Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak
Abstract
1. Introduction
2. Results and Discussion
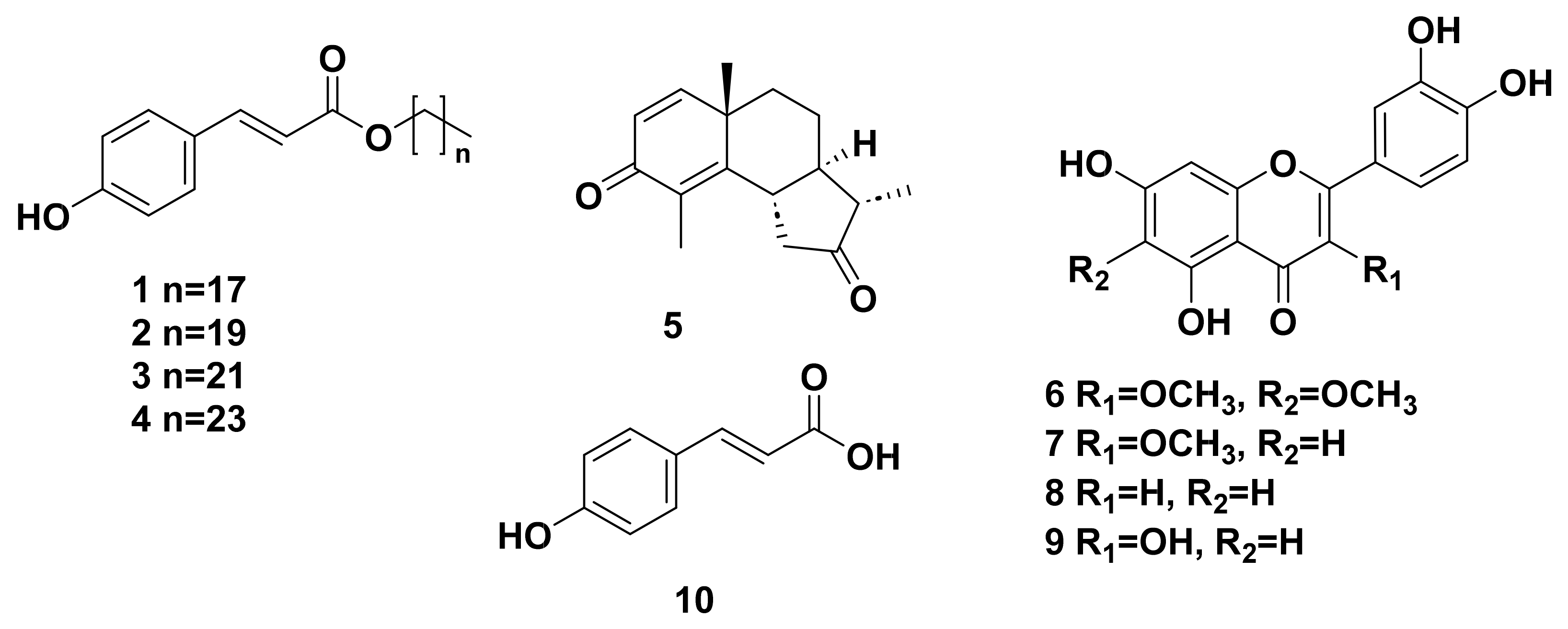
2.1. Identification of the Isolated Compounds
2.2. Antimicrobial Activity
2.3. Identification and Tentative Characterization of Secondary Metabolites Using LC-QToF
2.3.1. Sesquiterpene Lactones (1–22)
2.3.2. Flavonoids (23–36)
2.3.3. Others (37–43)
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Evaluation of Antimicrobial Activity
3.5. Liquid Chromatography-Diode Array Detector-Quadrupole Time-of-Flight Mass Spectrometry (LC-DAD-QToF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef]
- Ahuja, A.; Yi, Y.S.; Kim, M.Y.; Cho, J.Y. Ethnopharmacological properties of Artemisia asiatica: A comprehensive review. J. Ethnopharmacol. 2018, 28, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Willcox, M. Artemisia species: From traditional medicines to modern antimalarial and back again. J. Altern. Complem. Med. 2009, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Efferth, T. The activity of Artemisia spp. and their constituents against Trypanosomiasis Janine. Phytomedicine 2018, 47, 184–191. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional use, phytochemical profiles and pharmacological properties of Artemisia genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef]
- Nedel’ko, E.S.; Nikonov, G.K. Methyl ethers of quercetin from Artemisia heptapotamica. Chem. Nat. Compd. 1987, 23, 254–255. [Google Scholar] [CrossRef]
- Zhamilya, A.; Yuan, J.; Janar, J.; Tang, C.P.; Ye, Y. Monomeric and dimeric sesquiterpene lactones from Artemisia heptapotamica. Chin. J. Nat. Med. 2019, 17, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Takenaka, Y.; Kishi, M.; Tanahashi, T.; Yoshida, H.; Okuda, C.; Mizushina, Y. Synthesis and DNA polymerase alpha and beta inhibitory activity of alkyl p-coumarates and related compounds. Chem. Pharm. Bull. 2009, 57, 476–480. [Google Scholar] [CrossRef]
- Khan, H.; Saeed, M.; Khan, M.A.; Muhammad, N.; Khan, A.; Ullah, A.; Safiullah. Lipoxygenase and urease inhibition of extracts of Polygonatum verticillatum rhizome: Augmented by its isolated compound, santonin. J. Chem. Soc. Pak. 2014, 36, 865–869. [Google Scholar]
- Ata, A.; Nachtigall, J.A. Microbial Transformations of α-Santonin. Z. Naturforsch. 2004, 59c, 209–214. [Google Scholar] [CrossRef]
- Barberá, O.; Marco, J.A.; Sanz, J.F.; Sánchez-Parareda, J. 3-Methoxyflavones and coumarins from Artemisia incanescens. Phytochemistry 1986, 25, 2357–2360. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, H.J.; Song, Y.S.; Jin, C.; Lee, K.T.; Cho, J.; Lee, Y.S. Constituents of the stems and fruits of Opuntia ficus-indica var saboten. Arch. Pharm. Res. 2003, 26, 1018–1023. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Lee, M.-H.; Kim, H.-T.; Seo, W.D.; Kim, J.Y.; Baek, I.-Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar]
- Sinha, R.; Gadhwal, M.K.; Joshi, U.J.; Srivastava, S.; Govil, G. Modifying effect of quercetin on model biomembranes: Studied by molecular dynamic simulation, DSC and NMR. Int. J. Curr. Pharm. Res. 2012, 4, 70–79. [Google Scholar]
- Swisłocka, R.; Kowczyk-Sadowy, M.; Kalinowska, M.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H and 13CNMR) and theoretical studies of p-coumaric acid and alkali metal p-coumarates. Spectroscopy 2012, 27, 35–48. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar] [CrossRef]
- Wasada, N.; Tsuchiya, T.; Yoshi, E.; Watanabe, E. A study of santonins and their derivatives by mass spectrometry. Tetrahedron 1967, 23, 4623–4634. [Google Scholar] [CrossRef]
- Teresa, C.; Rossana, P.; Maria, P.A.; Paride, P.; Francesco, P.F.; Luciano, V.; Pinarosa, A. Phytochemical analysis of herbal tea from Artemisia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar]
- Piejie, Z.; Jun, J.; Ke, Z.; Wenjing, L.; Pengfei, T.; Jun, L.; Yuelin, S.; Jiao, Z.; Li, T. Shotgun chemome characterization of Artemisia rupestris L. using direct infusion-MS/MSALL. J. Chromatogr. B. 2021, 1176, 122735. [Google Scholar]
- Chunqing, F.; Ping, Y.; Manyuan, W.; Feng, Q. Phytochemical analysis and geographic assessment of flavonoids, coumarins and sesquiterpenes in Artemisia annua L. based on HPLC-DAD quantification and LC-ESI-QTOF-MS/MS confirmation. Food Chem. 2020, 312, 126070. [Google Scholar]
- Avula, B.; Katragunta, K.; Wang, Y.-H.; Ali, Z.; Khan, I.A. Simultaneous determination and characterization of flavonoids, sesquiterpene lactone, and other phenolics from Centaurea benedicta and dietary supplements using UHPLC-PDA-MS and LC-DAD-QToF. J. Pharm. Biomed. Anal. 2022, 216, 114806. [Google Scholar] [CrossRef]
- Dictionary of Natural Products v31.2. CRC Press: Boca Raton, FL, USA; Taylor Francis Group: Abingdon, UK, 1995.
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 83–100. [Google Scholar] [CrossRef]
- Huang, Z.S.; Pei, Y.H.; Liu, C.M.; Lin, S.; Tang, J.; Huang, D.S.; Song, T.F.; Lu, L.H.; Gao, Y.P.; Zhang, W.D. Highly oxygenated guaianolides from Artemisia dubia. Planta Med. 2010, 76, 1710–1716. [Google Scholar] [CrossRef]
- Irwin, M.A.; Geissman, T.A. Sesquiterpene lactones from Artemisia: Arbusculin-C, rothin-A and rothin-B. Phytochemistry 1971, 10, 637–645. [Google Scholar] [CrossRef]
- Uehara, A.; Kitajima, J.; Kokubugata, G.; Iwashina, T. Further characterization of foliar flavonoids in Crossostephium chinense and their geographic variation. Nat. Prod. Commun. 2014, 9, 163–164. [Google Scholar] [CrossRef]
- Nidiry, E.S.J. Tentative detection of some alkyl coumarates and alkyl ferulates in Lpomoea Carnea subsp. fistulosa by HRESIMS and comparison of these compounds among Convolvulaceae plants. Pharmacogn. Commun. 2013, 3, 12–15. [Google Scholar]
- Snook, M.E.; Data, E.S.; Kays, S.J. Characterization and quantitation of hexadecyl, octadecyl, and eicosyl esters of p-coumaric acid in the vine and root latex of sweet potato [Ipomoea batatas (L.) Lam.]. J. Agricult. Food Chem. 1994, 42, 2589–2595. [Google Scholar] [CrossRef]
- Samy, M.N.; Mahmoud, B.K.; Shady, N.H.; Abdelmohsen, U.R.; Ross, S.A. Bioassay-guided fractionation with antimalarial and antimicrobial activities of Paeonia officinalis. Molecules 2022, 27, 8382. [Google Scholar] [CrossRef] [PubMed]
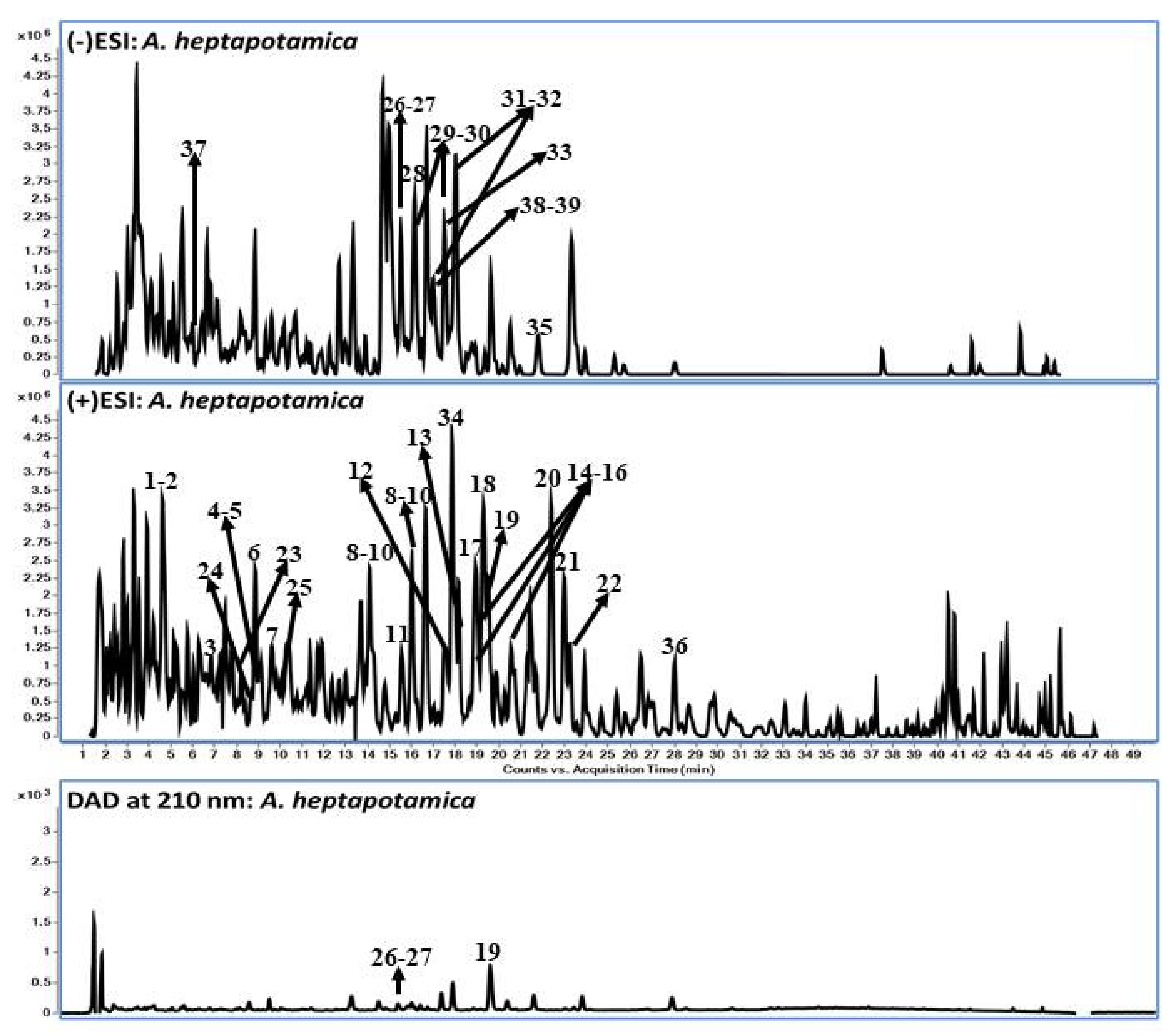
| # | RT (min) | Compound Name | Molecular Formula | Mass | Adduct (+ve Mode) | Fragment Ions (Positive Ion Mode) | Adduct (-ve Mode) | Fragment Ions (Negative Ion Mode) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Sesquiterpene lactones | |||||||||
| 1 | 4.6 | Artemisinin/Artemisinin G | C15H22O5 | 282.1467 | 300.1809 (300.1813) * [M + NH4]+ | 181.0845, 105.0685, 91.0534 | - | - | [22] |
| 2 | 4.7 | - | - | ||||||
| 3 | 6.8 | Artelavanolide A/ Austroyunnane B/C/E/ Artemdubolide I | C15H20O6 | 296.1260 | 314.1584 (314.1598) [M + NH4]+ | 183.1003, 153.0895, 107.0845 | - | - | [20] |
| 4 | 8.7 | Guaianolide derivative | C15H18O4 | 262.1205 | 263.1271 (263.1278) [M + H]+ 285.1095 (285.1097) [M + Na]+ | 245.1165, 233.1162, 91.0433, 772.0286 | - | - | [20,27] |
| 5 | 8.8 | Rupicoline B/ Hydroxyachillin | C15H20O4 | 264.1362 | 265.1423 (265.1434) [M + H]+ 287.1242 (287.1254) [M + Na]+ | - | 263.1294 (263.1289) [M-H]− | - | [20] |
| 6 | 8.9 | Artemdubolide I | C16H21O8 | 296.1260 | - | - | 295.1177 (295.1187) [M-H]− 341.1231 (341.1242) [M + COOH]− | - | [20] |
| 7 | 9.3 | Millifolide A | C30H34O9 | 538.2203 | - | - | 537.2119 (537.2130) [M-H]− 583.2145 (583.2185) [M + COOH]− | - | [20] |
| 8 | 14.1 | Dihydroxy-eudesmen-olide/ Dihydroxy-germacradien-olide | C15H22O4 | 266.1518 | 267.1586 (267.1591) [M + H]+ 284.1845 (284.1856) [M + NH4]+ | 249.1470 | - | - | [20,21] |
| 9 | 16.1 | - | - | ||||||
| 10 | 16.6 | - | - | ||||||
| 11 | 15.5 | Valerianin C | C17H24O7 | 340.1522 | 363.1403 (363.1414) [M + Na]+ | 323.1477, 305.1385, 281.1376, 169.1210, 151.1107, 109.1003 | - | - | [20] |
| 12 | 17.4 | Ezoartemin/Yamayomoginin (Guaianolide derivative) | C17H22O7 | 338.1366 | 356.1695 (356.1704) [M + NH4]+ | 279.1582, 261.1459, 247.1307. 205.1198, 173.0940, 153.0892 | - | - | [20,27] |
| 13 | 18.1 | 9-Acetoxy-5-hydroperoxy-4(15),11(13)- eudesmadien-12-oic acid | C17H24O6 | 324.1573 | 342.1898 (342.1911) [M + NH4]+ | 281.1726, 265.1430 | - | - | [20,21] |
| 14 | 18.8 | Trihydroxy-guaiadien-olide/ Eudesmanolide derivatives | C15H20O5 | 280.1311 | 298.1636 (298.1649) [M + NH4]+ 303.1199 (303.1203) [M + Na]+ | 263.1262, 155.1057, 109.1005 | - | - | [20,21,27] |
| 15 | 19.2 | - | - | ||||||
| 16 | 20.4 | - | - | ||||||
| 17 | 19.2 | Ajaniaolide B | C14H18O3 | 234.1256 | 235.1318 (235.1329) [M + H]+ | - | - | [20] | |
| 18 | 19.5 | 3β-Acetoxy-1β-hydroxyarbusculin | C17H24O6 | 324.1573 | 325.1640 (325.1646) [M + H]+ 347.1458 (347.1465) [M + Na]+ | 247.1314 [C15H18O3 + H]+ (arbusculin skeleton), 173.0947, 135.0794, 115.0538 | - | - | [28] |
| 19 | 19.7 | Santonin | C15H18O3 | 246.1256 | 247.1329 (247.1329) [M + H]+ | 229.1219 [M + H-H2O]+, 201.1271 [M + H-H2O-CO]+, 173.0954 [M + H-H2O-CO-C2H4]+, 157.0648 [M + H-H2O-CO-C2H4-CH4]+, 129.0700 [M + H-H2O-CO-C2H4-CH4-CO]+, 115.0542 [M + H-H2O-CO-C2H4-CH4-C2H2O]+, 105,0698 [C8H8 + H]+, 91.0544 [C7H6]+ | - | - | [19] |
| 20 | 22.4 | Arbusculin C/Taurin/Finitin | C15H20O3 | 248.1412 | 271.1305 (271.1295) [M + Na]+ | 231.1363, 141.0685, 128.0606, 115.0530 | - | - | [20,28] |
| 21 | 23.0 | 3-Acetyldihydroridentin/Nitrosin /Torrentin/Epitorrentin/Herbolide B/C/D/ | C17H24O5 | 308.1624 | 331.1505 (331.1516) [M + Na]+ | 291.1577, 249.1484, 231.1363, 105.0695, 91.0537 | - | - | [20] |
| 22 | 23.3 | Dihydroeudesmanomolide | C19H26O7 | 366.1679 | 389.1577 (389.1571) [M + Na]+ | 229.1203, 135.1152 | - | - | [20,21] |
| Flavonoids | |||||||||
| 23 | 8.1 | 3,3′,4′,5,7-pentahydroxy-6-methoxyflavone; 3-O-[α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside] | C28H32O17 | 640.1639 | 641.1703 (641.1712) [M + H]+ | 347.0745 | - | - | [20] |
| 24 | 8.5 | 3′,4′,5,7-tetrahydroxy-3-methoxyflavone; 7-O-β-D-glucopyranoside | C22H22O12 | 478.1111 | 479.1176 (479.1184) [M + H]+ | 302.0405 | - | - | [20] |
| 25 | 9.6 | 3′,4′,5,7-tetrahydroxy-3,6-dimethoxyflavone; 7-O-β-D-glucopyranoside | C23H24O13 | 508.1217 | 509.1284 (509.1290) [M + H]+ | 347.0762, 331.0434, 314.0487, 289.0329, 105.0687, 91.0534 | 507.1149 (507.1144) [M-H]− | 492.0915, 345.0626, 329.0311, 314.0069 | [20] |
| 26 | 15.3 | Luteolin | C15H10O6 | 286.0477 | 287.0548 (287.0550) [M + H]+ | - | 285.0406 (285.0405) [M-H]- | - | [29] |
| 27 | 15.4 | Quercetin | C15H10O7 | 302.0427 | 303.0491 (303.0499) [M + H]+ | 153.0145 | 301.0354 (301.0356) [M-H]− | 151.0033 | [21] |
| 28 | 15.9 | Tetrahydroxy-methoxyflavone | C16H12O7 | 316.0583 | 317.0652 (317.0656) [M + H]+ | 274.0463, 168.0054, 140.0100 | 315.0516 (315.0510) [M-H]− | - | [29] |
| 29 | 16.1 | 3′,4′,5,5′,7-pentahydroxyflavone; 3′-Me ether | C16H12O7 | 316.0583 | - | - | 315.0511 (315.0510) [M-H]− | 300.0279, 271.0250, 243.0304, 227.0349 | [25,29] |
| 30 | 17.4 | ||||||||
| 31 | 16.5 | 3′,4′,5,6-tetrahydroxy-3,7-dimethoxyflavone | C17H14O8 | 346.0689 | - | - | 345.0619 (345.0616) [M-H]− | 287.0195, 149.0246 | [25,29] |
| 32 | 18.0 | ||||||||
| 33 | 17.3 | Quercetin 3-O-methyl ether | C16H12O7 | 316.0583 | 317.0658 (317.0656) [M + H]+ | 301.0343, 274.0465, 137.0233 | 315.0512 (315.0510) [M-H]− | 300.0277, 271.0250, 243.0307, 227.0348, 199.0398 | [21] |
| 34 | 17.8 | Axillarin | C17H14O8 | 346.0689 | 347.0762 (347.0761) [M + H]+ | 289.0336, 269.0439, 203.0335, 137.0232 | - | - | [29] |
| 35 | 21.7 | 3′,4′,5′,6,7-pentahydroxyflavone; 3′,5′-dimethyl ether | C17H14O7 | 330.0740 | - | - | 329.0670 (329.0667) [M-H]− | 271.0252 | [25,29] |
| 36 | 28.0 | Dihydroxy-trimethoxyflavone | C18H16O7 | 344.0896 | 345.0961 (345.0969) [M + H]+ | 329.0652, 287.0536, 269.0436, 169.0129 | - | - | [25,29] |
| Others | |||||||||
| 37 | 5.8 | p-Coumaric acid | C9H8O3 | 164.0473 | (165.0546) 165.0546 [M + H]+ | - | 163.0398 (163.0401) [M-H]− | 119.0502 | [21] |
| 38 | 16.7 | 6-Methyl-2-methylene-6-octene-triol | C10H18O3 | 186.1256 | - | - | 185.1186 (185.1183) [M-H]− | 167.1077 | [20] |
| 39 | 16.9 | 3,4,5-tri-O-caffeoylquinic acid | C34H30O15 | 678.1585 | - | - | 677.1524 (677.1512) [M-H]− | 515.1198, 409.0245, 353.0822, 329.0673, 285.0407, 271.0257, 243.0302, 191.0571, 179.0351, 173.0458, 161.0243 | [22] |
| 40 | 46.2 | Docosyl p-coumarate | C31H52O3 | 472.3916 | - | - | 471.3848 (471.3844) [M-H]− | - | [30,31] |
| 41 | 49.1 | Icosy p-coumarate | C29H48O3 | 444.3603 | - | - | 443.3536 (443.3531) [M-H]− | - | [30,31] |
| 42 | 52.2 | Octadecyl p-coumarate | C27H44O3 | 416.3290 | - | - | 415.3226 (415.3218) [M-H]− | - | [30,31] |
| 43 | 56.5 | Tetracosyl p-coumarate | C33H56O3 | 500.4229 | - | - | 499.4159 (499.4157) [M-H]− | - | [30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukatay, U.; Samy, M.N.; Avula, B.; Katragunta, K.; Kemelbek, M.; Zhubanova, A.; Khan, I.A.; Ross, S.A. Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak. Molecules 2023, 28, 2908. https://doi.org/10.3390/molecules28072908
Mukatay U, Samy MN, Avula B, Katragunta K, Kemelbek M, Zhubanova A, Khan IA, Ross SA. Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak. Molecules. 2023; 28(7):2908. https://doi.org/10.3390/molecules28072908
Chicago/Turabian StyleMukatay, Umit, Mamdouh Nabil Samy, Bharathi Avula, Kumar Katragunta, Moldir Kemelbek, Azhar Zhubanova, Ikhlas A. Khan, and Samir Anis Ross. 2023. "Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak" Molecules 28, no. 7: 2908. https://doi.org/10.3390/molecules28072908
APA StyleMukatay, U., Samy, M. N., Avula, B., Katragunta, K., Kemelbek, M., Zhubanova, A., Khan, I. A., & Ross, S. A. (2023). Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak. Molecules, 28(7), 2908. https://doi.org/10.3390/molecules28072908










