Methods for Silk Property Analyses across Structural Hierarchies and Scales
Abstract
1. Introduction
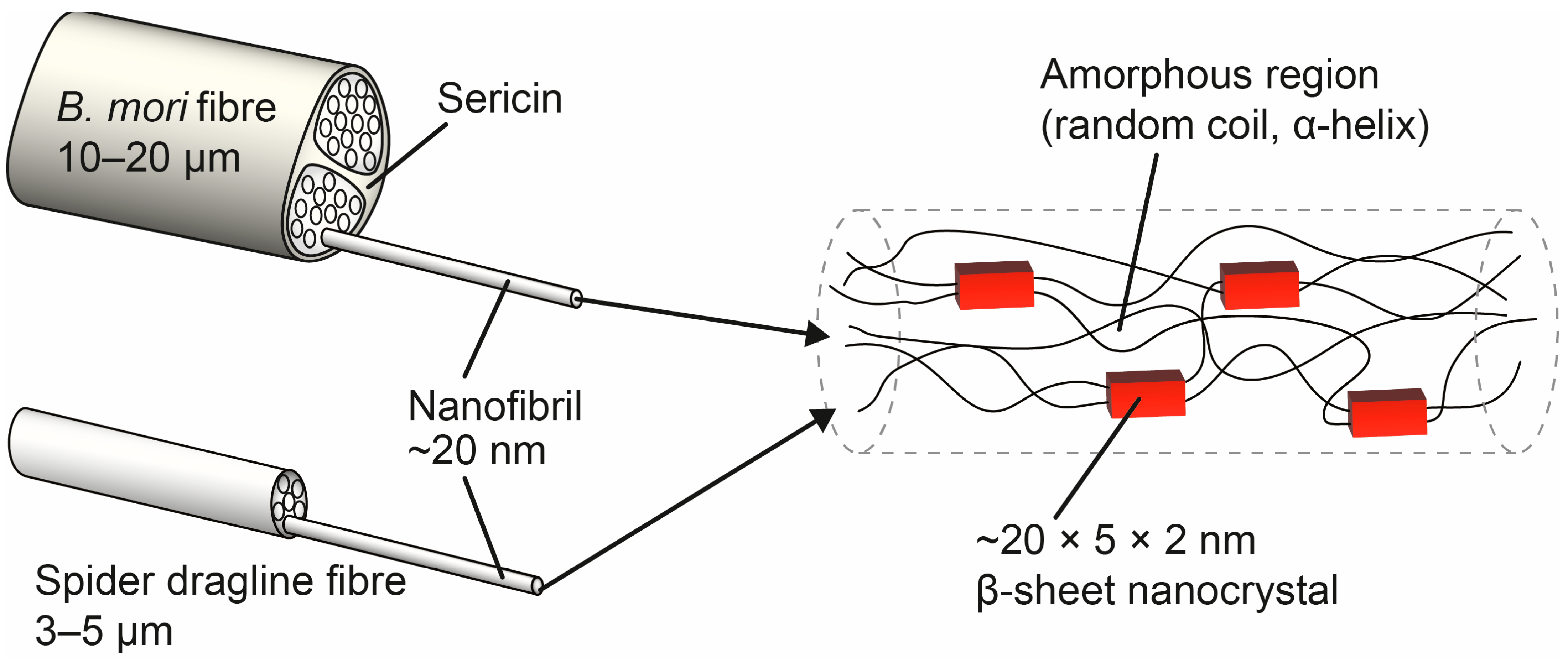
2. Structural Integrity and Characterization
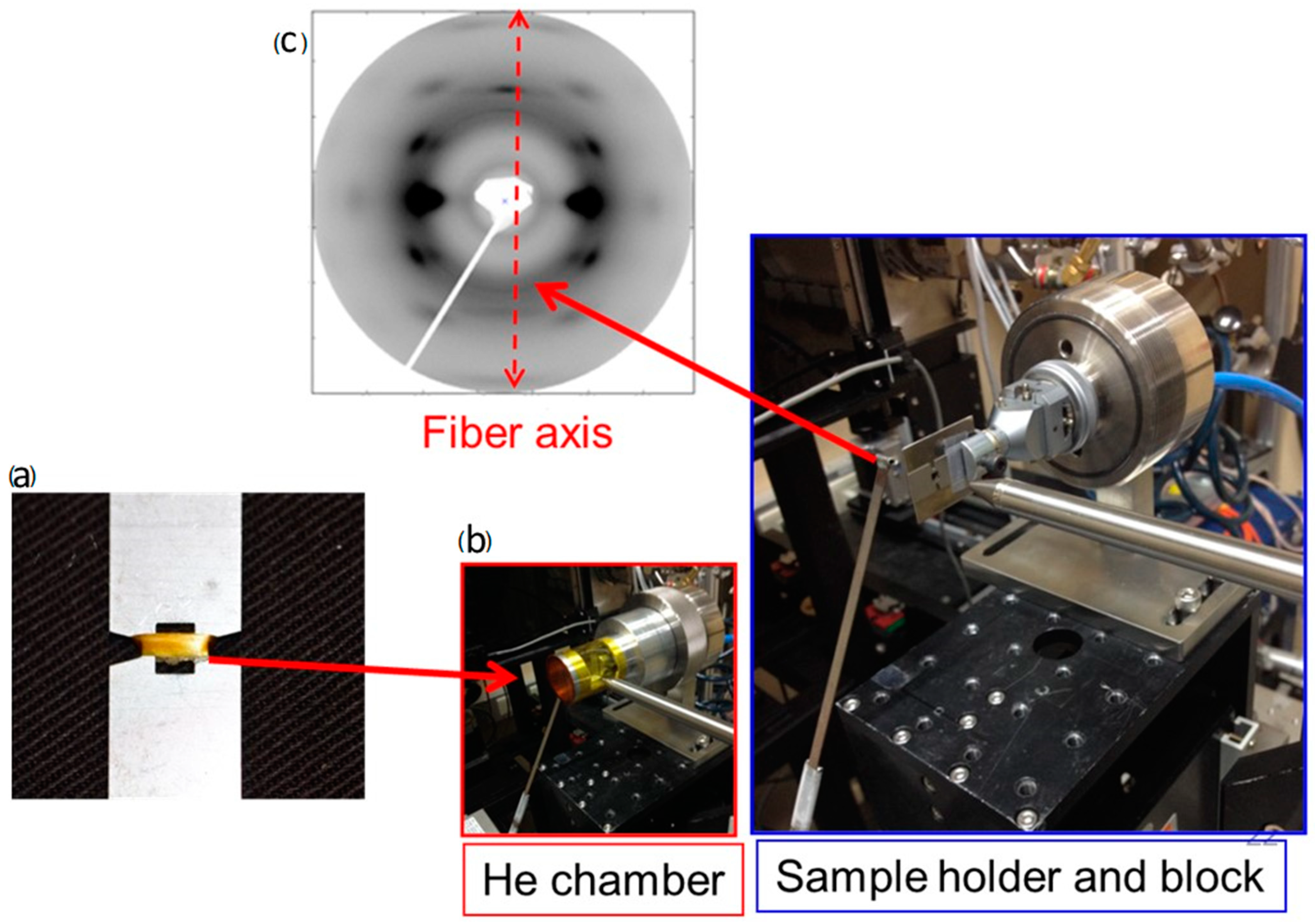
2.1. X-ray Scattering
2.2. Small-Angle X-ray Scattering Analyses of Silks
2.3. Wide-Angle X-ray Scattering Analyses of Silks
2.4. CD, FTIR, Vibrational, and Raman Spectroscopy
2.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
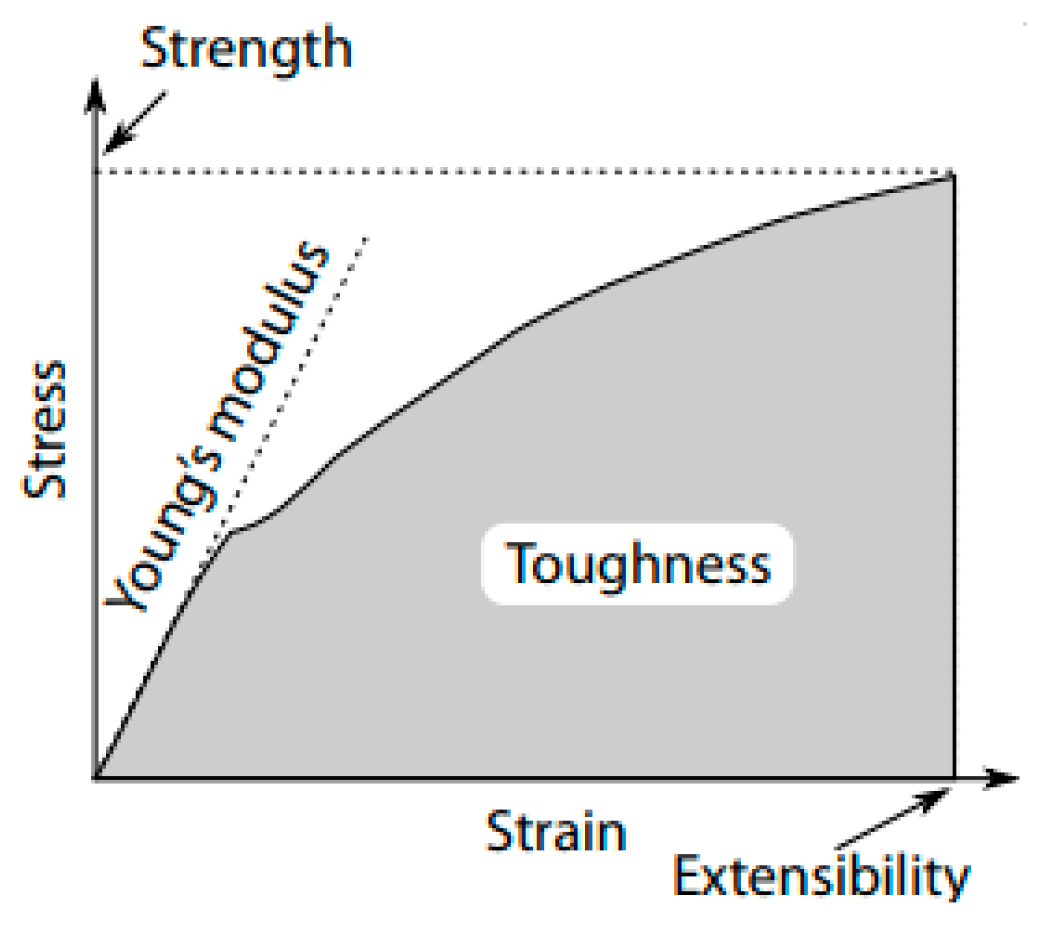
3. Mechanical Property Analyses
4. Vibrometry
5. Optical, Thermal, and Conductive Properties
5.1. Optical Measurements
5.2. Thermo-Gravitational Analyses, Differential Scanning Calorimetry, and Dynamic Mechanical Thermal Analysis
6. Microscopy
6.1. Scanning Electron Microscopy
6.2. Atomic Force Microscopy and Transmission Electron Microscopy
7. Amino Acid Profiling
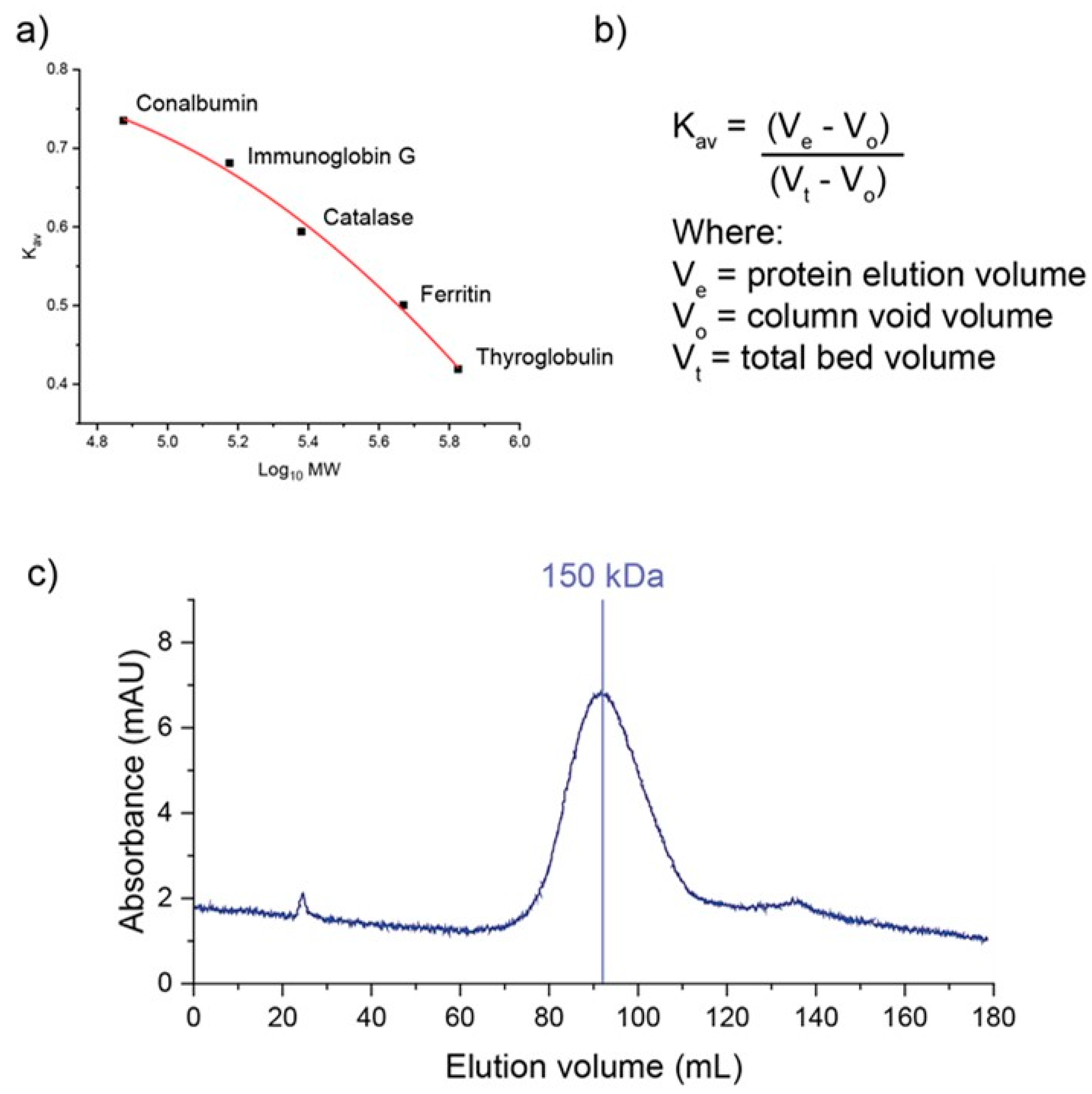
8. Determination of Molecular Weight
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanggaard, K.W.; Bechgaard, J.S.; Fang, X.; Duan, J.; Dyrlund, T.F.; Gupta, V.; Jiang, X.; Cheg, L.; Fan, D.; Feng, Y.; et al. Spider genomes provide insight into composition and evolution of venom and silk. Nat. Comms. 2014, 5, 3765. [Google Scholar] [CrossRef] [PubMed]
- Blamires, S.J.; Blackledge, T.A.; Tso, I.M. Physicochemical property variation in spider silk: Ecology, evolution, and synthetic production. Annu. Rev. Entomol. 2017, 62, 443–460. [Google Scholar]
- Kono, N.; Nakamura, H.; Ohtoshi, R.; Tomita, M.; Numata, K.; Arakawa, K. The bagworm genome reveals a unique fibroin gene that provides high tensile strength. Comms. Biol. 2019, 2, 148. [Google Scholar] [CrossRef]
- Blamires, S.J. Silk: Exploring Nature’s Superfibre; SSRL/XLIBRIS: Sydney, Australia, 2022. [Google Scholar]
- Porter, D.; Vollrath, F. Spider silk: Super material or thin fibre? Adv. Mater. 2012, 25, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Blamires, S.J.; Spicer, P.T.; Flanagan, P.J. Spider silk biomimetics programs to inform the development of new wearable technologies. Front. Mater. 2020, 7, 29. [Google Scholar] [CrossRef]
- Heidebrecht, A.; Eisoldt, L.; Diehl, J.; Scmidt, A.; Geffers, M.; Lang, G.; Scheibel, T.R. Biomimetic fibers made of recombinant spidroins with the same toughness as natural spider silk. Adv. Mater. 2015, 27, 2189–2194. [Google Scholar] [CrossRef]
- Wolff, J.O.; Wells, D.; Reid, C.R.; Blamires, S.J. Clarity of objectives and working principles enhances the success of biomimetic programs. Bioinspir. Biomim. 2017, 12, 051001. [Google Scholar] [CrossRef] [PubMed]
- Hsia, Y.; Gnesa, E.; Jeffery, F.; Tang, S.; Viera, C. Spider silk composites and applications. In Metal, Ceramic and Polymeric Composites for Various Uses; Cuppoletti, J., Ed.; In Tech: Rijeka, Croatia, 2021; pp. 303–324. [Google Scholar]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Health Mater. 2018, 8, 1800465. [Google Scholar] [CrossRef] [PubMed]
- Urie, R.; Guo, C.; Ghosh, D.; Thelakkaden, M.; Wong, V.; Lee, J.K.; Kilbourne, J.; Yarger, J.L.; Rege, K. Rapid soft tissue approximation and repair using laser-activated silk nanosealants. Adv. Funct. Mater. 2018, 28, 1802874–1802885. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef]
- Simmons, A.H.; Michal, C.; Jelenski, L.W. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science 1996, 271, 84–87. [Google Scholar] [CrossRef]
- Kono, N.; Nakamura, H.; Mori, M.; Yoshida, Y.; Ohtoshi, R.; Malay, A.D.; Pedrazzoli Moran, D.A.; Tomita, M.; Numata, K.; Arakawa, K. Multicomponent nature underlies the extraordinary mechanical properties of spider dragline silk. Proc. Nat. Acad. Sci. USA 2021, 118, e2107065118. [Google Scholar] [CrossRef]
- Agnarsson, I.; Kuntner, M.; Blackledge, T.A. Bioprospecting finds the toughest biological material: Extraordinary silk from a giant riverine orb spider. PLoS ONE 2010, 5, e11234. [Google Scholar] [CrossRef]
- Stengel, D.; Addison, B.A.; Onfrei, D.; Huyn, N.U.; Youssef, G.; Holland, G.P. Hydration-induced β-sheet crosslinking of α-helical-rich spider prey wrapping silk. Adv. Funt. Mater. 2021, 31, 2007161. [Google Scholar] [CrossRef]
- Chung, H.; Kim, T.Y.; Lee, S.Y. Recent advances in production of recombinant spider silk proteins. Curr. Opin. Biotechnol. 2012, 23, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Teule, F.; Miao, Y.G.; Sohn, B.H.; Kim, Y.S.; Hull, J.J.; Fraser, M.J., Jr.; Lewis, R.V.; Jarvis, D.L. Silkworms transformed with chimeric silkworm/spider silk genes spin composite silk fibers with improved mechanical properties. Proc. Nat. Acad. Sci. USA 2012, 109, 923–928. [Google Scholar] [CrossRef]
- Vollrath, F. Biology of spider silks. Inter. J. Biol. Macromol. 1999, 24, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef]
- Greving, I.; Cai, M.; Vollrath, F.; Schneipp, H.C. Shear-induced self-assembly of native silk proteins into fibrils studied by atomic force microscopy. Biomacromolecules 2012, 13, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Boulet-Audet, M.; Holland, C.; Gheysens, T.; Vollrath, F. Dry-spun silk produces native-like fibroin solutions. Biomacromolecules 2016, 17, 3198–3204. [Google Scholar] [CrossRef]
- Yonemura, N.; Sehnal, F. The design of silk fiber composition in moths has been conserved for more than 150 million years. J. Mol. Evol. 2006, 63, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, K.Q. Silk fiber–Molecular formation mechanism, structure- property relationship and advanced applications. In Oligomerization of Chemical and Biological Compounds; Lesieur, C., Ed.; In Tech: Rejika, Croatia, 2011; pp. 69–101. [Google Scholar]
- Rising, A.; Hjalm, G.; Engstrom, W.; Johansson, J. N-terminal nonrepetitive domain common to dragline, flagelliform, and cylindriform spider silk proteins. Biomacromolecules 2006, 7, 3120–3124. [Google Scholar] [CrossRef]
- Ittah, S.; Barak, N.; Gat, U. A proposed model for dragline spider silk self-assembly: Insights from the effect of the repetitive domain size on fiber properties. Biopolymers 2010, 93, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.W.; Vasanthavada, K.; Kohler, K.; McNary, S.; Moore, A.M.F.; Vierra, C.A. Molecular mechanisms of spider silk. Cell Mol. Life Sci. 2006, 63, 1986–1989. [Google Scholar] [CrossRef]
- Lewis, R.V. Spider silk: Ancient ideas for new biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Holland, G.P.; Lewis, R.V.; Yarger, J.L. WISE NMR characterization of nanoscale heterogeneity and mobility in supercontracted Nephila clavipes spider dragline silk. J. Am. Chem. Soc. 2004, 126, 5867–5872. [Google Scholar] [CrossRef]
- Yang, M.; Nakazawa, Y.; Yamauchi, K.; Knight, D.; Asakura, T. Structure of model peptides based on Nephila clavipes dragline silk spidroin (MaSp1) studied by 13C cross polarization/magic angle spinning NMR. Biomacromolecules 2005, 6, 3220–3226. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Yamauchi, K.; Gullion, T.; Asakura, T. Structural analysis of the Gly-rich region in spider dragline silk using stable-isotope labeled sequential model peptides and solid-state NMR. Chem. Comm. 2009, 28, 4176–4178. [Google Scholar] [CrossRef]
- Yarger, J.L.; Cherry, B.R.; Van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Brown, C.P.; MacLeod, J.; Amenitsch, H.; Cacho-Nerin, F.; Gill, H.S.; Price, A.J.; Traversa, E.; Licoccia, S.; Rosei, F. The critical role of water in spider silk and its consequence for protein mechanics. Nanoscale 2011, 3, 3805–3811. [Google Scholar] [CrossRef]
- Walker, A.A.; Weisman, S.; Trueman, H.E.; Merritt, D.J.; Sutherland, T.D. The other prey-capture silk: Fibres made by glow-worms (Diptera: Keroplatidae) comprise cross-β-sheet crystallites in an abundant amorphous fraction. Comp. Biochem. Physiol. B 2015, 187, 78–84. [Google Scholar] [CrossRef]
- Blamires, S.J.; Nobbs, M.; Martens, P.J.; Tso, I.M.; Chuang, W.S.; Chang, C.K. Multiscale mechanisms of nutritionally induced property variation in spider silks. PLoS ONE 2018, 13, e0192005. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tashiro, K.; Ohta, N. Molecular orientation enhancement of silk by the hot-stretching- induced transition from α-helix-HFIP complex to β-sheet. Biomacromolecules 2016, 17, 1437–1448. [Google Scholar] [CrossRef]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, F. Recent advances in development of functional spider silk-based hybrid materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef]
- Craig, H.C.; Yao, Y.; Ariotte, N.; Setty, M.; Remadevi, R.; Kasumovic, M.M.; Rajkhowa, R.; Rawal, A.; Blamires, S.J. Nanovoid formation induces variation within and across individual silkworm threads. J. Mater. Chem. B 2022, 10, 5561–5570. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials; John Wiley and Sons: New York, NY, USA, 1959. [Google Scholar]
- Hukins, W.L. X-ray Diffraction by Disordered and Ordered Systems; Pergamon Press: Oxford, UK, 1981. [Google Scholar]
- Trancik, J.E.; Czernuszka, J.T.; Bell, F.I.; Viney, C. Nanostructural features of a spider dragline silk as revealed by electron and X-ray diffraction studies. Polymer 2006, 47, 5633–5642. [Google Scholar] [CrossRef]
- Glisovic, A.; Salditt, T. Temperature dependent structure of spider silk by X-ray diffraction. Appl. Phys. A Mater. Sci. Proc. 2007, 87, 63–69. [Google Scholar] [CrossRef]
- Yang, Z.; Grubb, D.T.; Jelenski, L.W. Small-angle X-ray scattering of spider dragline silk. Macromolecules 1997, 30, 8254–8261. [Google Scholar] [CrossRef]
- Rossle, M.; Panine, P.; Urban, V.S.; Riekel, C. Structural evolution of regenerated silk fibroin under shear: Combined wide- and small-angle X-ray scattering experiments using synchrotron radiation. Biopolymers 2004, 74, 316–327. [Google Scholar] [CrossRef]
- Glisovic, A.; Vehoff, T.; Davies, R.J.; Salditt, T. Strain dependent structural changes of spider dragline silk. Macromolecules 2008, 41, 390–398. [Google Scholar] [CrossRef]
- McGill, M.; Holland, G.P.; Kaplan, D.L. Experimental methods for characterizing the secondary structure and thermal properties of silk proteins. Macromol. Rapid Comms. 2018, 2018, 1800390. [Google Scholar] [CrossRef] [PubMed]
- Riekel, C.; Burghammer, M.; Rosenthal, M. Nanoscale X-ray diffraction of silk fibres. Front. Mater. 2019, 6, 315. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tsubota, T.; Tashiro, K.; Jouraku, A.; Kameda, T. A study of the extraordinarily strong and tough silk produced by bagworms. Nat. Comms. 2019, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Benmore, C.J.; Izdebski, T.; Yarger, J.L. Total X-Ray scattering of spider dragline silk. Phys. Rev. Lett. 2012, 108, 178102. [Google Scholar] [CrossRef] [PubMed]
- Graewert, M.A.; Svergun, D.I. Impact and progress in small and wide angle X-ray scattering (SAXS and WAXS). Curr. Opin. Struct. Biol. 2013, 23, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J.F.; Jeon, S.M.; Lee, H.H.; Char, K.; Sohn, B.H. Orientation of lamellar nanostructures in the patterned thin films of a diblock copolymer. Macromolecules 2008, 41, 3401–3404. [Google Scholar] [CrossRef]
- Du, N.; Yang, Z.; Liu, Y.; Li, Y.; Xu, H.Y. Structural origin of the strain-hardening of spider silk. Adv. Funct. Mater. 2011, 21, 772–778. [Google Scholar] [CrossRef]
- Warwicker, J.O. Comparative studies of fibroins II. The crystal structures of various fibroins. J. Mol. Biol. 1960, 2, 350–362. [Google Scholar] [CrossRef]
- Miller, L.D.; Putthanarat, S.; Eby, R.K.; Adams, W.W. Investigation of the nanofibrillar morphology in silk fibers by small angle X-ray scattering and atomic force microscopy. Int. J. Biol. Macromol. 1999, 24, 159–165. [Google Scholar] [CrossRef]
- Numata, K.; Masunaga, H.; Hikima, T.; Sasaki, S.; Sekiyama, K.; Takata, M. Use of extension-deformation-based crystallisation of silk fibres to differentiate their functions in nature. Soft Matt. 2015, 11, 6335–6342. [Google Scholar] [CrossRef]
- Riekel, C.; Rossle, M.; Sapede, D.; Vollrath, F. Influence of CO2 on the micro-structural properties of spider dragline silk: X-ray microdiffraction results. Naturwissenschaften 2004, 91, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.B.; Solanas, C.; Marí-Buyé, N.; Madurga, R.; Agulló-Rueda, F.; Muinelo, A.; Riekel, C.; Burghammer, M.; Jorge, I.; Vázquez, J.; et al. The apparent variability of silkworm (Bombyx mori) silk and its relationship with degumming. Eur. Polym. J. 2016, 78, 129–140. [Google Scholar] [CrossRef]
- Riekel, C. New avenues in x-ray microbeam experiments. Rep. Progr. Phys. 2000, 3, 233–262. [Google Scholar] [CrossRef]
- Koch, M.H.J.; Bras, W. Synchrotron radiation studies of non-crystalline systems. Annu. Rep. C (Phys. Chem.) 2008, 104, 35–80. [Google Scholar] [CrossRef]
- Riekel, C.; Vollrath, F. Spider silk fibre extrusion: Combined wide- and small-angle X-ray microdiffraction experiments. Int. J. Biol. Macromol. 2001, 29, 203–210. [Google Scholar] [CrossRef]
- Rousseau, M.E.; Hernandez Cruz, D.; West, M.M.; Hitchcock, A.P.; Pezolet, M. Nephila clavipes spider dragline silk microstructure studied by scanning transmission X-ray microscopy. J. Am. Chem. Soc. 2007, 129, 3897–3905. [Google Scholar] [CrossRef]
- Sampath, S.; Isdebski, T.; Jenkins, J.E.; Ayon, J.V.; Henning, R.W.; Orgel, J.P.R.O.; Antipoa, O.; Yarger, J.L. X-ray diffraction study of nanocrystalline and amorphous structure within major and minor ampullate dragline spider silks. Soft Matt. 2012, 8, 6713–6722. [Google Scholar] [CrossRef]
- Blamires, S.J.; Wu, C.C.; Wu, C.L.; Sheu, H.W.; Tso, I.M. Uncovering spider silk nanocrystalline variations that facilitate wind- induced mechanical property changes. Biomacromolecules 2013, 14, 3484–3490. [Google Scholar] [CrossRef]
- Blamires, S.J.; Liao, C.P.; Chang, C.K.; Chuang, Y.C.; Wu, C.L.; Blackledge, T.A.; Sheu, H.S.; Tso, I.M. Mechanical performance of spider silk is robust to nutrient-mediated changes in protein composition. Biomacromolecules 2015, 16, 1218–1225. [Google Scholar] [CrossRef]
- Sibillano, T.; Terzi, A.; De Caro, L.; Ladisa, M.; Altamura, D.; Moliterni, A.; Lassandro, R.; Scattarella, F.; Siliqi, D.; Giannini, C. Wide angle X-Ray scattering to study the atomic structure of polymeric fibers. Crystals 2020, 10, 274. [Google Scholar] [CrossRef]
- Asakura, T.; Yamane, T.; Nakazawa, Y.; Kameda, T.; Ando, K. Structure of Bombyx mori silk fibroin before spinning in solid state studied with wide angle X-Ray scattering and 13C cross-polarization/magic angle spinning NMR. Biopolymers 2001, 58, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Madurga, R.; Blackledge, T.A.; Perea, B.; Plaza, G.R.; Riekel, C.; Burghammer, M.; Elices, M.; Guinea, G.V.; Perez-Rigueiro, J. Persistence and variation in microstructural design during the evolution of spider silk. Sci. Rep. 2015, 5, 14820. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural comparison of various silkworm silks: An insight into the structure−property relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef]
- Drummy, L.F.; Phillips, D.M.; Stone, M.O.; Farmer, B.L.; Naik, R.R. Thermally induced a-helix to b-sheet transition in regenerated silk fibers and films. Biomacromolecules 2005, 6, 3328–3333. [Google Scholar] [CrossRef] [PubMed]
- Anton, M.A.; Heidebrecht, A.; Mahmood, N.; Beiner, M.; Scheibel, T.R.; Kremer, F. Foundation of the outstanding toughness in biomimetic and natural spider silk. Biomacromolecules 2017, 18, 3954–3962. [Google Scholar] [CrossRef]
- Dicko, C.; Kennedt, J.M.; Knight, D.P.; Vollrath, V. Transition to a β-sheet-rich structure in spidroin in vitro: The effects of pH and cations. Biochemistry 2004, 43, 14080–14087. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, J.; Rising, A. Silk spinning in silkworms and spiders. Int. J. Mol. Sci. 2016, 17, 1290. [Google Scholar] [CrossRef]
- Percot, A.; Colomban, P.; Paris, C.; Dinh, H.M.; Wojcieszak, M.; Maucamp, B. Water dependent structural changes of silk from Bombyx mori gland to fibre as evidenced by Raman and IR spectroscopies. Vibrat. Spectr. 2014, 73, 79–89. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Biospinning by silkworms: Silk fiber matrices for tissue engineering applications. Acta Biomater. 2010, 6, 360–371. [Google Scholar] [CrossRef]
- Asakura, T.; Suzuki, Y.; Nakazawa, Y.; Holland, G.P.; Yarger, J.L. Elucidating silk structure using solid-state NMR. Soft Matt. 2013, 9, 11440–11450. [Google Scholar] [CrossRef]
- Asakura, T.; Suzuki, Y.; Nakazawa, Y.; Yazawa, K.; Holland, G.P.; Yarger, J.L. Silk structure studied with Nuclear Magnetic Resonance. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 69, 23–68. [Google Scholar] [CrossRef]
- Asakura, T. Structure and dynamics of spider silk studied with solid-state Nuclear Magnetic Resonance and Molecular Dynamics Simulation. Molecules 2020, 25, 2634. [Google Scholar] [CrossRef] [PubMed]
- Hibbler, R.C. Mechanics of Materials; Pearson: Bangkok, Thailand, 2008. [Google Scholar]
- Mortimer, B.; Vollrath, F. Diversity and properties of key spider silks and webs. Res. Knowl. 2015, 1, 32–42. [Google Scholar]
- Meyers, M.A.; Chawla, K.K. Mechanical Behavior of Materials; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Cole, M.R.; Hansel, M.H.; Seath, C.J. A quantitative study of the physical properties of nest paper in three species of Vespine wasps (Hymenoptera, Vespidae). Insect. Soc. 2001, 48, 33–39. [Google Scholar] [CrossRef]
- Blackledge, T.A.; Swindeman, J.E.; Hayashi, C.Y. Quasistatic and continuous dynamic characterization of the mechanical properties of silk from the cobweb of the black widow spider Latrodectus hesperus. J. Exp. Biol. 2005, 208, 1937–1949. [Google Scholar] [CrossRef]
- Dirks, J.H.; Taylor, D. Fracture toughness of locust cuticle. J. Exp. Biol. 2012, 215, 1502–1508. [Google Scholar] [CrossRef]
- Cetinkaya, M.; Xiao, X.; Market, B.; Stacklies, W.; Grater, F. Silk fiber mechanics from multiscale force distribution analysis. Biophys. J. 2011, 100, 1298–1305. [Google Scholar] [CrossRef]
- Parthasarathy, K.M.; Naresh, M.D.; Arumugam, V.; Subramaniam, V.; Sanjeevi, R. Study on the viscoelastic response of silk. J. Appl. Polym. Sci. 1996, 59, 2049–2053. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Gupta, V.B.; Kothari, V.K. Tensile stress–strain and recovery behavior of Indian silk fibers and their structural dependence. J. Appl. Polym. Sci. 2000, 77, 2418–2429. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.-Y.; Du, N.; Xu, G.; Li, B. Unraveled mechanism in silk engineering: Fast reeling induced silk toughening. Appl. Phys. Lett. 2009, 95, 093703. [Google Scholar] [CrossRef]
- Oberst, S.; Martin, R.; Halkon, B.J.; Lai, J.C.S.; Evans, T.A.; Saadatfar, M. Submillimeter mechanistic details of multi-scale termite-built functional structures. J. Roy. Soc. Interf. 2021, 18, 20200957. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.; King, S.W. Atomic force microscopy for nanoscale mechanical property characterization. J. Vac. Sci. Technol. B 2020, 38, 060801. [Google Scholar] [CrossRef]
- Blamires, S.J.; Nobbs, M.; Wolff, J.O.; Heu, C. Nutrient-induced nano-scale variability in spider silk structural and mechanical properties. J. Mech. Behav. Biomed. Mater. 2021, 125, 104873. [Google Scholar] [CrossRef] [PubMed]
- Piorkowski, D.; He, B.C.; Blamires, S.J.; Tso, I.M.; Kane, D.M. Nanoscale material heterogeneity of glowworm capture threads revealed by AFM. Molecules 2021, 26, 3500. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.S.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Osaki, S. Spider silk as a mechanical lifeline. Nature 1996, 384, 419. [Google Scholar] [CrossRef]
- Chen, Y.K.; Liao, C.P.; Tsai, F.Y.; Chi, K.J. More than a safety line: Jump-stabilizing silk of salticids. J. Roy. Soc. Interf. 2013, 10, 20130572. [Google Scholar] [CrossRef]
- Mortimer, B.; Soler, A.; Siviour, C.R.; Zaera, R.; Vollrath, F. Tuning the instrument: Sonic properties in the spider’s web. J. Roy. Soc. Interf. 2016, 13, 20160341. [Google Scholar] [CrossRef]
- Su, I.; Qin, Z.; Saraceno, T.; Krell, A.; Muhlethaler, R.; Bisshop, A.; Beuhler, M.J. Imaging and analysis of a three dimensional spider web architecture. J. Roy. Soc. Interf. 2018, 15, 20180193. [Google Scholar] [CrossRef]
- Mulder, T.; Mortimer, B.; Vollrath, F. Functional flexibility in a spider’s orb web. J. Exp. Biol. 2020, 223, jeb234070. [Google Scholar] [CrossRef]
- Kaewunruen, S.; Ngamkhanong, C.; Xu, S. Large amplitude vibrations of imperfect spider web structures. Sci. Rep. 2020, 10, 19161. [Google Scholar] [CrossRef] [PubMed]
- Kawano, A.; Morassi, A.; Zaera, R. The prey’s catching problem in an elastically supported spider orb-web. Mech. Syst. Sign. Proc. 2021, 151, 107310. [Google Scholar] [CrossRef]
- Blamires, S.J.; Little, D.J.; White, T.E.; Kane, D.M. Photoreflectance/scattering measurements of spider silks informed by standard optics. Roy. Soc. Open Sci. 2020, 7, 192174. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, D. Spider silk-structure, properties and spinning. J. Text. Appar. Technol. Manag. 2006, 5, 1–20. [Google Scholar]
- Little, D.J.; Kane, D.M. Image contrast immersion method for measuring refractive index applied to spider silks. Opt. Expr. 2011, 19, 19182–19188. [Google Scholar] [CrossRef] [PubMed]
- Freddi, G.; Gotoh, Y.; Ihihiko, T.; Tsukada, M. Chemical structure and physical properties of Antheraea assama silk. J. Appl. Polym. Sci. 1994, 52, 775–781. [Google Scholar] [CrossRef]
- Cavallini, S.; Toffanin, S.; Chieco, C.; Sagnella, A.; Formaggio, F.; Pistone, A.; Posati, T.; Natali, M.; Caprini, M.; Benfaniti, V.; et al. Naturally functionalized silk as useful material for photonics applications. Compos. B Eng. 2015, 71, 152–158. [Google Scholar] [CrossRef]
- Lawrence, B.D.; Cronin-Golomb, M.; Georgakoudi, I.; Kaplan, D.L.; Omenetto, F.G. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules 2008, 9, 1214–1220. [Google Scholar] [CrossRef]
- Holland, C.; Hawkins, N.; Frydrych, M.; Laity, P.R.; Porter, D.; Vollrath, F. Differential scanning calorimetry of native silk feedstock. Macromol. Biosci. 2018, 2018, 1800228. [Google Scholar] [CrossRef]
- Blamires, S.J.; Cerexhe, G.; White, T.E.; Herberstein, M.E.; Kasumovic, M.M. Spider silk colour covaries with thermal properties but not protein structure. J. Roy. Soc. Interf. 2019, 16, 20190199. [Google Scholar] [CrossRef]
- Cebe, P.; Hu, X.; Kaplan, D.L.; Zhuravlev, E.; Wurm, A.; Arbeiter, D.; Schick, C. Beating the heat—Fast scanning melts silk beta sheet crystals. Sci. Rep. 2013, 3, 1130. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.P.; Wang, H.; Ho, C.K.; Lau, K.T. A study on the dynamic mechanical properties of silk fibre composites. Adv. Mater. Res. 2012, 410, 106–109. [Google Scholar] [CrossRef]
- Huang, W.; Krishnaji, S.; Hu, X.; Kaplan, D.L.; Cebe, P. Heat capacity of spider silk-like block copolymers. Macromolecules 2011, 44, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J. The glass transition temperature Tg of polymers—Comparison of the values from differential thermal analysis (DTA, DSC) and dynamic mechanical measurements (torsion pendulum). Polym. Test. 2001, 20, 199–204. [Google Scholar] [CrossRef]
- Li, S.F.Y.; McGhie, A.J.; Tang, S.L. New internal structure of spider dragline silk revealed by atomic force microscopy. Biophys. J. 1994, 66, 1209–1212. [Google Scholar] [CrossRef]
- Stubbs, D.G.; Tillinghast, E.K.; Townley, M.A. Fibrous composite structure in spider silk. Naturwissenschaften 1992, 79, 231–234. [Google Scholar] [CrossRef]
- Augsten, K.; Muhlig, P.; Herrmann, C. Glycoproteins and skin-core structure in Nephila clavipes spider silk observed by light and electron microscopy. Scanning 2000, 22, 12–15. [Google Scholar] [CrossRef]
- Mao, Y.; Su, Y.; Hsiao, S. Probing structure and orientation using synchrotron small- and wide-angle X-ray scattering techniques. Eur. Polym. J. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Nony, P.; Prudhomme, J.-C.; Couble, P. Regulation of the P25 gene transcription in the silk gland of Bombyx. Biol. Cell 1995, 84, 43–52. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk fibroin of Bombyx mori is secreted assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucl. Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, J.; Wang, X.; Nguyen, A.T.; Liu, X.Y.; Kaplan, D.L. Comparative study of strain-dependent structural changes of silkworm silks: Insight into the structural origin of strain-stiffening. Small 2017, 13, 1702266. [Google Scholar] [CrossRef] [PubMed]
- Gulrajani, M.L. Degumming of silk. Rev. Progr. Color. Rel. Top. 1992, 22, 79–89. [Google Scholar] [CrossRef]
- Sashina, E.S.; Bochek, A.M.; Novoselov, N.P.; Kirichenko, D.A. Structure and solubility of natural silk fibroin. Russ. J. Appl. Chem. 2006, 79, 869–876. [Google Scholar] [CrossRef]
- Wray, L.S.; Hu, X.; Gallego, J.; Georgakoudi, I.; Omenetto, F.G.; Schmidt, D.; Kaplan, D.L. Effect of processing on silk-based biomaterials: Reproducibility and biocompatibility. J. Biomed. Mater. Res. B 2011, 99, 89–101. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; Des Rochers, T.M.; Burke, K.A.; Kaplan, D.L. The effect of sterilization on silk fibroin biomaterial properties. Macromol. Biosci. 2015, 15, 861–874. [Google Scholar] [CrossRef]
- Kluge, J.A.; Kahn, B.T.; Brown, J.E.; Omenetto, F.G.; Kaplan, D.L. Optimizing molecular weight of lyophilized silk as a shelf-stable source material. ACS Biomat. Sci. Eng. 2016, 2, 595–605. [Google Scholar] [CrossRef]
- Partlow, B.P.; Tabatabai, P.A.; Leisk, G.G.; Cebe, P.; Blair, D.L.; Kaplan, D.L. Silk fibroin degradation related to rheological and mechanical properties. Macromol. Biosci. 2016, 16, 666–675. [Google Scholar] [CrossRef]
- Yao, Y.; Allardyce, B.J.; Rajkhowa, R.; Hegh, D.; Sutti, A.; Subianto, S.; Gupta, S.; Rana, S.; Greenhill, S.; Venkatesh, S.; et al. Improving the tensile properties of wet spun silk fibers using rapid Bayesian algorithm. ACS Biomater. Sci. Eng. 2020, 6, 3197–3207. [Google Scholar] [CrossRef]
- Pawcenis, D.; Koperska, M.A.; Milczarek, J.M.; Łojewski, T.; Łojewska, J. Size exclusion chromatography for analyses of fibroin in silk: Optimization of sampling and separation conditions. Appl. Phys. A 2014, 114, 301–308. [Google Scholar] [CrossRef]
- Lei, C.; Zhu, H.; Li, J.; Feng, X.; Chen, J. Preparation and hemostatic property of low molecular weight silk fibroin. J. Biomater. Sci. Polym. Ed. 2016, 27, 403–418. [Google Scholar] [CrossRef]
- Freddi, G.; Berlin, A.; Tsukada, M.; Paglia, D.E. Use of HP-size exclusion chromatography to study the degree of polymerization of silk (Bombyx mori) fibroin fibres. Sericologia 2000, 40, 363–381. [Google Scholar]
- Freddi, G.; Allagra, G. Fractionation of silk proteins (Bombyx mori) by precipitation techniques and hydrophobic interaction chromatography. Séricologia 1997, 37, 659–682. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blamires, S.J.; Rawal, A.; Edwards, A.D.; Yarger, J.L.; Oberst, S.; Allardyce, B.J.; Rajkhowa, R. Methods for Silk Property Analyses across Structural Hierarchies and Scales. Molecules 2023, 28, 2120. https://doi.org/10.3390/molecules28052120
Blamires SJ, Rawal A, Edwards AD, Yarger JL, Oberst S, Allardyce BJ, Rajkhowa R. Methods for Silk Property Analyses across Structural Hierarchies and Scales. Molecules. 2023; 28(5):2120. https://doi.org/10.3390/molecules28052120
Chicago/Turabian StyleBlamires, Sean J., Aditya Rawal, Angela D. Edwards, Jeffrey L. Yarger, Sebastian Oberst, Benjamin J. Allardyce, and Rangam Rajkhowa. 2023. "Methods for Silk Property Analyses across Structural Hierarchies and Scales" Molecules 28, no. 5: 2120. https://doi.org/10.3390/molecules28052120
APA StyleBlamires, S. J., Rawal, A., Edwards, A. D., Yarger, J. L., Oberst, S., Allardyce, B. J., & Rajkhowa, R. (2023). Methods for Silk Property Analyses across Structural Hierarchies and Scales. Molecules, 28(5), 2120. https://doi.org/10.3390/molecules28052120










