Advances in the Study of Probiotics for Immunomodulation and Intervention in Food Allergy
Abstract
1. Introduction
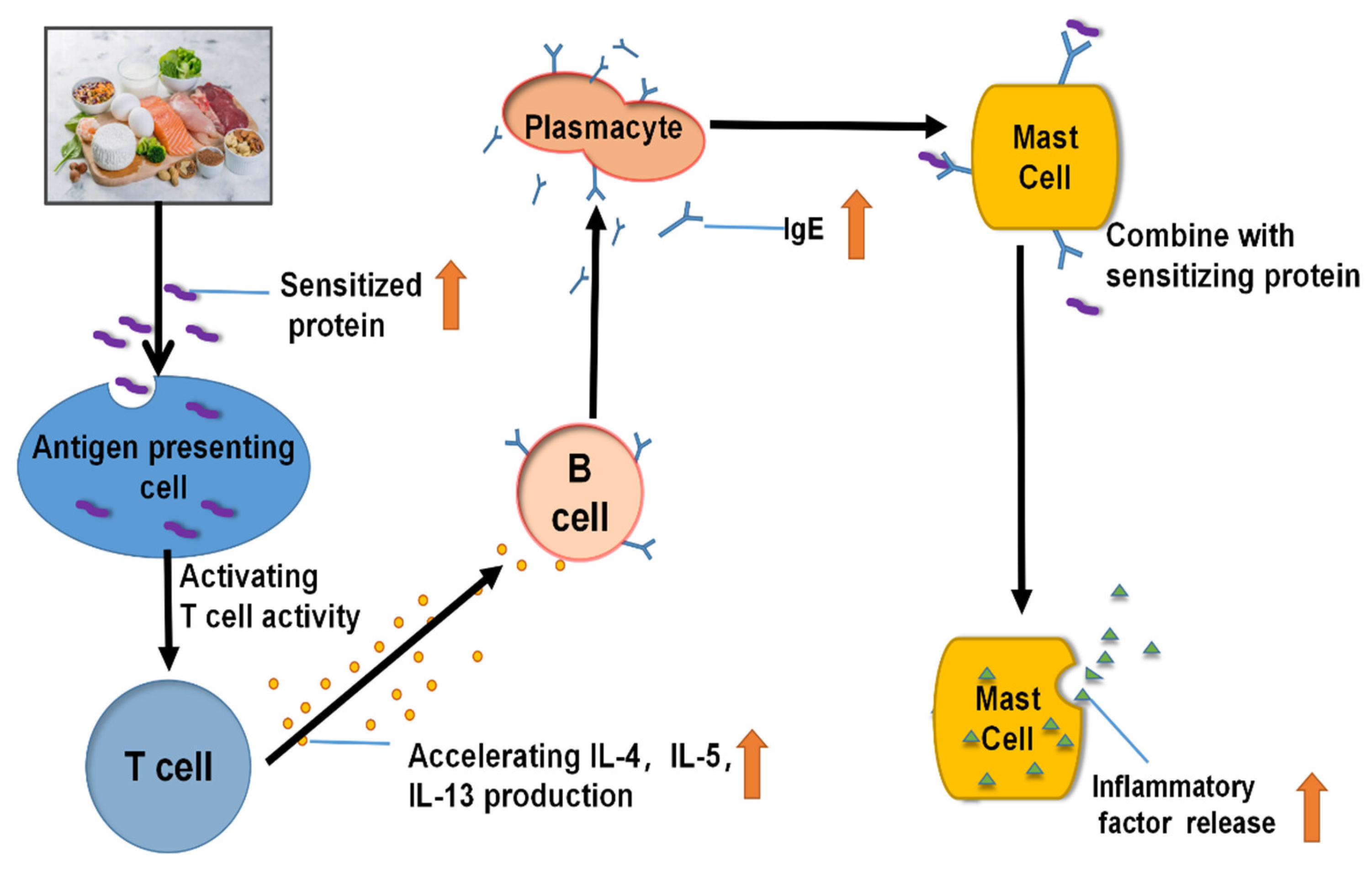
2. Allergy Mechanism of Various Food Allergens
2.1. Soy Products
2.2. Dairy Products
2.3. Aquatic Product
2.4. Poultry and Egg Products
2.5. Nut Products
3. Probiotics Regulate Immunity through Intestinal Flora
3.1. Relationship between Probiotics and Intestinal Flora
3.2. Probiotics Regulate the Immune Mechanism of Food Allergy through Intestinal Flora
3.3. Probiotics Regulate Immunity by Modulating the Intestinal Barrier
3.4. Probiotics Regulate Host Immune Active Cells
3.5. Probiotics Improve Host Amino Acid Metabolism to Influence Immunity
3.6. Probiotics Improve Host Lipid Metabolism to Influence Immunity
4. Postbiotics and Food Allergy
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sindher, S.B.; Long, A.; Chin, A.R.; Hy, A.; Sampath, V.; Nadeau, K.C.; Chinthrajah, R.S. Food allergy, mechanisms, diagnosis and treatment: Innovation through a multi-targeted approach. Allergy 2022, 77, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.L.; Mavoa, S.; Koplin, J.J. An Overview of Environmental Risk Factors for Food Allergy. Int. J. Environ. Res. Public Health 2022, 19, 722. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, Q.; Gu, Y.; Wang, J.; Liu, G.; He, T.; Che, H. Meta-Analysis: Prevalence of Food Allergy and Food Allergens—China, 2000–2021. China CDC Wkly. 2022, 4, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Sharma, J.; Babu, S.; Rizwanulla, S.A.; Singla, A. Role of probiotics in health and disease: A review. J. Pak. Med. Assoc. 2013, 63, 253–257. [Google Scholar] [PubMed]
- Di Costanzo, M.; Carucci, L.; Canani, R.B.; Biasucci, G. Gut Microbiome Modulation for Preventing and Treating Pediatric Food Allergies. Int. J. Mol. Sci. 2020, 21, 5275. [Google Scholar] [CrossRef] [PubMed]
- Steele, L.; Mayer, L.; Berin, M.C. Mucosal immunology of tolerance and allergy in the gastrointestinal tract. Immunol. Res. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Zhao, X.; Hogenkamp, A.; Li, X.; Chen, H.; Garssen, J.; Knippels, L.M.J. Role of selenium in IgE mediated soybean allergy development. Crit. Rev. Food Sci. Nutr. 2022, 1–9. [Google Scholar] [CrossRef]
- Brick, T.; Hettinga, K.; Kirchner, B.; Pfaffl, M.; Ege, M.J. The Beneficial Effect of Farm Milk Consumption on Asthma, Allergies, and Infections: From Meta-Analysis of Evidence to Clinical Trial. J. Allergy Clin. Immunol. Pract. 2019, 8, 878–889. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Betzel, C.; Singh, T.P. Structure and function of proteins involved in milk allergies. J. Chromatogr. B 2001, 756, 183–187. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Huang, Y.; Lin, H.; Xu, M.; Ahmed, I.; Chen, G.; Chen, Y.; Li, Z. Fish allergens of turbot (Scophthalmus maximus) parvalbumin triggers food allergy via inducing maturation of bone marrow derived dendritic cells and driving Th2 immune response. Food Funct. 2022, 13, 4194–4204. [Google Scholar] [CrossRef]
- Liu, R.; Krishnan, H.B.; Xue, W.; Liu, C. Characterization of Allergens Isolated from the Freshwater Fish Blunt Snout Bream (Megalobrama amblycephala). J. Agric. Food Chem. 2010, 59, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.-H.; Hou, T.-Y.; Fan, C.-K.; Chang, J.-H.; Lin, C.-L.; Huang, S.-C.; Lee, Y.-L. Catalpol exerts antiallergic effects in IgE/ovalbumin-activated mast cells and a murine model of ovalbumin-induced allergic asthma. Int. Immunopharmacol. 2021, 96, 107782. [Google Scholar] [CrossRef] [PubMed]
- Dona, D.W.; Suphioglu, C. Egg Allergy: Diagnosis and Immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Geng, T.; Stojsin, D.; Liu, K.; Schaalje, B.; Postin, C.; Ward, J.; Wang, Y.C.; Liu, Z.L.; Li, B.; Glenn, K. Natural Variability of Allergen Levels in Conventional Soybeans: Assessing Variation across North and South America from Five Production Years. J. Agric. Food Chem. 2017, 65, 463–472. [Google Scholar] [CrossRef]
- Breiteneder, H.; Mills, E.C. Plant food allergens—Structural and functional aspects of allergenicity. Biotechnol. Adv. 2005, 23, 395–399. [Google Scholar] [CrossRef]
- Lin, J.; Shewry, P.R.; Archer, D.B.; Beyer, K.; Niggemann, B.; Haas, H.; Wilson, P.; Alcocer, M.J. The Potential Allergenicity of Two 2S Albumins from Soybean (Glycine max): A Protein Microarray Approach. Int. Arch. Allergy Immunol. 2006, 141, 91–102. [Google Scholar] [CrossRef]
- Hao, Y.; Zhan, Z.; Guo, P.; Piao, X.; Li, D. Soybean β-conglycinin-induced gut hypersensitivity reaction in a piglet model. Arch. Anim. Nutr. 2009, 63, 188–202. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Petrova, S.Y.; Khlgatian, S.V.; Emel’Yanova, O.Y.; Pishulina, L.A.; Berzhets, V.M. Current Data about Milk Caseins. Russ. J. Bioorg. Chem. 2022, 48, 273–280. [Google Scholar] [CrossRef]
- Złotkowska, D.; Stachurska, E.; Fuc, E.; Wróblewska, B.; Mikołajczyk, A.; Wasilewska, E. Differences in Regulatory Mechanisms Induced by β-Lactoglobulin and κ-Casein in Cow’s Milk Allergy Mouse Model–In Vivo and Ex Vivo Studies. Nutrients 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-M.; Ni, W.-W.; Li, Y.; Zhang, X.; Hou, J.-C.; Meng, X.; Li, A.-L.; Jiang, Z.-M. Analysis of altered miRNA profiling in the colon of a mouse model with β-lactoglobulin allergy. Allergol. Immunopathol. 2020, 48, 666–674. [Google Scholar] [CrossRef]
- Ballegaard, A.-S.R.; Bøgh, K.L. Intestinal protein uptake and IgE-mediated food allergy. Food Res. Int. 2023, 163, 112150. [Google Scholar] [CrossRef]
- Smith-Norowitz, T.A.; Bluth, M.H. Probiotics and diseases of altered IgE regulation: A short review. J. Immunotoxicol. 2015, 13, 136–140. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Rabesona, H.; Drouet, M.; Choiset, Y.; Haertlé, T.; Mozzi, F.; de Valdez, G.F.; Chobert, J.-M. Proteolytic action of Lactobacillus delbrueckii subsp. bulgaricus CRL 656 reduces antigenic response to bovine beta-lactoglobulin. Food Chem. 2011, 127, 487–492. [Google Scholar] [CrossRef]
- Kliche, T.; Li, B.; Bockelmann, W.; Habermann, D.; Klempt, M.; de Vrese, M.; Wutkowski, A.; Clawin-Raedecker, I.; Heller, K.J. Screening for proteolytically active lactic acid bacteria and bioactivity of peptide hydrolysates obtained with selected strains. Appl. Microbiol. Biotechnol. 2017, 101, 7621–7633. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Huang, Y.; Wang, J.; Li, S.; Xu, M.; Lin, H.; Li, Z. Comparative Analysis of Glycosylation Affecting Sensitization by Regulating the Cross-Reactivity of Parvalbumins in Turbot (Scophthalmus maximus), Conger Eel (Conger myriaster) and Sea Bass (Micropterus salmoides). J. Agric. Food Chem. 2022, 70, 10611–10619. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Perng, K.; Hou, Y.-C.; Shen, C.-J.; Chen, I.-N.; Chen, Y.-T. Effect of species, muscle location, food processing and refrigerated storage on the fish allergens, tropomyosin and parvalbumin. Food Chem. 2023, 402, 134479. [Google Scholar] [CrossRef]
- Colquitt, A.S.; Miles, E.A.; Calder, P.C. Do Probiotics in Pregnancy Reduce Allergies and Asthma in Infancy and Childhood? A Systematic Review. Nutrients 2022, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Ugajin, T.; Kobayashi, Y.; Takayama, K.; Yokozeki, H. A parvalbumin allergy case was successfully treated with oral immunotherapy using hypoallergenic fish. Allergol. Int. 2021, 70, 509–511. [Google Scholar] [CrossRef]
- Kirjavainen, P.V.; Salminen, S.J.; Isolauri, E. Probiotic Bacteria in the Management of Atopic Disease: Underscoring the Importance of Viability. J. Craniofacial Surg. 2003, 36, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Fu, S.; Wang, C.; Xie, M.; Wang, Y. Yogurt-sourced probiotic bacteria alleviate shrimp tropomyosin-induced allergic mucosal disorders, potentially through microbiota and metabolism modifications. Allergol. Int. 2019, 68, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-G.; Kim, J.-N.; Jang, A.-S.; Shin, M. Combined Effects of Lactobacillus rhamnosus and Egg Oral Immunotherapy in a Mouse Model of Egg Allergy. Allergy Asthma Immunol. Res. 2020, 12, 701–711. [Google Scholar] [CrossRef]
- Metugriachuk, Y.; Tsuchiya, J.; Marotta, F.; Kawakita, S.; Okura, R.; Kuroi, O.; Minelli, E. Effect of a prebiotic-enriched phytocompound in improving ovalbumin allergenicity. Chin. J. Dig. Dis. 2006, 7, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhong, J.; Meng, X.; Gao, J.; Li, H.; Sun, J.; Li, X.; Chen, H. The gut microbiome-immune axis as a target for nutrition-mediated modulation of food allergy. Trends Food Sci. Technol. 2021, 114, 116–132. [Google Scholar] [CrossRef]
- Azemi, N.F.H.; Misnan, R.; Keong, B.P.; Mokhtar, M.; Kamaruddin, N.; Fah, W.C.; Yadzir, Z.H.M.; Yadzir, H.M.; Bakhtiar, F.; Abdullah, N.; et al. Molecular and allergenic characterization of recombinant tropomyosin from mud crab Scylla olivacea. Mol. Biol. Rep. 2021, 48, 6709–6718. [Google Scholar] [CrossRef]
- De Silva, C.; Dhanapala, P.; King, S.; Doran, T.; Tang, M.; Suphioglu, C. Immunological Comparison of Native and Recombinant Hen’s Egg Yolk Allergen, Chicken Serum Albumin (Gal d 5), Produced in Kluveromyces lactis. Nutrients 2018, 10, 757. [Google Scholar] [CrossRef]
- Rigaux, P.; Daniel, C.; Ratajczak, C.; Pot, B.; Pestel, J.; Jacquet, A. Immunomodulatory Properties of Recombinant Lactic Acid Bacteria Encoding a Major House-dust Mite Allergen. J. Allergy Clin. Immunol. 2006, 117, S220. [Google Scholar] [CrossRef]
- Fuhrmann, V.; Huang, H.-J.; Akarsu, A.; Shilovskiy, I.; Elisyutina, O.; Khaitov, M.; van Hage, M.; Linhart, B.; Focke-Tejkl, M.; Valenta, R.; et al. From Allergen Molecules to Molecular Immunotherapy of Nut Allergy: A Hard Nut to Crack. Front. Immunol. 2021, 12, 3641. [Google Scholar] [CrossRef] [PubMed]
- Borres, M.P.; Sato, S.; Ebisawa, M. Recent advances in diagnosing and managing nut allergies with focus on hazelnuts, walnuts, and cashew nuts. World Allergy Organ. J. 2022, 15, 100641. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Pasquato, N.; Folli, C.; Folloni, S.; Berni, R.; Cianci, M.; Helliwell, J.R.; Zanotti, G. The allergenic non-specific lipid transfer protein from peach: Structural studies. Acta Crystallogr. Sect. A Found. Crystallogr. 2005, 61, C252. [Google Scholar] [CrossRef]
- Kankaanpää, P. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol. Lett. 2001, 194, 149–153. [Google Scholar] [CrossRef]
- Liu, W.; Pu, X.; Sun, J.; Shi, X.; Cheng, W.; Wang, B. Effect of Lactobacillus plantarum on functional characteristics and flavor profile of fermented walnut milk. LWT 2022, 160, 113254. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Ponsonby, A.-L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744. [Google Scholar] [CrossRef]
- Hol, J.; van Leer, E.H.; Schuurman, B.E.E.; de Ruiter, L.F.; Samsom, J.N.; Hop, W.; Neijens, H.J.; de Jongste, J.C.; Nieuwenhuis, E.E. The acquisition of tolerance toward cow’s milk through probiotic supplementation: A randomized, controlled trial. J. Allergy Clin. Immunol. 2008, 121, 1448–1454. [Google Scholar] [CrossRef]
- Maiga, M.A.; Morin, S.; Bernard, H.; Rabot, S.; Adel-Patient, K.; Hazebrouck, S. Neonatal mono-colonization of germ-free mice with Lactobacillus casei enhances casein immunogenicity after oral sensitization to cow’s milk. Mol. Nutr. Food Res. 2017, 61, 1600862. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, Y.-C.; Jan, T.-R. Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J. Funct. Foods 2017, 31, 44–51. [Google Scholar] [CrossRef]
- Hyung, K.E.; Moon, B.S.; Kim, B.; Park, E.S.; Park, S.-Y.; Hwang, K.W. Lactobacillus plantarum isolated from kimchi suppress food allergy by modulating cytokine production and mast cells activation. J. Funct. Foods 2017, 29, 60–68. [Google Scholar] [CrossRef]
- Aoki-Yoshida, A.; Yamada, K.; Hachimura, S.; Sashihara, T.; Ikegami, S.; Shimizu, M.; Totsuka, M. Enhancement of Oral Tolerance Induction in DO11.10 Mice by Lactobacillus gasseri OLL2809 via Increase of Effector Regulatory T Cells. PLoS ONE 2016, 11, e0158643. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Song, J.; Wang, C.; Fu, S.; Wang, Y. Bifidobacterium infantis Potentially Alleviates Shrimp Tropomyosin-Induced Allergy by Tolerogenic Dendritic Cell-Dependent Induction of Regulatory T Cells and Alterations in Gut Microbiota. Front. Immunol. 2017, 8, 1536. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.; Nagy, A.; Stanton, C.; Ross, P.; Gelencsér, E.; Silva, C. Immunomodulatory activity of exopolysaccharide producing Leuconostoc citreum strain isolated from Pico cheese. J. Funct. Foods 2017, 33, 235–243. [Google Scholar] [CrossRef]
- Zhang, J.; Su, H.; Li, Q.; Wu, H.; Liu, M.; Huang, J.; Zeng, M.; Zheng, Y.; Sun, X. Oral administration of Clostridium butyricum CGMCC0313-1 inhibits β-lactoglobulin-induced intestinal anaphylaxis in a mouse model of food allergy. Gut Pathog. 2017, 9, 11. [Google Scholar] [CrossRef]
- Cahenzli, J.; Köller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef]
- Wang, W.; Luo, X.; Zhang, Q.; He, X.; Zhang, Z.; Wang, X. Bifidobacterium infantis Relieves Allergic Asthma in Mice by Regulating Th1/Th2. Med. Sci. Monit. 2020, 26, e920583. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376–385. [Google Scholar]
- Strugnell, R.A.; Wijburg, O.L.C. The role of secretory antibodies in infection immunity. Nat. Rev. Genet. 2010, 8, 656–667. [Google Scholar] [CrossRef]
- Deng, M.; Wu, X.; Duan, X.; Xu, J.; Yang, X.; Sheng, X.; Lou, P.; Shao, C.; Lv, C.; Yu, Z. Lactobacillus paracasei L9 improves colitis by expanding butyrate-producing bacteria that inhibit the IL-6/STAT3 signaling pathway. Food Funct. 2021, 12, 10700–10713. [Google Scholar] [CrossRef]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M.R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep. 2019, 26, 2509–2520. [Google Scholar] [CrossRef]
- Yin, Y. Differential proteome analysis along jejunal crypt-villus axis in piglets. Front. Biosci. Landmark 2016, 21, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Liu, L.; Cao, H.; Moore, D.; Washington, M.; Wang, B.; Peek, R.; Acra, S.; Polk, D. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. 2016, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, L.; Wu, Y.-P.; Cui, Z.-W.; Wang, Y.-Q.; Huang, Y.; Zhang, X.-P.; Li, W.-F. Oral administration of Lactobacillus rhamnosus GG to newborn piglets augments gut barrier function in pre-weaning piglets. J. Zhejiang Univ. B 2019, 20, 180–192. [Google Scholar] [CrossRef]
- Hsiao, K.; Ponsonby, A.; Ashley, S.; Lee, C.Y.Y.; Jindal, L.; Tang, M.L.K.; Loke, P.; Axelrad, C.; Pitkin, S.; Robinson, M.; et al. Longitudinal antibody responses to peanut following probiotic and peanut oral immunotherapy in children with peanut allergy. Clin. Exp. Allergy 2022, 52, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Pagnini, C.; Di Paolo, M.C.; Graziani, M.G.; Fave, G.D. Probiotics and Vitamin D/Vitamin D Receptor Pathway Interaction: Potential Therapeutic Implications in Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 747856. [Google Scholar] [CrossRef]
- Rajput, I.R.; Li, W.F. Potential Role of Probiotics in Mechanism of Intestinal Immunity. Pak. Vet. J. 2012, 32, 303–308. [Google Scholar]
- Yang, Y.-H.; Qian, W.; Hou, X.-H.; Dai, C.-B. Bifidobacterium bifidum and Bacteroides fragilis Induced Differential Immune Regulation of Enteric Glial Cells Subjected to Exogenous Inflammatory Stimulation. Inflammation 2022, 45, 2388–2405. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, F.; Zhou, G.; Sun, M.; Ai, F.; Liu, Z. Food-specific IgGs Are Highly Increased in the Sera of Patients with Inflammatory Bowel Disease and Are Clinically Relevant to the Pathogenesis. Intern. Med. 2018, 57, 2787–2798. [Google Scholar] [CrossRef]
- Lee, M.; Chang, E.B. Inflammatory Bowel Diseases (IBD) and the Microbiome—Searching the Crime Scene for Clues. Gastroenterology 2021, 160, 524–537. [Google Scholar] [CrossRef]
- Vanderpool, C.; Yan, F.; Polk, B.D. Mechanisms of probiotic action: Implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 2008, 14, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-O.; Nguyen, H.H.; Kwak, H.-S. The Role of the Immune System in the use of Probiotic Lactic Acid Bacteria in Preventing and Treating Allergic Diseases. Korean J. Food Sci. Anim. Resour. 2010, 30, 1–12. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851. [Google Scholar] [CrossRef]
- Srikham, K.; Thirabunyanon, M. Bioprophylactic potential of novel human colostrum probiotics via apoptotic induction of colon cancer cells and cell immune activation. Biomed. Pharmacother. 2022, 149, 112871. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2019, 58, 342–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.-Y.; Ji, G.E. Timing of Bifidobacterium Administration Influences the Development of Allergy to Ovalbumin in Mice. Biotechnol. Lett. 2005, 27, 1361–1367. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yang, C.-S.; Shin, A.-R.; Jeon, S.-R.; Park, J.-K.; Kim, H.-J.; Jo, E.-K. Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-κB, and MAPK Pathways. Immune Netw. 2011, 11, 123–133. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, X.; Sun, S.; Che, H. High-Fat Diet-Induced Obesity Aggravates Food Allergy by Intestinal Barrier Destruction and Inflammation. Int. Arch. Allergy Immunol. 2021, 183, 80–92. [Google Scholar] [CrossRef]
- Frontiers Production Office. Erratum: Taxonomic Characterization and Short-Chain Fatty Acids Production of the Obese Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 781260. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. From gut microbiota dysfunction to obesity: Could short-chain fatty acids stop this dangerous course? Hormones 2019, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Ranjbar, R.; Vahdati, S.N.; Tavakoli, S.; Khodaie, R.; Behboudi, H. Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections. Biomed. Pharmacother. 2021, 141, 111817. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Zhang, Z.; Liang, X.; Liu, T.; Yi, H.; Gong, P.; Wang, L.; Yang, W.; Zhang, X.; et al. Konjac glucomannan with probiotics acts as a combination laxative to relieve constipation in mice by increasing short-chain fatty acid metabolism and 5-hydroxytryptamine hormone release. Nutrition 2020, 84, 111112. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Farjadian, S.; Kashef, S.; Alyasin, S.; Afrasiabi, M.; Orooj, M. Specific IgE to Common Food Allergens in Children with Atopic Dermatitis. Iran. J. Immunol. 2012, 9, 32–38. [Google Scholar]
- Jang, H.; Kim, E.G.; Kim, M.; Kim, S.Y.; Kim, Y.H.; Sohn, M.H.; Kim, K.W. Metabolomic profiling revealed altered lipid metabolite levels in childhood food allergy. J. Allergy Clin. Immunol. 2021, 149, 1722–1731. [Google Scholar] [CrossRef]
- Sartorio, M.U.A.; Pendezza, E.; Coppola, S.; Paparo, L.; D’Auria, E.; Zuccotti, G.V.; Canani, R.B. Potential Role of Omega-3 Polyunsaturated Fatty Acids in Pediatric Food Allergy. Nutrients 2021, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Blaser, M.J.; Ley, R.E.; Knight, R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 2011, 140, 1713–1719. [Google Scholar] [CrossRef]
- Yordshahi, A.S.; Moradi, M.; Tajik, H.; Molaei, R. Design and preparation of antimicrobial meat wrapping nanopaper with bacterial cellulose and postbiotics of lactic acid bacteria—ScienceDirect. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef]
- Rad, A.H.; Maleki, L.A.; Kafil, H.S.; Abbasi, A. Postbiotics: A novel strategy in food allergy treatment. Crit. Rev. Food Sci. Nutr. 2021, 61, 492–499. [Google Scholar] [CrossRef]
- González, L.A.G.; Díez, F.A. Mucosal bacterial immunotherapy with MV130 highly reduces the need of tonsillectomy in adults with recurrent tonsillitis. Hum. Vaccines Immunother. 2019, 15, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Mckenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, H.; Dichiara, J.M.; Huck, O.; Amar, S. Pasteurized Akkermansia muciniphila reduces periodontal and systemic inflammation induced by Porphyromonas gingivalis in lean and obese mice. J. Clin. Periodontol. 2022, 49, 717–729. [Google Scholar] [CrossRef]
- Santos, S.S.; Miranda, V.C.; Trindade, L.M.; Cardoso, V.N.; Reis, D.C.; Cassali, G.D.; Nicoli, J.R.; Cara, D.C.; Martins, F.S. Bifidobacterium longum subsp. longum 51A Attenuates Signs of Inflammation in a Murine Model of Food Allergy. Probiotics Antimicrob. Proteins 2021, 15, 63–73. [Google Scholar] [CrossRef]
| Food Source of Allergy | Sensitized Protein | Allergy Mechanism | Isoelectric Points | KD (ku) | Reference |
|---|---|---|---|---|---|
| Soybean | 7S globulin | IgE, IgG co-mediate | 8.70 | 190 | [7] |
| Dairy products | Casein | IgG-mediated | 4.60~6.00 | 14 | [8] |
| lactalbumin | IgE-mediated | 4.80 | 14 | [9] | |
| Aquatic Products | Water-soluble Glycoprotein | IgE, IgG co-mediate | --- | 10~70 | [9] |
| Parvalbumin | IgE, IgG co-mediate | 3.90~5.50 | 10~14 | [10] | |
| Tropomyosin | IgE-mediated | 4.50 | 38~40 | [10] | |
| Aldolase A | IgE-mediated | --- | 40 | [10] | |
| β-enolase | IgE-mediate | --- | 47~50 | [11] | |
| Egg products of poultry | Ovomucoid | IgE-mediated | 3.83~5.50 | 28 | [12] |
| Ovalbumin | IgE-mediated | --- | 45 | [13] | |
| Ovotransferrin | IgE-mediated | 6.50 | 76.6 | [13] | |
| Lysozyme | IgE-mediated | --- | 14.3 | [13] | |
| Nut products | Serum C—reactive protein | IgE-mediated | 4.49~5.61 | 15~80 | [14] |
| Probiotics | Experimental Model | Effect on Immunity | References |
|---|---|---|---|
| Lactobacillus rhamnosus CGMCC 1.3724 | Peanut-allergic children | Decreased levels of peanut-specific IgE and increased levels of peanut-specific IgG | [47] |
| Lactobacillus casei CRL431 and Bifidobacterium lactis Bb-12 | Children with milk allergies | The proportion of lymphocytes increased | [48] |
| Lactobacillus casei BL23 | Cholera toxin-containing milk-sensitized mice | IL-17 secretion increased | [49] |
| Lactobacillus reuteri | Ovalbumin (OVA) sensitized BALB/c mice. | Allergic diarrhea, activation of mast cells, and the generation of serum IgE in allergic mice were alleviated. | [50] |
| Lactobacillus plantarum CJLP133 | Intestinal allergy in 6-week-old BALB/c mice | It inhibited the production of IL-4, IL-5, IL-13, and IL-17A cytokines in the spleen cells of OVA-sensitized mice. | [51] |
| Lactobacillus gasseri OLL2809 | OVA-sensitized mouse model | The proliferation rate and IL-2 production of CD4 T cells in OVA-fed mice were significantly reduced. | [52] |
| Bifidobacterium infantis 14.518 | A mouse model sensitized to shellfish tropomyosin | Stimulated the maturation of DC cells and the accumulation of tolerant DC cells in intestinal-associated lymphoid tissues. | [53] |
| Leuconostoc citreum L3C1E7, | Ovalbumin-sensitized rat model. | Significantly reducing plasma OVA-specific lgE may alleviate Th2-mediated allergic symptoms. | [54] |
| Clostridium butyricum CGMCC0313-1 | β-lactoglobulin (BLG) sensitized mouse model | SIgA increased, reversing the imbalance of Th1/Th2 and Th17/Treg. | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-Y.; Liang, Y.-T.; Wu, J.-M.; Wu, W.-T.; Liu, X.-T.; Ye, T.-T.; Chen, X.-R.; Zeng, X.-A.; Manzoor, M.F.; Wang, L.-H. Advances in the Study of Probiotics for Immunomodulation and Intervention in Food Allergy. Molecules 2023, 28, 1242. https://doi.org/10.3390/molecules28031242
Huang Y-Y, Liang Y-T, Wu J-M, Wu W-T, Liu X-T, Ye T-T, Chen X-R, Zeng X-A, Manzoor MF, Wang L-H. Advances in the Study of Probiotics for Immunomodulation and Intervention in Food Allergy. Molecules. 2023; 28(3):1242. https://doi.org/10.3390/molecules28031242
Chicago/Turabian StyleHuang, Yan-Yan, Yan-Tong Liang, Jia-Min Wu, Wei-Tong Wu, Xin-Tong Liu, Ting-Ting Ye, Xiao-Rong Chen, Xin-An Zeng, Muhammad Faisal Manzoor, and Lang-Hong Wang. 2023. "Advances in the Study of Probiotics for Immunomodulation and Intervention in Food Allergy" Molecules 28, no. 3: 1242. https://doi.org/10.3390/molecules28031242
APA StyleHuang, Y.-Y., Liang, Y.-T., Wu, J.-M., Wu, W.-T., Liu, X.-T., Ye, T.-T., Chen, X.-R., Zeng, X.-A., Manzoor, M. F., & Wang, L.-H. (2023). Advances in the Study of Probiotics for Immunomodulation and Intervention in Food Allergy. Molecules, 28(3), 1242. https://doi.org/10.3390/molecules28031242








