Abstract
Cancer is a severe threat to human life. Recently, various therapeutic strategies, such as chemotherapy, photodynamic therapy, and combination therapy have been extensively applied in cancer treatment. However, the clinical benefits of these therapeutics still need improvement. In recent years, supramolecular chemistry based on host–guest interactions has attracted increasing attention in biomedical applications to address these issues. In this review, we present the properties of the major macrocyclic molecules and the stimulus–response strategies used for the controlled release of therapeutic agents. Finally, the applications of supramolecular-macrocycle-based nanomaterials in cancer therapy are reviewed, and the existing challenges and prospects are discussed.
1. Introduction
Supramolecular chemistry has developed rapidly since Donald J. Cram, Jean-Marie Lehn, and Charles J. Pedersen won the Nobel Prize in 1987 for their contributions to host–guest systems. Supramolecular systems are assembled by non-covalent interactions such as hydrogen bonds, coordination bonds, hydrophobic interactions, electrostatic interactions, and π-π stacking [1,2]. Compared with covalent interaction, non-covalent interactions demonstrate several advantages. First, non-covalent interactions offer a facile strategy for constructing supramolecular materials, effectively avoiding complicated synthesis and purification processes [3]. Such supramolecular-based strategies are generally environmentally friendly and cost/time-saving, since supramolecular materials are readily prepared by simply mixing functional units in solution under ambient conditions [4]. In addition, this non-covalent interaction endows supramolecular materials with dynamic and reversible properties, especially the ability to respond to external stimuli, which provides great potential for the design and construction of stimulus–response materials based on supramolecular chemistry [5]. Most importantly, supramolecular chemistry allows for the manipulation of functional units at the molecular level, enabling a “bottom-up” strategy to control the size and morphology of supramolecular materials. In particular, the construction of supramolecular materials with uniform sizes in the nanometer range has attracted increasing attention in biomedical applications.
Among various non-covalent interactions, host–guest interactions have received increasing attention in biomedical applications by integrating macrocyclic molecules into supramolecular materials. During the past few decades, a series of macrocyclic hosts, including crown ethers, cyclodextrins, calix(n)arenes, cucurbit(n)urils, and pillar(n)arenes, have been developed [6,7,8,9]. Typically, these macrocyclic hosts possess a hydrophobic cavity and a hydrophilic outer shell, allowing the accommodation of guest molecules into their cavities. This unique host–guest interaction provides molecular-level encapsulation for drug molecules, thereby effectively protecting drug molecules from degradation or inactivation. In addition, most host–guest interactions have a host–guest stoichiometry of 1:1 at thermodynamic equilibrium and are determined by the characteristic constant of association (Ka) [10]. As a result, the drug loading content can be directly predicted at a given concentration and association constant.
Taking advantage of host–guest interactions, several limitations that hinder the clinical application of anticancer drugs can be effectively addressed. For example, the solubility and stability of anticancer drugs can be effectively improved by forming host–guest complexes with macrocyclic hosts [11,12]. In addition, a high accumulation of therapeutic agents can be guaranteed by forming supramolecular self-assembly, resulting in significantly enhanced therapeutic efficacy and reduced toxic effects [13]. Furthermore, the upper and lower rim of the macrocyclic hosts are easily modified to incorporate functional groups—for example, imaging agents, targeting ligands, and even therapeutic agents—thus endowing these macrocyclic hosts with therapeutic properties [14,15]. More importantly, the dynamic binding nature of host–guest interaction allows the macrocyclic host to precisely control the release of loaded cargo in response to abnormal biomarkers in tumor tissues—for example, acidic pH, GSH, ATP, and hypoxic. The dynamic nature of host–guest interactions makes macrocyclic host-based anticancer strategies more versatile than traditional nanomedicines that lack stimulus responsiveness.
This review introduces the properties of four major macrocyclic hosts, including cyclodextrins, calixarenes, pillararene, and cucurbiturils (Figure 1). Next, strategies for the controlled release of therapeutic agents based on stimulus–response strategies (hypoxic, acidic, GSH, and ATP) are demonstrated. Finally, we summarize the recent advances in supramolecular-macrocycle-based nanomaterials for cancer treatment, including supramolecular-macrocycle-based chemotherapy, supramolecular-macrocycle-based photodynamic therapy, and supramolecular-macrocycle-based combination therapy [16,17].
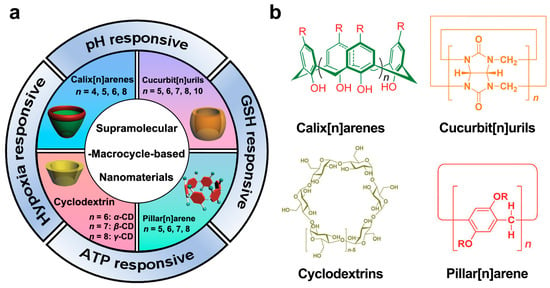
Figure 1.
(a) Recent advances in supramolecular-macrocycle-based nanomaterials in cancer treatment. (b) The chemical structure of four major macrocyclic molecules. Reproduced from ref. [6]. Copyright 2017 Royal Society of Chemistry.
2. Macrocyclic Host Molecules
Poor solubility and stability are two major factors restricting the general applications of 40–70% of newly developed anticancer drugs/probes in clinical cancer treatment. Encapsulating therapeutic agents into macrocyclic hosts offers a feasible strategy to address these issues. This section discusses four major types of macrocyclic hosts for fabricating supramolecular therapeutic agents, including cyclodextrins, calixarenes, and cucurbiturils.
2.1. Cyclodextrin
Cyclodextrins (CDs) have received increasing attention among the many macrocyclic host molecules, especially for their biological applications. CDs refer to a class of water-soluble macrocyclic oligosaccharides linked by multiple α-1,4 glycosidic bonds [18]. Since the French scientist Villiers accidentally discovered CDs in natural products in 1891, it has undergone hundreds of years of development [19]. Moreover, the easy accessibility of CDs from starch precursors such as corn, rice, and potato also facilitated its development [20]. To date, CDs have been extensively applied in various fields, including pharmaceutics, catalytic reactions, enzyme technology, and analytical chemistry [21,22,23]. The commercially available CDs have three major subtypes, γ, β, and α-CD, consisting of eight, seven, and six D-glucose units. CDs have a truncated cone-like molecular container structure with a hydrophilic external surface and a hydrophobic interior cavity. This unique structure enables CD to form host–guest complexes with various guest molecules, such as drugs, amino acid residues, and fluorescent probes, in an aqueous solution through van der Waals interaction, hydrogen binding, and hydrophobic interactions [24,25]. The resulting host–guest complex effectively improves the safety, stability, and solubility of the loaded drugs, thereby significantly reducing side effects and enhancing bioavailability [26,27]. In addition, the saccharide nature of CDs determines their excellent biocompatibility. Benefiting from these unique properties, more than 54 CD-based nanomedicines and prodrugs have been developed and applied in clinical and preclinical studies.
2.2. Calix(n)arenes
Calix(n)arenes (CAs), representing the third generation of macrocyclic hosts after CDs and crown ethers, are typically prepared by bridging multiple phenolic units with methylene groups at the 2- and 6-positions [28]. The CAs have a corn-like structure, with a hollow cavity and two rims on the upper and lower sides [29]. Due to the fully synthetic process, CAs exhibit several advantages over naturally available CDs, including ease of modification, controllable conformations, and tunable scaffolds (usually composed of 4, 5, 6, or 8 units) [30]. With these characteristics, CAs are considered macrocycle hosts with unlimited structure and possibilities [31]. As potential therapeutic modifiers and drug carriers, the water-soluble CAs were synthesized by introducing functional groups such as sulfonic acid, carboxylic acid, amino, or quaternary ammonium to the upper or lower rim of CAs [32,33]. Like CDs, CAs can load guest molecules such as drugs and fluorescent probes through host–guest interactions [34,35,36]. More importantly, their binding affinities can be adjusted in response to abnormal biomarkers in tumor tissues, allowing for precise control of drug release in tumor tissues.
2.3. Cucurbit(n)urils
Compared with CDs and CAs, the cucurbit(n)urils (CBs) have a relatively short history. As early as 1905, Behrend et al. reported the synthesis of CBs by condensing glycoluril and formaldehyde. However, it was only in 1981 that the Mock group gave the definite chemical structure of CBs, and finally, Kim et al. isolated CB members around 2000 [37]. CBs have a highly symmetric pumpkin-like structure with a central hydrophobic cavity and two hydrophilic carbonyl rims. The CB family possesses a uniform cavity depth of 0.91 nm, and the width depends on the number of glycoluril units [38,39]. Uniquely, the cavity of CBs is hydrophobic and nonpolar due to the absence of lone pairs of electrons or chemical bonds within the cavity [40]. Therefore, CBs are ideal macrocyclic hosts for encapsulating neutral and positively charged guest molecules. For example, CB(6) can host alkyl ammonium ions, while CB(7) can host adamantanamine. CB(8) is even capable of hosting two guest molecules (2,6-bis(4,5-diydro-1H-imidazol-2-yl)naphthalene) by forming 1:2 host–guest complexes [41]. Despite their extremely high binding affinities toward various guest molecules, the low water solubility of CBs is a significant bottleneck that limits their general application. To address these issues, functionalized CBs were developed by using aldehyde or glycoluril derivatives in the condensation process [42,43]. Although promising, the complicated synthesis and purification procedures still limit their applications. Therefore, developing straightforward and efficient functionalization strategies is crucial for CB’s applications.
2.4. Pillar(n)arene
Similar to CBs, pillar(n)arene (PAs) also have a short history. In 2008, Ogoshi synthesized a type of phenol para-bridged “pillar” supramolecular macrocyclic molecule called pillararenes [44]. Pillararenes have a rigid pillar structure and hydrophobic cavities. The size of the cavities can be regulated by changing the number of repeating units, which can be named pillar(n)arene (n = 5, 6, 7, 8) [6]. Moreover, water-soluble pillar(n)arene, such as water-soluble pillar(5)arene (WP5) and water-soluble pillar(6)arene (WP6), were developed through functional modification [45,46]. Similarly to CDs and CAs, pillar(n)arenes have excellent host–guest recognition ability to achieve effective loading of guest molecules [47]. Moreover, due to its electron-rich cavities and polyhydroxy or alkoxy structures, pillar(n)arene can form supramolecular complexes with pyridine salts, ferrocene, and quaternary ammonium salts through non-covalent interactions, which broadens the variety of host–guest complexes [48,49,50]. Benefiting from these unique properties, pillar(n)arene has attracted increasing attention in biomedical applications.
3. Strategies for Controlled Drug Release
The abnormal proliferation and metabolism of tumor cells lead to overexpressed physiological indicators in TME. In addition, the dynamic binding nature of host–guest interaction offers great potential for the macrocyclic host to respond to these indicators to release loaded cargo in tumor tissues. This section will briefly introduce the recently developed stimulus–response supramolecular-macrocycle-based nanomaterials for cancer treatment based on acidity, redox, ATP, and hypoxia conditions.
3.1. pH-Responsive Supramolecular-Macrocycle-Based Nanomaterials
Acidity is a typical hallmark for distinguishing tumors from normal tissues, since most solid tumors are accompanied by excessive accumulation of acidic metabolic organelles in the TME [51]. Many pH-responsive macrocyclic hosts have been developed for tumor-targeted delivery of anticancer drugs, which is mainly achieved by introducing ionizable functional groups into macrocyclic hosts [52,53]. In acidic TME, the physical properties, cavity size, and chemical structure of these functionalized macrocyclic hosts are changed due to the protonation effect, resulting in a significant decrease in binding affinity and the on-demand release of loaded drugs in the TME. For example, Li et al. presented an N, N-diisopropylenediamine (DPA)-grafted β-CD (β-CD-DPA) to deliver succinobucol (SCB) for the effective treatment of breast cancer [54]. SCB was loaded into the hydrophobic domain of β-CD-DPA. Upon reaching tumor tissue, DPA efficiently protonated and transformed β-CD-DPA to a hydrophilic state, leading to the release of SCB and tumor suppression in the 4T1 breast cancer mouse model. Similarly, Li et al. reported a carboxylatopillar(6)arene (CP6A) for the tumor-targeted delivery of oxaliplatin (OX) [55]. Under acidic conditions, the binding affinity of CP6A to OX was significantly reduced due to the protonation effect, thereby achieving the controlled release of OX in tumor tissues. In addition, introducing pH-responsive motifs in guest molecules is also a promising strategy for constructing pH-responsive supramolecular-macrocycle-based nanomaterials. For example, Liu et al. reported a pH-responsive double positive charged guest molecule, ADA2+ [56]. They constructed a supramolecular nanoparticle (ADA2+@HACD) by complexing with hyaluronic acid-modified β-CD (HACD) for efficient plasmid DNA (pDNA) delivery. The strong positive charge of ADA2+ enabled ADA2+@HACD to condense and encapsulate pDNA efficiently. Under the acidic conditions, the ester bonds of ADA2+ were degraded to carboxyl groups, resulting in the controlled release of loaded pDNA in tumor tissues.
3.2. GSH-Responsive Supramolecular-Macrocycle-Based Nanomaterials
GSH is one of the essential biomarkers in tumor tissues and is closely related to the occurrence and progression of tumors. In addition, the significantly different levels of GSH in extracellular space (1–10 μM) and inside cells (2–10 mM) make GSH an ideal and ubiquitous trigger for intracellular drug delivery [57,58]. For example, Wang et al. constructed a β-D-galactose-modified pillar(5)arene (GlaP5) for tumor-targeted delivery of camptothecin (CPT) [59]. Firstly, CPT was prepared as a prodrug by introducing trimethylammonium groups and disulfide bonds. The trimethylammonium groups function as the binding sites to efficiently deliver CPT prodrugs, and the disulfide bonds function as responsive units to allow the controlled release of CPT in tumor tissues. With this strategy, they greatly enhanced the cytotoxicity of CPT against HepG2 liver cancer cells. Xu et al. reported a GSH-responsive poly-cyclodextrin nanocage (PDOP NCs) to deliver doxorubicin (DOX) for enhanced cancer immunotherapy [60]. After systemic administration, PDOP NCs accumulated in tumor tissues via the enhanced permeability and retention (EPR) effect. Subsequently, DOX was released from PDOP NCs to activate immunogenic cell death (ICD) of 4T1 cells, thereby enhancing cancer immunotherapy. Aside from disulfide bonds, the ferrocenium cation is another commonly used functional group to construct GSH-responsive drug delivery systems. For example, Pei et al. reported a novel ferrocenium-capped pillar(5)arene (FCAP) to deliver siRNA and DOX [61]. Driven by iron ions, FCAP self-assembles into nanoparticles to facilitate the cellular uptake of DOX and siRNA. After internalization, the ferrocene cation was efficiently reduced to neutral ferrocene by GSH, leading to the disassembly of the nanoparticles and the release of the loaded cargoes. Similarly, Zhu et al. reported a ferrocenium-integrated supramolecular block polymer (SBC) and achieved intracellular delivery of pDNA with high transfection efficiency [62].
3.3. ATP-Responsive Supramolecular-Macrocycle-Based Nanomaterials
ATP is an essential compound for life, providing energy for most processes in living systems, such as chemical synthesis, dissolution of condensates, transmission of nerve impulses, and muscle contraction [63,64,65]. In particular, ATP is significantly elevated in tumor tissues due to its rapid proliferation and metabolism [66]. Recently, several ATP-responsive supramolecular-macrocycle-based nanomaterials have been developed for tumor diagnosis and targeted delivery. For example, Guo et al. presented a novel amphiphilic guanidinium-modified calix(5)arene (GC5A-12C) for tumor-targeted delivery of photosensitizers (PS) [67]. GC5A-12C is designed to contain multiple guanidine groups on its upper rim, which allows GC5A-12C to form a salt bridge with ATP/PS through electrostatic interactions and hydrogen bonds. During blood circulation, the strong binding affinity between GC5A-12C and PS effectively quenches PS fluorescence and avoids payload leakage. Upon reaching the tumor tissues, the PS is outcompeted by overexpressed ATP, accompanied by restoration of fluorescence and photoactivity. Similarly, Meng et al. presented a cationic water-soluble pillar(6)arene (WP6A)-based ATP-responsive supramolecular drug delivery system for tumor-targeted delivery of DOX [68]. In tumor tissue, a high concentration of ATP acts as the competitive guest molecule. It bound to WP6A competitively, leading to the disassembly of the supramolecular assembly and the release of DOX. In addition to being used as a biomarker for designing responsive nanomaterials, capturing ATP offers an alternative strategy for cancer treatment. For example, Huang et al. reported a trimethylammonium-modified pillar(6)arene (WP6A), which recognized and exhausted intracellular ATP to block the energy supply [69]. As a result, WP6A effectively overcame drug resistance and significantly enhanced the cytotoxicity of DOX on MCF-7/ADR cells.
3.4. Hypoxia-Responsive Supramolecular-Macrocycle-Based Nanomaterials
Hypoxia is another indicator of tumors. Tumor hypoxia is usually caused by abnormal angiogenesis and rapid proliferation of tumor cells [70]. The lack of oxygen in the tumor site causes an imbalance in the redox state of the cancer cells [71]. Taking advantage of this property, Guo et al. introduced azo groups into the upper rim of calixarenes and designed a series of hypoxia-responsive azocalixarenes [72,73,74]. For example, they reported a carboxylated azocalix(4)arene (CAC4A) for hypoxia-targeted drug delivery [75]. CAC4A exhibited a strong binding affinity to various anticancer drugs during blood circulation, effectively avoiding payload leakage and side effects. Upon reaching the hypoxic TME, the azo groups of CAC4A were effectively reduced by bio-reductase, resulting in significantly decreased binding affinity and the release of drugs in tumor tissues. Additionally, the same group also developed sulfonated azocalix(5)arene (SAC5A) and achieved tumor-targeted delivery of paclitaxel (PTX) [76]. By integrating macrocyclic hosts in nanosystems, our group reported a macrocyclic-amphiphile-based self-assembled nanoparticle (MASN) for ratiometric delivery of drug combinations to tumor tissues [77]. In hypoxic TME, MASN was reduced by bio-reductase, leading to the spontaneous release of drug combinations in tumor tissues. Through precise loading and ratiometric co-delivery of drug combinations, MASN achieved effective combination chemotherapy and significantly suppressed tumor growth in a 4T1 breast cancer mouse model. Similarly, we developed a calixarene-integrated nano-drug delivery system (CanD) and achieved tumor-targeted delivery and tracking of anticancer drugs in vivo [67].
4. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Chemotherapy
Chemotherapy refers to the use of cytotoxic drugs to control and kill tumors. Despite the remarkable success, common issues associated with low bioavailability and high cytotoxicity of anticancer drugs still limit their general applications in clinical cancer treatment [78,79]. In addition, increased interstitial fluid pressure (IFP) also limits the penetration of anticancer drugs into deep tumor tissues, further restricting the therapeutic efficacy of anticancer drugs [80]. Compared with traditional nanocarriers, supramolecular-macrocycle-based nanomaterials possess the following advantages: (i) precise loading and on-demand release of drugs; (ii) easy size manipulation at tumor sites due to the dynamic nature of host–guest interactions [29,81]. With these properties, supramolecular-macrocycle-based nanomaterials demonstrate great potential to improve the therapeutic efficacy of anticancer drugs by enhancing tumor accumulation and promoting tumor penetration. This chapter mainly discusses the progress of supramolecular-macrocycle-based nanomaterials in these aspects.
4.1. Enhancing Tumor Accumulation
Compared with conventional anticancer drugs, nanomedicines have relatively controllable biodistribution and can passively accumulate at tumor sites through the EPR effects [82]. In addition, active tumor targeting is also feasible by introducing specific targeting ligands. The mutation or overexpression of glycoproteins on the cell surface is a typical class of tumor markers–for example, carbohydrate antigen (CA125), CAl5-3, and CA50 [83,84,85]. Modifying the corresponding glycosylated ligands in drug delivery systems is an effective strategy for active tumor targeting. For example, Pei et al. constructed a mannose-modified GSH-responsive supramolecular vesicle (glycol-NV) for tumor-targeted delivery of DOX (Figure 2, Table 1) [86]. The glycol-NV was formed by self-assembly of mannose derivatives (Man-NH3+) and diselenium-bridged pillar(5)arene dimers (SeSe-(P5)2). The decorated mannose effectively guided glycol-NV to tumor cells through the interactions between mannose and mannose receptors. After cellular internalization, overexpressed GSH led to the cleavage of the Se-Se bonds in glycol-NV, resulting in the efficient release of DOX and enhanced antitumor efficacy. Similarly, Pei et al. presented a galactose-integrated supramolecular vesicle (CAAP5G) for systemic delivery of DOX and achieved significantly enhanced suppressions in HpeG2 cells [87]. Aside from glycosylated ligands, antigens and peptides are also commonly used as targeting ligands. For example, the tripeptide Arg-Gly-Asp (RGD) binds strongly to integrin αvβ3, which is overexpressed on the surface of various cancer cells, including melanoma, breast, and ovarian cancer. Taking advantage of RGD, Schmuck et al. reported size-controllable supramolecular nanocarriers (WP5-DOX⊃RGD-SG) for targeted delivery of DOX (Figure 3) [88]. By adjusting the host–guest molar ratio, WP5-DOX⊃RGD-SG was assembled into both vesicles and micelles, and it was found that the micellar WP5-DOX⊃RGD-SG demonstrated significantly enhanced antitumor efficacy in a HepG 2 liver cancer mouse model. Similarly, Zhang et al. developed an RGD-modified layer-by-layer film based on CD and Ada interactions for tumor-targeted delivery of DOX, and achieved significantly enhanced antitumor efficacy in an A549 tumor mouse model [89].
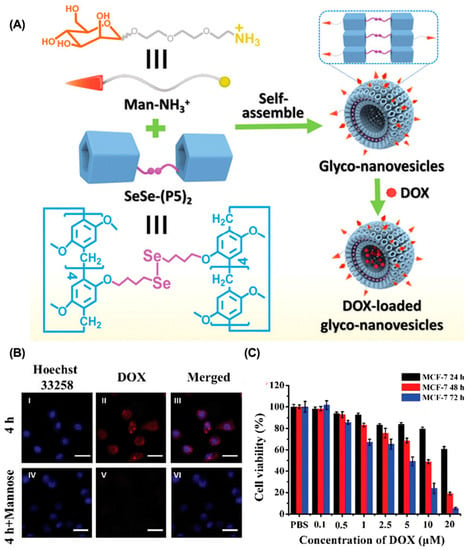
Figure 2.
Glycol-NV for targeted drug delivery to enhance tumor accumulation. (A) Schematic illustration of the construction of glycol-NV. (B) CLSM of MCF-7 cells incubated with glycol-NV with or without pre-incubation of mannose. (C) Cell viability of MCF-7 cells incubated with glycol-NV. Reproduced from ref. [86]. Copyright 2020 Royal Society of Chemistry.
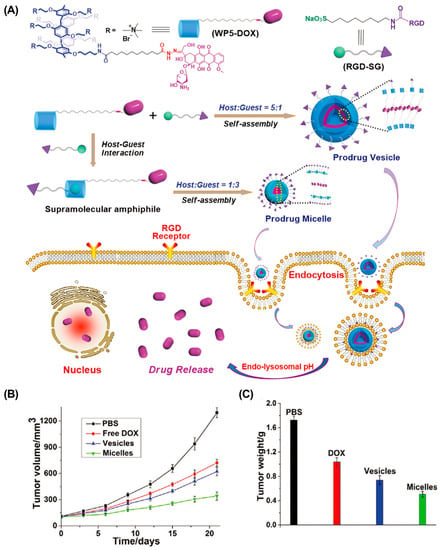
Figure 3.
RGD-modified size-controllable supramolecular nanocarriers for targeted drug delivery. (A) Schematic illustration of the construction of supramolecular vesicles and micelles. (B) Tumor volume and (C) tumor weight of different treated groups. Reproduced from ref. [88]. Copyright 2018 Wiley-VCH.
4.2. Enhancing Tumor Penetration
Although various strategies effectively enhance drug accumulation in tumors, their clinical benefits are still unsatisfactory. The inherent properties of solid tumors, including the dense extracellular matrix and high IFP, make it difficult for large-sized nanoparticles to penetrate deeply into the tumor tissues [90,91]. Therefore, constructing small-sized nanoparticles is a promising strategy to enhance tumor penetration. However, small-sized nanoparticles may undergo rapid clearance by the reticuloendothelial system (RES) during blood circulation [92]. Recently, several size-adjustable supramolecular-macrocycle-based drug delivery systems have been developed to solve this dilemma [93,94,95]. These nanoparticles usually have a relatively large size during blood circulation to ensure efficient tumor accumulation. Upon reaching tumor tissues, they transform into smaller nanoparticles, allowing for efficient tumor penetration. For example, Xu et al. constructed a size-convertible supramolecular nanocomponent (DCD SNs) for efficient tumor penetration (Figure 4) [96]. DCD SNs (126 nm) were prepared by self-assembly of β-CD-modified polyhydroxy dextran (DA-CD) and DOX-modified polyhydroxy dextran (DA-DOX). Upon reaching acidic TME, the DCD SNs dissociate into smaller particles (~30 nm), effectively penetrating deeply into the tumor to enhance chemotherapeutic efficacy. Similarly, Guo et al. constructed a size-switchable supramolecular self-assembly for DOX delivery via host–guest interactions between WP5 and polyethylene glycol-modified aniline tetramer (TAPEG) [97]. They achieved significantly enhanced tumor suppression in a CT26 colon cancer mouse model.
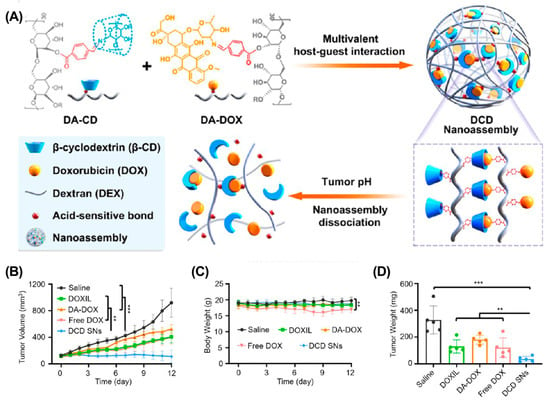
Figure 4.
DCD SN for deep tumor penetration to enhance cancer therapy. (A) Schematic illustration of the construction of DCD SNs. (B) Tumor growth curves, (C) body weight, and (D) tumor weight after different treatments. (** p < 0.01, *** p < 0.001). Reproduced from ref. [96]. Copyright 2021 American Chemical Society.
5. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Photodynamic Therapy
Photodynamic therapy (PDT), as a non-invasive, highly selective, and controllable strategy, has attracted more and more attention in cancer treatment [98]. PDT requires three essential elements: light, oxygen, and photosensitizers (PS). PDT employs PS to absorb light, transfer energy to the surrounding oxygen, and generate cytotoxic ROS to destroy proteins, DNA, and lipids in tumor cells, and also induce apoptosis [99]. Although PDT has made significant progress in clinical practice, it suffers from several limitations: (1) the inherent limitations of PS: for example, the aggregation-caused quenching (ACQ) effect and dark toxicity; (2) insufficient oxygen (O2) supply: oxygen, an important PDT element, provides the raw material for ROS generation [100]. However, abnormal neovascularization may lead to insufficient O2 supply, resulting in limited ROS generation and tumor suppression [101]. The rapid development of supramolecular-macrocycle-based nanomaterials offers great opportunities to address these issues in PDT. For example, these nanomaterials can efficiently improve PS’s water solubility and stability, thereby effectively avoiding the ACQ effect and dark toxicity [102,103]. In addition, supramolecular-macrocycle-based nanomaterials can carry O2 or O2-generation agents, thereby improving O2 supply and ROS generation. This section will discuss the recent advances in supramolecular-macrocycle-based nanomaterials for PDT.
5.1. Overcome the Inherent Defects of PS
The ACQ effect and dark toxicity are two defects of PS that restrict the general application of PDT. The following describes the application of supramolecular-macrocycle-based nanomaterials in overcoming these limitations for enhanced PDT.
ACQ effect. ACQ refers to the unique phenomenon in which a fluorophore is highly luminescent in the solution state, but becomes weak or non-luminescent in the aggregated state [104]. However, most PSs are hydrophobic and easily aggregated in an aqueous solution, significantly reducing singlet oxygen generation efficiency [105,106]. Therefore, increasing the water solubility of PS to avoid aggregation is a promising strategy to overcome the ACQ effect of PS and enhance the antitumor efficacy of PDT. For example, Zhang et al. reported a linear supramolecular polymer based on the host–guest interaction between β-CD and AD-modified porphyrin (Figure 5) [107]. The unique linear structure increases the water solubility of the porphyrin, and the introduction of β-CD increases the steric hindrance between PSs, which significantly inhibits the aggregation of the porphyrin and improves the efficacy of PDT. Similarly, introducing β-CD to tetraphenyl porphyrin also overcame the ACQ effect and greatly enhanced tumor suppression in a 4T1 breast cancer mouse model [108].
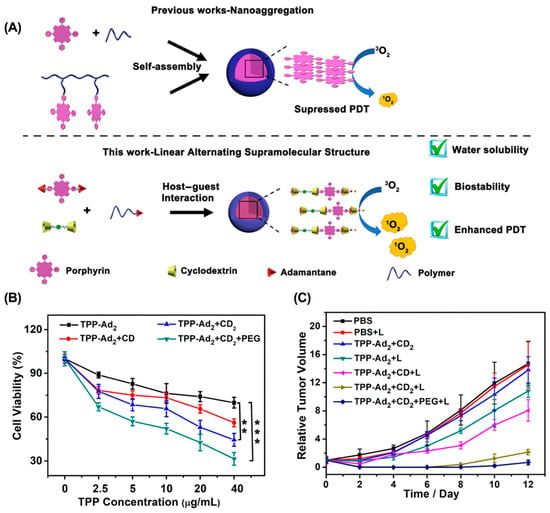
Figure 5.
Linear supramolecular polymer for overcoming the ACQ effect to enhance PDT. (A) Schematic for the preparation of supramolecular nanoparticles from linear supramolecular polymer. (B) In vitro phototoxicity toward 4T1 cells. (C) Tumor weight of 4T1 tumor-bearing mice after different treatments. (** p < 0.01, *** p < 0.005). Reproduced from ref. [107]. Copyright 2020 American Chemical Society.
Dark toxicity. Dark toxicity refers to the inherent toxicity of PS without irradiation. The in vivo dark toxicity of PSs is mainly associated with the non-specificity biodistribution of PSs [109,110]. Therefore, optimizing the biodistribution of PSs is a feasible strategy to address this issue. For example, Guo et al. developed an ATP-responsive supramolecular assembly (GC5A-12C) for enhanced PDT (Figure 6) [111]. During blood circulation, the PS was loaded into the cavity of GC5A-12C to avoid undesired dark toxicity, and was competed out by overexpressed ATP as tumor tissues were reached. As a result, GC5A-12C achieved a significantly enhanced antitumor effect in the 4T1 breast cancer mouse model without causing noticeable toxic effects. Similarly, Tang et al. reported a supramolecular PDT system based on the host–guest interaction between p-sulfonatocalix(4)arene and pyridinium-functionalized tetra phenylethylene, which effectively avoided the dark toxicity of PS and significantly suppressed tumor growth in a 4T1 breast cancer mouse model [112].
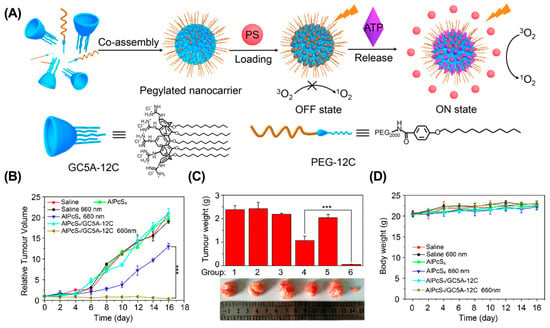
Figure 6.
ATP-responsive supramolecular assembly for enhanced PDT. (A) Schematic illustration of the preparation of GC5A-12C nanocarrier. The tumor volume (B), tumor weight (C), and body weight (D) in different groups. (*** p < 0.001). Reproduced from ref. [111]. Copyright 2018 American Chemical Society.
5.2. Alleviating Tumor Hypoxia
Oxygen, a critical PDT element, is essential for ROS generation and tumor ablation. However, abnormal proliferation of tumor cells and dysfunctional angiogenesis lead to the formation of a hypoxic microenvironment that significantly limits the efficacy of PDT [70,113]. Recent studies have shown that several types of nanomaterials can interact with, store, and release O2 in a controlled manner at tumor sites [113,114,115]. Integrating these nanomaterials into the supramolecular system is a promising strategy to alleviate tumor hypoxia for enhanced PDT. For example, metal–organic framework (MOF) is a porous nanomaterial that can adsorb and transport oxygen. Taking advantage of MOF, Pei et al. reported a zeolitic imidazolate frameworks-8 (ZIF-8)-based supramolecular system (OCZWM) for enhanced PDT (Figure 7) [116]. The OCZWM was formed by coordinating the host–guest complex formed by WP6 and methylene blue (MB) with ZIF-8. After internalization, the OCZWM efficiently decomposes to release loaded MB and O2 in response to acid. As a result, OCZWM effectively alleviated tumor hypoxia and significantly enhanced the anti-tumor efficacy of PDT.
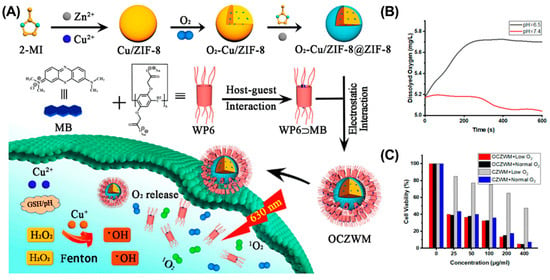
Figure 7.
OCZWM for alleviating tumor hypoxia to enhance PDT therapy. (A) Schematic illustration of the preparation of OCZWM. (B) The O2 content under the OCZWM (pH = 6.5 and 7.4). (C) Cell viability of HepG2 cells after treatments. Reproduced from ref. [116]. Copyright 2021 Licensee MDPI, Basel, Switzerland.
Although delivering O2 directly to the tumor can effectively increase the O2 content, O2 leakage limits its effectiveness. In situ oxygen generation is an alternative strategy to alleviate tumor hypoxia [117]. For example, Tian et al. reported ruthenium (II)-coordinated supramolecular complexes (RuSMDC) for in situ oxygen production to alleviate tumor hypoxia (Figure 8) [118]. RuSMDC was constructed by host–guest complexing of WP5 with Ru metal complexes. After internalization, Ru catalyzed hydrogen peroxide to generate O2, providing sufficient raw materials for ROS generation. In vitro and in vivo experiments showed that RuSMDC effectively alleviated tumor hypoxia and enhanced the efficacy of PDT. Furthermore, the Fenton reaction can also be used to generate O2 [119]. For example, Gao et al. reported the β-CD and Ce6-modified Cu2–xSe nanoparticles (CS–CD–Ce6 NPs) to alleviate tumor hypoxia via the Fenton reaction [120]. In tumor tissue, Cu+ ions effectively decomposed H2O2 to O2 and •OH, increasing the O2 concentration and providing substrates for Ce6 to generate ROS. As a result, the CS–CD–Ce6 NPs effectively alleviated tumor hypoxia and improved the antitumor effect of PDT in a 4T1 cancer mouse model. Similarly, Lu et al. constructed a supramolecular micelle by host–guest complexing β-CD with ferrocene (Fc) [121]. In tumor tissue, Fc is oxidized to Fc+ and catalyzes O2 generation, thereby significantly alleviating tumor hypoxia and enhancing PDT therapy.
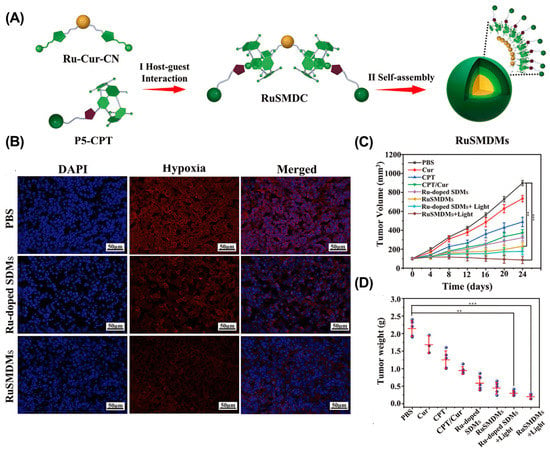
Figure 8.
RuSMDC for in situ oxygen supply to enhance PDT therapy. (A) Schematic illustration of the preparation of RuSMDC. (B) Immunofluorescence imaging of tumor tissue. (C) Tumor growth curves. (D) Tumor weight in different groups. (** p < 0.005; *** p < 0.001). Reproduced from ref. [118]. Copyright 2021 Wiley-VCH GmbH.
6. Supramolecular-Macrocycle-Based Nanomaterials for Enhanced Combination Therapy
Cancer is one of the most malignant diseases worldwide, involving various genetic alterations and cellular abnormalities and posing significant challenges to traditional monotherapy [122,123]. Common issues associated with monotherapy include low response rate, drug resistance, and side effects [124]. A combination therapy that employs two or more anticancer drugs is a promising strategy to solve these problems. Generally, conventional “cocktail” therapies were achieved by co-administrating multiple drugs [125]. However, different physicochemical properties of these drugs may lead to different pharmacokinetics (PK) and biodistribution in vivo, resulting in limited synergistic effects. The development of the drug delivery system offers great potential to solve these problems by co-loading multiple drugs within a single nanocarrier [126,127]. With this strategy, the PK and biodistribution of combined drugs can be effectively unified, ensuring the synergism of multiple drugs in tumor tissues. In this chapter, we will first introduce the rationale for combination therapies and then discuss the recent advances in supramolecular-macrocycle-based nanomaterials for enhanced combination therapy.
6.1. Rationales for Combination Therapy
Combination therapy is meaningful if it produces better clinical outcomes than a single drug [128]. To this end, the choice of drugs for combination therapy is the priority to be considered, which should be based on the following general principles: (1) the selected drugs should have non-overlapping toxicities so that they can be administered at near-maximal doses; (2) the selected drugs should have different mechanisms of action and minimal cross-resistance to avoid the development of multidrug resistance (MDR) [129]; (3) the selected drugs should be demonstrated to have synergistic or additive anticancer efficacy relative to single drugs, which is currently quantified by the Chou–Talalay method. In the Chou–Talalay method, the synergistic effect of drug combinations is usually expressed in terms of combination index (CI), in which CI < 0.9 represents synergism, 0.9 < CI < 1.1 represents additive effect, and CI > 1.1 represents antagonism [130].
6.2. Supramolecular-Macrocycle-Based Combination Therapy
Combination therapy can be roughly divided into three categories according to the molecule nature of anticancer therapeutics, including small molecule–small molecule, small molecule–biologics, and biologics–biologics. This chapter mainly introduces the recent progress of supramolecular-macrocycle-based nanomaterials in small molecule-small molecule combination therapy.
Chemo-Chemotherapy. In combination chemotherapy, the synergistic effect mainly depends on the concentration relationship, in which a certain proportion of the drug combination produces synergistic effects, while other proportions may be additive or antagonistic [131]. Therefore, ratiometric co-delivery of drug combinations in an optimized molar ratio to tumor tissues is essential for effective combination chemotherapy [132]. Macrocyclic molecules with defined chemical structures, predetermined cavity size, and characteristic binding affinity are ideal candidates for precise multidrug loading and ratiometric co-delivery. For example, our groups presented calixarene-modified albumin (CaMA) for the ratiometric delivery of multiple drugs in combination chemotherapy (Figure 9) [133]. Multiple hypoxia-responsive calixarenes (sulfonate azocalix(4)arene, SAC4A) were integrated into one albumin, which ensured the precise load and ratiometric co-delivery of multiple drugs to tumors via host–guest interaction. As it reached the hypoxic tumor microenvironment, SAC4A was reduced by reductase, leading to the spontaneous release of the drugs. By taking DOX and mitomycin C (MMC) as an example, delivering this drug combination at the optimal ratio via CaMA significantly enhanced tumor suppression compared to conventional cocktail therapy. Similarly, we developed a macrocyclic-amphiphile-based self-assembled nanoparticle and achieved the ratiometric co-delivery of PTX and NLG919 to tumor tissues [77].
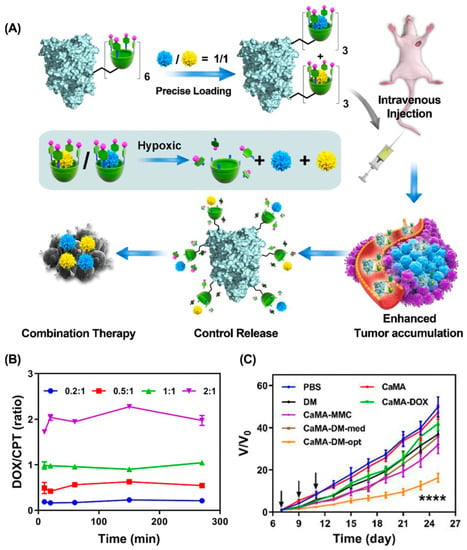
Figure 9.
Calixarene-modified albumin for the ratiometric delivery of multiple drugs in combination chemotherapy. (A) Schematic illustrations of CaMA for combination chemotherapy. (B) The molar ratios of DOX to CPT released from CaMA-DC at different time points. (C) The therapeutic effect of CaMA-DM-opt on 4T1-bearing mice. (**** p < 0.0001). Reproduced from ref. [133]. Copyright 2022 Ivyspring International Publisher.
Chemo-photodynamic therapy. Chemo-photodynamic therapy is a promising combination therapy strategy for cancer treatment, since (1) PDT usually has non-overlapping toxicity with chemotherapy; (2) PDT can overcome MDR by reducing the expression of P-gp to reduce drug efflux; and (3) ROS generated by PDT can be used as a stimulus to promote the drug’s release from the nanocarrier [134]. Loading chemotherapeutic agents and PS into nanocarriers is the most common method for combining these two therapies. For example, Wang et al. presented a supramolecular micelle to co-deliver Chlorin e6 (Ce6) and banoxantrone (AQ4N) for chemo-photodynamic therapy (Figure 10) [135]. Ce6 and AQ4N were loaded into the cavity of cucurbit(7)uril (CB(7)) and were precisely released in tumor tissues in response to overexpressed spermine. After irradiation, the hypoxic environment activated the prodrug AQ4N to chemotherapeutic AQ4, enabling synergistic chemo-photodynamic therapy. In another example, Xu et al. designed a ROS-responsive cyclodextrin-based polymeric micelle to co-deliver DOX and purpurin 18 (P18) [136]. After irradiation with a near-infrared laser, P18 in polymer micelles generated ROS, promoting DOX release and tumor cell apoptosis. Both in vitro and in vivo studies suggested that this strategy provided an effective method for the combination of chemotherapy and photodynamic therapy. Similarly, Yao et al. fabricated a WP6-based supramolecular polypeptide nanomedicine (BPC/DOX-ICG) for the co-delivery of DOX and indocyanine green (ICG), resulting in enhanced antitumor efficacy in an MCF-7/ADR tumor mouse model [137].
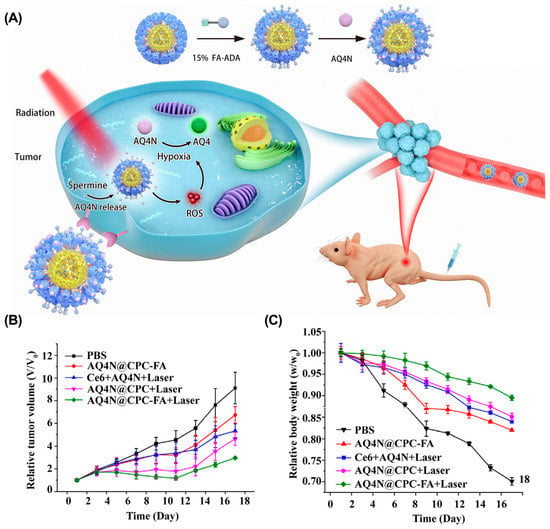
Figure 10.
Supramolecular micelle for synergistic chemo-photodynamic therapy. (A) Schematic illustrations of AQ4N@CPC-FA for chemo-photodynamic therapy. (B) The tumor volume and the relative body weight (C) of mice after different treatments. Reproduced from ref. [135]. Copyright 2021 Elsevier.
Photothermal immunotherapy. Photothermal therapy (PTT) is another promising cancer treatment strategy with the advantages of minimal invasiveness and high precision [138]. PTT utilizes photothermal reagents to absorb light, convert the light energy into local hyperthermia, disrupt cell membrane integrity, and induce tumor cell apoptosis or necrosis. In addition, PTT also promotes the release of damage-associated molecular patterns (DAMPs), including calreticulin (CRT), high mobility group box 1 (HMGB-1), and adenosine triphosphate (ATP), thus effectively triggering immunogenic cell death (ICD) and eliciting an antitumor immune response [139,140]. With this unique property, supramolecular-macrocycle-based nanomaterials are widely used in photothermal immunotherapy [141]. Nanogold is an ideal photothermal reagent with high photothermal conversion efficiency. For example, Yan et al. integrated gold nanoparticles into a supramolecular system and reported a gold nanorod-based nanosystem (GNR) for enhanced photothermal immunotherapy [142]. GNR was formed by layer assembly of γ-CD-crosslinked PEI on the surface of gold nanorods, and then the N6-methyladenosine demethylase inhibitor meclofenamic acid (MA) was loaded into the cavities of γ-CD to form GNR-CDP8MA. As it reached tumor tissues, the NIR-II induced the release of MA from γ-CD of GNR-CDP8MA, thus effectively decreasing the stability of PD-L1 transcripts. As a result, GNR-CDP8MA achieved effective photothermal immunotherapy in prostate cancer tumor-bearing (RM-1) mice. Similarly, Ping et al. reported a novel gold nanorod-based supramolecular nanomaterial (ANP/HSP) to disrupt the programming death-1/programming death ligand-1 pathway (PD-1/PD-L1) for enhanced photothermal immunotherapy [143]. The disruption of the PD-1/PD-L1 pathway synergized with PTT-induced ICD, resulting in significantly enhanced antitumor efficacy in B16F10 melanoma mice.
6.3. Supramolecular-Macrocycle-Based Combination Therapy in Overcoming Drug Resistance
Multidrug resistance (MDR) refers to the ability of cancer cells to develop resistance to other anticancer drugs while being induced by a single anticancer drug during cancer therapy [144]. MDR is mainly associated with the overexpression of channel proteins on the cell membrane, such as p-glycoprotein (P-gp) [145]. As a typical ATP energy-dependent efflux pump, P-gp can efflux anticancer drugs to the extracellular TME, thereby reducing the antitumor efficacy of chemotherapy [146,147,148]. Therefore, co-delivering P-gp inhibitors with anticancer drugs is a promising strategy to reverse MDR. For example, Tian et al. developed a supramolecular assembly (SDDNMs) to reverse MDR based on the host–guest interactions between curcumin-modified β-CD and ferrocene-CPT [149]. After internalization, high levels of H2O2 efficiently disrupted host–guest complexation, leading to the rapid release of CPT and curcumin derivatives. As a result, SDDNMs effectively reversed MDR and greatly enhanced the antitumor efficacy of CPT in a B16F10 melanoma mouse model. In addition to p-gp inhibitors, low levels of nitrogen monoxide (NO) can down-regulate p-gp expression, reduce drug efflux, and alleviate MDR. Yao et al. constructed a supramolecular polypeptide nanomedicine to co-deliver ICG, DOX, and S-nitrosothiol (SNO, NO donors) for enhanced combination cancer therapy (Figure 11) [137]. After internalization, the nanomedicine disintegrated in response to acid and released the ICG and DOX. Upon irradiation, S-nitrosothiol efficiently generates NO due to PTT-induced hyperthermia. As a result, this strategy effectively reversed MDR and suppressed tumor growth in an MCF-7/ADR breast mouse model. In addition, cutting off the energy supply to P-gp is another strategy to reverse MDR [150]. Wu et al. reported a supramolecular assembly based on the host–guest complexation of β-CD with 1-adamantaneacetic acid to co-deliver DOX and adjudin (ADD), which is a mitochondrial inhibitor [151]. The controlled and sustained release of DOX and ADD in response to internal tumor acidity inhibited mitochondrial function and reduced ATP synthesis, thereby reducing the efficiency of P-gp.
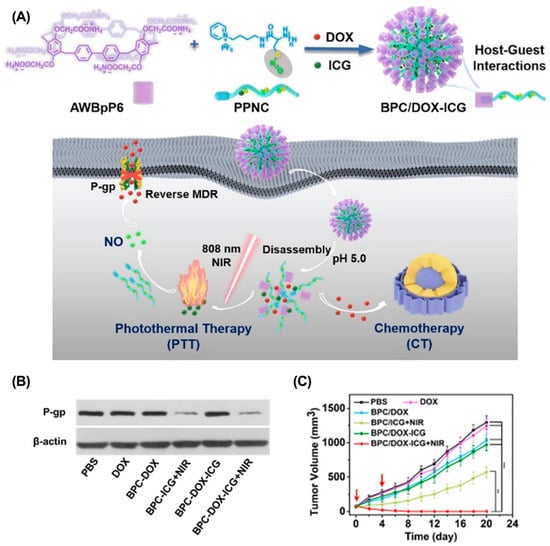
Figure 11.
Supramolecular polypeptide nanomedicine for enhanced combination cancer therapy to overcome MDR. (A) Schematic illustration of the preparation of supramolecular polypeptide nanomedicine for combination cancer therapy. (B) Western blot analyses of the expressions of P-pg. (C) The tumor volume in each treatment group. Reproduced from ref. [137]. Copyright 2022 The Royal Society of Chemistry.

Table 1.
Advanced supramolecular-macrocycle-based nanomaterials in cancer treatment.
Table 1.
Advanced supramolecular-macrocycle-based nanomaterials in cancer treatment.
| Types | Macrocycles | Formulations | Payloads | Tumor | Refs |
| Chemotherapy | WP5 | Supramolecular vesicles | DOX | MCF-7 | [86] |
| Chemotherapy | WP5 | Supramolecular vesicles | DOX | HpeG2 | [87] |
| Chemotherapy | WP5 | Vesicles or micelles | DOX | HpeG2 | [88] |
| Chemotherapy | β-CD | LbL films | DOX | A549 | [89] |
| Chemotherapy | β-CD | Supramolecular nanoparticles | DOX | 4T1 | [90] |
| Chemotherapy | WP5 | Supramolecular nanoparticles | DOX | CT26 | [96] |
| PDT | β-CD | Supramolecular nanoparticles | TPP | 4T1 | [107] |
| PDT | β-CD | Supramolecular organic framework | TPP | 4T1 | [108] |
| PDT | GC5A-12C | Supramolecular nanoparticles | AlPcS4 | 4T1 | [111] |
| PDT | SC4A | Supramolecular nanoparticles | TPE-PHO | 4T1 | [112] |
| PDT | WP6 | Supramolecular photosensitizer system | MB | HepG2 | [116] |
| PDT | WP5 | Supramolecular metallodrug micelles | Curcumin | B16 | [118] |
| PDT | β-CD | Supramolecular nanoparticles | Ce6 | 4T1 | [119] |
| PDT | β-CD | Supramolecular vesicles | Ce6 | 4T1 | [121] |
| Chemo-chemotherapy | SAC4A | Calixarene-modified albumin | DOX and MMC | 4T1 | [133] |
| Chemo-chemotherapy | QAAC4A-12C | Supramolecular nanoparticles | PTX and NLG919 | 4T1 | [77] |
| Chemo-photodynamic therapy | CB(7) | Supramolecular vesicles | Ce6 and AQ4N | MCF-7 | [135] |
| Chemo-photodynamic therapy | β-CD | Supramolecular micelles | DOX and P18 | 4T1 | [136] |
| Chemo-photodynamic therapy | WP6 | Supramolecular polypeptide nanomedicine | DOX and ICG | MCF-7/ADR | [137] |
| Chemo-chemotherapy | β-CD | Supramolecular micelles | CPT and Cur | B16 | [149] |
| Combination therapy | AWBpP6 | Supramolecular polypeptide nanomedicine | DOX and SNO | MCF-7/ADR | [137] |
| Chemo-chemotherapy | β-CD | Supramolecular nanoparticles | DOX and ADD | MCF-7/ADR | [151] |
| Photothermal immunotherapy | γ-CD | Gold nanorod-based supramolecular nanomaterial | MA | RM-1 | [142] |
| Photothermal immunotherapy | β-CD | Gold nanorod-based supramolecular nanomaterial | HSP-Cas9 plasmid | B16F10 | [143] |
7. Conclusions and Perspectives
With the rapid development of supramolecular chemistry, supramolecular macrocycle-based nanomaterials with diverse functions have shown great potential in biomedical applications. Because of their dynamic, reversible host–guest interactions and sensitivity to abnormal tumor indicators (acidic, redox, ATP, and hypoxic), supramolecular-macrocycle-based nanomaterials have been widely explored in chemotherapy, photodynamic therapy, and combination therapy for cancer treatment. Despite the remarkable success, the clinical benefits of supramolecular-macrocycle-based nanomaterials still face many obstacles, including (1) the introduction of chemical groups to modify the host or guest molecules is often accompanied by complex chemical synthesis and purification processes, and the resulting new host or guest molecules may have toxic effects; (2) dynamic and weak non-covalent interactions may lead to leakage of immature drug into the blood circulation, resulting in poor therapeutic efficacy and severe side effects; (3) the self-assembled nature of supramolecular-macrocycle-based nanomaterials may result in batch-to-batch variability.
For the better development of supramolecular-macrocycle-based nanomaterials in biomedical applications, the following aspects need to be considered in the future: (1) the potential toxicity of the introduced modification groups should be thoroughly analyzed to avoid unnecessary toxicity; (2) new supramolecular hosts with better biocompatibility should be developed; (3) diagnostic and imaging capabilities of supramolecular nano-systems should be enhanced for more accurate therapy; and (4) the assembly and preparation process should be optimized to reduce batch variance.
Author Contributions
Conceptualization, Z.P., Z.Z. and Y.L.; writing—original draft preparation, Z.P., X.Z. and Q.L.; writing—review and editing, Z.Z. and Y.L.; supervision, Y.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Programs of China (2018YFA0209700), Fundamental Research Funds for the Central Universities (Nankai University, 63206015), National Natural Science Foundation of China (Grant Nos. 22077073, 52203172), Frontiers Science Center for New Organic Matter (Nankai University, 63181206).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, T.; Zimmerman, S.C.; Nakashima, S. A highly stable quadruply hydrogen-bonded heterocomplex useful for supramolecular polymer blends. J. Am. Chem. Soc. 2005, 127, 6520–6521. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Rybtchinski, B. Adaptive supramolecular nanomaterials based on strong noncovalent interactions. ACS Nano 2011, 5, 6791–6818. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th anniversary article: Reversible and adaptive functional supramolecular materials: “noncovalent interaction” matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.D.; Barooah, N.; Aswal, V.K.; Pal, H.; Bhasikuttan, A.C.; Mohanty, J. Stimuli-responsive supramolecular micellar assemblies of cetylpyridinium chloride with cucurbit[5/7]urils. Soft Matter 2014, 10, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host-guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Lee, S.F.; Zhu, X.M.; Wang, Y.X.; Xuan, S.H.; You, Q.; Chan, W.H.; Wong, C.H.; Wang, F.; Yu, J.C.; Cheng, C.H.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Structural diversification of pillar[n]arene macrocycles. Angew. Chem. Int. Ed. 2020, 59, 6314–6316. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, W.; Liu, Y. Macrocyclic supramolecular assemblies based on hyaluronic acid and their biological applications. Acc. Chem. Res. 2022, 55, 3417–3429. [Google Scholar] [CrossRef]
- Hettiarachchi, G.; Nguyen, D.; Wu, J.; Lucas, D.; Ma, D.; Isaacs, L.; Briken, V. Toxicology and drug delivery by cucurbit[n]uril type molecular containers. PLoS ONE 2010, 5, e10514. [Google Scholar] [CrossRef]
- Shangguan, L.; Chen, Q.; Shi, B.; Huang, F. Enhancing the solubility and bioactivity of anticancer drug tamoxifen by water-soluble pillar[6]arene-based host-guest complexation. Chem. Commun. 2017, 53, 9749–9752. [Google Scholar] [CrossRef] [PubMed]
- Shetty, D.; Skorjanc, T.; Olson, M.A.; Trabolsi, A. Self-assembly of stimuli-responsive imine-linked calix[4]arene nanocapsules for targeted camptothecin delivery. Chem. Commun. 2019, 55, 8876–8879. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, Y.; Huang, Z.; Xu, J.F.; Sun, Z.; Zhang, X. Supramolecular chemotherapy: Carboxylated pillar[6]arene for decreasing cytotoxicity of oxaliplatin to normal cells and improving its anticancer bioactivity against colorectal cancer. ACS Appl. Mater. Interfaces 2018, 10, 5365–5372. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, J.W.; Liu, J.D.; Zhao, Z.M.; Song, Y.J. Design, preparation, and characterization of novel calix[4]arene bioactive carrier for antitumor drug delivery. Front. Chem. 2019, 7, 732. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, X.; Zhang, Z.; Han, C.; Mao, Z.; Gao, C.; Huang, F. Pillar[6]arene/paraquat molecular recognition in water: High binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J. Am. Chem. Soc. 2012, 134, 19489–19497. [Google Scholar] [CrossRef]
- Petronella, F.; De Biase, D.; Zaccagnini, F.; Verrina, V.; Lim, S.I.; Jeong, K.U.; Miglietta, S.; Petrozza, V.; Scognamiglio, V.; Godman, N.P.; et al. Label-free and reusable antibody-functionalized gold nanorod arrays for the rapid detection of Escherichia coli cells in a water dispersion. Environ. Sci. Nano 2022, 9, 3343–3360. [Google Scholar] [CrossRef]
- Montanarella, F.; Kovalenko, M.V. Three millennia of nanocrystals. ACS Nano 2022, 16, 5085–5102. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem. Soc. Rev. 2010, 39, 495–505. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug. Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Möller, N.; Ravoo, B.J. Stimulus-responsive assembly of nanoparticles using host-guest interactions of cyclodextrins. Chem. Eur. J. 2018, 24, 4741–4748. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Host-guest interactions mediated nano-assemblies using cyclodextrin-containing hydrophilic polymers and their biomedical applications. Nano Today 2010, 5, 337–350. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-based supramolecular nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Pan, Y.C.; Hu, X.Y.; Guo, D.S. Biomedical applications of calixarenes: State of the art and perspectives. Angew. Chem. Int. Ed. 2021, 60, 2768–2794. [Google Scholar] [CrossRef]
- Giuliani, M.; Morbioli, I.; Sansone, F.; Casnati, A. Correction: Moulding calixarenes for biomacromolecule targeting. Chem. Commun. 2015, 51, 15208. [Google Scholar] [CrossRef]
- Naseer, M.M.; Ahmed, M.; Hameed, S. Functionalized calix[4]arenes as potential therapeutic agents. Chem. Biol. Drug Des. 2017, 89, 243–256. [Google Scholar] [CrossRef]
- Perret, F.; Lazar, A.N.; Coleman, A.W. Biochemistry of the para-sulfonato-calix[n]arenes. Chem. Commun. 2006, 21, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.H.; Zhao, Y. Efficient synthesis of water-soluble calixarenes using click chemistry. Org. Lett. 2005, 7, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.S.; Liu, Y. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Kong, Y.; Zheng, Z.; Geng, W.C.; Zhao, Z.Y.; Sun, H.; Guo, D.S. Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response. Beilstein J. Org. Chem. 2019, 15, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Liu, S.; Zavalij, P.Y.; Isaacs, L. Cucurbit[10]uril. J. Am. Chem. Soc. 2005, 127, 16798–16799. [Google Scholar] [CrossRef]
- Moghaddam, S.; Yang, C.; Rekharsky, M.; Ko, Y.H.; Kim, K.; Inoue, Y.; Gilson, M.K. New ultrahigh affinity host-guest complexes of cucurbit[7]uril with bicyclo[2.2.2]octane and adamantane guests: Thermodynamic analysis and evaluation of M2 affinity calculations. J. Am. Chem. Soc. 2011, 133, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Samal, S.; Selvapalam, N.; Kim, H.J.; Kim, K. Cucurbituril homologues and derivatives: New opportunities in supramolecular chemistry. Acc. Chem. Res. 2003, 36, 621–630. [Google Scholar] [CrossRef]
- Jon, S.Y.; Selvapalam, N.; Oh, D.H.; Kang, J.K.; Kim, S.Y.; Jeon, Y.J.; Lee, J.W.; Kim, K. Facile synthesis of cucurbit[n]uril derivatives via direct functionalization: Expanding utilization of cucurbit[n]uril. J. Am. Chem. Soc. 2003, 125, 10186–10187. [Google Scholar] [CrossRef]
- Sasmal, S.; Sinha, M.K.; Keinan, E. Facile purification of rare cucurbiturils by affinity chromatography. Org. Lett. 2004, 6, 1225–1228. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Li, C.; Shu, X.; Li, J.; Chen, S.; Han, K.; Xu, M.; Hu, B.; Yu, Y.; Jia, X. Complexation of 1,4-bis(pyridinium)butanes by negatively charged carboxylatopillar[5]arene. J. Org. Chem. 2011, 76, 8458–8465. [Google Scholar] [CrossRef]
- Yu, G.; Xue, M.; Zhang, Z.; Li, J.; Han, C.; Huang, F. A water-soluble pillar[6]arene: Synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J. Am. Chem. Soc. 2012, 134, 13248–13251. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene host-guest complexation induced chirality amplification: A new way to detect cryptochiral compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef]
- Xia, W.; Hu, X.Y.; Chen, Y.; Lin, C.; Wang, L. A novel redox-responsive pillar[6]arene-based inclusion complex with a ferrocenium guest. Chem. Commun. 2013, 49, 5085–5087. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.Y.; Li, Y.; Zou, X.; Xiong, S.; Lin, C.; Shen, Y.Z.; Wang, L. Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, X.; Chen, D.; Yan, H.; Li, X.; Du, X. Supramolecular vesicles coassembled from disulfide-linked benzimidazolium amphiphiles and carboxylate-substituted pillar[6]arenes that are responsive to five stimuli. Angew. Chem. Int. Ed. 2017, 56, 2655–2659. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Yang, Y.; Zhang, Y.H.; Liu, Y. Polysaccharide nanoparticles for efficient siRNA targeting in cancer cells by supramolecular pKa shift. Sci. Rep. 2016, 6, 28848. [Google Scholar] [CrossRef]
- Kaizerman-Kane, D.; Hadar, M.; Tal, N.; Dobrovetsky, R.; Zafrani, Y.; Cohen, Y. pH-Responsive pillar[6]arene-based water-soluble supramolecular hexagonal boxes. Angew. Chem. Int. Ed. 2019, 58, 5302–5306. [Google Scholar] [CrossRef]
- Dan, Z.; Cao, H.; He, X.; Zhang, Z.; Zou, L.; Zeng, L.; Xu, Y.; Yin, Q.; Xu, M.; Zhong, D.; et al. A pH-responsive host-guest nanosystem loading succinobucol suppresses lung metastasis of breast cancer. Theranostics 2016, 6, 435–445. [Google Scholar] [CrossRef]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, X.; Zhang, Y.M.; Li, N.; Liu, Y. Supramolecular nanoparticles based on β-CD modified hyaluronic acid for DNA encapsulation and controlled release. Chem. Commun. 2018, 54, 8713–8716. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. Mitochondria-targeting nanomedicine self-assembled from GSH-responsive paclitaxel-ss-berberine conjugate for synergetic cancer treatment with enhanced cytotoxicity. J. Control. Release 2020, 318, 38–49. [Google Scholar] [CrossRef]
- Kang, Y.; Ju, X.; Ding, L.S.; Zhang, S.; Li, B.J. Reactive oxygen species and glutathione dual redox-responsive supramolecular assemblies with controllable release capability. ACS Appl. Mater. Interfaces 2017, 9, 4475–4484. [Google Scholar] [CrossRef]
- Liu, X.; Shao, W.; Zheng, Y.; Yao, C.; Peng, L.; Zhang, D.; Hu, X.Y.; Wang, L. GSH-Responsive supramolecular nanoparticles constructed by β-d-galactose-modified pillar[5]arene and camptothecin prodrug for targeted anticancer drug delivery. Chem. Commun. 2017, 53, 8596–8599. [Google Scholar] [CrossRef]
- Hu, J.; Liang, M.; Ye, M.; Xu, J.; Liu, H.; Zhang, X.; Sun, W.; Xue, P.; Kang, Y.; Xu, Z. Reduction-triggered polycyclodextrin supramolecular nanocage induces immunogenic cell death for improved chemotherapy. Carbohydr. Polym. 2023, 301, 120365. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: A redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. 2014, 53, 13126–13130. [Google Scholar] [CrossRef]
- Yasen, W.; Dong, R.; Zhou, L.; Wu, J.; Cao, C.; Aini, A.; Zhu, X. Synthesis of a cationic supramolecular block copolymer with covalent and noncovalent polymer blocks for gene delivery. ACS Appl. Mater. Interfaces 2017, 9, 9006–9014. [Google Scholar] [CrossRef]
- Lee, H.; Woo, S.M.; Jang, H.; Kang, M.; Kim, S.Y. Cancer depends on fatty acids for ATP production: A possible link between cancer and obesity. Semin. Cancer Biol. 2022, 86, 347–357. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, D.H.; Hong, J.I.; Yoon, J. Chemosensors for pyrophosphate. Acc. Chem. Res. 2009, 42, 23–31. [Google Scholar] [CrossRef]
- Fuentes, E.; Palomo, I. Extracellular ATP metabolism on vascular endothelial cells: A pathway with pro-thrombotic and anti-thrombotic molecules. Vasc. Pharmacol. 2015, 75, 1–6. [Google Scholar] [CrossRef]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- Xu, L.; Chai, J.; Wang, Y.; Zhao, X.; Guo, D.-S.; Shi, L.; Zhang, Z.; Liu, Y. Calixarene-integrated nano-drug delivery system for tumor-targeted delivery and tracking of anti-cancer drugs in vivo. Nano Res. 2022, 15, 7295–7303. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Zhao, L.; Zhang, Y.; Chen, L.; Ma, M.; Du, X.; Meng, Z.; Li, C.; Meng, Q. Supramolecular drug delivery system from macrocycle-based self-assembled amphiphiles for effective tumor therapy. ACS Appl. Mater. Interfaces 2021, 13, 53564–53573. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, J.; Shen, J.; Tang, G.; Huang, F. Cationic pillar[6]arene/ATP host-guest recognition: Selectivity, inhibition of ATP hydrolysis, and application in multidrug resistance treatment. Chem. Sci. 2016, 7, 4073–4078. [Google Scholar] [CrossRef]
- Zhang, T.X.; Hou, X.; Kong, Y.; Yang, F.; Yue, Y.X.; Shah, M.R.; Li, H.B.; Huang, F.; Liu, J.; Guo, D.S. A hypoxia-responsive supramolecular formulation for imaging-guided photothermal therapy. Theranostics 2022, 12, 396–409. [Google Scholar] [CrossRef]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew. Chem. Int. Ed. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Yao, S.Y.; Yue, Y.X.; Ying, A.K.; Hu, X.Y.; Li, H.B.; Cai, K.; Guo, D.S. An antitumor dual-responsive host-guest supramolecular polymer based on hypoxia-cleavable azocalix[4]arene. Angew. Chem. Int. Ed. 2023, 62, e202213578. [Google Scholar] [CrossRef]
- Li, J.J.; Hu, Y.; Hu, B.; Wang, W.; Xu, H.; Hu, X.Y.; Ding, F.; Li, H.B.; Wang, K.R.; Zhang, X.; et al. Lactose azocalixarene drug delivery system for the treatment of multidrug-resistant pseudomonas aeruginosa infected diabetic ulcer. Nat. Commun. 2022, 13, 6279. [Google Scholar] [CrossRef]
- Geng, W.C.; Jia, S.; Zheng, Z.; Li, Z.; Ding, D.; Guo, D.S. A noncovalent fluorescence turn-on strategy for hypoxia imaging. Angew. Chem. Int. Ed. 2019, 58, 2377–2381. [Google Scholar] [CrossRef]
- Zhang, T.X.; Zhang, Z.Z.; Yue, Y.X.; Hu, X.Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.S. A general hypoxia-responsive molecular container for tumor-targeted therapy. Adv. Mater. 2020, 32, e1908435. [Google Scholar] [CrossRef]
- Yue, Y.-X.; Zhang, Z.; Wang, Z.-H.; Ma, R.; Chen, M.-M.; Ding, F.; Li, H.-B.; Li, J.-J.; Shi, L.; Liu, Y.; et al. Promoting tumor accumulation of anticancer drugs by hierarchical carrying of exogenous and endogenous vehicles. Small Struct. 2022, 3, 2200067. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, Y.X.; Xu, L.; Wang, Y.; Geng, W.C.; Li, J.J.; Kong, X.L.; Zhao, X.; Zheng, Y.; Zhao, Y.; et al. Macrocyclic-amphiphile-based self-assembled nanoparticles for ratiometric delivery of therapeutic combinations to tumors. Adv. Mater. 2021, 33, e2007719. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, Z.; Sun, B.; Chen, Q.; Sun, J.; He, Z.; Luo, C. Nanotherapeutics for antimetastatic treatment. Trends Cancer 2020, 6, 645–659. [Google Scholar] [CrossRef]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Chen, B.; Dai, W.; Mei, D.; Liu, T.; Li, S.; He, B.; He, B.; Yuan, L.; Zhang, H.; Wang, X.; et al. Comprehensively priming the tumor microenvironment by cancer-associated fibroblast-targeted liposomes for combined therapy with cancer cell-targeted chemotherapeutic drug delivery system. J. Control. Release 2016, 241, 68–80. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X. Theoretical study of macrocyclic host molecules: From supramolecular recognition to self-assembly. Phys. Chem. Chem. Phys. 2022, 24, 19011–19028. [Google Scholar] [CrossRef] [PubMed]
- Mei, K.C.; Bai, J.; Lorrio, S.; Wang, J.T.; Al-Jamal, K.T. Investigating the effect of tumor vascularization on magnetic targeting in vivo using retrospective design of experiment. Biomaterials 2016, 106, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Liu, C.; Guo, M.; Cheng, H.; Lu, Y.; Jin, K.; Liu, L.; Long, J.; Xu, J.; Lu, R.; et al. Potential biomarkers in lewis negative patients with pancreatic cancer. Ann. Surg. 2017, 265, 800–805. [Google Scholar] [CrossRef]
- Kline, J.B.; Fernando, S.; Ross, E.N.; Grasso, L.; Nicolaides, N.C. Tumor-shed antigen CA125 blocks complement-mediated killing via suppression of C1q-antibody binding. Eur. J. Immunol. 2018, 48, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Babamiri, B.; Hallaj, R.; Salimi, A. Ultrasensitive electrochemiluminescence immunoassay for simultaneous determination of CA125 and CA15-3 tumor markers based on PAMAM-sulfanilic acid-Ru(bpy)(3)(2+) and PAMAM-CdTe@CdS nanocomposite. Biosens. Bioelectron. 2018, 99, 353–360. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, M.; Chen, Z.; Hu, X.; Pu, L.; Pei, Z.; Pei, Y. Tumor microenvironment responsive supramolecular glyco-nanovesicles based on diselenium-bridged pillar[5]arene dimer for targeted chemotherapy. Chem. Commun. 2020, 56, 10642–10645. [Google Scholar] [CrossRef]
- Lu, Y.; Hou, C.; Ren, J.; Yang, K.; Chang, Y.; Pei, Y.; Dong, H.; Pei, Z. A multifunctional supramolecular vesicle based on complex of cystamine dihydrochloride capped pillar[5]arene and galactose derivative for targeted drug delivery. Int. J. Nanomed. 2019, 14, 3525–3532. [Google Scholar] [CrossRef]
- Hu, X.Y.; Gao, L.; Mosel, S.; Ehlers, M.; Zellermann, E.; Jiang, H.; Knauer, S.K.; Wang, L.; Schmuck, C. From supramolecular vesicles to micelles: Controllable construction of tumor-targeting nanocarriers based on host-guest interaction between a pillar[5]arene-based prodrug and a RGD-sulfonate guest. Small 2018, 14, e1803952. [Google Scholar] [CrossRef]
- Huang, T.; Luan, X.; Xia, Q.; Pan, S.; An, Q.; Wu, Y.; Zhang, Y. Molecularly selective regulation of delivery fluxes by employing supramolecular interactions in layer-by-layer films. Chem. Asian J. 2018, 13, 1067–1073. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef]
- Li, Z.; Shan, X.; Chen, Z.; Gao, N.; Zeng, W.; Zeng, X.; Mei, L. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv. Sci. 2020, 8, 2002589. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Liu, Y.; Liu, X.; Su, K.; Shi, K. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm. Sin. B 2021, 11, 2265–2285. [Google Scholar] [CrossRef]
- Yan, X.; Ma, F.; Chen, Q.; Gou, X.; Li, X.; Zhang, L.; Gao, H. Construction of size-transformable supramolecular nano-platform against drug-resistant colorectal cancer caused by Fusobacterium nucleatum. Chem. Eng. J. 2022, 450, 137605. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface charge switchable supramolecular nanocarriers for nitric oxide synergistic photodynamic eradication of biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Q.; Yue, L.; Gao, C.; Wei, J.; Ding, Y.; Wang, Y.; Zheng, Y.; Wang, R. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz. 2021, 6, 907–912. [Google Scholar] [CrossRef]
- Liang, M.; Gao, Y.; Qiu, W.; Ye, M.; Hu, J.; Xu, J.; Xue, P.; Kang, Y.; Xu, Z. Acid-sensitive supramolecular nanoassemblies with multivalent interaction: Effective tumor retention and deep intratumor infiltration. ACS Appl. Mater. Interfaces 2021, 13, 37680–37692. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Sun, X.; Zhou, J.; Chen, X.; Xi, J.; Fan, L.; Han, J.; Guo, R. Supramolecular core-shell nanoassemblies with tumor microenvironment-triggered size and structure switch for improved photothermal therapy. Small 2022, 18, e2200588. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J., III; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Tang, X.L.; Wang, Z.; Zhu, Y.Y.; Xiao, H.; Xiao, Y.; Cui, S.; Lin, B.L.; Yang, K.; Liu, H.Y. Hypoxia-activated ROS burst liposomes boosted by local mild hyperthermia for photo/chemodynamic therapy. J. Control. Release 2020, 328, 100–111. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated fenton and fenton-like reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Yang, K.; Wen, J.; Chao, S.; Liu, J.; Yang, K.; Pei, Y.; Pei, Z. A supramolecular photosensitizer system based on the host-guest complexation between water-soluble pillar[6]arene and methylene blue for durable photodynamic therapy. Chem. Commun. 2018, 54, 5911–5914. [Google Scholar] [CrossRef]
- Khurana, R.; Kakatkar, A.S.; Chatterjee, S.; Barooah, N.; Kunwar, A.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular nanorods of (N-Methylpyridyl) porphyrin with captisol: Effective photosensitizer for anti-bacterial and anti-tumor activities. Front. Chem. 2019, 7, 452. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, Y.; Sun, C.; Bao, L.; Zhao, Y.; Yang, X.; Zhao, Y. A photosensitizer discretely loaded nanoaggregate with robust photodynamic effect for local treatment triggers systemic antitumor responses. ACS Nano 2022, 16, 3070–3080. [Google Scholar] [CrossRef]
- Li, X.; Kim, C.Y.; Shin, J.M.; Lee, D.; Kim, G.; Chung, H.M.; Hong, K.S.; Yoon, J. Mesenchymal stem cell-driven activatable photosensitizers for precision photodynamic oncotherapy. Biomaterials 2018, 187, 18–26. [Google Scholar] [CrossRef]
- Li, Y.; Lin, T.Y.; Luo, Y.; Liu, Q.; Xiao, W.; Guo, W.; Lac, D.; Zhang, H.; Feng, C.; Wachsmann-Hogiu, S.; et al. A smart and versatile theranostic nanomedicine platform based on nanoporphyrin. Nat. Commun. 2014, 5, 4712. [Google Scholar] [CrossRef]
- Tian, J.; Xia, L.; Wu, J.; Huang, B.; Cao, H.; Zhang, W. Linear alternating supramolecular photosensitizer for enhanced photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 32352–32359. [Google Scholar] [CrossRef]
- Tian, J.; Huang, B.; Cui, Z.; Wang, P.; Chen, S.; Yang, G.; Zhang, W. Mitochondria-targeting and ROS-sensitive smart nanoscale supramolecular organic framework for combinational amplified photodynamic therapy and chemotherapy. Acta Biomater. 2021, 130, 447–459. [Google Scholar] [CrossRef]
- Guo, L.; Niu, G.; Zheng, X.; Ge, J.; Liu, W.; Jia, Q.; Zhang, P.; Zhang, H.; Wang, P. Single near-infrared emissive polymer nanoparticles as versatile phototheranostics. Adv. Sci. 2017, 4, 1700085. [Google Scholar] [CrossRef]
- Lovell, J.F.; Liu, T.W.; Chen, J.; Zheng, G. Activatable photosensitizers for imaging and therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef]
- Gao, J.; Li, J.; Geng, W.C.; Chen, F.Y.; Duan, X.; Zheng, Z.; Ding, D.; Guo, D.S. Biomarker displacement activation: A general host-guest strategy for targeted phototheranostics in vivo. J. Am. Chem. Soc. 2018, 140, 4945–4953. [Google Scholar] [CrossRef]
- Feng, H.T.; Li, Y.; Duan, X.; Wang, X.; Qi, C.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Substitution activated precise phototheranostics through supramolecular assembly of AIEgen and calixarene. J. Am. Chem. Soc. 2020, 142, 15966–15974. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Weston, M.H.; Fuller, P.E.; Tovar, T.M.; Peterson, G.W.; LeVan, M.D.; Farha, O.K. Metal-organic frameworks for oxygen storage. Angew. Chem. Int. Ed. 2014, 53, 14092–14095. [Google Scholar] [CrossRef]
- Rummer, J.L.; McKenzie, D.J.; Innocenti, A.; Supuran, C.T.; Brauner, C.J. Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science 2013, 340, 1327–1329. [Google Scholar] [CrossRef]
- Hu, C.; Yu, Y.; Chao, S.; Zhu, H.; Pei, Y.; Chen, L.; Pei, Z. A supramolecular photosensitizer system based on nano-Cu/ZIF-8 capped with water-soluble pillar[6]arene and methylene blue host-guest complexations. Molecules 2021, 26, 3878. [Google Scholar] [CrossRef]
- Fang, X.; Cai, S.; Wang, M.; Chen, Z.; Lu, C.; Yang, H. Photogenerated holes mediated nitric oxide production for hypoxic tumor treatment. Angew. Chem. Int. Ed. 2021, 60, 7046–7050. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, M.; Li, P.; Bai, Y.; Pang, J.; Fan, L.; Tian, W. Ruthenium (II)-coordinated supramolecular metallodrug complex realizing oxygen self-supply in situ for overcoming hypoxic tumors. Adv. Fun. Mater. 2021, 31, 2105837. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, W.; Ke, W.; Chen, W.; He, C.; Ge, Z. Multifunctional polymeric micelles with amplified fenton reaction for tumor ablation. Biomacromolecules 2018, 19, 1990–1998. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Han, Y.; Liu, H.; Ren, F.; Zeng, J.; Sun, Q.; Li, Z.; Gao, M. Light-enhanced O(2)-evolving nanoparticles boost photodynamic therapy to elicit antitumor immunity. ACS Appl. Mater. Interfaces 2019, 11, 16367–16379. [Google Scholar] [CrossRef]
- Qin, Y.; Tong, F.; Zhang, W.; Zhou, Y.; He, S.; Xie, R.; Lei, T.; Wang, Y.; Peng, S.; Li, Z.; et al. Self-delivered supramolecular nanomedicine with transformable shape for ferrocene-amplified photodynamic therapy of breast cancer and bone metastases. Adv. Funct. Mater. 2021, 31, 2104645. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Huang, C.H.; Lee, K.C.; Doudna, J.A. Applications of CRISPR-Cas enzymes in cancer therapeutics and detection. Trends Cancer 2018, 4, 499–512. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Fang, F.; Xu, T.; Lan, M.; Zhang, J. Advances and perspectives in carrier-free nanodrugs for cancer chemo-monotherapy and combination therapy. Biomaterials 2021, 268, 120557. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Liang, J.L.; Luo, G.F.; Chen, W.H.; Zhang, X.Z. Recent advances in engineered materials for immunotherapy-involved combination cancer therapy. Adv. Mater. 2021, 33, e2007630. [Google Scholar] [CrossRef]
- Li, F.; Simon, M.C. Cancer cells don’t live alone: Metabolic communication within tumor microenvironments. Dev. Cell 2020, 54, 183–195. [Google Scholar] [CrossRef]
- Kemp, J.A.; Shim, M.S.; Heo, C.Y.; Kwon, Y.J. “Combo” nanomedicine: Co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv. Drug Deliv. Rev. 2016, 98, 3–18. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, L.; Li, X.; Song, W.; Liu, Y.; Huang, L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano 2019, 13, 12511–12524. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Zheng, Z.; Du, N.; Guan, S.; Guo, W.; Tang, X.; Cui, J.; Zhang, L.; Liu, K.; et al. Co-delivery of precisely prescribed multi-prodrug combination by an engineered nanocarrier enables efficient individualized cancer chemotherapy. Adv. Mater. 2022, 34, e2110490. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhao, X.; Xu, L.; Zheng, Y.; Li, H.B.; Guo, D.S.; Shi, L.; Liu, Y. Calixarene-modified albumin for stoichiometric delivery of multiple drugs in combination-chemotherapy. Theranostics 2022, 12, 3747–3757. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Hu, J.J.; Qin, S.Y.; Zhang, A.Q.; Zhang, X.Z. Recent advances in functional mesoporous silica-based nanoplatforms for combinational photo-chemotherapy of cancer. Biomaterials 2020, 232, 119738. [Google Scholar] [CrossRef]
- Huang, X.; Chen, T.; Mu, N.; Lam, H.W.; Sun, C.; Yue, L.; Cheng, Q.; Gao, C.; Yuan, Z.; Wang, R. Supramolecular micelles as multifunctional theranostic agents for synergistic photodynamic therapy and hypoxia-activated chemotherapy. Acta Biomater. 2021, 131, 483–492. [Google Scholar] [CrossRef]
- Jia, D.; Ma, X.; Lu, Y.; Li, X.; Hou, S.; Gao, Y.; Xue, P.; Kang, Y.; Xu, Z. ROS-responsive cyclodextrin nanoplatform for combined photodynamic therapy and chemotherapy of cancer. Chin. Chem. Lett. 2021, 32, 162–167. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Y.; Zhu, L.; Xu, Y.; Wang, C.; Lu, B.; Wang, Y.; Du, C.; Yao, Y. Nitric oxide-containing supramolecular polypeptide nanomedicine based on [2]biphenyl-extended-pillar[6]arenes for drug resistance reversal. J. Mater. Chem. B 2022, 10, 6181–6186. [Google Scholar] [CrossRef]
- Koo, M.; Oh, K.T.; Noh, G.; Lee, E.S. Gold nanoparticles bearing a tumor pH-sensitive cyclodextrin cap. ACS Appl. Mater. Interfaces 2018, 10, 24450–24458. [Google Scholar] [CrossRef]
- Shi, K.; Liu, X.; Liu, Y.; Liu, C.; Wang, Y.; Liu, Y. Supramolecular polypeptide self-assembly mediated in situ elicitation of robust innate and adaptive immune responses boosts immunogenic photothermal therapy toward "cold" tumor. Adv. Healthc. Mater. 2022, 12, e2202017. [Google Scholar] [CrossRef]
- De Angelis, B.; Depalo, N.; Petronella, F.; Quintarelli, C.; Curri, M.L.; Pani, R.; Calogero, A.; Locatelli, F.; De Sio, L. Stimuli-responsive nanoparticle-assisted immunotherapy: A new weapon against solid tumours. J. Mater. Chem. B 2020, 8, 1823–1840. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Lei, Q.; Qiu, W.X.; Liu, Y.X.; Cheng, Y.J.; Zhang, X.Z. A triple-collaborative strategy for high-performance tumor therapy by multifunctional mesoporous silica-coated gold nanorods. Adv. Fun. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Wang, Y.; Han, M.; Wang, C.; Yan, F. Cyclodextrin-functionalized gold nanorods loaded with meclofenamic acid for improving N(6)-methyladenosine-mediated second near-infrared photothermal immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 40612–40623. [Google Scholar] [CrossRef]
- Tang, H.; Xu, X.; Chen, Y.; Xin, H.; Wan, T.; Li, B.; Pan, H.; Li, D.; Ping, Y. Reprogramming the tumor microenvironment through second-near-infrared-window photothermal genome editing of PD-L1 mediated by supramolecular gold nanorods for enhanced cancer immunotherapy. Adv. Mater. 2021, 33, e2006003. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Q.; Wu, M.; Li, Y.; Tang, G.; Ping, Y.; Chu, P.K. Lanthanide-integrated supramolecular polymeric nanoassembly with multiple regulation characteristics for multidrug-resistant cancer therapy. Biomaterials 2017, 129, 83–97. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, Z.; Tan, L.; Zhu, W.; Tao, K.; Wang, G.; Shi, W.; Gao, J. Interferon regulatory factor-1 reverses chemoresistance by downregulating the expression of P-glycoprotein in gastric cancer. Cancer Lett. 2019, 457, 28–39. [Google Scholar] [CrossRef]
- Xia, L.L.; Tang, Y.B.; Song, F.F.; Xu, L.; Ji, P.; Wang, S.J.; Zhu, J.M.; Zhang, Y.; Zhao, G.P.; Wang, Y.; et al. DCTPP1 attenuates the sensitivity of human gastric cancer cells to 5-fluorouracil by up-regulating MDR1 expression epigenetically. Oncotarget 2016, 7, 68623–68637. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Liu, C.; Fan, L.; Tian, W. Supramolecular dual drug nanomicelles for circumventing multidrug resistance. ACS Biomater. Sci. Eng. 2021, 7, 5515–5523. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, C.; Wang, L.; Gao, X.; Wu, J.; Tan, S.; Wu, G. Doxorubicin and adjudin co-loaded pH-sensitive nanoparticles for the treatment of drug-resistant cancer. Acta Biomater. 2019, 94, 469–481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).