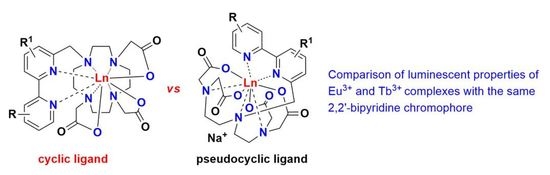
Comparison of Photophysical Properties of Lanthanide(III) Complexes of DTTA- or DO3A-Appended Aryl-2,2′-Bipyridines
Abstract
1. Introduction
2. Results
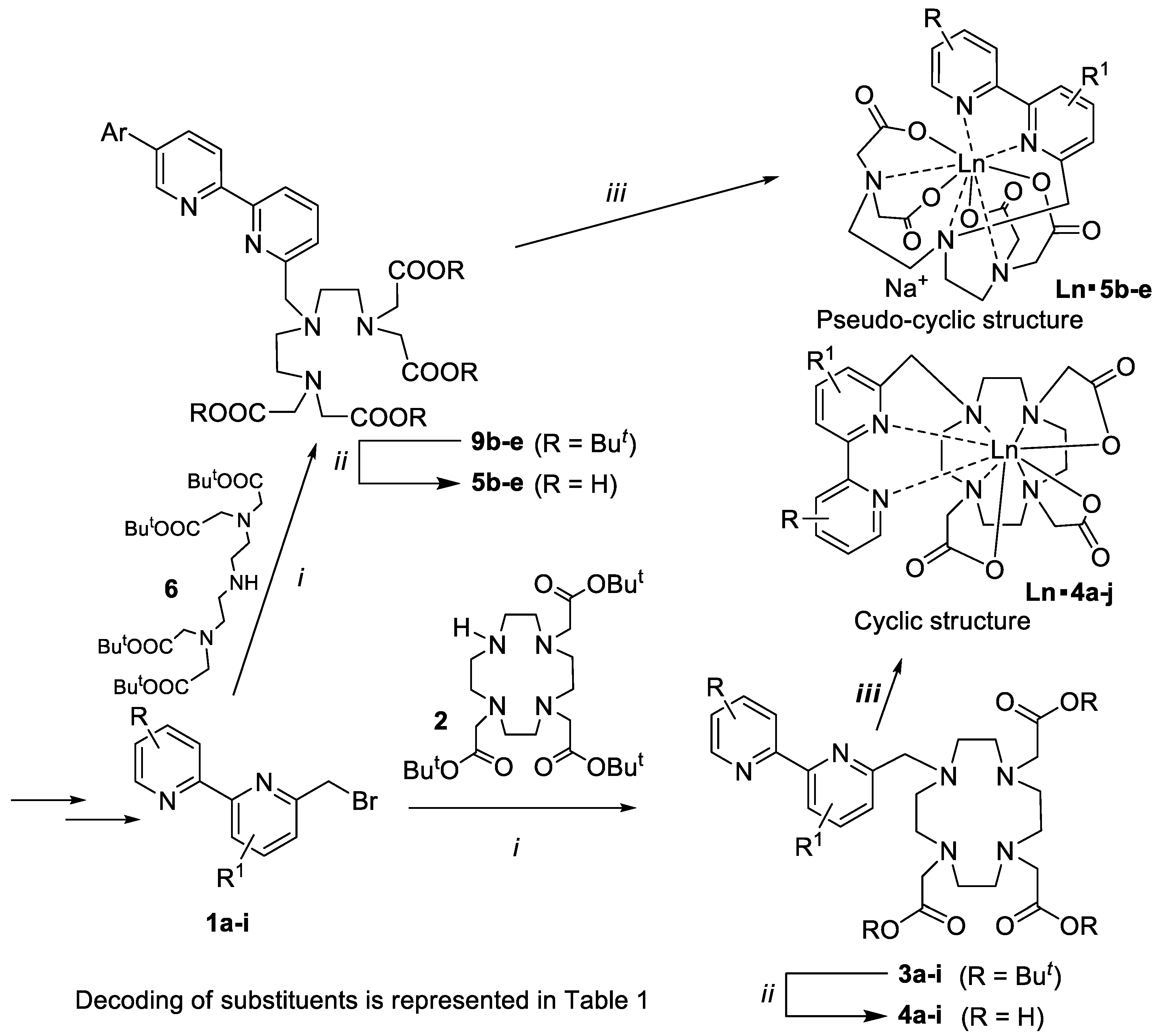
2.1. Synthesis of the New Water-Soluble Lanthanide(III) Complexes
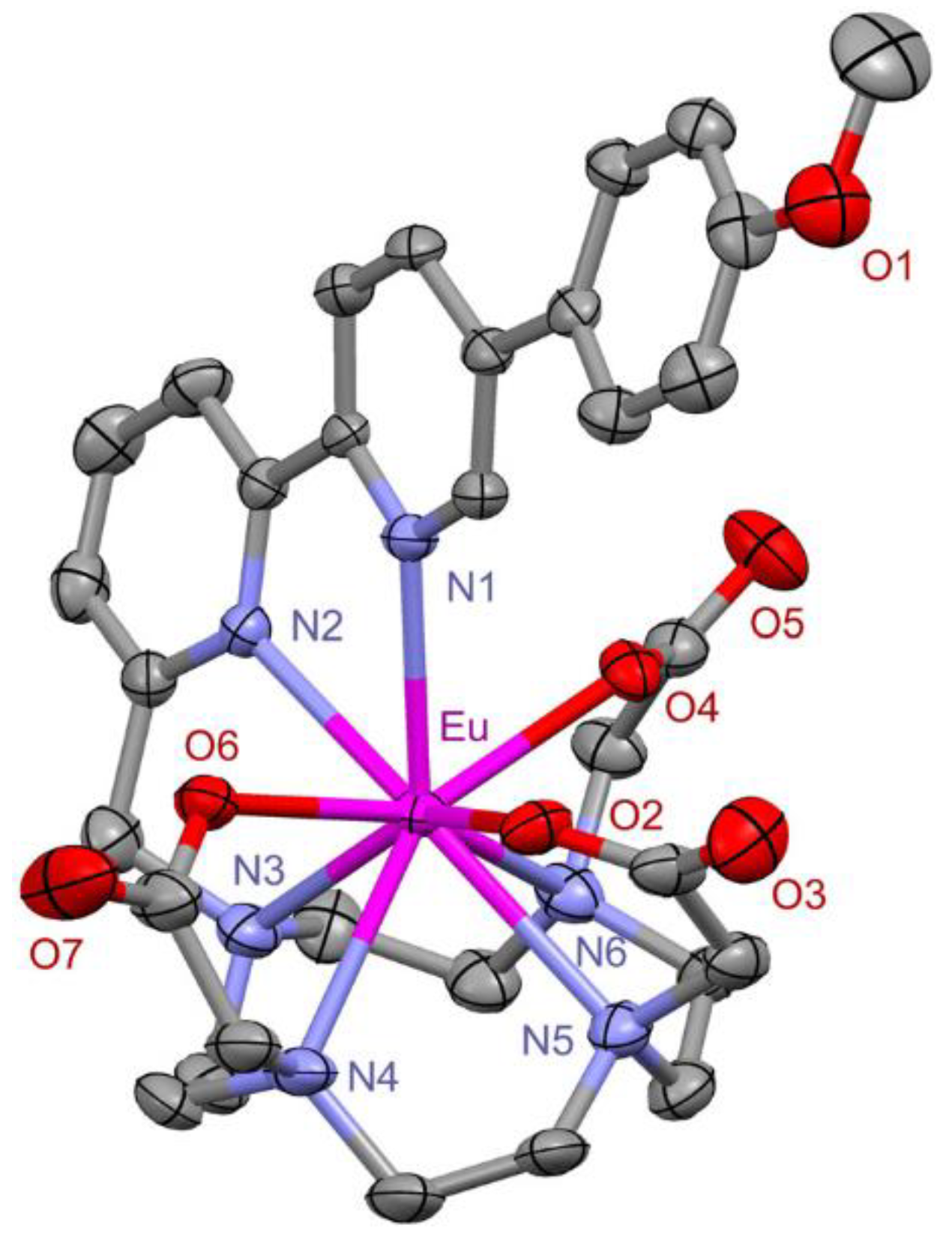
2.2. Photophysical Properties of the New Water-Soluble Lanthanide(III) Complexes
3. Experimental Methods
Materials and Equipment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Ohmagari, H.; Tanaka, H.; Machida, K. Luminescence of lanthanide complexes: From fundamental to prospective approaches related to water- and molecular-stimuli. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100484. [Google Scholar] [CrossRef]
- Hovinen, J.; Guy, P.M. Bioconjugation with stable luminescent lanthanide(III) chelates comprising pyridine subunits. Bioconjugate Chem. 2009, 20, 404–421. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, K.; Kikuchi, K.; Kojima, H.; Urano, Y.; Nagano, T. Development of a zinc ion-selective luminescent lanthanide chemosensor for biological applications. J. Am. Chem. Soc. 2004, 126, 12470–12476. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, G.; Chen, J.; Fu, X.; Zhang, W.; Yuan, J. Development of a novel terbium chelate-based luminescent chemosensor for time-resolved luminescence detection of intracellular Zn2+ ions. Biosens. Bioelectron. 2010, 26, 1043–1048. [Google Scholar] [CrossRef]
- Kotova, O.; Comby, S.; Gunnlaugsson, T. Sensing of biologically relevant d-metal ions using a Eu(III)-cyclen based luminescent displacement assay in aqueous pH 7.4 buffered solution. Chem. Commun. 2011, 47, 6810–6812. [Google Scholar] [CrossRef]
- Song, B.; Wang, G.; Tan, M.; Yuan, J. A Europium(III) Complex as an Efficient Singlet Oxygen Luminescence Probe. J. Am. Chem. Soc. 2006, 128, 13442–13450. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Cross, J.P.; Dadabhoy, A.; Sammes, P.G. The sensitivity of the Lehn cryptand-europium and terbium (III) complexes to anions compared to a coordinatively saturated systems. J. Lumin. 2004, 110, 113–124. [Google Scholar] [CrossRef]
- Montgomery, C.P.; Newa, E.J.; Palssona, L.O.; Parker, D.; Batsanov, A.S.; Lamarque, L. Emissive and Cell-Permeable 3-Pyridyl- and 3-Pyrazolyl-4-azaxanthone Lanthanide Complexes and Their Behavior in cellulo. Helv. Chim. Acta 2009, 92, 2186–2213. [Google Scholar] [CrossRef]
- Quici, S.; Marzanni, G.; Cavazzini, M.; Anelli, P.L.; Botta, M.; Gianolio, E.; Accorsi, G.; Armaroli, N.; Barigelletti, F. Highly Luminescent Eu3+ and Tb3+ Macrocyclic Complexes Bearing an Appended Phenanthroline Chromophore. Inorg. Chem. 2002, 41, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and Near-Infrared Intense Luminescence from Water-Soluble Lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] Complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Quici, S.; Scalera, C.; Cavazzini, M.; Accorsi, G.; Bolognesi, M.; Armelao, L.; Bottaro, G. Highly Photoluminescent Silica Layers Doped with Efficient Eu(III) and Tb(III) Antenna Complexes. Chem. Mater. 2009, 21, 2941–2949. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Gorbunov, E.B.; Kovalev, I.S.; Santra, S.; Zyryanov, G.V.; Majee, A.; Rusinov, V.L.; Chupakhin, O.N. Synthesis and luminescence of new water-soluble lanthanide complexes of DTTA-containing 4-(4-methoxyphenyl)-2,2′-bipyridine. Inorg. Chim. Acta 2018, 478, 49–53. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Ganebnykh, I.N.; Kovalev, I.S.; Santra, S.; Zyryanov, G.V.; Majee, A.; Rusinov, V.L.; Chupakhin, O.N. Highly-Luminescent DTTA-Appended Water-Soluble Lanthanide Complexes of 4-(Het)aryl-2,2-bipyridines: Synthesis and Photophysical Properties. Chem. Sel. 2019, 4, 6377–6381. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Shevyrin, V.A.; Egorov, I.N.; Santra, S.; Nosova, E.V.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N. Highly-Luminescent DTTA-Appended Lanthanide Complexes of 4-(Multi)fluoroaryl-2,2′-bipyridines: Synthesis and Photophysical Studies. Polyhedron 2021, 195, 114962. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Ganebnykh, I.N.; Kovalev, I.S.; Zyryanov, G.V.; Li, F.; Rusinov, V.L.; Chupakhin, O.N. DTTA-appended 6-phenyl- and 5,6-diphenyl-2,2′-bipyridines as new water soluble ligands for lanthanide cations. Polyhedron 2017, 134, 59–64. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Kim, G.A.; Shevyrin, V.A.; Santra, S.; Rahman, M.; Taniya, O.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Water-soluble luminescent lanthanide complexes based on C6-DTTA-appended 5-aryl-2,2′-bipyridines. Polyhedron 2020, 181, 114473. [Google Scholar] [CrossRef]
- Prokhorov, A.M.; Kozhevnikov, V.N.; Kopchuk, D.S.; Bernard, H.; Le Bris, N.; Tripier, R.; Handel, H.; Koenig, B.; Kozhevnikov, D.N. 1,2,4-Triazine method of bipyridine ligand synthesis for the preparation of new luminescent Eu(III) complexes. Tetrahedron 2011, 67, 597–607. [Google Scholar] [CrossRef]
- Krinochkin, A.P.; Kopchuk, D.S.; Shtaitz, Y.K.; Starnovskaya, E.S.; Savchuk, M.I.; Zyryanov, G.V.; Chupakhin, O.N.; Charushin, V.N. Synthesis of Water-Soluble Gadolinium(III) Complexes Based on 5-Aryl-2,2′-bipyridine with a DTTA Residue in Position C6′. AIP Conf. Proc. 2021, 2388, 030017-1–030017-5. [Google Scholar]
- Kopchuk, D.S.; Pavlyuk, D.E.; Kovalev, I.S.; Zyryanov, G.V.; Rusinov, V.L.; Chupakhin, O.N. Synthesis of a new DTTA-and 5-phenyl-2,2′-bipyridine-based ditopic ligand and its Eu3+ complex. Can. J. Chem. 2016, 94, 599–603. [Google Scholar] [CrossRef]
- Platzek, J.; Niedballa, U.; Radeuchel, B. Process for the Production of DTPA-Tetraesters of Terminal Carboxylic. U.S. Patent No. 5,514,810, 7 May 1996. [Google Scholar]
- Kopchuk, D.S.; Krinochkin, A.P.; Kozhevnikov, D.N.; Slepukhin, P.A. Novel neutral lanthanide complexes of 5-aryl-2,2′-bipyridine-6′-carboxylic acids with improved photophysical properties. Polyhedron 2016, 118, 30–36. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishic, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef] [PubMed]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; De Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc., Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Parker, D. Luminescent lanthanide sensors for pH, pO2 and selected anions. Coord. Chem. Rev. 2000, 205, 109–130. [Google Scholar] [CrossRef]






 | |||||||||
| Entry | Ligand, [Ref.] | Ar | Ln | λmax, nm a | E b, 10−3 M−1·cm−1 | ΦLn, % c | τH2O, ms d | τD2O, ms e | q f |
| 1 | 10 [21] | Ph | Eu | 304 | 7.95 | 0.34 | 0.369 | 1.734 | 2.02 |
| 2 | 5a [19] | Ph | Eu | 278, 327 | 12.02 | 9.3 | 0.80 | 1.30 | 0.27 |
| 3 | 4a [19] | Ph | Eu | 325 | 7.505 | 7.3 | 1.00 | 1.10 | −0.19 |
| 4 | 5b | 4-MeOC6H4 | Eu | 261, 339 | 12.1 | 10.7 | 0.95 | 1.53 | 0.18 |
| 5 | 4b [19] | 4-MeOC6H4 | Eu | 341 | 9.5 | 1.1 | 0.27 | 0.30 | 0.15 |
| 6 | 5c | 4-Tol | Eu | 281, 331 | 11.5 | 14.8 | 1.10 | 1.90 | 0.16 |
| 7 | 4c | 4-Tol | Eu | 261, 282, 332 | 10.8 | 24.9 | 1.094 | 1.586 | 0.05 |
| 8 | 5d | 4-ClC6H4 | Eu | 280, 328 | 11.64 | 17.6 | 1.106 | 1.936 | 0.17 |
| 9 | 4d | 4-ClC6H4 | Eu | 258sh, 280, 331 | 12.87 | 24.1 | 1.20 | 1.67 | −0.02 |
| 10 | 5e | 3-ClC6H4 | Eu | 274, 325 | 11.72 | 12.2 | 1.08 | 1.927 | 0.19 |
| 11 | 4e | 3-ClC6H4 | Eu | 255sh, 273, 326 | 11.02 | 31.1 | 1.14 | 1.58 | 0 |
| 12 | 5f [14] | 4-MeOC6H4 | Eu | 260sh, 278, 311 | 9.2 | 23.0 | 0.98 | 1.02 | −0.25 |
| 13 | 4f | 4-MeOC6H4 | Eu | 253, 279, 315 | 9.78 | 15.6 | 1.13 | 1.61 | 0.02 |
| 14 | 5f | 4-MeOC6H4 | Tb | 261sh, 278, 311 | 9.3 | 11.3 | 0.35 | 0.37 | 0.26 |
| 15 | 4f | 4-MeOC6H4 | Tb | 253, 278, 316 | 9.5 | 13.4 | 0.368 | 0.387 | 0.32 |
| 16 | 5g [15] | 3-ClC6H4 | Eu | 254, 259sh, 265, 276sh, 314, 329sh | 8.6 | 25.2 | 1.03 | 1.71 | 0.17 |
| 17 | 4g | 3-ClC6H4 | Eu | 250, 269, 317sh | 9.1 | 21.8 | 1.12 | 1.62 | 0.03 |
| 18 | 5g [15] | 3-ClC6H4 | Tb | 254, 259sh, 265, 276sh, 313, 330sh | 8.6 | 56.1 | 1.10 | 1.23 | 0.17 |
| 19 | 4g | 3-ClC6H4 | Tb | 253, 268sh, 317sh | 9.2 | 18.1 | 0.473 | 0.503 | 0.28 |
| 20 | 5h [16] | 2-FC6H4 | Eu | 251, 267, 274sh, 312, 322sh | 8.9 | 16.2 | 1.07 | 1.86 | 0.18 |
| 21 | 4h | 2-FC6H4 | Eu | 251, 266sh, 314,321sh | 9.5 | 26.2 | 1.13 | 1.66 | 0.04 |
| 22 | 5h [16] | 2-FC6H4 | Tb | 251, 267, 274sh, 312, 322sh | 9.2 | 49.2 | 0.99 | 1.27 | 0.68 |
| 23 | 4h | 2-FC6H4 | Tb | 251, 267sh, 312, 322sh | 9.9 | 20.9 | 0.527 | 0.567 | 0.32 |
| 24 | 5i [18] | Ph | Eu | 254, 316 | 9.7 | 12.8 | 1.09 | 1.88 | 0.17 |
| 25 | 4i | Ph | Eu | 253, 262sh, 316 | 9.6 | 25.2 | 1.16 | 1.70 | 0.03 |
| 26 | 5i [18] | Ph | Tb | 254, 317 | 9.8 | 0.43 | - | - | - |
| 27 | 4i | Ph | Tb | 254, 262sh, 317 | 10.2 | <0.1 | - | - | - |
| 28 | 5j [12] | - | Eu | 279 | - | 24 | 1.25 | 1.88 | 0.02 |
| 29 | 4j [11] | - | Eu | 295 | - | 21 | 1.24 | 1.77 | −0.01 |
| 30 | 5j [12] | - | Tb | 279 | - | 15 | 0.78 | 0.82 | 0.01 |
| 31 | 4j [11] | - | Tb | 295 | - | 11 | 0.31 | 0.32 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krinochkin, A.P.; Valieva, M.I.; Starnovskaya, E.S.; Shtaitz, Y.K.; Kopchuk, D.S.; Taniya, O.S.; Kim, G.A.; Shevyrin, V.A.; Zyryanov, G.V.; Chupakhin, O.N. Comparison of Photophysical Properties of Lanthanide(III) Complexes of DTTA- or DO3A-Appended Aryl-2,2′-Bipyridines. Molecules 2023, 28, 724. https://doi.org/10.3390/molecules28020724
Krinochkin AP, Valieva MI, Starnovskaya ES, Shtaitz YK, Kopchuk DS, Taniya OS, Kim GA, Shevyrin VA, Zyryanov GV, Chupakhin ON. Comparison of Photophysical Properties of Lanthanide(III) Complexes of DTTA- or DO3A-Appended Aryl-2,2′-Bipyridines. Molecules. 2023; 28(2):724. https://doi.org/10.3390/molecules28020724
Chicago/Turabian StyleKrinochkin, Alexey P., Maria I. Valieva, Ekaterina S. Starnovskaya, Yaroslav K. Shtaitz, Dmitry S. Kopchuk, Olga S. Taniya, Grigory A. Kim, Vadim A. Shevyrin, Grigory V. Zyryanov, and Oleg N. Chupakhin. 2023. "Comparison of Photophysical Properties of Lanthanide(III) Complexes of DTTA- or DO3A-Appended Aryl-2,2′-Bipyridines" Molecules 28, no. 2: 724. https://doi.org/10.3390/molecules28020724
APA StyleKrinochkin, A. P., Valieva, M. I., Starnovskaya, E. S., Shtaitz, Y. K., Kopchuk, D. S., Taniya, O. S., Kim, G. A., Shevyrin, V. A., Zyryanov, G. V., & Chupakhin, O. N. (2023). Comparison of Photophysical Properties of Lanthanide(III) Complexes of DTTA- or DO3A-Appended Aryl-2,2′-Bipyridines. Molecules, 28(2), 724. https://doi.org/10.3390/molecules28020724