Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter
Abstract
1. Introduction
2. Results
2.1. Physiological Parameters
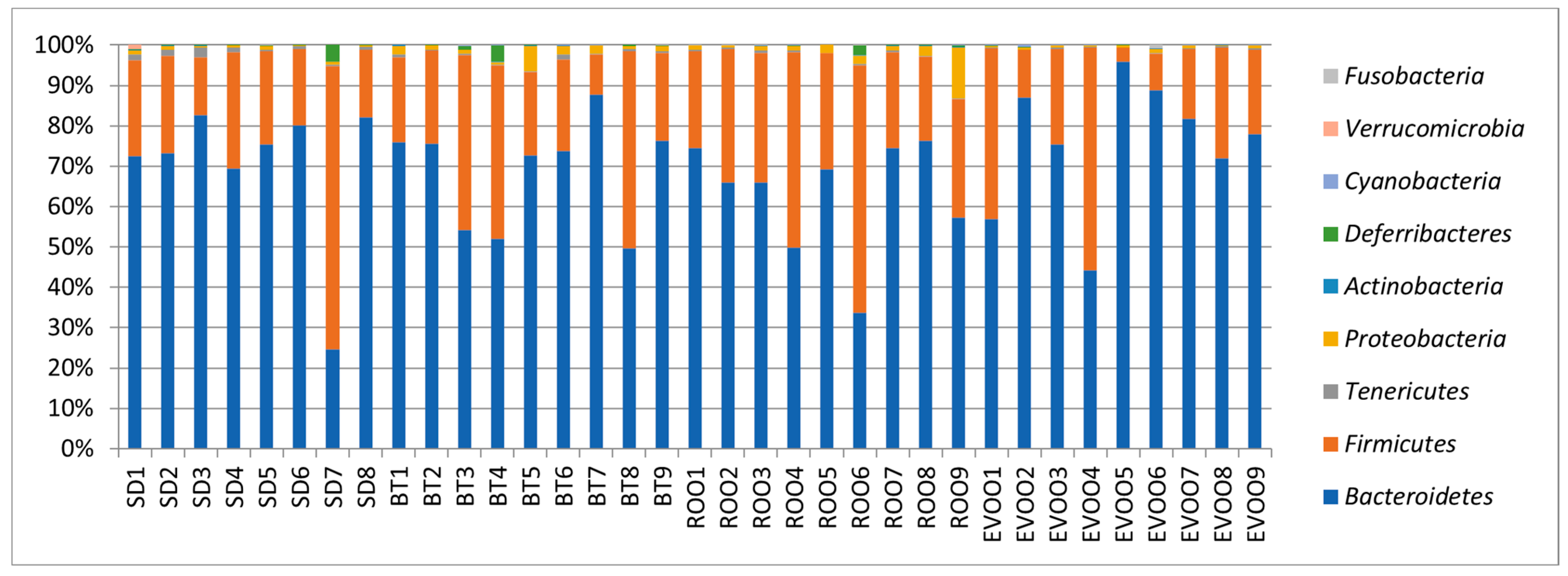
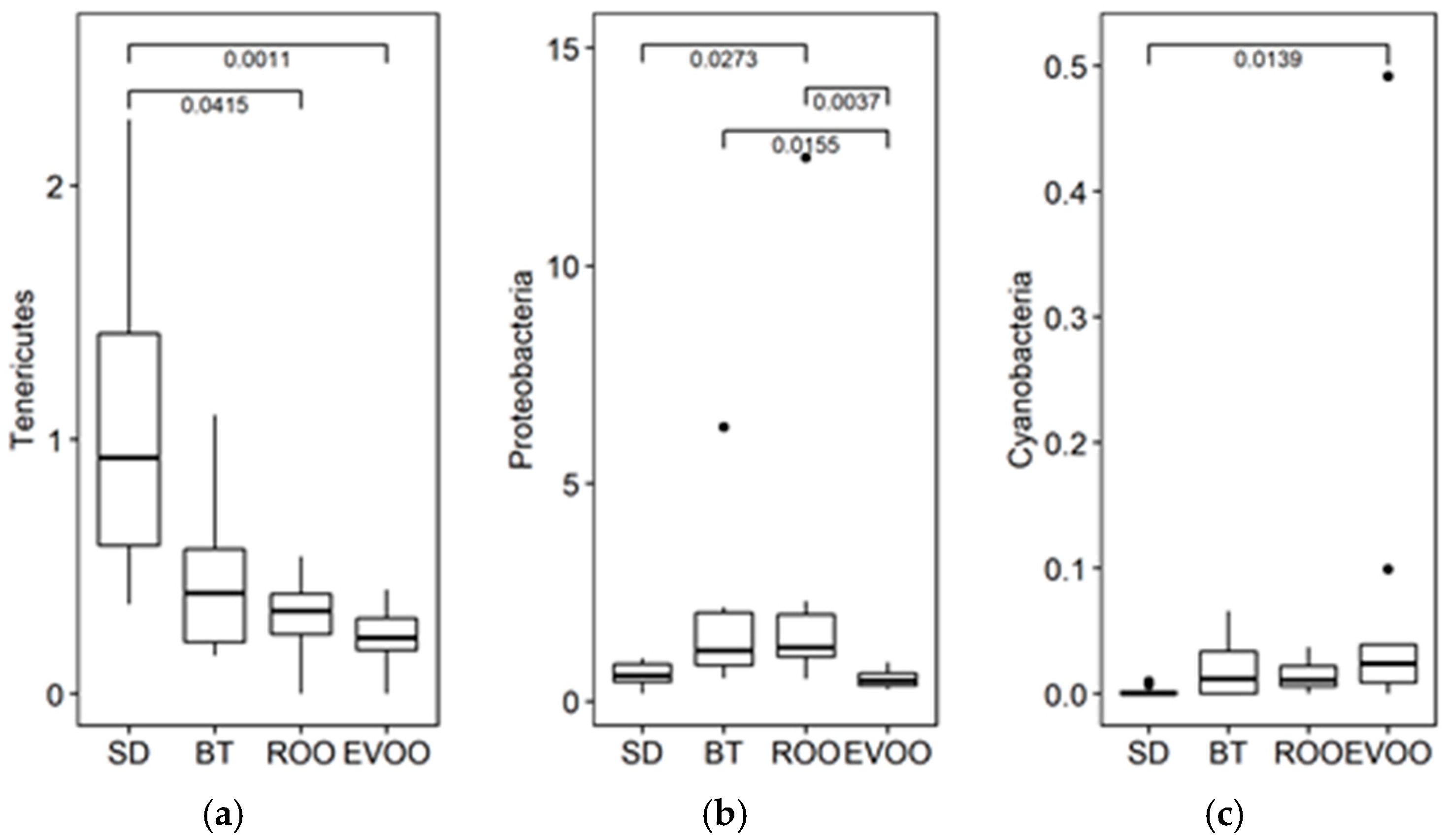
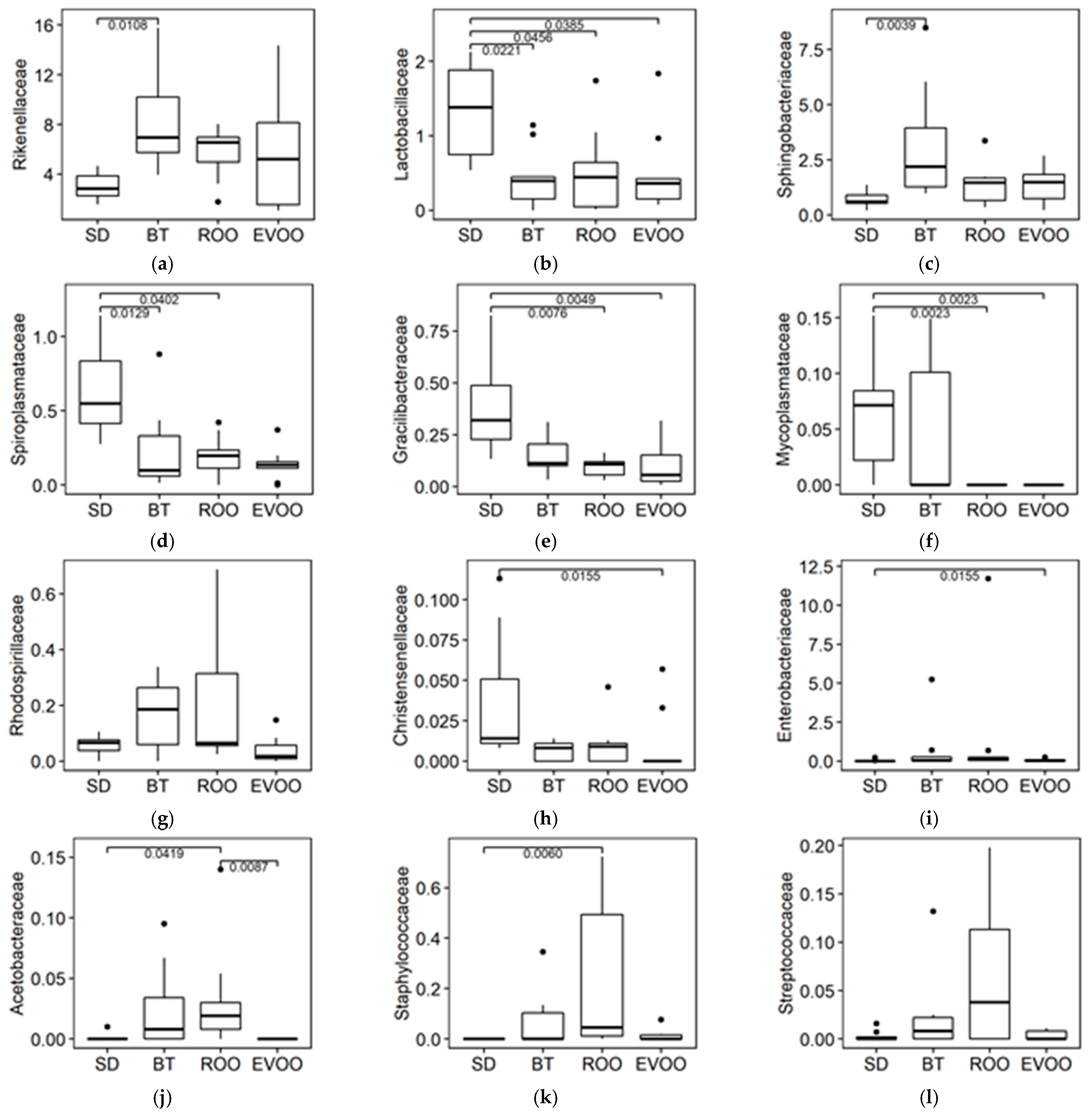
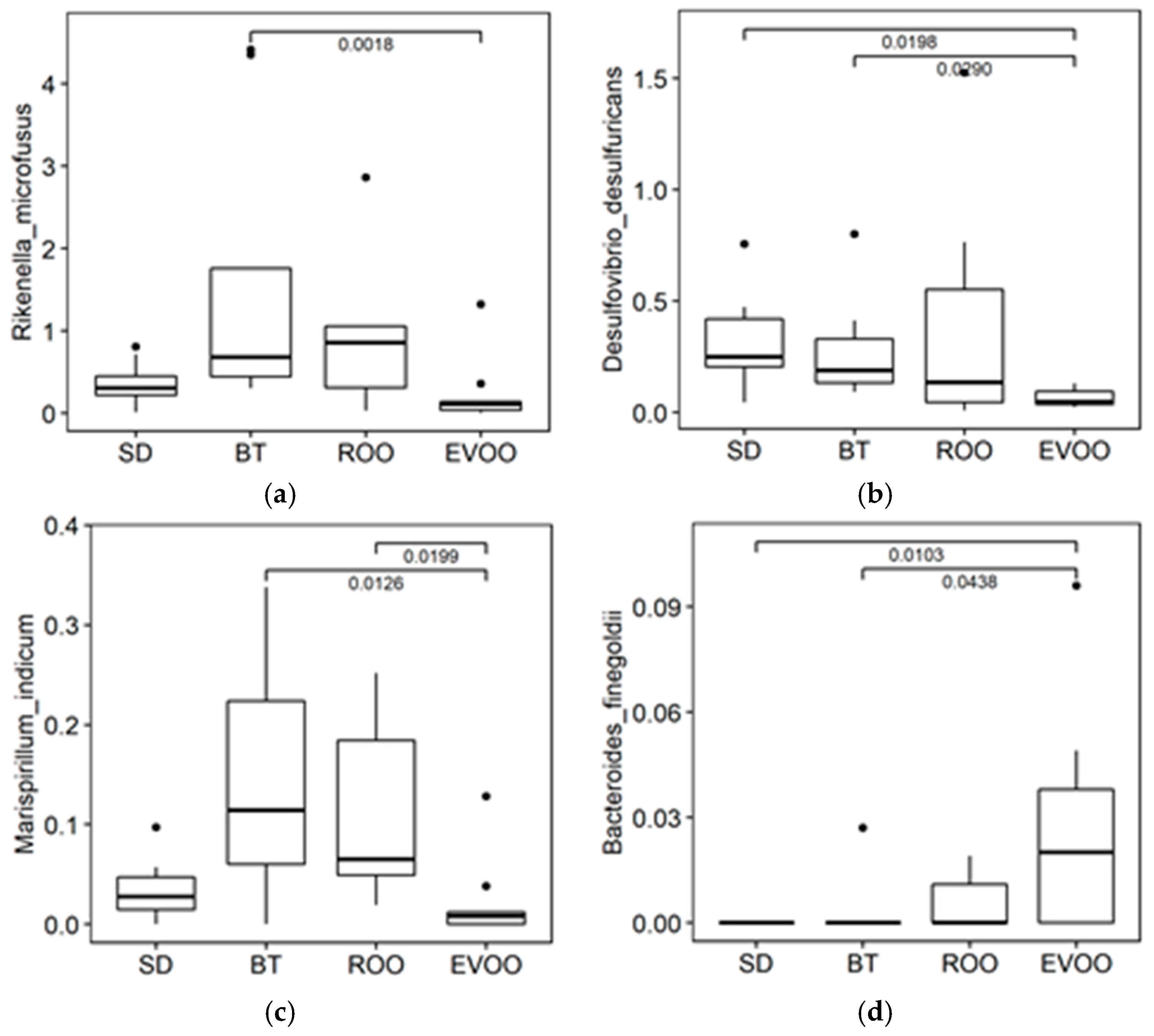
2.2. Sequencing, Taxa Adscription, Percentage Comparison and Multiple Regression Models
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Bacterial Biodiversity
4.3. Statistical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Zhang, M.; Wang, S.; Han, R.; Cao, Y.; Hua, W. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010, 4, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Miri, S.; Yeo, J.; Abubaker, S.; Hammami, R. Neuromicrobiology, an emerging neurometabolic facet of the gut microbiome? Front. Microbiol. 2023, 14, 1098412. [Google Scholar] [CrossRef] [PubMed]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-virgin olive oil and the gut-brain axis: Influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Martín-Calvo, N. The major European dietary patterns and metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 265–271. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abriouel, H.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Effect of virgin and refined olive oil consumption on gut microbiota. Comparison to butter. Food Res. Int. 2014, 64, 553–559. [Google Scholar] [CrossRef]
- Hidalgo, M.; Prieto, I.; Abrioue, H.; Villarejo, A.B.; Ramírez, M.; Cobo, A.; Benomar, N.; Gálvez, A.; Martínez-Cañamero, M. Changes in Gut Microbiota Linked to a Reduction in Systolic Blood Pressure in Spontaneously Hypertensive Rats Fed an Extra Virgin Olive Oil-Enriched Diet. Plant Foods Hum. Nutr. 2018, 73, 1–6. [Google Scholar] [CrossRef]
- Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Abriouel, H.; Gálvez, A.; Martínez-Cañamero, M. Influence of a diet enriched in virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS ONE 2018, 13, e0190368. [Google Scholar] [CrossRef]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Kumric, M.; Vilovic, M.; Martinovic, D.; Tomic, I.J.; Krnic, M.; Ticinovic Kurir, T.; Bozic, J. Effects of Olive Oil and Its Components on Intestinal Inflammation and Inflammatory Bowel Disease. Nutrients 2022, 14, 757. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Urbano, G.; López-Jurado, M.; Nestares, T.; Gomez, M.C.; Mir, A.; Ros, E.; Mataix, J.; Gil, A. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J. Nutr. 1999, 129, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Bemúdez, B.; López, S.; Abia, R.; Villar, J.; Muriana, F.J. Minor compounds of olive oil have postprandial anti-inflammatory effects. Br. J. Nutr. 2007, 98, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filesi, C.; Varì, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin olive oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Investig. 2016, 39, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.; Prieto, I.; Hidalgo, M.; Segarra, A.B.; Martínez-Rodríguez, A.M.; Cobo, A.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. Refined versus Extra Virgin Olive Oil High-Fat Diet Impact on Intestinal Microbiota of Mice and Its Relation to Different Physiological Variables. Microorganisms 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Andújar-Tenorio, N.; Prieto, I.; Cobo-Molinos, A.; Martínez-Rodríguez, A.M.; Hidalgo, M.; Segarra, A.B.; Ramírez, M.; Gálvez, A.; Martínez-Cañamero, M. High fat diets induce early changes in gut microbiota that may serve as markers of ulterior altered physiological and biochemical parameters related to metabolic syndrome. Effect of virgin olive oil in comparison to butter. PLoS ONE 2022, 17, e0271634. [Google Scholar] [CrossRef]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef]
- Heaver, S.L.; Johnson, E.L.; Ley, R.E. Sphingolipids in host–microbial interactions. Curr. Opin. Microbiol. 2018, 43, 92–99. [Google Scholar] [CrossRef]
- Harrison, P.J.; Dunn, T.M.; Campopiano, D.J. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep. 2018, 35, 921–954. [Google Scholar] [CrossRef]
- Alves, E.; Domingues, M.R.M.; Domingues, P. Polar Lipids from Olives and Olive Oil: A Review on Their Identification, Significance and Potential Biotechnological Applications. Foods 2018, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, G.; Piochi, M.; Pacetti, D.; Frega, N.G.; Bartolucci, E.; Scortichini, S.; Fiorini, D. Eleven Monovarietal Extra Virgin Olive Oils from Olives Grown and Processed under the Same Conditions: Effect of the Cultivar on the Chemical Composition and Sensory Traits. Foods 2020, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Rey, F.E.; Gonzalez, M.D.; Cheng, J.; Wu, M.; Ahern, P.P.; Gordon, J.I. Metabolic niche of a prominent sulfatereducing human gut bacterium. Proc. Natl. Acad. Sci. USA 2013, 110, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Devkota, S.; Chang, E.B. Interactions between Diet, Bile Acid Metabolism, Gut Microbiota, and Inflammatory Bowel Diseases. Dig. Dis. 2015, 33, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Cadby, I.T.; Faulkner, M.; Cheneby, J.; Long, J.; van Helden, J.; Dolla, A.; Cole, J.A. Coordinated response of the Desulfovibrio desulfuricans 27774, transcriptome to nitrate, nitrite and nitric oxide. Sci. Rep. 2017, 7, 16228. [Google Scholar] [CrossRef]
- Aranda-Caño, L.; Sánchez-Calvo, B.; Begara-Morales, J.C.; Chaki, M.; Mata-Pérez, C.; Padilla, M.N.; Valderrama, R.; Barroso, J.B. Post-Translational Modification of Proteins Mediated by Nitro-Fatty Acids in Plants: Nitroalkylation. Plants 2019, 8, 82. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Adhikari, K.; Chambers, E.; Chambers, D.H.; Carbonell-Barrachina, A.A. Cross-cultural perception of six commercial olive oils: A study with Spanish and US consumers. Food Sci. Technol. Int. 2015, 21, 454–466. [Google Scholar] [CrossRef]
- Uemura, K.; Mori, N. Influence of age and sex on high-fat diet-induced increase in blood pressure. Nagoya J. Med. Sci. 2006, 68, 109–114. [Google Scholar]
- Tan, Y.; Li, M.; Wu, G.; Lou, J.; Feng, M.; Xu, J.; Zhou, J.; Zhang, P.; Yang, H.; Dong, L.; et al. Short-term but not long-term high fat diet feeding protects against pressure erload-induced heart failure through activation of mitophagy. Life Sci. 2021, 272, 119242. [Google Scholar] [CrossRef]
- Oltra, L.; Reverte, V.; Tapia, A.; Moreno, J.M.; Salazar, F.J.; Llina’s, M.T. Cardiac, renal and uterine hemodynamics changes throughout pregnancy in rats with a prolonged high fat diet from an early age. PLoS ONE 2020, 15, e0234861. [Google Scholar] [CrossRef]
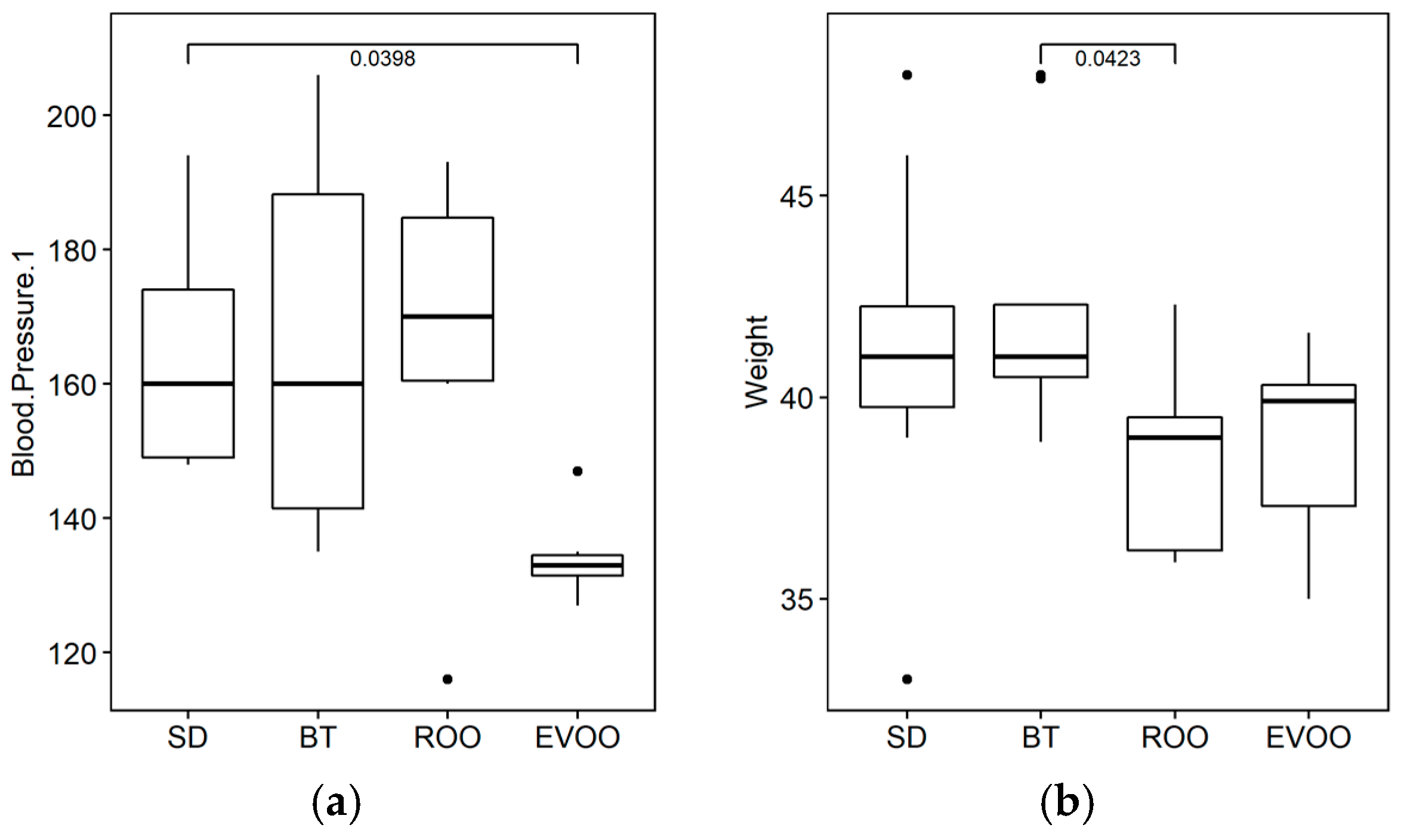

| BW | FI | WI | DIU | SBP | Glu | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 w | 12 w | 6 w | 12 w | 6 w | 12 w | 6 w * | 6 w | 12 w | 12 w | |
| 0.36 (0.0007) | 0.20 (0.0333) | 0.27 (0.0065) | 0.49 (0.0013) | 0.15 (0.0309) | 0.38 (0.0143) | 0.14 (0.0470) | 0.56 (0.0012) | 0.45 (0.0002) | 0.34 (0.0165) | |
| Rikenellaceae | 0.33 ±0.14 (0.0309) | 0.57 ±0.26 (0.0359) | −5.61 ±2.53 (0.0344) | |||||||
| Lactobacillaceae | 1.16 ±0.36 (0.0032) | 1.28 ±0.37 (0.0019) | ||||||||
| Sphingobacteriaceae | 0.61 ± 0.27 (0.0304) | −1.52 ± 0.58 (0.0143) | 8.53 ±3.16 (0.0134) | 11.83 ±2.56 (0.0000) | ||||||
| Spiroplasmataceae | 3.72 ± 1.76 (0.0426) | 39.50 ±13.03 (0.0064) | ||||||||
| Gracillibacteraceae | 10.66 ±2.80 (0.0006) | −3.54 ±1.31 (0.0110) | −5.42 ±1.38 (0.0005) | |||||||
| Rhodospirillaceae | 7.48 ±2.66 (0.0094) | 24.39 ±10.24 (0.0252) | 2.01 ±0.96 (0.0469) | |||||||
| Christensenellaceae | 18.90 ±7.79 (0.0220) | 345.67 ±147.60 (0.0265) | ||||||||
| Acetobacteraceae | 39.77 ±15.86 (0.0174) | −37.33 ±11.97 (0.0042) | −136.62 ±43.73 (0.0045) | |||||||
| Staphylococcaceae | 68.92 ±23.83 (0.0087) | |||||||||
| Streptococcaceae | 543.38 ±119.17 (0.0002) | 891.41 ±235.38 (0.0007) | ||||||||
| BW | FI | WI | SBP | Glucose | HDL/LDL | Leptin | |||
|---|---|---|---|---|---|---|---|---|---|
| 6 w | 12 w | 6 w | 12 w | 12 w * | 12 w * | 12 w | 12 w * | 12 w | |
| 0.36 (0.0008) | 0.20 (0.0327) | 0.39 (0.0013) | 0.31 (0.0097) | 0.19 (0.017) | 0.51 (0.0002) | 0.15 (0.0246) | 0.53 (0.0001) | 0.37 (0.0003) | |
| Alistipes | 0.14 ±0.06 (0.0188) | 0.22 ±0.05 (0.0004) | |||||||
| Lactobacillus | 1.26 ±0.34 (0.0008) | 1.11 ±0.38 (0.0072) | |||||||
| Parapedobacter | 0.64 ±0.28 (0.0298) | 0.06 ±0.02 (0.0011) | |||||||
| Spiroplasma | 3.56 1.74 (0.0497) | 0.18 ±0.07 (0.0209) | |||||||
| Gracillibacter | 0.68 ±0.07 (0.0009) | −3.21 ±1.22 (0.0134) | −4.36 ±1.47 (0.0059) | ||||||
| Rikenella | 0.06 ±0.02 (0.0075) | ||||||||
| Christensenella | 18.90 ±8.64 (0.0366) | ||||||||
| Anaerophaga | 3827.14 ±918.48 (0.0003) | ||||||||
| Oleomonas | 1.01 ±0.39 (0.0151) | −6.83 ±2.69 (0.0171) | |||||||
| Staphylococcus | 145.68 ±61.76 (0.0246) | ||||||||
| Diet | SD | EVOO | ROO | BT | ||||
|---|---|---|---|---|---|---|---|---|
| g/100 g | % Energy | g/100 g | % Energy | g/100 g | % Energy | g/100 g | % Energy | |
| Protein | 16.5 | 20 | 16.5 | 14 | 16.5 | 14 | 16.5 | 14 |
| Carbohydrates | 60 | 72 | 55 | 48 | 55 | 48 | 55 | 48 |
| Fat | 3 | 8 | 20 | 35 | 20 | 35 | 20 | 35 |
| Total Energy (kJ/g) | 14.2 | 19.6 | 19.6 | 19.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olid, M.C.; Hidalgo, M.; Prieto, I.; Cobo, A.; Martínez-Rodríguez, A.M.; Segarra, A.B.; Ramírez-Sánchez, M.; Gálvez, A.; Martínez-Cañamero, M. Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter. Molecules 2023, 28, 2265. https://doi.org/10.3390/molecules28052265
Olid MC, Hidalgo M, Prieto I, Cobo A, Martínez-Rodríguez AM, Segarra AB, Ramírez-Sánchez M, Gálvez A, Martínez-Cañamero M. Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter. Molecules. 2023; 28(5):2265. https://doi.org/10.3390/molecules28052265
Chicago/Turabian StyleOlid, María Collado, Marina Hidalgo, Isabel Prieto, Antonio Cobo, Ana M. Martínez-Rodríguez, Ana Belén Segarra, Manuel Ramírez-Sánchez, Antonio Gálvez, and Magdalena Martínez-Cañamero. 2023. "Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter" Molecules 28, no. 5: 2265. https://doi.org/10.3390/molecules28052265
APA StyleOlid, M. C., Hidalgo, M., Prieto, I., Cobo, A., Martínez-Rodríguez, A. M., Segarra, A. B., Ramírez-Sánchez, M., Gálvez, A., & Martínez-Cañamero, M. (2023). Evidence Supporting the Involvement of the Minority Compounds of Extra Virgin Olive Oil, through Gut Microbiota Modulation, in Some of the Dietary Benefits Related to Metabolic Syndrome in Comparison to Butter. Molecules, 28(5), 2265. https://doi.org/10.3390/molecules28052265






