Abstract
Steroids and their derivatives have been the subject of extensive research among investigators due to their wide range of pharmacological properties, in which steroidal oximes are included. Oximes are a chemical group with the general formula R1R2C=N−OH and they exist as colorless crystals and are poorly soluble in water. Oximes can be easily obtained through the condensation of aldehydes or ketones with various amine derivatives, making them a very interesting chemical group in medicinal chemistry for the design of drugs as potential treatments for several diseases. In this review, we will focus on the different biological activities displayed by steroidal oximes such as anticancer, anti-inflammatory, antibacterial, antifungal and antiviral, among others, as well as their respective mechanisms of action. An overview of the chemistry of oximes will also be reported, and several steroidal oximes that are in clinical trials or already used as drugs are described. An extensive literature search was performed on three main databases—PubMed, Web of Science, and Google Scholar.
Keywords:
steroids; oximes; chemistry; antitumor; anti-inflammatory; antibacterial; antifungal; antiviral 1. Introduction
Steroids belong to a class of natural or synthetic organic compounds, whose basic molecular structure is typically composed of 17 carbon atoms, bonded in four “fused” rings: three six-member cyclohexane rings (rings A, B and C) and one five-member cyclopentane ring (the D ring). (Figure 1). They play a crucial role in the human body, being responsible for the regulation of several biological processes. This fact, together with their interesting biochemical properties, such as the ability to penetrate cell membranes and bind to the nuclear and membrane receptors, makes them extremely attractive in the design of new potential drugs for the treatment of several diseases [1,2]. In fact, since their discovery in 1935, steroids have been widely used in the treatment of several conditions in the most variable areas of medicine, for example, for the treatment of autoimmune and inflammatory diseases and for the treatment of cancer [3,4]. Given the privileged scaffold of steroids and their suitability for structural modifications, steroidal derivatives have been arousing interest among medicinal chemists in the hunt for novel drug candidates. Slight alterations in the basic ring structure of steroids can elicit an enormous change in biological activity, giving rise to steroidal derivatives with a wide range of therapeutic activities [5,6].
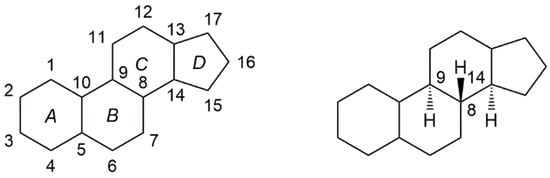
Figure 1.
General structure of steroidal scaffold (left). Unless implied or stated to the contrary, the configuration of hydrogen atoms at the bridgehead positions 8, 9 and 14 are oriented as shown in the right formula (i.e., 8β, 9α, 14α).
Oximes are one of the most popular and extensively hailed nitrogen-containing biological compounds, presenting several biological and pharmacological applications [7]. They have achieved popularity due to their application as antidotes against nerve agents, which is attained by their capacity to reactivate acetylcholinesterase (AChE) [8]. Since that, these hydroxy-imine derivatives have also been associated with several other biological activities such as antibacterial, antifungal, anti-inflammatory, antioxidant, and finally, anticancer as described [7,9].
Employing the hydrophobic steroid core with a hydroxyimino group constitutes an advantage since this chemical group can increase the molecules’ ability to interact with cell membranes, paving the way for enhanced biological activity [10]. For these reasons, in the last 20 years, a reasonable number of new steroidal oximes has been designed and synthesized and then evaluated for their biological activity. This review will focus mainly on the most active steroidal oximes developed as antitumor and antimicrobial agents. Additionally, a few examples of steroidal oximes with anti-inflammatory activity are also considered.
2. Chemistry of Steroidal Oximes
Oximes (R1R2C=N−OH) (Figure 2) are a nitrogen-rich group of compounds, which are produced in nature in the plant and animal kingdom. In plants, oximes and their derivatives play a fundamental role in the metabolism of plant growth and development and in a variety of biosynthetic pathways [9,11]. In animals, oximes are most commonly known for their participation in the olfactory communication between animals [12]. Oximes exist as colorless crystals, are poorly soluble in water, and are easily accessible in laboratories and in industry, which makes them very appealing [13,14]. Additionally, they are extensively used not only as protectors of carbonyl groups but also as intermediates in the Beckmann rearrangement to synthesize several lactam derivatives [9,15,16]. Furthermore, oximes have the particularity of being easily transformed into different chemical groups such as amines, nitro, and other heterocyclic compounds [16,17].
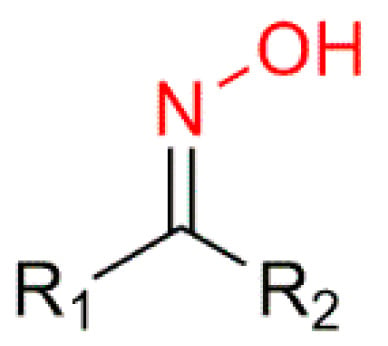
Figure 2.
General structure of the hydroxyimino group of the oximes.
There are several ways to produce oxime derivatives and some reviews have been published regarding the chemistry of oximes [13,16,18]. The most classical method of oxime synthesis, which is the most used in the synthesis of steroidal oximes, involves the reaction of a carbonyl compound, a ketone or an aldehyde with hydroxylamine (NH2OH) or a hydroxylammonium salt in the presence of a base (Figure 3). This type of reaction with aldehydes and non-symmetrical ketones can originate the two E or Z isomeric oxime forms, which can exist both as single compounds and or in mixture. Such chemical aspects can have a great impact on biological activity [7,12].
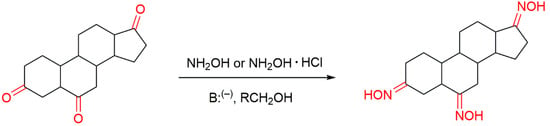
Figure 3.
Classical synthesis of steroidal oximes which can take part in different positions in the steroidal scaffold.
Apart from the most commonly used synthetic strategy, there are other methods to prepare oximes involving non-carbonyl compounds, which consist of the reduction of nitroalkenes to create aldoximes and ketoximes. The reduction of α,β-unsaturated nitroalkenes gives rise to different oxime derivatives, depending if the nitro group is terminal or internal. More specifically, when the nitro group is terminal, aldoximes are produced, in mildly acidic conditions, in good yields. On the contrary, if the nitro group is internal, under basic conditions ketoximes are formed also in good yields [9,16]. Other variants of oxime synthesis include oxime ethers, esters, and amidoximes, all of which are of great biological and pharmacological importance. Oximes can act both as weak acids and weak bases. For this reason, the oxime anions can behave as ambident nucleophiles, which means that they can attack through two different sites, allowing them to be widely used for the synthesis of the above-mentioned class of compounds (ethers, nitrones, etc.) [13].
Another aspect of this chemical group is that it can behave both as hydrogen-bond donor (via OH group) and as hydrogen-bond acceptor (via nitrogen and oxygen atoms), which together with the high polarity of the oxime moiety can have a tremendous impact on the interaction with the receptor binding sites, enhancing biological activity, when compared to the carbonyl group [7,9]. This premise will be the focus of our review, in which we discuss several synthesized steroidal oximes with enhanced biological activity when compared with the parent carbonyl compounds.
3. Steroidal Oximes as Antitumor Agents
Steroidal compounds have been associated with antitumor activity for many years. Several reports focusing on their interesting properties as anticancer agents have been published [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. The oxyimino group of oxime compounds is also a structural feature that confers very interesting biological properties among them antitumor activity [7,34]. For this reason, cytotoxic steroidal oximes have been extensively studied throughout the years [14,34]. In this review, we focused on the most active steroidal oximes, described in the literature, against several types of cancer. They are divided according to their steroidal motif: androstane, estrane, pregnane, cholestane, diosgenin, and bile acid derivatives.
3.1. Androstane Derivatives
5α-Reductase inhibitors have been widely studied for the treatment of diseases that are exacerbated by 5α-dihydrotestosterone (DHT). Finasteride and dutasteride are two 5α-reductase inhibitors approved by the FDA with several therapeutic applications such as for the treatment of benign prostatic hyperplasia and prostate cancer. Bearing this in mind, Dhingra et al. developed a series of 17-oxyimino -5-androsten-3β-yl esters and evaluated their cytotoxic activity against prostate cancer cells [35]. Compound 1a (Figure 4) was the most active in DU145 prostate cancer cells with a percentage of growth inhibition of almost 91% at 5 µg/mL and an IC50 value of 3.8 µM (Table 1), being more active than finasteride (78.51% of growth inhibition and IC50 = 3.9 µM). Moreover, the authors also evaluated the compounds’ acute toxicity using mouse macrophages, which indirectly allowed them to test for the compounds’ selectivity towards cancer cells. The toxicity index value (LC50) presented by compound 1a was very high (LC50 = 89.4 µM), proving that 1a was non-toxic to mouse macrophages.
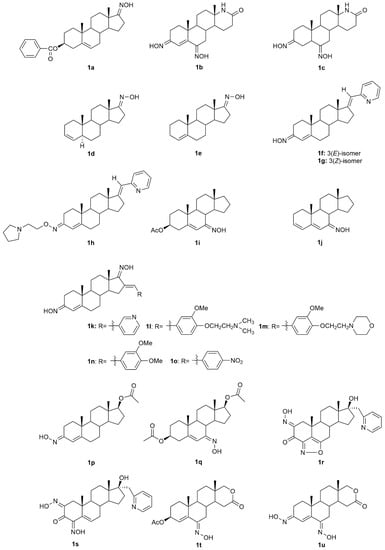
Figure 4.
Androstane oxime derivatives with anticancer activity in several types of cancer cells.
The introduction of a heteroatom or the substitution of one or more carbon atoms in the steroid scaffold by a heteroatom can have a great impact on the compound’s biological activity. Aza-homosteroids are a class of compounds with unusual structures, which have been associated with a wide range of biological activities such as antiparasitic, antifungal, and anticancer [36,37,38]. Following this line, Huang et al. synthesized a series of 17a-aza-D-homoandrostan-17-one derivatives, namely oximes 1b and 1c (Figure 4) [39]. Further cytotoxicity analysis by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method revealed that both compounds were active against HeLa and SMMC7404 cells, being that compound 1b stood out in HeLa cells with an IC50 of 15.1 µM (Table 1). Moreover, these oxime derivatives were in general more active than the compounds without the oxyimino group in their structure, which proves that this chemical group is important in conferring cytotoxicity.
Gomes et al. [34] designed and synthesized two novel steroidal oxime derivatives and evaluated them and two other previously synthesized oximes [40,41] in several cancer cell lines to assess their antiproliferative profile. Initial screening in WiDr, PC3, HepG2, and H1299 cancer cell lines revealed that oximes 1d and 1e (Figure 4) were able to decrease all cancer cells proliferation, being especially active against PC3 (IC50 = 13.8 µM for 1d and 14.5 µM for 1e) and WiDr (IC50 = 9.1 for 1d and 16.1 µM for 1e) cells (Table 1). Moreover, both oximes were even more potent than some of the chemotherapeutic drugs currently in clinical use for these types of cancer. Both oximes were able to induce cell cycle arrest at different phases, accompanied by cell death by apoptosis/necroptosis and oxidative stress (detected by an increase in ROS production) in both cell lines. Selectivity against cancer cells was also assessed by testing the compounds in normal human colon cells. Results demonstrated that both compounds are selective toward colon cancer cells [34].
Several novel oxime derivatives, such as (17E)-(pyridin-2-yl)methylidene 3-oximes 1f–1h were designed and synthesized (Figure 4) [42]. After the synthesis, the antiproliferative activity of both compounds was assessed in a series of several human cancer cell lines (MCF7, MDA-MB-231, PC3, HeLa, HT29, A549) and a normal human cell line, MRC5. A549, HT29, and MDA-MB-231 cancer cells were the most sensitive cell lines to both oximes being that A549 was the one with the best IC50 values for all compounds, 1f (IC50 = 1.5 µM) 1g (IC50 = 1.8 µM) and 1h (IC50 = 2.0 µM) (Table 1). Apoptosis induction analysis showed that these oximes induced apoptosis in A549 cells, while at the same time being non-toxic to normal lung fibroblasts MRC5. These results were very encouraging since lung cancer remains one of the most difficult cancers to treat.
Some steroidal compounds with a hydroxyimino group at position C-7 conjugated with an α,β-double bond in position C-5 were designed and synthesized [43]. After the synthesis, their antitumor activity was evaluated against several types of cancer such as cervical, gastric, epidermoid, and breast cancer. Results demonstrated that compounds 1i and 1j (Figure 4) were both able to decrease KB, HeLa, MKN-28, and MCF7 cancer cell proliferation (Table 1) and were more active than the corresponding parent ketones. Moreover, compound 1i was especially active against MCF7 cells presenting an IC50 value of 10.2 µM.
Dubey et al. designed and synthesized novel dioximes of 16-benzylidene substituted derivatives [44]. The antitumor activity of these dioximes was then evaluated in terms of the percentage of growth inhibition of NCI-H460, MCF7, and SF268 cancer cells. Results demonstrated that compounds 1k–1o (Figure 4) were considered active against these three cell lines, which encourages the need for further and more detailed studies.
A group of investigators focused their attention on androstene oximes and their O-alkylated derivatives [45]. These compounds and two previously synthesized oxime derivatives, compounds 1p [46] and 1q [47] (Figure 4) were evaluated in leukemia, colon, melanoma, and renal cancer cell lines. Only compounds 1p and 1q showed considerable cytotoxicity in all cell lines (percentages of growth of 10.07 to 75.01% at 10 µM), being this effect more pronounced in the leukemia cell lines, K562, HL60, and SR.
Aiming to evaluate the combined effect of the 17-heterocyclic ring and hydroxyimino function, a group of investigators designed and synthesized modified 17α-picolyl and 17(E)-picolinylidene androstane derivatives and their antiproliferative activity against breast, prostate, cervical, colon and lung adenocarcinoma, as well as normal fetal lung fibroblasts was assessed [48]. MTT assay results (Table 1) demonstrated that compound 1r (Figure 4) was the most active compound in PC3 cells (IC50 = 6.6 µM), while compound 1s (Figure 4) was more active, not only against PC3 cells (IC50 = 8.7 µM) but also against MCF7 cells (IC50 = 1.7 µM). Given these encouraging results, the authors went further ahead and studied deeply the potential mechanisms of action of compound 1s in MCF7 cells. Results showed that 1s induced apoptosis in breast cancer cells, assessed by alterations in the cells morphology, such as nuclear condensation, vacuolated cytoplasm, degradation of nuclei and cytoplasm, membrane blebbing, and apoptotic bodies formation [48].
Savić et al., designed and synthesized some novel D-homo lactone androstane derivatives and evaluated their antiproliferative activity against several cancer cell lines [49]. Among these compounds, the oxime derivative 1t (Figure 4), demonstrated to have high activity (Table 1). Moreover, 1t also revealed selectivity towards cancer cells since it presents a much higher IC50 in the normal human cell line, MRC5. A few years later, the same group of investigators designed, synthesized and evaluated the antitumor activity of some more new D-homo lactone androstane derivatives [50]. In vitro cytotoxicity assessment against cancer cells revealed that the steroidal oxime 1u (Figure 4) was able to decrease the proliferation of PC3 (IC50 = 27.94 µM) and HeLa (IC50 = 13.86 µm) cells (Table 1).

Table 1.
IC50 values (µM) of the synthesized androstane oxime derivatives.
Table 1.
IC50 values (µM) of the synthesized androstane oxime derivatives.
| Cell Line | Compounds | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | 1r | 1s | 1t | 1u | |
| DU145 | 3.9 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HeLa | 15.1 | 75.7 | - | - | >100 | >100 | 22.6 | 12.8 | 22.2 | >100 | 81.8 | 36.0 | 13.9 | |
| SMMC7404 | - | >200 | 184 | - | - | - | - | - | - | - | - | - | - | - |
| WiDr | - | - | - | 9.1 | 16.1 | - | - | - | - | - | - | - | - | - |
| PC3 | - | - | - | 13.8 | 14.5 | >100 | 57.7 | 77.1 | - | - | 6.6 | 8.7 | 36.7 | 27.9 |
| HepG2 | - | - | - | 23.9 | 18.2 | - | - | - | - | - | - | - | - | - |
| H1299 | - | - | - | 18.6 | 19.2 | - | - | - | - | - | - | - | - | - |
| MCF7 | - | - | - | - | - | 41.0 | 44.9 | >100 | 10.2 | 19.8 | 50.4 | 1.7 | 81.3 | >100 |
| MDA-MB-231 | - | - | - | - | - | 47.3 | 5.2 | 4.7 | - | - | 25.3 | 40.1 | 11.9 | >100 |
| HT29 | - | - | - | - | - | 4.4 | 10.6 | 3.3 | - | - | >100 | 10.3 | 4.0 | >100 |
| A549 | - | - | - | - | - | 1.5 | 1.8 | 2.0 | - | - | >100 | 56.0 | - | - |
| MRC5 | - | - | - | - | - | >100 | >100 | >100 | - | - | >100 | >100 | >100 | >100 |
| CEM | - | - | - | - | - | >50 | >50 | 30.4 | - | - | - | - | - | - |
| G361 | - | - | - | - | - | 45.3 | 46.6 | 8.9 | - | - | - | - | - | - |
| BJ | - | - | - | - | - | >50 | >50 | 25.3 | - | - | - | - | - | - |
| KB | - | - | - | - | - | - | - | - | 26 | 28.5 | - | - | - | - |
| MKN-28 | - | - | - | - | - | - | - | - | 18.1 | 36.1 | - | - | - | - |
| Ref. | [35] | [39] | [34] | [42] | [43] | [48] | [49] | [50] | ||||||
These values were obtained through different techniques such as MTT and SRB assays.
3.2. Estrane Derivatives
Estrogen-3-O-sulfamates (EMATEs) are a class of steroidal compounds, which act as irreversible inhibitors of steroid sulfatase (STS), an enzyme involved in the development of estrogen-dependent breast cancer [51]. Given this, Leese et al. decided to design and synthesize D ring-modified 2-substituted EMATEs and evaluated their in vitro anticancer activity in MCF7 cancer cells [52]. Antiproliferative activity evaluation of the steroidal oximes 2a–2j (Figure 5) revealed that in general, all oximes were very effective in inhibiting MCF7 cancer cell proliferation (Table 2). These results reinforce the importance of the modifications in the 17-position of the 2-substituted EMATEs, particularly the introduction of an oxyimino group, which increased the antiproliferative activity of this class of compounds [52].
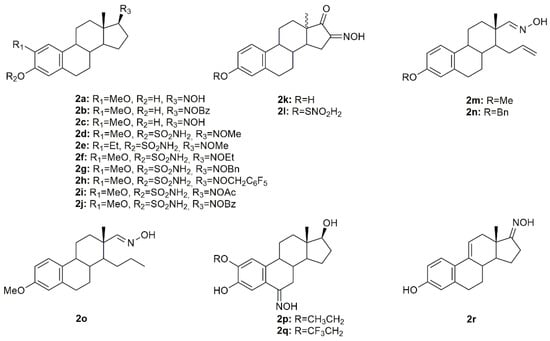
Figure 5.
Steroidal estrane oxime derivatives with anticancer activity in several types of cancer cells.
A series of novel estrone-16-oxime ethers were designed and synthesized, and their antitumor activity was evaluated in HeLa, MCF7, and A431 cancer cells [53]. From all the synthesized compounds, oxime 2k and 2l (Figure 5) decreased cancer cell proliferation in a more pronounced way, being HeLa cells the most susceptible to both compounds (IC50 = 4.41 µM for 2k and 4.04 µM for 2l) (Table 2). Further evaluation to characterize the mechanisms of action of these compounds revealed both of them interfered with the cell cycle at G1 phase and induced apoptosis in HeLa cells, by the activation of caspase-3. Following this study, the same authors continued their research on this topic and designed and synthesized a series of novel D-secooxime derivatives in the 13β- and 13α-methyl-estrone series [54]. After MTT assays to assess antiproliferative activity in HeLa, MCF7, A2780, and A431 cancer cell lines, compounds 2m–2o (Figure 5) stand out for displaying high cytotoxicity against all cell lines and being, generally, even more active than cisplatin as it can be seen in Table 2. Furthermore, compound 2n was selected for additional analysis in A2780 cells, namely cell cycle analysis. Results showed that 2n induced cell cycle arrest at the S phase, which in turn might be responsible for apoptosis induction [54].
Cushman et al. investigated several estradiol analogs to improve the anticancer activity of 2-methoxyestradiol, a naturally occurring tubulin polymerization inhibitor [55]. Starting from 2-ethoxyestradiol, an analog previously synthesized by the same group, from 2-methoxyestradiol [56], two novel steroidal oximes 2p and 2q (Figure 5) were designed and synthesized and their antitumor activity was assessed. Results revealed that both compounds were extremely toxic to all the cell lines studied (HOP62, HCT116, SF539, UACC62, OVCAR-3, SN12C and DU145) with GI50 (half growth inhibition) at values ranging from 0.010 to 0.066 µM (Table 2). Furthermore, 2p and 2q were able to inhibit tubulin polymerization. The oxime derivatives were the most active among all compounds synthesized, which points out the importance of the oxyimino functionality.
Aiming to develop new steroidal oximes with potential application in cancer treatment, a group of scientists designed and synthesized a series of estrone oxime derivatives and evaluated them in six cancer cell lines [57]. MTT results proved that compound 2r (Figure 5) is the most active against all cell lines, being LNCaP cells the most sensitive to this molecule (IC50 = 3.59 µM) (Table 2). Given this, the cytotoxicity of this steroidal oxime was mediated by a cell cycle arrest at G2/M phases accompanied by cell death by apoptosis, with evidence of condensed and fragmented nuclei. Moreover, the authors speculated that this compound might interfere with β-tubulin [57].

Table 2.
IC50 values (µM) of the synthesized estrane oxime derivatives.
Table 2.
IC50 values (µM) of the synthesized estrane oxime derivatives.
| Cell Line | Compounds | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 2e | 2f | 2g | 2h | 2i | 2j | 2k | 2l | 2m | 2n | 2o | 2p | 2q | 2r | |
| MCF7 | 5.87 | 6.47 | 0.24 | 0.17 | 0.23 | 0.23 | 1.33 | 5.14 | 0.17 | >30 | >30 | 2.6 | 2.6 | >30 | 2.1 | - | - | 25.63 |
| HeLa | - | - | - | - | - | - | - | - | - | - | - | - | 1.2 | 7.1 | 1.7 | - | - | - |
| A431 | - | - | - | - | - | - | - | - | - | - | - | - | 0.8 | 0.9 | 0.9 | - | - | - |
| A2780 | - | - | - | - | - | - | - | - | - | - | - | - | 0.9 | 1.4 | 0.7 | - | - | - |
| HOP62 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.017 | 1.2 | - |
| HCT116 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.031 | 2.1 | - |
| SF539 | 0.021 | 2.8 | ||||||||||||||||
| UACC62 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.015 | 0.88 | - |
| OVCAR3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.016 | 5.4 | - |
| SN12C | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.045 | 17 | - |
| DU145 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.049 | 17 | - |
| MDA-MB-231 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.010 | 6.5 | - |
| MGM | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.066 | 4.2 | - |
| T47D | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 43.45 |
| LNCaP | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 3.59 |
| HepaRG | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 18.35 |
| Caco | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 24.33 |
| NHDF | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 30.84 |
| Ref. | [52] | [53] | [54] | [55] | [57] | |||||||||||||
These values were obtained through different techniques such as MTT assays and specific assay kits.
3.3. Pregnane Derivatives
Bearing in mind the importance of pregnenolone in biological systems, Choudhary et al. synthesized a series of novel pregnenolone derivatives, being that some of which were oximes [58]. After synthesis, the authors came up with compound 3a (Figure 6) which was further evaluated in HepG2 and MDA-MB-231 cancer cells. Results demonstrated that this compound was quite active against both cell lines with IC50 values of 4.50 and 6.76 µM in HepG2 and MDA-MB-231 (Table 3), respectively, which makes it very promising to be further analyzed.
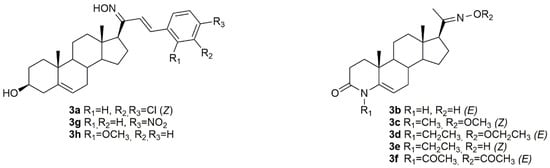
Figure 6.
Steroidal pregnane oxime derivatives with antitumor activity in several types of cancer cells.

Table 3.
IC50 values (µM) of the synthesized pregnane oxime derivatives.
Chen and collaborators did their research in 4-azasteroidal derivatives, namely 4-azasteroidal-20-oxime derivatives using progesterone as starting material [10]. The compounds were then evaluated by the MTS assay for their anticancer activity in human bladder carcinoma. After the synthesis, the authors came up with five very active oxime compounds (3b–3f, Figure 6), in T24 cells (Table 3). After structure-activity relationships (SAR) analysis, Chen et al. concluded that the methyl and ethyl oxime-ether derivatives were more active against the referred cell line when compared with the aryl oxime-ester derivatives.
Using pregnenolones as precursors, a group of scientists designed and synthesized a series of benzylidene pregnenolones and their oximes and further evaluated their potential antitumor activity in several cancer cell lines, namely HT29, HCT15, SF-295, HOP62, A549 and MCF-7 [59]. From all the synthesized oximes, compounds 3g and 3h (Figure 6) were the most active against HCT15 (IC50 = 0.31 µM for 3g and 0.65 µM for 3h) and MCF7 (IC50 = 0.60 µM for 3g and 1.91 µM for 3h) (Table 3) cancer cells, revealing a cell specificity. Moreover, the oxime derivatives were more potent than the corresponding precursors, which points out the importance of the oxyimino functionality in conferring cytotoxicity against cancer cells.
3.4. Cholestane Derivatives
Krstić et al. reported the design and synthesis of two novel steroidal oximes from cholesterol, compounds 4a and 4b (Figure 7) [60]. A few years later, the same group of scientists decided to evaluate the potential antitumor activity of these two oximes [61] against two human cancer cell lines (HeLa and K-562) and against non-stimulated and PHA-stimulated peripheral blood mononuclear cells (PBMC’s) from healthy donors to assess selectivity. Both compounds showed a dose-dependent decrease in the proliferation of HeLa cancer cells (IC50 = 35.24 ± 4.09 for 4a and IC50 = 20.68 ± 3.10 for 4b) and K-562 cancer cells (IC50 = 28.05 ± 10.18 for 4a and IC50 = 11.16 ± 1.24 for 4b), while showing almost no effects in normal immunocompetent cells (Table 4). Further evaluation revealed that compound 4b exerted its cytotoxicity by inducing apoptosis in HeLa cells. On the contrary, compound 4a showed no evidence of apoptosis or necrosis, which can be explained by the difference in the Z/E stereochemistry in the hydroxyimino group of both compounds [61].
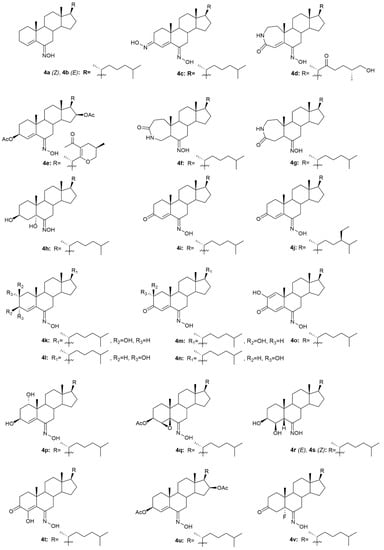
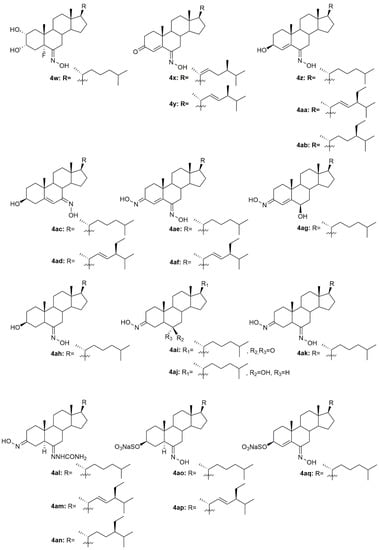
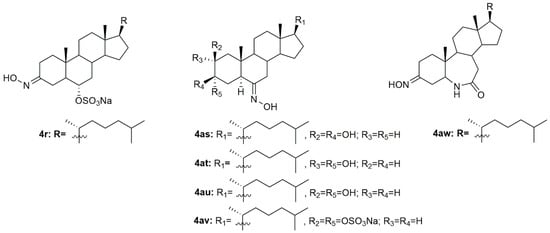
Figure 7.
Steroidal cholestane oxime derivatives with antitumor activity in several types of cancer cells.

Table 4.
IC50 values (µM) of the synthesized cholestane oxime derivatives.
A group of researchers investigated in aza-homosteroids derived from diosgenin and cholesterol-containing hydroxyimino and lactam groups in the A/B ring with four types of side chains: cholestane, spirostane, 22-oxocholestane and 22,26-epoxycholestene [62]. These compounds were further evaluated as potential antitumor agents in MCF7 cancer cells. Antitumor activity assessment revealed that from all the synthesized compounds, oximes 4c–4e (Figure 7) were the most promising ones with an IC50 of 8.2, 9.5, and 7.9 µM, respectively (Table 4). MCF7 cancer cells were more sensitive to the compounds containing a hydroxyimino group in comparison with the compounds without this chemical function. Moreover, Mora-Medina et al., evaluated these three oximes against PBMCs to test for the selectivity index of these compounds. Results showed that 4c-4e were all remarkably selective for MCF-7 cells, which makes these compounds very promising [62].
Huang et al. synthesized a series of 6-hydroxyimino-substituted-3-aza- and 4-aza-A-homo-3-oxycholestanes using cholesterol as starting material [63]. Oximes 4f and 4g (Figure 7) were further evaluated in three different cancer cell lines, namely HeLa, SMMC7404, and MGC7901. Results showed that both compounds displayed cytotoxicity against these cell lines (Table 4), being more active than the referenced drug, cisplatin, in HeLa and SMMC7404 cells. Altogether, these results demonstrated that the introduction of the hydroxyimino group at position 6 was crucial for the compounds’ cytotoxicity against cancer cells. Given the good outcomes obtained, these investigators decided to further analyze compound 4f (Figure 7) and evaluated its antitumor activity in five more cancer cell lines (GNE2, SPC-A, Tu686, PC3, and HT29) [64]. Results indicate that oxime 4f was also quite active in all cell lines with IC50 values ranging from 10.6 to 74.5 µM (Table 4). Furthermore, the molecular mechanisms by which 4f decreased cancer cell proliferation were studied. Results unveiled that compound 4f induced cancer cell apoptosis by activation of the intrinsic pathway, which was demonstrated by the annexin V labeling, activation of caspase-3, and release of cytochrome C. Moreover, this oxime was also evaluated in an in vivo model and proved to be able to inhibit tumor growth [64].
Oxysterols are a group of lipids derived from cholesterol, particularly interesting in the medicinal chemistry field due to their diverse biological effects [65]. Given this, Carvalho et al. designed and synthesized a series of oxysterols in which oxime 4h (Figure 7), a 3β,5α,6β-trihydroxycholestanol derivative, was included [66]. After the synthesis of 4h, the compound’s cytotoxicity was studied in five human cancer cell lines (HT29, SH-SY5Y, HepG2, A549, PC3) and two human normal cell lines (ARPE-19 and BJ). Oxime 4h was quite active in all cancer cell lines being that HT29 was the most sensitive (IC50 = 11.9 µM). Moreover, the compound revealed some selectivity towards HT29 cells (selectivity index of 1.99) since the IC50 displayed was higher in the normal cell lines (Table 4). This work contributed to deepening the understanding of oxysterols’ cytotoxicity and shed some light on the SAR of these classes of compounds.
In 1997, two steroidal molecules with very interesting and unusual structures, (6E)- hydroxyiminocholest-4-en-3-one (4i, Figure 7) and its 24-ethyl analog (4j, Figure 7), were isolated from Cinachyrella marine sponges [67]. Analysis of their antitumor activity revealed that only compound 4i was able to decrease the proliferation of P388 (IC50 = 1.25 µg/mL), A549 (IC50 = 1.25 µg/mL), HT29 (IC50 = 1.25 µg/mL), and MEL28 (IC50 = 2.5 µg/mL) cells (Table 5). Following this discovery, Deive et al. [68] further explored the SAR of this type of compound and prepared several derivatives of 4i and 4j with different structural features, namely with different side chains and degrees of unsaturation on ring A. From all the synthesized compounds, 4k–4o (Figure 7) stood out with an IC50 ranging from 0.125 to 1.25 µg/mL (Table 5) in the above-mentioned cancer cell lines. These results allowed to shed some light regarding SAR analysis and demonstrated that the presence of a cholesterol-type side chain, a ketone group at C-3 and a high degree of oxidation in ring A might play a major role in the compounds’ cytotoxicity [68]. A few years later, the same group continued their investigation in 6-hydroxyiminosteroids [69] and, bearing in mind the information about the SAR of these compounds, a series of new steroidal oximes were synthesized and evaluated as potential antitumor agents. After biological evaluation in four different cancer cell lines (A549, HCT116, PSN1, T98G), compounds 4p–4w (Figure 7) were the most effective in decreasing cancer cell proliferation, demonstrating good IC50 values (Table 4). Once again, the oxygenation of ring A turned out to be very important in the increased cytotoxicity of the compounds against cancer cells [69].

Table 5.
IC50 values (µg/mL) of the synthesized cholestane oxime derivatives.
Another study by Cui et al. [70] also reported the synthesis and further biological evaluation of 6-hydroxyiminosteroidal cholestane derivatives. They not only developed a facile and efficient synthetic method for the synthesis of the natural compounds 4i and 4j (Figure 7) but also designed and synthesized a new steroidal oxime (4x, Figure 7). Further antitumor activity evaluation in four human cancer cell lines, namely Sk-Hep-1, H292, PC3, and Hey1B revealed that compound 4x presented modest cytotoxicity against these cell lines with IC50 values ranging from 37 to 59.5 µg/mL (Table 5). Once again and compared to the cytotoxicity displayed by compounds 4i and 4j, the structure of the side chains impacts the cytotoxicity of the compounds. The cholesterol-type side chain seems to be important for biological activity, which goes accordingly to the results obtained by other research groups [68]. The same group of investigators continued their research on this topic and synthesized more steroidal oximes with the hydroxyimino groups in different locations (A ring or B ring) and with different types of side chains at position 17 [71]. From all the derivatives, compounds 4y–4ag (Figure 7) were the ones with the best antitumor activity against Sk-Hep-1, H292, PC3, and Hey1B (Table 5). This study reinforced the conclusions obtained in the previous ones [68,69,70], where it is stated that for enhanced cytotoxicity the compounds must have a cholesterol-type side chain, a hydroxyimino group on the B ring, and a hydroxy group on the A or B ring. The same authors went further ahead in the SAR and synthesized a series of derivatives similar to the ones synthesized in the previous study but without the 4,5-double bond [72]. After biological activity evaluation, compounds 4ah–4ak (Figure 7) proved to be the most effective in decreasing Sk-Hep-1, H292, PC3, and Hey1B cancer cells proliferation with IC50 values ranging from 35.4 to 103 µg/mL (Table 5). These compounds (without the 4,5-double bond) were more active than the equivalent ones with the 4,5-double bond [71], which indicates that the double bond in this particular position confers a negative effect in the biological activity displayed by these derivatives. All these studies were very important in unraveling the SAR of 6-hydroxyiminosteroids and might help shed some light on the design of novel chemotherapeutic drugs for the treatment of different types of cancer.
Gan et al. designed and synthesized some steroidal hydrazone derivatives with 3,6-disubstituted structure and different side chains at 17-position, being the hydroxyimino group one of these substitutions, namely in the 3-position [73]. Antiproliferative activity evaluation was carried out in vitro against gastric and liver cancer cells after 72 h of incubation. The synthetic routes gave rise to a series of novel molecules among them, compounds 4al–4an (Figure 7), which were remarkably active against the two cancer cell lines used, Bel7404 and SGC7901 (Table 6). Compound 4am was particularly active to Bel7404 cells (IC50 = 7.4 µM) being three times more active than cisplatin (IC50 = 22.3 µM), which makes 4am a potential antitumor drug.

Table 6.
- IC50 values (µM) of the synthesized cholestane oxime derivatives.
Huang et al. published a study describing the synthesis of new sulfated hydroxyiminosterols as potential antitumor agents [74]. In vitro antiproliferative activity, assessed in HeLa, SMMC7404 and MGC7901 cancer cell lines revealed that compounds 4ao–4ar (Figure 7) were all able to decrease all cancer cell lines proliferation (Table 6). Please note that compound 4ao was even more cytotoxic than cisplatin against all the cancer cell lines studied.
A group of investigators from Argentina synthesized three new 6E-hydroxyiminosteroids and then assessed their antitumor activity against prostate cancer cells (PC3 and LNCaP) [75]. After antiproliferative activity evaluation, compounds 4as–4av (Figure 7) proved to be quite active in both cancer cell lines with IC50 values ranging from 10.8 to 44.8 µM (Table 6).
Using analogs of compounds 4i and 4j as precursors, Huang et al. designed and synthesized novel steroidal oximes and then evaluated their anticancer efficacy against a panel of six cancer cell lines [76]. Of all the synthesized compounds, oxime 4aw (Figure 7) was the most powerful oxime, especially in HeLa and GNE2 cancer cell lines with IC50 values of 9.1 and 11.3 µM, respectively (Table 6). Remarkably, this compound was even more active than cisplatin (IC50 = 10.1 and 16.8 µM in HeLa and GNE2 cells, respectively).
3.5. Diosgenin Derivatives
Diosgenin is a natural steroidal sapogenin, which was first isolated from Discorea tokoro by Takeo Tsukamato in 1936 [77]. This molecule is widely used in the pharmaceutical industry as the main precursor in the synthesis of steroids. Given this, several investigators have been focusing their attention on diosgenin derivatives. Sánchez et al. designed and synthesized two novel oxime derivatives (5a and 5b, Figure 8) using diosgenin as a precursor and then tested their antitumor activity against HeLa and CaSki cancer cell lines [78]. Results revealed that both compounds caused a dose-dependent decrease in HeLa and CaSki cell proliferation with IC50 ranging from 10.9 to 48.18 µM (Table 7) and compound 5a was even more active than diosgenin, which reinforces the importance of the hydroxyimino group, positioned in the side chain, in conferring cytotoxicity. Further analysis of both compounds demonstrated that they exerted their antitumor activity by interfering with the cell cycle, and causing cell death by apoptosis mediated by the activation of caspase-3, which, in turn, is responsible for DNA fragmentation [78].
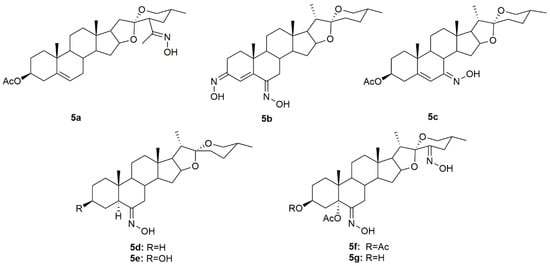
Figure 8.
Steroidal diosgenin oxime derivatives with antitumor activity in several types of cancer cells.

Table 7.
IC50 values (µM) of the synthesized diosgenin oxime derivatives.
Another group of investigators synthesized a series of diosgenin derivatives by introducing new modifications in rings A and B [79]. Among all these compounds, oxime 5c (Figure 8) was evaluated in HeLa, MDA-MB-231, and HCT-15 cancer cells to assess its antitumor activity. Results showed that 5c was quite active in all cell lines with IC50 of 18.23, 10.83, and 17.56 µg/mL in HeLa, MDA-MB-231, and HCT-15 (Table 7), respectively. Furthermore, this compound was not toxic to PBMC which can point to a selectivity towards cancer cells.
Carballo et al. designed and synthesized a series of novel hydroxyimino steroidal derivatives and evaluated their antiproliferative activity in MCF7 and MDA-MB-231 breast cancer cells [80]. Compounds 5d–5e (Figure 8) were able to decrease cell proliferation with IC50 values ranging from 9.3 to 11.8 µM (Table 7). Interestingly, compounds 5d and 5e (spirostan derivatives) were more active against the triple-negative cells, a subtype of breast cancer associated with a worse prognosis and fewer therapeutic options.
More recently, a group of scientists designed and synthesized a series of steroidal oximes from diosgenin and evaluated their antitumor activity against six cancer cell lines (A549, HBL100, HeLa, SW1573, T47D, and WiDr) [81]. Of all the compounds synthesized, 5f and 5g (Figure 8) were the most active against all cancer cell lines tested (Table 7). Moreover, compound 5f was more active than cisplatin in T47D and WiDr cells and 5g was more active only in WiDr cells. These results encourage more research to unravel the mechanisms of action behind the cytotoxicity displayed by these compounds against cancer cells.
3.6. Bile Acids Derivatives
Bile acids have shown good biological and chemical properties, which make them very useful in the designing of new pharmacological entities [82]. Given this, a group of investigators reported the synthesis of new 3-aza-A-homo-4-one bile acid and 7-deoxycholic acid derivatives, among them some with the hydroxyimino group in their structure [83]. Of all the compounds, 6a and 6b (Figure 9) were the most promising ones being very effective in decreasing the MGC7901, HeLa, and SMMC7404 cells proliferation. Please note that the most sensitive cell line to both 6a and 6b was the HeLa, ovarian cancer cell line, with an IC50 of 14.3 and 24.3 µM, respectively. Compound 6a was even more cytotoxic against cancer cells than the reference drug, cisplatin (IC50 = 20.6 µM). These results enlighten, once again, the relationship between the hydroxyimino group and biological activity [83].
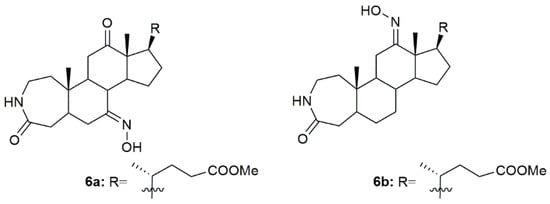
Figure 9.
Steroidal bile acid oxime derivatives with antitumor activity in several types of cancer cells.
4. Steroidal Oximes as Antimicrobial Agents
Infectious diseases are a public health problem and are among the top ten leading causes of death worldwide according to WHO [84]. The need for novel therapeutic options to treat this type of disease is of great importance. Steroids and hydroxyimino group-bearing compounds have been shown to be effective against bacteria, fungi, and some viruses [12,85]. In this section, we will describe the most potent steroidal oximes with antibacterial, antifungal, and antiviral activity.
4.1. Androstane Derivatives
A series of androstane derivatives containing the hydroxyimino group (7a–7e, Figure 10) were designed, synthesized, and further analyzed as antibacterial and antifungal drug candidates [86] against different strains of bacteria and fungi. Results demonstrated that all oximes presented excellent antibacterial and antifungal activity, being in general more toxic to the pathogens than the referenced drugs, as seen by the minimal inhibitory (MIC) and minimal bactericidal/fungicidal (MBC/MFC) concentrations described in Table 8 and Table 9.
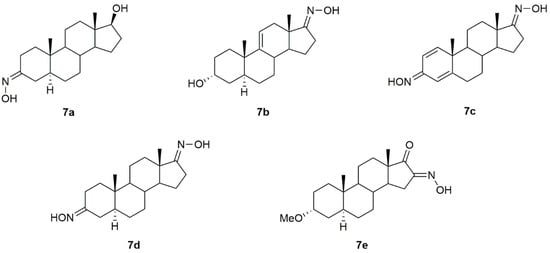
Figure 10.
Steroidal androstane oxime derivatives with antibacterial and antifungal activity.

Table 8.
MIC and MBC (mg/mL) values of the synthesized androstane oxime derivatives. Adapted from [86].

Table 9.
MIC and MFC (mg/mL) values of the synthesized androstane oxime derivatives. Adapted from [86].
4.2. Pregnane Derivatives
Using pregnenolone as starting material, Prabpayak et al. synthesized a novel oxime derivative (8a, Figure 11) and evaluated its antibacterial activity against a series of different strains of bacteria [87]. Results revealed that 8a was able to decrease bacterial growth, as seen by the calculation of the zone of inhibition which was 12.5 mm for S. mutans ATCC 1275 and 25 mm for C. diptheriae.
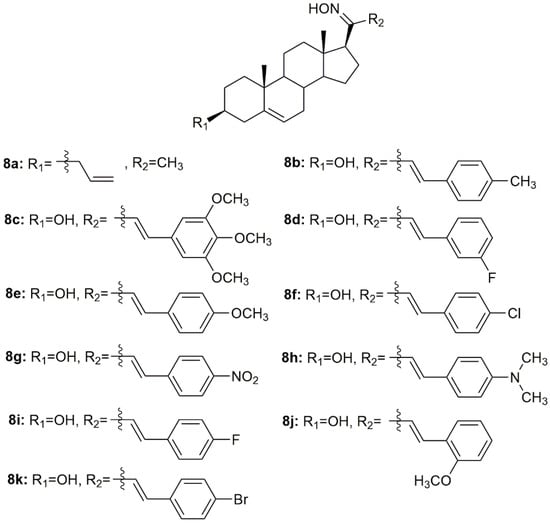
Figure 11.
Pregnane oxime derivatives with antimicrobial activity.
Lone et al. designed and synthesized nine oximes of steroidal chalcones (8b–8k, Figure 11) and screened them for in vitro antimicrobial activity against different strains of bacteria and fungi [88]. All compounds displayed good antimicrobial activity against all strains studied (Table 10). Moreover, the oximes showed enhanced antimicrobial activity when compared with the corresponding chalcones with a carbonyl group instead of a hydroxyimino group. These results highlight the importance of this hydroxyimino group for the toxicity displayed against pathogens.

Table 10.
MIC values of (µg/mL) of the synthesized pregnane oxime derivatives. Adapted with permission from Ref [88], 2023, Ana S. Pires.
4.3. Cholestane Derivatives
A group of scientists designed and synthesized three novel steroidal oximes (9a–9c, Figure 12) from cholestane [89]. After synthesis, the antibacterial and antifungal activity in different strains of bacteria (S. pyogenes, S. aureus, S. typhi, P. aeruginosa and E. coli) and fungi (P. marneffei, A. fumigatus, T. mentagrophytes, C. albicans and C. krusei) was assessed through analysis of the diameter of zone of inhibition (mm). Anthelmintic activity was also analyzed against earthworms. Generally, all compounds presented good antibacterial and antifungal activity with compound 9c being the most active against both bacterial (zone of inhibition ranging from 17.3 to 23.9 mm) and fungi strains (zone of inhibition ranging from 15.1 and 25.5 mm). This compound was also the most effective anthelmintic compound showing great early paralysis and lethal times. Further docking studies demonstrated that 9c had not only better affinity to the receptor, but also presented the best docking score, making it a very promising antimicrobial [89].
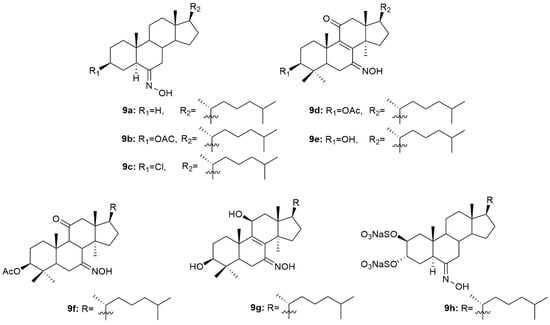
Figure 12.
Cholestane oxime derivatives with antimicrobial activity.
Oxysterols apart from displaying antitumor activity have also been associated with antimicrobial activity [90]. Compounds 9d–9g (Figure 12) were designed and synthesized and further evaluated against a series of fungal strains [91]. In general, all compounds displayed good antifungal activity against all the strains used in this study (MIC values ranging from 2 to 64 µg/mL, Table 11). C. neoformans (CN1) was particularly sensitive to all oximes synthesized, being the MIC values (2–4 µg/mL) presented even lower than the referenced drugs fluconazole (16 µg/mL) and amphotericin B (32 µg/mL).

Table 11.
MIC values (µg/mL) of cholestane oxime derivatives.
Aiming to develop novel therapeutic options for the treatment of herpes simplex virus, Pujol et al. synthesized a series of sulfated steroids derived from 5α-cholestanes [92]. The oxime 9h (Figure 12) was the most promising compound, showing the best inhibitory values of HSV-1, HSV-2, and pseudorabies virus (PrV) strains, including acyclovir-resistant strains in human and monkey cell lines (EC50 values ranging from 16.7 to 25 µg/mL, Table 11). Moreover, the authors went further ahead and decided to investigate the mechanism of action of 9h in HSV-1. After using the virucidal assay, authors concluded that the 9h was not able to affect the initial steps of virus entry but rather inhibited an ensuing event in the infection process of this virus [92].
4.4. Bile Acids Derivatives
Hepatitis B is a major global health problem and is caused by the hepatitis B virus (HBV). This condition affects the liver, leading to cirrhosis and liver cancer, which can lead to patient death [93]. In this context, a group of scientists focused their attention on designing and synthesizing oxime derivatives of dehydrocholic acid as potential HBV drugs [94]. After anti-hepatitis B virus activity evaluation in HepG 2.2.15 cells, compounds 10a–10c (Figure 13) exhibited more cytotoxicity against the virus than the referenced drug, entecavir, as seen by the most effective inhibition of HBeAg (hepatitis B -antigen). Compound 10b was the most active compound showing significant anti-HBV activity on inhibition secretion of HBeAg (IC50 = 96.64 µM) when compared, once again, with entecavir (IC50 = 161.24 µM). Moreover, docking studies were also performed to evaluate the potential mechanisms behind the activity of these compounds. Results point out a possible interaction with protein residues of heparan sulfate proteoglycan (HSPG) in host hepatocytes and bile acid receptors [94].
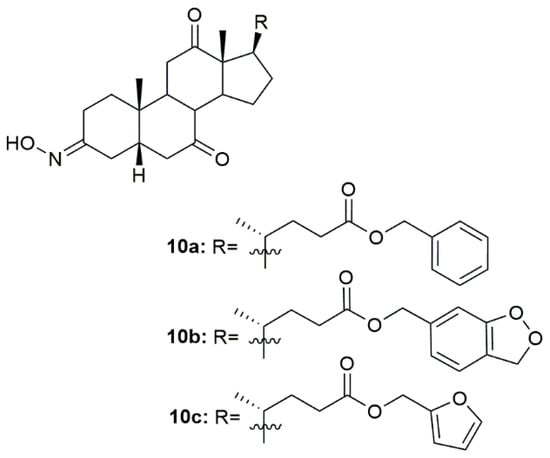
Figure 13.
Bile acid oxime derivatives with antiviral activity.
5. Steroidal Oximes as Anti-Inflammatory Agents
5.1. Cholestane Derivatives
Since inflammation plays a critical role in tumor progression, Díaz et al. designed and synthesized a series of 22-oxocholestane oximes as potential anti-inflammatory agents in an acute inflammation mouse ear model [95]. Five oxime derivatives (11a–11e, Figure 14) stood out from the rest as being the most promising, being able to reduce ear inflammation and edema. Moreover, these compounds also repressed the expression of pro-inflammatory genes such as TNF-α, COX-2, and IL-6, making them very promising lead candidates for further assessment.
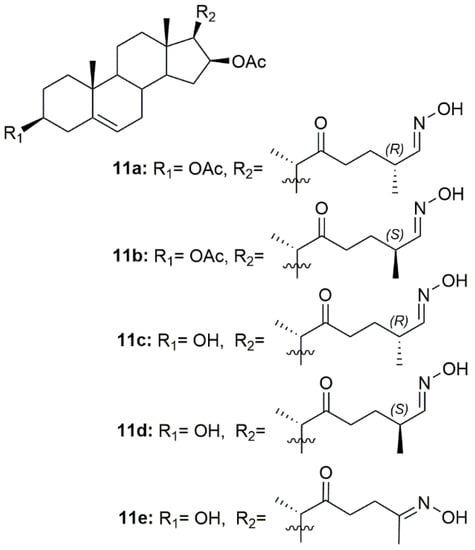
Figure 14.
Cholestane oxime derivatives with anti-inflammatory activity.
5.2. Bile Acid Derivatives
The need for anti-inflammatory drugs such as glucocorticoids has been increasing, especially since the COVID-19 pandemic, because this type of compounds is the standard treatment for this disease. Bearing this in mind, Bjedov and collaborators designed, synthesized, and screened the binding activity of a series of bile acid derivatives for the ligand-binding domain of glucocorticoid receptor (GR-LBD), the main receptor in the anti-inflammatory process [96]. Of all the synthesized compounds, oxime 12a (Figure 15) presented the best relative binding affinity for GR-LBD. Even though the authors were not able to perform molecular docking and predict the interactions between compound 12a and the enzyme, they suggest that this binding affinity could be attributed not only to the C-24 carboxylic group but also to the hydroxyimino groups. Moreover, the C-12 hydroxyimino group could also be used as an alternative hydrogen donor to the 11β-OH group of the glucocorticoids and the hydroxyimino group at C-3 may also be beneficial for glucocorticoid receptor affinity through the establishment of a hydrogen bond. This study was very important and helped shed some light on the possible interactions of steroidal oximes with their targets.
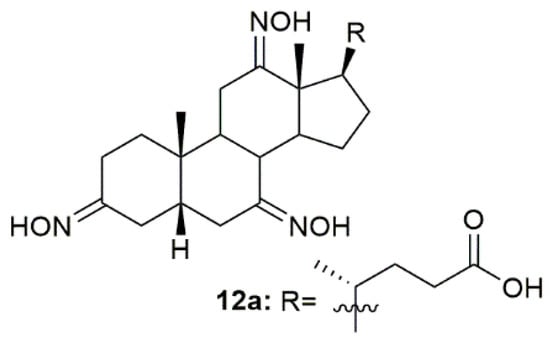
Figure 15.
Bile acid derivative with anti-inflammatory activity.
6. Steroidal Oximes in Clinical Development
Istaroxime
Istaroxime (Figure 16) is an inhibitor of sodium/potassium adenosine triphosphatase (Na+/K+ ATPase), which is currently under phase 2 clinical trial development for the treatment of acute decompensated heart failure [97,98]. Apart from this, istaroxime has also been evaluated as a potential antitumor agent in several types of cancer [99,100].
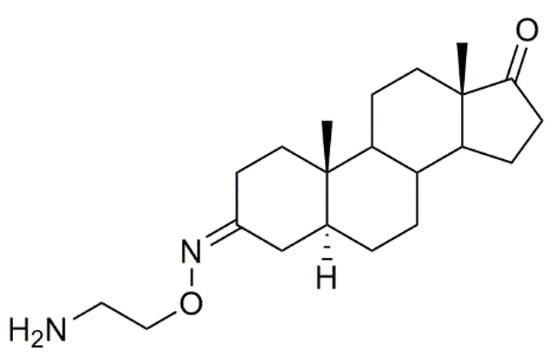
Figure 16.
Istaroxime.
7. Steroidal Oximes in Clinical Use
7.1. Norgestimate
Norgestimate (Figure 17), a steroidal oxime, (brand names, Ortho Tri-Cyclen or, Previfem, among others) is a progestin, which is used in hormonal contraception and menopausal hormone therapy for the treatment of some menopausal symptoms [101]. Norgestimate is not available as a single therapy, being used together with ethinylestradiol in birth control pills and in combination with estradiol in menopausal hormone therapy [102]. It was first introduced in the USA in 1999 and is one of the most prescribed birth control pills worldwide. This compound is sold as a mixture of the two E and Z conformers.
Figure 17.
Norgestimate.
This oxime has been subject to further biological evaluation such as antibacterial assessment and proved to be a promising lead compound to treat biofilm-associated infections and to resensitize bacterial strains resistant to some antibiotics [103].
7.2. Norelgestromin
Norelgestromin (Figure 18), or norelgestromine (brand names, Evra or Ortho Evra, among others) is also a progestin used for birth control. Like norgestimate, norelgestromin is not available as a single drug but rather in combination with an estrogen, ethinyl estradiol in the form of contraceptive patches [104]. It was first introduced to the market in 2002. This compound is also sold as a mixture of two E and Z isomers.
Figure 18.
Norelgestromin.
8. Mechanisms of Action of Steroidal Oximes
Throughout this review, we came upon a wide variety of synthesized steroidal oximes, starting from androstane to estrane, pregnane, cholestane, diosgenin, and bile acids derivatives with very different mechanisms of action.
Most of the compounds summarized here are being evaluated as potential antitumor agents and exert their cytotoxicity against cancer cells mainly by inducing apoptosis; however, the pathways leading to it differ from compounds. For example, the androstane derivatives appear to induce apoptosis [34,42,48] by cell cycle arrest at different phases and increased ROS production [34]. Necroptosis might also be a mechanism involved in the compounds’ cytotoxicity against cancer cells [34]. Oxime estrane derivatives induced cell death by apoptosis through cell cycle interference at G1 [53], S [54] and G2/M phases [57] and through activation of caspase-3 [53]. This class of compounds also interferes with microtubules by interfering with β-tubulin [55,57]. These are often related to cell cycle arrest and, consequently, a decrease in cell division and cell death. Concerning oxime pregnane, diosgenin, and some cholestane derivatives, they cause cell cycle alterations, and cell death by the activation of the apoptotic intrinsic pathway mediated by caspase-3 activation [64,78] and release of cytochrome C [61,64,78]. Additionally, and since inflammation plays an important role in carcinogenesis, inhibition of pro-inflammatory genes such as TNF-α, COX, and IL6 is also another important mechanism displayed by these steroidal oximes [95]. Additionally, for some of the cholestane derivatives, their mechanism of action is not well elucidated since most of the studies were conducted mainly to infer about SAR, rather than go deeper into their mode of action. However, the breakthroughs reached in this field are of great importance and encourage the need to further analyze their mechanisms of action.
Aside from antitumor activity, steroidal oximes also display antimicrobial activity. However, little is known about the mode of action of these compounds. Regarding antiviral activity, it seems that steroidal oximes exert their effect by inhibition of the infection process of the virus after it enters the cells [92] and by a possible interaction with protein residues of heparan sulfate proteoglycan (HSPG) in host hepatocyte and bile acid receptor [94].
Concerning the compounds in clinical trials and already in clinical use, their mechanisms are very different. Istaroxime is an inhibitor of the Na+/K+ ATPase pump [98], whereas norgestimate and norelgestromin, which are already in the market for birth control, act as progesterone agonists, inhibiting ovulation [101,102].
9. Structure-Activity Relationships of Steroidal Oximes
The biological activity of a compound is closely related to its chemical structure. Steroidal oximes exert several types of biological activities, which can vary depending on the position of the oxime functionality on the steroid scaffold. Given this, some SAR can be elucidated especially for the antitumor and antimicrobial activity to help in the design and synthesis of novel molecules with pharmaceutical potential (Figure 19, Figure 20 and Figure 21). However, the conclusions that can be drawn from Figure 19, Figure 20 and Figure 21 must be analyzed carefully, as they are just trends that were observed from the compounds studied in this review article, which were the most active compounds of each paper, and were evaluated with different methodologies, different times, and in different cancer cells.
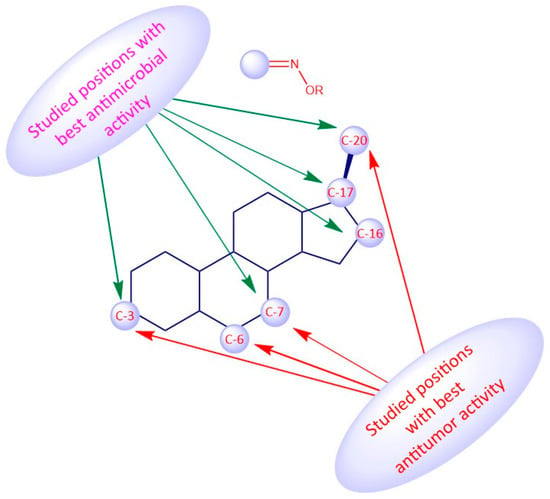
Figure 19.
Overview of the most common SAR of steroidal oximes with antitumor and antimicrobial activity.
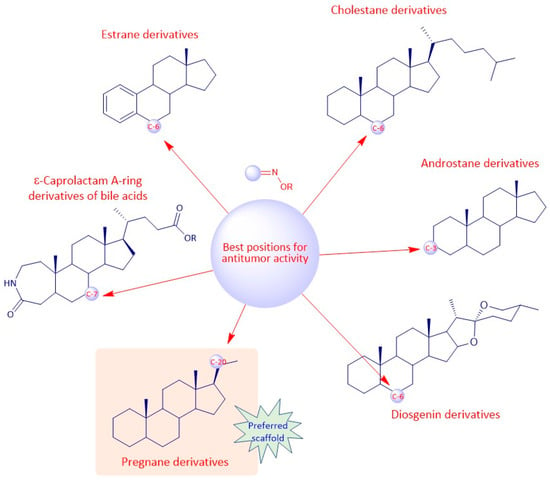
Figure 20.
Most common SAR of steroidal oximes with antitumor activity.
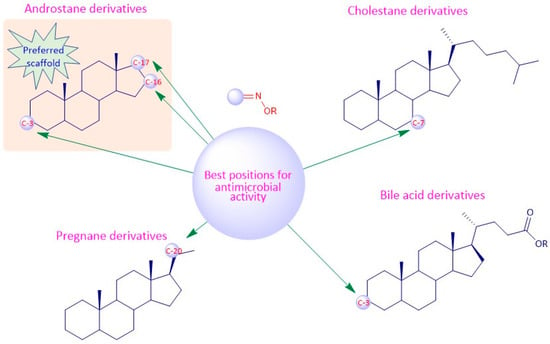
Figure 21.
Most common SAR of steroidal oximes with antimicrobial activity.
Regarding the antitumor activity, when comparing the different IC50 values displayed by the androstane oxime derivatives, and despite the presence of other functional groups, it seems that position C-3 of the steroidal scaffold is preferable since the compounds with a hydroxyimino group in this position were, in general, the ones with the lower IC50 values (1f, 1g, 1h). Compound 1h, which has a substituted oxyimino group at position C-3 was generally the compound with the best IC50 values in all cell lines reported in Table 1. Interestingly, the compounds that, in addition to having a hydroxyimino group at position C-3, also had another hydroxyimino group at position C-6 (1b, 1c and 1u) or at position C-17 (1k–1o) were slightly less active, suggesting that only a single hydroxyimino group at position C-3 is preferable and might be enough for the antitumor activity. Furthermore, in some types of cancer, namely in breast and prostate cancers, a hydroxyimino group at position C-2 was also important for the cytotoxicity displayed against cancer cells (compound 1r and 1s). Compound 1s combines another hydroxyimino group at position C-4, which proved to be better than with just a single hydroxyimino group at position C-2. Positions C-17 and C-7 seem to be less favorable than the other positions mentioned above. Regarding the estrane series, position C-6 seems to be the most preferable concerning antitumor activity since compounds 2p and 2q were the ones with best IC50 values. In general, compounds with a hydroxyimino group at position C-17 were also very active. Concerning the pregnane series, all oximes (substituted or not) present high activity with very low IC50 values (IC50 values ranging from 0.31 to 7.17 µM. A hydroxyimino group at position C-6 appears to be the most advantageous position, regarding the cholestane series, since they present the lowers IC50s. With regard to the diosgenin series, the compounds with just a single hydroxyimino group were slightly more cytoxic against cancer cells than the ones containing two hydroxyimino groups (compounds 5b, 5f and 5g present higher IC50 values than compounds 5a, 5d and 5e). Finally, introduction of a hydroxyimino group in the B ring was clearly more beneficial than at C-ring, considering that compound 6a presented a lower IC50 value than compound 6b, for the case of bile acid derivatives.
Considering the antimicrobial activity, the androstane oxime derivatives with just a single hydroxyimino group were slightly more potent than the compounds with two hydroxyimino groups, suggesting that a single hydroxyimino group is better for antimicrobial activity rather than two hydroxyimino groups. Furthermore, a hydroxyimino group at position C-7 might be a better option when designing and synthesizing oximes in the cholestane series, since the compounds with a hydroxyimino group at this position were slightly more toxic against pathogens than compounds with a hydroxyimino group at position C-6. Pregnane oxime derivatives were more active than the parent pregnane compounds.
To sum up, for the antitumor activity, SAR analysis points out that the pregnane scaffold might be the best option when designing novel molecules with antitumor activity. This steroidal oxime series presented the best IC50 values against the cancer cell lines studied when compared with the other above-mentioned series. As for the antimicrobial activity, and since the number of studies is considerably less, it turns out to be more difficult to establish robust SAR, but it seems that the androstane scaffold may be the most favorable option. In Figure 19 we can see an overview of the most common SAR of steroidal oximes for the antitumor and antimicrobial biological activities.
As mentioned at the beginning of this chapter, SAR are extremely import for the design and synthesis of novel drugs. Given this, it is our hope that this review might help guide researchers to design more potent and selective chemical entities and to help them understand the pharmaceutical potential of steroidal oximes.
10. Conclusions
This review sums up the work that has been developed regarding the most promising steroidal oximes in recent years. The oxyimino group is a privileged chemical function being, for that reason, very popular among the medicinal chemistry community.
Most steroidal oximes in preclinical settings present antitumor activity. In vitro and in vivo studies have demonstrated the significant antiproliferative activity and elucidated some of the mechanisms by which steroidal oximes display cytotoxicity against cancer cells, which include induction of cell cycle arrest, apoptosis, necroptosis, increased ROS production and interference with microtubules. The described targets for steroidal oximes exerting antitumor activity are caspase-3 and β-tubulin. Moreover, steroidal oximes also exert antimicrobial activity, sometimes by interfering with HSPG, resulting in tremendous cytotoxicity against bacteria, fungi, and viruses. The anti-inflammatory activity is associated with TNF-α, COX-2, and IL-6 genes expression. Finally, there are currently two steroidal oximes in the market used as birth control drugs and one in clinical trials for acute heart failure, acting by progesterone agonists and Na+/K+ ATPase pump inhibitors, respectively.
The acquired knowledge about the diversified biological activities of steroidal oximes only emphasizes the need to deeper understand the mechanisms behind their activity and consequently, unravel which are their targets. In addition, molecular interactions with targets need to be elucidated, robust SAR must be constructed, and ADME properties have to be studied in order to design more potent and selective compounds. Steroidal oximes can be, in fact, very promising lead compounds for the development of drugs for the treatment of several diseases and deserve to be better explored.
Author Contributions
Conceptualization, F.M.F.R., E.J.T.-d.-S. and A.R.G., Writing—original draft, A.R.G., Writing—review and editing, F.M.F.R., E.J.T.-d.-S., A.R.G. and A.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation for Science and Technology (Portugal) through the Strategic Projects UIDB/04539/2020 and UIDP/04539/2020 (CIBB) and through PhD grant of Ana R. Gomes (UI/BD/150865/2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Foundation for Science and Technology (FCT), Portugal for financial support through the PhD grant of Ana R. Gomes (UI/BD/150865/2021).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| A2780 | human ovarian cancer cell line |
| A431 | human skin epidermoid cancer cell line |
| A549 | human lung carcinoma cell line |
| Bel7404 | human liver cancer cell line |
| Ca Ski | human cervical cancer cell line |
| DU145 | human prostate cancer cell line |
| GNE2 | human nasopharyngeal cancer cell line |
| H1299 | human non-small lung cancer cell line |
| H292 | human lung cancer cell line |
| HBL100 | human normal breast cell line |
| HCT116 | human colon cancer cell line |
| HCT15 | human colorectal cancer cell line |
| HeLa | human cervical cancer cell line |
| HepG2 | human liver cancer cell line |
| Hey1B | human ovarian cancer cell line |
| HL60 | human leukemic cell line |
| HOP62 | human lung cancer cell line |
| HT29 | human colorectal cancer cell line |
| K562 | human leukemic cell line |
| KB | human nasopharyngeal cancer cell line |
| LNCaP | human prostate cancer cell line |
| MCF7 | human breast cancer cell line |
| MDA-MB-231 | human breast triple-negative cancer cell line |
| MEL28 | human melanoma cancer cell line |
| MGC7901 | human gastric cancer cell line |
| MKN-28 | human gastric cancer cell line |
| NCI-H460 | human lung large cell |
| OVCAR-3 | human ovarian cancer cell line |
| P388 | human lymphoma cell line |
| PC3 | human prostate cancer cell line |
| PSN1 | human pancreatic cancer cell line |
| SF268 | human astrocytoma cancer cell line |
| SF539 | human gliosarcoma cancer cell line |
| SGC7901 | human papillomavirus-related endocervical adenocarcinoma cell line |
| SH-SY5Y | human neuroblastoma cell line |
| SK-Hep-1 | human liver cancer cell line |
| SMMC7404 | human liver cancer cell line |
| SN12C | human renal cancer cell line |
| SPC-A | human papillomavirus-related endocervical adenocarcinoma cell line |
| SR | human lymphoma cell line |
| SW1573 | human lung cancer cell line |
| T24 | human urinary bladder cancer cell line |
| T47D | human breast ductal carcinoma cell line |
| T98G | human glioblastoma cancer cell line |
| Tu686 | human laryngeal squamous cell carcinoma cell line |
| UACC62 | human skin cancer cell line |
| WiDr | human colorectal cancer cell line |
References
- Bhatti, H.N.; Khera, R.A. Biological transformations of steroidal compounds: A review. Steroids 2012, 77, 1267–1290. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Ericson-Neilsen, W.; Kaye, A.D. Steroids: Pharmacology, complications, and practice delivery issues. Ochsner J. 2014, 14, 203–207. [Google Scholar] [PubMed]
- Rasheed, A.; Qasim, M. A Review of natural steroids and their applications. Int. J. Pharm. Sci. Res. 2013, 4, 520–531. [Google Scholar]
- Bansal, R.; Suryan, A. A Comprehensive Review on Steroidal Bioconjugates as Promising Leads in Drug Discovery. ACS Bio Med Chem Au 2022, 2, 340–369. [Google Scholar] [CrossRef]
- Bai, C.; Schmidt, A.; Freedman, L.P. Steroid Hormone Receptors and Drug Discovery: Therapeutic Opportunities and Assay Designs. ASSAY Drug Dev. Technol. 2003, 1, 843–852. [Google Scholar] [CrossRef]
- Schepetkin, I.; Plotnikov, M.; Khlebnikov, A.; Plotnikova, T.; Quinn, M. Oximes: Novel Therapeutics with Anticancer and Anti-Inflammatory Potential. Biomolecules 2021, 11, 777. [Google Scholar] [CrossRef]
- Grob, D.; Johns, R.J. Use of oximes in the treatment of intoxication by anticholinesterase compounds in normal subjects. Am. J. Med. 1958, 24, 497–511. [Google Scholar] [CrossRef]
- Dhuguru, J.; Zviagin, E.; Skouta, R. FDA-Approved Oximes and Their Significance in Medicinal Chemistry. Pharmaceuticals 2022, 15, 66. [Google Scholar] [CrossRef]
- Chen, S.-R.; Shen, F.-J.; Feng, G.-L.; Yuan, R.-X. Synthesis and Anticancer Activity of 4-azasteroidal-20-oxime Derivatives. J. Chem. Res. 2015, 39, 527–530. [Google Scholar] [CrossRef]
- Sørensen, M.; Neilson, E.H.; Møller, B.L. Oximes: Unrecognized Chameleons in General and Specialized Plant Metabolism. Mol. Plant 2017, 11, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Surowiak, A.K.; Lochyński, S.; Strub, D.J. Unsubstituted oximes as potential therapeutic agents. Symmetry 2020, 12, 575. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Sinha, A.S.; Epa, K.N.; Chopade, P.D.; Smith, M.M.; Desper, J. Structural Chemistry of Oximes. Cryst. Growth Des. 2013, 13, 2687–2695. [Google Scholar] [CrossRef]
- Canario, C.; Silvestre, S.; Falcão, A.; Alves, G. Steroidal Oximes: Useful Compounds with Antitumor Activities. Curr. Med. Chem. 2018, 25, 660–686. [Google Scholar] [CrossRef]
- Donaruma, L.G.; Heldt, W.Z. The Beckmann rearrangement. Org. React. 2011, 15, 1–156. [Google Scholar]
- Ãbele, E.; Lukevics, E. Recent advances in the chemistry of oximes. Org. Prep. Proced. Int. 2000, 32, 235–264. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Bokach, N.A.; Demakova, M.Y.; Kukushkin, V.Y. Metal-Involving Synthesis and Reactions of Oximes. Chem. Rev. 2017, 117, 13039–13122. [Google Scholar] [CrossRef]
- Elliott, M.C. Imines and Their N-Substituted Derivatives: Hydrazones and Other N,N-Derivatives Including Diazo Compounds. Cheminform 2005, 36, 469–523. [Google Scholar] [CrossRef]
- Savić, M.P.; Ajduković, J.J.; Plavša, J.J.; Bekić, S.S.; Ćelić, A.S.; Klisurić, O.R.; Jakimov, D.S.; Petri, E.T.; Djurendić, E.A. Evaluation of A-ring fused pyridine ᴅ-modified androstane derivatives for antiproliferative and aldo–keto reductase 1C3 inhibitory activity. Medchemcomm 2018, 20, 969–981. [Google Scholar] [CrossRef]
- Savić, M.P.; Škorić, D.Đ.; Kuzminac, I.Z.; Jakimov, D.S.; Kojić, V.V.; Rárová, L.; Strnad, M.; Djurendić, E.A. New A-homo lactam ᴅ-homo lactone androstane derivative: Synthesis and evaluation of cytotoxic and anti-inflammatory activities in vitro. Steroids 2020, 157, 108596. [Google Scholar] [CrossRef]
- Savić, M.P.; Kuzminac, I.Z.; Škorić, D.; Jakimov, D.S.; Rárová, L.; Sakač, M.N.; Djurendić, E.A. New oxygen-containing androstane derivatives: Synthesis and biological potential. J. Chem. Sci. 2020, 132, 1–10. [Google Scholar] [CrossRef]
- Kulmány, Á.E.; Herman, B.E.; Zupkó, I.; Sinreih, M.; Rižner, T.L.; Savić, M.; Oklješa, A.; Nikolić, A.; Nagy, V.; Ocsovszki, I.; et al. Heterocyclic androstane and estrane d-ring modified steroids: Microwave-assisted synthesis, steroid-converting enzyme inhibition, apoptosis induction, and effects on genes encoding estrogen inactivating enzymes. J. Steroid Biochem. Mol. Biol. 2021, 214, 105997. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, S.Z.; Podunavac-Kuzmanović, S.O.; Jevrić, L.R.; Jovanov, P.T.; Djurendić, E.A.; Ajduković, J.J. Comprehensive QSRR modeling as a starting point in characterization and further development of anticancer drugs based on 17α-picolyl and 17(E)-picolinylidene androstane structures. Eur. J. Pharm. Sci. 2016, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jójárt, R.; Ali, H.; Horváth, G.; Kele, Z.; Zupkó, I.; Mernyák, E. Pd-catalyzed Suzuki–Miyaura couplings and evaluation of 13α-estrone derivatives as potential anticancer agents. Steroids 2020, 164, 108731. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.S.; Varela, C.L.; Marques, I.A.; Abrantes, A.M.; Gonçalves, C.; Rodrigues, T.; Matafome, P.; Botelho, M.F.; Roleira, F.M.; Tavares-Da-Silva, E. Oxymestane, a cytostatic steroid derivative of exemestane with greater antitumor activity in non-estrogen-dependent cell lines. J. Steroid Biochem. Mol. Biol. 2021, 212, 105950. [Google Scholar] [CrossRef]
- Augusto, T.V.; Amaral, C.; Varela, C.L.; Bernardo, F.; da Silva, E.T.; Roleira, F.F.; Costa, S.; Teixeira, N.; Correia-Da-Silva, G. Effects of new C6-substituted steroidal aromatase inhibitors in hormone-sensitive breast cancer cells: Cell death mechanisms and modulation of estrogen and androgen receptors. J. Steroid Biochem. Mol. Biol. 2019, 195, 105486. [Google Scholar] [CrossRef] [PubMed]
- Roleira, F.M.F.; Varela, C.; Amaral, C.; Costa, S.C.; Correia-da-Silva, G.; Moraca, F.; Costa, G.; Alcaro, S.; Teixeira, N.A.A.; da Silva, E.J.T. C-6α- vs C-7α-substituted steroidal aromatase inhibitors: Which is better? Synthesis, biochemical evaluation, docking studies, and structure–activity relationships. J. Med. Chem. 2019, 62, 3636–3657. [Google Scholar] [CrossRef]
- Amaral, C.; Augusto, T.V.; Tavares-Da-Silva, E.; Roleira, F.M.; Correia-Da-Silva, G.; Teixeira, N. Hormone-dependent breast cancer: Targeting autophagy and PI3K overcomes Exemestane-acquired resistance. J. Steroid Biochem. Mol. Biol. 2018, 183, 51–61. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.L.; Maurício, J.; Sobral, A.F.; Costa, S.C.; Roleira, F.M.; da Silva, E.T.; Correia-Da-Silva, G.; Teixeira, N. Anti-tumor efficacy of new 7α-substituted androstanes as aromatase inhibitors in hormone-sensitive and resistant breast cancer cells. J. Steroid Biochem. Mol. Biol. 2017, 171, 218–228. [Google Scholar] [CrossRef]
- Amaral, C.; Lopes, A.; Varela, C.L.; da Silva, E.T.; Roleira, F.M.; Correia-Da-Silva, G.; Teixeira, N. Exemestane metabolites suppress growth of estrogen receptor-positive breast cancer cells by inducing apoptosis and autophagy: A comparative study with Exemestane. Int. J. Biochem. Cell Biol. 2015, 69, 183–195. [Google Scholar] [CrossRef]
- Varela, C.L.; Amaral, C.; Correia-Da-Silva, G.; Carvalho, R.A.; Teixeira, N.A.; Costa, S.C.; Roleira, F.M.; Tavares-Da-Silva, E.J. Design, synthesis and biochemical studies of new 7α-allylandrostanes as aromatase inhibitors. Steroids 2013, 78, 662–669. [Google Scholar] [CrossRef]
- Amaral, C.; Varela, C.; Correia-Da-Silva, G.; da Silva, E.T.; Carvalho, R.A.; Costa, S.C.; Cunha, S.C.; Fernandes, J.O.; Teixeira, N.; Roleira, F.M. New steroidal 17β-carboxy derivatives present anti-5α-reductase activity and anti-proliferative effects in a human androgen-responsive prostate cancer cell line. Biochimie 2013, 95, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Varela, C.; Borges, M.; da Silva, E.T.; Roleira, F.M.F.; Correia-Da-Silva, G.; Teixeira, N. Steroidal aromatase inhibitors inhibit growth of hormone-dependent breast cancer cells by inducing cell cycle arrest and apoptosis. Apoptosis 2013, 18, 1426–1436. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.R.; Pires, A.S.; Abrantes, A.M.; Gonçalves, A.C.; Costa, S.C.; Varela, C.L.; Silva, E.T.; Botelho, M.F.; Roleira, F.M. Design, synthesis, and antitumor activity evaluation of steroidal oximes. Bioorg. Med. Chem. 2021, 46, 116360. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, N.; Bhardwaj, T.R.; Mehta, N.; Mukhopadhyay, T.; Kumar, A.; Kumar, M. 17-Oximino-5-androsten-3β-yl esters: Synthesis, antiproliferative activity, acute toxicity, and effect on serum androgen level. Med. Chem. Res. 2011, 20, 817–825. [Google Scholar] [CrossRef]
- Gros, L.; Lorente, S.O.; Jimenez, C.J.; Yardley, V.; Rattray, L.; Wharton, H.; Little, S.; Croft, S.L.; Ruiz-Perez, L.M.; Gonzalez-Pacanowska, D.; et al. Evaluation of Azasterols as Anti-Parasitics. J. Med. Chem. 2006, 49, 6094–6103. [Google Scholar] [CrossRef]
- Burbiel, J.; Bracher, F. Azasteroids as antifungals. Steroids 2003, 68, 587–594. [Google Scholar] [CrossRef]
- Birudukota, N.; Mudgal, M.M.; Shanbhag, V. Discovery and development of azasteroids as anticancer agents. Steroids 2019, 152, 108505. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Zhong, Z.; Gan, C.; Zhang, W.; Song, H. Synthesis and cytotoxicity of 17a-aza-d-homo-androster-17-one derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 3641–3643. [Google Scholar] [CrossRef]
- Cepa, M.; Tavares da Silva, E.J.; Correia-da-Silva, G.; Roleira, F.M.F.; Teixeira, N.A.A. Synthesis and biochemical studies of 17-substituted androst-3-enes and 3,4-epoxyandrostanes as aromatase inhibitors. Steroids 2008, 73, 1409–1415. [Google Scholar] [CrossRef]
- Andrade, L.C.R.; de Almeida, M.J.M.; Roleira, F.M.F.; Varela, C.L.; da Silva, E.J.T. 5α-Androst-3-en-17-one oxime. Acta Cryst. C 2008, 64, o508–o510. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, J.J.; Jakimov, D.S.; Rárová, L.; Strnad, M.; Dzichenka, Y.U.; Usanov, S.; Škorić, D.; Jovanović-Šanta, S.S.; Sakač, M.N. Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: Synthesis and evaluation of cytotoxic effects. RSC Adv. 2021, 11, 37449–37461. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ye, W.; Liu, J.; Liu, J.; Wang, C. Synthesis and cytotoxicity of (3β)-3-acetyloxy-5(6)-androsten-7-one oxime and 3,5(6)-androstadien-7-one oxime. Med. Chem. Res. 2014, 23, 1839–1843. [Google Scholar] [CrossRef]
- Dubey, P.P.A.D.P.J.S.; Piplani, P.; Jindal, D. Synthesis and Evaluation of Some 16-Benzylidene Substituted 3,17- Dioximino Androstene Derivatives as Anticancer Agents. Lett. Drug Des. Discov. 2005, 2, 537–545. [Google Scholar] [CrossRef]
- Acharya, P.C.; Bansal, R. Synthesis and Antiproliferative Activity of Some Androstene Oximes and TheirO-Alkylated Derivatives. Arch. Pharm. 2013, 347, 193–199. [Google Scholar] [CrossRef]
- Jindal, D.P.; Chattopadhaya, R.; Guleria, S.; Gupta, R. Synthesis and antineoplastic activity of 2-alkylaminoethyl derivatives of various steroidal oximes. Eur. J. Med. Chem. 2003, 38, 1025–1034. [Google Scholar] [CrossRef]
- Bansal, R.; Guleria, S.; Ries, C.; Hartmann, R.W. Synthesis and Antineoplastic Activity of O-Alkylated Derivatives of 7-Hydroximinoandrost-5-ene Steroids. Arch. Pharm. 2010, 343, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ajduković, J.J.; Penov Gaši, K.M.; Jakimov, D.S.; Klisurić, O.R.; Jovanović-Šanta, S.S.; Sakač, M.N.; Aleksić, L.D.; Djurendić, E.A. Synthesis, structural analysis and antitumor activity of novel 17α-picolyl and 17(E)-picolinylidene A-modified androstane derivatives. Bioorg. Med. Chem. 2015, 23, 1557–1568. [Google Scholar] [CrossRef]
- Savić, M.P.; Djurendić, E.A.; Petri, E.T.; Ćelić, A.; Klisurić, O.R.; Sakač, M.N.; Jakimov, D.S.; Kojićd, V.V.; Gašia, K.M.P. Synthesis, structural analysis and antiproliferative activity of some novel ᴅ-homo lactone androstane derivatives. RSC Adv. 2013, 3, 10385–10395. [Google Scholar] [CrossRef]
- Savić, M.P.; Klisurić, O.R.; Penov Gaši, K.M.; Jakimov, D.S.; Sakač, M.N.; Djurendić, E.A. Synthesis, structural analysis and cytotoxic activity of novel A- and B-modified ᴅ-homo lactone androstane derivative. J. Chem. Crystallogr. 2016, 46, 84–92. [Google Scholar] [CrossRef]
- Purohit, A.; Woo, L.; Chander, S.; Newman, S.; Ireson, C.; Ho, Y.; Grasso, A.; Leese, M.; Potter, B.; Reed, M. Steroid sulphatase inhibitors for breast cancer therapy. J. Steroid Biochem. Mol. Biol. 2003, 86, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Leese, M.P.; Leblond, B.; Newman, S.P.; Purohit, A.; Reed, M.J.; Potter, B.V. Anti-cancer activities of novel D-ring modified 2-substituted estrogen-3-O-sulfamates. J. Steroid Biochem. Mol. Biol. 2005, 94, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Berényi, Á.; Minorics, R.; Iványi, Z.; Ocsovszki, I.; Ducza, E.; Thole, H.; Messinger, J.; Wölfling, J.; Mótyán, G.; Mernyák, E.; et al. Synthesis and investigation of the anticancer effects of estrone-16-oxime ethers in vitro. Steroids 2013, 78, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Mernyák, E.; Fiser, G.; Szabó, J.; Bodnár, B.; Schneider, G.; Kovács, I.; Ocsovszki, I.; Zupkó, I.; Wölfling, J. Synthesis and in vitro antiproliferative evaluation of d-secooxime derivatives of 13β- and 13α-estrone. Steroids 2014, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; He, H.-M.; Katzenellenbogen, J.A.; Varma, R.K.; Hamel, E.; Lin, C.M.; Ram, S.; Sachdeva, Y.P. Synthesis of Analogs of 2-Methoxyestradiol with Enhanced Inhibitory Effects on Tubulin Polymerization and Cancer Cell Growth. J. Med. Chem. 1997, 40, 2323–2334. [Google Scholar] [CrossRef]
- Cushman, M.; He, H.M.; Katzenellenbogen, J.A.; Lin, C.M.; Hamel, E. Synthesis, antitubulin and antimitotic activity, and cytotoxicity of analogs of 2-methoxyestradiol, an endogenous mammalian metabolite of estradiol that inhibits tubulin polymerization by binding to the colchicine binding site. J. Med. Chem. 1995, 38, 2041–2049. [Google Scholar] [CrossRef]
- Canário, C.; Matias, M.; Brito, V.; Santos, A.; Falcão, A.; Silvestre, S.; Alves, G. New Estrone Oxime Derivatives: Synthesis, Cytotoxic Evaluation and Docking Studies. Molecules 2021, 26, 2687. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Alam, M.S.; Yousuf, S.; Wu, Y.C.; Lin, A.S.; Shaheen, F. Pregnenolone derivatives as potential anticancer agents. Steroids 2011, 76, 1554–1559. [Google Scholar] [CrossRef]
- Banday, A.H.; Akram, S.; Shameem, S.A. Benzylidine pregnenolones and their oximes as potential anticancer agents: Synthesis and biological evaluation. Steroids 2014, 84, 64–69. [Google Scholar] [CrossRef]
- Krstić, N.M.; Bjelaković, M.S.; Dabović, M.M.; Lorenc, L.B.; Pavlović, V.D. Photochemical and Beckmann rearrangement of (Z)-cholest-4-en-6-one oxime. J. Serb. Chem. Soc. 2004, 69, 413–420. [Google Scholar] [CrossRef]
- Krstić, N.M.; Bjelaković, M.S.; Žižak, Ž.; Pavlović, M.D.; Juranić, Z.D.; Pavlović, V.D. Synthesis of some steroidal oximes, lactams, thiolactams and their antitumor activities. Steroids 2007, 72, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Mora-Medina, T.L.; Martínez-Pascual, R.; Peña-Rico, M.; Viñas-Bravo, O.; Montiel-Smith, S.; Pérez-Picaso, L.; Moreno-Díaz, H. Preparation and cytotoxic evaluation of new steroidal oximes and aza-homosteroids from diosgenin and cholesterol. Steroids 2022, 182, 109012. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cui, J.; Chen, S.; Gan, C.; Zhou, A. Synthesis and antiproliferative activity of some steroidal lactams. Steroids 2011, 76, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cui, J.; Zheng, Q.; Zeng, C.; Chen, Q.; Zhou, A. 6-Hydroximino-4-aza-A-homo-cholest-3-one and related analogue as a potent inducer of apoptosis in cancer cells. Steroids 2012, 77, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Vejux, A.; Lizard, G. Cytotoxic effects of oxysterols associated with human diseases: Induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol. Asp. Med. 2009, 30, 153–170. [Google Scholar] [CrossRef]
- Carvalho, J.F.S.; Silva, M.M.C.; Moreira, J.N.; Simões, S.; e Melo, M.L.S. Selective Cytotoxicity of Oxysterols through Structural Modulation on Rings A and B. Synthesis, in Vitro Evaluation, and SAR. J. Med. Chem. 2011, 54, 6375–6393. [Google Scholar] [CrossRef]
- Rodriguez, J.; Nufiez, L.; Peixinho, S.; Jimnez, C. Isolation and synthesis of the first natural 6-hydroximino 4-en-3-one-steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Deive, N.; Rodríguez, J.; Jiménez, C. Synthesis of Cytotoxic 6E-Hydroximino-4-ene Steroids: Structure/Activity Studies. J. Med. Chem. 2001, 44, 2612–2618. [Google Scholar] [CrossRef]
- Poza, J.; Rega, M.; Paz, V.; Alonso, B.; Rodríguez, J.; Salvador, N.; Fernández, A.; Jiménez, C. Synthesis and evaluation of new 6-hydroximinosteroid analogs as cytotoxic agents. Bioorg. Med. Chem. 2007, 15, 4722–4740. [Google Scholar] [CrossRef]
- Cui, J.; Huang, L.; Fan, L.; Zhou, A. A facile and efficient synthesis of some (6E)-hydroximino-4-en-3-one steroids, steroidal oximes from Cinachyrella spp. sponges. Steroids 2008, 73, 252–256. [Google Scholar] [CrossRef]
- Cui, J.G.; Fan, L.; Huang, L.L.; Liu, H.L.; Zhou, A.M. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (I). Steroids 2009, 74, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Fan, L.; Huang, Y.; Xin, Y.; Zhou, A. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (II). Steroids 2009, 74, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Cui, J.; Su, S.; Lin, Q.; Jia, L.; Fan, L.; Huang, Y. Synthesis and antiproliferative activity of some steroidal thiosemicarbazones, semicarbazones and hydrozones. Steroids 2014, 87, 99–107. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Li, Y.; Fan, L.; Jiao, Y.; Su, S. Syntheses and antiproliferative activity of some sulfated hydroximinosterols. Med. Chem. Res. 2012, 22, 409–414. [Google Scholar] [CrossRef]
- Richmond, V.; Careaga, V.P.; Sacca, P.; Calvo, J.C.; Maier, M.S. Synthesis and cytotoxic evaluation of four new 6E-hydroximinosteroids. Steroids 2014, 84, 7–10. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, J.; Chen, S.; Lin, Q.; Song, H.; Gan, C.; Su, B.; Zhou, A. Synthesis and Evaluation of Some New Aza-B-homocholestane Derivatives as Anticancer Agents. Mar. Drugs 2014, 12, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Tsukomoto, T.; Ueno, Y.; Ohta, Z. Constitution of diosgenin I. Glucoside of Dioscorea tokoro Makino. J. Pharm. Soc. Jpn. 1936, 57, 985–991. [Google Scholar]
- Sánchez-Sánchez, L.; Hernández-Linares, M.G.; Escobar, M.L.; López-Muñoz, H.; Zenteno, E.; Fernández-Herrera, M.A.; Guerrero-Luna, G.; Carrasco-Carballo, A.; Sandoval-Ramírez, J. Antiproliferative, Cytotoxic, and Apoptotic Activity of Steroidal Oximes in Cervicouterine Cell Lines. Molecules 2016, 21, 1533. [Google Scholar] [CrossRef]
- Martínez-Gallegos, A.A.; Guerrero-Luna, G.; Ortiz-González, A.; Cárdenas-García, M.; Bernès, S.; Hernández-Linares, M.G. Azasteroids from diosgenin: Synthesis and evaluation of their antiproliferative activity. Steroids 2020, 166, 108777. [Google Scholar] [CrossRef]
- Carrasco-Carballo, A.; Guadalupe Hernández-Linares, M.; Cárdenas-García, M.; Sandoval-Ramírez, J. Synthesis and biological in vitro evaluation of the effect of hydroxyimino steroidal derivatives on breast cancer cells. Steroids 2021, 166, 108787. [Google Scholar] [CrossRef]
- Martínez-Pascual, R.; Meza-Reyes, S.; Vega-Baez, J.L.; Merino-Montiel, P.; Padrón, J.M.; Mendoza, Á.; Montiel-Smith, S. Novel synthesis of steroidal oximes and lactams and their biological evaluation as antiproliferative agents. Steroids 2017, 122, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.; Hazra, B.G.; Pore, V.S. Steroidal Conjugates and Their Pharmacological Applications. Curr. Med. Chem. 2006, 13, 813–847. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Cui, J.; Gan, C.; Liu, Z.; Wei, Y.; Song, H. Synthesis and cytotoxicity of A-homo-lactam derivatives of cholic acid and 7-deoxycholic acid. Steroids 2011, 76, 690–694. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death—Factsheet. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 25 October 2022).
- Hanson, J.R. Steroids: Partial synthesis in medicinal chemistry. Nat. Prod. Rep. 2010, 27, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Amiranashvili, L.; Nadaraia, N.; Merlani, M.; Kamoutsis, C.; Petrou, A.; Geronikaki, A.; Pogodin, P.; Druzhilovskiy, D.; Poroikov, V.; Ciric, A.; et al. Antimicrobial Activity of Nitrogen-Containing 5-α-Androstane Derivatives: In Silico and Experimental Studies. Antibiotics 2020, 9, 224. [Google Scholar] [CrossRef]
- Prabpayak, W.; Charoenying, P.; Laosinwattana, C.; Aroonrerk, N. Antibacterial activity of pregnenolone derivatives. Curr. Appl. Sci. Technol. 2006, 6, 466–470. [Google Scholar]
- Lone, I.H.; Khan, K.Z.; Fozdar, B.I.; Hussain, F. Synthesis antimicrobial and antioxidant studies of new oximes of steroidal chalcones. Steroids 2013, 78, 945–950. [Google Scholar] [CrossRef]
- Alam, M.; Lee, D.-U. Green synthesis, biochemical and quantum chemical studies of steroidal oximes. Korean J. Chem. Eng. 2014, 32, 1142–1150. [Google Scholar] [CrossRef]
- Brunel, J.; Loncle, C.; Vidal, N.; Dherbomez, M.; Letourneux, Y. Synthesis and antifungal activity of oxygenated cholesterol derivatives. Steroids 2005, 70, 907–912. [Google Scholar] [CrossRef]
- Shingate, B.B.; Hazra, B.G.; Salunke, D.B.; Pore, V.S.; Shirazi, F.; Deshpande, M.V. Synthesis and antimicrobial activity of novel oxysterols from lanosterol. Tetrahedron 2013, 69, 11155–11163. [Google Scholar] [CrossRef]
- Pujol, C.A.; Sepúlveda, C.S.; Richmond, V.; Maier, M.S.; Damonte, E.B. Polyhydroxylated sulfated steroids derived from 5α-cholestanes as antiviral agents against herpes simplex virus. Arch. Virol. 2016, 161, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- You, C.R.; Lee, S.W.; Jang, J.W.; Yoon, S.K. Update on hepatitis B virus infection. World J. Gastroenterol. WJG 2014, 20, 13293. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Tan, J.; Cui, X.; Zhou, M.; Huang, Y.; Zang, N.; Chen, Z.; Wei, W. Design, Synthesis and Bioactive Evaluation of Oxime Derivatives of Dehydrocholic Acid as Anti-Hepatitis B Virus Agents. Molecules 2020, 25, 3359. [Google Scholar] [CrossRef] [PubMed]
- Zeferino-Díaz, R.; Olivera-Castillo, L.; Dávalos, A.; Grant, G.; Kantún-Moreno, N.; Rodriguez-Canul, R.; Bernès, S.; Sandoval-Ramírez, J.; Fernández-Herrera, M.A. 22-Oxocholestane oximes as potential anti-inflammatory drug candidates. Eur. J. Med. Chem. 2019, 168, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Bjedov, S.; Bekic, S.; Marinovic, M.; Skoric, D.; Pavlovic, K.; Celic, A.; Petri, E.; Sakac, M. Screening the binding affinity of bile acid derivatives for the glucocorticoid receptor ligand-binding domain. J. Serb. Chem. Soc. 2023, 88, 123–139. [Google Scholar] [CrossRef]
- The Safety and Efficacy of Istaroxime for Pre-Cardiogenic Chock—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04325035?term=istaroxime&draw=2&rank=2 (accessed on 26 October 2022).
- Aditya, S.; Rattan, A. Istaroxime: A rising star in acute heart failure. J. Pharmacol. Pharmacother. 2012, 3, 353–355. [Google Scholar] [CrossRef]
- Stagno, M.J.; Zacharopoulou, N.; Bochem, J.; Tsapara, A.; Pelzl, L.; Al-Maghout, T.; Kallergi, G.; Alkahtani, S.; Alevizopoulos, K.; Dimas, K.; et al. Istaroxime Inhibits Motility and Down-Regulates Orai1 Expression, SOCE and FAK Phosphorylation in Prostate Cancer Cells. Cell. Physiol. Biochem. 2017, 42, 1366–1376. [Google Scholar] [CrossRef]
- Alevizopoulos, K.; Dimas, K.; Papadopoulou, N.; Schmidt, E.M.; Tsapara, A.; Alkahtani, S.; Honisch, S.; Prousis, K.C.; Alarifi, S.; Calogeropoulou, T.; et al. Functional characterization and anti-cancer action of the clinical phase II cardiac Na+/K+ ATPase inhibitor istaroxime: In vitro and in vivo properties and cross talk with the membrane androgen receptor. Oncotarget 2016, 7, 24415–24428. [Google Scholar] [CrossRef]
- Bringer, J. Norgestimate: A clinical overview of a new progestin. Am. J. Obstet. Gynecol. 1992, 166, 1969–1979. [Google Scholar] [CrossRef]
- Henzl, M.R. Norgestimate. From the laboratory to three clinical indications. J. Reprod Med. 2001, 46, 647–661. [Google Scholar]
- Yoshii, Y.; Okuda, K.I.; Yamada, S.; Nagakura, M.; Sugimoto, S.; Nagano, T.; Okabe, T.; Kojima, H.; Iwamoto, T.; Kuwano, K.; et al. Norgestimate inhibits staphylococcal biofilm formation and resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. NPJ Biofilms Microbiomes 2017, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Philibert, D.; Bouchoux, F.; Degryse, M.; Lecaque, D.; Petit, F.; Gaillard, M. The pharmacological profile of a novel norpregnane progestin (trimegestone). Gynecol. Endocrinol. 1999, 13, 316–326. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).