Abstract
Chemical investigation of the stems of Micromelum falcatum (Lour.) Tan. led to the isolation of two new lactam derivatives, named 3-(hydroxy(10-hydroxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (1) and 3-(hydroxy(10-hydroxy-9-methoxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (2), along with five known compounds, trans-4-hydroxycinnamic acid (3), 4-hydroxybenzaldehyde (4), m-hydroxybenzoic acid (5), p-hydroxybenzoic acid (6), and gallic acid (7). Their structures were determined on the basis of spectroscopic studies, including nuclear magnetic resonance (NMR) spectrum, mass spectrometry (MS) data, ultraviolet (UV) spectrum, infrared (IR) data, and comparison with the literature. All compounds were evaluated for toxicity against brine shrimp larvae and cytotoxicity to HeLa and HepG-2 cells. Compounds 1–2 exhibited moderate brine shrimp larvae toxicity with an LC50 value of 50.6 and 121.8 μg mL−1, respectively.
1. Introduction
Micromelum falcatum (Lour.) Tan. (M. falcatum) is a mangrove-associated plant of the Rutaceae family and the genus Micromelum. Its flowers have unpleasant aromas. Fruits can be green, orange, or red due to their varying degrees of maturity. M. falcatum is mainly distributed in the southwest of Guangdong Province, Hainan Province, Guangxi Province, and the southeast of Yunnan Province [1,2,3,4,5,6]. It is also found in Vietnam, Laos, Cambodia, and Thailand [3]. Micromelum is a genus of the Rutaceae family, including approximately 10 species, most of which have medicinal values and are usually used to treat various diseases in traditional folk medicine [1,3,4]. For example, people use the leaves, stems, and roots of Micromelum integerrimum as medicine to dissipate blood stasis, relieve pain, and treat conditions such as stomachaches and rheumatic bone pain, among others [1,6]. M. falcatum is used to treat injuries, such as traumatic injury, snakebite, and rheumatism [7]. Since the 1960s, many researchers have studied the chemical composition of this genus of plants, and more than 100 compounds have been isolated and identified from the extracts of the roots, bark, and leaves, including coumarins, alkaloids, flavonoids, triterpenoids, and sterol, etc. [1,6,7,8,9].
Our interest in this plant was stimulated by the discovery of the potent anti-implantation alkaloid yuehchukene isolated from M. falcatum [10]. The dimeric indole alkaloid yuehchukene showed a strong ability to completely prevent implantation up to day 5 of pregnancy at 2 mg kg−1 in rats [11]. In order to search for new toxic agents, we further investigated the chemical constituents of M. falcatum collected from Sanya, Hainan Province. Herein, we report the structural elucidation and brine shrimp toxicity assessment of two new lactam derivatives (1–2). Additionally, five known compounds were identified through a combination of NMR, MS and comparison with the literature. These compounds were characterized as trans-4-hydroxycinnamic acid (3) [12], 4-hydroxybenzaldehyde (4) [13], m-hydroxybenzoic acid (5) [14], p-hydroxybenzoic acid (6) [15], and gallic acid (7) [16]. The isolated compounds 1 and 2 exhibited pronounced to moderate toxic activity against the brine shrimp larvae with LC50 values of 50.6 and 121.8 μg mL−1, respectively. Cytotoxicity was evaluated by determining the IC50 value against two human cancer cells (HeLa and HepG-2) using the CCK-8 assay. None of the compounds exhibited good cytotoxicity.
2. Results
The ethyl alcohol (EtOH) extract from the stems of M. falcatum was partitioned with n-hexane and ethyl acetate (AcOEt), as described in Section 4. The AcOEt extract was subjected to silica gel column chromatography, Sephadex LH-20, and semi-preparative HPLC to yield two new lactam derivatives, 1–2, in addition to the five known compounds: trans-4-hydroxycinnamic acid (3), 4-hydroxybenzaldehyde (4), m-hydroxybenzoic acid (5), p-hydroxybenzoic acid (6), and gallic acid (7). The structures of compounds 1–7 were elucidated through the analysis of spectroscopic data, including MS, UV, IR, and NMR spectra, as well as by comparison with previously published data.
Compound 1 was obtained as a yellow oil with [α-12.2 (c = 0.1, MeOH). The molecular formula of 1 was determined to be C18H19O4N via high-resolution electrospray ionization mass spectrometry (HR-ESI-MS) m/z: 336.1216 (calcd for C18H19O4NNa+[M + Na]+, 336.1206). The UV spectrum showed absorption bands at λmax 228 and 276 nm. Its IR spectrum revealed absorption bands at vmax = 3262, 1651, 1614, and 1516 cm−1, which indicated the presence of hydroxyl, amide carbonyl, and aromatic groups. The 1H-NMR spectrum of 1 (Table 1) exhibited two aromatic A2B2 systems (δH 7.13 (2H, d, J = 8.5 Hz), 6.68 (2H, d, J = 8.5 Hz), and δH 6.64 (2H, d, J = 8.5 Hz), 6.88 (2H, d, J = 8.5 Hz)), one methyl (δH 2.80 (3H, s, N-CH3)), and one methylene (δH 3.15 (2H, d, J = 2.5 Hz)). The 13C-NMR and DEPT spectra of 1 showed the presence of one methyl group (δC 29.7 (N-CH3)), one methylene (δC 57.4), eleven methines (δC 39.7, 59.0, 75.3, 115.8, 115.8, 116.4, 116.4, 129.1, 129.1, 129.1, and 129.1), and five quaternary carbons (δC 132.6, 135.7, 157.1, 158.3, and 176.9). These above data were similar to those for lepiotins A and B [17] and suggested that 1 has γ-lactam and two aromatic rings (Figure 1 and Figure 2).

Table 1.
NMR spectral data for 1–2 (500 MHz for 1H-NMR and 125 MHz for 13C-NMR in CD3OD).
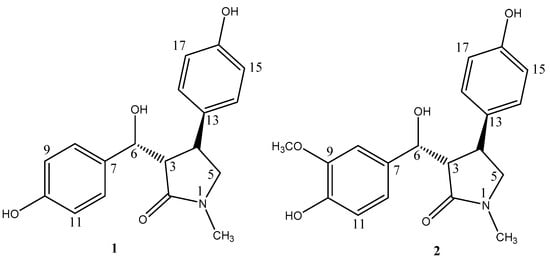
Figure 1.
Structures of compounds 1–2.
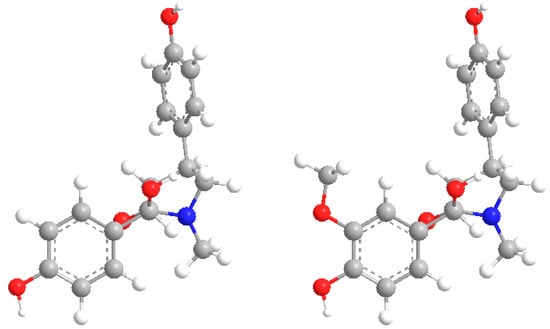
Figure 2.
Three-dimensional structures of compounds 1–2.
In the HMBC spectrum, the correlations between δH 2.80 (s, N-CH3) and δC 176.9 (C-2), 57.4 (C-5), between δH 2.93 (1H, dd, J = 6.0, 5.5 Hz, H-3) and δC 176.9 (C-2), 39.7 (C-4), and between δH 3.15 (2H, d, 2.5 Hz, H-5) and δC 176.9 (C-2), 39.7 (C-4) indicated the presence of a γ-lactam. Meanwhile, the HMBC correlations of δH 4.95 (1H, d, 6.0 Hz, H-6) with δC 59.0 (C-3), 39.7 (C-4), 132.6 (C-7), and 129.1 (C-8, 12) revealed one aromatic ring connecting with the γ-lactam skeleton through C-6. The HMBC correlations of δH 3.25 (1H, m, H-4) with δC 59.0 (C-3), 57.4 (C-5), 135.7 (C-13), and 129.1 (C-14, 18) revealed the other aromatic ring locating at C-4 of the γ-lactam skeleton (Figure 3). The 13C chemical shifts of C-6, 10, and 16 at δC 75.3, 158.3, and 157.1, respectively, indicated hydroxyl groups as their neighboring substituents, which was also confirmed by the HR-ESI-MS data. Based on these data, the structure of 1 was concluded and named 3-(hydroxy(10-hydroxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one.
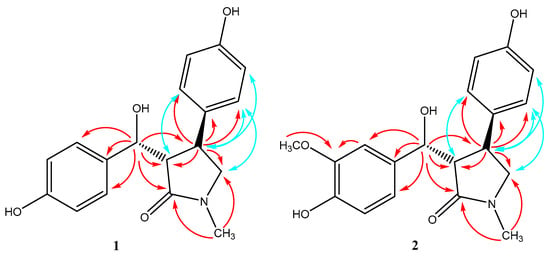
Figure 3.
Key HMBC (red) and NOESY (blue) correlations of compounds 1–2.
The relative configuration of compound 1 was deduced from the results of the NOESY experiment. The cross peaks between H-3 at δH 2.93 and H-18 at δH 6.88, H-4 at δH 3.25 and H-14 at δH 6.88, H-5 at δH 3.15 and H-14 at δH 6.88, H-4 at δH 3.25 and H-15 at δH 6.64 were observed, and the trans configurations between H-3 and H-4 could be established.
Compound 2 was obtained as a yellow oil with [α-12.8 (c = 0.1, MeOH). The molecular formula of 2 was established as C19H21NO5 via HR-ESI-MS m/z: 366.1317 (calcd for C19H21NO5Na+ [M + Na]+, 366.1312).The 1H- and 13C-NMR spectra of 2 (Table 1) were similar to those of 1, with the only difference being an aromatic proton (δH 6.68 (1H, d, 8.5 Hz, H-9)) for 1, substituted by a methoxy group (δH 3.75 (3H, s), δC 56.4) for 2 (Figure 1). This suggested that compound 2 was also a γ-lactam with two aromatic rings, which was proved by the HMBC spectrum, showing correlations of δH 2.84 (s, N-CH3) with C-2/C-5, δH 4.91 (1H, d, 7.0 Hz, H-6) with C-2/C-3/C-4/C-7/C-8/C-12, and δH 3.21 (1H, m, H-4) with C-3/C-5/C-13/C-14/C-18 (Figure 3). So, the structure of 2 was assigned as 3-(hydroxy(10-hydroxy-9-methoxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one.
The relative configuration of compound 2 was also deduced from the results of the NOESY experiment. The cross peaks between H-3 at δH 2.97 and H-18 at δH 6.86, H-4 at δH 3.21 and H-14 at δH 6.86, H-5 at δH 3.22 and H-14 at δH 6.86, and H-4 at δH 3.21 and H-15 at δH 6.63 were observed. These signals demonstrated that compounds 2 and 1 had the same relative configurations.
The toxic activity of all isolated compounds from M. falcatum was evaluated against brine shrimp larvae using a 96-well plate assay. Compounds 1–2 had moderate activity and exhibited LC50 values of 50.6 and 121.8 μg mL−1, respectively (Table 2).

Table 2.
The LC50 values of compounds 1–2 against brine shrimp larvae.
In addition, cytotoxicity was evaluated based on the IC50 value against two human cancer cells (Hela and HepG-2) using the CCK-8 assay. None of the compounds showed good cytotoxicity.
3. Discussion
Lactams are a class of compounds with the cyclic structure of R1-CONH-R2 (the R group is generally a hydrocarbon group). The lactam rings have four to seven members, which can be synthesized using methods, such as acylation reactions, aminolysis reactions of amides, and cyclization reactions. The lactam skeleton is widely found in many natural products and synthetic molecules [18,19,20,21]. For example, lajollamycin, a natural nitro-tetraene spiro-β-lactone-γ-lactam antibiotic isolated from a strain of Streptomyces nodosus, exhibits antimicrobial activity against both drug-sensitive and drug-resistant microorganisms, and it shows cytotoxicity to the B16-F10 cell line with an EC50 of 9.6 µM [19]. Rolipram, a γ-lactam synthetic compound, was developed as an inhibitor of phosphodiesterase iv(pde-4), which is used for depression, with better antidepressant effects and tolerance than tricyclic antidepressants [22]. In recent decades, chemists have made many efforts to achieve the precise construction of chiral lactams using organic asymmetric catalysis and enzyme catalysis. Lactams have extensive applications in various fields, including pharmaceuticals, polymer materials, and pesticides [20,23]. Additionally, 2-azabicyclo[2.2.1]hept-5-en-3-one (γ-lactam) is also a key chiral synthon in the synthesis of the antiviral drugs carbovir and abacavir [24]. The two new lactam derivatives reported in this study are γ-lactam structures with substituents at positions 3 and 4, which theoretically have the potential to be developed.
In 2014, five phenethyl cinnamides isolated from M. falcatum [6], all of which have a core of micrometal C, were found in which micrometam C significantly decreased the elevation of reactive oxygen species levels, increase catalase, glutathione, and superoxide dismutase levels, and reduced the inflammation-associated migration of immune cells [25]. In terms of biosynthetic pathways, two new lactam derivatives(γ-lactams) 1–2 may be synthesized from these five phenethyl cinnamides via the cyclization of phenethyl cinnamides into a ring.
4. Materials and Methods
4.1. General Experimental Procedures
LC-ESI-MS was recorded on a SHIMADZU PR-LC MS-2020 LC/MS/MS mass spectrometer. HRESI-MS was performed using a VG Auto Spec-3000 MS spectrometer and an Agilent G6540A UHD Accurate-Mass Q-TOF LC/MS with a 1260 HPLC System (Agilent Technologies, Santa Clara, CA, USA). NMR spectra were recorded on a Bruker DRX-500MHz and Bruker Avance Ⅲ HD 400 MHz spectrometer with tetramethylsilane(TMS) as the internal standard (Bruker, Billerica, MA, USA). Optical rotation and IR spectra were measured on a Polaptronic-HNQW5 high-resolution polarimeter and a SHIMADZU IRPrestige-21 Fourier-Transform Infrared Spectrometer. Silica gel (200–300 mesh; Qingdao Haiyang Chemical Plant, Qingdao, China) and Sephadex LH-20 (GE Healthcare, Chicago, IL, USA) were used for the column chromatography. Thin-layer chromatography (TLC) was performed on pre-coated silica gel G plates (300–400 mesh, Qingdao Haiyang Chemical Plant, Qingdao, China), and spots were visualized by spraying the plates with a 50% H2SO4 solution, followed by heating. A Waters e2695 HPLC system (Phenomenex Luna 5u C18(2) 100 A, 250 nm × 4.60 mm) was equipped with a Waters 2998 photodiode array detector. Semi-preparative RP-HPLC was performed on ODS columns (YMC-Pack ODS-5-A, 250 mm × 10 mm, i.d., 5 μm, YMC) using a CH3OH–H2O solvent system as the eluent. Deuterated dimethyl sulfoxide-d6 (DMSO-d6), Methanol-d4 (CD3OD), and chloroform-d (CDCl3) were supplied by Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). DMEM and fetal bovine serum were supplied by Gibco Inc. (New York, NY, USA). The CCK-8 reagent was obtained from Beyotime Biotechnology (Shanghai, China). The cytotoxicity assay data were read using a microplate reader (BioTek EL808, Highland Park, VT, USA). By means of HPLC-MS, 1H-NMR, and ESI-MS, the purity of all compounds was investigated, and the degree of purity of all the tested compounds was >95%. All other reagents and solvents used were of reagent or HPLC analytical grade.
4.2. Plant Material
Micromelum falcatum (Lour.) Tan. collected from Sanya, Hainan Province, China, in January 2018, was authenticated by Prof. Jun Wu, Guangdong Medical University, and a voucher specimen was deposited at the Guangdong Provincial Key Laboratory of Pharmaceutical Bioactive Substances (No. MA-20180916-02).
4.3. Extraction and Isolation
The dried aerial parts of Micromelum falcatum (Lour.) Tan. were ground to a fine powder to obtain a 3.5 kg sample, which was extracted with 95% EtOH (15.0 L) three times. After the solvent was evaporated under reduced pressure, the residue (511.3 g) was extracted with n-hexane and EtOAc (4 × 2 L each). The EtOAc extract (85.0 g) was separated on silica gel (815 g, 200–300 mesh) using solvents of increasing polarity, 10–70% acetone in n-hexane, followed by 5–100% MeOH in CHCl3, to obtain 107 fractions. According to the TLC analysis, the fraction 15 (1.59 g) was subjected to CC (chloroform: acetone, 15:1) to yield a crude component, which was further purified using Sephadex LH-20 (MeOH) to yield 4 (15.0 mg). Fractions 23–25 (3.75 g) were subjected to CC (chloroform: acetone, 10:1) to yield fractions A-F. Fraction B was purified by Sephadex LH-20 (MeOH) to give 3 (13.7 mg) and 5 (11.3 mg), and fraction C was purified by semi-preparative HPLC (250 mm × 10 mm i.d., 5 μm, MeOH/H2O, 40:60, flow rate 3 mL min−1, UV detection at 254 and 280 nm) to yield 6.8 mg of 6 and 13.6 mg of 7. Fractions 43–44 were combined to give a 2.6 g mixture. The mixture was fractionated on silica gel (350 g, 200–300 mesh) with chloroform-acetone (8:2), and a total of 22 sub-fractions (ca. 180 mL each) were collected and combined using TLC. After purification using Sephadex LH-20, sub-fractions 17–20 (75–375 mL) were further separated via semi-preparative HPLC (250 × 10 mm i.d., 5 μm, MeOH/H2O, 50:50, flow rate 3 mL min−1, UV detection at 254 and 280 nm) to generate 6.2 mg of 1 and 14.2 mg of 2 [26,27,28].
3-(Hydroxy(10-hydroxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (1): yellow oil; [α-12.2 (c = 0.1, MeOH); UV-Vis (EtOH) λ/nm 228, 276; IR (KBr) v/cm−1:3262, 1651, 1614, 1516, 1248, 833; Positive ESI-MS m/z, observed: 649 [2M + Na]+ (45), 336 [M + Na]+ (93), 141 (100); HR-ESI-MS m/z, observed: 336.1216 (calcd for C18H19NO4Na+ [M + Na]+, 336.1206); 1H- and 13C-NMR data, see Table 1.
3-(Hydroxy(10-hydroxy-9-methoxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (2): yellow oil; [α-12.8 (c = 0.1, MeOH); UV-Vis (EtOH) λ/nm 228, 278; IR (KBr) v/cm−1: 3363, 1653, 1603, 1514, 1269, 1250, 831; Positive ESI-MS m/z, observed: 709 [2M + Na]+ (28), 366 [M + Na]+ (100); HR-ESI-MS m/z, observed: 366.1317; (calcd for C19H21NO5Na+ [M + Na]+, 366.1312); 1H- and 13C-NMR data, see Table 1.
4.4. Brine Shrimp Larvae Lethality Assays
Brine shrimp spawns were incubated in a beaker containing seawater and cultivated for 48 h at room temperature (22–29 °C). Brine shrimp larvae flocked together on one side of the container with the assistance of a luminous source, which was readily assembled for the brine shrimp larval lethality assay. Compounds 1–7 were dissolved in DMSO at a concentration of 50 mg mL−1, then diluted in a 96-well plate with 200 µL of seawater to test at final concentrations of 5, 50, and 500 μg mL−1. Each test was conducted in triplicate with approximately ten larvae. After 24 h of cultivation, the brine shrimp larvae were tallied under a luminous source in 96-well plates using a magnifier. LC50 values for each assay were calculated using a Finney Probit analysis program on a Dell computer [29].
4.5. Cytotoxicity Assays
In brief, 100 µL of the cell suspension was seeded into each well of 96-well plates (6 × 103 per well) and then incubated for 12–24 h to complete cell attachment. Fresh medium containing concentrations of test compounds was added to each well after removing the medium. Each concentration was tested in triplicate. After culturing for 24 h, 10 µL of the CCK-8 reagent was added to each well and incubated in a CO2 incubator for 1 h. The absorbance was measured at 450 nm using a microplate reader (BioTek EL808, Highland Park, VT, USA). The cytotoxicity assay data were read using Gen5 CHS V2.01 software [30].
5. Conclusions
The present phytochemical investigation of M. falcatum afforded two new lactam derivatives, 3-(hydroxy(10-hydroxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (1) and 3-(hydroxy(10-hydroxy-9-methoxyphenyl)methyl)-4-(16-hydroxyphenyl)-1-methylpyrrolidin-2-one (2), and five known compounds, trans-4-hydroxycinnamic acid (3), 4-hydroxybenzaldehyde (4), m-hydroxybenzoic acid (5), p-hydroxybenzoic acid (6), and gallic acid (7). All the isolated compounds were evaluated for their brine shrimp larvae and cytotoxic activities against HeLa and HepG-2 cells. Unfortunately, no compounds showed good cytotoxicity. The brine shrimp larvae lethality bioassay of the isolated compounds 1–2 showed moderate activity, exhibiting LC50 values of 50.6 and 121.8 μg mL−1, respectively. After determining the relative configurations of compounds 1–2 through the NOESY spectrum, we attempted to determine the absolute configurations. However, the Mosher reaction did not succeed. Compounds 1–2 also cannot easily form crystalline states. Due to the limited weight of the separated compounds, we only tested two activities and did not screen out compounds with good activity. Future work can start from multiple directions, such as determining the absolute configuration of compounds 1–2 through other methods (for instance, circular dichroism calculation), separating more weight of these compounds and testing other activities, or studying their drug synthesis pathways, and so on.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28207157/s1, Figures S1–S33 including 1H-NMR, 3C-NMR and mass figures of all isolated compounds as well as 2D NMR and IR figures for compounds 1-2.
Author Contributions
X.L. designed the study. B.L. isolated the compounds. X.L. and B.L. carried out the structure elucidation and edited the paper. X.C. and W.Z. carried out the biological activities assay. X.J. and X.W. provided experimental instrument support. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31670349) and the Young Scientists Fund of the National Natural Science Foundation of China (81802678) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained in the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of compounds 1–7 are available from the authors.
Abbreviations
The following abbreviations are used in this manuscript:
| EtOAc | Ethyl acetate |
| EtOH | Ethyl alcohol |
| AcOEt | Ethyl acetate |
| MeOH | Methanol |
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESI-MS | High resolution electrospray ionization mass spectroscopy |
| DEPT | Distortionless Enhancement by Polarization Transfer |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift Correlation Spectroscopy |
| NOESY | Nuclear Overhauser effect spectroscopy |
| ODS | Octadecyl silane |
| HPLC | High performance liquid chromatography |
| DMSO | Dimethyl sulfoxide |
| CCK-8 | Cell Counting Kit-8 |
References
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Qi, S.H.; Yin, H.; Gao, C.H.; Zhang, S. Alkaloids from the Stem Bark of Micromelum falcatum. Chem. Pharm. Bull. 2009, 57, 600. [Google Scholar] [CrossRef] [PubMed]
- Kamperdick, C.; Phuong, N.M.; Sung, T.V.; Schmidt, J.; Adam, G. Coumarins and dihydrocinnamic acid derivatives from Micromelum falcatum. Phytochemistry 1999, 52, 1671. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Ombito, J.O.; Chi, G.F.; Wansi, J.D. Ethnomedicinal uses, phytochemistry, and pharmacology of the genus Vepris (Rutaceae): A review. J. Ethnopharmacol. 2021, 267, 113622. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.; He, W.J.; Zhao, S.X.; Yin, Z.Q.; Tan, N.H. Studies on Cyclic Peptides and Other Chemical Constituents in the Stem and Leaves of Xiaoyunmu. In Proceedings of the 10th National Natural Organic Chemistry Academic Conference of the Chinese Chemical Society—Part 1: Natural Product Isolation and Structural Identification, Guangzhou, China, 21–23 November 2014; p. 193. [Google Scholar]
- Suthiwong, J.; Sriphana, U.; Thongsri, Y.; Promsuwan, P.; Prariyachatigul, C.; Yenjai, C. Coumarinoids from the fruits of Micromelum falcatum. Fitoterapia 2014, 4, 134–141. [Google Scholar] [CrossRef]
- Luo, X.M.; Huang, Y.; Zhang, S.; Yin, H.; Li, C.R.; Li, Q.X. Five new phenethyl cinnamides from the mangrove associates Micro-melum falcatum. Biochem. Syst. Ecol. 2014, 56, 191–195. [Google Scholar] [CrossRef]
- Cao, N.K.; Chen, Y.M.; Zhu, S.S.; Zeng, K.W.; Zhao, M.B.; Li, J.; Tu, P.F.; Jiang, Y. Isolation and structure characterization of cytotoxic alkaloids from Micromelum integerrimum. Phytochemistry 2020, 178, 112463. [Google Scholar] [CrossRef]
- Kong, Y.C.; But, P.P.H.; Ng, K.H.; Li, Q.; Cheng, K.F.; Waterman, P.G. Micromelum: A key genus in the chemosystematics of the Clauseneae. Biochem. Syst. Ecol. 1988, 16, 485. [Google Scholar] [CrossRef]
- Ng, P.C.; Ho, D.D.; Ng, K.H.; Kong, Y.C.; Cheng, K.F.; Stone, G. Mixed estrogenic and anti-estrogenic activities of yuehchukene—A bis-indole alkaloid. Eur. J. Pharmacol. 1994, 264, 1–12. [Google Scholar]
- Oliveira, D.R.; Nepomuceno, D.D.; Castro, R.N.; Braz, R.F.; Carvalho, M.G. Special metabolites isolated from Urochloa humidicola (Poaceae). An. Acad. Bras. Cienc. 2017, 89, 789–797. [Google Scholar] [CrossRef]
- Orfali, R.; Perveen, S.; Khan, M.F.; Ahmed, A.F.; Wadaan, M.A.; Al-Taweel, A.M.; Alqahtani, A.S.; Nasr, F.A.; Tabassum, S.; Luciano, P.; et al. Antiproliferative Illudalane Sesquiterpenes from the Marine Sediment Ascomycete Aspergillus oryzae. Mar. Drugs 2021, 19, 333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; He, X.F.; Jiang, C.X.; Zhang, W.; Shi, Z.N.; Li, H.F.; Zhu, Y. Two novel bioactive sulfated guaiane sesquiterpenoid salt alkaloids from the aerial parts of Scorzonera divaricata. Fitoterapia 2018, 124, 113–119. [Google Scholar] [CrossRef]
- Elhady, S.S.; Abdelhameed, R.F.A.; El-Ayouty, M.M.; Ibrahim, A.K.; Habib, E.S.; Elgawish, M.S.; Hassanean, H.A.; Safo, M.K.; Nafie, M.S.; Ahmed, S.A. New Antiproliferative Triflavanone from Thymelaea hirsuta-Isolation, Structure Elucidation and Molecular Docking Studies. Molecules 2021, 26, 739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Woo, S.; Sung, S.H.; Yang, H. A new phenolic compound from Phedimus middendorffianus with antiproliferative activity. Nat. Prod. Res. 2020, 34, 1663–1668. [Google Scholar] [CrossRef]
- Ohta, T.; Inoue, H.; Kusano, G.; Oshima, Y. Lepiotins A and B, New Alkaloids from the Mushrooms, Macrolepiota neomastoidea and Chlorophyllum molybdites. Heterocycles 1998, 47, 883. [Google Scholar] [CrossRef] [PubMed]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef] [PubMed]
- Manam, R.R.; Teisan, S.; White, D.J.; Nicholson, B.; Grodberg, J.; Neuteboom, S.T.; Lam, K.S.; Mosca, D.A.; Lloyd, G.K.; Potts, B.C. Lajollamycin, a nitro-tetraene spiro-beta-lactone-gamma-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J. Nat. Prod. 2005, 68, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Lin, S.; Chen, M.; Liu, T.; Wang, A.; Li, J.; Guo, Q.; Shang, X. Monascustin, an Unusual γ-Lactam from Red Yeast Rice. J. Nat. Prod. 2017, 80, 201–204. [Google Scholar] [CrossRef]
- Fong, A.; Ross, M.; Boudreau, J.; Nokhbeh, R.; Tilbe, K.; Lee, H. Raja 42, a novel gamma lactam compound, is effective against Clostridioides difficile. PLoS ONE 2021, 9, e0257143. [Google Scholar] [CrossRef]
- Cheol, H.Y.; Advait, N.; Chiliu, C.; Drashti, G.; Kyung, W.J. γ-Lactam Synthesis via C−H Insertion: Elaboration of N-Benzyl Protecting Groups for High Regioselectivity toward the Total Synthesis of Rolipram. Org. Lett. 2003, 5, 2259–2262. [Google Scholar]
- Sflakidou, E.; Dalezis, P.; Trafalis Dimitrios, T.; Sarli, V. Synthesis and antiproliferative activities of steroidal lactam conjugates bearing a new nitrogen mustard. Eur. J. Med. Chem. 2023, 5, 249. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, S.; Huang, R.; Lu, Y.; Zheng, G. Efficient synthesis of the intermediate of abacavir and carbovir using a novel (+)-γ-lactamase as a catalyst. Bioorg. Med. Chem. Lett. 2015, 25, 3878–3881. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ge, H.; Chen, Z.B.; Luo, X.M.; Su, F.J.; Liang, Y.B.; Li, Z.Y.; Wu, J.G.; Yang, Q.; Zeng, L.J.; et al. Micrometam C Protects against Oxidative Stress in Inflammation Models in Zebrafish and RAW264.7 Macrophages. Mar. Drugs 2015, 13, 5593–5605. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.D.; Rodenburg, D.L. Preparative chromatography and natural products discovery. Curr. Opin. Biotechnol. 2014, 25, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Periat, A.; Guillarme, D.; Veuthey, J.L.; Boccard, J.; Moco, S.; Barron, D.; Grand-Guillaume Perrenoud, A. Optimized selection of liquid chromatography conditions for wide range analysis of natural compounds. J. Chromatogr. A 2017, 1504, 91–104. [Google Scholar] [CrossRef]
- Namera, A.; Ota, S.; Tomioka, Y.; Saito, T.; Nagao, M. Facile determination of natural cannabinoids in cannabis products using a conventional fully porous particle column and isocratic high-performance liquid chromatography with diode-array detector. Forensic Toxicol. 2022, 40, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Wanyoike, G.N.; Chhabra, S.C.; Lang’at-Thoruwa, C.C.; Omar, S.A. Brine shrimp toxicity and antiplasmodial activity of five Kenyan medicinal plants. J. Ethnopharmacol. 2004, 90, 129–133. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Zhang, K.; Li, X.; Liu, J.; Zhang, W.J.; Yu, X.X. Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera. Molecules 2021, 26, 284. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).