Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes
Abstract
1. Introduction
- Influences on the genetic apparatus, repression of genes or inhibition of nucleic acid synthases;
- Impairments of the membrane bilayer and membrane proteins, changes in membrane structure, fluidity and permeability, membrane pore formation and depolarization and ion leakage;
- Changes in bacterial metabolism, perturbation in bacterial homeostasis or enzyme inhibition (e.g., DNA gyrase inhibition);
- Inhibition of adhesions and microbial growth;
- Reactive oxygen species (ROS) generation;
- Controlling multidrug resistance, inactivation of bacterial efflux pump transporters (multidrug-resistance pumps) in bacteria and increasing susceptibility to antibiotics;
- Inhibition of binding to target cells;
- Metal ion chelation;
- Perturbation in cell envelope metabolism and envelope synthesis through the inhibition of fatty acids synthesis and;
- Damage to the bacterial respiratory chain, energy transduction mechanism uncoupling, inhibition of ATP synthase and disruption of bioenergetic status.
- Prevention of toxin secretion, inactivation of toxins and bacterial lipopolysaccharides by interacting with the toxins and changing their conformation and activities [30];
- Inhibition of biofilm formation and cell adhesion by action on target cells to enhance their toxin resistance [31] and;
- Perturbation in organization of bacterial quorum and intercellular communication [32].
2. Results
2.1. Antimicrobial Effects of the Flavonoids
2.2. Effects of Flavonoids on Diameter, Zeta-potential and Membrane Structure of the Bacterial Cell
2.3. Antihemolytic Activities of Flavonoids During Sheep Erythrocyte Hemolysis Caused by S. aureus (NCTC 5655 Strain) and Flavonoid Effects on Erythrocyte and Liposomal Membrane Structures
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Bacterial Strain and Growth Condition
5.3. Antibacterial Activity of Flavonoids
5.4. Inhibition of Sheep Erythrocyte Hemolysis by Flavonoids
5.5. Measurements of Erythrocyte Membrane Fluidity
5.6. Measurements of S. aureus Membrane Fluidity
5.7. S. aureus Nanoscale Cell Diameter and Zeta-Potential
5.8. Interaction of Flavonoids with Liposomal Membranes
5.9. Calculations of Flavonoid and S. aureus α-Hemolysin Molecular Geometries
5.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Assessment of the Antibacterial Activity of Galangin against 4-Quinolone Resistant Strains of Staphylococcus aureus. Phytomedicine 2006, 13, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Detection of Galangin-Induced Cytoplasmic Membrane Damage in Staphylococcus aureus by Measuring Potassium Loss. J. Ethnopharmacol. 2005, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.J.M.; Afolayan, A.J.; Taylor, M.B.; Erasmus, D. Antiviral Activity of Galangin Isolated from the Aerial Parts of Helichrysum Aureonitens. J. Ethnopharmacol. 1997, 56, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18 (Part B), 820–897. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Ruviaro, A.R.; Barbosa, P.d.P.M.; da Silva, I.F.; de Ávila, A.R.A. Chapter 7—Hesperetin and Naringenin: Protective Effects against Metabolic Syndrome–Associated Inflammation. In Discovery and Development of Anti-Inflammatory Agents from Natural Products; Brahmachari, G., Ed.; Natural Product Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–239. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of Catechins and Their Applications. Biomed. Dermatol. 2020, 4, 8. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, e162750. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Cruz-Martins, N.; Nepovimova, E.; Oleksak, P.; Dhanjal, D.S.; Bhardwaj, S.; Singh, R.; Chopra, C.; Verma, R.; et al. Applications of Fruit Polyphenols and Their Functionalized Nanoparticles Against Foodborne Bacteria: A Mini Review. Molecules 2021, 26, 3447. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef] [PubMed]
- Donadio, G.; Mensitieri, F.; Santoro, V.; Parisi, V.; Bellone, M.L.; De Tommasi, N.; Izzo, V.; Dal Piaz, F. Interactions with Microbial Proteins Driving the Antibacterial Activity of Flavonoids. Pharmaceutics 2021, 13, 660. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Elmasri, W.A.; Zhu, R.; Peng, W.; Al-Hariri, M.; Kobeissy, F.; Tran, P.; Hamood, A.N.; Hegazy, M.F.; Paré, P.W.; Mechref, Y. Multitargeted Flavonoid Inhibition of the Pathogenic Bacterium Staphylococcus aureus: A Proteomic Characterization. J. Proteome Res. 2017, 16, 2579–2586. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of Anti-Staphylococcus aureus Activity of Quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simões, M. Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Eumkeb, G.; Chukrathok, S. Synergistic Activity and Mechanism of Action of Ceftazidime and Apigenin Combination against Ceftazidime-Resistant Enterobacter Cloacae. Phytomedicine 2013, 20, 262–269. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Si, X.; Liu, X.; Deng, X.; Niu, X.; Jin, Y.; Wang, D.; Wang, J. Quercetin Protects Rats from Catheter-related Staphylococcus aureus Infections by Inhibiting Coagulase Activity. J. Cell. Mol. Med. 2019, 23, 4808–4818. [Google Scholar] [CrossRef]
- Tomar, A.; Broor, S.; Kaushik, S.; Bharara, T.; Arya, D.S. Synergistic Effect of Naringenin with Conventional Antibiotics Against Methicillin Resistant Staphylococcus aureus. Eur. J. Mol. Clin. Med. 2021, 8, 1770–1784. [Google Scholar]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure-Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lin, Y.; Zhang, Z.; Shen, J.; Yang, C.; Jiang, M.; Hou, Y. Systematic Analysis of Bacteriostatic Mechanism of Flavonoids Using Transcriptome and Its Therapeutic Effect on Vaginitis. Aging 2020, 12, 6292–6305. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent Advances in Understanding the Antibacterial Properties of Flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Osonga, F.J.; Akgul, A.; Miller, R.M.; Eshun, G.B.; Yazgan, I.; Akgul, A.; Sadik, O.A. Antimicrobial Activity of a New Class of Phosphorylated and Modified Flavonoids. ACS Omega 2019, 4, 12865–12871. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Sekowski, S.; Bitiucki, M.; Dobrzynska, I.; Shlyonsky, V.; Ionov, M.; Burzynski, P.; Roszkowska, A.; Swiecicka, I.; Abdulladjanova, N.; et al. Inhibition of Interaction between Staphylococcus aureus α-Hemolysin and Erythrocytes Membrane by Hydrolysable Tannins: Structure-Related Activity Study. Sci. Rep. 2020, 10, 11168. [Google Scholar] [CrossRef]
- Chang, E.H.; Huang, J.; Lin, Z.; Brown, A.C. Catechin-Mediated Restructuring of a Bacterial Toxin Inhibits Activity. Biochim. Biophys. Acta BBA Gen. Subj. 2019, 1863, 191–198. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Swiecicka, I.; Andreeva-Kovaleskaya, Z.; Solonin, A.; Bonarska-Kujawa, D.; Kleszczyńska, H.; Mavlyanov, S.; Zamaraeva, M. Role of Structural Changes Induced in Biological Membranes by Hydrolysable Tannins from Sumac Leaves (Rhus typhina L.) in Their Antihemolytic and Antibacterial Effects. J. Membr. Biol. 2014, 247, 533–540. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.-P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-Sensing Receptors *. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of Antibacterial, Antitoxin, Antiviral, and Antifungal Activities of Tea Flavonoids and Teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Meng, X.-M.; Li, B.; Wang, C.-Q.; Wang, S.-Q.; Wang, T.-L.; Tian, Y.-M. Inhibition of Alpha-Hemolysin Expression by Resveratrol Attenuates Staphylococcus aureus Virulence. Microb. Pathog. 2019, 127, 85–90. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and Let Die. Front. Cell Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef]
- Berube, B.J.; Wardenburg, J.B. Staphylococcus aureus α-Toxin: Nearly a Century of Intrigue. Toxins 2013, 5, 1140–1166. [Google Scholar] [CrossRef]
- Gouaux, E. α-Hemolysin from Staphylococcus aureus: An Archetype of β-Barrel, Channel-Forming Toxins. J. Struct. Biol. 1998, 121, 110–122. [Google Scholar] [CrossRef]
- He, S.; Deng, Q.; Liang, B.; Yu, F.; Yu, X.; Guo, D.; Liu, X.; Dong, H. Suppressing Alpha-Hemolysin as Potential Target to Screen of Flavonoids to Combat Bacterial Coinfection. Molecules 2021, 26, 7577. [Google Scholar] [CrossRef]
- Wen, J.; Liu, B.; Yuan, E.; Ma, Y.; Zhu, Y. Preparation and Physicochemical Properties of the Complex of Naringenin with Hydroxypropyl-β-Cyclodextrin. Molecules 2010, 15, 4401–4407. [Google Scholar] [CrossRef]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria Bassiana Culture and Its Interaction with Liposome Membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, K.; King, J.W.; Howard, L.R.; Monrad, J.K. Solubility of Gallic Acid, Catechin, and Protocatechuic Acid in Subcritical Water from (298.75 to 415.85) K. J. Chem. Eng. Data 2010, 55, 3101–3108. [Google Scholar] [CrossRef]
- Mykytczuk, N.C.S.; Trevors, J.T.; Leduc, L.G.; Ferroni, G.D. Fluorescence Polarization in Studies of Bacterial Cytoplasmic Membrane Fluidity under Environmental Stress. Prog. Biophys. Mol. Biol. 2007, 95, 60–82. [Google Scholar] [CrossRef]
- El Khoury, M.; Swain, J.; Sautrey, G.; Zimmermann, L.; Van Der Smissen, P.; Décout, J.-L.; Mingeot-Leclercq, M.-P. Targeting Bacterial Cardiolipin Enriched Microdomains: An Antimicrobial Strategy Used by Amphiphilic Aminoglycoside Antibiotics. Sci. Rep. 2017, 7, 10697. [Google Scholar] [CrossRef] [PubMed]
- Jasniewski, J.; Cailliez-Grimal, C.; Younsi, M.; Millière, J.-B.; Revol-Junelles, A.-M. Functional Differences in Leuconostoc Sensitive and Resistant Strains to Mesenterocin 52A, a Class IIa Bacteriocin. FEMS Microbiol. Lett. 2008, 289, 193–201. [Google Scholar] [CrossRef]
- Seel, W.; Baust, D.; Sons, D.; Albers, M.; Etzbach, L.; Fuss, J.; Lipski, A. Carotenoids Are Used as Regulators for Membrane Fluidity by Staphylococcus xylosus. Sci. Rep. 2020, 10, 330. [Google Scholar] [CrossRef]
- National Collection of Type Cultures UK: Staphylococcus Aureus NCTC 5655. Available online: https://www.culturecollections.org.uk/products/bacteria/detail.jsp?refId=NCTC+5655&collection=nctc (accessed on 2 September 2022).
- Bernheimer, A.W.; Schwartz, L.L.Y. Isolation and Composition of Staphylococcal Alpha Toxin. Microbiology 1963, 30, 455–468. [Google Scholar] [CrossRef]
- Gurnev, P.A.; Nestorovich, E.M. Channel-Forming Bacterial Toxins in Biosensing and Macromolecule Delivery. Toxins 2014, 6, 2483–2540. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids—Food Sources, Health Benefits, and Mechanisms Involved. In Bioactive Molecules in Food; Mérillon, J.-M., Ramawat, K.G., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A Structure-Activity Relationship Study of Flavonoids as Inhibitors of E. Coli by Membrane Interaction Effect. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Júnior, S.D.d.C.; Santos, J.V.d.O.; Campos, L.A.d.A.; Pereira, M.A.; Magalhães, N.S.S.; Cavalcanti, I.M.F. Antibacterial and Antibiofilm Activities of Quercetin against Clinical Isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with Resistance Profile. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1948–1958. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Gómez-Mora, J.A.; Meraz-Rodríguez, M.A.; Flores-Sánchez, M.A.; Ortiz-Miranda, L.F. Effect of Flavonoids on Antimicrobial Activity of Microorganisms Present in Dental Plaque. Heliyon 2019, 5, e0313. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Song, Z.; Li, L.; Chang, H.; Li, S.; Zhou, W. Quercetin Inhibits Virulence Properties of Porphyromas gingivalis in Periodontal Disease. Sci. Rep. 2020, 10, 18313. [Google Scholar] [CrossRef] [PubMed]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A Natural Dietary Flavone Myricetin as an α-Hemolysin Inhibitor for Controlling Staphylococcus aureus Infection. Front. Cell Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Adnan, S.-N.-A.; Ibrahim, N.; Yaacob, W.A. Disruption of Methicillin-Resistant Staphylococcus aureus Protein Synthesis by Tannins. Germs 2017, 7, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Baikar, S.; Malpathak, N.; Malpathak, N.; Malpathak, N. Secondary Metabolites as DNA Topoisomerase Inhibitors: A New Era Towards Designing of Anticancer Drugs. Pharmacogn. Rev. 2010, 4, 12–26. [Google Scholar] [CrossRef]
- Plaper, A.; Golob, M.; Hafner, I.; Oblak, M.; Šolmajer, T.; Jerala, R. Characterization of Quercetin Binding Site on DNA Gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal Catechins Damage the Lipid Bilayer. Biochim. Biophys. Acta BBA Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of Antibacterial Action of Retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, C.; Song, L.; Li, T.; Cui, S.; Zhang, L.; Jia, Y. Antimicrobial Activity and Mechanism of Larch Bark Procyanidins against Staphylococcus aureus. Acta Biochim. Biophys. Sin. 2017, 49, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Hirai, C.; Sugiyama, Y.; Utsumi, M.; Yanagida, A.; Murata, M.; Ohashi, N.; Masuda, S. Interaction between Various Apple Procyanidin and Staphylococcal Enterotoxin A and Their Inhibitory Effects on Toxin Activity. Toxins 2017, 9, 243. [Google Scholar] [CrossRef]
- Choi, O.; Yahiro, K.; Morinaga, N.; Miyazaki, M.; Noda, M. Inhibitory Effects of Various Plant Polyphenols on the Toxicity of Staphylococcal α-Toxin. Microb. Pathog. 2007, 42, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tombola, F.; Campello, S.; De Luca, L.; Ruggiero, P.; Del Giudice, G.; Papini, E.; Zoratti, M. Plant Polyphenols Inhibit Vac A, a Toxin Secreted by the Gastric Pathogen Helicobacter Pylori. FEBS Lett. 2003, 543, 184–189. [Google Scholar] [CrossRef]
- Ostroumova, O.S.; Efimova, S.S.; Schagina, L.V. 5- and 4′-Hydroxylated Flavonoids Affect Voltage Gating of Single Alpha-Hemolysin Pore. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 2051–2058. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Johnson, B.J.; Hickey, T.E.; Pons, T.; Ligler, F.S. Binding and Neutralization of Lipopolysaccharides by Plant Proanthocyanidins. J. Nat. Prod. 2007, 70, 1718–1724. [Google Scholar] [CrossRef]
- Satoh, E.; Ishii, T.; Shimizu, Y.; Sawamura, S.; Nishimura, M. The Mechanism Underlying the Protective Effect of the Thearubigin Fraction of Black Tea (Camellia sinensis) Extract against the Neuromuscular Blocking Action of Botulinum Neurotoxins. Pharmacol. Toxicol. 2002, 90, 199–202. [Google Scholar] [CrossRef]
- Morinaga, N.; Iwamaru, Y.; Yahiro, K.; Tagashira, M.; Moss, J.; Noda, M. Differential Activities of Plant Polyphenols on the Binding and Internalization of Cholera Toxin in Vero Cells. J. Biol. Chem. 2005, 280, 23303–23309. [Google Scholar] [CrossRef]
- Shah, S.; Stapleton, P.D.; Taylor, P.W. The Polyphenol (−)-Epicatechin Gallate Disrupts the Secretion of Virulence-Related Proteins by Staphylococcus aureus. Lett. Appl. Microbiol. 2008, 46, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A Dual Antibacterial Mechanism Involved in Membrane Disruption and DNA Binding of 2R,3R-Dihydromyricetin from Pine Needles of Cedrus Deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ulhuq, F.R.; Mariano, G. Bacterial Pore-forming Toxins. Microbiology 2022, 168, 001154. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Banerjee, A.; Basu, K.; Sengupta, B.; Sengupta, P.K. Interaction of Flavonoids with Red Blood Cell Membrane Lipids and Proteins: Antioxidant and Antihemolytic Effects. Int. J. Biol. Macromol. 2007, 41, 42–48. [Google Scholar] [CrossRef]
- Schwiering, M.; Brack, A.; Stork, R.; Hellmann, N. Lipid and Phase Specificity of α-Toxin from S. Aureus. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative Analysis of Molecular Properties and Reactions with Oxidants for Quercetin, Catechin, and Naringenin. Mol. Cell Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Chudík, S.; Klouček, P.; Marek, R.; Cvačka, J.; Urbanová, M.; Julínek, O.; Kokoška, L.; Šlapetová, T.; Holubová, P.; et al. Antibacterial C-Geranylflavonoids from Paulownia Tomentosa Fruits. J. Nat. Prod. 2008, 71, 706–709. [Google Scholar] [CrossRef]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of Staphylococcal α-Hemolysin, a Heptameric Transmembrane Pore. Science 1996, 274, 1859–1865. [Google Scholar] [CrossRef]
- Merzlyak, P.G.; Yuldasheva, L.N.; Rodrigues, C.G.; Carneiro, C.M.; Krasilnikov, O.V.; Bezrukov, S.M. Polymeric Nonelectrolytes to Probe Pore Geometry: Application to the Alpha-Toxin Transmembrane Channel. Biophys. J. 1999, 77, 3023–3033. [Google Scholar] [CrossRef]
- Stefureac, R.; Long, Y.-T.; Kraatz, H.-B.; Howard, P.; Lee, J.S. Transport of Alpha-Helical Peptides through Alpha-Hemolysin and Aerolysin Pores. Biochemistry 2006, 45, 9172–9179. [Google Scholar] [CrossRef]
- DeGuzman, V.S.; Lee, C.C.; Deamer, D.W.; Vercoutere, W.A. Sequence-Dependent Gating of an Ion Channel by DNA Hairpin Molecules. Nucleic Acids Res. 2006, 34, 6425–6437. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Mies, F.; Sekowski, S.; Dubis, A.; Laurent, P.; Zamaraeva, M.; Swiecicka, I.; Shlyonsky, V. Enzymatic Synthesis and Characterization of Aryl Iodides of Some Phenolic Acids with Enhanced Antibacterial Properties. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184011. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of Membrane Fluidity of Multidrug-Resistant Isolates of Escherichia coli and Staphylococcus aureus in Presence and Absence of Antibiotics. J. Photochem. Photobiol. B. Biology 2018, 181, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Muzafarov, E.N.; Kim, Y.A. Rafts Making and Rafts Braking: How Plant Flavonoids May Control Membrane Heterogeneity. Mol. Cell Biochem. 2008, 314, 65. [Google Scholar] [CrossRef] [PubMed]
- Valeva, A.; Hellmann, N.; Walev, I.; Strand, D.; Plate, M.; Boukhallouk, F.; Brack, A.; Hanada, K.; Decker, H.; Bhakdi, S. Evidence that Clustered Phosphocholine Head Groups Serve as Sites for Binding and Assembly of an Oligomeric Protein Pore. J. Biol. Chem. 2006, 281, 26014–26021. [Google Scholar] [CrossRef]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.; Zavodnik, I.B. Flavonoids Modulate Liposomal Membrane Structure, Regulate Mitochondrial Membrane Permeability and Prevent Erythrocyte Oxidative Damage. Biochim. Biophys. Acta BBA Biomembr. 2020, 1862, 183442. [Google Scholar] [CrossRef]
- Onishi, T. Quantum Computational Chemistry; Springer: Singapore, 2018; ISBN 978-981-10-5933-9. [Google Scholar]
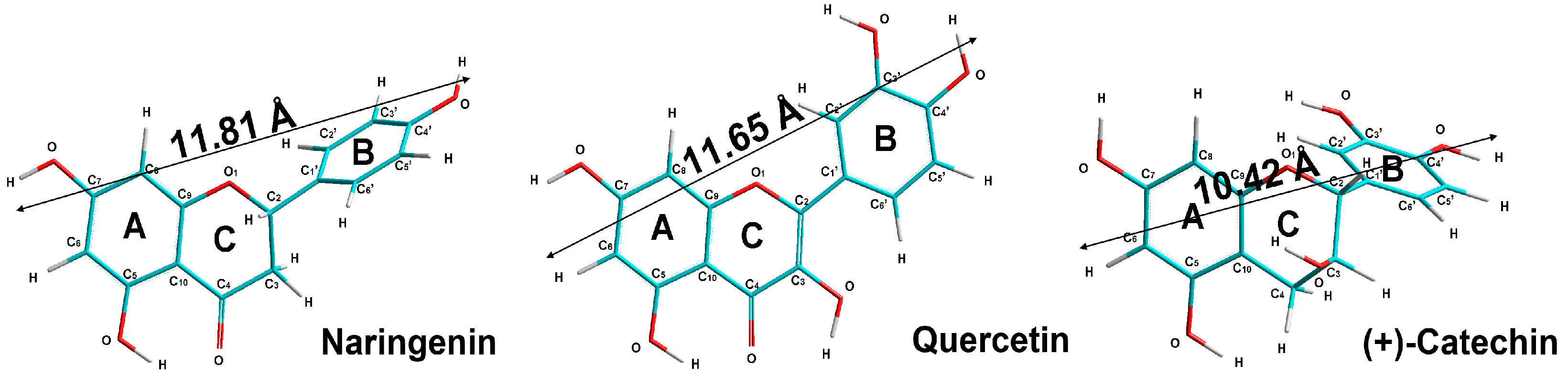
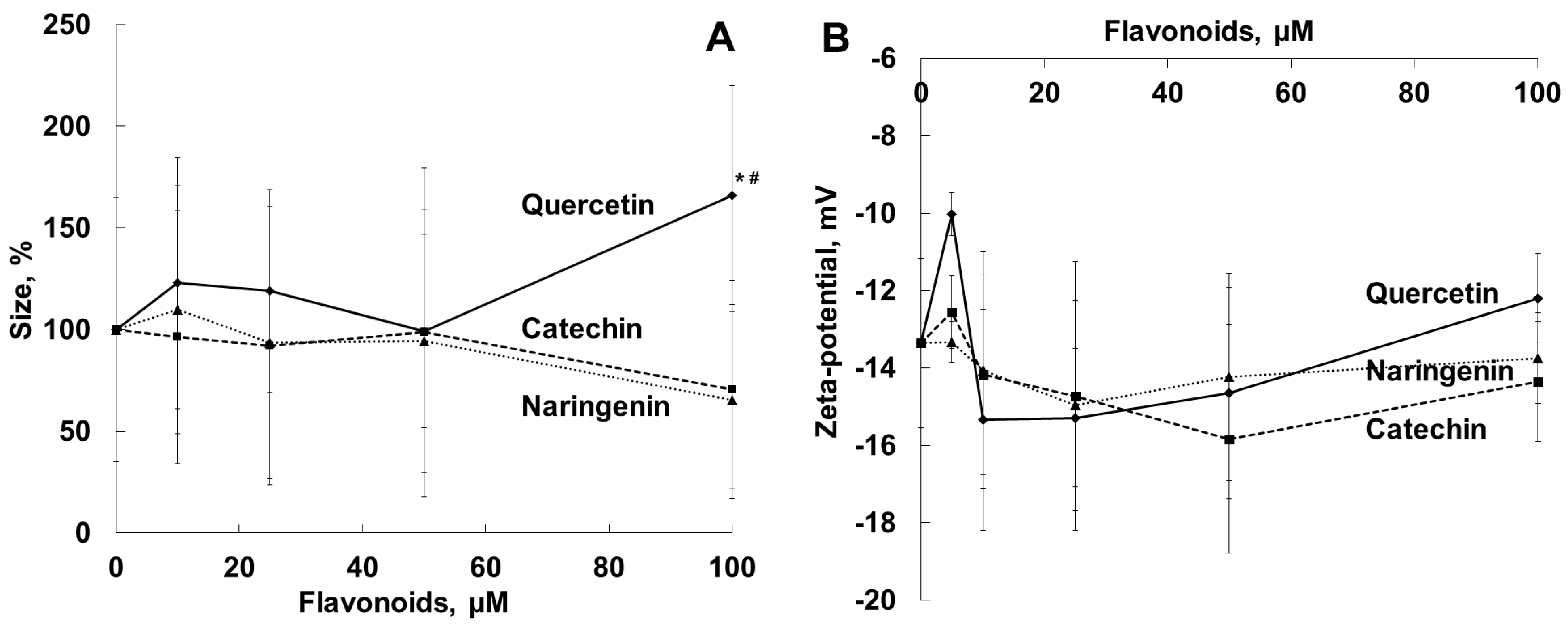
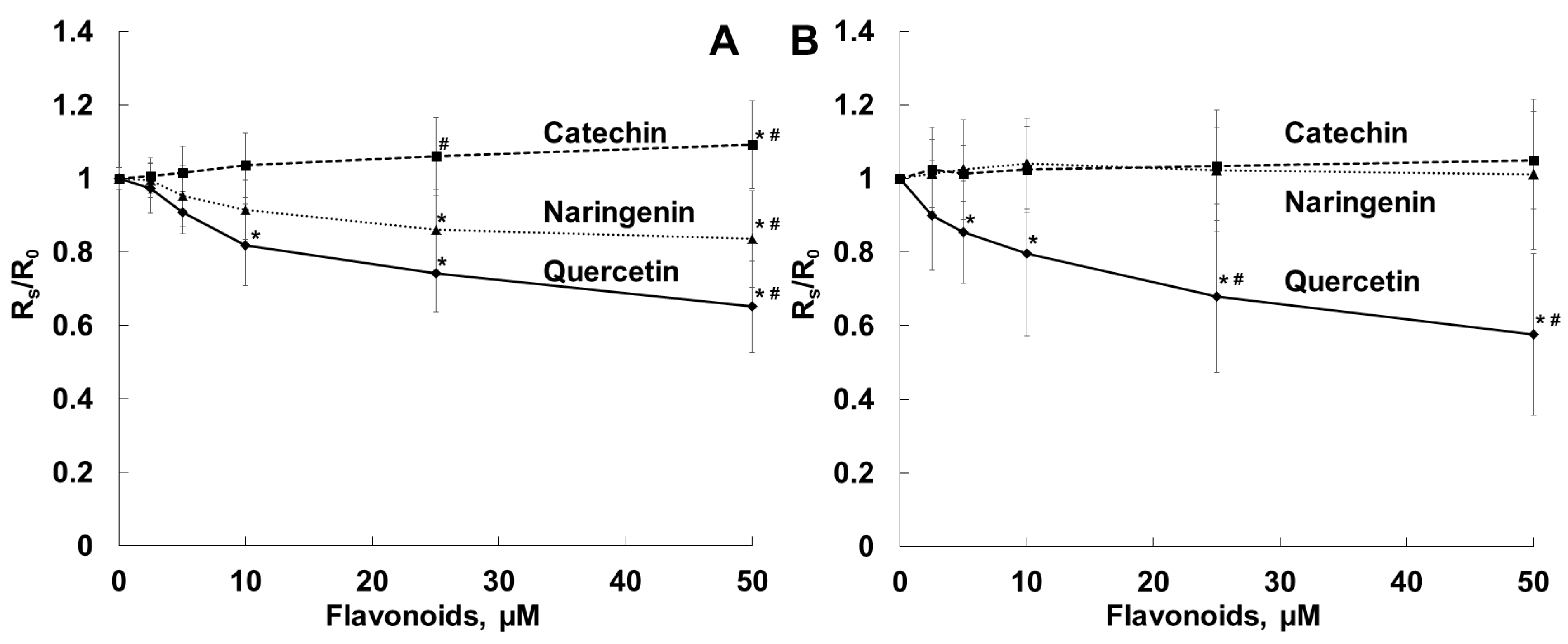

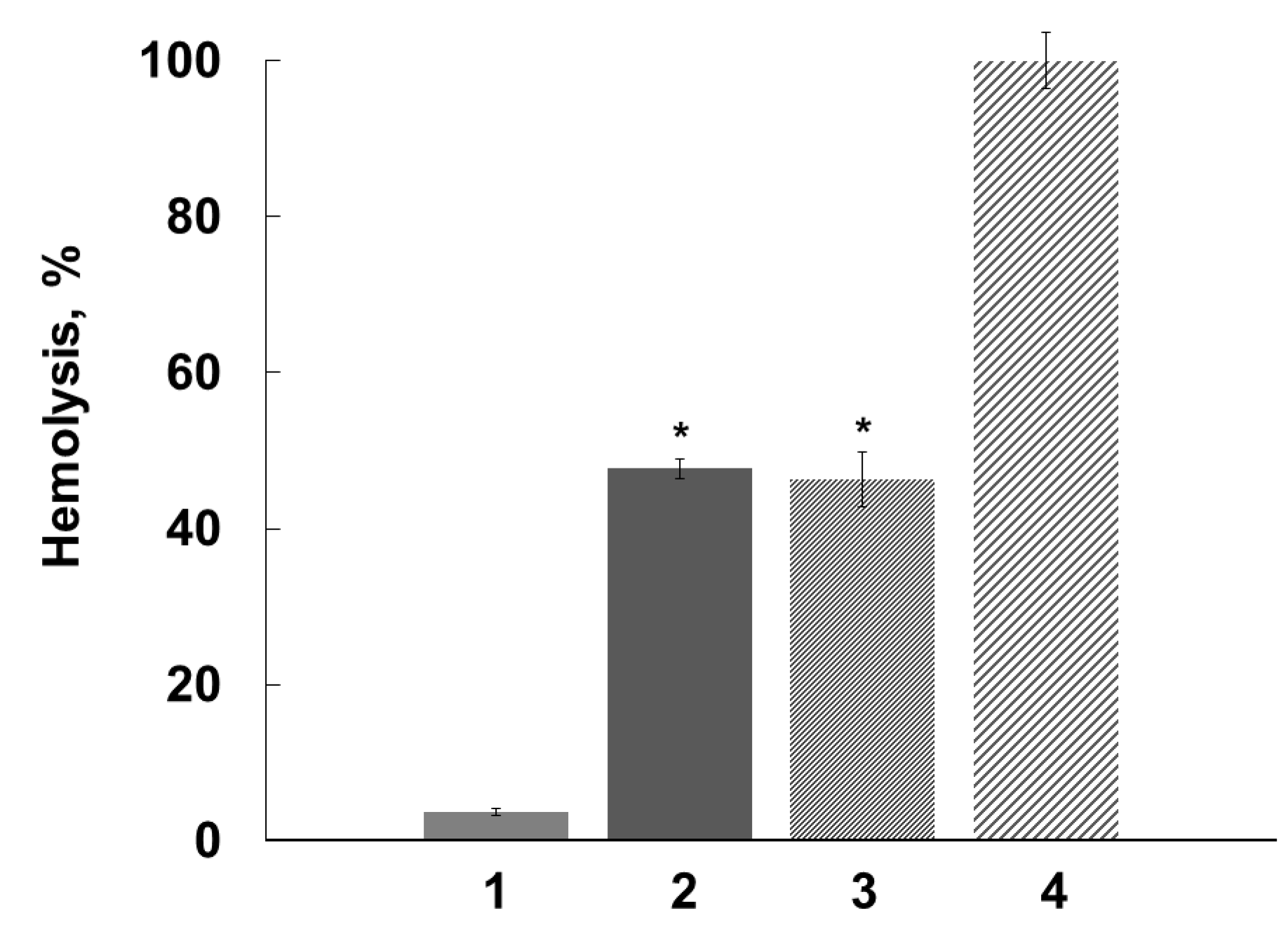
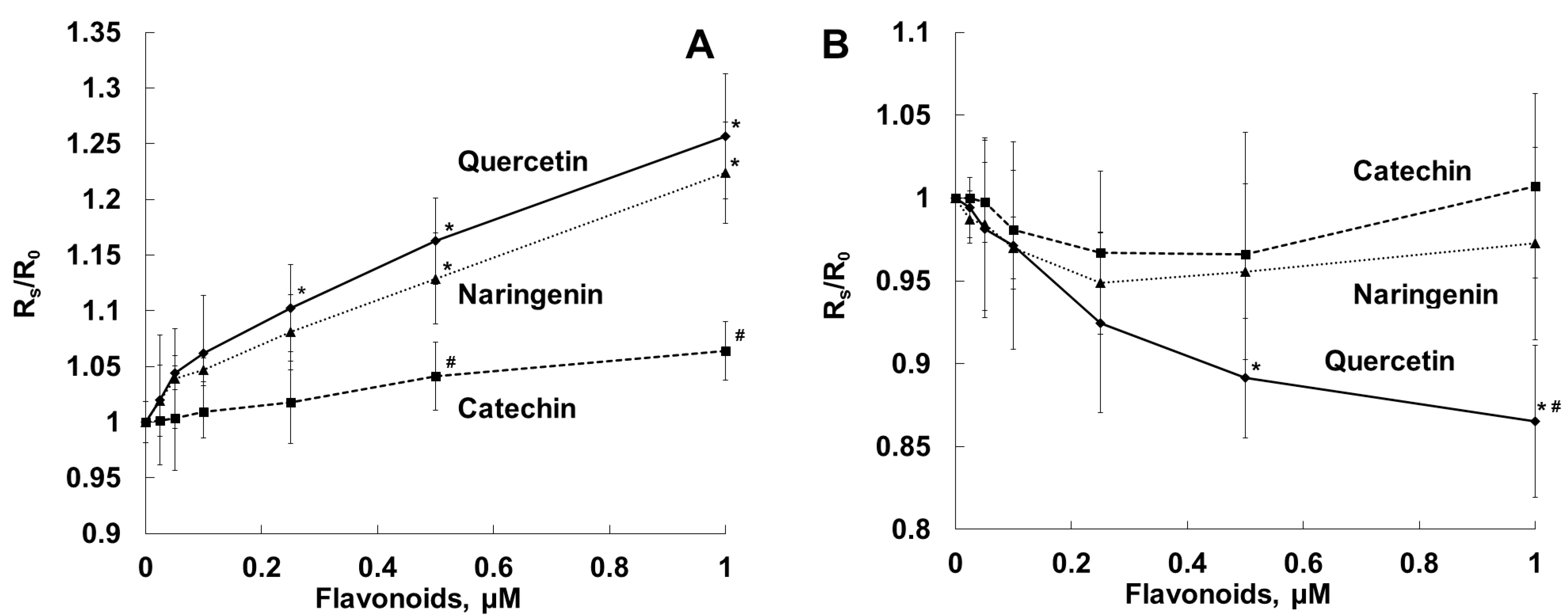
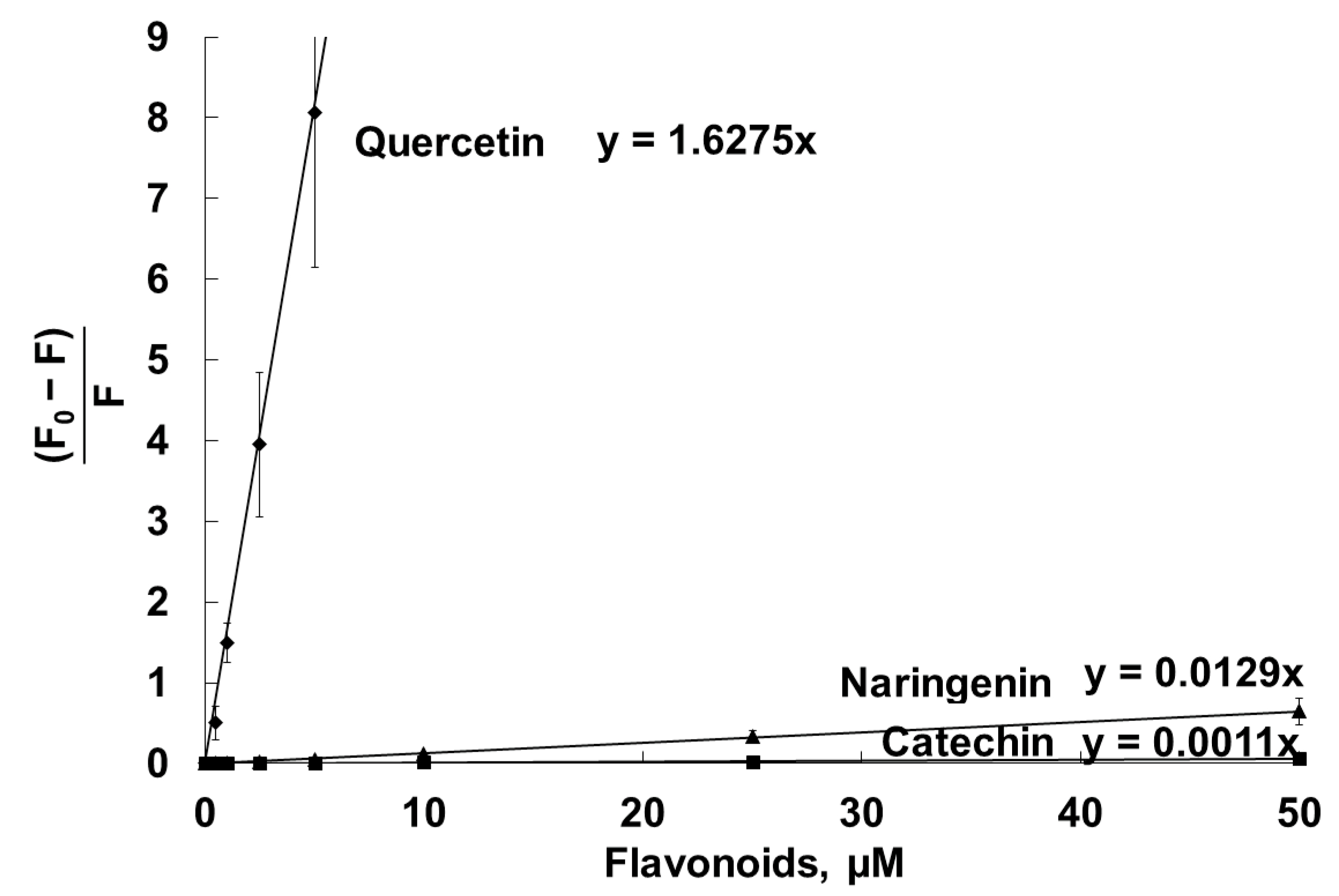
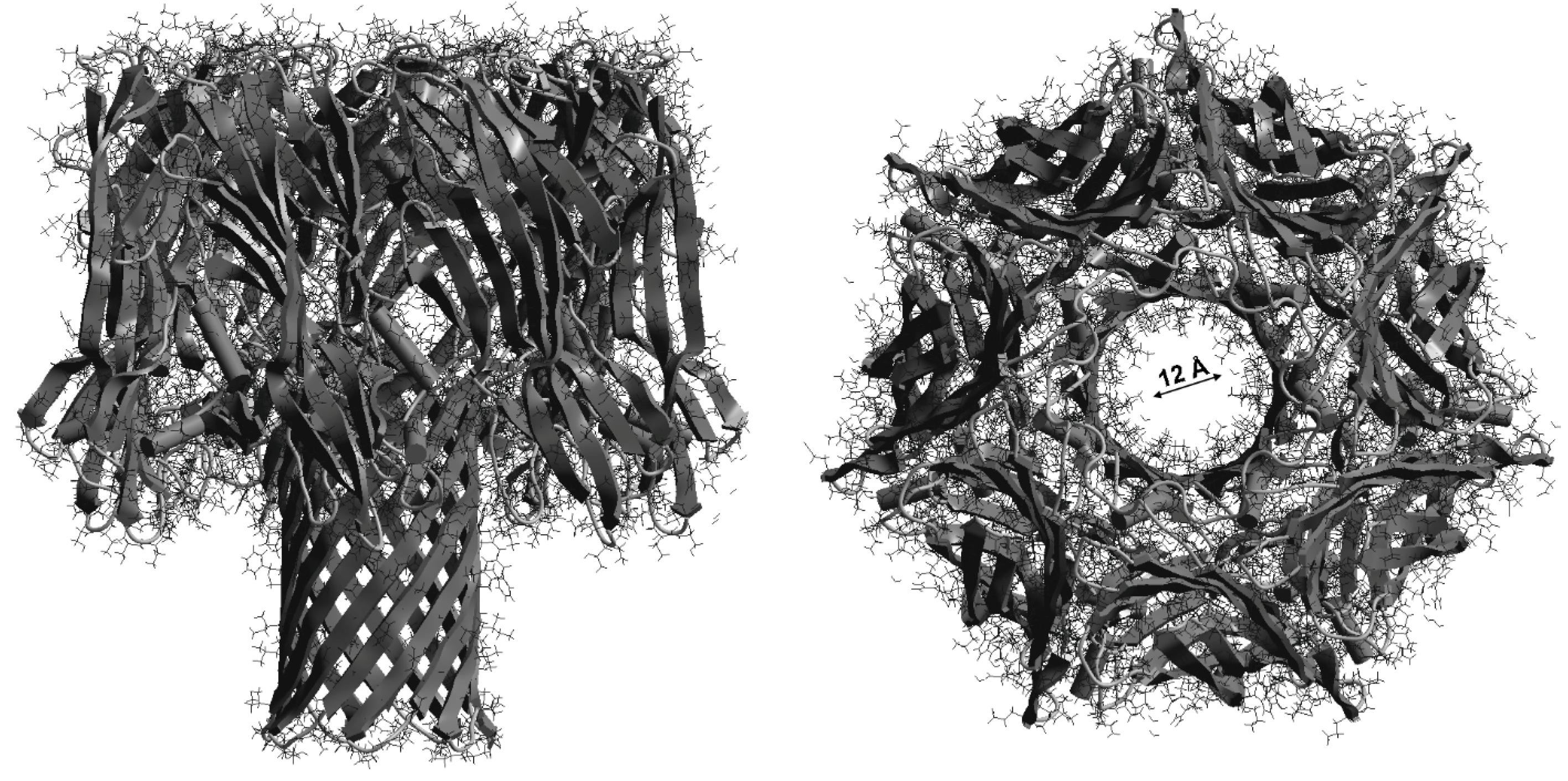
| Parameter | Naringenin | Quercetin | (+)-Catechin |
|---|---|---|---|
| Minimal inhibitory concentrations (MICs), μM | 200 | 100 | 150 |
| Dipole moments, D | 1.602 | 0.986 | 2.107 |
| Torsion angles (C3-C2-C1′-C2′) | 86 | 180 | 118 |
| Stern–Volmer constants of DPH fluorescence quenching in liposomal membranes, µM−1 | 0.012 ± 0.003 | 1.66 ± 0.20 | 0.0012 ± 0.0002 |
| Water solubility, mg/L | 4.38 | 0.51 | 2260 |
| Flavonoid | MICs | Bacterial Strain | References |
|---|---|---|---|
| Quercetin | 120 µM (35.76 µg/mL) > 3410 µM (> 1024 μg/mL) 830 µM (250 µg/mL) 1670 µM (500 µg/mL) > 3330 µM (> 1000 µg/mL) 1670 µM (500 µg/mL) 170 µM (50 µg/mL) 200 μM (60 µg/mL) | E. coli S. aureus strain Newman methicillin-susceptible S. aureus methicillin-resistant S. aureus S. aureus E. coli S. aureus P. gingivalis | [52] [20] [53] [54] [55] [55] [56] [57] |
| Naringenin Glycoside naringin | 460 µM (125 μg/mL) 1720 µM (1000 µg/mL) | methicillin-resistant S. aureus S. aureus | [21] [56] |
| Catechin | 3550 µM (1000 µg/mL) | S. aureus | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules 2023, 28, 1252. https://doi.org/10.3390/molecules28031252
Veiko AG, Olchowik-Grabarek E, Sekowski S, Roszkowska A, Lapshina EA, Dobrzynska I, Zamaraeva M, Zavodnik IB. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules. 2023; 28(3):1252. https://doi.org/10.3390/molecules28031252
Chicago/Turabian StyleVeiko, Artem G., Ewa Olchowik-Grabarek, Szymon Sekowski, Anna Roszkowska, Elena A. Lapshina, Izabela Dobrzynska, Maria Zamaraeva, and Ilya B. Zavodnik. 2023. "Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes" Molecules 28, no. 3: 1252. https://doi.org/10.3390/molecules28031252
APA StyleVeiko, A. G., Olchowik-Grabarek, E., Sekowski, S., Roszkowska, A., Lapshina, E. A., Dobrzynska, I., Zamaraeva, M., & Zavodnik, I. B. (2023). Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules, 28(3), 1252. https://doi.org/10.3390/molecules28031252







