Abstract
Recently, polyolefin thermoplastic elastomers can be obtained directly using ethylene as a single feedstock via α-diimine nickel-catalyzed ethylene chain walking polymerization. Here, a new range of bulky acenaphthene-based α-diimine nickel complexes with hybrid o-phenyl and -diarylmethyl anilines were constructed and applied to ethylene polymerization. All the nickel complexes under the activation of excess Et2AlCl exhibited good activity (level of 106 g mol−1 h−1) and produced polyethylene with high molecular weight (75.6–352.4 kg/mol) as well as proper branching densities (55–77/1000C). All the branched polyethylenes obtained exhibited high strain (704–1097%) and moderate to high stress (7–25 MPa) at break values. Most interestingly, the polyethylene produced by the methoxy-substituted nickel complex exhibited significantly lower molecular weights and branching densities, as well as significantly poorer strain recovery values (48% vs. 78–80%) than those by the other two complexes under the same conditions.
1. Introduction
As a kind of high-performance polyolefin material, polyolefin thermoplastic elastomers (TPE) can be processed at high temperatures and exhibit rubbery properties at room temperature. Such polyolefin materials thus have the advantages of both rubber and plastic. They are widely used in the automotive industry as high-performance accessory materials and photovoltaic film fields [1,2]. Most polyolefin TPEs in the industry today are available through metallocene-catalyzed copolymerization of ethylene with α-olefins [3,4]. Recently, it is possible to prepare polyolefin TPEs directly by using ethylene as a single raw material via α-diimine nickel-catalyzed ethylene chain walking polymerization [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. The direct preparation of polyolefin TPEs with only ethylene feedstock is extremely attractive and shows great application potential. For example, recently, Chen, Jian, Sun and our group have designed a series of novel unsymmetrical nickel α-diimine catalysts (Scheme 1A–D) to catalyze the polymerization of ethylene to obtain high-performance polyethylene TPEs [5,6,8,11,15,20,21]. Controlling the ratio of chain walking to chain growth by the reaction temperature and ethylene pressure to obtain polyethylene of a certain crystallinity is the key to preparing high-performance polyolefin TPEs. High molecular weight is one of the necessary requirements [17]. Moreover, some symmetrical bulky nickel α-diimine catalysts (Scheme 1E–G) have also been shown to catalyze the polymerization of ethylene to obtain polyethylene material with high elastic recovery [7,9,10]. The carbon spectrum analysis of the obtained polyethylene shows that the distribution of branching in these branched polyethylenes is random and methyl branching dominates. Different from α-diimine nickel catalysts, the corresponding α-diimine palladium catalysts tend to possess excessive chain-walking ability, producing fully amorphous polyethylene [22,23,24,25,26,27,28,29,30,31,32,33]. We have recently succeeded in suppressing chain walking in palladium-catalyzed ethylene polymerization using a bulky o-aryl substitution strategy [34,35]. By selecting suitable o-aryl substituents, the α-diimine palladium catalysts (Scheme 1H) can also catalyze the polymerization of ethylene to obtain the corresponding polyethylene TPEs [36]. What is more, polar functionalized polyethylene TPEs can also be prepared by co-polymerization of ethylene with polar monomers [36]. In this study, a series of new hybrid bulky acenaphthene-based α-diimine Ni(II) catalysts were synthesized and applied to prepare polyethylene TPEs with excellent recovery performance.
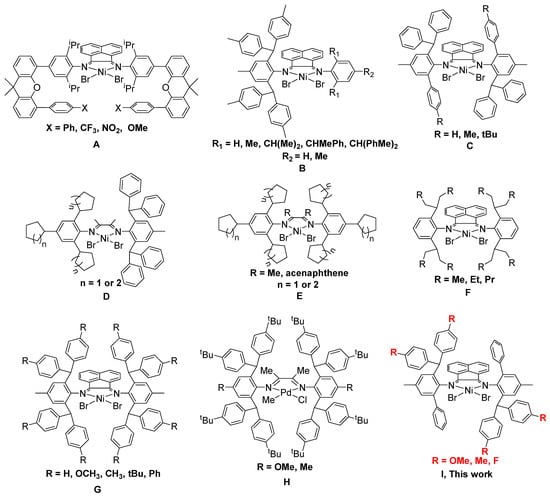
Scheme 1.
Bulky α-diimine Ni(II) and Pd(II) catalysts for the preparation of polyethylene TPEs in previous works (A–H) and our current work (I).
2. Results and Discussion
2.1. Synthesis and Characterization of α-Diimine Ni(II) Complexes
The hybrid bulky acenaphthene-based α-diimine ligands (L1-L3) were obtained based on previously reported literature [37]. The target nickel complexes (Ni1-Ni3) were yielded by reacting the ligands with equivalent (1,2-dimethoxyethane)nickel dibromide (DMENiBr2) in dichloromethane (DCM) at an ambient temperature (Scheme 2). Ideal yields (63–82%) could be achieved and all the nickel complexes were characterized by elemental analysis and Infrared Spectrum (Figures S13–S15). As shown in Figure 1, the single crystal of Ni2 was fortunately obtained by layering its DCM solution with diethyl ether in the glove box at room temperature. The nickel center adopts a slightly distorted square planar geometry, which is inconsistent with the classical tetrahedral conformation adopted by most previous α-diimine nickel complexes [38,39,40,41,42]. This may be caused by the squeezing of the surrounding bulky o-aryl substituents. Moreover, the Ni(II) complex adopts the anti-configuration with ortho-phenyl groups located on the opposite side.
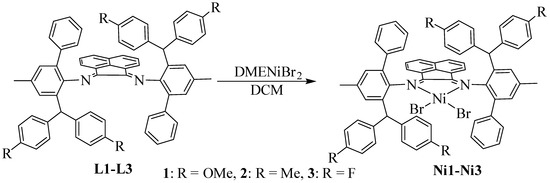
Scheme 2.
Synthesis of hybrid bulky acenaphthene-based α-diimine Ni(II) complexes.
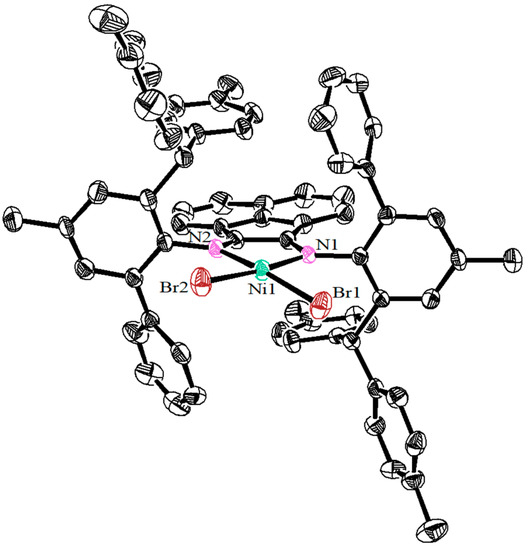
Figure 1.
Single crystal structure of Ni2 (2233726) presented by ORETP (Oak Ridge Thermal Ellipsoid Plot) with 30% probability level, and H atoms were omitted for clarity.
2.2. Ethylene Polymerization
With the activator of 300 eq. Et2AlCl, all the nickel complexes exhibited high catalytic activities in ethylene polymerization in the level of 106 g mol−1 h−1, over a wide temperature range from 30 °C to 70 °C (Table 1). The polymerization activity gradually decreased with raising temperature in most trials, where the highest activity was obtained by Ni1 at 30 °C (Figure 2a). High-molecular-weight (Figures S10–S12) polyethylene with moderate to high branching density (Figures S1–S3 and S16) were yielded and the molecular weight gradually decreased with raising temperatures in most trials, where the highest molecular weight was obtained by Ni3 at 30 °C (Figure 2b). The above phenomena could be explained that higher temperatures promote more chain transfer than chain growth in polymerization process. Most interestingly, the polyethylene generated by the methoxy-substituted Ni1 exhibited significantly lower molecular weights and branching densities than those by the other two complexes. This may be caused by electron-rich aryl-metal weak neighbor–group interactions, which are described in many known reports [43,44,45,46,47]. The weak neighbor–group interactions between p-methoxyphenyl and nickel center promote the chain transfer and retard the chain walking by suppressing β-H elimination in ethylene polymerization (Figure 3).

Table 1.
Ethylene Polymerization a.
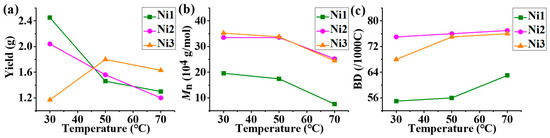
Figure 2.
The comparison of polyethylene catalyzed by Ni1-3 within a certain temperature range (30–70 °C): (a) yield; (b) molecular weight; (c) branching density.
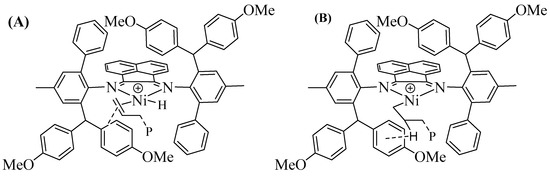
Figure 3.
The weak neighbor–group interactions promote the chain transfer (A) and suppress β-H elimination (B) in ethylene polymerization.
We further analyzed the mechanical properties of all the branched polyethylenes generated by Ni1-3. The polyethylene products generated by Ni1 showed both high stress (18–25 MPa) and high strain (943–1019%) at break values while those yielded by Ni2-3 displayed moderate stress (7–15 MPa) and high strain (704–1097%) at break values (Table 2, Figure 4). The lower branching density and a higher melting point (Figures S4–S9) of polyethylene produced by Ni1 are conducive to having higher tensile strength. Similar phenomena have also been reported in other literature [17,36]. A deeper reason may be that a higher melting point and lower branching density are conducive to increasing the crystallinity of the polymer and thus enhancing its physical crosslinking strength. These polyethylene mechanical parameters are susceptible to variations in polymerization temperature. Typically, polyethylene obtained at high temperatures tends to have a lower Young’s modulus and a higher strain at break values (Table 2, Figure 4). This is primarily because high temperatures facilitate the chain walking of the catalyst and result in higher-branched polyethylene with correspondingly lower polyethylene crystallinity. Hysteresis experiments were carried out to investigate strain recovery (SR) values of the polyethylene samples obtained at 70 °C by Ni1-3. As shown in Figure 5, the polyethylene produced by Ni1 at 70 °C presented a moderate recovery performance (SR = 48%) while those yielded by Ni2-3 displayed better ones (SR = 78–80%). This is also the result of the higher melting point and lower branching density of the polyethylene obtained by Ni1 than those by Ni2-3. The above results indicate that we can obtain polyethylene TPEs with an excellent performance by Ni2-3-catalyzed ethylene polymerization. The 13C analysis of the sample from entry 9, Table 1 indicates that the polyethylene contains a variety of branching, with methyl branching dominating and more than 11% of the branching above C3+. The presence of significantly more proportional long-chain branching may help improve elastic recovery.

Table 2.
Mechanical Properties for Different Polyethylene Samples a.
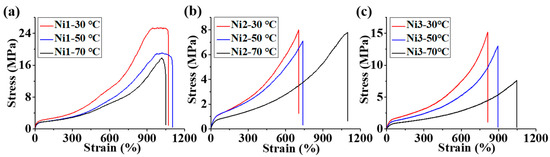
Figure 4.
The comparisons of stress–strain curves for polyethylene samples produced by Ni1-3 at different temperatures (a–c).
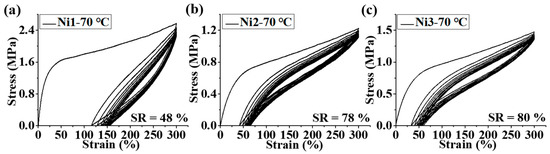
Figure 5.
Hysteresis experiment plots of polyethylene samples produced by Ni1-3 at 70 °C for ten cycles at 300% strain (a–c).
3. Conclusions
A series of bulky unsymmetrical acenaphthene-based α-diimine nickel complexes were synthesized and employed for the ethylene polymerization in this study. These complexes all showed high catalytic activity (~106 g mol−1 h−1) and the obtained polyethylene products possessed high molecular weights (75.6–352.4 kg/mol) and proper branching densities (55–77/1000C). Most interestingly, the polyethylene produced by the methoxy-substituted Ni1 exhibited much lower molecular weights and branching densities than those by Ni2-3. All the branched polyethylenes produced by Ni1-3 exhibited moderate to high stress (7–25 MPa) as well as high strain (704–1097%) at break values, and moderate to high strain recovery (48–80%) in the tensile tests. Overall, polyethylene TPEs with ideal performance were successfully prepared by Ni2-3-catalyzed ethylene polymerization, which has great potential in the automotive industry and photovoltaic film fields.
4. Experiments
4.1. General Considerations
Unless otherwise stated, all the chemicals were purchased commercially. Polymerization reactions in this work were all performed via standard Schlenk techniques or in a glove box with N2 atmosphere. Deuterated solvents were dried and distilled before being used for NMR. A JEOL JNM-ECZ600R 600 spectrometer (JEOL, Tokyo, Japan) or JEOL JNM-ECZ400R 400 spectrometer (JEOL, Tokyo, Japan) was used to get 1H and 13C NMR spectra at room temperature. The chemical shifts of the 1H and 13C NMR spectra were referenced to the residual solvent; the coupling constants are in Hz. Elemental analysis was performed by the Analytical Center of Anhui University. X-ray diffractometer (XRD) (Bruker Smart CCD) (Bruker, Billerica, MA, USA) was applied to characterize the crystal structure at 298(2) K with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Size exclusion chromatography (SEC) was used to determine the samples’ molecular weight and its distribution at 150 °C with a PL 210 equipped with three columns one Shodex AT-803S and two Shodex AT-806MS (Agilent Technologies, Santa Clara, CA, USA). Differential scanning calorimetry (DSC) analysis was carried out on a TA Instruments Q25 (TA Instruments, Newcastle, DE, USA).
4.2. Synthesis of Nickel Complexes
Typically, 1 equivalent (DME) NiBr2 and 0.2 mmol ligand were fully dissolved in DCM by vigorous stirring overnight. Subsequently, the brown powders were collected after removing the solvent and washed with hexanes (5 mL) for two times. The resultant product was vacuum dried, finally giving the nickel complexes.
- Ni1 (149 mg, 63 %). Anal. Calcd for (C68H56Br2N2NiO4): C, 69.00; H, 4.77; N, 2.37. Found: C, 69.12; H, 4.67; N, 2.31. IR: C=N (1613, 1646 cm−1).
- Ni2 (184 mg, 82 %). Anal. Calcd for (C68H56Br2N2Ni): C, 72.94; H, 5.04; N, 2.50. Found: C, 72.87; H, 5.12; N, 2.63. IR: C=N (1630, 1663 cm−1).
- Ni3 (166 mg, 73 %). Anal. Calcd for (C64H44Br2F4N2Ni): C, 67.69; H, 3.91; N, 2.47. Found: C, 67.76; H, 3.87; N, 2.53. IR: C=N (1606–1658 cm−1).
4.3. Preparation of Polyethylene TPEs by Nickel Complexes
At first, we dried the glass reactor (350 mL) connected with a high gas pressure line in a blast oven at 60 °C, over 24 h. Then, 300 eq. Et2AlCl and 40 mL toluene were mixed in the 350 mL flask. 2 μmol Ni catalyst was dissolved in 2 mL DCM and added to the reaction system by injection. Subsequently, the polymerization was carried out with vigorously stirring at a proper temperature and ethylene pressure of 6 atm for 30 min. It is worth noting that all the above procedures were performed in the glove box with N2 atmosphere. Finally, the polyethylene products were precipitated in ethanol, followed by vacuum drying at 25 °C for about 24 h.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28052266/s1, Figures S1–S3: 1H NMR spectrum of the polymer. Figures S4–S9: DSC of the polymer. Figures S10–S12: GPC of the polymer. Figures S13–S15: IR of the complex. Figure S16: 13C NMR spectrum of the polymer. CCDC number of Ni2 is 2233726. The data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 January 2023).
Author Contributions
Conceptualization, S.D.; methodology, H.W.; validation, S.D., H.W. and W.L.; formal analysis, S.D.; investigation, H.W.; resources, S.D. and H.W.; data curation, S.D.; writing—original draft preparation, S.D.; writing—review and editing, S.D. and H.W.; supervision, S.D.; project administration, S.D.; funding acquisition, M.Z. and S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the open fund of Information Materials and Intelligent Sensing Laboratory of Anhui Province (IMIS202208), Research Start-up Foundation of Hefei Normal University (2022rcjj56), and Natural Science Foundation of Anhui Province (2108085Y06). And The APC was funded by Natural Science Foundation of Anhui Province (2108085Y06).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no competing financial interests.
Sample Availability
Samples of the compounds are available from the authors upon reasonable request.
References
- Holden, G. Applied Plastics Engineering Handbook: Processing and Materials; Kutz, M., Ed.; William Andrew: Norwich, NY, USA, 2011; pp. 77–91. ISBN 978-1-4377-3514-7. [Google Scholar]
- Craver, C.D.; Carraher, C.E. Applied Polymer Science: 21st Century; Elsevier Science: Amsterdam, The Netherlands, 2000; ISBN 978-0-08-043417-9. [Google Scholar]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H.; Deplace, F.; Vo, G.D.; LaPointe, A.M.; Shimizu, F.; Sugano, T.; Kramer, E.J.; Fredrickson, G.H.; Coates, G.W. Allyl-terminated polypropylene macromonomers: A route to polyolefin elastomers with excellent elastic behavior. Macromolecules 2015, 48, 7489–7494. [Google Scholar] [CrossRef]
- Lian, K.B.; Zhu, Y.; Li, W.M.; Dai, S.Y.; Chen, C.L. Direct synthesis of thermoplastic polyolefin elastomers from nickel-catalyzed ethylene polymerization. Macromolecules 2017, 50, 6074–6080. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.; Li, Y.; Chen, C. Synthesis of polyolefin elastomers from unsymmetrical α-diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 9, 4143–4149. [Google Scholar] [CrossRef]
- Guo, L.; Lian, K.; Kong, W.; Xu, S.; Jiang, G.; Dai, S. Synthesis of various branched ultra-high-molecular-weight polyethylenes using sterically hindered acenaphthene-based α-diimine Ni (II) Catalysts. Organometallics 2018, 37, 2442–2449. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Gong, Q.; Zhang, S.; Liu, B.; Dai, S. Systematic investigations of ligand steric effects on α-diimine nickel catalyzed olefin polymerization and copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Dai, S.; Li, S.; Xu, G.; Wu, C.; Liao, Y.; Guo, L. Flexible cycloalkyl substituents in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 1393–1400. [Google Scholar] [CrossRef]
- Guo, L.; Sun, W.; Li, S.; Xu, G.; Dai, S. Bulky yet flexible substituents in insertion polymerization with α-diimine nickel and palladium systems. Polym. Chem. 2019, 10, 4866–4871. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Ma, Y.; Guo, C.Y.; Solan, G.A.; Sun, Y.; Sun, W. Elastomeric polyethylenes accessible via ethylene homo-polymerization using an unsymmetrical α-diimino-nickel catalyst. Polym. Chem. 2017, 8, 2785–2795. [Google Scholar] [CrossRef]
- Chen, S.; Pan, R.; Liu, Y.; Lu, X. Bulky o-phenylene-bridged bimetallic α-diimine Ni(II) and Pd(II) catalysts in ethylene (Co)polymerization. Organometallics 2021, 40, 3703–3711. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, G.; Wang, H.; Dai, S. Synthesis of thermoplastic polyethylene elastomers and ethylene-methyl acrylate copolymers using methylene-bridged binuclear bulky dibenzhydryl α-diimine Ni(II) and Pd(II) catalysts. Eur. Polym. J. 2022, 168, 111105. [Google Scholar] [CrossRef]
- Wang, H.; Duan, G.; Fan, H.; Dai, S. Second coordination sphere effect of benzothiophene substituents on chain transfer and chain walking in ethylene insertion polymerization. Polymer 2022, 245, 24707. [Google Scholar] [CrossRef]
- Hai, Z.; Lu, Z.; Li, S.; Cao, Z.; Dai, S. Synergistic effect of rigid and flexible substituents in insertion polymerization with α-diimine nickel and palladium catalysts. Polym. Chem. 2021, 12, 4643–4653. [Google Scholar] [CrossRef]
- Dai, S.; Li, G.; Lu, W.; Liao, Y.; Fan, W. Suppression of chain transfer via restricted rotation effect of dibenzosuberyl substituents in polymerization catalysis. Polym. Chem. 2021, 12, 3240–3249. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Dai, S. Synthesis of polyethylene thermoplastic elastomer by using robust α-diimine Ni(II) catalysts with abundant tBu substituents. J. Polym. Sci. 2021, 59, 638–645. [Google Scholar] [CrossRef]
- Li, S.; Xu, G.; Dai, S. A remote nonconjugated electron effect in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 2692–2699. [Google Scholar] [CrossRef]
- Lu, W.; Fan, W.; Dai, S. A Rigid-flexible double-layer steric strategy promoting ethylene polymerization and copolymerization in alkane solvents. Inorg. Chem. Front. 2023, 10, 108–117. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Jian, Z. Comprehensive studies of the ligand electronic effect on unsymmetrical α-diimine nickel(II) promoted ethylene (Co)polymerizations. Polym. Chem. 2020, 11, 4005–4012. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Zhang, Y.; Jian, Z. Asymmetrical strategy makes significant differences in α-diimine nickel and palladium catalyzed ethylene (Co)polymerizations. ChemCatChem 2020, 12, 2497–2505. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)-and Ni(II)-based catalysts for polymerization of ethylene and α-olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-metal catalysts for ethylene homo-and copolymerization. Chem. Rev. 2000, 100, 1169–1204. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, C. (α-Diimine) palladium catalyzed ethylene polymerization and (Co)polymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Popeney, C.; Guan, Z. Ligand electronic effects on late transition metal polymerization catalysts. Organometallics 2005, 24, 1145–1155. [Google Scholar] [CrossRef]
- Popeney, C.S.; Camacho, D.H.; Guan, Z. Efficient incorporation of polar comonomers in copolymerizations with ethylene using a cyclophane-based Pd(II) α-diimine catalyst. J. Am. Chem. Soc. 2007, 129, 10062–10063. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Campos, J.; Daugulis, O.; Brookhart, M. Living polymerization of ethylene and copolymerization of ethylene/methyl acrylate using “sandwich” diimine palladium catalysts. ACS Catal. 2015, 5, 456–464. [Google Scholar] [CrossRef]
- Zhong, S.; Tan, Y.; Zhong, L.; Gao, J.; Liao, H.; Jiang, L.; Gao, H.; Wu, Q. Precision synthesis of ethylene and polar monomer copolymers by palladium-catalyzed living coordination copolymerization. Macromolecules 2017, 50, 5661–5669. [Google Scholar] [CrossRef]
- Xiang, P.; Ye, Z.; Subramanian, R. Synthesis and characterization of low-and medium-molecular-weight hyperbranched polyethylene by chain walking ethylene polymerization with Pd-diimine catalysts. Polymer 2011, 52, 5027–5039. [Google Scholar] [CrossRef]
- Dall’Anese, A.; Rosar, V.; Cusin, L.; Montini, T.; Balducci, G.; D’Auria, I.; Pellecchia, C.; Fornasiero, P.; Felluga, F.; Milani, B. Palladium-catalyzed ethylene/methyl acrylate copolymerization: Moving from the acenaphthene to the phenanthrene skeleton of α-diimine ligands. Organometallics 2019, 38, 3498–3511. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Mecking, S.; Jian, Z. Ultrahigh branching of main-chain-functionalized polyethylenes by inverted insertion selectivity. Angew. Chem. Int. Ed. 2020, 59, 14296–14302. [Google Scholar] [CrossRef]
- Alberoni, C.; D’Alterio, M.C.; Balducci, G.; Immirzi, B.; Polentarutti, M.; Pellecchia, C.; Milani, B. Tunable “in-chain” and “at the end of the branches” methyl acrylate incorporation in the polyolefin skeleton through Pd(II) catalysis. ACS Catal. 2022, 12, 3430–3443. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, Y.; Cui, L.; Mu, H.; Jian, Z. Pentiptycenyl substituents in insertion polymerization with α-diimine nickel and palladium Species. Organometallics 2019, 38, 2075–2083. [Google Scholar] [CrossRef]
- Dai, S.; Sui, X.; Chen, C. Highly robust palladium (II) α-diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefin polymerization and copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Dai, S.; Li, S.; Xu, G.; Chen, C. Direct synthesis of polar functionalized polyethylene thermoplastic elastomer. Macromolecules 2020, 53, 2539–2546. [Google Scholar] [CrossRef]
- Lu, W.; Xu, G.; Chang, G.; Wang, H.; Dai, S. Synthesis of highly branched polyethylene and ethylene-ma copolymers using hybrid bulky α-diimine Pd(II) catalysts. J. Organomet. Chem. 2021, 953, 122118. [Google Scholar] [CrossRef]
- Zheng, H.; Li, Y.; Du, W.; Cheung, C.S.; Li, D.; Gao, H.; Deng, H.; Gao, H. Unprecedented square-planar α-diimine dibromonickel complexes and their ethylene polymerizations modulated by Ni-phenyl interactions. Macromolecules 2022, 55, 3533–3540. [Google Scholar] [CrossRef]
- Zhong, L.; Li, G.; Liang, G.; Gao, H.; Wu, Q. Enhancing thermal stability and living fashion in α-diimine–nickel-catalyzed (Co)polymerization of ethylene and polar monomer by increasing the steric bulk of ligand backbone. Macromolecules 2017, 50, 2675–2682. [Google Scholar] [CrossRef]
- Kanai, Y.; Foro, S.; Plenio, H. Bispentiptycenyl-diimine-nickel complexes for ethene polymerization and copolymerization with polar monomers. Organometallics 2019, 38, 544–551. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Luo, Y.; He, S.; Fan, W.; Dai, S. The unexpected effect of catalyst’s structural symmetry on branching microstructure of polyethylene in late transition metal polymerization catalysis. ACS Catal. 2023, 13, 725–734. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Fu, Z.; Qin, Y.; Zhang, Q.; Fan, Z. Combining 1,2-diketopyracene with bulky benzhydryl-substituted anilines to obtain highly active α-diimine nickel catalysts at elevated temperature. J. Cat. 2022, 413, 311–320. [Google Scholar] [CrossRef]
- Falivene, L.; Wiedemann, T.; Göttker-Schnetmann, I.; Caporaso, L.; Cavallo, L.; Mecking, S. Control of chain walking by weak neighboring group interactions in unsymmetrical catalysts. J. Am. Chem. Soc. 2018, 140, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.J.; McInnis, J.P.; Chen, C.; Weberski, M.P.; Motta, A.; Delferro, M.; Marks, T.J. Ni(II) phenoxyiminato olefin polymerization catalysis: Striking coordinative modulation of hyperbranched polymer microstructure and stability by a proximate sulfonyl group. ACS Catal. 2014, 4, 999–1003. [Google Scholar] [CrossRef]
- Fan, H.; Xu, G.; Wang, H.; Dai, S. Direct synthesis of hyperbranched ethene oligomers and ethene-MA Co-oligomers using iminopyridyl systems with weak neighboring group interactions. J. Polym. Sci. 2022, 60, 1944–1953. [Google Scholar] [CrossRef]
- Fan, H.; Chang, G.; Wang, H.; Dai, S. Second coordination sphere effect of benzothiophene groups enhancing chain transfer in ethylene (Co)oligomerization. New J. Chem. 2023, 47, 2488–2494. [Google Scholar] [CrossRef]
- Fan, H.; Chang, G.; Bi, H.; Gui, X.; Wang, H.; Xu, G.; Dai, S. Facile synthesis of hyperbranched ethylene oligomers and ethylene-methyl acrylate Co-oligomers with different microscopic chain architectures. ACS Polym. Au 2022, 2, 88–96. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).