Abstract
The epoxidation process of semi-synthetic triterpenoids 2-methyl-3-oxo-19β,28-epoxy- 18α-olean-1-ene, and its allylic alcohol derivatives were examined. 1,2α-epoxide, as the main product, was found to be formed from the starting enone exposed to m-chloroperbenzoic acid (mCPBA). In the case of hydroxy-directed mCPBA-oxidation of triterpenic allyl alcohols and their 3α-alkyl-substituted derivatives, inversion of C1 and C2 asymmetric centers with the formation of 1,2β-epoxyalcohols took place. The synthesis of 2,3α-epoxides was fulfilled from 2,3-dialkyl-substituted C(3) allyl alcohols by the action of pyridinium chlorochromate under [1,3]-oxidative rearrangement conditions. The transformations brought about enabled chiral oleanane derivatives with an oxygen-containing substituent at the C1, C2, and C3 atoms to be obtained. The study also provides information on in silico PASS prediction of pharmacological effects and in vitro evaluation of the cytotoxic activity of the synthesized compounds.
1. Introduction
Biologically active secondary plant metabolites, in particular pentacyclic triterpenoids with different carbon skeletons (dammarane, lupane, oleanane, ursane, etc.), have been widely used as promising candidates for developing drugs against various pathologies, especially metabolic and neurological disorders, and infectious and cardiovascular diseases [1,2,3,4,5,6]. Although a huge number of native and semi-synthetic triterpenoids with significant in vitro biological activity, an extremely low bioavailability inhibits the progress of hydrophobic triterpenoids as drug candidates. Usually, the relative bioavailability of a drug candidate is a function of the presence of both lipophilic and hydrophilic fragments within its structure, which determines the extent of interaction of the organic medicinal agent with lipid and/or aqueous phases [7]. Generally, introducing additional hydrophilic functional groups into triterpenic molecules with a non-polar lipophilic ring system is believed to render the nature of these compounds more hydrophilic and to play a key role in manifesting biological activity (e.g., in the case of polyoxygenated derivatives) [8,9,10]. Oxidative transformations of triterpenoids are most often focused on ring A, which already has a 3-hydroxyl group as a synthetic handle. At the same time, plant cytochrome P450 monooxygenases decorate basic pentacyclic triterpenoids (α-amyrin, β-amyrin, and lupeol) by the regioselective introduction of hydroxyl, ketone, aldehyde, carboxyl, or epoxy groups, at the typical C12, C13, C24, C28, and C30 positions [11]. There has also been shown a possibility of site-selective oxidation of pentacyclic triterpenoids at С1, C2, C7, C11, C15, C16, C28, C29, C30 positions by microbial biotransformation [12,13] as well as hydroxylation at C2, C6, C15, C16, C20, C21, C22, C23 positions using chemical C-H oxidation [9,14,15].
Although the A ring of pentacyclic triterpenoids is the main target for functionalization in many synthetic schemes, the examples of obtaining simultaneously oxidized C1, C2, and C3 atoms are limited [16]. At the same time, semi-synthetic 28/30-ester derivatives of 1α,2β,3β-trihydroxy-18β-olean-12-en-28-oic and 1α,2β,3β-trihydroxy-11-oxo-18β-Olean-12-en-30-oic acids, as well as a native compound 1β,2β,3β-trihydoxy-18β-urs-12-ene-23-oic-rhamnoside, which had effectively inhibited Gram-positive bacteria growth by regulating the metabolism and virulence gene expression, have been described recently [17,18,19]. Moreover, there have been synthesized new examples of antibacterial 1α,2α-epoxy-3β-hydroxy 18β-oleanolic acid ester derivatives that presumably regulate the metabolism, hemolysis, and β-lactamase gene expression [20]. The above findings prompted us to pay more attention to the transformation of the α-alkyl-substituted enone (2) previously synthesized from the triterpenoid allobetulone (1) in a few synthetic steps [21]. Introducing new oxygen-containing groups at the C1 and C2 positions of the enone (2) simultaneously can be attained through an epoxy group. Here, we have investigated the possibility of regio- and stereoselective epoxidation of the 2-methyl-3-oxo-19β,28-epoxy-18α-olean-1-ene (2) and its 3-alkylated derivatives (allylic alcohols). In addition, the in silico PASS prediction of pharmacological effects and in vitro evaluation of the cytotoxic activity of the synthesized compounds were conducted.
2. Results and Discussion
There have been numerous examples of successful strategies for using polycyclic epoxides as intermediates in the synthesis of new biologically active compounds. Epoxidation of all trans-fused unsaturated steroids is generally accepted to proceed with the predominant formation of α-epoxide as a single product [22]. Introducing the oxirane fragment into triterpenic di-, tri-, or tetrasubstituted alkenes most often also proceeds stereoselectively and give rise to forming a least hindered isomeric α-epoxide as a single product [23,24,25,26,27,28,29]. Concurrently, some examples of epoxidizing triterpenic trisubstituted alkenes have been described with mCPBA resulting in a mixture of isomeric epoxides [25,30,31,32]. On the other hand, taking into account the structure of the starting enone 2 with trans-fused A/B, B/C, and C/D rings and the syn-stereodirecting effect of the allylic 3β-hydroxyl group in the case of triterpenic 2-methyl-1-alkenes 3 and 7–9, the epoxidation process of the alkenes investigated here (Scheme 1) may involve an attack of the achiral mCPBA oxidant from both α- and β-sides.
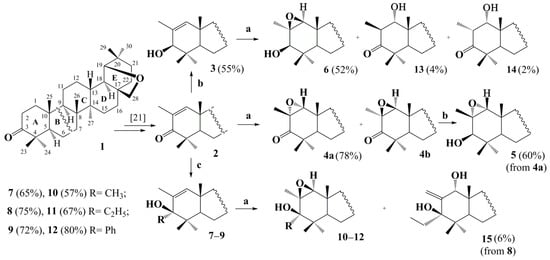
Scheme 1.
Synthesis and mCPBA-mediated epoxidation of 3β-hydroxy- (3) and 3α-alkyl/aryl-3β-hydroxy derivatives 7–9. Reaction conditions: (a) mCPBA, CH2Cl2, rt; (b) NaBH4, MeOH, rt; (c) RMgX, (C2H5)2O, THF,110 °C.
The transformation procedure for allobetulone (1) into 2-methyl-3-oxo-19β,28-epoxy-18α-olean-1-ene (2) was described by us earlier [21]. To expand the range of the substrates under study, we carried out the reduction of α,β-unsaturated ketone 2 using NaBH4, as well as its reductive alkylation using an appropriate Grignard reagent with the formation of a secondary 3 or tertiary 7–9 allylic alcohols (up to 75% yield), respectively. The 13C NMR data confirmed the structures of the obtained allylic alcohols 3, 7–9 by recording and identifying a typical signal given by a C3 atom bounded to the hydroxyl group at δC 77.56–82.59 ppm and followed by the characteristic signals of a trisubstituted double bond between C1 and C2 atoms (δC 130.35–142.98 and δC 133.72–154.93 ppm) accompanied by the signal of H1 olefinic proton at δH 5.53–5.90 ppm in the 1H NMR spectra. The structures of compounds 3, 7–9 with the assignment of the absolute configuration of a new C3 asymmetric carbon center were finally confirmed by the X-ray crystallography technique of alcohols 3, 7, 8 (Figure 1a,c,d). Thus, the β-orientation of the 3-hydroxy group of compounds 3, 7–9 agrees with the previously obtained data when, during the reductive alkylation of 3-oxotriterpenoids, the Grignard reagent ensured attack exclusively on the α-side to afford an α-oriented 3-alkyl/aryl substituent, due to the complexation of the Grignard reagent with the solvent THF and steric loading of axially oriented angular methyl groups at С4 and С10 atoms [33,34,35,36].
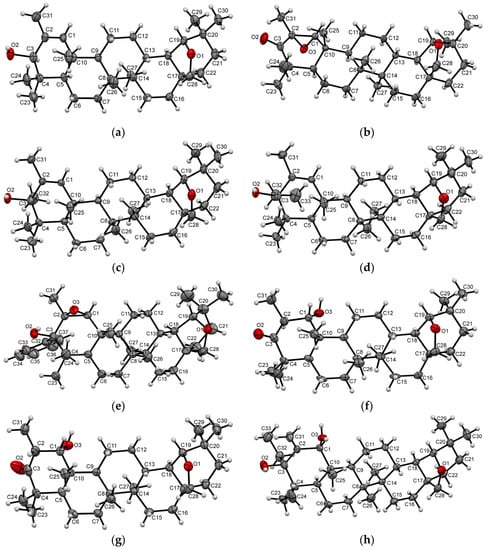
Figure 1.
Molecular structures of compounds 3 (a), 4a (b), 7 (c), 8 (d), 12 (e), 13 (f), 14 (g), 15 (h) with atoms represented as thermal vibration ellipsoids, with 50% probability.
According to the 1H NMR spectrum, the mCPBA-mediated oxidation of triterpenic α,β-unsaturated enone 2 proceeded with the formation of a mixture of two epimeric epoxides (87:13). The signal of the H1 proton of the minor epoxide 4b (δH 3.40 ppm) was observed in a low-field region of the 1H NMR spectrum of the mixture of epoxides 4a,b as compared with the signal of main isomer 4a (δH 3.38 ppm). The major product 4a was obtained in ~80% yield after column chromatography, and its structure with a traditionally α-oriented 1,2-oxirane ring was confirmed by the single crystal X-ray diffraction analysis (Figure 1b). Thus, the β-oriented bulky angular methyl groups at the C4 and C10 atoms on the front side of triterpenoid 2 had mainly sterically prevented the attack of peracid from the β-face. Reducing epoxide 4a by NaBH4 led to the corresponding 3β-hydroxy 1,2α-epoxide 5 (60% yield), the shift of the 1H proton signal in the 1H NMR spectrum of which was recorded at δH 3.06 ppm.
The reaction of mCPBA with secondary allylic alcohol 3 (the product of the reductive conversion of ketone 2) led to forming epoxide 6 (52% yield) with a β-oriented oxirane ring, whose NMR signal of 1H proton appeared in a higher field region at 2.99 ppm. In this case, the possibility of the diastereofacial selectivity of the epoxidation process on the syn-side to the hydroxyl group provided a peracid approach from the sterically hindered β-side due to forming an intermolecular hydrogen bond between alcohol and mCPBA [37]. P. Kočovský showed [38] an increased steric hindrance (e.g., an axial alkyl group in a vicinal position to the hydroxy group) to impair, to some extent, the hydroxyl-directed syn-epoxidation of cyclic allylic alcohols with peracid, leading to forming, in some cases, a mixture of diastereoisomeric epoxy alcohols. According to NMR spectroscopy data, during mCPBA-mediated oxidation of allylic alcohols 3, 7–9, the corresponding epoxides 6, 10–12 were obtained as the sole diastereoisomeric product of the reaction. Stereochemistry of the epoxidation process was confirmed using the data of a set of two-dimensional NMR spectra (1H–13C HMBC, 1H–13C HSQC, NOESY) for compound 10 and the result of X-ray diffraction analysis of epoxide 12 (Figure 1e). The correlations observed in the two-dimensional spectra confirmed the structure of the epoxide 10 and enabled the relative configuration of the C1 and C2 atoms to be defined. For example, the obvious NOE correlation between protons Н1 and Н5, Н1 and Н32, Н5 and Н32, Н6 and Н32, Н24 and Н31, Н31 and Н32, H5 and H9 in compound 10 favored the β-orientation of the oxirane ring (See Supporting Information). Comparing the 1H NMR spectra enabled the proton at the C1 atom of the discussed epoxides 6, 10–12 to be determined as being equally α-oriented: it resonated similarly in the δH 2.72–2.99 ppm region of spectra of the epoxides 6, 10 and 11, and only in case of epoxide 12, this signal had a downfield shift caused by the phenyl substituent at the С3 atom. Thus, the 3β-hydroxyl substituent turned out to be a highly effective syn-stereo-directing group for the semi-synthetic triterpenoids under the study, and the direction of the 2,3-epoxidation process was insensitive to the gem-dimethyl group located in a vicinal position to the hydroxyl group. The ability of the C3 geminal alkyl/aryl substituent to influence the reaction evinced the relatively bulky substituent to be more favorable for the epoxidation process, as evidenced by a higher yield in the epoxide series (CH3 < C2H5 < C6H5).
The unexpected side products 13–15 (yields 2–6%) were also isolated from the reaction mixtures obtained by epoxidation of allylic alcohols 3 and 8 (Scheme 1). The structures 13–15 were explicitly verified by X-ray diffraction analysis, confirming the presence of a 1α-hydroxyl group combined with a 3-oxo moiety or a 2,31-exo-methylene group (Figure 1f,g,h). With the hints taken into account from the earlier reports on the possibility of the ring-opening of the epoxides, which proceeded through the anti-dihydroxylation stage [39] under the action of benzoic acid formed as a by-product during mCPBA-epoxidation, as well as the formation of β-hydroxyketones or allyl alcohols as a result of the isomerization of polyfunctional epoxides [40], the reaction pathways for transforming epoxides 6 and 11 to 1α-hydroxy derivatives 13–15 have been proposed (Scheme 2).
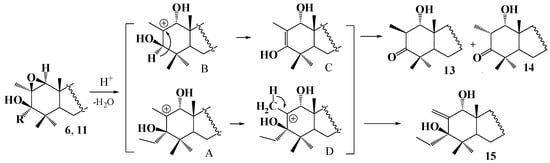
Scheme 2.
Possible reaction mechanism for isomerization of epoxides 6 and 11 to β-hydroxyketones 13, 14 and allyl alcohol 15.
A possible mechanism for forming compounds 13–15 includes the stage of the acid-catalyzed ring-opening of epoxides 6, 11 with forming 1α,2β-diol intermediate followed by the C2 dehydration to a carbocation A; the next stage involves a hydride shift for forming enol C, acid-catalyzed enolization of which yields β-hydroxyketones 13 and 14. In the case of intermediate D, the shift of a proton from the methyl group to C2 proceeds with forming allyl alcohol 15. Thus, isolating the compounds 13–15 may indirectly indicate the use of acid catalysis for opening the studied epoxides 6, 10–12 as being suitable for preparing 1,3-dihydroxy but not 1,2,3-trihydroxytriterpenic derivatives.
An alternative synthesis of oxidized C1 derivatives from allyl alcohols was also tested. Tertiary allylic alcohols are known as tending to undergo [1,3]-oxidative rearrangement, being often used to obtain biologically active compounds or their key intermediates. Oxochromium (VI) based reagents are the most commonly used reagent systems for the [1,3]-oxidative transposition, in particular, pyridinium chlorochromate (PCC), which enables the desired products to be obtained in high yields under mild conditions [41,42]. The β-substituted α,β-unsaturated ketones are preferentially registered as the rearrangement products of the [1,3]-transposition reaction [42]. On the other hand, there have been described examples of forming β-substituted α,β-epoxy carbonyl compounds with the participation of a Collins reagent or PCC [43,44,45]. Among the studied tertiary alcohols 7–9, only alcohols 7 and 8 are involved in the [1,3]-transposition reaction: the corresponding 2,3α-epoxy rearrangement products 16 and 17 were isolated as single reaction products (~60% yield) (Scheme 3). The formation of epoxides 16 and 17 having an oxo group at position C1 was evidenced by the absence of a signal of olefinic H1 proton in the 1H NMR spectra and also by the disappearance of signals of the trisubstituted double 1,2-bond and a signal of a C3 carbon atom bound to the hydroxyl group against the background of the presence of a carbonyl signal of C1 atom at δC 208.84–208.93 ppm in the 13C NMR spectra. X-ray diffraction analysis of compounds 16 and 17 (Figure 2a,b) also supported the proposed structures with α-orientated oxirane moiety at C2 and C3 atoms. The oxidation of the alkyl allylic alcohols 7 and 8 by PCC, which yields epoxides 16 and 17, most likely occurs as a result of the formation of a chromate ester as a key intermediate A. This reaction is followed by 3,3-sigmatropic rearrangement to 1-oxychromate B, then 2,3-epoxychromate C is formed, hydrolysis and subsequent oxidation of which lead to α-keto-epoxide. A plausible mechanism for forming 1-oxo-2,3α-epoxides 16 and 17 is similar to that previously described for diterpenoid methyl dihydroisopimarate [45] and is represented in Scheme 3. In the case of compound 9, the reaction fails to occur, as the formation of the α-oriented 1-chromate derivative is sterically hindered due to the C3 aryl fragment. Thus, the characteristic tendency of triterpenoids to form sterically less hindered α-oriented oxirane ring structures persists in the case of [1,3]-oxidative transposition as well.
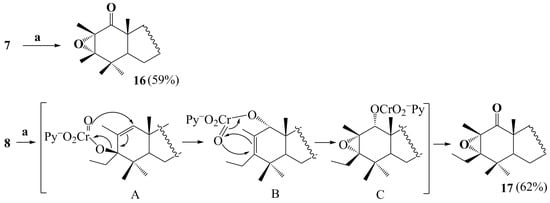
Scheme 3.
Synthesis and plausible mechanism for the formation of 2,3α-epoxides 16 and 17. Reaction conditions: (a) CrO3·Py·HCl (PCC), CH2Cl2, rt.
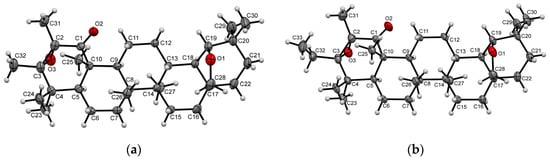
Figure 2.
Molecular structures of compounds 16 (a) and 17 (b) with atoms represented as thermal vibration ellipsoids, with 50% probability.
Ring A plays an important role in realizing anticancer, antiviral, anti-inflammatory, antibacterial, antifungal, and antiparasitic activities by pentacyclic triterpenoids [1,2,3,4,5,6,16,32,33,34,35,36]. At the same time, the modification of ring A with the 1,2-oxirane fragment most often improves the antibacterial, antifungal, and anticancer properties of triterpenoids [17,46,47]. Taking into account the presence of a pharmacophoric oxirane fragment [48], different biological activities could be expected manifested by novel triterpenic epoxides 4–6, 10–12, 16, and 17. To predict appropriate pharmacological effects of the synthesized derivatives 2–17, there was used an openly accessible in silico tool, the PASS (prediction of activity spectra for substances) software [49]. The current version of the PASS program (2019) predicts 5066 pharmacological effects, mechanisms of action, side effects and toxic effects, influence on gene expression, etc., with an average invariant accuracy of 0.9645 prediction values of probable activity (Pa) or probable inactivity (Pi) [50]. Among the pharmacological effects calculated by the PASS program for the tested structures 2–17, the probability coefficient (Pa) of antineoplastic properties’ manifestation was the highest (Pa 0.911–0.983), especially against such cancer types as colorectal (Pa 0.837–0.914), colon (Pa 0.833–0.911), and lung (Pa 0.747–0.852) cancer (Table 1).

Table 1.
PASS-predicted biological activities of the compounds 2–17.
In the next stage, the MTT method [51] was used to test the cytotoxic activity of the synthesized compounds 3, 4a, 5, 9–12, and 17 against six human tumor cell lines, including hepatocellular carcinoma HEpG2, colorectal carcinoma HCT 116, melanoma MS, rhabdomyosarcoma RD TE32, non-small cell lung carcinoma A549, and estrogen-dependent breast adenocarcinoma MCF7. Table 2 shows that the tested compounds were generally non-toxic (IC50 > 200 μM) against most cell lines, including those of colorectal and lung carcinomas. Concurrently, on the breast cancer cell line MCF-7, there was a selective cytotoxic effect of the synthesized compounds, with allylic alcohol 9 and epoxides 11 and 12 being especially active against MCF-7 cells with an IC50 value of 37.08–45.88 μM. At the same time, epoxide 10 with a methyl substituent at position C3 was cytotoxically inactive. Structure-activity relationship (SAR) analysis revealed a threefold increase in the activity against MS cells resulting from the reduction of the oxo group of the starting α,β-unsaturated ketone 2 with the ensuing forming of the hydroxy derivative 3. The opposite trend was noted after α-epoxidation of the double bond of compounds 2, 3: hydroxy-epoxide 5 was inactive against MS cells, while the activity of epoxide 4a was slightly higher than that of compound 3. The most notable increase in cytotoxicity was achieved by adding a phenyl group at position C3 of compounds 2 and 3, when cytotoxicity reached the values of 45.27 and 37.08 μM for compounds 9 and 12, respectively, as compared with the parent enone 2 with IC50 60.94 μM.

Table 2.
In vitro cytotoxic activity of the compounds 2, 3, 4a, 5, 9–12, and 17.
3. Conclusions
Alkyl enones are valuable intermediates for the total synthesis of natural products or biologically active compounds. Herein, we have shown that employing inexpensive reagents enables carrying out the regio- and stereoselective transformations of 2-methyl-3-oxo-19β,28-epoxy-18α-olean-1-ene with the formation of various chiral hydroxy epoxides, epoxy-ketones, β-hydroxy ketones, or 1,3-dihydroxy derivatives bearing C1, C2, and C3 asymmetric centers in the triterpenic A-ring, which, if manipulated further, can furnish compounds of interest and useful.
The in silico PASS evaluation predicted the highest probability of antineoplastic properties of the new compounds. According to an in vitro study, allylic alcohol 9 and epoxides 11 and 12 have selective cytotoxicity against the breast cancer cell line MCF-7.
4. Materials and Methods
The IR spectra of the compounds dissolved in CHCl3 were recorded on a Bruker 66/S IFS Fourier spectrometer (Bruker, Ettlingen, Germany). The 1H, 13C, DEPT NMR spectra of the compounds dissolved in CDCl3 were recorded on a Bruker AVANCE II spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) at 400 and 100 MHz, respectively. Structural assignments of compound 10 were also supported by 2D HMR (COSY 1H–1H, HSQC 1H–13C, HMBC 1H–13C, and NOESY 1H–1H) spectra. Chemical shifts (δ) were expressed in parts per million (ppm) relative to TMS as an internal standard. Optical rotation was measured on a Perkine Elmer 341 polarimeter (Perkin Elmer, Waltham, MA, USA) using the sodium D line (589 nm) as a light source for CHCl3 solutions. Mass spectra (MS) were determined on an Agilent 6890N/5975B chromatograph (Agilent Technologies, Wilmington, NC, USA) equipped with an HP-5ms UI capillary column (4 m × 0.25 mm, 0.25 µm; 70 eV electron impact). GC-MS analysis was performed for solutions of compounds in CH2Cl2 at 1–2 mg/mL concentration under the following conditions: an initial temperature of 100 °С, ramped to 300 °С at 40 °С/min, evaporator temperature of 310 °С, and the retention time of analyzed compounds was 3–7 min. Melting points were measured using an OptiMelt MPA100 (Stanford Research Systems, Sunnyvale, CA, USA) instrument at a heating rate of 1 °C/min. Column chromatography was carried out using 60 Å, 200–400 mesh particle size silica gel purchased from Macherey-Nagel (Duren, Germany) and the solvent mixtures of light petroleum (b.p. 40–60 °C) and ethyl acetate as an eluent. The reactions were monitored by TLC using Sorbfil plates (Sorbpolymer, Krasnodar, Russia). The solvents were purified and dried according to standard procedures [52].
The unit cell parameters and the X-ray diffraction intensities were measured on an Xcalibur Ruby diffractometer (Agilent Technologies, Cheadle, UK). Empirical absorption correction was introduced by a multi-scan method using the SCALE3 ABSPACK algorithm [53]. Using OLEX2 [54], the structures were solved with the SHELXS [55], SUPERFLIP [56], or SHELXT [57] programs and refined by the full-matrix least-squares minimization in anisotropic approximation for all non-hydrogen atoms with the SHELXL program. Hydrogen atoms bound to carbon were positioned geometrically and refined using the riding model. The OH groups’ hydrogen atoms were refined freely with isotropic displacement parameters. The contribution of the solvent electron density for 12 was removed using the SQUEEZE routine in PLATON [58].
4.1. General Procedure for Preparing Compounds , , –, –
To a solution of compound 2 (4.4 mmol), 3 (4.3 mmol), 7 (4.2 mmol), 8 (4.1 mmol), or 9 (3.7 mmol) in CH2Cl2 (20 mL) a sixfold excess of m-chloroperbenzoic acid was added. The reaction mixture was stirred at room temperature for 48 h. Completeness of the reaction was monitored by TLC. The reaction mixture was diluted with a 10% aqueous solution of NaOH. The reaction products were extracted with ethyl acetate (3 × 50 mL) and then washed with H2O to a neutral pH of the flushing waters. The organic layer was dried over anhydrous MgSO4, the solvent was evaporated, and the residue was purified by column chromatography on silica gel to obtain corresponding epoxides 4, 6, 10–12, and compounds 13–15 as by-products.
(1R,2R)-2-Methyl-3-oxo-(1,2),(19β,28)-diepoxy-18αН-oleanane (4a): Yield: 78%, Rf value 0.44 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 250.0 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+66.4 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 1698 (С=О). 1H NMR (400 MHz, CDCl3) δ: 0.81, 0.89, 0.93, 0.96, 0.98, 1.01, 1.08 (21H, 7s, CH3 × 7); 1.41 (3H, s, 3Н-31); 3.38 (1Н, s, Н-1); 3.45 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.53 (1Н, s, Н-19). 1H NMR (400 MHz, CDCl3) δ (4b): 3.40 (1Н, s, Н-1); 3.43 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.52 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.31, 15.86, 16.07, 16.79, 19.13, 21.31, 21.90, 24.53, 26.12, 26.22, 26.43, 28.11, 28.78, 32.72, 33.02, 34.32, 36.27, 36.73, 38.38, 40.90, 41.02, 41.47, 43.81, 44.74, 46.74, 46.80, 61.28, 70.63, 71.26, 87.89, 213.34. GC-MS (m/z): 468.3 (M+).
(1S,2R,3R)-3β-Hydroxy-2-methyl-(1,2),(19β,28)-diepoxy-18αН-oleanane (6): Yield: 52%, Rf value 0.15 (light petroleum (b.p. 40–60 °C) /ethyl acetate 5:1), colorless crystals, m.p. 213.6 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+38.0 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3409 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.79, 0.82, 0.92, 0.93, 0.95, 0.99, 1.05 (21H, 7s, CH3 × 7); 1.40 (3H, s, 3Н-31); 2.99 (1Н, s, Н-1); 3.31 (1Н, s, Н-3); 3.45 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.54 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.58, 14.70, 17.04, 17.50, 21.69, 21.94, 24.68, 24.70, 26.48, 26.59, 26.60, 28.96, 29.84, 32.90, 34.60, 34.63, 36.44, 36.93, 37.30, 37.98, 41.19, 41.65, 41.97, 46.95, 47.10, 55.30, 62.92, 71.30, 71.45, 79.73, 88.01. GC-MS (m/z): 470.3 (M+).
(1S,2S,3R)-3β-Hydroxy-2,3-dimethyl-(1,2),(19β,28)-diepoxy-18αН-oleanane (10): Yield: 57%, Rf value 0.15 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 165.4 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+7.4 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3466 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.81, 0.83, 0.85, 1.03, 1.08 (15H, 5s, CH3 × 5); 0.97 (6H, s, CH3 × 2); 1.02 and 1.66 (2H, 2m, 2H-15); 1.04 (1H, m, H-5); 1.08 and 1.57 (2H, 2m, 2H-12); 1.22 and 1.52 (2H, 2m, 2H-21); 1.28 and 1.41 (2H, 2m, 2H-22); 1.30 and 1.43 (2H, 2m, 2H-11); 1.37 (3H, s, 3Н-31); 1.40 and 1.44 (2H, 2m, 2H-7); 1.49 (1H, m, H-18); 1.51 (1H, m, H-13); 1.60 and 2.06 (2H, 2m, 2H-16); 1.70 (3H, s, 3Н-32); 1.85 and 1.88 (1Н, dd, J = 4.0, 14.0 Hz, Н-9); 2.07 and 2.09 (2H, 2m, 2H-6); 2.87 (1Н, s, Н-1); 3.47 and 3.80 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.56 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.53 (C27), 15.95 (C26), 17.75 (C25), 20.50 (C31), 20.88 (C24), 23.28 (C32), 23.45 (C23), 24.47 (C30), 26.06 (C15), 26.27 (C16), 26.40 (C11), 26.49 (C12), 28.76 (C29), 32.22 (C6), 32.82 (C21), 34.00 (C7), 34.24 (C13), 36.26 (C20), 36.83 (C22), 37.86 (C10), 39.77 (C4), 40.84 (C14), 40.93 (C8), 41.51 (C17), 44.84 (C9), 44.96 (C5), 46.94 (C18), 63.88 (C2), 68.34 (C1), 71.26 (C28), 75.31 (C3), 88.00 (C19). GC-MS (m/z): 484.3 (M+).
(1S,2S,3R)-3-Ethyl-3β-hydroxy-2-methyl-(1,2),(19β,28)-diepoxy-18αН-oleanane (11): Yield: 67%, Rf value 0.40 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 151.7 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+15.8 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3428 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.73, 0.78, 0.81, 0.92, 0.93, 0.98, 1.03 (21H, 7s, CH3 × 7); 1.12 (3Н, t, J = 8.0 Hz, 3Н-33); 1.38 (3Н, s, 3Н-31); 2.72 (1Н, s, Н-1); 3.43 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.51 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 9.44, 13.66, 16.02, 18.23, 21.35, 21.47, 23.39, 23.50, 24.49, 26.03, 26.26, 26.41, 26.48, 28.55, 28.80, 32.22, 32.77, 34.18, 36.27, 36.80, 37.97, 40.38, 40.80, 40.91, 41.52, 44.69, 44.93, 46.90, 62.85, 67.55, 71.29, 77.15, 88.01. GC-MS (m/z): 498.3 (M+).
(1S,2S,3R)-3β-Hydroxy-2-methyl-3-phenyl-(1,2),(19β,28)-diepoxy-18αН-oleanane (12): Yield: 80%, Rf value 0.41 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 232.2 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+18.0 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3440 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.31, 0.82, 0.95, 1.04, 1.09, 1.14 (18H, 6s, CH3 × 6); 1.10 (6H, 2s, CH3 × 2); 3.27 (1Н, s, Н-1); 3.46 and 3.78 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.58 (1Н, s, Н-19); 7.23 (3Н, m, Н-33, Н-37, Н-36); 7.33-7.37 (1Н, m, Н-34); 7.74 (1Н, d, J = 8.0 Hz, Н-35). 13C NMR (100 MHz, CDCl3) δ: 13.17, 15.32, 17.25, 17.27, 20.26, 20.84, 21.56, 24.52, 26.36 (2C), 26.43, 27.71, 28.81, 32.74, 34.25, 34.90, 36.29, 36.82, 37.27, 41.21, 41.30, 41.51, 41.93, 46.50, 46.87, 49.77, 67.22, 70.84, 71.32, 79.94, 87.84, 126.17, 126.65, 127.72, 127.89, 128.40, 142.79. GC-MS (m/z): 546.4 (M+).
(1S,2S)-1α-Hydroxy-2-methyl-3-oxo-19β,28-epoxy-18αН-oleanane (13): Yield: 4%, Rf value 0.20 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 243.1 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+3.0 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 1737 (С=О), 3447 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.58, 0.79, 0.93, 0.98, 1.00, 1.04, 1.07 (21H, 7s, CH3 × 7); 1.19 (3H, d, J = 8.0 Hz, 3Н-31); 2.58 (1Н, dk, J = 8.0, 4.0 Hz, Н-2); 3.21 (1Н, d, J = 4.0 Hz, Н-1); 3.44 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.51 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.28, 16.17, 16.78, 19.66, 21.37, 21.47, 24.49, 26.28, 26.37, 26.42, 28.32, 28.75, 29.61, 32.78, 33.48, 33.86, 34.49, 36.26, 36.81, 38.90, 41.05, 41.50, 41.51, 44.48, 45.50, 46.84, 53.73, 71.27, 79.10, 87.89, 215.40. GC-MS (m/z): 470.3 (M+).
(1S,2R)-1α-Hydroxy-2-methyl-3-oxo-19β,28-epoxy-18αН-olean-1-еne (14): Yield: 2%, Rf value 0.28 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 192.9 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), IR ν (CHCl3) cm−1: 1732 (С=О), 3452 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.78, 0.92, 0.93, 1.03, 1.15 (15H, 5s, CH3 × 5); and 1.05 (6H, 2s, CH3 × 2); 1.09 (3H, d, J = 8.0 Hz, 3Н-31); 3.16 (1Н, dk, J = 8.0, 4.0 Hz, Н-1); 3.77 (1Н, d, J = 4.0 Hz, Н-1); 3.44 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.51 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 12.12, 13.58, 16.08, 16.35, 20.74, 21.34, 22.67, 24.53, 24.55, 26.23, 26.27, 26.49, 28.79, 29.69, 31.92, 32.75, 33.35, 34.25, 36.28, 36.77, 40.86, 41.31, 41.49, 41.65, 41.95, 46.91, 49.30, 71.28, 80.18, 88.00, 216.39. GC-MS (m/z): 452.3 (M-H2O).
(1S,3S)-1α,3β-Dihydroxy-3-ethyl-2-methylene-19β,28-epoxy-18αН-oleanane (15): Yield: 6%, Rf value 0.32 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 180.7 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+18.2 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 1687 (C=CH2), 3444 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.73, 0.77, 0.78, 0.91, 0.92, 0.96, 0.98 (21H, 7s, CH3 × 7); 1.01 (3Н, t, J = 8.0 Hz, 3Н-33); 3.43 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.52 (1Н, s, Н-19); 3.92 (1Н, s, Н-1); 5.15 and 5.25 (2H, 2d, J = 4.0 Hz, 2Н-31). 13C NMR (100 MHz, CDCl3) δ: 9.01, 13.80, 15.67, 19.16, 19.44, 21.14, 23.39, 24.54, 26.23, 26.29, 26.41, 26.61, 28.81, 29.68, 32.22, 32.78, 34.42, 36.29, 36.79, 40.53, 40.86, 41.13, 41.52, 41.73, 43.00, 46.29, 46.90, 71.30, 80.04, 80.30, 88.00, 116.13, 148.66. GC-MS (m/z): 498.3 (M+).
4.2. General Procedure for Preparing Compounds and
To a solution of compound 2 (4.4 mmol) or 4 (4.3 mmol) in MeOH (20 mL), the 10-fold excess of NaBH4 was added. The reaction mixture was stirred at room temperature for 40 min and for 5 min while boiled. Completeness of the reaction was monitored by TLC. Then MeOH was evaporated, and the resulting precipitate was diluted with 100 mL of 10% HCl. The products were extracted with ethyl acetate (3 × 50 mL) and then washed with H2O to a neutral pH of the flushing waters. The organic layer was dried over anhydrous MgSO4, the solvent was evaporated, and the residue was purified by column chromatography on silica gel to obtain corresponding compounds 3 and 5.
(3S)-3β-Hydroxy-2-methyl-19β,28-epoxy-18αН-olean-1-еne (3): Yield: 55%, Rf value 0.33 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 153.7 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+67.0 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3425 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.79, 0.80, 0.90, 0.92, 0.96, 0.987, 0.995 (21H, 7s, CH3 × 7); 1.71 (3H, s, 3Н-31); 3.43 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.52 (1Н, s, Н-19); 3.73 (1H, s, H-3); 5.67 (1Н, s, H-1). 13C NMR (100 MHz, CDCl3) δ: 13.45, 16.33, 17.77, 17.84, 19.10, 20.26, 21.04, 24.53, 26.32, 26.33, 28.01, 28.81, 32.76, 34.16, 34.32, 36.27, 36.80, 37.51, 38.85, 41.01, 41.41, 41.49 (2C), 46.86, 47.71, 54.13, 71.29, 79.94, 87.92, 130.35, 134.24. GC-MS (m/z): 454.4 (M+).
(1R,2S,3R)-3β-Hydroxy-2-methyl-(1,2),(19β,28)-diepoxy-18αН-oleanane (5): Yield: 60%, Rf value 0.28 (light petroleum (b.p. 40–60 °C) /ethyl acetate 5:1), colorless crystals, m.p. 192.9 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+13.0 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3437 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.74, 0.79, 0.85, 0.927, 0.934, 0.985, 0.992 (21H, 7s, CH3 × 7); 1.44 (3H, s, 3Н-31); 3.06 (1Н, s, Н-1); 3.22 (1Н, s, Н-3); 3.44 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.52 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.54, 15.96, 17.63, 21.26, 23.05, 23.39, 23.94, 24.53, 25.96, 26.04, 26.23, 26.41, 28.80, 32.22, 32.76, 34.14, 36.28, 36.75, 36.77, 37.87, 40.92, 40.96, 41.52, 41.61, 44.58, 46.86, 61.38, 69.96, 71.28, 77.07, 88.01. GC-MS (m/z): 470.4 (M+).
4.3. General Procedure for Preparing Compounds –
Compound 2 (6.6 mmol) in small portions was added to a freshly prepared solution of CH3MgI (13.2 mmol), С2Н5MgBr (13.2 mmol), or С6Н5MgI (13.2 mmol) in anhydrous Et2O (20 mL), and then an anhydrous mixture of Et2O and THF in a ratio of 2:1 (15 mL) was dropwise added additionally. The reaction mixture was heated and stirred, and 1 h later, the solution was cooled to 20 °C, diluted dropwise with ice water (25 mL), then with a mixture of HCl: H2O (1:1, 20 mL) and stirred until the precipitate was completely dissolved (approximately 1 h). The reaction products were extracted with ethyl acetate (3 × 20 mL). The organic layer was separated and washed sequentially with a saturated solution of NaHSO3 and NaHCO3, then with a small amount of H2O, and dried over anhydrous MgSO4. The solvent was evaporated. The residue was purified by column chromatography on silica gel to obtain corresponding compounds 7–9.
(3S)-3β-Hydroxy-2,3-dimethyl-19β,28-epoxy-18αН-olean-1-еne (7): Yield: 65%, Rf value 0.31 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 106.1 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+35.4 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3456 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.78, 0.84, 0.92, 0.95, 0.98 (15Н, 5s, CH3 × 5); 0.90 (6H, s, CH3 × 2); 1.22 (3H, s, 3Н-32); 1.71 (3H, s, 3Н-31); 3.43 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.52 (1Н, s, Н-19); 5.53 (1Н, s, H-1). 13C NMR (100 MHz, CDCl3) δ: 13.47, 16.51, 18.16, 18.77, 19.41, 20.70, 21.01, 23.46 (2C), 24.49, 26.21, 26.32, 26.35, 28.79, 32.75, 34.05, 34.65, 36.25, 36.80, 38.99, 39.90, 40.97, 41.27, 41.48, 46.88, 48.20, 51.43, 71.28, 77.56, 87.91, 132.36, 133.72. GC-MS (m/z): 468.3 (M+).
(3S)-3-Ethyl-3β-hydroxy-2-methyl-19β,28-epoxy-18αН-olean-1-еne (8): Yield: 75%, Rf value 0.41 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 155.8 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+30.4 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3456 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.79, 0.83, 0.89, 0.92, 0.93, 0.96, 0.99 (21H, 7s, CH3 × 7); 0.96 (3Н, t, J = 8.0 Hz, 3Н-33); 1.70 (3H, s, 3Н-31); 3.44 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.53 (1Н, s, Н-19); 5.67 (1Н, s, H-1). 13C NMR (100 MHz, CDCl3) δ: 11.35, 13.32, 16.49, 18.07, 19.25, 19.44, 21.14, 21.82, 23.29, 24.46, 26.26, 26.30, 26.32, 28.78, 30.08, 32.72, 34.03, 34.75, 36.22, 36.79, 38.91, 40.68, 40.96, 41.22, 41.45, 46.87, 48.27, 50.54, 71.25, 79.37, 87.86, 131.96, 134.10. GC-MS (m/z): 482.4 (M+).
(3R)-3β-Hydroxy-2-methyl-3-phenyl-19β,28-epoxy-18αН-olean-1-еne (9): Yield: 72%, Rf value 0.46 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 83.5 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+16.4 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 3432 (-ОН). 1H NMR (400 MHz, CDCl3) δ: 0.53, 0.81, 0.93, 0.94, 1.01, 1.02, 1.05 (21H, 7s, CH3 × 7); 1.48 (3H, s, 3Н-31); 3.44 and 3.77 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.56 (1Н, s, Н-19); 5.90 (1Н, s, H-1); 7.23 (1Н, m, Н-33); 7.27–7.30 (3Н, m, Н-34, Н-36, Н-37); 7.69 (1Н, dd, J = 1.2, 8.0 Hz, Н-35). 13C NMR (100 MHz, CDCl3) δ: 13.24, 16.53, 17.57, 19.30, 21.08, 21.82, 24.51, 26.26, 26.29, 26.31, 26.39, 28.80, 32.75, 34.10, 34.46, 36.27 (2С), 36.82, 38.84, 40.53, 41.08, 41.25, 41.49, 46.93, 47.80, 49.31, 71.29, 82.59, 87.90, 126.66, 126.97, 128.25, 131.00, 131.72, 134.30, 142.98, 154.93. GC-MS (m/z): 530.4 (M+).
4.4. General Procedure for Preparing Compounds and
To a solution of compound 7 (4.2 mmol) or 8 (4.1 mmol) in CH2Cl2 (20 mL), a threefold excess of PCC was added. The reaction mixture was stirred at room temperature for 24 h. Completeness of the reaction was monitored by TLC. The solvent was distilled off, and the residue was diluted with water and extracted with ethyl acetate (3 × 50 mL). The organic layer was dried over anhydrous MgSO4, the solvent was evaporated, and the residue was purified by column chromatography on silica gel to obtain corresponding compounds 15 and 16.
(2S,3S)-2,3-Dimethyl-(2,3),(19β,28)-diepoxy-18αН-olean-1-one (16): Yield: 59%, Rf value 0.35 (light petroleum (b.p. 40–60 °C)/ethyl acetate, 5:1), colorless crystals, m.p. 172.5 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+34.8 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 1716 (C=O). 1H NMR (400 MHz, CDCl3) δ: 0.79, 0.98, 1.06, 1.07, 1.10 (15H, 5s, CH3 × 5); 0.92 (6H, s, CH3 × 2); 1.33 (3H, s, 3Н-32); 1.41 (3H, s, 3Н-31); 3.43 and 3.75 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.54 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 13.27, 14.06, 14.33, 15.60, 16.58, 19.46, 20.99, 24.54, 25.09, 26.12, 26.26, 26.41, 26.51, 28.79, 32.66, 32.78, 34.82, 36.28, 36.75, 37.00, 40.17, 40.41, 41.02, 41.49, 46.63, 46.66, 49.65, 63.74, 68.85, 71.35, 87.91, 208.84. GC-MS (m/z): 482.3 (M+).
(2S,3S)-3-Ethyl-2-methyl-(2,3),(19β,28)-diepoxy-18αН-olean-1-one (17): Yield: 62%, Rf value 0.48 (light petroleum (b.p. 40–60 °C)/ethyl acetate 5:1), colorless crystals, m.p. 175.1 °С (light petroleum (b.p. 40–60 °C)/ethyl acetate 7:1), [α]+43.2 (c 0.5, CHCl3). IR ν (CHCl3) cm−1: 1715 (С=О). 1H NMR (400 MHz, CDCl3) δ: 0.80, 0.92, 0.923, 0.99, 1.05, 1.148, 1.152 (21H, 7s, CH3 × 7); 1.08 (3Н, t, J = 8.0 Hz, 3Н-33); 1.44 (3H, s, 3Н-31); 3.43 and 3.76 (2Н, 2d, J = 8.0 Hz, 2Н-28); 3.54 (1Н, s, Н-19). 13C NMR (100 MHz, CDCl3) δ: 11.27, 13.26, 13.36, 14.77, 16.55, 19.42, 21.12, 23.58, 24.53, 25.54, 26.12, 26.25, 26.40, 26.50, 28.79, 32.61, 32.77, 34.81, 36.27, 36.75, 37.78, 40.13, 40.41, 41.02, 41.47, 46.62, 46.68, 49.58, 63.91, 71.35, 71.55, 87.89, 208.93. GC-MS (m/z): 496.4 (M+).
CCDC 2167086 (3), 2167084 (4a), 2167087 (7), 2167085 (8), 2167091 (12), 2167088 (13), 2169665 (14), 2167089 (15), 2167092 (16), and 2167090 (17) contain the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 3 January 2023).
4.5. Screening for Cytotoxic Activity of Compounds , , , –, , and
The cytotoxic activity of the tested compounds was determined by MTT assay [51] on HEpG2, HCT116, MS, RD TE32, A549, and MCF-7 cancer cell lines. The standardized ATCC cell lines were obtained from the N.N. Blokhin National Medical Research Center of Oncology (the Ministry of Health of the Russian Federation, Moscow, Russia). The cells were maintained in DMEM (MCF-7, HCT116, HEpG2) or RPMI 1640 (RD TE32, MS, A549) medium (PanEco, Moscow, Russia) with 10% fetal bovine serum (Biosera, Nuaille, France), 2 mM L-glutamine (PanEco, Moscow, Russia), and 1% penicillin/streptomycin (50 U/mL; 50 µg/mL) (PanEco, Russia). The cells were seeded in 96-well plates at a density of 1 × 104 cells/well and incubated for 24 h in a humidified CO2 incubator (model 460-СЕ, Thermo Fisher Scientific, Waltham, MA, USA) at +37 °C and 5% CO2. The stock solutions (1 × 10−2 M) of the tested compounds were prepared by dissolving in DMSO and then added to the wells by a micromethod of serial twofold dilutions at the concentration range of 0.3125 to 100 µM. The cells were cultivated with the compounds for 72 h, then 20 µL of MTT solution (5 mg/mL) was added to each well, and the cells were incubated for 3 h. After incubation, the medium with the compounds was removed, and the formed formazan crystals were dissolved in 100 μL of DMSO. 1% DMSO was considered to be safe for the cells and used as a control. Doxorubicin (Tocris Bioscience, Bristol, UK) was used as a reference drug. The optical density of the DMSO solutions was measured using a microplate reader FLUOstar Optima at 544 nm (BMG Labtech, Ortenberg, Germany). The IC50 value was determined on the basis of dose-dependent curves with the use of the Prism 6.0 program (GraphPad Software, San Diego, CA, USA). All the experiments were reiterated thrice, and the findings were presented as a mean ± standard deviation (SD).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28020550/s1, NMR spectra of synthesized compounds; Table S1: crystal data and structure refinement for 3; Table S2: crystal data and structure refinement for 4a; Table S3: crystal data and structure refinement for 7; Table S4: crystal data and structure refinement for 8; Table S5: crystal data and structure refinement for 12; Table S6: crystal data and structure refinement for 13; Table S7: crystal data and structure refinement for 14; Table S8: crystal data and structure refinement for 15; Table S9: crystal data and structure refinement for 16; Table S10: crystal data and structure refinement for 17.
Author Contributions
Conceptualization, V.G.; methodology, G.K., M.D. and Y.B.; software, M.D. and G.K.; investigation, G.K., M.D. and Y.B.; writing—original draft preparation, V.G. and G.K.; writing—review and editing, V.G.; supervision, V.G.; funding acquisition, V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the State Contractual Order Nr. 122012400109-8. The work was carried out using the equipment of The Core Facilities Center “Research of materials and matter” at the PFRC UB RAS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The crystallographic data of the synthesized derivatives (CCDC: 2167086 (3), 2167084 (4a), 2167087 (7), 2167085 (8), 2167091 (12), 2167088 (13), 2169665 (14), 2167089 (15), 2167092 (16), 2167090 (17)) can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Markov, A.V.; Zenkova, M.A.; Logashenko, E.B. Modulation of tumour-related signaling pathways by natural pentacyclic triterpenoids and their semisynthetic derivatives. Curr. Med. Chem. 2017, 24, 1277–1320. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xu, J.; Lin, A.; Wu, X.; Wu, L.; Xie, W. Recent advances for the synthesis of selenium-containing small molecules as potent antitumor agents. Curr. Med. Chem. 2018, 25, 2009–2033. [Google Scholar] [CrossRef]
- Wu, H.F.; Morris-Natschke, S.L.; Xu, X.D.; Yang, M.H.; Cheng, Y.Y.; Yu, S.S.; Lee, K.H. Recent advances in natural anti-HIV triterpenoids and analogs. Med. Res. Rev. 2020, 40, 2339–2385. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Şoica, C.; Voicu, M.; Ghiulai, R.; Dehelean, C.; Racoviceanu, R.; Trandafirescu, C.; Roșca, O.-J.; Nistor, G.; Mioc, M.; Mioc, A. Natural compounds in sex hormone-dependent cancers: The role of triterpenes as therapeutic agents. Front. Endocrinol. 2021, 11, 612396. [Google Scholar] [CrossRef]
- Nadendla, R.R. Principles of Organic Medicinal Chemistry; New Age Publishers: New Delhi, India, 2005; 322p. [Google Scholar]
- Urban, M.; Klinot, J.; Tislerova, I.; Biedermann, D.; Hajduch, M.; Cisarova, I.; Sarek, J. Reactions of activated lupane oxo-compounds with diazomethane: An approach to new derivatives of cytotoxic triterpenes. Synthesis 2006, 23, 3979–3986. [Google Scholar] [CrossRef]
- Michaudel, Q.; Journot, G.; Regueiro-Ren, A.; Goswami, A.; Guo, Z.; Tully, T.P.; Zou, L.; Ramabhadran, R.O.; Houk, K.N.; Baran, P.S. Improving physical properties via C-H oxidation: Chemical and enzymatic approaches. Angew. Chem. Int. Ed. 2014, 53, 12091–12096. [Google Scholar] [CrossRef]
- Huang, R.Z.; Jin, L.; Wang, C.G.; Xu, X.J.; Du, Y.; Liao, N.; Ji, M.; Liao, Z.X.; Wang, H.S. A pentacyclic triterpene derivative possessing polyhydroxyl ring A suppresses growth of HeLa cells by reactive oxygen species-dependent NF-κB pathway. Eur. J. Pharmacol. 2018, 838, 157–169. [Google Scholar] [CrossRef]
- Guo, H.; Wang, H.; Huo, Y.X. Engineering critical enzymes and pathways for improved triterpenoid biosynthesis in yeast. ACS Synth. Biol. 2020, 9, 2214–2227. [Google Scholar] [CrossRef]
- Shah, S.; Tan, H.; Sultan, S.; Faridz, M.; Shah, M.; Nurfazilah, S.; Hussain, M. Microbial-catalyzed biotransformation of multifunctional triterpenoids derived from phytonutrients. Int. J. Mol. Sci. 2014, 15, 12027–12060. [Google Scholar] [CrossRef]
- Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of oleanane and ursane triterpenic acids. Molecules 2020, 25, 5526. [Google Scholar] [CrossRef]
- Berger, M.; Knittl-Frank, C.; Bauer, S.; Winter, G.; Maulide, N. Application of relay C−H oxidation logic to polyhydroxylated oleanane triterpenoids. Chem 2020, 6, 1183–1189. [Google Scholar] [CrossRef]
- Mu, T.; Wei, B.; Zhu, D.; Yu, B. Site-selective C-H hydroxylation of pentacyclic triterpenoids directed by transient chiral pyridine-imino groups. Nat. Commun. 2020, 11, 4371. [Google Scholar] [CrossRef]
- Borkova, L.; Hodon, J.; Urban, M. Synthesis of betulinic acid derivatives with modified A-ring and their application as potential drug candidates. Asian J. Org. Chem. 2018, 7, 1542–1560. [Google Scholar] [CrossRef]
- Huang, L.; Luo, H.; Li, Q.; Wang, D.; Zhang, J.; Hao, X.; Yang, X. Pentacyclic triterpene derivatives possessing polyhydroxyl ring A inhibit Gram-positive bacteria growth by regulating metabolism and virulence genes expression. Eur. J. Med. Chem. 2015, 95, 64–75. [Google Scholar] [CrossRef]
- Huang, L.R.; Hao, X.J.; Li, Q.J.; Wang, D.P.; Zhang, J.X.; Luo, H.; Yang, X.S. 18β-glycyrrhetinic acid derivatives possessing a trihydroxylated a ring are potent gram-positive antibacterial agents. J. Nat. Prod. 2016, 79, 721–731. [Google Scholar] [CrossRef]
- Motlhanka, D.; Houghton, P.; Miljkovic-Brake, A.; Habtemariam, S. A novel pentacyclic triterpene glycoside from a resin of Commiphora glandulosa from Botswana. Afr. J. Pharm. Pharmacol. 2010, 4, 549–554. [Google Scholar]
- Liang, Z.M.; Wang, X.H.; Huang, L.R.; Li, Q.J.; Guan, T.Q.; Hao, X.J.; Luo, H.; Yang, X.S. 1α,2α-Epoxy-3β-hydroxy oleanolic acid derivatives regulation of the metabolism, haemolysis and β-lactamase gene expression in vitro and their structure–microbicidal activity relationship. Bioorganic Med. Chem. Lett. 2016, 26, 3870–3875. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Zhukova, A.E.; Dmitriev, M.V.; Grishko, V.V. Synthesis and intramolecular cyclization of a 2,3-seco-oleanane triterpenoid with an ethylketone fragment. Chem. Nat. Compd. 2018, 54, 1094–1099. [Google Scholar] [CrossRef]
- Hanson, J.R.; Hitchcock, P.B.; Kiran, I. The stereochemistry of epoxidation of steroidal 4,6-dienes. J. Chem. Res.—Part S 1999, 3, 198–199. [Google Scholar] [CrossRef]
- Kinot, J.; Krumpolc, M.; Vystrčil, A. Triterpenes. IX. Reaction of isomeric 2,3-epoxides with Grignard reagent. Collect. Czechoslov. Chem. Commun. 1966, 31, 3174–3181. [Google Scholar] [CrossRef]
- Kehrli, A.R.H.; Taylor, D.A.H.; Niven, M. The synthesis of a 1α,2α,3α-triacetoxy limonoid. J. Chem. Soc. Perkin Trans. 1990, 7, 2057–2065. [Google Scholar] [CrossRef]
- García-Granados, A.; López, P.E.; Melguizo, E.; Parra, A.; Simeó, Y. Oxidation of several triterpenic diene and triene systems. Oxidative cleavage to obtain chiral intermediates for drimane and phenanthrene semi-synthesis. Tetrahedron 2004, 60, 3831–3845. [Google Scholar] [CrossRef]
- Amer, H.; Mereiter, K.; Stanetty, C.; Hofinger, A.; Czollner, L.; Beseda, I.; Jordis, U.; Kueenburg, B.; Claßen-Houben, D.; Kosma, P. Synthesis and crystal structures of ring A modified glycyrrhetinic acid derivatives derived from 2,3-oxirane and 2,3-thiirane intermediates. Tetrahedron 2010, 66, 4390–4402. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Khusnutdinova, E.F.; Korlyukov, A.A. Stereospecific epoxidation of an olean-18(19)-ene-type triterpenoid. Chem. Nat. Compd. 2011, 46, 900–901. [Google Scholar] [CrossRef]
- Mikhailova, L.R.; Budaev, A.S.; Spirikhin, L.V.; Baltina, L.A. Oxidation of licorice-root triterpene-acid derivatives by m-chloroperbenzoic acid. Chem. Nat. Compd. 2019, 55, 88–91. [Google Scholar] [CrossRef]
- Pakulski, Z.; Cmoch, P.; Korda, A.; Luboradzki, R.; Gwardiak, K.; Karczewski, R. Rearrangements of the betulin core. Synthesis of terpenoids possessing the bicyclo[3.3.1]nonane fragment by rearrangement of lupane-type epoxides. J. Org. Chem. 2021, 86, 1084–1095. [Google Scholar] [CrossRef]
- Paryzek, Z. Epoxidation of lanost-9(11)-enes. The effect of a β-carbonyl group upon the stereochemistry of epoxidation. J. Chem. Soc. Perkin Trans. 1978, 1, 329–3369. [Google Scholar] [CrossRef]
- Kvasnica, M.; Tislerova, I.; Sarek, J.; Sejbal, J.; Cisarova, I. Preparation of new oxidized 18-α-oleanane derivatives. Collect. Czech. Chem. Commun. 2005, 70, 1447–1464. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Shelepen’kina, L.N.; Shashkov, A.S.; Grishko, V.V.; Glushkov, V.A.; Tolstikov, A.G. Reaction of 3-acetoxy-(2,3),(19β,28)-diepoxyoleanane with cyclic and linear amines. Chem. Nat. Compd. 2007, 43, 153–158. [Google Scholar] [CrossRef]
- Csuk, R.; Nitsche, C.; Sczepek, R.; Schwarz, S.; Siewert, B. Synthesis of antitumor-active betulinic acid-derived hydroxypropargylamines by copper-catalyzend mannich reactions. Arch. Pharm. 2013, 346, 232–246. [Google Scholar] [CrossRef]
- Pereslavtseva, A.V.; Tolmacheva, I.A.; Slepukhin, P.A.; El’tsov, O.S.; Kucherov, I.I.; Eremin, V.F.; Grishko, V.V. Synthesis of A-pentacyclic triterpene α,β-alkenenitriles. Chem. Nat. Compd. 2014, 49, 1059–1066. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Nebogatikov, V.O.; Tolmacheva, I.A.; Dmitriev, M.V.; Grishko, V.V. Synthesis of cytotoxically active derivatives based on alkylated 2,3-seco-triterpenoids. Eur. J. Med. Chem. 2017, 140, 74–83. [Google Scholar] [CrossRef]
- Konysheva, A.V.; Eroshenko, D.V.; Grishko, V.V. Synthesis, Cyclization, and cytotoxic activity of 2,3-secolupane triterpenoids with an ethylketone fragment. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A: Structure and Mechanisms, 5th ed.; Springer: New York, NY, USA, 2007; 1199p. [Google Scholar]
- Kočovský, P. Stereochemistry of epoxidation of allylic and homoallylic cyclohexene alcohols. J. Chem. Soc. Perkin Trans. 1994, 13, 1759–1763. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Afonso, C.A.M. Brønsted acid-catalyzed dihydroxylation of olefins in aqueous medium. Adv. Synth. Catal. 2011, 353, 2920–2926. [Google Scholar] [CrossRef]
- Jat, J.L.; Kumar, G. Isomerization of Epoxides. Adv. Synth. Catal. 2019, 361, 4426–4441. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ganesh, V. Oxidation adjacent to oxygen of alcohols by chromium reagents. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, pp. 277–294. [Google Scholar]
- Luzzio, F.A. 1,3-Oxidative transpositions of allylic alcohols in organic synthesis. Tetrahedron 2012, 68, 5323–5339. [Google Scholar] [CrossRef]
- Papillaud, B.; Tiffon, F.; Taran, M.; Miguel, B.A.S.; Delmond, B. Part I. epoxydes diterpeniques: Synthese et reactivite d’epoxydes derives d’acides resiniques. Tetrahedron 1985, 41, 1845–1857. [Google Scholar] [CrossRef]
- Ley, S.V.; Madin, A. Oxidation adjacent to oxygen of alcohols by chromium reagents. In Comprehensive Organic Synthesis; Trost, B.M., Ed.; Plenum: New York, NY, USA, 1991; Volume 7, 251p. [Google Scholar]
- Kharitonov, Y.V.; Shul’ts, E.E.; Shakirov, M.M. Synthetic transformations of higher terpenoids. XXXIII.*Preparation of 15,16-dihydroisopimaric acid and methyl dihydroisopimarate and their transformations. Chem. Nat. Compd. 2014, 49, 2857–2899. [Google Scholar] [CrossRef]
- Singha, R.; Ghosh, P. Phytochemical investigation of Sapium baccatum: Identification of 3α-hydroxy-1α, 2α-epoxy lupan. J. Indian Chem. Soc. 2018, 95, 549–552. [Google Scholar] [CrossRef]
- Salomatina, O.V.; Sen’kova, A.V.; Moralev, A.D.; Savin, I.A.; Komarova, N.I.; Salakhutdinov, N.F.; Zenkova, M.A.; Markov, A.V. Novel Epoxides of Soloxolone Methyl: An Effect of the Formation of Oxirane Ring and Stereoisomerism on Cytotoxic Profile, Anti-Metastatic and Anti-Inflammatory Activities In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 6214. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Molina, M.T.; Anjum, S. Naturally occurring cyclohexane epoxides: Sources, biological activities and synthesis. Chem. Rev. 2004, 104, 2857–2899. [Google Scholar] [CrossRef] [PubMed]
- Way2Drug Predictive Services. PASS Online. Available online: http://www.pharmaexpert.ru/passonline/index.php (accessed on 6 April 2022).
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biomed. Chem. 2020, 66, 30–41. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Keil, B. Laboratory Technika Organicke Chemie; Nakladatelstvi ČSAV: Praha, Czech Republic, 1963; 751p. [Google Scholar]
- CrysAlisPro, Version 1.171.37.33 (Release 27-03-2014 CrysAlis171.NET); Agilent Technologies: Yarnton, UK, 2014.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. SUPERFLIP - A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).