Abstract
Hydrogen can be stored in the interstitial sites of the lattices of intermetallic compounds. To date, intermetallic compound LaNi5 or related LaNi5-based alloys are known to be practical hydrogen storage materials owing to their higher volumetric hydrogen densities, making them a compact hydrogen storage method and allowing stable reversible hydrogen absorption and desorption reactions to take place at room temperature below 1.0 MPa. By contrast, gravimetric hydrogen density is required for key improvements (e.g., gravimetric hydrogen density of LaNi5: 1.38 mass%). Although hydrogen storage materials have typically been evaluated for their hydrogen storage properties below 10 MPa, reactions between hydrogen and materials can be facilitated above 1 GPa because the chemical potential of hydrogen dramatically increases at a higher pressure. This indicates that high-pressure experiments above 1 GPa could clarify the latent hydrogen absorption reactions below 10 MPa and potentially explore new hydride phases. In this study, we investigated the hydrogen absorption reaction of LaNi5 above 1 GPa at room temperature to understand their potential hydrogen storage capacities. The high-pressure experiments on LaNi5 with and without an internal hydrogen source (BH3NH3) were performed using a multi-anvil-type high-pressure apparatus, and the reactions were observed using in situ synchrotron radiation X-ray diffraction with an energy dispersive method. The results showed that 2.07 mass% hydrogen was absorbed by LaNi5 at 6 GPa. Considering the unit cell volume expansion, the estimated hydrogen storage capacity could be 1.5 times higher than that obtained from hydrogen absorption reaction below 1.0 MPa at 303 K. Thus, 33% of the available interstitial sites in LaNi5 remained unoccupied by hydrogen atoms under conventional conditions. Although the hydrogen-absorbed LaNi5Hx (x < 9) was maintained below 573 K at 10 GPa, LaNi5Hx began decomposing into NiH, and the formation of a new phase was observed at 873 K and 10 GPa. The new phase was indexed to a hexagonal or trigonal unit cell with a ≈ 4.44 Å and c ≈ 8.44 Å. Further, the newly-formed phase was speculated to be a new hydride phase because the Bragg peak positions and unit cell parameters were inconsistent with those reported for the La-Ni intermetallic compounds and La-Ni hydride phases.
1. Introduction
The utilization of renewable energy is key to reducing the emission of greenhouse gases. Thus, a hydrogen-based society comprising hydrogen production by renewable energy, hydrogen storage, and hydrogen utilization is required for the effective utilization of renewable energy. This indicates that efficient and safe hydrogen storage methods are indispensable in a hydrogen-based society. Pressurized gaseous hydrogen, liquid hydrogen, and metal hydrides (hydrogen storage materials) are prime candidates for hydrogen storage methods [1,2,3,4,5]. Among them, hydrogen in molecular states (H2) is stored in the pressurized gaseous and liquid hydrogen, and hydrogen in atomic states (H) is stored in the hydrogen storage materials. Hydrogen storage capacity, an important factor in evaluating hydrogen storage methods, can be defined by its volumetric and gravimetric hydrogen densities. The total amount of hydrogen per volume is referred to as the volumetric hydrogen density, where a larger value indicates a more compact hydrogen storage method. The total amount of hydrogen per mass or weight is referred to as the gravimetric hydrogen density, where a larger value indicates a lighter hydrogen storage method. Notably, the volumetric hydrogen densities of pressurized hydrogen and liquid hydrogen cannot exceed 70 kgH2/m3, owing to the repulsion between hydrogen molecules. However, the volumetric hydrogen densities of most hydrogen storage materials can exceed 90 kgH2/m3 because hydrogen storage materials are stored hydrogen as atoms, which avoids repulsive interactions between the hydrogen molecules [1,2]. The high volumetric hydrogen densities of hydrogen storage materials are one of the advantages of the three methods. In addition, hydrogen storage materials exhibit reversible hydrogen absorption and desorption reactions under moderate pressure and temperature conditions (e.g., room temperature and below 1 MPa of hydrogen pressure). In the case of pressurized gaseous hydrogen, gaseous hydrogen is compressed under 70 MPa of hydrogen pressure for fuel-cell vehicles. Liquid hydrogen is obtained at 20–30 K. However, practically used hydrogen storage materials, such as LaNi5-based alloys, can undergo hydrogen absorption and desorption reactions at room temperature below 1 MPa of hydrogen pressure. Although hydrogen storage materials have disadvantages, such as low gravimetric hydrogen densities (e.g., gravimetric hydrogen density of LaNi5: 1.38 mass%), they are still considered efficient and safe hydrogen storage methods compared to gaseous or liquid hydrogen storage methods, owing to their high volumetric hydrogen densities and capability to undergo reversible hydrogen absorption and desorption reactions in moderate conditions. Therefore, many hydrogen storage materials have been reported [1,2,3,4,5].
The hydrogen absorption and desorption reactions of hydrogen storage materials involve the reaction of materials under hydrogen gas pressure. Hydrogen absorption reactions correspond to the formation of hydride phases, and hydrogen desorption reactions correspond to the decomposition of the hydride phases [1,2]. In hydrides, hydrogen basically exists in various states, including elemental hydrogen (hydrogen with charge neutral), ionic (protons (H+) and hydride ions (H−)), and covalently bonded hydrogen. For this reason, hydrogen storage materials are classified by their respective hydrogen states in their hydrides, of which there are three types: interstitial hydrides with elemental hydrogen; ionic hydrides with hydride ions; and complex hydrides with covalently bonded hydrogen [1,2,3,4,5,6,7,8,9,10,11,12]. In addition, porous materials adsorb hydrogen on their surfaces as molecular hydrogen (physisorption) [13,14,15].
Among the hydrides, interstitial hydrides are typical hydrogen storage materials, and some of them (e.g., LaNi5-based alloys) are practically used. The reasons are that ionic hydrides (e.g., MgH2) and complex hydrides (e.g., NaAlH4) can undergo hydrogen absorption and desorption reactions at relatively higher temperatures (typically several hundred degrees Celsius) and pressures (above several MPa of hydrogen gas pressures) because of thermodynamically stable phases, slow hydrogen absorption, and desorption reaction kinetics although their gravimetric hydrogen densities are higher than interstitial hydrides. Intermetallic compounds are often considered to form interstitial hydrides, which can also undergo stable hydrogen absorption and desorption reactions under moderate conditions, as mentioned above. During the hydrogen absorption reactions of intermetallic compounds, hydrogen atoms are located at the interstitial sites, which are typically octahedral and tetrahedral sites coordinated by six and four metal atoms in their lattices, respectively, while maintaining their atomic metal configurations [1,2,3,4,5]. The absorbed hydrogen atoms in the hydrides are released as hydrogen gas by controlling the pressure and/or temperature of the hydrogen gas.
Among hydrogen storage materials, LaNi5-based alloys of the AB5 alloy type (A and B are typically lanthanides with high hydrogen affinities and transition metals with low hydrogen affinities, respectively) are well-known as practical hydrogen storage materials because of their ability to undergo reversible hydrogen absorption and desorption reactions at room temperature and below 1 MPa of hydrogen gas pressure. Therefore, many studies have investigated the hydrogen storage properties of LaNi5-based alloys, including the hydrogen absorption and desorption reaction cycles, crystal structures, microstructures, and morphologies [5,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The crystal structures of LaNi5 before and after hydrogen absorption are shown in Figure 1 [18,21]. LaNi5 has a hexagonal structure with a = 5.013 Å and c = 3.987 Å in the space group P6/mmm (No. 191) [21]. Although crystal structures for the hydrogen-absorbed phase LaNi5H6 with different space groups and unit cell parameters have been reported, we refer to a trigonal unit cell with a = 5.410 Å and c = 4.293 Å in the space group P31m (No. 157) [18] in this paper. In the crystal structure of LaNi5H6, hydrogen atoms are located at octahedral sites coordinated by two La and four Ni atoms; the tetrahedral sites are coordinated by two La and two Ni atoms. In the octahedral and tetrahedral sites, the site occupancies of the hydrogen atoms are 96% at 3c (octahedral sites) and 52% at 6d (tetrahedral sites) in the space group P31m (No. 157), respectively. The gravimetric and volumetric hydrogen densities of LaNi5 are 1.38 mass% and 92 kgH2/m3, respectively. Despite the higher volumetric hydrogen density of LaNi5 compared to that of gaseous and liquid hydrogen (less than 70 kgH2/m3), it is necessary to improve the low gravimetric hydrogen density.
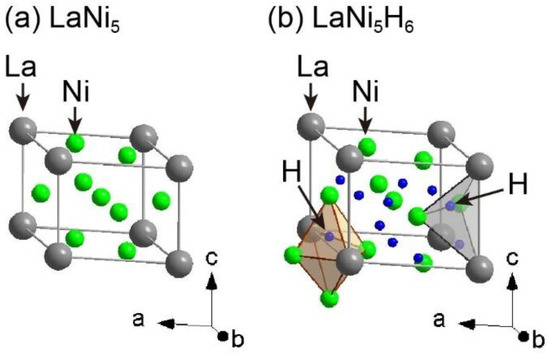
Figure 1.
Crystal structures of (a) LaNi5 with a = 5.013 Å and c = 3.987 Å in the space group P6/mmm (No. 191) [21], and (b) LaNi5H6 with a = 5.410 Å and c = 4.293 Å in the space group P31m (No. 157) [18]. Gray, green, and blue spheres indicate La, Ni, and H atoms, respectively. The brown octahedron and gray tetrahedron denote H atomic sites at 3c and 6d, respectively.
Although studies on hydrogen absorption and desorption reactions in hydrogen storage materials are typically conducted below 10 MPa, hydrogen absorption reactions can be facilitated above 1 GPa because of the drastic increase in the chemical potential of hydrogen [31]. To apply pressures above 1 GPa, multi-anvil-type high-pressure apparatus or diamond anvil cells have often been employed. In the multi-anvil-type high-pressure apparatus experiments, internal hydrogen sources, which supply hydrogen during the high-pressure experiments, are usually enclosed for the hydrogenation reaction (hydrogen absorption) because it is difficult to supply such high pressures using gaseous hydrogen. Hydrogen sources must release hydrogen above 1 GPa at the lowest possible temperature. In addition, the released hydrogen should not be absorbed by the hydrogen sources but by the sample instead. This indicates that the internal hydrogen source did not exhibit a reversible reaction between hydrogen desorption and absorption above 1 GPa. To date, BH3NH3 (hydrogen content: 19.6 mass%), a mixture of NaBH4 and Ca(OH)2 (hydrogen content: 6.3 mass%), AlH3 (hydrogen content: 10.1 mass%), and LiAlH4 (hydrogen content: 10.6 mass%) have often been used as the internal hydrogen sources. BH3NH3 has the highest hydrogen content and releases hydrogen at approximately 373–473 K at ambient pressure, and has the lowest hydrogen release temperature among the candidate internal hydrogen sources. Furthermore, hydrogen sources are generally separated by BN with a hexagonal crystal structure because the released hydrogen from the hydrogen sources can pass through the BN. A high-pressure experiment above 1 GPa enables the synthesis of new hydrides with the potential for hydrogen storage, which is otherwise difficult to synthesize under conventional conditions. Using a multi-anvil-type high-pressure apparatus, in which samples are enclosed with hydrogen sources, novel hydrides such as Mg7TMHx (TM: transition metals in groups 4 and 5) and transition metal complex hydrides with alkali or alkaline-earth metals and Al3FeH4 have been successfully synthesized [32,33,34,35,36,37,38,39].
Furthermore, synchrotron X-ray and neutron diffraction are important characterization techniques for observing the hydrogen absorption reaction processes and elucidating their mechanisms under high pressure, as reported for hydride formation processes [37,38,39]. As mentioned above, many intermetallic compounds classified as interstitial hydrides undergo reversible hydrogen absorption and desorption reactions at room temperature and above ambient pressure. This indicates that the hydrogen-absorbed phases (hydride phases) must be observed under hydrogen pressure because the absorbed hydrogen atoms are released from the intermetallic compounds at ambient pressure. To date, many new hydrides with high hydrogen contents have been reported. Most are ionic or complex hydrides, such as hydrogen with intermediate hydrogen states, which can be recovered at ambient pressure because they are thermodynamically stable hydrides. To our knowledge, a typical hydrogen storage material, LaNi5, has not yet been investigated for hydrogen absorption reactions above 1 GPa, although a TiFe with reversible hydrogen absorption and desorption reactions below 10 GPa has been reported [40].
In this study, we conducted hydrogen absorption reactions on LaNi5, a typical hydrogen storage material, above 1 GPa at room temperature, focusing on investigating its potential hydrogen storage capacity.
2. Results and Discussion
2.1. Hydrogen Absorption and Desorption Reactions below 1 MPa
In this study, the hydrogen absorption and desorption of LaNi5 were measured using a Pressure Composition Temperature (PCT) apparatus at 303 K below 1 MPa (Figure 2). The observed hydrogen storage capacity (1.40 mass%) and equilibrium hydrogen absorption and desorption pressures (0.3 and 0.2 MPa, respectively), including hysteresis, which would originate from strain in atomic arrangements, were consistent with those reported by Liang et al. in [24]. Then, the samples were characterized by a conventional X-ray diffraction diffractometer, as shown in Figure 2. Although the preferred orientation (001) at 2θ ≈ 22.2°, and (002) at 2θ ≈ 45.4°, and Bragg peak broadening were observed, the Bragg peak positions and unit cell parameters, hexagonal unit cells with a = 5.013(2) Å, and c = 3.988(1) Å obtained by the indexing program TREOR97 [41], were consistent with the simulated X-ray diffraction pattern of LaNi5 with hexagonal unit cells (a = 5.013 Å, and c = 3.987 Å) [21]. This strain was observed by broadening the Bragg peaks in the X-ray diffraction pattern after the hydrogen absorption and desorption reactions, as reported in [22,24,25]. Therefore, this sample was used to observe the hydrogen absorption reactions of LaNi5 at pressures above 1 GPa, which will be discussed in the following section.
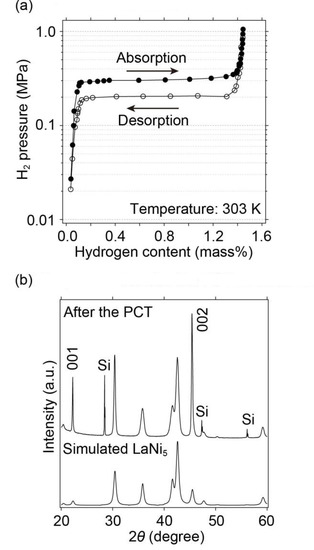
Figure 2.
(a) PCT curves of LaNi5 measured at 303 K, and (b) X-ray diffraction pattern after the PCT measurements with simulated X-ray diffraction pattern of LaNi5. In the X-ray diffraction pattern after the PCT measurement, strong intensities of (001) and (002) reflections originated into a preferred orientation of (00l) reflection. Si is added as an internal standard in the X-ray diffraction pattern after the PCT measurement.
2.2. Hydrogen Absorption Reactions at 1.6 GPa and 473 K
LaNi5 with an internal hydrogen source BH3NH3 was compressed to 1.6 GPa at room temperature and subsequently heated to 473 K at a heating rate of 100 K/min to release hydrogen. During heating, synchrotron radiation X-ray diffraction patterns were collected every 1 min using the energy-dispersive method, as shown in Figure 3. After 5 min of reaction at 473 K and 1.6 GPa, the Bragg peaks shifted to larger d-spacings. This indicates that BH3NH3 released hydrogen, which was then absorbed by LaNi5 without yielding byproducts from the reaction between LaNi5 and BN capsules. The amount of absorbed hydrogen is estimated to be approximately 1.70 mass%, corresponding to the formation of LaNi5H7.3, considering the unit cell volume expansion. The details of the hydrogen content considered in this study are discussed later. After the hydrogen absorption reaction was completed, the temperature was lowered to room temperature while maintaining the pressure at 1.6 GPa to observe the hydrogen absorption reaction at room temperature with increasing pressure.
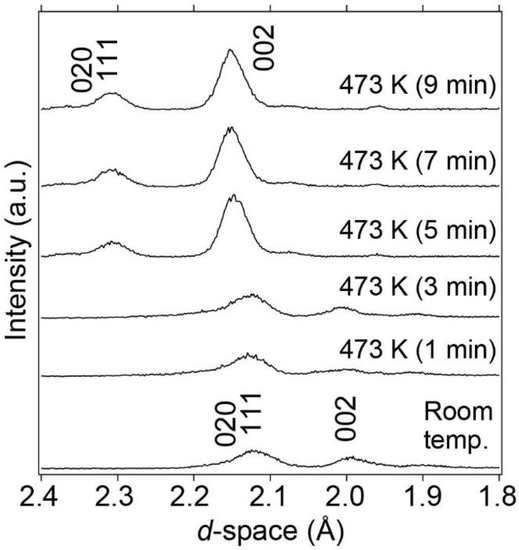
Figure 3.
Synchrotron radiation X-ray diffraction patterns of LaNi5 at 473 K and 1.6 GPa focusing on Bragg peaks of the (020), (111), and (002) reflections.
2.3. Hydrogen Absorption Reactions in the Range of 1–10 GPa
Figure 4 shows the synchrotron radiation X-ray diffraction patterns of LaNi5 with and without BH3NH3 in the 1.6–10 GPa range at room temperature. High-pressure experiments with and without BH3NH3 were performed to observe the hydrogen absorption reaction of LaNi5 and study the unit cell volume expansion of pure LaNi5 with increasing pressure. Although it is difficult to evaluate Bragg peak intensities and widths due to data collection of the X-ray diffraction by the energy-dispersive method and sample positions, the Bragg peak positions of LaNi5 without BH3NIH3 shifted to smaller d-spacings with increasing pressure, corresponding to the decrease in the unit cell volume as higher pressure was applied. By contrast, the Bragg peak positions of LaNi5 with BH3NH3 either remained constant or shifted to larger d-spacing despite increasing pressure (1.6 to 6 GPa). This indicated that LaNi5 absorbed hydrogen during compression. Using the X-ray diffraction pattern fitting program, PDindexer [42], the experimentally obtained synchrotron radiation X-ray diffraction patterns were fitted to that of LaNi5H6 with a hexagonal structure in the space group P31m (No. 157) [18], and unit cell volumes at each pressure were estimated based on the fitting results. Figure 5 shows the pressure dependence of the unit cell volume. The unit cell volumes of LaNi5Hx (LaNi5 with BH3NH3), predicted LaNi5H6, and LaNi5 (LaNi5 without BH3NH3) were plotted as a function of pressure. The unit cell volume of the predicted LaNi5H6 was estimated using the unit cell volume of LaNi5 (black data points in Figure 5) and the volume of the hydrogen contribution to LaNi5H6. The volume of the hydrogen contribution (22.05 Å3/f.u.) was calculated by subtracting the unit cell volume of LaNi5 (87.76 Å3/f.u. [21]) from that of LaNi5H6 (108.81 Å3/f.u. [18]). As shown in Figure 5, gaps were observed in the unit cell volumes of LaNi5Hx and the predicted LaNi5H6. These gaps were proposed to hold extra hydrogen in LaNi5H6 for the high-pressure experiment. Because the volume of hydrogen contribution was 22.05 Å3/f.u. in LaNi5H6, each hydrogen atomic contribution was estimated to be 3.68 Å3/f.u. Therefore, the maximum hydrogen storage capacity reaches 2.07 mass% (LaNi5H9) at 6 GPa. Notably, a similar intermetallic compound, TiFe, with reversible hydrogen absorption and desorption reactions below 10 GPa, also showed the same hydrogen content under normal conditions and higher pressures [40].
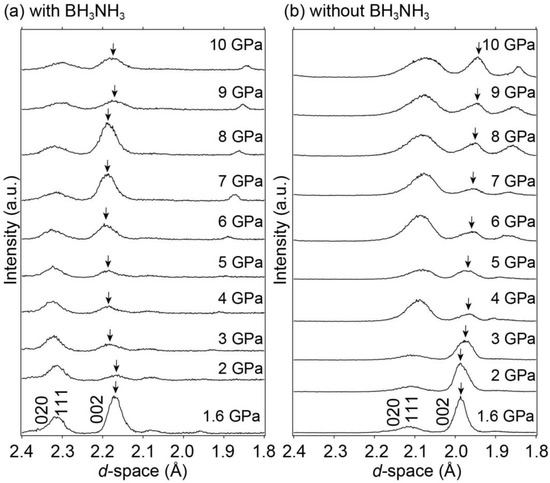
Figure 4.
Synchrotron radiation X-ray diffraction patterns of LaNi5 (a) with and (b) without the internal hydrogen source BH3NH3 at room temperature and 1.6–10 GPa focusing on Bragg peaks of the (020), (111), and (002) reflections. Arrows indicate the Bragg peak of (002) at each pressure.

Figure 5.
(a) Changes in the unit cell volume of LaNi5Hx (LaNi5 with BH3NH3) predicted LaNi5H6 and LaNi5 without BH3NH3, and (b) estimated hydrogen content in LaNi5Hx as a function of pressure.
Subsequently, the sample was heated from room temperature to 873 K at a pressure of 10 GPa to further investigate hydrogen absorption and decomposition (into La hydride (LaHx) and Ni) reactions at higher temperatures. X-ray diffraction patterns were simultaneously collected during heating. Bragg peaks corresponding to NiH and unknown Bragg peaks were observed at 873 K and 10 GPa although the hydrogen-absorbed LaNi5Hx (x < 9) was maintained below 573 K at 10 GPa (Figure 6). Using the indexing program TREOR97 [41], the unknown Bragg peaks were indexed to a hexagonal or trigonal unit cell with a ≈ 4.44 Å and c ≈ 8.44 Å. The new phase with the hexagonal or trigonal unit cell was speculated to be a new hydride phase because the Bragg peak positions and the phase with the hexagonal or trigonal unit cell did not match those of the La-Ni hydrides and La-Ni intermetallic compounds. Because the new phase could be recovered at an ambient pressure, it might not be classified as an interstitial hydride. The details of the new phase, including its atomic positions and hydrogen content, are currently being investigated.
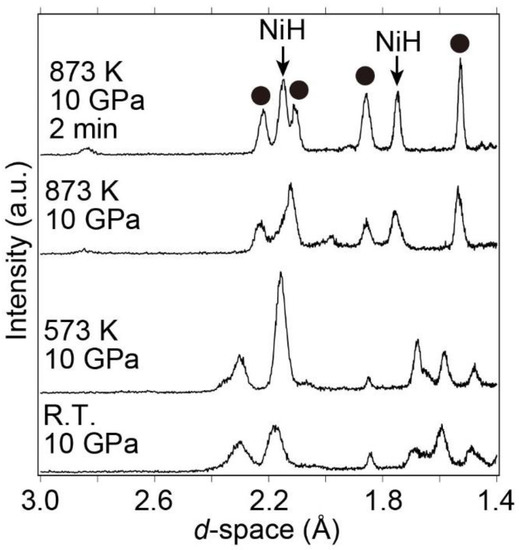
Figure 6.
Synchrotron radiation X-ray diffraction patterns from room temperature to 873 K at 10 GPa. The Bragg peaks at 873 K and 10 GPa (2 min) are identified as NiH and a new phase with a hexagonal unit cell (closed circles).
Thus, the high-pressure experiments on LaNi5 suggest that its maximum hydrogen storage capacity of LaNi5 could be 2.07 mass% at 6 GPa, indicating that 33% of the available interstitial sites in LaNi5 remain unoccupied under practical operating conditions. Although hydrogen atoms in LaNi5 occupy octahedral sites coordinated by two La and four Ni atoms and tetrahedral sites coordinated by two La and two Ni atoms (see Figure 1), a small number of hydrogen atoms in LaNi5 with Al (LaNi4.5Al0.5) occupied other tetrahedral sites, coordinated by one La and three Ni atoms and by four Ni atoms, as reported by atomic pair distribution function analysis [30]. Therefore, it can be speculated that the hydrogen storage capacities might improve if more hydrogen atoms could occupy the respective tetrahedral sites coordinated by one La, three Ni atoms, and four Ni atoms. To increase the hydrogen atomic occupancies at the tetrahedral sites, replacing multiple metal atoms, which have a larger radius and higher affinity for hydrogen than Ni atoms, with Ni atomic sites might lead to increased amounts of hydrogen atoms in such tetrahedral sites.
3. Materials and Methods
Pieces of LaNi5 (Japan Metals & Chemicals Co., Ltd., Tokyo, Japan) were used in this study. The hydrogen storage properties of LaNi5 at 303 K and below 1 MPa of hydrogen gas pressure were confirmed by PCT measurements (Japan Metals & Chemicals Co., Ltd.). Before the PCT measurement, five cycles of hydrogen absorption and desorption reactions on LaNi5 were performed at room temperature under 1 MPa of hydrogen gas pressure and subsequently under vacuum for activation after heat treatment at 623 K for 3 h. After the PCT measurements, the samples were characterized by powder X-ray diffraction, which was conducted on a Rigaku SmartLab X-ray diffractometer (Tokyo, Japan) using Cu Kα radiation (λ = 1.540593 Å for Kα1 and λ = 1.544414 Å for Kα2). The samples were handled in an Ar-gas-filled glovebox with an O2 content < 1 ppm to prevent (hydro)oxidation.
The hydrogen absorption reactions of LaNi5 at room temperature and above 1 GPa were observed using a multi-anvil-type high-pressure apparatus combined with synchrotron radiation X-ray diffraction at the BL14B1 beamline of SPring-8 in Japan. Before the high-pressure experiment, LaNi5 was activated by heat treatment at 623 K for 3 h, and hydrogen absorption and desorption reactions as the same processes with the PCT measurement at 303 K, as mentioned above. Activated LaNi5 was placed in a BN capsule enclosed by an internal hydrogen source, BH3NH3 (Sigma-Aldrich, 97%, St. Louis, MO, USA). The weight ratio of LaNi5 to BH3NH3 was approximately 3:2. The sample and the internal hydrogen source were enclosed in a NaCl hydrogen-sealing capsule. The sample cell used in this study was based on that used by Saitoh et al. [38]. The hydrogen release reactions of BH3NH3 above 1 GPa have been described in [43,44]. Synchrotron radiation X-ray diffraction patterns were collected during high-pressure experiments using energy-dispersive X-ray diffraction. In high-pressure experiments, diffracted X-rays were obtained using a Ge solid-state detector mounted on a goniometer. The d-space of the lattice was obtained using Bragg’s law:
where:
h: Planck constant;
c: Speed of light;
E: Energies of the diffracted X-ray;
θ: 3° (diffraction angle 2θ was fixed at 6° in this experiment).
High-pressure experiments on LaNi5 without an internal hydrogen source were also performed using the same procedure to compare the hydrogen absorption reactions. For these cases, BN was used instead of the internal hydrogen source.
In addition, the Bragg peaks in the experimentally obtained synchrotron radiation X-ray diffraction patterns were indexed using TREOR97 [41] and identified using PDindexer [42].
4. Conclusions
In this study, we investigated the hydrogen absorption and desorption reactions of LaNi5 below 1 MP at 303 K and above 1 GPa at room temperature to determine its potential hydrogen storage capacities. High pressures above 1 GPa were induced using a multi-anvil-type high-pressure apparatus, and the hydrogen absorption reactions of LaNi5 above 1 GPa were observed using in situ synchrotron radiation X-ray diffraction with an energy dispersive method at the BL14B1 beamline of SPring-8 in Japan. Although LaNi5 absorbed up to 1.40 mass% hydrogen, of which LaNi5H6 was formed below 1 MPa at 303 K, the results suggested that the hydrogen storage capacity increased with increasing pressure up to 6 GPa. Considering the unit cell volumes of hydrogen-absorbed LaNi5 phase LaNi5Hx (LaNi5 with an internal hydrogen source), predicted LaNi5H6 and LaNi5 (without an internal hydrogen source). The final amount of LaNi5 reached 2.07 mass%, corresponding to the formation of LaNi5H9 at 6 GPa and room temperature. This indicated that approximately 33% of the available interstitial sites in LaNi5 remained unoccupied by hydrogen atoms under practical operating conditions. The locations of the unoccupied hydrogen atomic sites were speculated to be in tetrahedral sites coordinated by one La, three Ni atoms, and four Ni atoms because small amounts of hydrogen could be located at the tetrahedral sites when a part of Ni was replaced with Al. To increase the hydrogen atomic occupancies at the tetrahedral sites, replacing multiple metal atoms, which have a larger radius and higher affinity for hydrogen than Ni atoms, with Ni atomic sites might lead to increased amounts of hydrogen atoms in unoccupied hydrogen atomic sites under normal conditions.
While increasing the temperature from room temperature to 873 K at 10 GPa, the hydrogen-absorbed phase LaNi5Hx (x < 9) decomposed to form NiH and a new phase at 873 K. The new phase was indexed to a hexagonal or trigonal unit cell with a ≈ 4.44 Å and c ≈ 8.44 Å. It was speculated to be a new hydride phase because its Bragg peak positions were inconsistent with La-Ni Intermetallic compounds and La-Ni hydrides.
Author Contributions
Conceptualization, T.S., H.S., S.T. and S.O.; methodology, T.S. and H.S.; formal analysis, T.S., H.S. and J.I.; investigation, T.S., H.S., R.U., J.I., K.O. and Y.N.; data curation, T.S., H.S., R.U. and J.I.; writing—original draft preparation, T.S.; writing—review and editing, T.S., H.S., R.U., J.I., K.O., Y.N., S.T. and S.O.; visualization, T.S.; supervision, T.S.; project administration, T.S.; funding acquisition, T.S., H.S. and S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MEXT/JSPS KAKENHI (22H01817, JP18H05513 (“Hydrogenomics”)), GIMRT Program of the Institute for Materials Research, Tohoku University (Proposal No. 202112-RDKGE-0012), and JST SICORP (JPMJSC 1802).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by MEXT/JSPS KAKENHI (22H01817, JP18H05513 (“Hydrogenomics”)) and the GIMRT Program of the Institute for Materials Research, Tohoku University (Proposal No. No. 202112-RDKGE-0012) and JST SICORP (JPMJSC 1802). Synchrotron X-ray radiation experiments at SPring-8 were supported by the QST Advanced Characterization Nanotechnology Platform under the remit of the Nanotechnology Platform of the Ministry of Education, Culture, Sports, Science, and Technology MEXT, Japan (Proposal No. JPMXP09A21QS0031, No. JPMXP1222QS0007, and no. JPMXP1222QS0117). The synchrotron radiation X-ray diffraction experiments were performed using a QST experimental station at the QST beamline BL14B1 and SPring-8, with the approval of the Japan Synchrotron Radiation Research Institute JASRI (Proposal No. 2021B3694, No. 2022A3694, and No. 2022B3694).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A. Materials for Hydrogen Storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Crivello, J.-C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of Magnesium Hydride–Based Materials: Development and Optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for Hydrogen-Based Energy Storage Past, Recent Progress and Future Outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Pasquini, L.; Sakaki, S.; Akiba, E.; Allendorf, M.D.; Cho, Y.W.; Alvares, E.; Ares, J.D.; Badai, D.; Bricco, M.; von Colbe, J.B.; et al. Magnesium- and Intermetallic Alloys-Based Hydrides for Energy Storage: Modelling, Synthesis and Properties. Prog. Energy 2022, 4, 032007. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Takagi, S.; Orimo, S. Recent Progress in Hydrogen-Rich Materials from the Perspective of Bonding Flexibility of Hydrogen. Scr. Mater. 2015, 109, 1–5. [Google Scholar] [CrossRef]
- Sato, T.; Takagi, S.; Deledda, S.; Hauback, B.C.; Orimo, S. Extending the Applicability of the Goldschmidt Tolerance Factor to Arbitrary Ionic Compounds. Sci. Rep. 2016, 6, 23592. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Mochizuki, T.; Ikeda, K.; Honda, T.; Otomo, T.; Sagayama, H.; Yang, H.; Luo, W.; Lombardo, L.; Züttel, A.; et al. Crystal Structural Investigations for Understanding the Hydrogen Storage Properties of YMgNi4–Based Alloys. ASC Omega 2020, 5, 31192–31198. [Google Scholar] [CrossRef]
- Sato, T.; Orimo, S. The Crystal Structures in Hydrogen Absorption Reactions of REMgNi4-Based Alloys (RE: Rare-earth metals). Energies 2021, 14, 8163. [Google Scholar] [CrossRef]
- Sato, T.; Ikeda, K.; Honda, T.; Daemen, L.L.; Cheng, Y.; Otomo, T.; Sagayama, H.; Ramirez–Cuesta, A.J.; Takagi, S.; Kono, T.; et al. Effect of Co Substitution on Hydrogen Absorption and Desorption Reactions of YMgNi4-based Alloys. J. Phys. Chem. C 2022, 126, 16943–16951. [Google Scholar] [CrossRef]
- Zhang, L.; Allendorf, M.D.; Balderas–Xicohténcatl, R.; Broom, D.P.; Fanourgakis, G.S.; Froudakis, G.E.; Gennett, T.; Hurst, K.E.; Ling, S.; Milanese, C.; et al. Fundamentals of Hydrogen Storage in Nanoporous Materials. Prog. Energy 2022, 4, 042013. [Google Scholar] [CrossRef]
- Mehrabi, M.; Parvin, P.; Reyhani, A.; Mortazavi, S.Z. Hydrogen Storage in Multi-Walled Carbon Nanotubes Decorated with Palladium Nanoparticles using Laser Ablation/Chemical Reduction Methods. Mater. Res. Express 2017, 4, 095030. [Google Scholar] [CrossRef]
- Mortazavi, S.Z.; Reyhani, A.; Mirershadi, S. Hydrogen Storage Properties of Multi-Walled Carbon Nanotubes and Carbon Nano-Onions Grown on Single and Bi–Catalysts including Fe, Mo, Co and Ni Supported by MgO. Int. J. Hydrogen Energy 2017, 41, 24885–24896. [Google Scholar] [CrossRef]
- Van Mal, H.H.; Buschow, K.H.J.; Miedema, A.R. Hydrogen Absorption in LaNi5 and Related Compounds: Experimental Observations and Their Explanation. J. Less-Common Met. 1974, 35, 65–76. [Google Scholar] [CrossRef]
- Boser, O. Hydrogen Sorption in LaNi5. J. Less-Common Met. 1976, 46, 91–99. [Google Scholar] [CrossRef]
- Fischer, P.; Furrer, A.; Busch, G.; Schlapbach, L. Neutron Scattering Investigations of the LaNi5 Hydrogen Storage System. Halv. Phys. Acta 1977, 50, 421–430. [Google Scholar]
- Percheron-Guégan, A.; Lartigue, C.; Achard, J.C. Correlations Between the Structural Properties, the Stability and the Hydrogen Content of Substituted LaNi5 Compounds. J. Less-Common Met. 1985, 109, 287–309. [Google Scholar] [CrossRef]
- Sakai, T.; Oguro, K.; Miyamura, H.; Kuriyama, N.; Kato, A.; Ishikawa, H.; Iwakura, C. Some Factors Affecting the Cycling Lives of LaNi5-based Alloy Electrodes of Hydrogen Batteries. J. Less-Common Met. 1990, 161, 193–202. [Google Scholar] [CrossRef]
- Kisi, E.H.; Buckley, C.E.; Gray, E.M.A. The Hydrogen Activation of LaNi5. J. Alloys Compd. 1992, 185, 369–384. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oguro, K.; Uehara, I.; Akiba, E. X-ray Diffraction Peak Broadening and Degradation in LaNi5-Based Alloys. Int. J. Hydrog. Energy 2000, 25, 531–537. [Google Scholar] [CrossRef]
- Černý, R.; Joubert, J.-M.; Latroche, M.; Percheron-Guégan, A.; Yvon, K. Anisotropic Diffraction Peak Broadening and Dislocation Substructure in Hydrogen-Cycled LaNi5 and Substitutional Derivatives. J. Appl. Cryst. 2000, 33, 999–1005. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Scchulz, R. Hydrogen Storage Properties of the Mechanically Alloyed LaNi5-based Materials. J. Alloys Compd. 2001, 320, 133–139. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Latroche, M.; Černý, R.; Percheron-Guégan, A.; Yvon, K. Hydrogen Cycling Induced Degradation in LaNi5–type Materials. J. Alloys Compd. 2002, 330–332, 208–214. [Google Scholar] [CrossRef]
- Décampsa, B.; Joubert, J.-M.; Cérny, R.; Percheron–Guégan, A. TEM Study of the Dislocations Generated by Hydrogen Absorption/Desorption in LaNi5 and Derivatives. J. Alloys Compd. 2005, 404–406, 570–575. [Google Scholar] [CrossRef]
- Liu, W.; Aguey-Zinsou, K.-F. Low Temperature Synthesis of LaNi5 Nanoparticles for Hydrogen Storage. Int. J. Hydrogen Energy 2015, 41, 1679–1687. [Google Scholar] [CrossRef]
- Faisal, M.; Balani, K.; Subramaniam, A. Cross–Sectional TEM Investigation of Mg–LaNi5–Soot Hybrids for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 5507–5519. [Google Scholar] [CrossRef]
- Joubert, J.-M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 Related AB5 Compounds: Structure, Properties and Applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Ikeda, K.; Ohshita, H.; Otomo, T.; Sakaki, K.; Kim, H.; Nakamura, Y.; Machida, A.; Von Dreele, R.B. Pressure Cells for in situ Neutron Total Scattering: Time and Real–Space Resolution during Deuterium Absorption. J. Appl. Cryst. 2022, 55, 1631–1639. [Google Scholar] [CrossRef]
- Sugimoto, H.; Fukai, Y. Solubility of Hydrogen in Metals under High Hydrogen Pressures: Thermodynamical Calculations. Acta Metall. Mater. 1992, 40, 2327–2336. [Google Scholar] [CrossRef]
- Moser, D.; Bull, D.J.; Sato, T.; Noréus, D.; Kyoi, D.; Sakai, T.; Kitamura, N.; Yusa, H.; Taniguchi, T.; Kalisvaart, W.P.; et al. Structure and Stability of High Pressure Synthesized Mg–TM Hydrides (TM = Ti, Zr, Hf, V, Nb and Ta) as Possible New Hydrogen Rich Hydrides for Hydrogen Storage. J. Mater. Chem. 2009, 19, 8150–8161. [Google Scholar] [CrossRef]
- Saitoh, H.; Takagi, S.; Matsuo, M.; Iijima, Y.; Endo, N.; Aoki, K.; Orimo, S. Li4FeH6: Iron-Containing Complex Hydride with High Gravimetric Hydrogen Density. APL Mater. 2014, 2, 076103. [Google Scholar] [CrossRef]
- Takagi, S.; Iijima, Y.; Sato, T.; Saitoh, H.; Ikeda, K.; Otomo, T.; Miwa, K.; Ikeshoji, T.; Aoki, K.; Orimo, S. True Boundary for the Formation of Homoleptic Transition-Metal Hydride Complexes. Angew. Chem. Int. Ed. 2015, 54, 5650–5653. [Google Scholar] [CrossRef]
- Takagi, S.; Iijima, Y.; Sato, T.; Saitoh, H.; Ikeda, K.; Otomo, T.; Miwa, K.; Ikeshoji, T.; Orimo, S. Formation of Novel Transition Metal Hydride Complexes with Ninefold Hydrogen Coordination. Sci. Rep. 2017, 7, 44253. [Google Scholar] [CrossRef]
- Spektor, K.; Crichton, W.A.; Filippov, S.; Klarbring, J.; Simak, S.I.; Häussermann, U. Na-Ni-H Phase Formation at High Pressures and High Temperatures: Hydrido complexes [NiH5]3− Versus the perovskite NaNiH3. ACS Omega 2020, 5, 8730–8743. [Google Scholar] [CrossRef] [PubMed]
- Spektor, K.; Crichton, W.A.; Filippov, S.; Simak, S.I.; Häussermann, U. Na3FeH7 and Na3CoH6: Hydrogen-Rich Complex Transition Metal Hydrides from High Pressure Synthesis. Inorg. Chem. 2020, 59, 16467–16473. [Google Scholar] [CrossRef]
- Saitoh, H.; Sato, T.; Tanikami, M.; Ikeda, K.; Machida, A.; Watanuki, T.; Taguchi, T.; Yamamoto, S.; Yamaki, T.; Takagi, S.; et al. Hydrogen Storage by Earth-Abundant Metals, Synthesis and Characterization of Al3FeH3.9. Mater. Des. 2021, 208, 109953. [Google Scholar] [CrossRef]
- Utsumi, R.; Morimoto, M.; Saitoh, H.; Watanuki, T.; Sato, T.; Takagi, S.; Orimo, S. In situ Synchrotron Radiation X-ray Diffraction Measurements of Fe–Mo Alloy Hydrides Formed under High Pressure and High Temperature. J. Alloys Compd. 2022, 893, 162300. [Google Scholar] [CrossRef]
- Endo, N.; Saita, I.; Nakamura, Y.; Saitoh, H.; Machida, A. Hydrogenation of a TiFe-based alloy at High Pressure and Temperature. Int. J. Hydrogen Energy 2015, 40, 3283–3287. [Google Scholar] [CrossRef]
- Werner, P.-E.; Eriksson, L.; Westdahl, M. TREOR, A Semi–Exhaustive Trial–and–Error Powder Indexing Program for All Symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Seto, Y.; Haname, D.; Nagai, T.; Sata, N. Development of a Software Suite on X-ray Diffraction Experiments. Rev. High Pressure Sci. Technol. 2010, 20, 269–276. [Google Scholar] [CrossRef]
- Nylén, J.; Sato, T.; Soignard, E.; Yarger, J.L.; Stoyanov, E.; Häussermann, U. Thermal Decomposition of Ammonia Borane at High Pressures. J. Chem. Phys. 2009, 131, 104506. [Google Scholar] [CrossRef]
- Nylén, J.; Eriksson, L.; Benson, D.; Häussermann, U. Characterization of a High pressure, High Temperature Modification of Ammonia Borane (BH3NH3). J. Chem. Phys. 2013, 139, 054507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).