Pharmacokinetic Study of Triptolide Nanocarrier in Transdermal Drug Delivery System—Combination of Experiment and Mathematical Modeling
Abstract
1. Introduction
2. Results
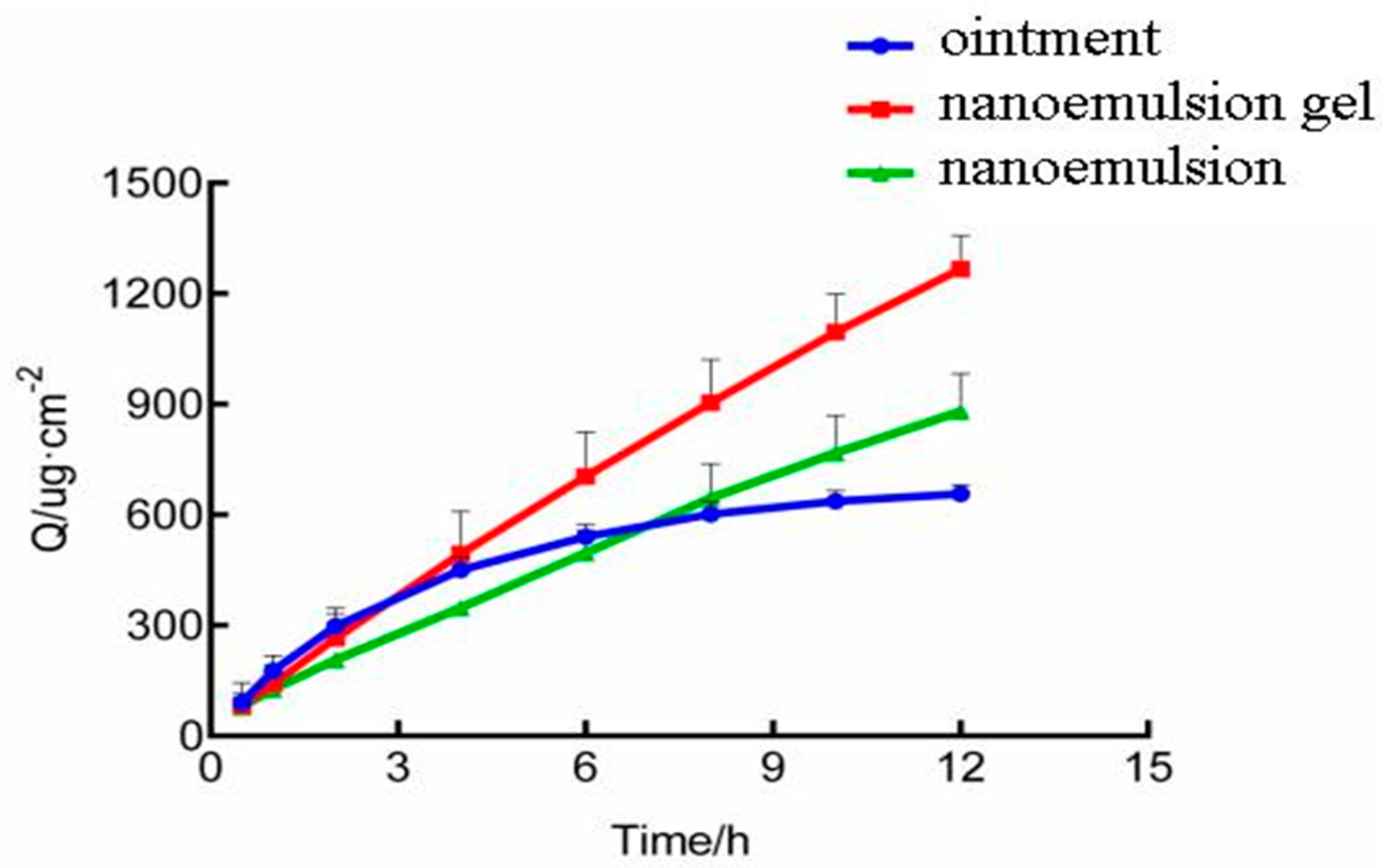
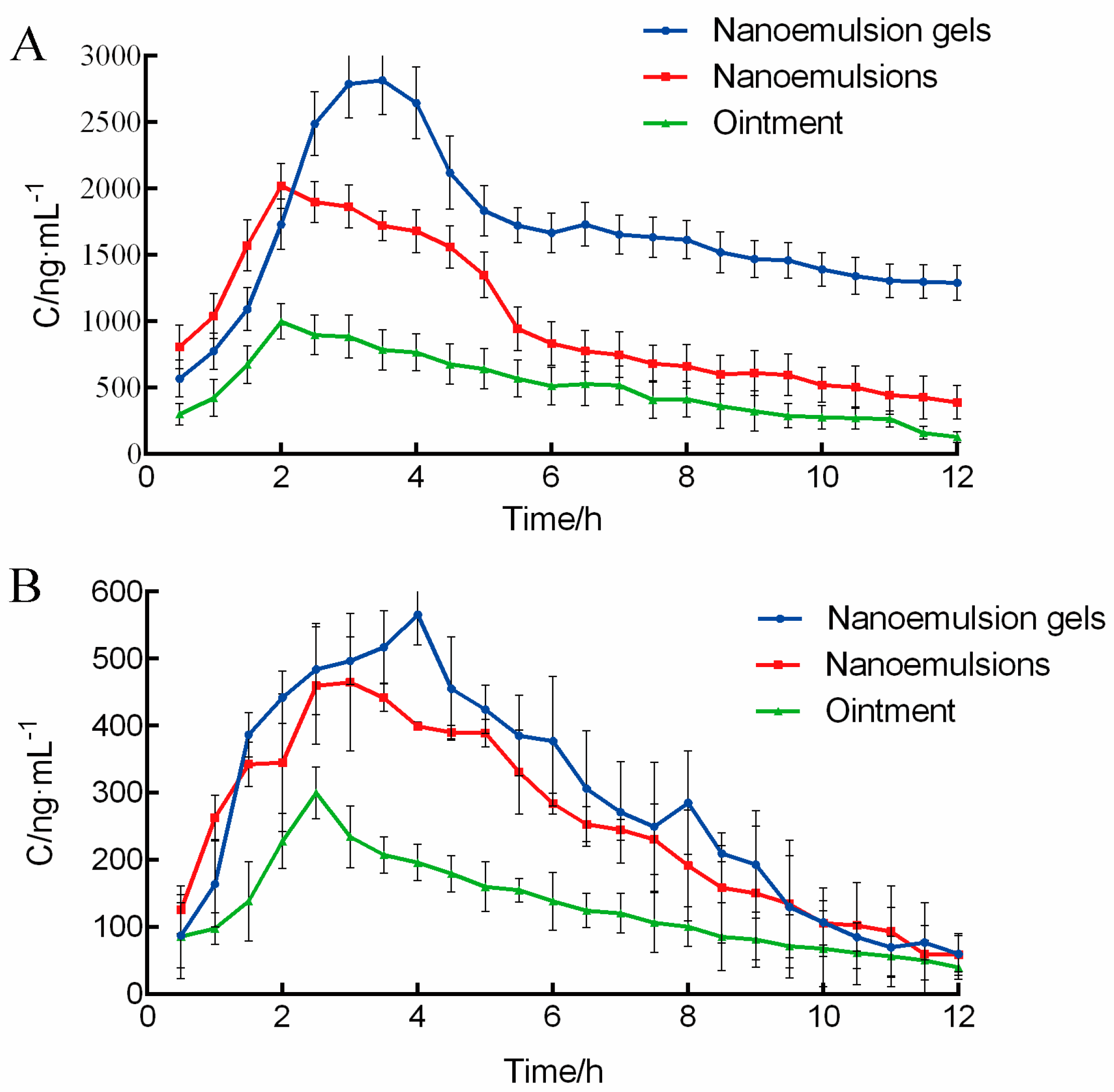
2.1. Pharmacokinetis Study of Triptolide
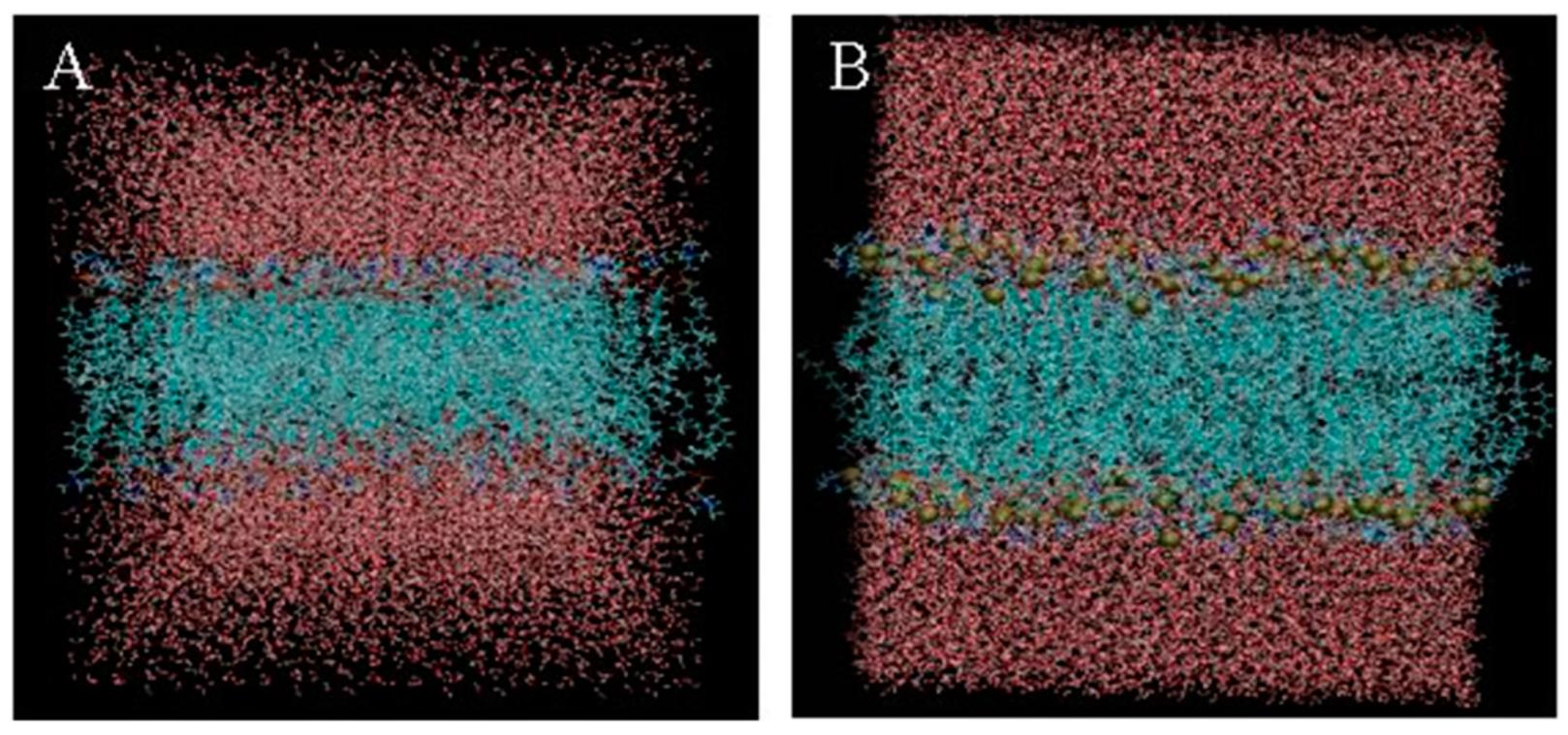
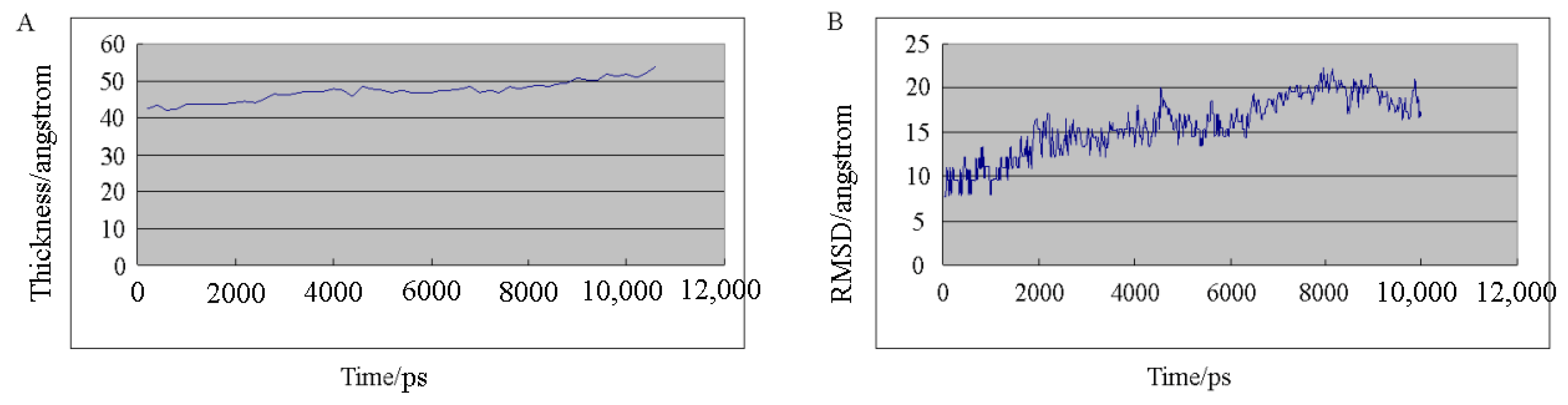
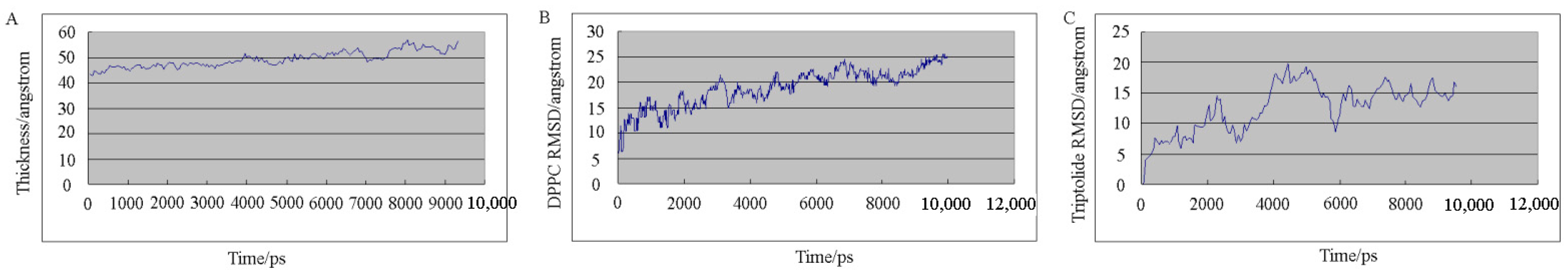
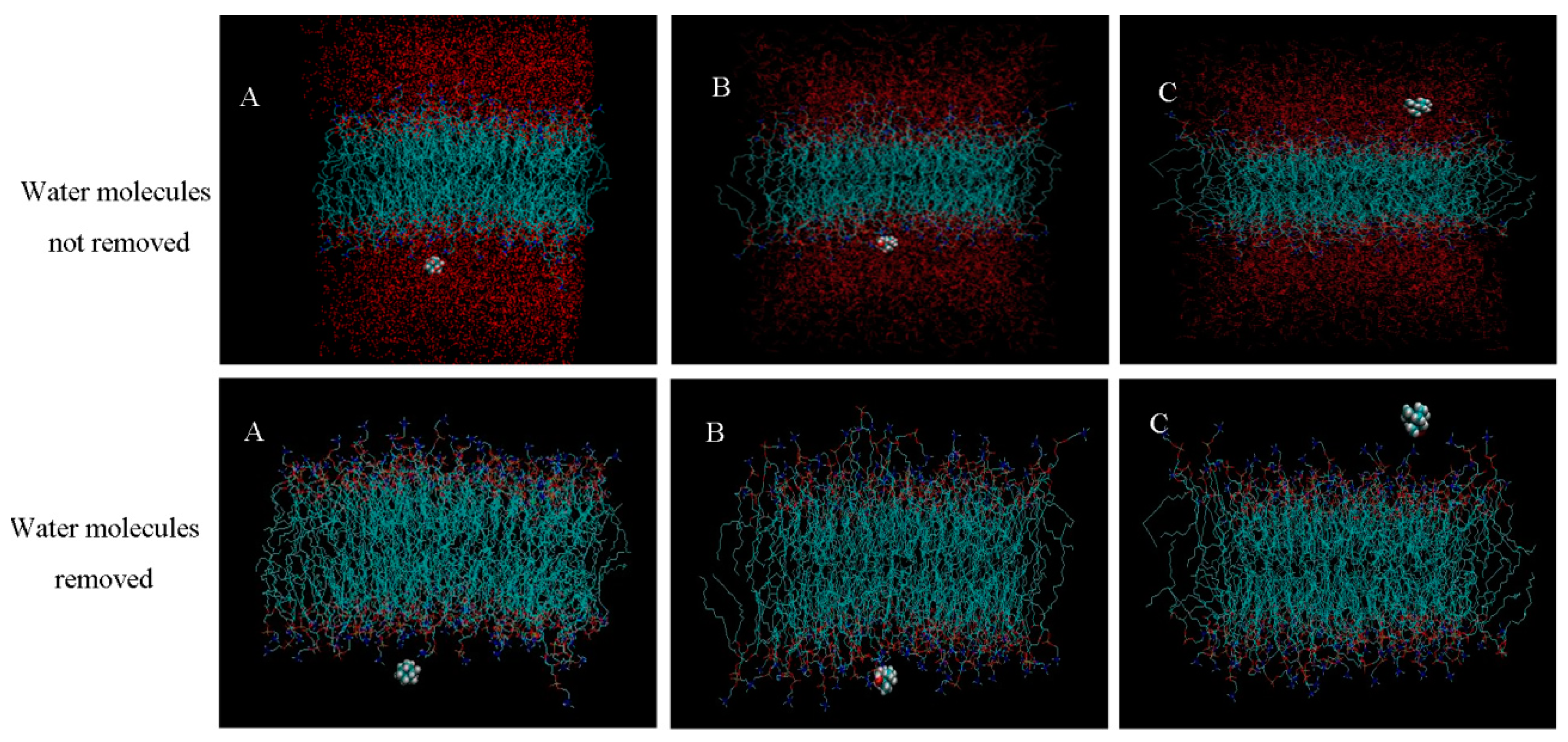
2.2. Molecular Dynamics Simulation
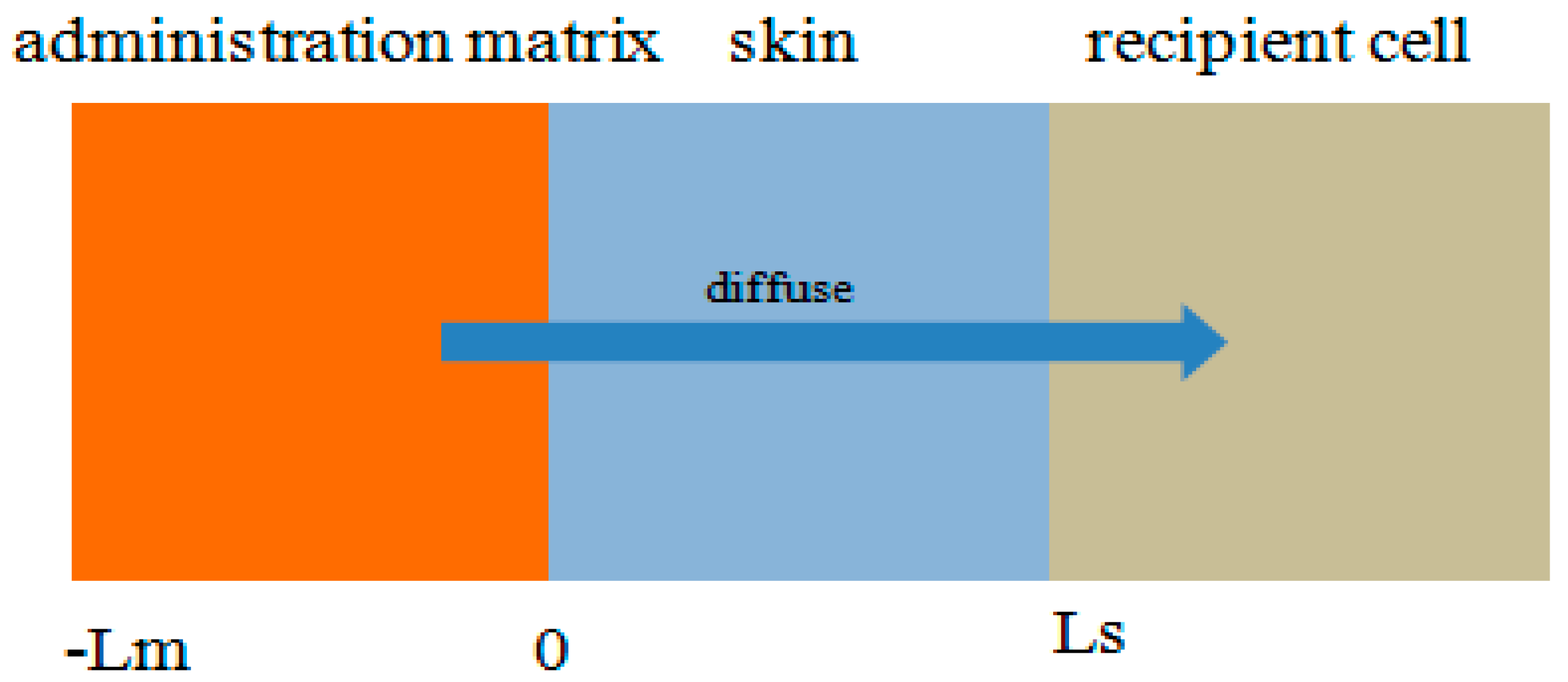
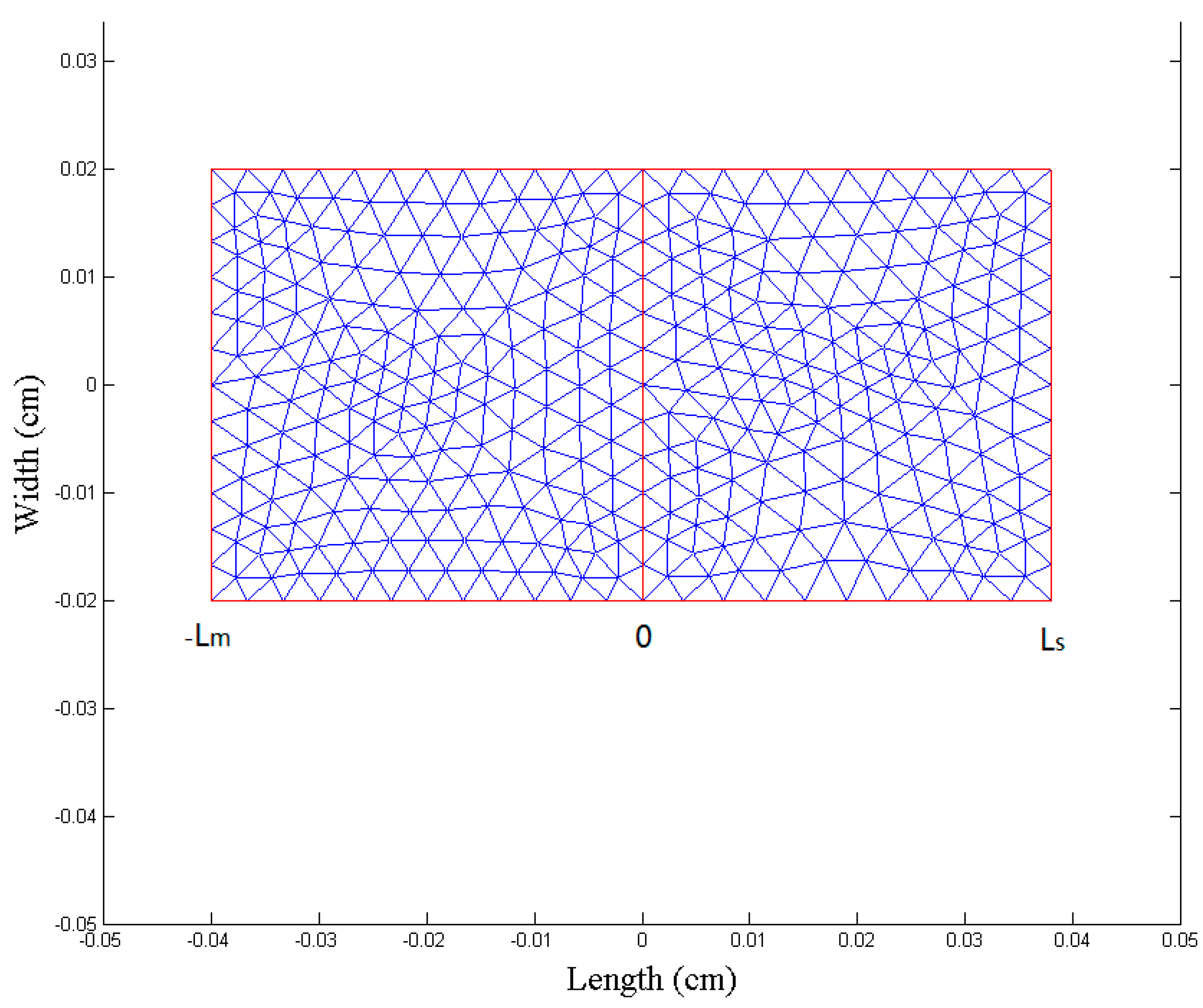
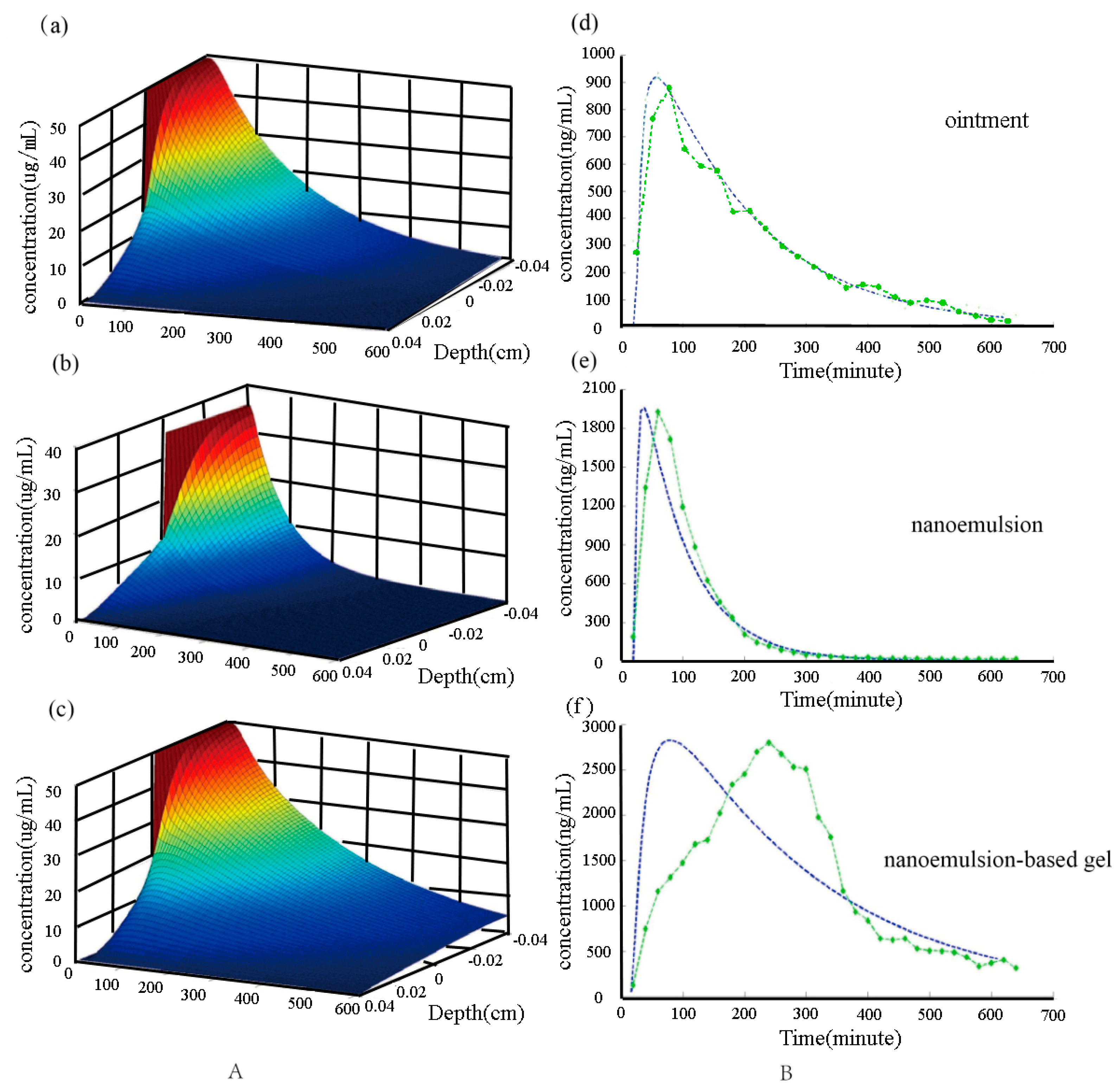
2.3. Numerical Simulation
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Experimental Method
3.3.1. LC–MS Analysis [4,5]
3.3.2. Preparation of Nanoemulsion and Nanoemulsion−Based Gels
3.3.3. Ex Vivo Percutaneous Penetration
3.3.4. In Vivo Microdialysis Studies
3.3.5. Pharmacokinetic Analysis
3.4. Molecular Dynamics Simulation
3.4.1. Construction of DPPC Bilayer Biofilms
3.4.2. Construction of a New Biofilm System of Triptolide and DPPC
3.5. Numerical Simulation
3.5.1. Establishment of Multilayer Structure Model
3.5.2. Selection of Model Parameters
3.5.3. Numerical Methods
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Gu, Y.; Tang, X.; Wang, T.; Liu, J. Advancement of Lipid-Based Nanocarriers and Combination Application with Physical Penetration Technique. Curr. Drug. Deliv. 2019, 16, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug. Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Güngör, S.; Kahraman, E. Nanocarriers Mediated Cutaneous Drug Delivery. Eur. J. Pharm. Sci. 2021, 158, 105638. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of triptolide-nanoemulsion gels for percutaneous administration: Physicochemical, transport, pharmacokinetic and pharmacodynamic characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, M.; Tang, X.; Wang, T.; Yang, D.; Zhai, G.; Liu, J. Lipid nanoparticles loading triptolide for transdermal delivery: Mechanisms of penetration en-hancement and transport properties. J. Nanobiotechnol. 2018, 16, 68. [Google Scholar] [CrossRef]
- Lan, J.; Liu, L.; Zeng, R.; Qin, Y.; Hou, J.; Xie, S.; Yue, S.; Yang, J.; Rodney, J.; Ding, Y.; et al. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021, 407, 127212. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Gulati, M.; Singh, S.K.; Kapoor, B.; Dureja, H.; Chellappan, D.K.; Anand, K.; Dua, K.; Khursheed, R.; et al. Nanocarriers for treatment of dermatological diseases: Principle, perspective and practices. Eur. J. Pharm. 2021, 890, 173691. [Google Scholar] [CrossRef]
- Pierre, M.B.R. Nanocarriers for Photodynamic Therapy Intended to Cutaneous Tumors. Curr. Drug Targets 2021, 22, 1090–1107. [Google Scholar] [CrossRef]
- Fan, Y.; Marioli, M.; Zhang, K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J. Pharm. Biomed. Anal. 2021, 192, 113642. [Google Scholar] [CrossRef]
- Omidi, Y.; Kianinejad, N.; Kwon, Y.; Omidian, H. Drug delivery and targeting to brain tumors: Considerations for crossing the blood-brain barrier. Expert Rev. Clin. Pharm. 2021, 14, 357–381. [Google Scholar] [CrossRef]
- Spencer, P.; Jiang, Y.; Liu, N.; Han, J.; Li, Y.; Vodovoz, S.; Dumont, A.S.; Wang, X. Update: Microdialysis for Monitoring Cerebral Metabolic Dysfunction after Subarachnoid Hemorrhage. J. Clin. Med. 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspensions. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Mechanisms of sustained action mediation. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 41, 6–12. [Google Scholar] [CrossRef]
- Liu, X.; Shi, D.; Zhou, S.; Liu, H.; Liu, H.; Yao, X. Molecular dynamics simulations and novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 23–37. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular Dynamics Simulation for All. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Jeřábek, J.; Rinderer, M.; Gessler, A.; Weiler, M. Xylem sap phosphorus sampling using microdialysis-a non-destructive high sampling frequency method tested under laboratory and field conditions. Tree Physiol. 2020, 40, 1623–1638. [Google Scholar] [CrossRef]
- Altendorfer-Kroath, T.; Schimek, D.; Eberl, A.; Rauter, G.; Ratzer, M.; Raml, R.; Sinner, F.; Birngruber, T. Comparison of cerebral Open Flow Microperfusion and Microdialysis when sampling small lipophilic and small hydrophilic substances. J. Neurosci. Methods 2019, 311, 394–401. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.P.; Harikumar, S.L. Development and optimization of nanoemulsion based gel for enhanced transdermal delivery of nitrendipine using box-behnken statistical design. Drug Dev. Ind. Pharm. 2020, 46, 329–342. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, X.; Yang, M.; Yang, D.; Liu, J. Transdermal drug delivery of triptolide-loaded nanostructured lipid carriers: Preparation, pharmacokinetic, and evaluation for rheumatoid arthritis. Int. J. Pharm. 2019, 554, 235–244. [Google Scholar] [CrossRef]
- Li, Z.; Biswas, A.; Finkelstein, J.; Kapoor, Y.; Milewski, M.; Queisser, G. Modeling Drug Absorption from the Dermis after an Injection. J. Pharm. Sci. 2021, 110, 1279–1291.e1. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, L.; Ghate, V.M.; Lewis, S.A. Derma rollers in therapy: The transition from cosmetics to transdermal drug delivery. Biomed. Microdevices 2020, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Nagra, U.; Zaman, M.; Mahmood, A.; Barkat, K. Lipid Vesicles and Nanoparticles for Non-invasive Topical and Transdermal Drug De-livery. Curr. Pharm. Des. 2020, 26, 2149–2166. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.K.; Kim, S.; Lee, J.; Im, W. CHARMM-GUI Membrane Builder for Lipid Nanoparticles with Ionizable Cationic Lipids and PEGylated Lipids. J. Chem. Inf. Model. 2021, 61, 5192–5202. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Lee, J.; Klauda, J.B.; Im, W. CHARMM-GUI Nanodisc Builder for modeling and simulation of various nanodisc systems. J. Comput. Chem. 2019, 40, 893–899. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with Glyco-lipids and Lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kern, N.R.; Kim, S.; Kanhaiya, K.; Afshar, Y.; Jeon, S.H.; Jo, S.; Brooks, B.R.; Lee, J.; Tadmor, E.B.; et al. CHARMM-GUI Nanomaterial Modeler for Modeling and Simulation of Nanomaterial Systems. J. Chem. Theory Comput. 2022, 18, 479–493. [Google Scholar] [CrossRef]
- Meli, R.; Biggin, P.C. Spyrmsd: Symmetry-corrected RMSD calculations in Python. J. Cheminform 2020, 12, 49. [Google Scholar] [CrossRef]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A web server for automatic structure matching and RMSD calculations among identical and similar compounds in protein-ligand docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Coutsias, E.A.; Wester, M.J. RMSD and Symmetry. J. Comput. Chem. 2019, 40, 1496–1508. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Zhang, L.; Yang, Y.; Liu, R.; Li, Z. Comparison of Different Chitosan Lipid Nanoparticles for Improved Ophthalmic Tetrandrine De-livery: Formulation, Characterization, Pharmacokinetic and Molecular Dynamics Simulation. J. Pharm. Sci. 2020, 109, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Cametti, C. Fractional derivatives in the diffusion process in heterogeneous systems: The case of transdermal patches. Math. Biosci. 2017, 291, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.S.; Skirtach, A.G.; Volodkin, D. Hybrids of Polymer Multilayers, Lipids, and Nanoparticles: Mimicking the Cellular Microenvironment. Langmuir 2019, 35, 8565–8573. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Gu, Q.; Yang, Q.; Yang, M.; Wang, S.; Liu, J. Finite Element Analysis for Predicting Skin Pharmacokinetics of Nano Transdermal Drug Delivery System Based on the Multilayer Geometry Model. Int. J. Nanomed. 2020, 15, 6007–6018. [Google Scholar] [CrossRef] [PubMed]
- Defraeye, T.; Bahrami, F.; Rossi, R.M. Inverse Mechanistic Modeling of Transdermal Drug Delivery for Fast Identification of Op-timal Model Parameters. Front. Pharm. 2021, 12, 641111. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Liu, J.; Huang, S.; Liang, B.; Huang, X.; Yang, C.; Chen, M.; Liu, J.; Zhang, T.; Xie, X.; et al. Determination of Transdermal Rate of Metallic Microneedle Array through an Impedance Measurements-Based Numerical Check Screening Algorithm. Micromachines 2022, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.M.; Rzhevskiy, A.S.; Anissimov, Y.G. Numerical Investigation of Analytical Models of Drug Flux Through Microporated Skin. J. Pharm. Sci. 2019, 108, 358–363. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Li, T.S.; Su, H.L.; Lee, P.C.; Wang, H.M.D. Transdermal Delivery Systems of Natural Products Applied to Skin Therapy and Care. Molecules 2020, 25, 5051. [Google Scholar] [CrossRef]
- Varma, L.T.; Singh, N.; Gorain, B.; Choudhury, H.; Tambuwala, M.M.; Kesharwani, P.; Shukla, R. Recent Advances in Self-Assembled Nanoparticles for Drug Delivery. Curr. Drug Deliv. 2020, 17, 279–291. [Google Scholar] [CrossRef]
- Kho, C.M.; Enche Ab Rahim, S.K.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2017, 54, 3506–3527. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.F.; Kwasnicki, A.; Lakka, S.S.; Engelhard, H.H. Cerebral Microdialysis as a Tool for Assessing the Delivery of Chemotherapy in Brain Tumor Patients. World Neurosurg. 2021, 145, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Al Hanbali, O.A.; Khan, H.M.S.; Sarfraz, M.; Arafat, M.; Ijaz, S.; Hameed, A. Transdermal patches: Design and current approaches to painless drug delivery. Acta Pharm. 2019, 69, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Cametti, C. Diffusion through skin in the light of a fractional derivative approach: Progress and challenges. J. Pharm. Pharm. 2021, 48, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, P.; Azimi, A. Numerical simulation of fractional non-Fourier heat conduction in skin tissue. J. Biol. 2019, 84, 274–284. [Google Scholar] [CrossRef]
- Bora, D.J.; Dasgupta, R. Numerical simulation of iontophoresis for in-silico prediction of transdermal drugs in the dermal layers using skin impedance values. Comput. Methods Programs Biomed. 2021, 214, 106551. [Google Scholar] [CrossRef] [PubMed]
| Concentration (ng/mL) | Linear Recovery (%) | Concentric Cannula Recovery (%) | ||
|---|---|---|---|---|
| Gain Method | Loss Method | Gain Method | Loss Method | |
| 52.5 (low) | 61.4 ± 2.4 | 59.2 ± 3.1 | 48.4 ± 6.3 | 48.9 ± 3.8 |
| 210 (middle) | 61.4 ± 1.9 | 63.8 ± 1.8 | 53.7 ± 1.8 | 59.1 ± 4.5 |
| 1050 (high) | 62.1 ± 2.2 | 64.4 ± 3.8 | 49.1 ± 0.5 | 54.1 ± 1.5 |
| Concentration (ng/mL) | Linear Recovery (%) | Concentric Cannula Recovery (%) |
|---|---|---|
| 21 | / | 46.4 ± 9.9 |
| 52.5 | 64.8 ± 5.5 | 54.1 ± 4.7 |
| 210 | 70.9 ± 3.5 | 54.4 ± 3.7 |
| 1050 | 72.4 ± 2.2 | / |
| Parameters | Nanoemulsion | Nanoemulsion Gel | Ointment | |||
|---|---|---|---|---|---|---|
| Skin | Blood | Skin | Blood | Skin | Blood | |
| Cmax (ng/mL) | 2020.21 ± 170.27 | 464.98 ± 102.54 | 2645.43 ± 269.45 | 565.92 ± 45.66 | 998.72 ± 133.70 | 300.01 ± 38.82 |
| Tmax (min) | 120.0 | 180.0 | 210.0 | 240.0 | 120.0 | 150.0 |
| T1/2 (min) | 363.8 ± 20.5 | 223.5 ± 60.1 | 185.6 ± 29.1 | 234.8 ± 19.2 | 189.2 ± 35.1 | 234.6 ± 54.7 |
| AUC0–660 (ng·min/mL) | 13,412,917 ± 109,463.9 | 116,743.4 ± 98,612.4 | 17,674,876.1 ± 132,437.9 | 202,961.8 ± 18,672.8 | 8,764,515.3 ± 23,977.3 | 97,210.4 ± 20,966.4 |
| AUC0–∞ (ng·min/mL) | 18,932,186.3 ± 119,216.3 | 134,865.2 ± 89,192.0 | 21,126,376.4 ± 152,863.4 | 247,136.4 ± 17,604.1 | 9,007,619.4 ± 22,409.1 | 108,876.2 ± 36,214.6 |
| Name | Parameter |
|---|---|
| System | NTP (constant temperature and pressure, and the number of particles in the system remains unchanged) |
| Periodic boundary condition box | 6 nm × 6 nm × 6 nm |
| Force field | GROMACA force field |
| Temperature | 323 K, Nose Hoover temperature coupling, coupling constant 0.1 ps |
| Pressure | 1 bar, Parrinello Rahman pressure coupling, coupling constant 1 ps |
| Time interval of each step | 2 fs |
| Covalent bond constraint Electrostatic interaction Van der Waals interaction | LINCS algorithm Particle mesh Ewald scheme with 10 Å cut−off Lennard−Jones interactions with 10 Å cut−off |
| Dosage Form | Dm (× 10−6 cm2/h) | Ds (× 10−6 cm2/h) |
|---|---|---|
| Ointment | 9.21 | 20.25 |
| Nanoemulsion | 15.72 | 46.15 |
| Nanoemulsion−based gel | 7.42 | 10.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Meng, J.; Han, L.; Yu, X.; Fan, Z.; Yuan, Y. Pharmacokinetic Study of Triptolide Nanocarrier in Transdermal Drug Delivery System—Combination of Experiment and Mathematical Modeling. Molecules 2023, 28, 553. https://doi.org/10.3390/molecules28020553
Yang M, Meng J, Han L, Yu X, Fan Z, Yuan Y. Pharmacokinetic Study of Triptolide Nanocarrier in Transdermal Drug Delivery System—Combination of Experiment and Mathematical Modeling. Molecules. 2023; 28(2):553. https://doi.org/10.3390/molecules28020553
Chicago/Turabian StyleYang, Meng, Jianxia Meng, Lu Han, Xiaoyan Yu, Zhimin Fan, and Yongfang Yuan. 2023. "Pharmacokinetic Study of Triptolide Nanocarrier in Transdermal Drug Delivery System—Combination of Experiment and Mathematical Modeling" Molecules 28, no. 2: 553. https://doi.org/10.3390/molecules28020553
APA StyleYang, M., Meng, J., Han, L., Yu, X., Fan, Z., & Yuan, Y. (2023). Pharmacokinetic Study of Triptolide Nanocarrier in Transdermal Drug Delivery System—Combination of Experiment and Mathematical Modeling. Molecules, 28(2), 553. https://doi.org/10.3390/molecules28020553




