Cellular Localization of Selected Porphyrins and Their Effect on the In Vitro Motility of Human Colon Tumors and Normal Cells
Abstract
1. Introduction
2. Results
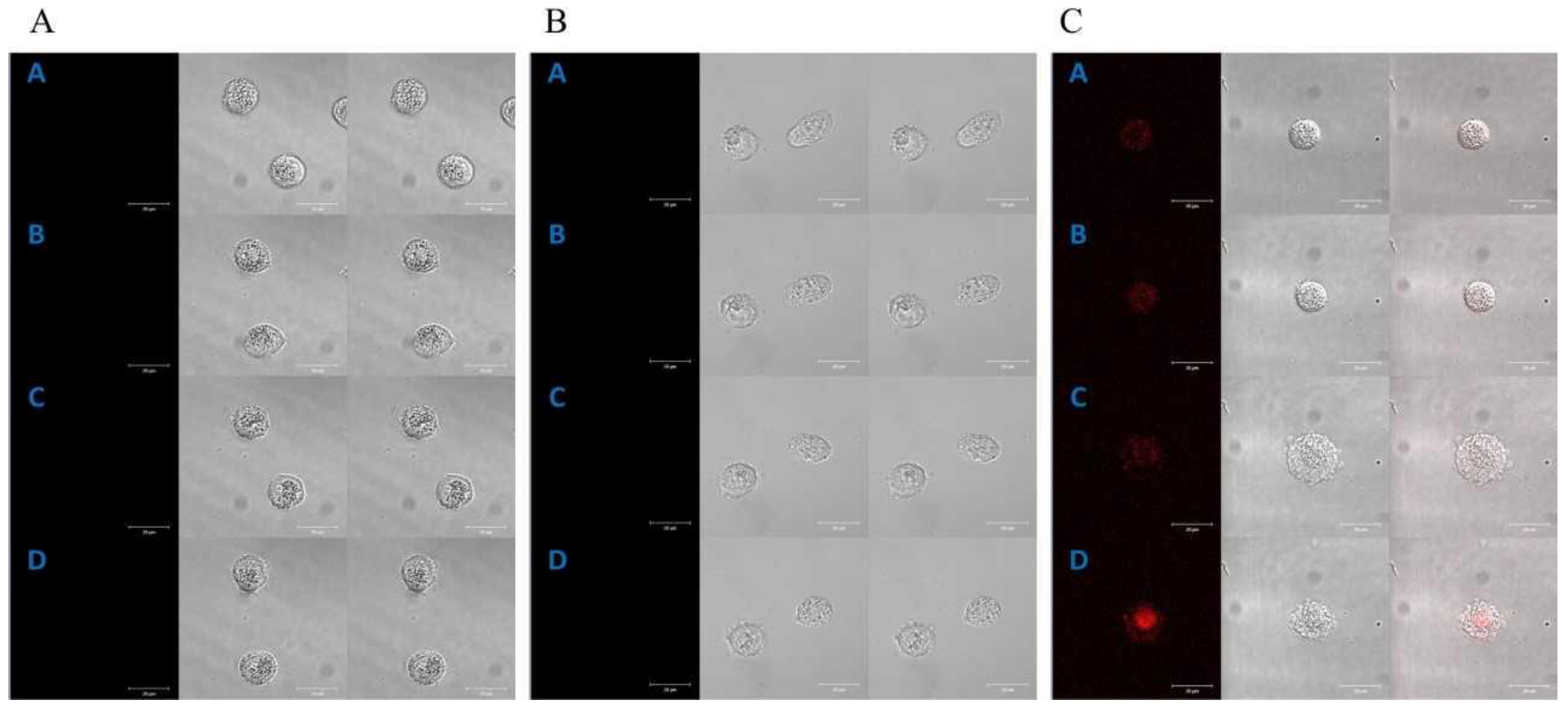
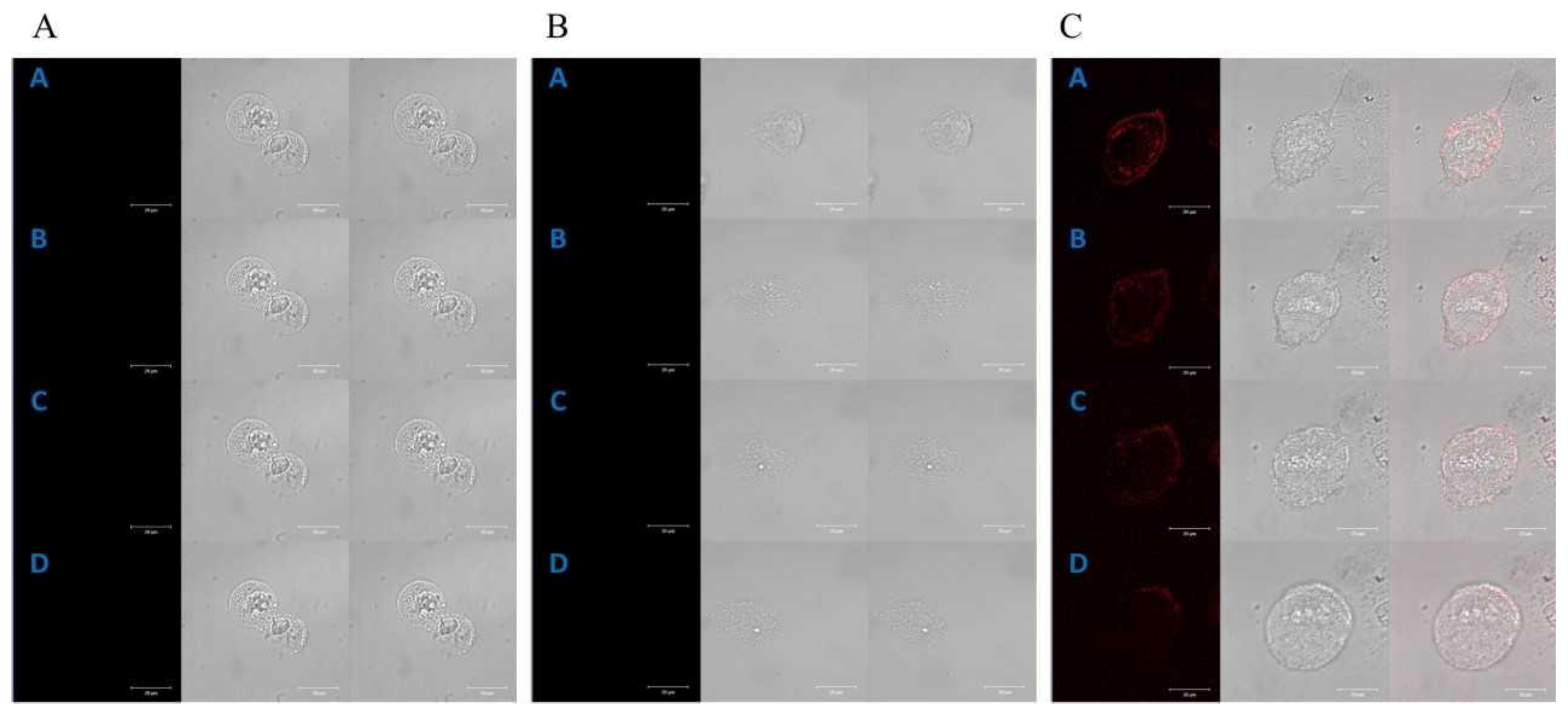
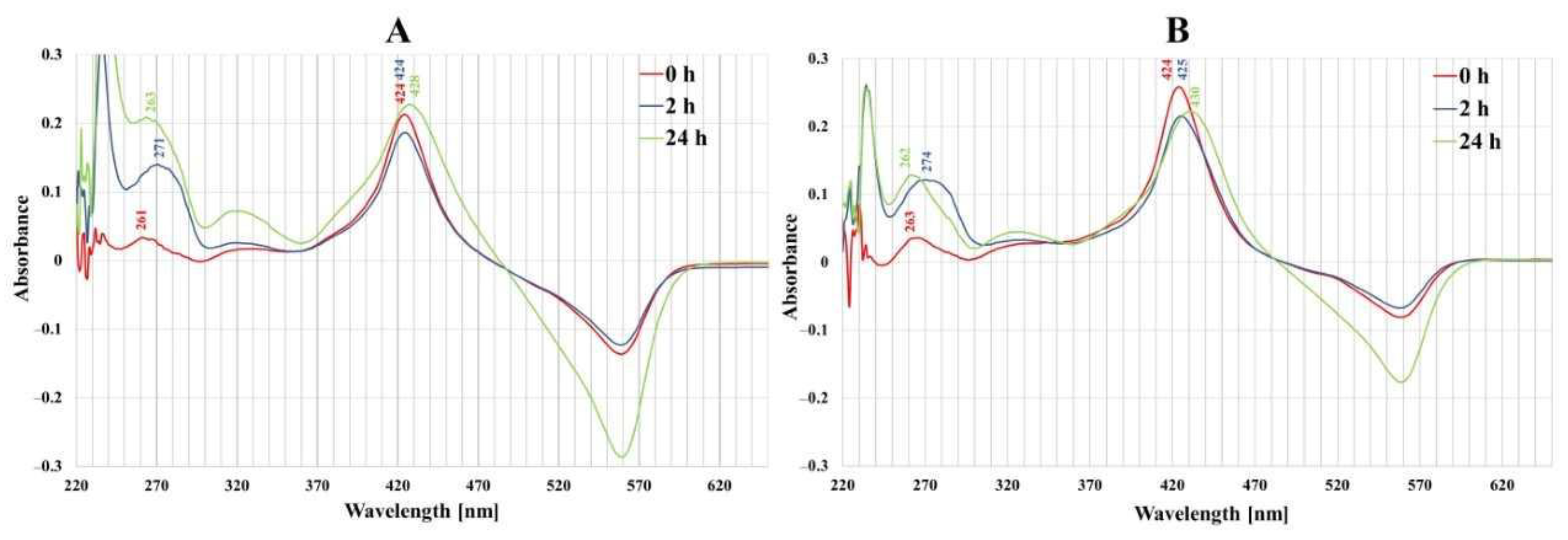
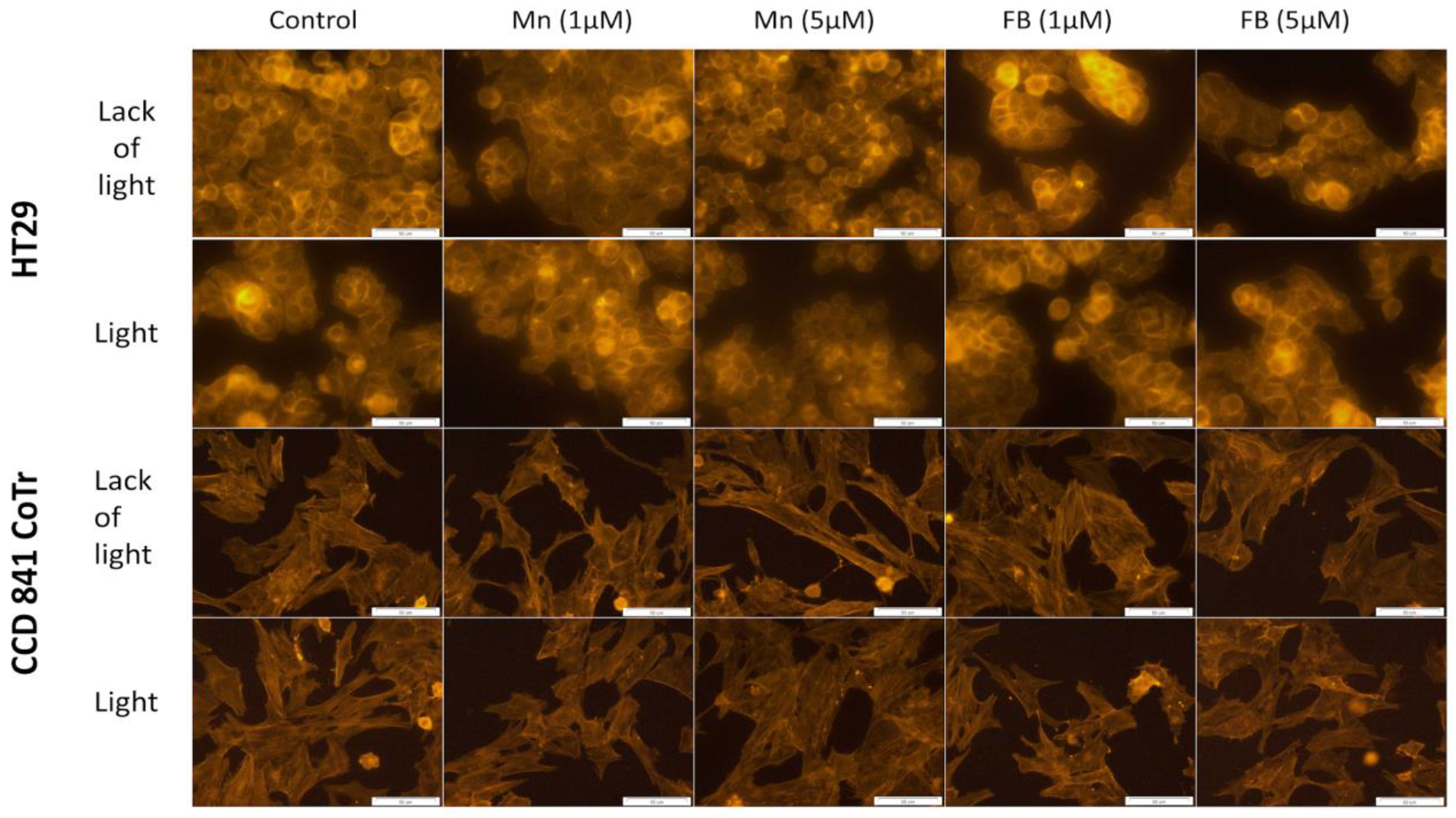
2.1. Cellular Localization of Porphyrins
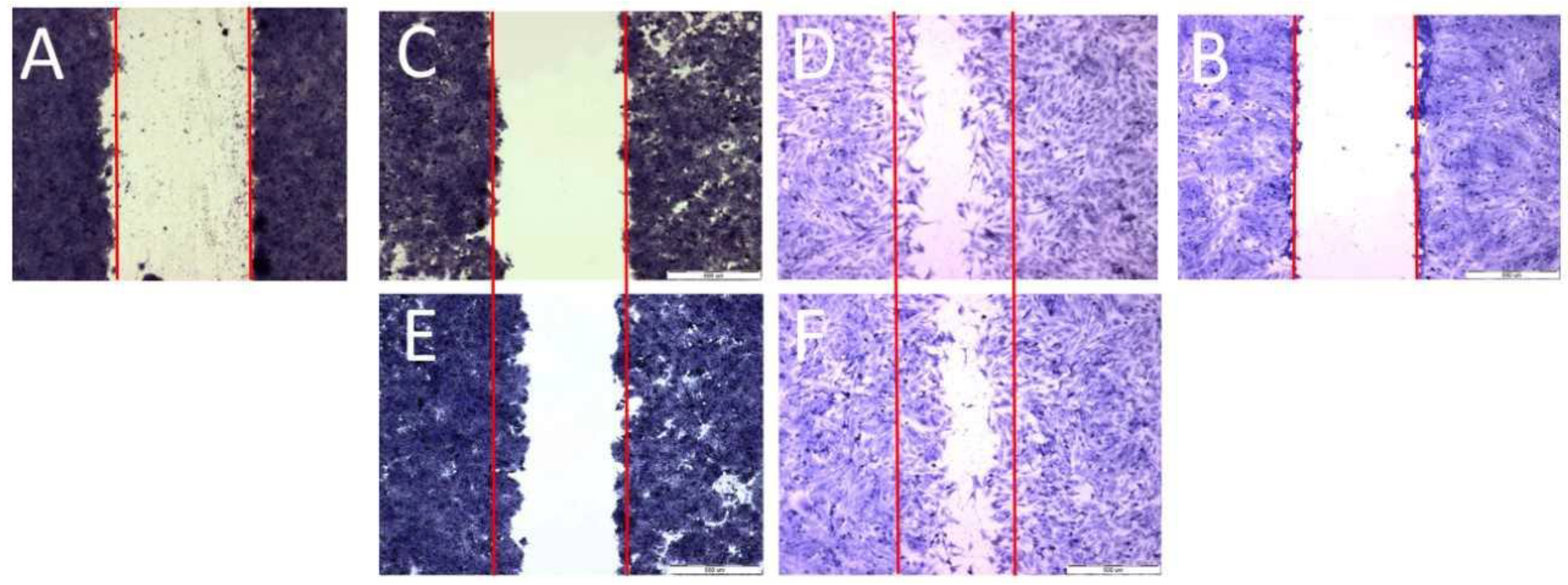
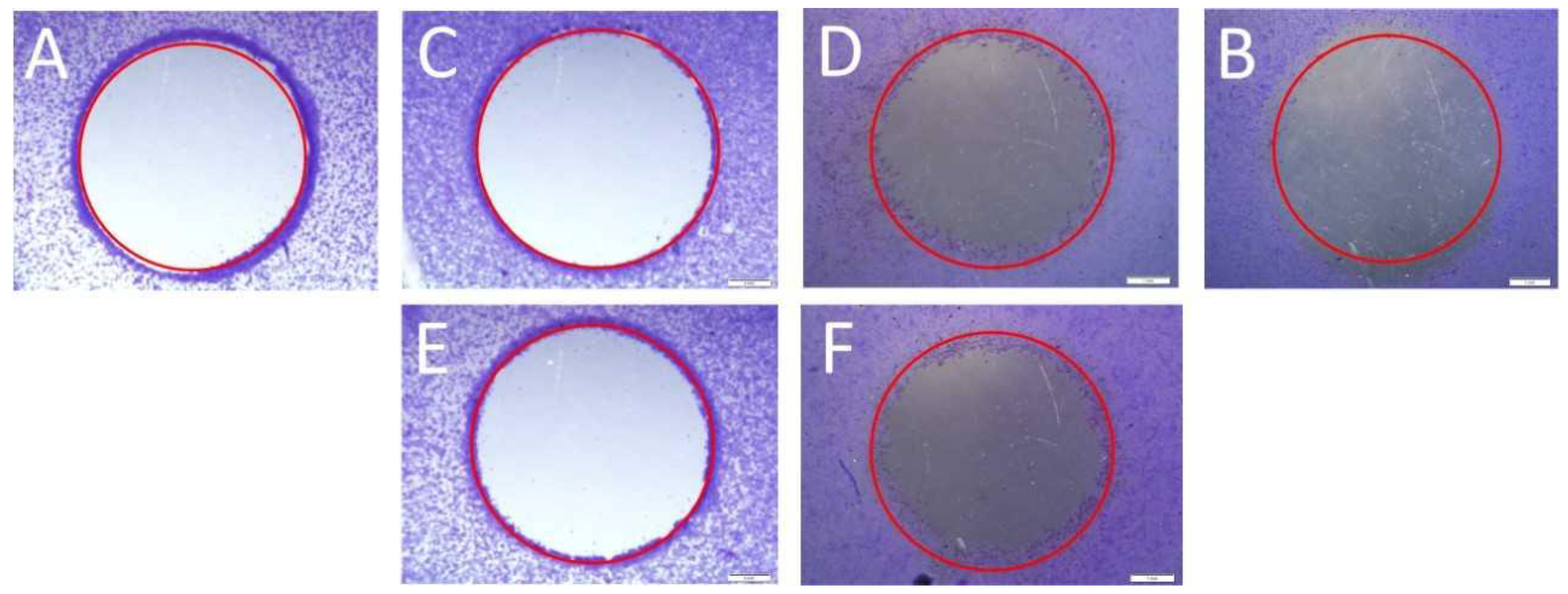
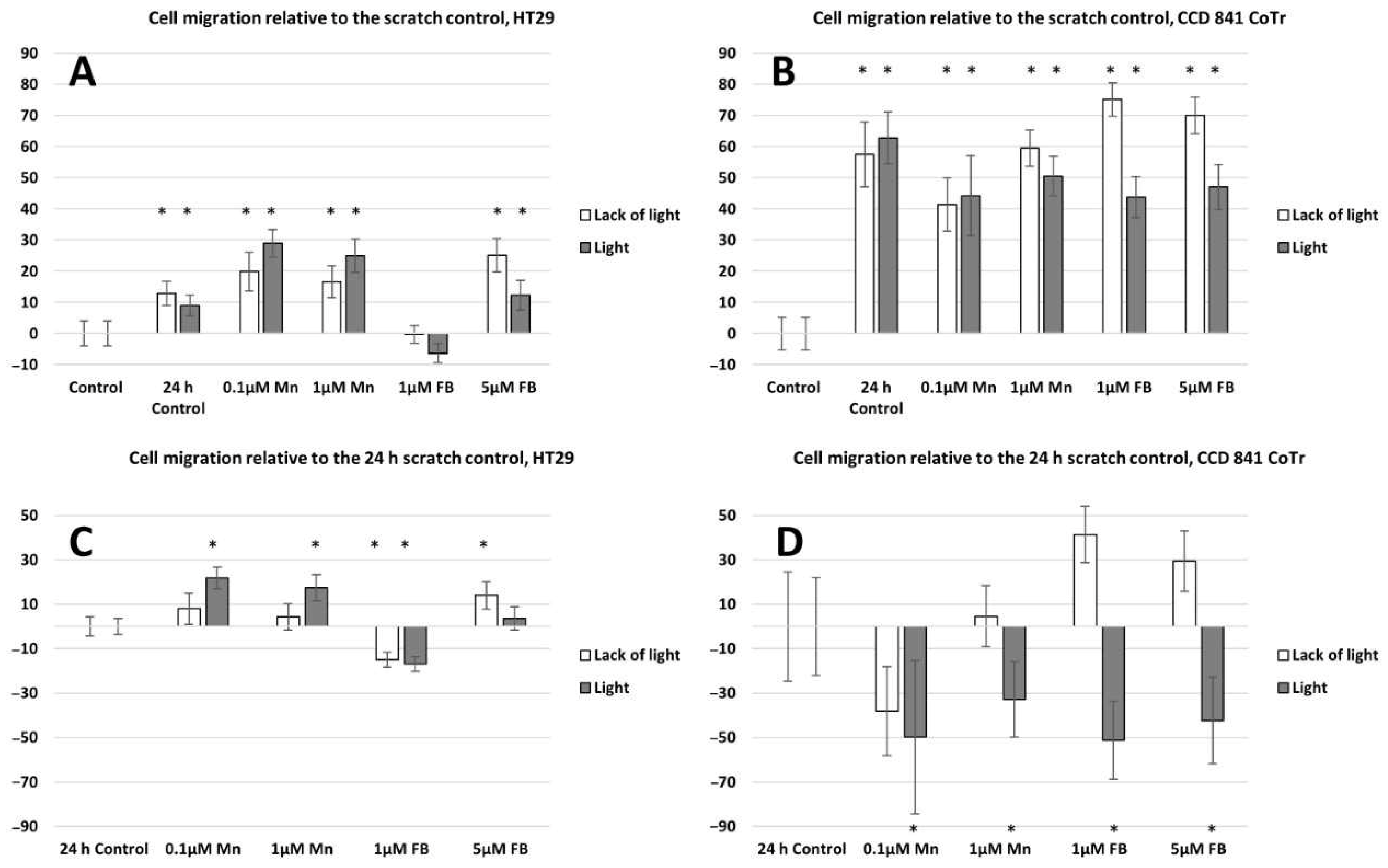
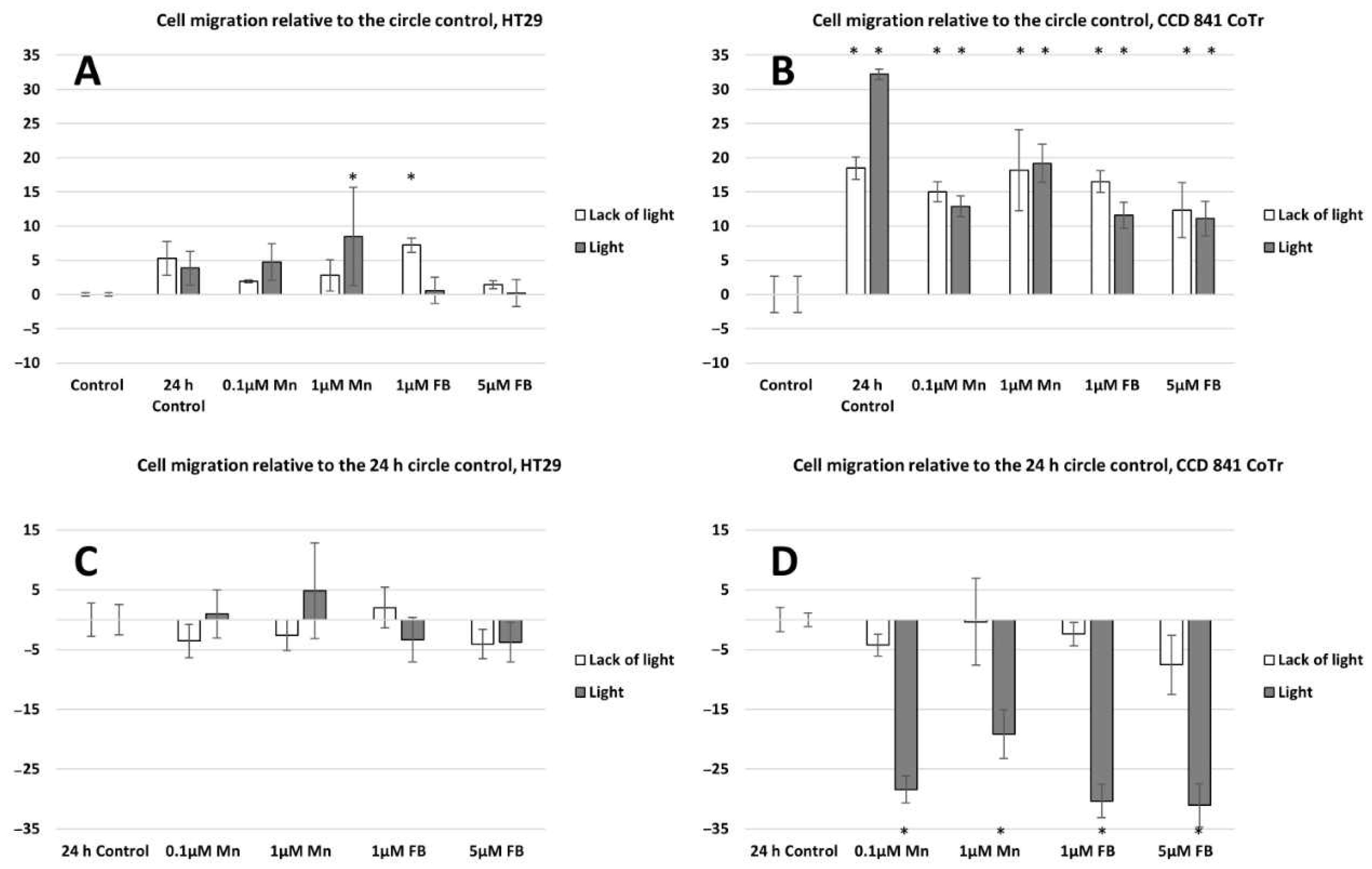
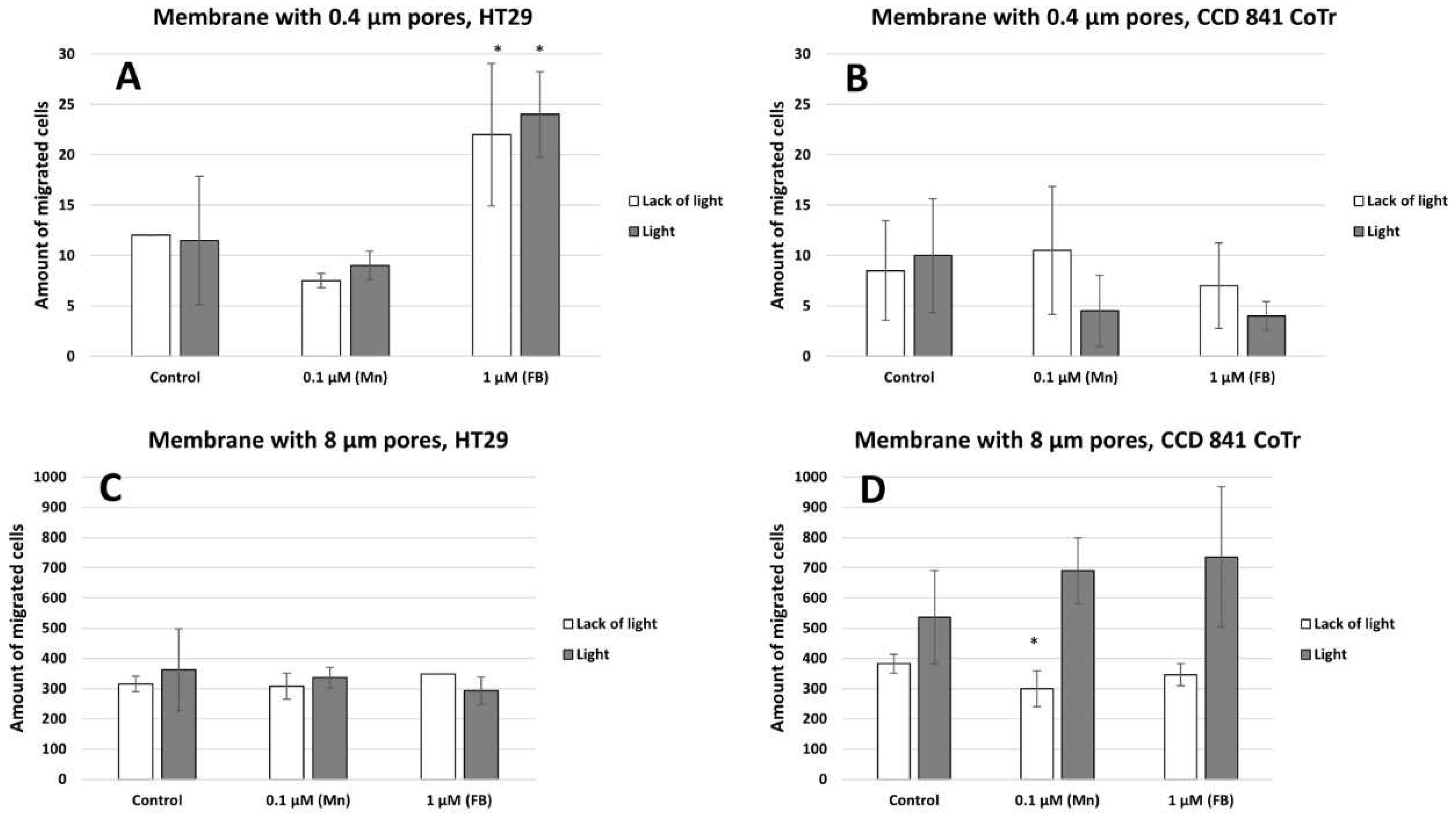
2.2. Cell Migration Analysis
3. Discussion
4. Materials and Methods
4.1. Tested Substances and Experimental Conditions
4.2. Cell Cultures
4.3. Study of Porphyrin Uptake by Colon Cells
4.4. Cell Migration into the Free Space (May-Grünwald-Giemsa Staining) (MGG Staining)
4.4.1. Scratch Assay
4.4.2. Circle Assay
4.5. Vertical Migration Analyses
4.6. F-Actin Staining
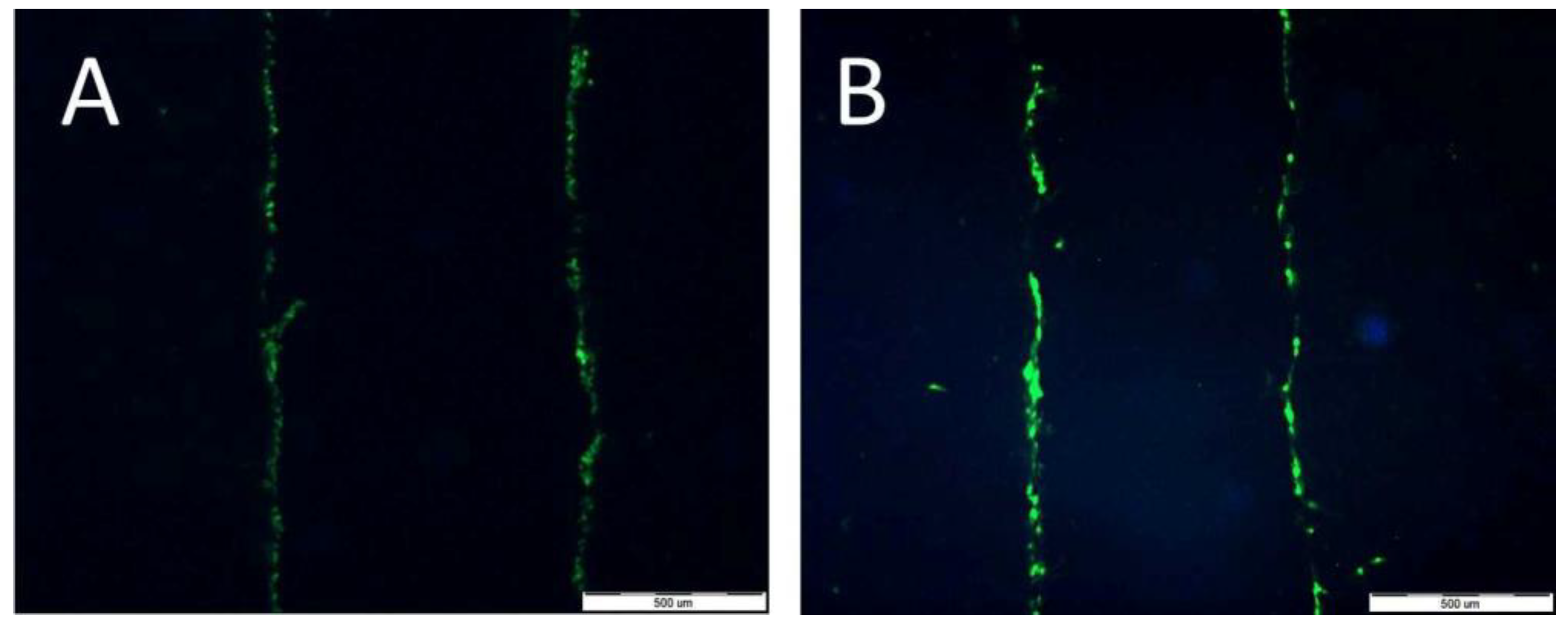
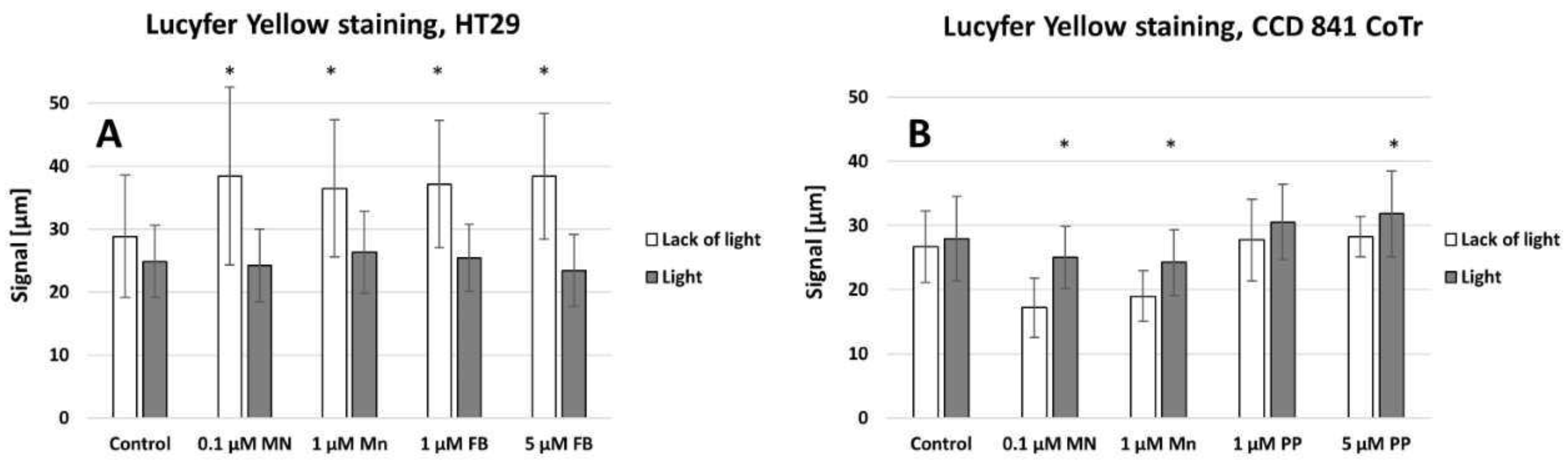
4.7. Analyses of Gap Junction (Lucifer Yellow Staining)
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, G.; Sundquist, J.; Sundquist, K.; Ji, J. Colorectal cancer risk in association with colorectal cancer as a second malignancy in relatives: A nationwide cohort study. BMC Cancer 2022, 22, 902. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Gao, Y.; Su, J.; Feng, Y. By characterizing metabolic and immune microenvironment reveal potential prognostic markers in the development of colorectal cancer. Front. Bioeng. Biotechnol. 2022, 10, 822835. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Wang, K.; Ding, Y.; Li, H.; Liu, Y.; Ding, L. Which patients are prone to sufer liver metastasis? A review of risk factors of metachronous liver metastasis of colorectal cancer. Eur. J. Med. Res. 2022, 27, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Zeng, X.L.; Guan, Y.L.; Lu, J.X.; Tu, K.; Liu, F.Y. Verticillin A inhibits colon cancer cell migration and invasion by targeting c-Met. J. Zhejiang Univ. Sci. B 2020, 21, 779–795. [Google Scholar] [CrossRef]
- Lai, X.; Li, Q.; Wu, F.; Lin, J.; Chen, J.; Zheng, H.; Guo, L. Epithelial-Mesenchymal Transition and metabolic switching in cancer: Lessons from somatic cell reprogramming. Front. Cell Dev. Biol. 2020, 8, 760. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sofany, W.I.; Al-Harbi, R.A.K.; El-Shahat, M. Development of a novel series of anticancer and antidiabetic: Spirothiazolidines analogs. Molecules 2019, 24, 2511. [Google Scholar] [CrossRef]
- Abdelhameed, R.M.; Abu-Elghait, M.; El-Shahat, M. Engineering titanium-organic framework decorated silver molybdate and silver vanadate as antimicrobial, anticancer agents, and photo-induced hydroxylation reactions. J. Photochem. Photobiol. A Chem. 2022, 423, 113572. [Google Scholar] [CrossRef]
- El-Sofany, W.I.; El-sayed, W.A.; Abd-Rabou, A.A.; El-Shahat, M. Synthesis of new imidazole-triazole-glycoside hybrids as anti-breast cancer candidates. J. Mol. Struct. 2022, 1270, 133942. [Google Scholar] [CrossRef]
- Park, J.M.; Hong, K.; Lee, H.; Jang, W. Bioinspired applications of porphyrin derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The course of immune stimulation by photodynamic therapy: Bridging fundamentals of photochemically-induced immunogenic cell death to the enrichment of T cell repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Chen, X.; Gao, J.; Duan, Y.; Wei, D.; Zhang, J.; Ge, K.; Liang, X.-J.; Huang, Y.; et al. Nano-herb medicine and PDT induced synergistic immunotherapy for colon cancer treatment. Biomaterials 2021, 269, 120654. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Czubab, Z.P.; Kwiatek, B.; Kwiatek, S.; Krupka, M.; Sieroń, A. The effect of ALA-PDT under normoxia and cobalt chloride (CoCl2)-induced hypoxia on adhesion molecules (ICAM-1, VCAM-1) secretion by colorectal cancer cells. Photodiagn. Photodyn. Ther. 2017, 19, 103–115. [Google Scholar] [CrossRef]
- Nkune, N.W.; Kruger, C.A.; Abrahamse, H. Possible enhancement of photodynamic therapy (PDT) colorectal cancer treatment when combined with cannabidiol. Anti-Cancer Agents Med. Chem. 2021, 21, 137–148. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Dandash, F.; Leger, D.Y.; Diab-Assaf, M.; Sol, V.; Liagre, B. Porphyrin/chlorin derivatives as promising molecules for therapy of colorectal cancer. Molecules 2021, 26, 7268. [Google Scholar] [CrossRef]
- Janas, K.; Boniewska-Bernacka, E.; Dyrda, G.; Słota, R. Porphyrin and phthalocyanine photosensitizers designed for targeted photodynamic therapy of colorectal cancer. Bioorg. Med. Chem. 2021, 30, 115926. [Google Scholar] [CrossRef]
- Bretin, L.; Pinon, A.; Bouramtane, S.; Ouk, C.; Richard, L.; Perrin, M.L.; Chaunavel, A.; Carrion, C.; Bregier, F.; Sol, V.; et al. Photodynamic therapy activity of new porphyrin-xylan-coated silica nanoparticles in human colorectal cancer. Cancers 2019, 11, 1474. [Google Scholar] [CrossRef]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J. Microsc. 2005, 218 Pt 2, 133–147. [Google Scholar] [CrossRef]
- Skwor, T.A.; Klemm, S.; Zhang, H.; Schardt, B.; Blaszczyk, S.; Bork, M.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus and Escherichia coli: A metalloporphyrin comparison. J. Photochem. Photobiol. B 2016, 165, 51–57. [Google Scholar] [CrossRef]
- Wang, F.-X.; Liu, J.-W.; Hong, X.-Q.; Tan, C.-P.; Zhang, L.; Chen, W.-H.; Sadler, P.J.; Mao, Z.-W. Anion-responsive manganese porphyrin facilitates chloride transport and induces immunogenic cell death. CCS Chem. 2021, 3, 2527–2537. [Google Scholar] [CrossRef]
- Chen, J.; Jin, X.; Mei, Y.; Shen, Z.; Zhu, J.; Shi, H.; Wang, M.; Zheng, X.; Liang, G. The different biological effects of TMPyP4 and cisplatin in the inflammatory microenvironment of osteosarcoma are attributed to G-quadruplex. Cell Prolif. 2021, 54, e13101. [Google Scholar] [CrossRef] [PubMed]
- Vongsvivut, J.; Itoh, T.; Ikehata, A.; Ekgasit, S.; Ozaki, Y. Surface-enhanced infrared spectra of manganese (III) tetraphenylporphine chloride physisorbed on gold island films. Sci. Asia 2006, 32, 261–269. [Google Scholar] [CrossRef]
- Celli, J.; Spring, B.; Rizvi, I.; Evans, C.; Samkoe, K.; Verma, S.; Pogue, B.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Alhamami, M.; Cheng, W.; Lyu, Y.; Allen, C.; Zhang, X.; Cheng, H.-L.M. Manganese-porphyrin-enhanced MRI for the detection of cancer cells: A quantitative in vitro investigation with multiple clinical subtypes of breast cancer. PLoS ONE 2018, 13, e0196998. [Google Scholar] [CrossRef] [PubMed]
- Frant, M.P.; Trytek, M.; Paduch, R. Assessing the in vitro activity of selected porphyrins in human colorectal cancer cells. Molecules 2022, 27, 2006. [Google Scholar] [CrossRef] [PubMed]
- Gjuroski, I.; Furrer, J.; Vermathen, M. Probing the interactions of porphyrins with macromolecules using NMR spectroscopy techniques. Molecules 2021, 26, 1942. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Babayan, N.; Poghosyan, D.; Margaryan, K.; Harutyunyan, B.; Grigoryan, R.; Sarkisyan, N.; Spasojevic, I.; Mamyan, S.; Sahakyan, L.; et al. Novel amphiphilic cationic porphyrin and its Ag(II) complex as potential anticancer agents. J. Inorg. Biochem. 2014, 140, 94–103. [Google Scholar] [CrossRef]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-based cationic amphiphilic photosensitisers as potential anticancer, antimicrobial and immunosuppressive agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef]
- Jensen, T.; Vicente, M.; Luguya, R.; Norton, J.; Fronczek, F.; Smith, K. Effect of overall charge and charge distribution on cellular uptake, distribution and phototoxicity of cationic porphyrins in HEp2 cells. J. Photochem. Photobiol. B Biol. 2010, 100, 100–111. [Google Scholar] [CrossRef]
- Kramer-Marek, G.; Serpa, C.; Szurko, A.; Widel, M.; Sochanik, A.; Snietura, M.; Kus, P.; Nunes, R.; Arnaut, L.; Ratuszna, A. Spectroscopic properties and photodynamic effects of new lipophilic porphyrin derivatives: Efficacy, localisation and cell death pathways. J. Photochem. Photobiol. B Biol. 2006, 84, 1–14. [Google Scholar] [CrossRef]
- Kulbacka, J.; Kotulska, M.; Rembiałowska, N.; Choromańska, A.; Kamińska, I.; Garbiec, A.; Rossowska, J.; Daczewska, M.; Jachimska, B.; Saczko, J. Cellular stress induced by photodynamic reaction with CoTPPS and MnTMPyPCl5 in combination with electroporation in human colon adenocarcinoma cell lines (LoVo and LoVoDX). Cell Stress Chaperones 2013, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Khadria, A.; Fleischhauer, J.; Boczarow, I.; Wilkinson, J.D.; Kohl, M.M.; Anderson, H.L. Porphyrin Dyes for Nonlinear Optical Imaging of Live Cells. iScience 2018, 4, 153–163. [Google Scholar] [CrossRef]
- Temizel, E.; Sagir, T.; Ayan, E.; Isik, S.; Ozturk, R. Delivery of lipophilic porphyrin by liposomevehicles: Preparation and photodynamic therapy activity against cancer cell lines. Photodiagn. Photodyn. 2014, 11, 537–545. [Google Scholar] [CrossRef]
- Lue, W.; Liu, R.; Zhu, J.; Li, Y.; Liu, H. Subcellular location and photodynamic therapeutic effect of chlorin e6 in the human tongue squamous cell cancer Tca8113 cell line. Oncol. Lett. 2015, 9, 551–556. [Google Scholar] [CrossRef]
- Valachová, K.; Rapta, P.; Moura, N.M.M.; Batinic-Haberle, I.; Šoltés, L. Ortho isomeric Mn(III) N-Alkyl- and Alkoxyalkylpyridylporphyrins—Enhancers of hyaluronan degradation induced by ascorbate and cupric ions. Int. J. Mol. Sci. 2021, 22, 8608. [Google Scholar] [CrossRef]
- Shah, M.H.; Liu, G.-S.; Thompson, E.W.; Dusting, G.J.; Peshavariya, H.M. Differential effects of superoxide dismutase and superoxide dismutase/catalase mimetics on human breast cancer cells. Breast Cancer Res. Treat. 2015, 150, 523–534. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sdium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Liu, Q.; Wingnang Leung, A.; Wang, P.; Xu, C. Sinoporphyrin sodium mediated photodynamic therapy inhibits the migration associated with collapse of F-actin filaments cytoskeleton in MDA-MB-231 cells. Photodiagn. Photodyn. Ther. 2016, 13, 58–65. [Google Scholar] [CrossRef]
- Wufuer, R.; Ma, H.-X.; Luo, M.-Y.; Xu, K.-Y.; Kang, L. Downregulation of Rac1/PAK1/LIMK1/cofilin signaling pathway in colon cancer SW620 cells treated with Chlorin e6 photodynamic therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102143. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Kang, L.; Lu, Y. Effects of chlorin e6-mediated photodynamic therapy on human colon cancer SW480 cells. Int. J. Clin. Exp. Med. 2014, 7, 4867–4876. [Google Scholar]
- Tong, Q.; Weaver, M.R.; Kosmacek, E.A.; O’Connor, B.P.; Harmacek, L.; Venkataraman, S.; Oberley-Deegan, R.E. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic. Biol. Med. 2016, 94, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberled, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, P.; Yan, R.; Wang, Q.; Fang, E.; Wu, H.; Li, S.; Tan, H.; Zhou, X.; Ma, X.; et al. MnTE-2-PyP attenuates TGF-β-induced epithelial-mesenchymal transition of colorectal cancer cells by inhibiting the Smad2/3 signaling pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8639791. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H.; Chen, Q.; Yu, L.; Bai, D. MPPa-PDT suppresses breast tumor migration/invasion by inhibiting Akt-NF-κB dependent MMP-9 expression via ROS. BMC Cancer 2019, 19, 1159. [Google Scholar] [CrossRef]
- Costa, J.G.; Saraiva, N.; Batinic-Haberle, I.; Castro, M.; Oliveira, N.G.; Fernandes, A.S. The SOD mimic MnTnHex-2-PyP5+ reduces the viability and migration of 786-O human renal cancer cells. Antioxidants 2019, 8, 490. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, H.; Tao, Y.; Zhong, S.; Yu, H.; Li, J.; Bai, Z.; Ou, Y. Antitumor effects and mechanisms of pyropheophorbide-α methyl ester-mediated photodynamic therapy on the human osteosarcoma cell line MG-63. Int. J. Mol. Med. 2020, 45, 971–982. [Google Scholar] [CrossRef]
- Shin, S.-W.; Choi, C.; Kim, H.; Kim, Y.; Park, S.; Kim, S.-Y.; Batinic-Haberle, I.; Park, W. MnTnHex-2-PyP5+, coupled to radiation, suppresses metastasis of 4T1 and MDA-MB-231 breast cancer via AKT/Snail/EMT pathways. Antioxidants 2021, 10, 1769. [Google Scholar] [CrossRef]
- Zheng, X.-H.; Xin Nie, X.; Liu, H.-Y.; Fang, Y.-M.; Zhao, Y.; Xia, L.-X. TMPyP4 promotes cancer cell migration at low doses, but induces cell death at high doses. Sci. Rep. 2016, 6, 26592. [Google Scholar] [CrossRef]
- Konieczna, N.; Romaniuk-Drapała, A.; Lisiak, N.; Totoń, E.; Paszel-Jaworska, A.; Kaczmarek, M.; Rubiś, B. Telomerase inhibitor TMPyP4 alters adhesion and migration of breast cancer cells MCF7 and MDA-MB-231. Int. J. Mol. Sci. 2019, 20, 2670. [Google Scholar] [CrossRef]
- Costantini, F.; Di Leo, F.; Di Sano, C.; Fiore, T.; Pellerito, C.; Barbieri, G. Dibutyltin(IV) and Tributyltin(IV) derivatives of meso-Tetra(4-sulfonatophenyl)porphine inhibit the growth and the migration of human melanoma cells. Cells 2019, 8, 1547. [Google Scholar] [CrossRef]
- Von der Ecken, J.; Müller, M.; Lehman, W.; Manstein, D.J.; Penczek, P.A.; Raunser, S. Structure of the F–actin–tropomyosin complex. Nature 2015, 519, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, K.; Li, H.; Luo, M.; Wufuer, R.; Kang, L. Photodynamic effect of chlorin e6 on cytoskeleton protein of human colon cancer SW480 cells. Photodiagn. Photodyn. Ther. 2021, 33, 102201. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.; Hamblin, M.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Casas, A.; Di Venosa, G.; Hasan, T.; Batlle, A. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef]
- Casas, A.; Sanz-Rodriguez, F.; Di Venosa, G.; Rodriguez, L.; Mamone, L.; Blázquez, A.; Jaén, P.; Batlle, A.; Stockert, J.C.; Juarranz, A. Disorganisation of cytoskeleton in cells resistant to photodynamic treatment with decreased metastatic phenotype. Cancer Lett. 2008, 270, 56–65. [Google Scholar] [CrossRef]
- Li, D.; Li, L.; Li, P.; Li, Y.; Chen, X. Apoptosis of HeLa cells induced by a new targeting photosensitizer-based PDT via a mitochondrial pathway and ER stress. OncoTargets Ther. 2015, 8, 703–711. [Google Scholar] [CrossRef]
- Trosko, J.; Chang, C.-C.; Wilson, M.; Upham, B.; Hayashi, T.; Wade, M. Gap junctions and the regulation of cellular functions of stem cells during development and differentiation. Methods 2000, 20, 245–264. [Google Scholar] [CrossRef]
- Rutkowski, R.; Kosztyła-Hojna, B.; Kańczuga-Koda, L.; Sulkowska, M.; Sulkowski, S.; Rutkowski, K. Struktura i fizjologiczna funkcja białek koneksynowych. Postęp. Hig. Med. Dośw. 2008, 62, 632–641. [Google Scholar]
- Buczek, K.; Trytek, M.; Deryło, K.; Borsuk, G.; Rybicka-Jasińska, K.; Gryko, D.; Cytryńska, M.; Tchórzewski, M. Bioactivity studies of porphyrinoids against microsporidia isolated from honeybees. Sci. Rep. 2020, 10, 11553. [Google Scholar] [CrossRef]
- De Brauwer, E.; Jacobs, J.; Nieman, F.; Bruggeman, C.; Drent, M. Test characteristics of acridine orange, gram, and May-Grünwald-Giemsa stains for enumeration of intracellular organisms in bronchoalveolar lavage fluid. J. Clin. Microbiol. 1999, 37, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Pirilä, E.; Väyrynen, O.; Sundquist, E.; Päkkilä, K.; Nyberg, P.; Nurmenniemi, S.; Pääkkönen, V.; Pesonen, P.; Dayan, D.; Vered, M.; et al. Macrophages modulate migration and invasion of human tongue squamous cell carcinoma. PLoS ONE 2015, 10, e0120895. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Tang, Y.; Wang, L.; Ladt, K.; Loi, J.; Dargent, B.; Leterrier, C.; Roy, S. A dynamic formin-dependent deep F-actin network in axons. J. Cell Biol. 2015, 210, 401–417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frant, M.P.; Trytek, M.; Deryło, K.; Kutyła, M.; Paduch, R. Cellular Localization of Selected Porphyrins and Their Effect on the In Vitro Motility of Human Colon Tumors and Normal Cells. Molecules 2023, 28, 2907. https://doi.org/10.3390/molecules28072907
Frant MP, Trytek M, Deryło K, Kutyła M, Paduch R. Cellular Localization of Selected Porphyrins and Their Effect on the In Vitro Motility of Human Colon Tumors and Normal Cells. Molecules. 2023; 28(7):2907. https://doi.org/10.3390/molecules28072907
Chicago/Turabian StyleFrant, Maciej P., Mariusz Trytek, Kamil Deryło, Mateusz Kutyła, and Roman Paduch. 2023. "Cellular Localization of Selected Porphyrins and Their Effect on the In Vitro Motility of Human Colon Tumors and Normal Cells" Molecules 28, no. 7: 2907. https://doi.org/10.3390/molecules28072907
APA StyleFrant, M. P., Trytek, M., Deryło, K., Kutyła, M., & Paduch, R. (2023). Cellular Localization of Selected Porphyrins and Their Effect on the In Vitro Motility of Human Colon Tumors and Normal Cells. Molecules, 28(7), 2907. https://doi.org/10.3390/molecules28072907





