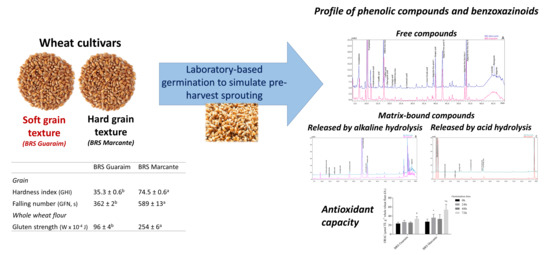
Grain Germination Changes the Profile of Phenolic Compounds and Benzoxazinoids in Wheat: A Study on Hard and Soft Cultivars
Abstract
1. Introduction
2. Results and Discussion
2.1. Grain Characteristics, Alveography and Germination Follow-Up in Wheat Cultivars
2.2. Tentative Identification of Phytochemical Compounds
2.3. Changes in the Phytochemical Profile of Wheat during Germination
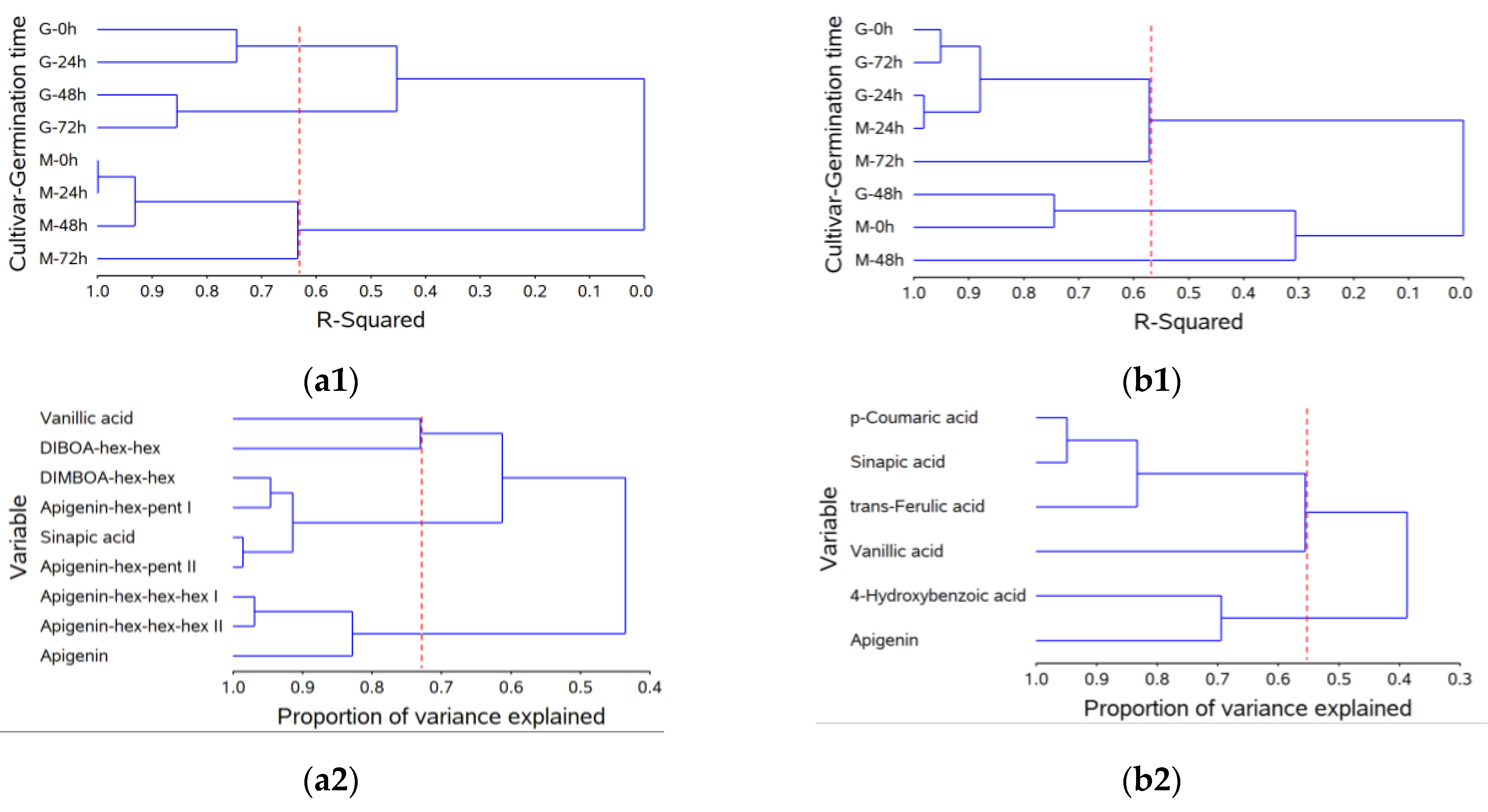
2.4. Multivariate Analysis of Phytochemical Changes in during Wheat Germination
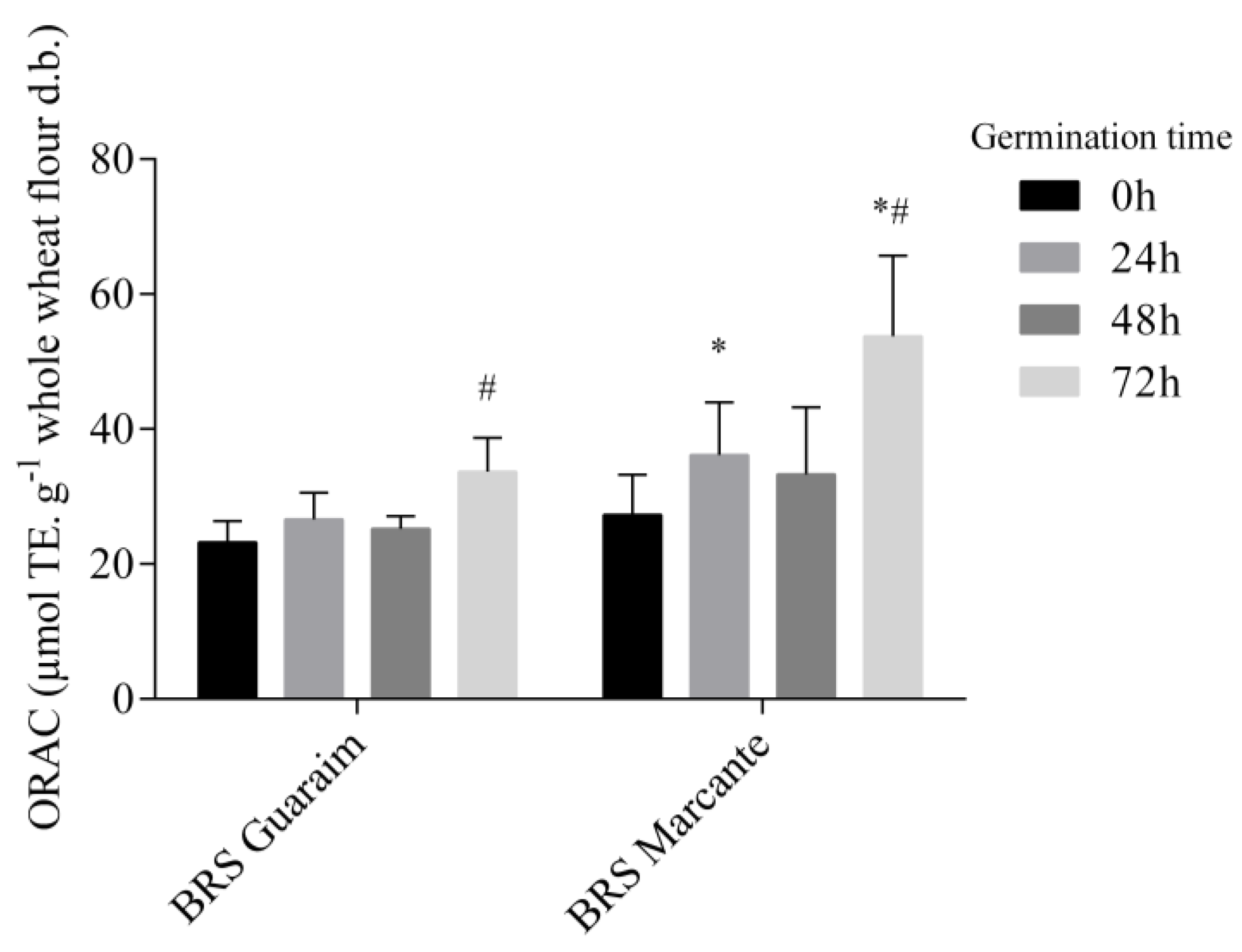
2.5. Antioxidant Capacity of Wheat Cultivars during Germination
3. Materials and Methods
3.1. Wheat Samples
3.2. Samples Characterization
3.3. Germination
3.4. Grain Milling
3.5. Germination Marker
3.6. Extraction of Free and Matrix-Bound Phytochemical Compounds
3.7. Phytochemical Compounds Identification
3.8. Quantification of Phytochemical Compounds
3.9. Oxygen Radical Absorbance Capacity (ORAC)
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Nonogaki, H.; Barrero, J. Seed Dormancy, Germination and Pre-Harvest Sprouting; Balestrazzi, A., Ed.; University of Pavia: Pavia, Italy, 2019; Volume 9, ISBN 9782889457625. [Google Scholar]
- Baranzelli, J.; Kringel, D.H.; Colussi, R.; Paiva, F.F.; Aranha, B.C.; de Miranda, M.Z.; Zavareze, E.d.R.; Dias, A.R.G. Changes in Enzymatic Activity, Technological Quality and Gamma-Aminobutyric Acid (GABA) Content of Wheat Flour as Affected by Germination. LWT-Food Sci. Technol. 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Olaerts, H.; Courtin, C.M. Impact of Preharvest Sprouting on Endogenous Hydrolases and Technological Quality of Wheat and Bread: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Olaerts, H.; Roye, C.; Derde, L.J.A.; Sinnaeve, G.; Meza, W.R.; Bodson, B.; Courtin, C.M. Impact of Preharvest Sprouting of Wheat (Triticum Aestivum) in the Field on Starch, Protein and Arabinoxylan Properties. J. Agric. Food Chem. 2016, 64, 8324–8332. [Google Scholar] [CrossRef] [PubMed]
- Baranzelli, J.; Kringel, D.H.; Mallmann, J.F.; Bock, E.; el Halal, S.L.M.; Prietto, L.; Zavareze, E.d.R.; de Miranda, M.Z.; Dias, A.R.G. Impact of Wheat (Triticum aestivum L.) Germination Process on Starch Properties for Application in Films. Starch/Staerke 2019, 71, 1800262. [Google Scholar] [CrossRef]
- Kringel, D.H.; Baranzelli, J.; Schöffer, J.D.N.; el Halal, S.L.M.; de Miranda, M.Z.; Dias, A.R.G.; Zavareze, E.D.R. Germinated Wheat Starch as a Substrate to Produce Cyclodextrins: Application in Inclusion Complex to Improve the Thermal Stability of Orange Essential Oil. Starch/Staerke 2020, 72, 1–7. [Google Scholar] [CrossRef]
- Hefni, M.; Witthöft, C.M. Increasing the Folate Content in Egyptian Baladi Bread Using Germinated Wheat Flour. LWT-Food Sci. Technol. 2011, 44, 706–712. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of Germination on Chemical, Functional and Nutritional Characteristics of Wheat, Brown Rice and Triticale: A Comparative Study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Nelson, K.; Mathai, M.L.; Ashton, J.F.; Donkor, O.N.; Vasiljevic, T.; Mamilla, R.; Stojanovska, L. Effects of Malted and Non-Malted Whole-Grain Wheat on Metabolic and Inflammatory Biomarkers in Overweight/Obese Adults: A Randomised Crossover Pilot Study. Food Chem. 2016, 194, 495–502. [Google Scholar] [CrossRef]
- Adhikari, K.B.; Tanwir, F.; Gregersen, P.L.; Steffensen, S.K.; Jensen, B.M.; Poulsen, L.K.; Nielsen, C.H.; Høyer, S.; Borre, M.; Fomsgaard, I.S. Benzoxazinoids: Cereal Phytochemicals with Putative Therapeutic and Health-Protecting Properties. Mol. Nutr. Food Res. 2015, 59, 1324–1338. [Google Scholar] [CrossRef]
- Kadiri, O. A Review on the Status of the Phenolic Compounds and Antioxidant Capacity of the Flour: Effects of Cereal Processing. Int. J. Food Prop. 2017, 20, S798–S809. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, E.; Moroni, A.v.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.-A.; van den Broeck, H.C.; Brouns, F.J.P.H.; de Brier, N.; et al. Impact of Cereal Seed Sprouting on Its Nutritional and Technological Properties: A Critical Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Kowalska, I.; Jędrejek, D. Benzoxazinoid and Alkylresorcinol Content, and Their Antioxidant Potential, in a Grain of Spring and Winter Wheat Cultivated under Different Production Systems. J. Cereal Sci. 2020, 95, 103063. [Google Scholar] [CrossRef]
- de Bruijn, W.J.C.; Gruppen, H.; Vincken, J.P. Structure and Biosynthesis of Benzoxazinoids: Plant Defence Metabolites with Potential as Antimicrobial Scaffolds. Phytochemistry 2018, 155, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Tanwir, F.; Dionisio, G.; Adhikari, K.B.; Fomsgaard, I.S.; Gregersen, P.L. Biosynthesis and Chemical Transformation of Benzoxazinoids in Rye during Seed Germination and the Identification of a Rye Bx6-like Gene. Phytochemistry 2017, 140, 95–107. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Merillon, J.-M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, pp. 1543–1580. ISBN 9783642221446. [Google Scholar]
- Saura-Calixto, F. Concept and Health-Related Properties of Nonextractable Polyphenols: The Missing Dietary Polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive Compounds and Bioactivities of Germinated Edible Seeds and Sprouts: An Updated Review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S. Bioactive Components and Functional Properties of Biologically Activated Cereal Grains: A Bibliographic Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3051–3071. [Google Scholar] [CrossRef]
- Makowska, B.; Bakera, B.; Rakoczy-Trojanowska, M. The Genetic Background of Benzoxazinoid Biosynthesis in Cereals. Acta Physiol. Plant 2015, 37, 176. [Google Scholar] [CrossRef]
- AACCI. American Association of Cereal Chemists, 11th ed.; Approved Methods of the AACC International: St. Paul, MI, USA, 2010. [Google Scholar]
- Caierão, E.; e Silva, M.S.; Scheeren, P.L.; de Castro, R.L.; Eichelberger, L.; do Nascimento Junior, A.; Guarienti, E.M.; de Miranda, M.Z.; Pires, J.L.F.; Maciel, J.L.N.; et al. Resultados Agronômicos e Qualitativos Da Nova Cultivar de Trigo ‘BRS Marcante’. Ciência Rural 2015, 45, 644–646. [Google Scholar] [CrossRef]
- Scheeren, P.L.; Caierão, E.; e Silva, M.S.; de Castro, R.L.; Caetano, V.d.R.; Bassoi, M.C.; Pires, J.L.F.; Eichelberger, L.; de Miranda, M.Z.; Guarienti, E.M.; et al. BRS-Guaraim-Cultivar de Trigo Brando e Farinha Branqueadora. XI Reun. Comissão Bras. Pesqui. Trigo Triticale 2017, 11, 1–5. [Google Scholar]
- Sharma, S.; Katyal, M.; Singh, N.; Singh, A.M.; Ahlawat, A.K. Comparison of Effect of Using Hard and Soft Wheat on the High Molecular Weight-Glutenin Subunits Profile and the Quality of Produced Cookie. J. Food Sci. Technol. 2022, 59, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, M.; Nonogaki, H. Germination. In Encyclopedia of Applied Plant Sciences; Thomas, B., Ed.; Academic Press: Salem, OR, USA, 2017; Volume 1, pp. 509–512. ISBN 9780123948083. [Google Scholar]
- Olaerts, H.; Roye, C.; Derde, L.J.A.; Sinnaeve, G.; Meza, W.R.; Bodson, B.; Courtin, C.M. Evolution and Distribution of Hydrolytic Enzyme Activities during Preharvest Sprouting of Wheat (Triticum aestivum) in the Field. J. Agric. Food Chem. 2016, 64, 5644–5652. [Google Scholar] [CrossRef]
- Miã, Â.A.; Grundas, S. Wheat Grain Hardness Modified by the Laboratory Sprouting Test. Int. Agrophys. 2012, 16, 283–288. [Google Scholar]
- You, S.-Y.; Oh, S.-G.; Han, H.M.; Jun, W.; Hong, Y.-S.; Chung, H.-J. Impact of Germination on the Structures and in vitro Digestibility of Starch from Waxy Brown Rice. Int. J. Biol. Macromol. 2016, 82, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zilic, S. Phenolic Compounds of Wheat. Their Content, Antioxidant Capacity and Bioaccessibility. MOJ Food Process. Technol. 2016, 2, 37. [Google Scholar] [CrossRef]
- Gammoh, S.; Alu’datt, M.H.; Alhamad, M.N.; Rababah, T.; Ereifej, K.; Almajwal, A.; Ammari, Z.A.; al Khateeb, W.; Hussein, N.M. Characterization of Phenolic Compounds Extracted from Wheat Protein Fractions Using High-Performance Liquid Chromatography/Liquid Chromatography Mass Spectrometry in Relation to Anti-Allergenic, Anti-Oxidant, Anti-Hypertension, and Anti-Diabetic Propertie. Int. J. Food Prop. 2017, 20, 2383–2395. [Google Scholar] [CrossRef]
- Sharma, M.; Rahim, M.S.; Kumar, P.; Mishra, A.; Sharma, H.; Roy, J. Large-Scale Identification and Characterization of Phenolic Compounds and Their Marker–Trait Association in Wheat. Euphytica 2020, 216, 127. [Google Scholar] [CrossRef]
- Koistinen, V.M.; Hanhineva, K. Mass Spectrometry-Based Analysis of Whole-Grain Phytochemicals. Crit. Rev. Food Sci. Nutr. 2017, 57, 1688–1709. [Google Scholar] [CrossRef]
- Zhang, P.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.T.; Lei, K.M.K.; Poon, T.C.W. Revisiting Fragmentation Reactions of Protonated α-Amino Acids by High-Resolution Electrospray Ionization Tandem Mass Spectrometry with Collision-Induced Dissociation. Sci. Rep. 2019, 9, 6453. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Keski-Rahkonen, P.; Lappi, J.; Katina, K.; Pekkinen, J.; Savolainen, O.; Timonen, O.; Paananen, J.; Mykkänen, H.; Poutanen, K. The Postprandial Plasma Rye Fingerprint Includes Benzoxazinoid-Derived Phenylacetamide Sulfates. J. Nutr. 2014, 144, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, W.J.C.; Vincken, J.P.; Duran, K.; Gruppen, H. Mass Spectrometric Characterization of Benzoxazinoid Glycosides from Rhizopus-Elicited Wheat (Triticum aestivum) Seedlings. J. Agric. Food Chem. 2016, 64, 6267–6276. [Google Scholar] [CrossRef]
- Santos, M.C.B.; Lima, L.R.d.S.; Nascimento, F.R.; do Nascimento, T.P.; Cameron, L.C.; Ferreira, M.S.L. Metabolomic Approach for Characterization of Phenolic Compounds in Different Wheat Genotypes during Grain Development. Food Res. Int. 2018, 124, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Pihlava, J.-M.; Hellstrom, J.; Kurtelius, T.; Mattila, P. Flavonoids, Anthocyanins, Phenolamides, Benzoxazinoids, Lignans and Alkylresorcinols in Rye (Secale cereale) and Some Rye Products. J. Cereal Sci. 2018, 79, 183–192. [Google Scholar] [CrossRef]
- Zhu, Y.; Sang, S. Phytochemicals in Whole Grain Wheat and Their Health-Promoting Effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef]
- Tian, C.; Wang, Y.; Yang, T.; Sun, Q.; Ma, M.; Li, M. Evolution of Physicochemical Properties, Phenolic Acid Accumulation, and Dough-Making Quality of Whole Wheat Flour During Germination Under UV-B Radiation. Front. Nutr. 2022, 1, 877324. [Google Scholar] [CrossRef]
- Kim, M.J.; Kwak, H.S.; Kim, S.S. Effects of Germination on Protein, γ-Aminobutyric Acid, Phenolic Acids, and Antioxidant Capacity in Wheat. Molecules 2018, 23, 2244. [Google Scholar] [CrossRef]
- Tanwir, F.; Fredholm, M.; Gregersen, P.L.; Fomsgaard, I.S. Comparison of the Levels of Bioactive Benzoxazinoids in Different Wheat and Rye Fractions and the Transformation of These Compounds in Homemade Foods. Food Chem. 2013, 141, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Dihm, K.; Lind, M.V.; Sundén, H.; Ross, A.; Savolainen, O. Quantification of Benzoxazinoids and Their Metabolites in Nordic Breads. Food Chem. 2017, 235, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Rogachev, I.; Aura, A.M.; Aharoni, A.; Poutanen, K.; Mykkänen, H. Qualitative Characterization of Benzoxazinoid Derivatives in Whole Grain Rye and Wheat by LC-MS Metabolite Profiling. J. Agric. Food Chem. 2011, 59, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Rao, J.; Chen, B. Phenolic Compounds in Germinated Cereal and Pulse Seeds: Classification, Transformation, and Metabolic Process. Crit. Rev. Food Sci. Nutr. 2020, 60, 740–759. [Google Scholar] [CrossRef]
- Zilic, S.; Basic, Z.; Sukalovic, V.H.-T.; Maksimovic, V.; Jankovic, M.; Filipovic, M. Can the Sprouting Process Applied to Wheat Improve the Contents of Vitamins and Phenolic Compounds and Antioxidant Capacity of the Flour? Int. J. Food Sci. Technol. 2014, 49, 1040–1047. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Nichelle, S.M.; Minuzzi, N.M.; Teixeira, C.F.; da Cruz, I.B.M.; Júnior, M.R.M.; Emanuelli, T. Dietary Fiber and Fiber-Bound Polyphenols of Grape Peel Powder Promote GSH Recycling and Prevent Apoptosis in the Colon of Rats with TNBS-Induced Colitis. J. Funct. Foods 2020, 64, 103644. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Nichelle, S.M.; Lamas, C.d.A.; Cagnon, V.H.A.; Morari, J.; Velloso, L.A.; Júnior, M.R.M.; et al. Grape Peel Powder Attenuates the Inflammatory and Oxidative Response of Experimental Colitis in Rats by Modulating the NF-ΚB Pathway and Activity of Antioxidant Enzymes. Nutr. Res. 2020, 76, 52–70. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019; Volume Chapter 2, pp. 33–50. ISBN 9780128147757. [Google Scholar]
- Naumenko, N.; Potoroko, I.; Kalinina, I. Stimulation of Antioxidant Activity and γ-Aminobutyric Acid Synthesis in Germinated Wheat Grain Triticum Aestivum L. by Ultrasound: Increasing the Nutritional Value of the Product. Ultrason. Sonochem. 2022, 86, 106000. [Google Scholar] [CrossRef]
- Miyahira, R.F.; Pena, F.d.L.; Fabiano, G.A.; Lopes, J.d.O.; Ponte, L.G.S.; da Cunha, D.T.; Bezerra, R.M.N.; Antunes, A.E.C. Changes in Phenolic Compound and Antioxidant Activity of Germinated Broccoli, Wheat, and Lentils during Simulated Gastrointestinal Digestion. Plant Foods Hum. Nutr. 2022, 77, 233–240. [Google Scholar] [CrossRef]
- Yang, B.; Yin, Y.; Liu, C.; Zhao, Z.; Guo, M. Effect of Germination Time on the Compositional, Functional and Antioxidant Properties of Whole Wheat Malt and Its End-Use Evaluation in Cookie-Making. Food Chem. 2021, 349, 129125. [Google Scholar] [CrossRef]
- Dhillon, B.; Choudhary, G.; Sodhi, N.S. A Study on Physicochemical, Antioxidant and Microbial Properties of Germinated Wheat Flour and Its Utilization in Breads. J. Food Sci. Technol. 2020, 57, 2800–2808. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Weng, Y.; Ma, Y.; Gu, Z.; Yang, R. Comparison of Phenolic Profiles, Antioxidant Capacity and Relevant Enzyme Activity of Different Chinese Wheat Varieties during Germination. Food Biosci. 2017, 20, 159–167. [Google Scholar] [CrossRef]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Costa, E.L.; Morari, J.; Velloso, L.A.; Leal, R.F.; Rodrigues, E.; Bochi, V.C.; et al. Grape Peel Powder Promotes Intestinal Barrier Homeostasis in Acute TNBS-Colitis: A Major Role for Dietary Fiber and Fiber-Bound Polyphenols. Food Res. Int. 2019, 123, 425–439. [Google Scholar] [CrossRef]
- Brasil, M.A.P.A. Regras Para Análise de Sementes; Ministério da Agricultura Pecuária e Abastecimento: Brasília, Brazil, 2009; Volume 1, ISBN 1932-6203.
- Zhang, Y.; Wang, L.; Yao, Y.; Yan, J.; He, Z. Phenolic Acid Profiles of Chinese Wheat Cultivars. J. Cereal Sci. 2012, 56, 629–635. [Google Scholar] [CrossRef]
- Astudillo-Pascual, M.; Domínguez, I.; Aguilera, P.A.; Frenich, A.G. New Phenolic Compounds in Posidonia Oceanica Seagrass: A Comprehensive Array Using High Resolution Mass Spectrometry. Plants 2021, 10, 864. [Google Scholar] [CrossRef]
- Quatrin, A.; Pauletto, R.; Maurer, L.H.; Minuzzi, N.; Nichelle, S.M.; Carvalho, J.F.C.; Maróstica, M.R.; Rodrigues, E.; Bochi, V.C.; Emanuelli, T. Characterization and Quantification of Tannins, Flavonols, Anthocyanins and Matrix-Bound Polyphenols from Jaboticaba Fruit Peel: A Comparison between Myrciaria Trunciflora and M. Jaboticaba. J. Food Compos. Anal. 2019, 78, 59–74. [Google Scholar] [CrossRef]
- Mallmann, L.P.; Tischer, B.; Vizzotto, M.; Rodrigues, E.; Manfroi, V. Comprehensive Identification and Quantification of Unexploited Phenolic Compounds from Red and Yellow Araçá (Psidium cattleianum Sabine) by LC-DAD-ESI-MS/MS. Food Res. Int. 2020, 131, 108978. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]

| Germination Time | BRS Guaraim | BRS Marcante | Time Mean |
|---|---|---|---|
| 0 h | 352 ± 12 aB | 389 ± 6 aA | 370 ± 8 |
| 24 h | 216 ± 7 bA | 162 ± 7 bB | 189 ± 9 |
| 48 h | 96 ± 5 cA | 69 ± 2 cB | 82 ± 4 |
| 72 h | 62 ± 0 dA | 62 ± 0 cA | 62 ± 0 |
| Cultivar mean | 181 ± 21 | 171 ± 24 |
| Cultivar (C) | Germination Time (G) | Mean | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | C | G | C × G | ||
| Vanillic acid | 0.1977 | 0.0233 | 0.0035 | |||||
| BRS Guaraim | 0.18 Aa | 0.00 b | 0.00 b | 0.00 b | 0.04 | |||
| BRS Marcante | 0.00 B | 0.00 | 0.00 | 0.06 | 0.02 | |||
| Mean | 0.09 | 0.00 | 0.00 | 0.03 | SEM = | 0.01330 | ||
| DIBOA-hex-hex * | 0.0006 | 0.0001 | 0.7841 | |||||
| BRS Guaraim | 1.39 | 1.50 | 4.09 | 10.28 | 4.31 B | |||
| BRS Marcante | 3.97 | 3.97 | 8.66 | 14.63 | 7.81 A | |||
| Mean | 2.68 c | 2.73 c | 6.37 b | 12.45 a | SEM = | 0.79561 | ||
| DIMBOA-hex-hex * | 0.7277 | 0.0001 | 0.8312 | |||||
| BRS Guaraim | 0.69 | 0.54 | 3.64 | 7.61 | 3.12 | |||
| BRS Marcante | 0.79 | 0.95 | 2.75 | 7.23 | 2.93 | |||
| Mean | 0.74 c | 0.74 c | 3.19 b | 7.42 a | SEM = | 0.47198 | ||
| Apigenin-hex-pent I # | 0.048 | 0.0159 | 0.0414 | |||||
| BRS Guaraim | 4.05 a | 2.29 Bb | 4.06 a | 4.62 a | 3.75 | |||
| BRS Marcante | 4.16 | 4.25 A | 4.38 | 4.47 | 4.31 | |||
| Mean | 4.10 | 3.27 | 4.22 | 4.54 | SEM = | 0.16399 | ||
| Sinapic acid | 0.0001 | 0.8075 | 0.9454 | |||||
| BRS Guaraim | 0.50 | 0.49 | 0.52 | 0.47 | 0.49 B | |||
| BRS Marcante | 0.92 | 0.93 | 0.89 | 0.85 | 0.89 A | |||
| Mean | 0.71 | 0.71 | 0.70 | 0.66 | SEM = | 0.03594 | ||
| Apigenin-hex-pent II # | 0.0001 | 0.937 | 0.3065 | |||||
| BRS Guaraim | 6.27 | 6.49 | 6.37 | 6.79 | 6.48 B | |||
| BRS Marcante | 7.77 | 7.75 | 7.63 | 7.43 | 7.65 A | |||
| Mean | 7.02 | 7.12 | 7.00 | 7.11 | SEM = | 0.11631 | ||
| Apigenin-hex-hex-hex I # | 0.0001 | 0.0347 | 0.5372 | |||||
| BRS Guaraim | 4.11 | 4.22 | 4.75 | 4.49 | 4.39 B | |||
| BRS Marcante | 5.14 | 5.19 | 5.38 | 5.23 | 5.23 A | |||
| Mean | 4.62 b | 4.70 ab | 5.06 a | 4.86 ab | SEM = | 0.08466 | ||
| Apigenin-hex-hex-hex II # | 0.0001 | 0.101 | 0.2309 | |||||
| BRS Guaraim | 5.20 | 5.53 | 5.44 | 6.48 | 5.66 B | |||
| BRS Marcante | 8.38 | 8.60 | 8.92 | 8.70 | 8.65 A | |||
| Mean | 6.79 | 7.06 | 7.18 | 7.59 | SEM = | 0.25354 | ||
| Apigenin # | 0.8968 | 0.0001 | 0.9937 | |||||
| BRS Guaraim | 3.89 | 3.99 | 1.94 | 3.83 | 3.41 | |||
| BRS Marcante | 3.91 | 3.83 | 1.92 | 3.86 | 3.38 | |||
| Mean | 3.90 a | 3.91 a | 1.93 b | 3.84 a | SEM = | 0.18772 | ||
| Sum of free phenolic acids | 0.0001 | 0.3897 | 0.4308 | |||||
| BRS Guaraim | 0.68 | 0.49 | 0.52 | 0.47 | 0.54 B | |||
| BRS Marcante | 0.92 | 0.93 | 0.89 | 0.91 | 0.91 A | |||
| Mean | 0.8 | 0.71 | 0.7 | 0.69 | SEM = | 0.03671 | ||
| Sum of free benzoxazinoids | 0.022 | 0.0001 | 0.9849 | |||||
| BRS Guaraim | 2.08 | 2.03 | 7.72 | 17.88 | 7.43 B | |||
| BRS Marcante | 4.76 | 4.92 | 11.41 | 21.86 | 10.74 A | |||
| Mean | 3.42 c | 3.48 c | 9.56 b | 19.87 a | SEM = | 1.22276 | ||
| Sum of free flavonoids | 0.0001 | 0.0368 | 0.2241 | |||||
| BRS Guaraim | 23.5 | 22.5 | 22.6 | 26.2 | 23.7 B | |||
| BRS Marcante | 29.4 | 29.6 | 28.2 | 29.7 | 29.2 A | |||
| Mean | 26.4 ab | 26.1 ab | 25.4 b | 27.9 a | SEM = | 0.53165 | ||
| Sum of free phytochemical compounds | 0.0001 | 0.0001 | 0.9583 | |||||
| BRS Guaraim | 26.3 | 25.0 | 30.8 | 44.5 | 31.7 B | |||
| BRS Marcante | 35.0 | 35.5 | 40.5 | 52.4 | 40.9 A | |||
| Mean | 30.7 c | 30.2 c | 35.7 b | 48.5 a | SEM = | 1.55007 | ||
| Cultivar (C) | Germination Time (G) | Mean | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | C | G | C × G | ||
| Bound phytochemical compounds—Alkaline hydrolysis | ||||||||
| 4-Hydroxybenzoic acid | 0.489 | 0.0008 | 0.003 | |||||
| BRS Guaraim | 0.27 | 0.61 | 1.03 B | 1.64 A | 0.89 | |||
| BRS Marcante | 0.00 b | 0.00 b | 2.71 Aa | 0.00 Bb | 0.68 | |||
| Mean | 0.14 | 0.30 | 1.87 | 0.82 | SEM = | 0.19072 | ||
| Vanillic acid | 0.029 | 0.0044 | 0.004 | |||||
| BRS Guaraim | 0.00 b | 0.00 b | 0.09 Aa | 0.00 b | 0.02 | |||
| BRS Marcante | 0.00 | 0.00 | 0.00 B | 0.00 | 0.00 | |||
| Mean | 0.00 | 0.00 | 0.05 | 0.00 | SEM = | 0.00673 | ||
| p-Coumaric acid | 0.317 | 0.1711 | 0.374 | |||||
| BRS Guaraim | 2.85 | 2.97 | 3.03 | 2.76 | 2.90 | |||
| BRS Marcante | 3.24 | 2.93 | 3.04 | 2.78 | 3.00 | |||
| Mean | 3.04 | 2.95 | 3.04 | 2.77 | SEM = | 0.04886 | ||
| trans-Ferulic acid | 0.61 | 0.2318 | 0.027 | |||||
| BRS Guaraim | 44.2 | 45.7 | 50.9 A | 43.9 | 46.2 | |||
| BRS Marcante | 56.5 a | 50.8 a | 27.9 Bb | 40.9 ab | 44.0 | |||
| Mean | 50.3 | 48.3 | 39.4 | 42.4 | SEM = | 2.24618 | ||
| Sinapic acid | 0.046 | 0.0143 | 0.319 | |||||
| BRS Guaraim | 3.80 | 4.70 | 5.82 | 4.24 | 4.64 B | |||
| BRS Marcante | 5.68 | 5.44 | 6.15 | 4.30 | 5.39 A | |||
| Mean | 4.74 ab | 5.07 ab | 5.99 a | 4.27 b | SEM = | 0.20523 | ||
| Apigenin # | 0.016 | 0.0015 | 0.004 | |||||
| BRS Guaraim | 11.0 | 11.0 | 11.1 | 10.8 A | 11.0 | |||
| BRS Marcante | 11.0 a | 10.8 a | 11.0 a | 5.4 Bb | 9.5 | |||
| Mean | 11.0 | 10.9 | 11.0 | 8.1 | SEM = | 0.38845 | ||
| Sum of bound phenolic acids—Alkaline hydrolysis | 0.734 | 0.461 | 0.0463 | |||||
| BRS Guaraim | 51.1 | 54.0 | 60.9 A | 52.5 | 54.6 | |||
| BRS Marcante | 65.4 a | 59.2 ab | 39.8 Bb | 48.0 ab | 53.1 | |||
| Mean | 58.2 | 56.6 | 50.4 | 50.2 | SEM = | 2.33542 | ||
| Sum of bound flavonoids—Alkaline hydrolysis | 0.016 | 0.002 | 0.0035 | |||||
| BRS Guaraim | 11.0 | 11.0 | 11.1 | 10.8 A | 11.0 | |||
| BRS Marcante | 11.0 a | 10.8 a | 11.0 a | 5.4 Bb | 9.5 | |||
| Mean | 11.0 | 10.9 | 11.0 | 8.1 | SEM = | 0.38845 | ||
| Sum of bound phytochemical compounds—Alkaline hydrolysis | 0.522 | 0.309 | 0.0449 | |||||
| BRS Guaraim | 62.1 | 65.0 | 72.0 A | 63.3 | 65.6 | |||
| BRS Marcante | 76.4 a | 70.0 ab | 50.8 Bb | 53.3 b | 62.6 | |||
| Mean | 69.2 | 67.5 | 61.4 | 58.3 | SEM = | 2.43190 | ||
| Bound phytochemical compounds—Acid hydrolysis | ||||||||
| p-Coumaric acid | 0.9534 | 0.5427 | 0.0309 | |||||
| BRS Guaraim | 8.56 | 7.47 B | 9.39 | 8.72 | 8.53 | |||
| BRS Marcante | 7.73 | 9.36 A | 8.11 | 9.04 | 8.56 | |||
| Mean | 8.14 | 8.41 | 8.75 | 8.88 | SEM = | 0.20815 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranzelli, J.; Somacal, S.; Monteiro, C.S.; Mello, R.d.O.; Rodrigues, E.; Prestes, O.D.; López-Ruiz, R.; Garrido Frenich, A.; Romero-González, R.; Miranda, M.Z.d.; et al. Grain Germination Changes the Profile of Phenolic Compounds and Benzoxazinoids in Wheat: A Study on Hard and Soft Cultivars. Molecules 2023, 28, 721. https://doi.org/10.3390/molecules28020721
Baranzelli J, Somacal S, Monteiro CS, Mello RdO, Rodrigues E, Prestes OD, López-Ruiz R, Garrido Frenich A, Romero-González R, Miranda MZd, et al. Grain Germination Changes the Profile of Phenolic Compounds and Benzoxazinoids in Wheat: A Study on Hard and Soft Cultivars. Molecules. 2023; 28(2):721. https://doi.org/10.3390/molecules28020721
Chicago/Turabian StyleBaranzelli, Julia, Sabrina Somacal, Camila Sant’Anna Monteiro, Renius de Oliveira Mello, Eliseu Rodrigues, Osmar Damian Prestes, Rosalía López-Ruiz, Antonia Garrido Frenich, Roberto Romero-González, Martha Zavariz de Miranda, and et al. 2023. "Grain Germination Changes the Profile of Phenolic Compounds and Benzoxazinoids in Wheat: A Study on Hard and Soft Cultivars" Molecules 28, no. 2: 721. https://doi.org/10.3390/molecules28020721
APA StyleBaranzelli, J., Somacal, S., Monteiro, C. S., Mello, R. d. O., Rodrigues, E., Prestes, O. D., López-Ruiz, R., Garrido Frenich, A., Romero-González, R., Miranda, M. Z. d., & Emanuelli, T. (2023). Grain Germination Changes the Profile of Phenolic Compounds and Benzoxazinoids in Wheat: A Study on Hard and Soft Cultivars. Molecules, 28(2), 721. https://doi.org/10.3390/molecules28020721