 2018, 140, 15827–15841. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Sun, P.; Liu, Y.; Zhang, H.; Hu, W.; Zhang, W.; Liu, Z.; Fan, Q.; Li, L.; Huang, W. Novel Aza-Bodipy Based Small Molecular Nir-Ii Fluorophores for in Vivo Imaging. Chem. Commun. 2019, 55, 10920–10923. [Google Scholar] [CrossRef]
- Bhattacharyya, U.; Verma, B.; Saha, R.; Mukherjee, N.; Raza, M.; Sahoo, S.; Kondaiah, P.; Chakravarty, A.R. Structurally Characterized Bodipy-Appended Oxidovanadium(Iv) Beta-Diketonates for Mitochondria-Targeted Photocytotoxicity. ACS Omega 2020, 5, 4282–4292. [Google Scholar] [CrossRef]
- Xiong, H.; Kos, P.; Yan, Y.; Zhou, K.; Miller, J.; Elkassih, S.; Siegwart, D.J. Activatable Water-Soluble Probes Enhance Tumor Imaging by Responding to Dysregulated Ph and Exhibiting High Tumor-to-Liver Fluorescence Emission Contrast. Bioconjug. Chem. 2016, 27, 1737–1744. [Google Scholar] [CrossRef]
- Wang, K.; Xiao, Y.; Wang, Y.; Feng, Y.; Chen, C.; Zhang, J.; Zhang, Q.; Meng, S.; Wang, Z.; Yang, H. Self-Assembled Hydrophobin for Producing Water-Soluble and Membrane Permeable Fluorescent Dye. Sci. Rep. 2016, 6, 23061. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Panja, S.; Ryu, J.Y.; Lee, J.; Mandal, N.; Lee, C.Y. Selective Cytotoxicity of Self-Assembled Bodipy Metalla-Rectangles: Evidence of P53-Dependent Apoptosis via Both Intrinsic and Extrinsic Pathways. Dye. Pigment. 2020, 180, 108478. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, B.; Liu, Y.; Hu, D.; Sheng, Z.; Zhang, X.; Yuan, Z. Molecular Engineering of near-Infrared Light-Responsive Bodipy-Based Nanoparticles with Enhanced Photothermal and Photoacoustic Efficiencies for Cancer Theranostics. Theranostics 2019, 9, 5315–5331. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Feng, W.; Zhai, Q.; Feng, G. Selenocysteine Detection and Bioimaging in Living Cells by a Colorimetric and near-Infrared Fluorescent Turn-on Probe with a Large Stokes Shift. Biosens. Bioelectron. 2017, 87, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural Modification Strategies for the Rational Design of Red/Nir Region Bodipys. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, F.; Zhang, J.; Liu, J.; Li, B.; Ouyang, R.; Miao, Y.; Sun, Y.; Li, Y. Pegylated Iridium-Based Nano-Micelle: Self-Assembly, Selective Tumor Fluorescence Imaging and Photodynamic Therapy. Dye. Pigment. 2020, 182, 108651. [Google Scholar] [CrossRef]
- Yuan, Y.-X.; Wu, B.-X.; Xiong, J.-B.; Zhang, H.-C.; Hu, M.; Zheng, Y.-S. Exceptional Aggregation-Induced Emission from One Totally Planar Molecule. Dye. Pigment. 2019, 170, 107556. [Google Scholar] [CrossRef]
- Gorbatov, S.A.; Uvarov, D.Y.; Scherbakov, A.M.; Zavarzin, I.V.; Volkova, Y.A.; Romieu, A. A Novel Water-Soluble Bodipy Dye as Red Fluorescent Probe for Imaging Hypoxic Status of Human Cancer Cells. Mendeleev Commun. 2020, 30, 750–752. [Google Scholar] [CrossRef]
- Hoji, A.; Turghun, M.; Muyasier, W.; Mukhtar, I.; Li, H.; Zulihumaer, A.; Peng, X. Syntheses of Bodipy-Incorporated Polymer Nanoparticles with Strong Fluorescence and Water Compatibility. Eur. Polym. J. 2020, 141, 110058. [Google Scholar] [CrossRef]
- Quan, L.; Liu, S.; Sun, T.; Guan, X.; Lin, W.; Xie, Z.; Huang, Y.; Wang, Y.; Jing, X. Near-Infrared Emitting Fluorescent Bodipy Nanovesicles for in Vivo Molecular Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 16166–16173. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Xiong, Z.; Ran, X.; Tang, H.; Cao, D. Design, Synthesis and Applications of Nir-Emissive Scaffolds of Diketopyrrolopyrrole-Aza-Bodipy Hybrids. Chem. Commun. 2022, 58, 5996–5999. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, X.; Zhang, L.; Yang, Z.; Chen, L.; Wang, L.; Liu, S.; Xie, Z. Red Fluorescent Pyrazoline-Bodipy Nanoparticles for Ultrafast and Long-Term Bioimaging. Org. Biomol. Chem. 2020, 18, 707–714. [Google Scholar] [CrossRef]


| λabs (nm) a | λem (nm) b | ΦF (%) c | τ (ns) d | Δν (nm) e | |
|---|---|---|---|---|---|
| BDP-A | 566 | 605 | 93.2 | 4.9 | 39 |
| BDP-A NPs | 576 | 631 | 19.8 | -- | 55 |
| BDP-B | 645 | 729 | 62.1 | 5.7 | 84 |
| BDP-B NPs | 657 | 748 | 12.3 | -- | 91 |
| Materials | Λem (nm) | ΦF (%) | Δν (nm) | Size (nm) |
|---|---|---|---|---|
| NBB [46] | 722 | 15 | 63 | - |
| π2PhAA [47] | 652 | 0.3 | 11 | 255.13 |
| Mpb NPs [48] | 673 | 8 | 30 | 57 |
| LAB-TH4 [49] | 660 | - | 27 | 86.5 |
| PZL-BDP [50] | 620 | 15.8 | 111 | 230.5 |
| BDP-A NPs | 631 | 19.8 | 55 | 90 ± 10 |
| BDP-B NPs | 748 | 12.3 | 91 | 90 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jiang, Z.; Huang, C.; Zhao, S.; Zhu, S.; Liu, R.; Zhu, H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules 2023, 28, 2997. https://doi.org/10.3390/molecules28072997
Wang J, Jiang Z, Huang C, Zhao S, Zhu S, Liu R, Zhu H. Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules. 2023; 28(7):2997. https://doi.org/10.3390/molecules28072997
Chicago/Turabian StyleWang, Jiale, Zhao Jiang, Cheng Huang, Shimao Zhao, Senqiang Zhu, Rui Liu, and Hongjun Zhu. 2023. "Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging" Molecules 28, no. 7: 2997. https://doi.org/10.3390/molecules28072997
APA StyleWang, J., Jiang, Z., Huang, C., Zhao, S., Zhu, S., Liu, R., & Zhu, H. (2023). Self-Assembled BODIPY Nanoparticles for Near-Infrared Fluorescence Bioimaging. Molecules, 28(7), 2997. https://doi.org/10.3390/molecules28072997