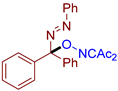
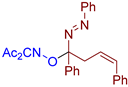
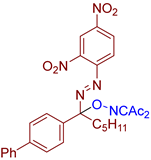
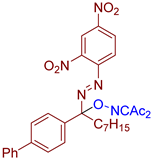
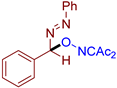
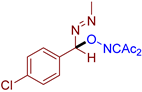
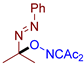
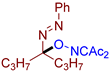
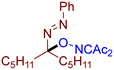
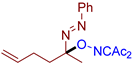
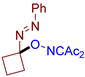
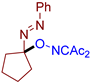
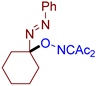
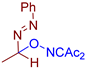
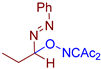
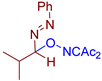
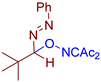
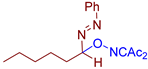
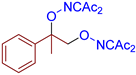
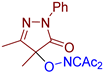
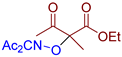
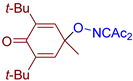
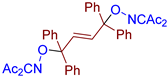
Abstract
Selective oxidative C–O coupling of hydrazones with diacetyliminoxyl is demonstrated, in which diacetyliminoxyl plays a dual role. It is an oxidant (hydrogen atom acceptor) and an O-partner for the oxidative coupling. The reaction is completed within 15–30 min at room temperature, is compatible with a broad scope of hydrazones, provides high yields in most cases, and requires no additives, which makes it robust and practical. The proposed reaction leads to the novel structural family of azo compounds, azo oxime ethers, which were discovered to be highly potent fungicides against a broad spectrum of phytopathogenic fungi (Venturia inaequalis, Rhizoctonia solani, Fusarium oxysporum, Fusarium moniliforme, Bipolaris sorokiniana, Sclerotinia sclerotiorum).
1. Introduction
The functionalization of organic compounds employing free radicals has emerged as a powerful tool in modern organic chemistry [1,2]. In particular, N-oxyl radicals [3] have gained much attention as key agents in oxidative functionalization due to their mild conditions of generation, relatively high stability combined with high reactivity towards organic substrates, and outstanding structural diversity, allowing for control of their properties. However, N-oxyl radicals are usually generated in situ from corresponding N-hydroxy compounds and thus their usage demands oxidants or catalysts and other additives. Frequently, these additional reagents contain transition-metal salts, pose limitations on the substrate scope, and do not correspond to the principles of green chemistry. The peculiar feature of the present work is the use of diacetyliminoxyl [4] as a single ready-to-use free-radical reagent which plays the role of both oxidant and coupling partner for the oxidative functionalization reaction of hydrazones (Scheme 1C). Previously, free-radical chemistry of hydrazones was associated mainly with addition and hydrogen substitution reactions of aldehyde hydrazones [5,6,7] (Scheme 1A) and cyclizations of hydrazone-derived N-radicals [7,8,9] (Scheme 1B). However, ionic mechanisms were proposed for oxidative cyclizations of α,β-unsaturated N-tosylhydrazones in some cases [8,10]. It should also be noted that in some functionalizations of type A (Scheme 1A), an additional synthetic step of chelate complex formation was necessary for effective radical functionalization of hydrazones [11,12,13]. Hydrazones are reported to undergo peroxidation by t-BuOOH in the presence of cobalt–salen complexes with the formation of geminal azoperoxides and geminal azoxyperoxides [14]. Unstable geminal azohydroperoxides [15] are formed as a result of hydrazone autoxidation by molecular oxygen [16,17,18,19]. In general, free-radical functionalization of hydrazones with the formation of azocompounds is less developed compared to methods based on electrophilic attack of hydrazone carbon atoms, such as Michael-type reactions [20,21,22,23], chlorination [24], alkoxylation, or cyanation [25]. Geminal azoacetates are synthesized by the oxidation of hydrazones with Pb(OAc)4 [26,27,28]. In the present work (Scheme 1C), diacetyliminoxyl was used as the only necessary reagent for high-yielding oxidative C–O coupling with the broad scope of both ketohydrazones and aldehyde-derived hydrazones at room temperature. It should be noted that none of the products of oxidative functionalization of hydrazones mentioned above were considered as fungicidal compounds. Unexpectedly, synthesized C–O coupling products were discovered as a new structural family of fungicides with activity against phytopathogenic fungi at the level of commercially used crop-protection compounds. This finding is very important in the light of the continuous development of strains of phytopathogenic fungi which are resistant against known synthetic fungicide types [29,30,31].
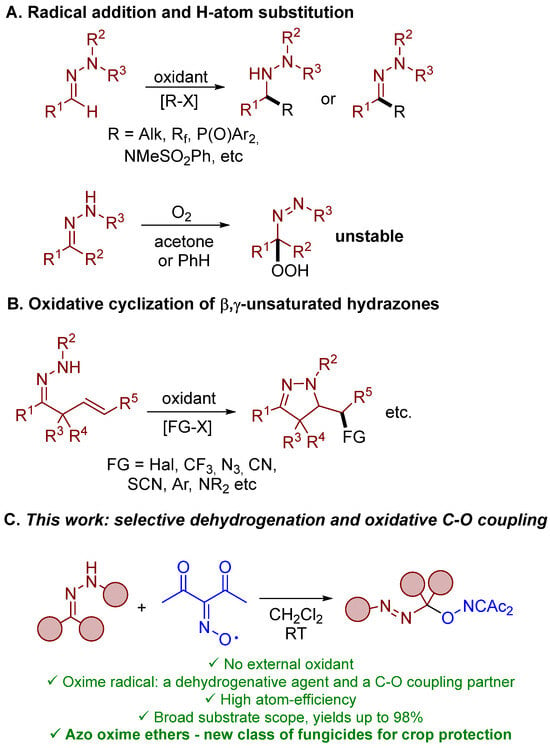
Scheme 1.
Radical functionalization of hydrazones.
2. Results and Discussion
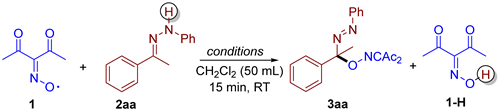
Hydrazone 2aa was used for the initial experiments with diacetyliminoxyl 1 (Table 1). CH2Cl2 was used as a solvent because it is a convenient medium for the synthesis and storage of diacetyliminoxyl 1. The reaction of 2aa with two equivalents of diacetyliminoxyl under air afforded C–O coupling product 3aa with an 85% yield (Table 1, entry 1), along with diacetyl oxime 1-H. The reaction completed in 15 min, as evidenced by the disappearance of the dark red color characteristic of diacetyliminoxyl (for UV-Vis spectrum of 1, see [32]) and TLC. To check the possible involvement of oxygen as an oxidant [18] in the discovered process, or its possible negative impact on the yield, an experiment under argon was conducted (Table 1, entry 2). However, carrying out the reaction under inert conditions did not lead to a significant change in the yield of 3aa. The increase in the amount of 1 above the stoichiometric ratio increased the yield of 3aa by 10% (entry 3 compared to entry 1). Finally, the reaction with excess of hydrazone 2aa resulted in almost the same yield as in the case of the stoichiometric amount of 2aa (entry 4).

Table 1.
Screening of the reaction parameters.
The conditions of entry 1 of Table 1 were used to test the scope of the discovered C–O coupling (Scheme 2). The discovered C–O coupling is compatible with a wide range of hydrazones derived from aromatic ketones (Scheme 2A), aromatic aldehydes (Scheme 2B), aliphatic ketones (Scheme 2C), and aliphatic aldehydes (Scheme 2D).
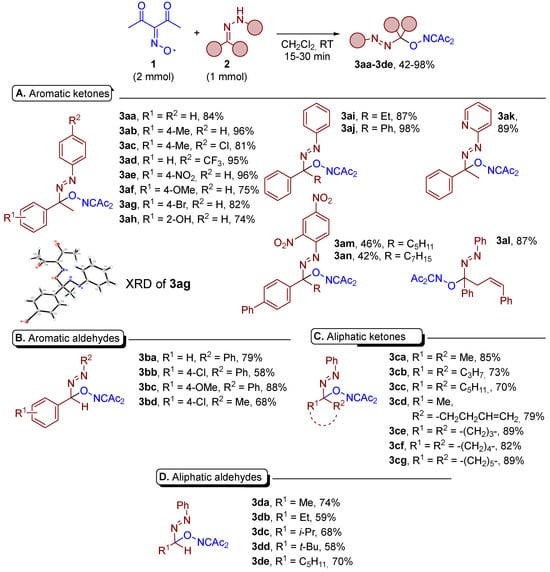
Scheme 2.
Oxidative C–O coupling of various hydrazones 2 with diacetyliminoxyl 1.
Good yields of C–O coupling products 3aa–3ah (74–96%) were observed for N-arylhydrazones of methylarylketones containing electron-donating or electron-withdrawing substituents at benzene rings. The structure of 3ag was unambiguously confirmed by XRD analysis (see the ESI). The replacement of a methyl group by ethyl did not affect the reaction yield significantly (product 3ai compared to 3aa, yields 84–87%). Hydrazone of benzophenone gave an almost quantitative yield of 3aj despite steric hindrance and the expected low energy of the formed C–O bond [33] due to the steric and electronic effects of phenyl rings. 2-Pyridyl moiety at the nitrogen atom of ketohydrazone was also tolerated (product 3ak). The reaction took place even in the case of bulky biphenylalkyl hydrazones with long-chain alkyl groups and a 2,4-dinitrophenyl group at the nitrogen atom, albeit with moderate yields of 42–46% (products 3am, 3an). The reaction of diacetyliminoxyl with β,γ-unsaturated phenylhydrazone 2al delivered the C–O coupling product 3al at 87% with the intact double C=C bond, despite the possible radical cyclization reactions typical of β,γ-unsaturated phenylhydrazones [9]. Moreover, diacetyliminoxyl 1 is known to undergo addition to C=C double bonds at room temperature [34]. Hydrazones derived from aromatic aldehydes also furnish C–O coupling products (3ba–3bd) in moderate to high yields. Of note, 3bd was obtained at a 68% yield employing N-methyl-substituted hydrazone 2bd. The reaction proceeded with high yields with acetone phenylhydrazone (product 3ca), and somewhat lower yields were obtained with higher homologues of acetone (products 3cd, 3cd). As in the case of unsaturated hydrazone 2al, allylacetone phenylhydrazone 2cd underwent oxidative C–O coupling with the formation of product 3cd containing intact C=C bond. Cyclic phenylhydrazones with ring sizes of 4–6 furnished the corresponding products 3ce–3cg at a 82–89% yield. The hydrazones of aldehydes reacted smoothly with diacetyliminoxyl, providing azocompounds 3da–3de with a 58–74% yield.
Scheme 3 demonstrates the practical applicability of the developed protocol for the synthesis at a 4 mmol scale without chromatographic purification or recrystallization (Scheme 3, (1)). Due to the instability of some phenylhydrazones in their pure form, we developed a one-pot procedure delivering the in situ generation of hydrazone that was sequentially added to the solution of diacetyliminoxyl (Scheme 3, (2)). Employing this protocol, the corresponding C–O coupling product 3ca was obtained at a yield of 71%.
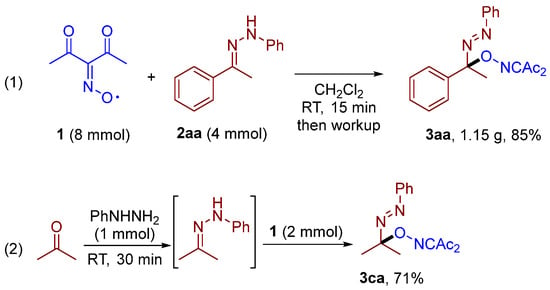
Scheme 3.
Gram-scale synthesis of 3aa (1) and developed one-pot procedure for the synthesis of 3ca (2).
Control experiments were conducted to support the plausible reaction mechanism (Scheme 4). N,N-diphenyl phenylhydrazone 2ao was introduced in the reaction with diacetyliminoxyl at standard reaction conditions (Scheme 4, (1)). There was no C–O coupling product observed by 1H-NMR monitoring of the crude reaction mixture after 24 h, indicating that hydrogen atom abstraction from the nitrogen atom is a possible crucial step rather than the addition of an oxime radical at the C=N double bond. The experiment with TEMPO (Scheme 4, (2)) is a typical control reaction which is usually employed to intercept possible C-centered radical intermediates. The introduction of two equivalents of TEMPO into the reaction of diacetyliminoxyl 1 with hydrazone 2aa did not lead to significant changes; product 3aa was obtained without yield loss (Scheme 4, (2)). Moreover, no formation of a TEMPO adduct with C-centered radical was observed (TEMPO recovery 91%), highlighting the exceptionally high efficiency of diacetyliminoxyl in scavenging stabilized C-centered radicals [33].
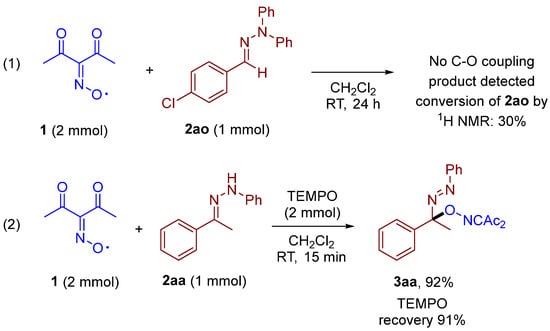
Scheme 4.
Control experiments: (1) reaction of 1 with N,N-disubstituted hydrazone, (2) TEMPO scavenging experiment.
Two possible reaction pathways can be proposed for the discovered C–O coupling of diacetyliminoxyl with hydrazones (Scheme 5). In path I, the hydrogen atom abstraction from hydrazone 2ca by diacetyliminoxyl 1 is followed by the coupling of the resultant hydrazyl radical A with 1. In path II, diacetyliminoxyl is added to hydrazone 2ca first, then hydrogen atom from adduct B is abstracted. In both cases, the first stage is expected to be rate determining, whereas the second is expected to be very fast or even barrierless. In order to evaluate which path is more plausible, DFT calculations were performed by employing the low-cost but robust B97-3c composite method [35]. The calculations revealed that path I is favored, both kinetically and thermodynamically, compared to path II; however, both pathways demonstrate activation barriers less than 20 kcal·mol−1, which are acceptable for room temperature reactions. The fact that path I is energetically more favored than path II is in agreement with the published data on C–O coupling of diacetyliminoxyl with pyrazolones, isoxazolones, and phenols [33]. However, it should be noted that diacetyliminoxyl addition reactions to π-systems were reported recently [34].
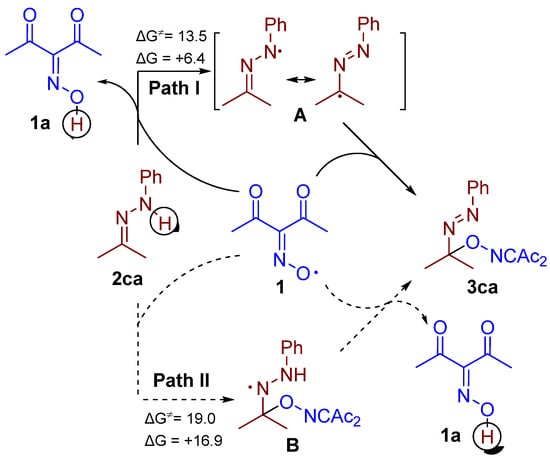
Scheme 5.
Possible reaction pathway of the oxidative C–O coupling of diacetyliminoxyl with hydrazones (ΔG and ΔG≠ values are calculated by B97-3c composite DFT method [35] and given in kcal·mol−1).
3. In Vitro Fungicidal Activity of the Synthesized Azo Compounds
In the second part of our research, the synthesized azo oxime ethers 3 were discovered as a new class of fungicides. Fungal diseases of agricultural crops represents one of the major threats to crop production [36,37,38,39]. Phytopathogenic fungi contribute significantly to reductions in crop yield [37,38,39,40] and produce mycotoxins, which can be extremely dangerous food contaminants [41,42,43,44,45,46] (for example, aflatoxins produced by Aspergillus genus, trichothecenes by Fusarium species, and ergot alkaloids produced by fungi of Claviceps genus). Fungicides remain the most effective tool for crop protection against fungal diseases [47]; however, fungicidal resistance development against known active compound classes [29,30,31,48] is a serious threat to crop production, forcing scientists to search for new types of fungicides. Currently, despite the large number of fungicidal compounds used in agriculture, most of them belong to a limited number of classes and share a common mode of action. Namely, succinate dehydrogenase inhibitors (SDHIs), demethylation inhibitors (DMIs, imidazoles and triazoles), quinone outside inhibitors (QoI, or strobilurins), and quinone inside inhibitors (QiI) dominate the fungicide global market and development [49,50,51]. Thus, the discovery of novel antifungal agents with unforeseen modes of action is a primary scientific goal [52,53,54,55,56,57,58].
Synthesized products 3 were tested for fungicidal activity at concentrations of 10–30 μg/mL against six phytopathogenic fungi from different taxonomic classes: V. i.—Venturia inaequalis, R. s.—Rhizoctonia solani, F. o.—Fusarium oxysporum, F. m.—Fusarium moniliforme, B. s.—Bipolaris sorokiniana, S. s.—Sclerotinia sclerotiorum (Table 2). Triadimefon and kresoxim-methyl—commercially available fungicides—were used as reference compounds.

Table 2.
In vitro fungicidal activity of the synthesized azo oxime ethers 3.
As can be seen from Table 2, compounds 3da and 3dc exhibit the greatest activity against phytopathogenic fungi. In general, azo oxime ethers with small aliphatic substituents at the quaternary carbon atom (3ca–cg and 3da–de) possess a higher activity compared to azo oxime ethers bearing aromatic substituents at the quaternary center (3aa, 3ae, 3ag, and 3ba). Compounds 3aj, 3al, 3am, and 3an with bulky substituents at quaternary carbon atom do not show significant fungicidal activity, as well as azo oxime ether 3bd with a Me substituent at the nitrogen atom. Aldehyde-derived azo oxime ethers (3ba and 3da–de), in general, are superior to ketone derivatives (3aa, 3ae, 3ag, and 3ca–cg). In the series of long-chain alkyl or cyclic azo compounds, activity decreases with increasing alkyl chain (3ca, 3cb, 3cc) or ring size (3ce, 3cf, 3cg). AIBN, an alkyl azo derivative frequently used as a radical initiator taken for comparison, does not show significant activity. Compounds with a diacetyl oxime moiety, obtained by oxidative C–O coupling of diacetyliminoxyl with alkenes [34], pyrazolones [33], phenols [33], and dicarbonyl compounds [59], were also tested for fungicidal activity. None of them show essential mycelium growth inhibition, indicating that diacetyl oxime moiety itself is not sufficient for the manifestation of the observed fungicidal activity. It is noteworthy that the activity of the synthesized azo compounds in the present study was not predictable due to their structural novelty. The closest related fungicidal compounds are generally diaryl azo derivatives [60,61,62] and substituted oxime derivatives with a RO–N=C–N=N-Ar fragment at the oxime moiety [63]. In contrast to these fungicides, the azo oxime ethers reported in the present work contain a tertiary C(sp3) atom at the azo group. The activity of the azo oxime ethers 3ca, 3cd–cf, and 3da–de is comparable to that of triadimefon and kresoxim-methyl, which are commercially available fungicides widely used in crop protection.
EC50 values were measured for the most promising azo compounds, 3ca and 3da, and reference compound kresoxim-methyl (Table 3).

Table 3.
EC50 Values for mycelium growth inhibition by the most active azo oxime ethers 3ca and 3da in comparison with kresoxim-methyl.
Synthesized azo compounds 3ca and 3da have a similar activity spectrum that greatly differs from that of kresoxim-methyl. Overall, the EC50 values of 3ca and 3da are comparable to those of kresoxim-methyl; however, at higher concentrations, these azo compounds demonstrate stronger mycelium growth inhibition (Table 2).
4. Materials and Methods
In all experiments RT stands for 22–25 °C. 1H and 13C NMR spectra were recorded on Bruker AVANCE II 300 and Bruker Fourier 300HD (300.13 for 1H and 75.47 MHz for 13C, respectively) spectrometers in CDCl3. Chemical shifts were reported in parts per million (ppm), and the residual solvent peak was used as an internal reference: 1H (CDCl3 δ = 7.26 ppm); 13C (CDCl3 δ = 77.16 ppm). Multiplicity was indicated as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet). Coupling constants were reported in Hertz (Hz). FT-IR spectra were recorded on Bruker Alpha instrument. High resolution mass spectra (HR-MS) were measured on a Bruker maXis instrument using electrospray ionization (ESI). The measurements were performed in a positive ion mode (interface capillary voltage—4500 V); mass range from m/z 50 to m/z 3000 Da; external calibration with Electrospray Calibrant Solution (Fluka). A syringe injection was used for all acetonitrile solutions (flow rate 3 µL/min). Nitrogen was applied as a dry gas; interface temperature was set at 180 °C.
Phenylhydrazine 97%, p-tolylhydrazine hydrochloride 98%, 4-chlorophenylhydrazine hydrochloride 97%, 4-(trifluoromethyl) phenylhydrazine 96%, 2-hydrazinopyridine 98%, 2,4-dinitrophenylhydrazine 97%, methylhydrazine 98%, acetophenone 98%, 4-methylacetophenone 95%, 4-nitroacetophenone 98%, 4-methoxyacetophenone 98%, 4-bromoacetophenone 98%, 2-hydroxyacetophenone 98%, propiophenone 99%, benzophenone 99%, benzaldehyde 98%, 4-chlorobenzaldehyde 98%, 4-methoxybenzaldehyde 99%, 4-heptanone 98%, 6-undecanone 97%, 5-hexen-2-one 98%, cyclobutanone 98%, cyclopentanone 99%, cyclohexanone 99%, acetaldehyde 99.5%, propionaldehyde 98%, isobutyraldehyde 99+%, pivaldehyde 96%, hexanal 96%. Hydrazones 2 were synthesized by condensation with the corresponding carbonyl compounds [64,65,66,67,68,69]. Ketones and corresponding hydrazones 2l–n were synthesized according to published procedures [70,71,72,73]. Compounds 4 and 8 [34], 5 [4,33], 7 [33], and 6 [59] with a diacetyl oxime moiety were synthesized by oxidative C–O coupling according to published procedures. CH2Cl2 was distilled prior to use. Acetone was distilled over KMnO4. The preparation of diacetyliminoxyl radical is described earlier in [4]. Then, Pb(OAc)4 (469 mg, 1.0 mmol) was added to a stirred solution of diacetyl oxime (258 mg, 2 mmol) in CH2Cl2 (4 mL) with vigorous stirring. Stirring was continued for 10 min; then, the reaction mixture was chromatographed on silica gel using CH2Cl2 as eluent. The fraction corresponding to the dark-red spot was collected, so that the volume of the fraction was 50 mL.
General reaction conditions for Table 1
Hydrazone 2aa (1–2 mmol) was added to a stirred solution of diacetyliminoxyl radical 1 (2–3 mmol) in CH2Cl2 (50 mL), prepared as described earlier in [4], at room temperature. The reaction mixture was stirred for 15 min under air (entries 1, 3, 4) or under argon (entry 2) atmosphere, until the dark red color of diacetyliminoxyl disappeared. After that, the reaction mixture was rotary evaporated under a water-jet vacuum. Yields were determined by 1H NMR using 1,1,2,2-tetrachloroethane as an internal standard.
Experimental details for Scheme 2
Hydrazone 2 (1 mmol, 134–460 mg) was added to a stirred solution of diacetyliminoxyl 1 (2 mmol) in CH2Cl2 (50 mL) at room temperature. The reaction mixture was stirred at RT for 15–30 min until the red color of diacetyliminoxyl disappeared. After that, the reaction mixture was rotary evaporated under a water-jet vacuum. C–O coupling products 3 were isolated by column chromatography on silica gel.
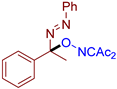
- (E)-3-((1-phenyl-1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3aa, was synthesized as a yellow oil (84%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.81–7.69 (m, 2H), 7.56–7.43 (m, 5H), 7.43–7.28 (m, 3H), 2.48 (s, 3H), 2.27 (s, 3H), 2.05 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.8, 194.7, 156.6, 151.6, 139.4, 131.6, 129.2, 128.7, 128.6, 126.5, 122.9, 105.2, 30.5, 25.9, 23.8. FT-IR (thin layer): νmax = 1725, 1690, 1363, 960, 695. HR-MS (ESI): m/z = 360.1313, calcd. for C19H19N3O3+Na+: 360.1319.
- (E)-3-((1-(phenyldiazenyl)-1-(p-tolyl)ethoxy)imino)pentane-2,4-dione, 3ab, was synthesized as a yellow oil (96%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.81–7.71 (m, 2H), 7.54–7.44 (m, 3H), 7.41 (d, J = 8.2 Hz, 2H), 7.19 (d, J = 8.2 Hz, 2H), 2.49 (s, 3H), 2.35 (s, 3H), 2.29 (s, 3H), 2.06 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.7, 194.7, 156.5, 151.6, 138.6, 136.4, 131.4, 129.24, 129.18, 126.5, 122.8, 105.2, 30.5, 25.8, 23.5, 21.2. FT-IR (thin layer): νmax = 1725, 1687, 1363, 1305, 968. HR-MS (ESI): m/z = 352.1654, calcd. for C20H21N3O3+H+: 352.1656.
- (E)-3-((1-((4-chlorophenyl)diazenyl)-1-(p-tolyl)ethoxy)imino)pentane-2,4-dione, 3ac, was synthesized as a yellow oil (81%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.70 (d, J = 8.7 Hz, 2H), 7.44 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 8.3 Hz, 2H), 7.19 (d, J = 8.3 Hz, 2H), 2.47 (s, 3H), 2.35 (s, 3H), 2.29 (s, 3H), 2.04 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.6, 194.6, 156.7, 149.9, 138.7, 137.5, 136.2, 129.4, 129.3, 126.4, 124.1, 105.3, 30.5, 25.8, 23.4, 21.2. FT-IR (thin layer): νmax = 1726, 1690, 1362, 1300, 1088, 959. HR-MS (ESI): m/z = 386.1252, 388.1230, calcd. for C20H20ClN3O3+H+: 386.1266, 388.1238.
- (E)-3-((1-phenyl-1-((4-(trifluoromethyl)phenyl)diazenyl)ethoxy)imino)pentane-2,4-dione, 3ad, was synthesized as a yellow oil (95%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.85 (d, J = 8.4 Hz, 2H), 7.75 (d, J = 8.4 Hz, 2H), 7.58–7.49 (m, 2H), 7.47–7.32 (m, 3H), 2.49 (s, 3H), 2.29 (s, 3H), 2.10 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.5, 194.5, 156.7, 153.3, 138.8, 132.89 (q, J = 32.7 Hz), 128.9, 128.7, 126.42 (q, J = 3.6 Hz), 123.84 (q, J = 272.5 Hz), 123.0, 105.5, 30.5, 25.7, 23.6. FT-IR (thin layer): νmax = 1726, 1692, 1364, 1324, 1169, 1131, 1066, 959. HR-MS (ESI): m/z = 428.1198, calcd. for C20H18F3N3O3+Na+: 428.1192.
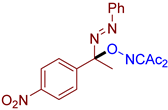
- (E)-3-((1-(4-nitrophenyl)-1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3ae, was synthesized as a yellow oil (98%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 8.25 (d, J = 8.9 Hz, 2H), 7.81–7.67 (m, 4H), 7.56–7.45 (m, 3H), 2.48 (s, 3H), 2.24 (s, 3H), 2.03 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.2, 194.3, 157.1, 151.3, 148.0, 146.6, 132.2, 129.4, 127.8, 123.8, 123.0, 104.1, 30.4, 25.9, 24.4. FT-IR (thin layer): νmax = 1727, 1693, 1605, 1522, 1350, 1300, 1142, 1109, 1079, 1067, 958, 855, 769, 758, 693. HR-MS (ESI): m/z = 405.1161, calcd. for C19H18N4O5+Na+: 405.1169.
- (E)-3-((1-(4-methoxyphenyl)-1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3af, was synthesized as a yellow oil (75%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.80–7.67 (m, 2H), 7.55–7.38 (m, 5H), 6.95–6.82 (m, 2H), 3.80 (s, 3H), 2.47 (s, 3H), 2.28 (s, 3H), 2.04 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.8, 194.7, 159.9, 156.5, 151.6, 131.4, 129.2, 128.0, 122.8, 113.9, 105.1, 55.4, 30.5, 25.8, 23.3. FT-IR (thin layer): νmax = 1725, 1690, 1608, 1514, 1363, 1303, 1253, 1185, 1109, 1030, 960, 834, 769. HR-MS (ESI): m/z = 390.1423, calcd. for C20H21N3O4+Na+: 390.1424.
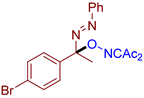
- (E)-3-((1-(4-bromophenyl)-1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3ag, was synthesized as yellow crystals (82%, purified by column chromatography with DCM as eluent). Mp = 90–91 °C. 1H NMR (300.13 MHz, CDCl3): δ = 7.81–7.70 (m, 2H), 7.55–7.45 (m, 5H), 7.44–7.35 (m, 2H), 2.47 (s, 3H), 2.27 (s, 3H), 2.01 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.6, 194.6, 156.8, 151.4, 138.6, 131.8, 129.3, 128.4, 123.1, 122.9, 104.6, 102.8, 30.5, 25.9, 23.8. FT-IR (thin layer): νmax = 1773, 1484, 1397, 1362, 1302, 1135, 1078, 1010, 966, 920, 828, 685, 550. HR-MS (ESI): m/z = 416.0608, 418.0592, calcd. for C19H18BrN3O3+H+: 416.0604, 418.0585. Single crystal X-ray analysis is available (see Supplementary Figure S1, page S15).
- (E)-3-((1-(2-hydroxyphenyl)-1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3ah, was synthesized as a pale yellow solid (74%, purified by column chromatography with DCM as eluent) Mp = 103–104 °C. 1H NMR (300.13 MHz, CDCl3): δ = 8.16 (s, 1H), 7.74–7.71 (m, 2H), 7.54–7.49 (m, 3H), 7.36–7.27 (m, 2H), 6.97–6.90 (m, 2H), 2.46 (s, 3H), 2.25 (s, 3H), 2.09 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.2, 194.4, 157.0, 155.4, 150.9, 132.5, 131.3, 129.6, 127.3, 124.0, 123.0, 120.3, 118.5, 106.9, 30.6, 25.9, 22.8. FT-IR (thin layer): νmax = 1727, 1692, 1483, 1458, 1364, 1299, 1246, 1201, 1105, 957, 939, 76. HR-MS (ESI): m/z = 376.1260, cald. for C19H19N3O4+Na+ = 376.1268.
- (E)-3-((1-phenyl-1-(phenyldiazenyl)propoxy)imino)pentane-2,4-dione, 3ai, was synthesized as a yellow oil (87%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.85–7.69 (m, 2H), 7.57–7.53 (m, 2H), 7.51–7.47 (m, 3H), 7.44–7.30 (m, 3H), 2.52 (s, 3H), 2.60–2.35 (m, 2H), 2.23 (s, 3H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.8, 194.7, 156.7, 151.6, 138.2, 131.4, 129.2, 128.5, 128.3, 126.9, 122.8, 106.9, 31.1, 30.3, 25.8, 7.7. FT-IR (thin layer): νmax =2979, 1726, 1691, 1450, 1363, 1296, 1138, 1070, 963, 763, 699, 691. HR-MS (ESI): m/z = 374.1472, calcd. for C20H21N3O3+Na+: 374.1475.
- (E)-3-((diphenyl(phenyldiazenyl)methoxy)imino)pentane-2,4-dione, 3aj, was synthesized as a slightly yellow solid (98%, purified by column chromatography with DCM as eluent). Mp = 103–104 °C. 1H NMR (300.13 MHz, CDCl3): δ = 7.86–7.75 (m, 2H), 7.58–7.45 (m, 7H), 7.43–7.30 (m, 6H), 2.56 (s, 3H), 2.05 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.7, 194.7, 156.3, 151.5, 139.9, 131.6, 129.3, 128.6, 128.4, 128.0, 123.0, 105.6, 30.1, 25.7. FT-IR (thin layer): νmax = 1725, 1686, 1300, 1013, 976, 941, 762, 695. HR-MS (ESI): m/z = 422.1461, calcd. for C24H21N3O3+Na+: 422.1475.
- (E)-3-((1-phenyl-1-(pyridin-2-yldiazenyl)ethoxy)imino)pentane-2,4-dione, 3ak, was synthesized as a yellow oil (89%, purified by column chromatography with PE/EtOAc = 2/5 as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 8.70 (d, J = 4.2 Hz, 1H), 7.85 (td, J = 7.7, 1.8 Hz, 1H), 7.60–7.48 (m, 3H), 7.46–7.29 (m, 4H), 2.48 (s, 3H), 2.28 (s, 3H), 2.12 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.6, 194.6, 162.2, 156.8, 149.6, 138.6, 138.5, 129.0, 128.7, 126.5, 125.8, 114.3, 106.0, 30.6, 25.9, 23.5. FT-IR (thin layer): νmax = 1725, 1690, 1583, 1455, 1425, 1363, 1299, 1261, 1194, 1145, 1119,1069, 955, 791, 770, 699. HR-MS (ESI): m/z = 339.1448, calcd. for C18H18N4O3+H+: 339.1452.
- 3-((((Z)-1,4-diphenyl-1-((E)-phenyldiazenyl)but-3-en-1-yl)oxy)imino)pentane-2,4-dione, 3al, was synthesized as a slightly yellow viscous gum (87%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.79–7.70 (m, 2H), 7.58–7.45 (m, 5H), 7.45–7.17 (m, 8H), 6.54 (d, J = 11.8 Hz, 1H), 5.58 (dt, J = 11.8, 7.2 Hz, 1H), 3.57 (dd, J = 7.2, 1.8 Hz, 2H), 2.50 (s, 3H), 1.98 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.6, 194.7, 156.6, 151.5, 137.3, 137.2, 132.5, 131.6, 129.2, 128.74, 128.67, 128.61, 128.4, 127.0, 126.9, 124.9, 122.9, 106.3, 36.2, 30.3, 25.6. FT-IR (thin layer): νmax = 1725, 1690, 1600, 1494, 1449, 1363, 1301, 1193, 1059, 1018, 1003, 950, 765, 699. HR-MS (ESI): m/z = 462.1781, calcd. for C27H25N3O3+Na+: 462.1788.
- (E)-3-(((1-([1,1′-biphenyl]-4-yl)-1-((2,4-dinitrophenyl)diazenyl)hexyl)oxy)imino)pentane-2,4-dione, 3am, was synthesized as a viscous orange gum (46%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 8.83 (d, J = 2.3 Hz, 1H), 8.51 (dd, J = 8.7, 2.3 Hz, 1H), 7.73–7.56 (m, 6H), 7.52–7.41 (m, 3H), 7.41–7.32 (m, 1H), 2.60–2.47 (m, 2H), 2.44 (s, 3H), 2.33 (s, 3H), 1.42–1.20 (m, 6H), 0.94–0.77 (m, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.5, 194.4, 157.1, 148.6, 148.1, 146.1, 141.7, 140.2, 135.6, 129.0, 128.4, 127.9, 127.5, 127.18, 127.15, 120.5, 120.3, 108.1, 37.3, 31.8, 30.2, 25.8, 22.6, 22.4, 14.0. FT-IR (thin layer): νmax = 3103, 2957, 2931, 2869, 1726, 1692, 1608, 1536, 1487, 1346, 1298, 1147, 954, 836, 766, 744, 698. HR-MS (ESI): m/z = 582.1955, calcd. for C29H29N5O7+Na+: 582.1959.
- (E)-3-(((1-([1,1′-biphenyl]-4-yl)-1-((2,4-dinitrophenyl)diazenyl)octyl)oxy)imino)pentane-2,4-dione, 3an, was synthesized as a viscous orange gum (42%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 8.82 (d, J = 2.3 Hz, 1H), 8.51 (dd, J = 8.7, 2.3 Hz, 1H), 7.78–7.55 (m, 6H), 7.54–7.40 (m, 3H), 7.39–7.29 (m, 1H), 2.66–2.49 (m, 2H), 2.45 (s, 3H), 2.34 (s, 3H), 1.55–1.09 (m, 10H), 0.86 (t, J = 6.5 Hz, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.5, 194.4, 157.1, 148.6, 148.1, 146.1, 141.8, 140.2, 135.6, 129.0, 128.4, 127.9, 127.5, 127.2, 120.5, 120.3, 108.1, 37.4, 31.8, 30.3, 29.6, 29.1, 25.8, 23.0, 22.7, 14.2. FT-IR (thin layer): νmax = 1724, 1691, 1607, 1545, 1541, 1346, 1297, 1194, 1146, 963, 835, 766, 747, 698. HR-MS (ESI): m/z = 605.2712, calcd. for C31H33N5O7+H+: 605.2718.
- (E)-3-((phenyl(phenyldiazenyl)methoxy)imino)pentane-2,4-dione, 3ba, was synthesized as a yellow oil (79%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.79–7.76 (m, 2H), 7.55–7.51 (m, 2H), 7.50–7.47 (m, 3H), 7.44–7.39 (m, 3H), 6.50 (s, 1H), 2.46 (s, 3H), 2.35 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.9, 194.4, 157.2, 151.6, 134.4, 131.9, 130.7, 129.8, 129.3, 129.0, 127.9, 123.7, 107.3, 30.6, 25.9. FT-IR (thin layer): νmax =1725, 1693, 1453, 1419, 1360, 1195, 1098, 1019, 952, 766, 695. HR-MS (ESI): m/z = 346.1162 cald. for C18H17N3O3+Na+ = 346.1162.
- (E)-3-(((4-chlorophenyl)(phenyldiazenyl)methoxy)imino)pentane-2,4-dione, 3bb, was synthesized as a yellow powder (58%, purified by column chromatography with DCM as eluent). Mp = 49–50 °C. 1H NMR (300.13 MHz, CDCl3): δ = 7.84–7.69 (m, 2H), 7.57–7.42 (m, 5H), 7.42–7.35 (m, 2H), 6.45 (s, 1H), 2.45 (s, 3H), 2.35 (s, 3H) 13C NMR (75.47 MHz, CDCl3): δ = 197.7, 194.3, 157.3, 151.4, 135.9, 132.9, 132.1, 129.3, 129.23, 129.19, 123.1, 106.5, 30.6, 26.0. FT-IR (thin layer): νmax = 1725, 1697, 1488, 1413, 1363, 1296, 1091, 1049, 1019, 939, 821, 768, 691. HR-MS (ESI): m/z = 380.0770, cald. for C18H16ClN3O3+Na+: 380.0772.
- (E)-3-(((4-methoxyphenyl)(phenyldiazenyl)methoxy)imino)pentane-2,4-dione, 3bc, was synthesized as a yellow solid (88%, purified by column chromatography with DCM as eluent). Mp = 69–70 °C 1H NMR (300 MHz, CDCl3): δ = 7.81–7.69 (m, 2H), 7.51–7.38 (m, 5H), 6.98–6.89 (m, 2H), 6.44 (s, 1H), 3.81 (s, 3H), 2.45 (s, 3H), 2.35 (s, 3H). 13C NMR (76 MHz, CDCl3): δ = 198.0, 194.5, 160.8, 157.0, 151.5, 131.8, 129.3, 129.2, 126.6, 123.0, 114.4, 107.2, 55.4, 30.6, 25.9. FT-IR (thin layer): νmax = 1725, 1692, 1515, 1360, 1300, 1253, 1027, 951. HR-MS (ESI): m/z = 376.1261 cald. for C19H19N3O4+Na+ = 376.1268.
- (E)-3-(((4-chlorophenyl)(methyldiazenyl)methoxy)imino)pentane-2,4-dione, 3bd, was synthesized as a yellow oil (68%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, DMSO-d6): δ = 7.53 (d, J = 8.6 Hz, 2H), 7.47 (d, J = 8.6 Hz, 1H), 6.36 (s, 1H), 3.85 (s, 3H), 2.35 (s, 3H), 2.32 (s, 3H). 13C NMR (75.47 MHz, DMSO-d6): δ = 198.2, 193.7, 156.9, 134.5, 133.1, 129.5, 128.9, 104.5, 57.0, 30.1, 25.6. FT-IR (thin layer): νmax = 1727, 1693, 1493, 1363, 1298, 1090, 977, 950. HR-MS (ESI): m/z = 318.0611, cald. for C13H14ClN3O3+Na+: 318.0616.
- (E)-3-(((2-(phenyldiazenyl)propan-2-yl)oxy)imino)pentane-2,4-dione, 3ca, was synthesized as a yellow oil (85%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.76–7.67 (m, 2H), 7.52–7.43 (m, 3H), 2.44 (s, 3H), 2.35 (s, 3H), 1.62 (s, 6H). 13C NMR (75.47 MHz, CDCl3): δ = 198.9, 194.8, 156.3, 151.6, 131.3, 129.2, 122.6, 104.7, 30.6, 25.8, 23.5. FT-IR (thin layer): νmax =1726, 1690, 1384, 1303, 1196, 1173, 1145, 1070, 963, 767, 691. HR-MS (ESI): m/z = 298.1160, calcd. for C14H17N3O3+Na+: 298.1162.
- (E)-3-(((4-(phenyldiazenyl)heptan-4-yl)oxy)imino)pentane-2,4-dione, 3cb, was synthesized as a yellow oil (73%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.75–7.63 (m, 2H), 7.54–7.41 (m, 3H), 2.44 (s, 3H), 2.34 (s, 3H), 2.15–1.91 (m, 4H), 1.55–1.19 (m, 4H), 0.90 (t, J = 7.3 Hz, 6H). 13C NMR (75.47 MHz, CDCl3): δ = 199.0, 194.8, 156.2, 151.6, 131.2, 129.2, 122.5, 107.5, 37.2, 30.4, 25.8, 16.3, 14.6. FT-IR (thin layer): νmax = 2964, 2934, 2875, 1726, 1690, 1363, 1303, 960, 768, 691. HR-MS (ESI): m/z = 354.1782, calcd. for C18H25N3O3+Na+: 354.1788.
- (E)-3-(((6-(phenyldiazenyl)undecan-6-yl)oxy)imino)pentane-2,4-dione, 3cc, was synthesized as a yellow oil (70%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.73–7.64 (m, 2H), 7.53–7.42 (m, 3H), 2.44 (s, 3H), 2.34 (s, 3H), 2.13–1.93 (m, 4H), 1.49–1.18 (m, 12H), 0.86 (t, J = 6.8 Hz, 6H). 13C NMR (75.47 MHz, CDCl3): δ = 198.9, 194.8, 156.3, 151.7, 131.1, 129.2, 122.5, 107.6, 34.8, 32.2, 30.4, 25.8, 22.5, 22.4, 14.1. FT-IR (thin layer): νmax = 2957, 2932, 2870, 1727, 1692, 1363, 1301, 960, 767. HR-MS (ESI): m/z = 410.2402, calcd. For C22H33N3O3+Na+: 410.2414.
- (E)-3-(((2-(phenyldiazenyl)hex-5-en-2-yl)oxy)imino)pentane-2,4-dione, 3cd, was synthesized as a slightly yellow viscous gum (79%, purified by column chromatography with PE/EA = 10/1 as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.76–7.66 (m, 2H), 7.54–7.44 (m, 3H), 5.93–5.62 (m, 1H), 5.21–4.77 (m, 2H), 2.45 (s, 3H), 2.35 (s, 3H), 2.27–1.98 (m, 4H), 1.63 (s, 3H). 13C NMR (75.47 MHz, CDCl3): δ = 198.9, 194.8, 156.4, 151.6, 137.7, 131.4, 129.2, 122.6, 115.1, 106.0, 36.4, 30.5, 27.4, 25.9, 21.4. FT-IR (thin layer): νmax = 1726, 1690, 1420, 1367, 1303, 982, 960, 826. HR-MS (ESI): m/z = 338.1475, calcd. for C17H21N3O3+Na+: 338.1475.
- (E)-3-((1-(phenyldiazenyl)cyclobutoxy)imino)pentane-2,4-dione, 3ce, was synthesized as a slightly yellow viscous gum (89%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.81–7.70 (m, 2H), 7.56–7.42 (m, 3H), 2.69–2.50 (m, 4H), 2.46 (s, 3H), 2.35 (s, 3H), 2.09–1.84 (m, 2H).13C NMR (75.47 MHz, CDCl3): δ = 198.7, 194.7, 157.3, 151.7, 131.4, 129.2, 122.8, 105.2, 31.9, 30.7, 25.9, 12.0. FT-IR (thin layer): νmax = 1727, 1690, 1364, 1304, 1251, 1143, 954, 768, 690. HR-MS (ESI): m/z = 310.1163, calcd. for C15H17N3O3+Na+: 310.1162.
- (E)-3-(((1-(phenyldiazenyl)cyclopentyl)oxy)imino)pentane-2,4-dione, 3cf, was synthesized as a slightly yellow viscous gum (82%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.78–7.63 (m, 2H), 7.55–7.40 (m, 3H), 2.44 (s, 3H), 2.35 (s, 3H), 2.30–2.12 (m, 4H), 1.96–1.81 (m, 4H). 13C NMR (75.47 MHz, CDCl3): δ = 198.8, 194.8, 156.8, 151.7, 131.2, 129.2, 122.6, 115.6, 36.4, 30.5, 25.9, 24.8. FT-IR (thin layer): νmax = 2959, 1725, 1685, 1363, 1302, 1188, 959, 766, 690. HR-MS (ESI): m/z = 340.1059, calcd. for C16H19N3O3+K+: 340.1058.
- (E)-3-(((1-(phenyldiazenyl)cyclohexyl)oxy)imino)pentane-2,4-dione, 3cg, was synthesized as a slightly yellow viscous gum (89%, purified by column chromatography with DCM as eluent). 1H NMR (300.13 MHz, CDCl3): δ = 7.74–7.64 (m, 2H), 7.55–7.39 (m, 3H), 2.46 (s, 3H), 2.45 (s, 3H), 2.19–2.06 (m, 2H), 1.92–1.68 (m, 5H), 1.67–1.46 (m, 2H), 1.45–1.27 (m, 1H). 13C NMR (75.47 MHz, CDCl3): δ = 198.9, 194.8, 156.6, 151.7, 131.2, 129.2, 122.6, 105.2, 32.1, 30.6, 25.9, 25.0, 21.9. FT-IR (thin layer): νmax = 2938, 2863, 1727, 1689, 1599, 1450, 1420, 1363, 1304, 1275, 1256, 1195, 1159, 1146, 1069, 1023, 983, 960, 928, 911, 766, 691. HR-MS (ESI): m/z = 316.1654, calcd. for C17H21N3O3+H+: 316.1656.
- (E)-3-((1-(phenyldiazenyl)ethoxy)imino)pentane-2,4-dione, 3da, was synthesized as a pale brown gum (74%, purified by column chromatography with DCM as eluent). 1H NMR (300 MHz, CDCl3): δ = 7.79–7.69 (m, 2H), 7.54–7.44 (m, 3H), 5.67 (q, J = 6.3 Hz, 1H), 2.45 (s, 3H), 2.34 (s, 3H), 1.59 (d, J = 6.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ = 198.2, 194.5, 156.8, 151.5, 131.7, 129.3, 122.8, 103.5, 30.6, 25.9, 17.5. FT-IR (thin layer): νmax = 1727, 1691, 1365, 1299, 1107, 1088, 1060, 965, 770, 691. HR-MS (ESI): m/z = 300.0733, calcd. for C13H15N3O3+K+: 300.0745.
- (E)-3-((1-(phenyldiazenyl)propoxy)imino)pentane-2,4-dione, 3db, was synthesized as a pale yellow gum (59%, purified by column chromatography with DCM as eluent). 1H NMR (300 MHz, CDCl3): δ = 7.83–7.64 (m, 2H), 7.60–7.39 (m, 3H), 5.55–5.41 (m, 1H), 2.45 (s, 3H), 2.33 (s, 3H), 2.15–1.88 (m, 2H), 1.05 (t, J = 7.5 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ = 198.2, 194.6, 156.9, 151.5, 131.6, 129.3, 122.8, 107.9, 30.6, 25.9, 25.3, 8.7. FT-IR (thin layer): νmax = 1726, 1691, 1363, 1301, 1022, 988, 950, 769, 691. HR-MS (ESI): m/z = 298.1152, calcd. for C14H17N3O3+Na+: 298.1162.
- (E)-3-((2-methyl-1-(phenyldiazenyl)propoxy)imino)pentane-2,4-dione, 3dc, was synthesized as a yellow gum (68%, purified by column chromatography with DCM as eluent). 1H NMR (300 MHz, DMSO-d6): δ = 7.77–7.68 (m, 2H), 7.62–7.49 (m, 3H), 5.42 (d, J = 5.5 Hz, 1H), 2.40 (s, 3H), 2.39–2.28 (m, 1H), 2.26 (s, 3H), 0.99 (t, J = 7.5 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ = 198.3, 193.7, 156.7, 150.9, 131.8, 129.4, 122.3, 109.1, 31.1, 30.0, 25.5, 17.5, 16.6. FT-IR (thin layer): νmax = 2970, 1726, 1691, 1364, 1299, 1020, 998, 959, 769, 691. HR-MS (ESI): m/z = 290.1500, calcd. For C15H19N3O3+H+: 290.1499.
- (E)-3-((2,2-dimethyl-1-(phenyldiazenyl)propoxy)imino)pentane-2,4-dione, 3dd, was synthesized as a pale yellow gum (21%, purified by column chromatography with DCM as eluent). 1H NMR (300 MHz, CDCl3): δ = 7.80–7.69 (m, 2H), 7.53–7.44 (m, 3H), 5.27 (s, 1H), 2.44 (s, 3H), 2.29 (s, 3H), 1.08 (s, 9H). 13C NMR (75 MHz, CDCl3): δ = 198.1, 194.5, 156.8, 151.6, 131.6, 129.3, 122.9, 112.1, 36.0, 30.4, 25.7. FT-IR (thin layer): νmax = 2973, 1727, 1693, 1365, 1300, 1021, 999, 959. HR-MS (ESI): m/z = 326.1474, calcd. for C16H21N3O3+Na+: 326.1475.
- (E)-3-(((1-(phenyldiazenyl)hexyl)oxy)imino)pentane-2,4-dione, 3de, was synthesized as a yellow gum (70%, purified by column chromatography with DCM as eluent). 1H NMR (300 MHz, CDCl3): δ = 7.80–7.67 (m, 2H), 7.55–7.42 (m, 3H), 5.55 (dd, J = 7.7, 5.1 Hz, 1H), 2.45 (s, 3H), 2.33 (s, 3H), 2.11–1.76 (m, 2H), 1.55–1.40 (m, 2H), 1.40–1.23 (m, 4H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (75 MHz, CDCl3): δ = 198.2, 194.6, 156.8, 151.5, 131.6, 129.2, 122.8, 107.0, 31.8, 31.6, 30.6, 25.9, 23.9, 22.5, 14.1. FT-IR (thin layer): νmax = 2956, 2931, 1727, 1691, 1363, 1299, 964. HR-MS (ESI): m/z = 318.1810, calcd. for C17H23N3O3+H+: 318.1812.
Experimental details for gram-scale synthesis of 3aa (Scheme 3)
Hydrazone 2aa (4 mmol, 840 mg) was added to a stirred solution of diacetyliminoxyl 1 (8 mmol) in CH2Cl2 (200 mL) at room temperature. The reaction mixture was stirred at RT for 15 min, then rotary evaporated under a water-jet vacuum to an approximate volume of 40 mL. The reaction mixture was successively washed with 50 mL of saturated solution of NaHCO3, 50 mL of water, dried over MgSO4, and rotatory evaporated under a water-jet vacuum. The obtained C–O coupling product 3aa (1.15 g, 3.41 mmol) was analytically pure, which was further confirmed by 1H and 13C NMR spectroscopy.
Experimental details for one-pot procedure for the synthesis of 3ca (Scheme 3)
Phenylhydrazine (1 mmol, 108 mg) was dissolved in acetone (5 mL) and stirred at room temperature for 30 min. Then, the resulting solution was added dropwise to a stirred solution of diacetyliminoxyl 1 (2 mmol) in CH2Cl2 (50 mL). The obtained reaction mixture was stirred at RT for 15 min, and was then rotary evaporated under a water-jet vacuum. The C–O coupling product 3ca was purified by column chromatography on silica gel as described in the experimental details of Scheme 2.
Hydrazone 2ao (1 mmol, 306 mg) was added to a stirred solution of diacetyliminoxyl 1 (2 mmol) in CH2Cl2 (50 mL) at room temperature. The reaction mixture was stirred at RT for 24 h and analyzed by 1H-NMR spectroscopy using 1,1,2,2-tetrachloroethane as an internal standard.
Experimental details for TEMPO scavenging experiment (Scheme 4).
TEMPO (2 mmol, 312 mg) and hydrazone 2aa (1 mmol, 210 mg) were added to a stirred solution of diacetyliminoxyl radical 2 (2 mmol) in DCM (50 mL). The reaction mixture was stirred for 15 min at room temperature and was then rotary evaporated under water-jet vacuum. Column chromatography on silica gel afforded 3aa (310 mg, 0.92 mmol, 92%) and TEMPO (284 mg, 1.81 mmol, 91% recovery).
Fungicidal activity tests (experimental details for Table 2 and Table 3). The standard poison food technique [58,74,75,76,77,78] was used for fungicidal activity measurements against six phytopathogenic fungi of different taxonomic classes: V.i.—Venturia inaequalis MRA-16-2, R.s.—Rhizoctonia solani 100063, F.o.—Fusarium oxysporum FO-8, F.m.—Fusarium moniliforme 100146, B.s.—Bipolaris sorokiniana MRB(V)-1, S.s.—Sclerotinia sclerotiorum 100033. The strains used in this work were obtained from the collection of the All-Russian Research Institute for Phytopathology (B. Vyazemy, Moscow reg., Russia). The tested substances were dissolved in acetone (concentration 1 mg/mL) and introduced into liquid sugar-potato agar at 50–55 °C, so that the final substance concentration in the nutrient medium was 10 mg/L, and mixed thoroughly. Then, the agar containing the tested substance was poured into sterile Petri dishes. After the cooling of agar to room temperature, pieces of mycelium from the peripheral growth zone of a 3–5 day old culture of the fungus were transferred to test Petri dishes using a needle. A colony grown in the same medium without the addition of a fungicidal substance (same volume of acetone without any substance was added) was used as a control. The diameters of the formed fungal colonies were measured 72 h after inoculation. Each experiment was repeated 3 times, except for tests with V.i. culture which were conducted in 5 replicates. The suppression of mycelium growth in comparison with the control was calculated as ((Dc – Ds)/Dc) × 100%, where Dc is an average fungus colony diameter in control medium, and Ds is an average fungus colony diameter in the presence of the tested substance. Serial two-fold dilution experiments were conducted for EC50 determination.
Computational details (Scheme 5). DFT calculations were conducted by the B97-3c composite method [35] including D3 dispersion correction [79,80], as implemented in the Orca 5.0.4 program [81]. The main conformers of diacetyliminoxyl 1 and hydrazone 2ca were considered in all calculations. The presented results correspond to 218.15 K and 1 atm. See the supporting information for the cartesian coordinates and energy values of optimized structures of 1a, 1, 2ca, A, B, and transition states for path I and path II. Optimized geometries were visualized by the Avogadro 1.2 program [82].
5. Conclusions
In summary, we have disclosed the oxidative C–O coupling of hydrazones with diacetyliminoxyl as a ready-to-use radical reagent playing two roles: the role of a hydrogen atom acceptor and the role of a partner for the C–O coupling. The developed protocol is compatible with both aromatic and aliphatic keto- and aldehyde-derived hydrazones. Synthesized azo oxime ethers were discovered as a novel structural fungicide type with activity against phytopathogenic fungi that is comparable to the activity of commercial fungicides (triadimefon and kresoxim-methyl).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28237863/s1, 1H and 13C NMR spectra of the synthesized compounds, XRD of 3ag, computational details. References [83,84,85,86] are cited in the supplementary materials.
Author Contributions
Conceptualization, I.B.K. and A.O.T.; investigation, A.S.B., A.V.L., O.O.S., M.I.S., I.B.K. and A.L.A.; writing—original draft preparation, A.S.B.; writing—review and editing, I.B.K., A.I.I. and A.O.T.; supervision, A.I.I., G.I.N. and A.O.T.; project administration, A.I.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (Grant number 19-73-20190).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castellino, N.J.; Montgomery, A.P.; Danon, J.J.; Kassiou, M. Late-Stage Functionalization for Improving Drug-like Molecular Properties. Chem. Rev. 2023, 123, 8127–8153. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, G.; Wang, H.; Huang, Z.; Wang, J.; Singh, A.K.; Lei, A. Recent Advances in Radical C–H Activation/Radical Cross-Coupling. Chem. Rev. 2017, 117, 9016–9085. [Google Scholar] [CrossRef] [PubMed]
- Leifert, D.; Studer, A. Organic Synthesis Using Nitroxides. Chem. Rev. 2023, 123, 10302–10380. [Google Scholar] [CrossRef] [PubMed]
- Krylov, I.B.; Paveliev, S.A.; Shelimov, B.N.; Lokshin, B.V.; Garbuzova, I.A.; Tafeenko, V.A.; Chernyshev, V.V.; Budnikov, A.S.; Nikishin, G.I.; Terent’ev, A.O. Selective Cross-Dehydrogenative C–O Coupling of N-Hydroxy Compounds with Pyrazolones. Introduction of the Diacetyliminoxyl Radical into the Practice of Organic Synthesis. Org. Chem. Front. 2017, 4, 1947–1957. [Google Scholar] [CrossRef]
- Prieto, A.; Bouyssi, D.; Monteiro, N. Radical-Mediated Formal C(Sp2)–H Functionalization of Aldehyde-Derived N, N-Dialkylhydrazones. Eur. J. Org. Chem. 2018, 2018, 2378–2393. [Google Scholar] [CrossRef]
- Van Der Worp, B.A.; Kosobokov, M.D.; Levin, V.V.; Dilman, A.D. Photoredox Fluoroalkylation of Hydrazones in Neutral and Reductive Modes. Adv. Synth. Catal. 2021, 363, 1152–1158. [Google Scholar] [CrossRef]
- Latrache, M.; Hoffmann, N. Photochemical Radical Cyclization Reactions with Imines, Hydrazones, Oximes and Related Compounds. Chem. Soc. Rev. 2021, 50, 7418–7435. [Google Scholar] [CrossRef]
- Lv, Y.; Meng, J.; Li, C.; Wang, X.; Ye, Y.; Sun, K. Update on the Synthesis of N-Heterocycles via Cyclization of Hydrazones (2017–2021). Adv. Synth. Catal. 2021, 363, 5235–5265. [Google Scholar] [CrossRef]
- Si, Y.; Lv, Q.; Yu, B. Radical Cascade Reactions of β,γ-Unsaturated Hydrazones/Oximes. Adv. Synth. Catal. 2021, 363, 4640–4666. [Google Scholar] [CrossRef]
- Paveliev, S.A.; Segida, O.O.; Bityukov, O.V.; Tang, H.; Pan, Y.; Nikishin, G.I.; Terent’ev, A.O. Electrocatalytic Synthesis of Substituted Pyrazoles via Hypervalent Iodine Mediated Intramolecular C−N Coupling. Adv. Synth. Catal. 2022, 364, 3910–3916. [Google Scholar] [CrossRef]
- Rubanov, Z.M.; Supranovich, V.I.; Levin, V.V.; Dilman, A.D. BF 2 -Chelates of N-Acylhydrazones as Versatile Coupling Partners in Photoredox Promoted Reactions. Eur. J. Org. Chem. 2023, 26, e202300247. [Google Scholar] [CrossRef]
- Rubanov, Z.M.; Levin, V.V.; Dilman, A.D. Zinc Chelate Complexes of N-Acyl Hydrazones as Substrates for Addition of Alkyl and Fluorinated Radicals. Adv. Synth. Catal. 2023, 365, 2636–2642. [Google Scholar] [CrossRef]
- Dmitriev, I.A.; Levin, V.V.; Dilman, A.D. Boron Chelates Derived from N -Acylhydrazones as Radical Acceptors: Photocatalyzed Coupling of Hydrazones with Carboxylic Acids. Org. Lett. 2021, 23, 8973–8977. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, A.; Yamazakhi, S.; Nogusa, H.; Shimoyam, T.; Matsuura, T. Oxidation of Phenols and Hydrazones with T-Butyl Hydroperoxide and Catalysis by Co(Salen). Chem. Informationsdienst 1985, 1985, 378–386. [Google Scholar] [CrossRef][Green Version]
- Tezuka, T.; Ando, S. Novel Substituent Effect Controlling the Stability of α-Azohydroperoxides. Chem. Lett. 1986, 15, 1671–1674. [Google Scholar] [CrossRef]
- Schulz, M.; Missol, U.; Bohm, H. Azoperoxide. I Synthese von trans-α-Hydroxy-dialkyldiazenen Aus α-Alkylazo-alkylhydroperoxiden. J. Prakt. Chem. 1974, 316, 47–53. [Google Scholar] [CrossRef]
- Baumstark, A.L.; Vasquez, P.C. Oxygen-Atom Transfer Chemistry of α-AZO Hydroperoxides: Effect of Competitive Intramolecular Hydrogen Bonding and α-Methyl Substitution. J. Phys. Org. Chem. 1988, 1, 259–265. [Google Scholar] [CrossRef]
- Harej, M.; Dolenc, D. Autoxidation of Hydrazones. Some New Insights. J. Org. Chem. 2007, 72, 7214–7221. [Google Scholar] [CrossRef]
- Nazran, A.S.; Warkentin, J. Concerted Homolysis in Thermal Decomposition of Peresters from. Alpha.-Hydroperoxydiazenes. J. Am. Chem. Soc. 1982, 104, 6405–6407. [Google Scholar] [CrossRef]
- Fernández, M.; Uria, U.; Vicario, J.L.; Reyes, E.; Carrillo, L. Enantioselective Conjugate Addition of Donor–Acceptor Hydrazones to α,β-Unsaturated Aldehydes through Formal Diaza–Ene Reaction: Access to 1,4-Dicarbonyl Compounds. J. Am. Chem. Soc. 2012, 134, 11872–11875. [Google Scholar] [CrossRef]
- Mondal, B.; Maiti, R.; Yang, X.; Xu, J.; Tian, W.; Yan, J.-L.; Li, X.; Chi, Y.R. Carbene-Catalyzed Enantioselective Annulation of Dinucleophilic Hydrazones and Bromoenals for Access to Aryl-Dihydropyridazinones and Related Drugs. Chem. Sci. 2021, 12, 8778–8783. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Takeda, N.; Yasui, M.; Ito, Y.; Konishi, K.; Ueda, M. Synthesis of Pyrazoles Utilizing the Ambiphilic Reactivity of Hydrazones. Org. Lett. 2020, 22, 9249–9252. [Google Scholar] [CrossRef] [PubMed]
- De Gracia Retamosa, M.; Matador, E.; Monge, D.; Lassaletta, J.M.; Fernández, R. Hydrazones as Singular Reagents in Asymmetric Organocatalysis. Chem. A Eur. J. 2016, 22, 13430–13445. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.W. Chlorination of Aldehyde and Ketone Phenylhydrazones. J. Org. Chem. 1972, 37, 383–385. [Google Scholar] [CrossRef]
- Okimoto, M.; Takahashi, Y.; Kakuchi, T. Electrochemical Formation of Methoxy- and Cyano(Phenylazo)Alkanes from Aldehyde and Ketone Phenylhydrazones. Synthesis 2003, 13, 2057–2063. [Google Scholar] [CrossRef]
- Zheng, J.; Meng, S.; Wang, Q.; Wang, J. Synthesis of Antimicrobial Benzo[1,2,4]Triazoloazepinium Salts and Tetrahydronaphtho[1,2-e][1,2,4]Triazines by Polar [3+ + 2] and [4 + 2]-Cycloaddition Reactions. J. Org. Chem. 2022, 87, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Luan, L.; Song, Z.; Li, Z.; Wang, Q.; Wang, J. Synthesis of Triazolodiazepinium Salts: Sequential [3+ +2] Cycloaddition/Rearrangement Reaction of 1-Aza-2-Azoniaallenium Cation Intermediates Generated from Piperidin-4-Ones. J. Org. Chem. 2018, 83, 3441–3452. [Google Scholar] [CrossRef]
- Iffland, D.C.; Salisbury, L.; Schafer, W.R. The Preparation and Structure of Azoacetates, a New Class of Compounds 1. J. Am. Chem. Soc. 1961, 83, 747–749. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide Resistance Management: Maximizing the Effective Life of Plant Protection Products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Budnikov, A.S.; Krylov, I.B.; Ushakov, I.E.; Subbotina, I.R.; Monin, F.K.; Nikishin, G.I.; Efimov, N.N.; Gorbunov, D.E.; Gritsan, N.P.; Tretyakov, E.V.; et al. Two Discoveries in One Crystal: σ-Type Oxime Radical as an Unforeseen Building Block in Molecular Magnetics and Its Spatial Structure. Inorg. Chem. 2023, 62, 10965–10972. [Google Scholar] [CrossRef] [PubMed]
- Budnikov, A.S.; Krylov, I.B.; Kuzmin, I.V.; Segida, O.O.; Lastovko, A.V.; Shevchenko, M.I.; Nikishin, G.I.; Terent’ev, A.O. Diacetyliminoxyl as a Selective Radical Reagent for Organic Synthesis: Dehydrogenation and Dehydrogenative C–O Coupling Reactions. Org. Chem. Front. 2023, 10, 388–398. [Google Scholar] [CrossRef]
- Budnikov, A.S.; Krylov, I.B.; Lastovko, A.V.; Dolotov, R.A.; Shevchenko, M.I.; Terent’ev, A.O. The Diacetyliminoxyl Radical in Oxidative Functionalization of Alkenes. Org. Biomol. Chem. 2023, 21, 7758–7766. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.G.; Bannwarth, C.; Hansen, A.; Grimme, S. B97-3c: A Revised Low-Cost Variant of the B97-D Density Functional Method. J. Chem. Phys. 2018, 148, 064104. [Google Scholar] [CrossRef]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging Fungal Threats to Animal, Plant and Ecosystem Health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.S.S.L.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent Advances and Remaining Barriers to Producing Novel Formulations of Fungicides for Safe and Sustainable Agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Xu, J. Assessing Global Fungal Threats to Humans. mLife 2022, 1, 223–240. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Richard, J.L. Some Major Mycotoxins and Their Mycotoxicoses—An Overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Li, J. Updating Techniques on Controlling Mycotoxins—A Review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary Mycotoxins, Co-Exposure, and Carcinogenesis in Humans: Short Review. Mutat. Res. Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef]
- Ingenbleek, L.; Sulyok, M.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; Eyangoh, S.; Verger, P.; et al. Regional Sub-Saharan Africa Total Diet Study in Benin, Cameroon, Mali and Nigeria Reveals the Presence of 164 Mycotoxins and Other Secondary Metabolites in Foods. Toxins 2019, 11, 54. [Google Scholar] [CrossRef]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.-N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The Benefits of Pesticides to Mankind and the Environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- Thind, T.S. New Insights into Fungicide Resistance: A Growing Challenge in Crop Protection. Indian Phytopathol. 2022, 75, 927–939. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of Novel Pesticides in the 21st Century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Crop Protection Compounds—Trends and Perspective. Pest Manag. Sci. 2021, 77, 3608–3616. [Google Scholar] [CrossRef]
- Jeschke, P. Progress of Modern Agricultural Chemistry and Future Prospects: Progress of Modern Agricultural Chemistry and Future Prospects. Pest Manag. Sci. 2016, 72, 433–455. [Google Scholar] [CrossRef]
- Blokhina, S.V.; Sharapova, A.V.; Ol’khovich, M.V.; Doroshenko, I.A.; Levshin, I.B.; Perlovich, G.L. Synthesis and Antifungal Activity of New Hybrids Thiazolo[4,5-d]Pyrimidines with (1H-1,2,4)Triazole. Bioorg. Med. Chem. Lett. 2021, 40, 127944. [Google Scholar] [CrossRef]
- Yang, Y.-D.; He, Y.-H.; Ma, K.-Y.; Li, H.; Zhang, Z.-J.; Sun, Y.; Wang, Y.-L.; Hu, G.-F.; Wang, R.-X.; Liu, Y.-Q. Design and Discovery of Novel Antifungal Quinoline Derivatives with Acylhydrazide as a Promising Pharmacophore. J. Agric. Food Chem. 2021, 69, 8347–8357. [Google Scholar] [CrossRef]
- Xia, D.; Cheng, X.; Liu, X.; Zhang, C.; Wang, Y.; Liu, Q.; Zeng, Q.; Huang, N.; Cheng, Y.; Lv, X. Discovery of Novel Pyrazole Carboxylate Derivatives Containing Thiazole as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 8358–8365. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Gao, W.; Liu, X.; Lv, Y.; Hao, Z.; Tang, L.; Li, K.; Zhao, B.; Fan, Z. Design, Synthesis, and Evaluation of Novel Isothiazole-Purines as a Pyruvate Kinase-Based Fungicidal Lead Compound. J. Agric. Food Chem. 2021, 69, 9461–9471. [Google Scholar] [CrossRef]
- Long, Z.-Q.; Yang, L.-L.; Zhang, J.-R.; Liu, S.-T.; Xie, J.; Wang, P.-Y.; Zhu, J.-J.; Shao, W.-B.; Liu, L.-W.; Yang, S. Fabrication of Versatile Pyrazole Hydrazide Derivatives Bearing a 1,3,4-Oxadiazole Core as Multipurpose Agricultural Chemicals against Plant Fungal, Oomycete, and Bacterial Diseases. J. Agric. Food Chem. 2021, 69, 8380–8393. [Google Scholar] [CrossRef]
- Obydennov, K.L.; Kalinina, T.A.; Galieva, N.A.; Beryozkina, T.V.; Zhang, Y.; Fan, Z.; Glukhareva, T.V.; Bakulev, V.A. Synthesis, Fungicidal Activity, and Molecular Docking of 2-Acylamino and 2-Thioacylamino Derivatives of 1 H-Benzo[d]Imidazoles as Anti-Tubulin Agents. J. Agric. Food Chem. 2021, 69, 12048–12062. [Google Scholar] [CrossRef]
- Budnikov, A.S.; Lopat’eva, E.R.; Krylov, I.B.; Segida, O.O.; Lastovko, A.V.; Ilovaisky, A.I.; Nikishin, G.I.; Glinushkin, A.P.; Terent’ev, A.O. 4-Nitropyrazolin-5-Ones as Readily Available Fungicides of the Novel Structural Type for Crop Protection: Atom-Efficient Scalable Synthesis and Key Structural Features Responsible for Activity. J. Agric. Food Chem. 2022, 70, 4572–4581. [Google Scholar] [CrossRef]
- Budnikov, A.S.; Krylov, I.B.; Lastovko, A.V.; Paveliev, S.A.; Romanenko, A.R.; Nikishin, G.I.; Terent’ev, A.O. Stable and Reactive Diacetyliminoxyl Radical in Oxidative C–O Coupling with β-Dicarbonyl Compounds and Their Complexes. Org. Biomol. Chem. 2021, 19, 7581–7586. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, X. Synthesis of Diaryl-Azo Derivatives as Potential Antifungal Agents. Bioorg. Med. Chem. Lett. 2010, 20, 4193–4195. [Google Scholar] [CrossRef]
- Lv, M.; Ma, J.; Li, Q.; Xu, H. Discovery of Benzotriazole-Azo-Phenol/Aniline Derivatives as Antifungal Agents. Bioorg. Med. Chem. Lett. 2018, 28, 181–187. [Google Scholar] [CrossRef]
- Lizard, G.; Latruffe, N.; Vervandier-Fasseur, D. Aza- and Azo-Stilbenes: Bio-Isosteric Analogs of Resveratrol. Molecules 2020, 25, 605. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, H.; Trah, S. Azo Oxime Ethers and Their Use as Fungicides. WO/1993/016986, 2 September 1993. [Google Scholar]
- Andleeb, H.; Tehseen, Y.; Ali Shah, S.J.; Khan, I.; Iqbal, J.; Hameed, S. Identification of Novel Pyrazole–Rhodanine Hybrid Scaffolds as Potent Inhibitors of Aldose Reductase: Design, Synthesis, Biological Evaluation and Molecular Docking Analysis. RSC Adv. 2016, 6, 77688–77700. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.; Dong, W.; Miao, M.; Ren, H. I2-Catalyzed Oxidative Coupling Reactions of Hydrazones and Amines and the Application in the Synthesis of 1,3,5-Trisubstituted 1,2,4-Triazoles. Org. Lett. 2016, 18, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Nguyen, H.D.; Lv, L.; Chen, S.; Li, Z. Chemo-, Stereo- and Regioselective Fluoroallylation/Annulation of Hydrazones with Gem -Difluorocyclopropanes via Tunable Palladium/NHC Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202303271. [Google Scholar] [CrossRef]
- Su, Y.-M.; Hou, Y.; Yin, F.; Xu, Y.-M.; Li, Y.; Zheng, X.; Wang, X.-S. Visible Light-Mediated C–H Difluoromethylation of Electron-Rich Heteroarenes. Org. Lett. 2014, 16, 2958–2961. [Google Scholar] [CrossRef]
- Yang, X.-L.; Peng, X.-X.; Chen, F.; Han, B. TEMPO-Mediated Aza-Diels–Alder Reaction: Synthesis of Tetrahydropyridazines Using Ketohydrazones and Olefins. Org. Lett. 2016, 18, 2070–2073. [Google Scholar] [CrossRef]
- Zhang, G.; Miao, J.; Zhao, Y.; Ge, H. Copper-Catalyzed Aerobic Dehydrogenative Cyclization of N-Methyl-N-Phenylhydrazones: Synthesis of Cinnolines. Angew. Chem. Int. Ed. 2012, 51, 8318–8321. [Google Scholar] [CrossRef]
- Kašpar, M.; Hamplová, V.; Novotná, V.; Pacherová, O. The Effect of the Alkyl Chain Length on the Mesomorphic Properties of New Lactic Acid Derivatives. Liq. Cryst. 2014, 41, 1179–1187. [Google Scholar] [CrossRef]
- Katagiri, T.; Ota, S.; Ohira, T.; Yamao, T.; Hotta, S. Synthesis of Thiophene/Phenylene Co-Oligomers. V. Functionalization at Molecular Terminals toward Optoelectronic Device Applications. J. Heterocycl. Chem. 2007, 44, 853–862. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Schmidt, E.Y.; Zorina, N.V.; Ivanova, E.V.; Ushakov, I.A. Transition-Metal-Free Superbase-Promoted Stereoselective α-Vinylation of Ketones with Arylacetylenes: A General Strategy for Synthesis of β,γ-Unsaturated Ketones. J. Org. Chem. 2012, 77, 6880–6886. [Google Scholar] [CrossRef] [PubMed]
- Pünner, F.; Sohtome, Y.; Sodeoka, M. Solvent-Dependent Copper-Catalyzed Synthesis of Pyrazoles under Aerobic Conditions. Chem. Commun. 2016, 52, 14093–14096. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods; CRC Press: Boca Raton, FL, USA, 1985; ISBN 978-0-8493-5921-7. [Google Scholar]
- Xu, H.; Fan, L. Antifungal Agents. Part 4: Synthesis and Antifungal Activities of Novel Indole[1,2-c]-1,2,4-Benzotriazine Derivatives against Phytopathogenic Fungi in Vitro. Eur. J. Med. Chem. 2011, 46, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K. Synthesis and Fungicidal Activity of Novel 3-(Substituted/Unsubstituted Phenylselenonyl)-1-Ribosyl/Deoxyribosyl-1 H -1,2,4-Triazole. J. Agric. Food Chem. 2012, 60, 5813–5818. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Kajino, H.; Tsukiyama, T.; Tobitsuka, J.; Ohta, H.; Takahi, Y.; Tsuda, M.; Takeshiba, H. Synthesis of Silicon-Containing Azole Derivatives with Magnesium Bromide Diethyl Etherate, and an Investigation of Their Fungicidal Activities. Bioorg. Med. Chem. 2002, 10, 4029–4034. [Google Scholar] [CrossRef]
- Popkov, S.V.; Kovalenko, L.V.; Bobylev, M.M.; Molchanov, O.Y.; Krimer, M.Z.; Tashchi, V.P.; Putsykin, Y.G. The Synthesis and Fungicidal Activity of 2-Substituted 1-Azol-1-Ylmethyl-6-Arylidenecyclohexanols. Pestic. Sci. 1997, 49, 125–129. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).