Saturated Cannabinoids: Update on Synthesis Strategies and Biological Studies of These Emerging Cannabinoid Analogs
Abstract
1. Introduction
2. Saturated Tricyclic Hexahydrocannabinol Homologs
2.1. Synthesis of Hexahydrocannabinol and Its Analogs
2.2. Pathways to Obtain Natural Machaeriols and Their Synthetic Analogs
2.3. Partial and Total Synthesis of 9R-11-Hydroxyhexahydrocannabinol and Its Derivatives
2.4. C-9 Ketocannabinoids: Different Enantioselective Synthetic Routes
2.5. Cannabinoid Lactones Modified in the C-Ring
3. Hydrogenated Bicyclic Cannabidiol Analogs
3.1. Hydrogenation of CBD and Its Derivatives
3.2. Machaeridiols and Their Synthetic Analogs
4. Non-Classic Hydrated Phytocannabinoids and Their Synthetic Analogs
4.1. Cannabielsoin: A Metabolite of Cannabidiol
4.2. Cannabimovone, Anhydrocannabimovone, and Their Non-Natural Analogs
5. Saturated Quinonoid Cannabinoid
5.1. Different Oxidation Pathways of Hydrogenated Cannabidiol and Tetrahydrocannabinol Derivatives
5.2. Applying the Domino Knoevenagel Intramolecular Hetero Diels–Alder Reaction to Obtain Benzoquinone Derivatives
6. Bi-, Tri-, and Tetra-Cyclic Hydrogenated Natural Cannabinoid Scaffolds
6.1. Cannabicitran
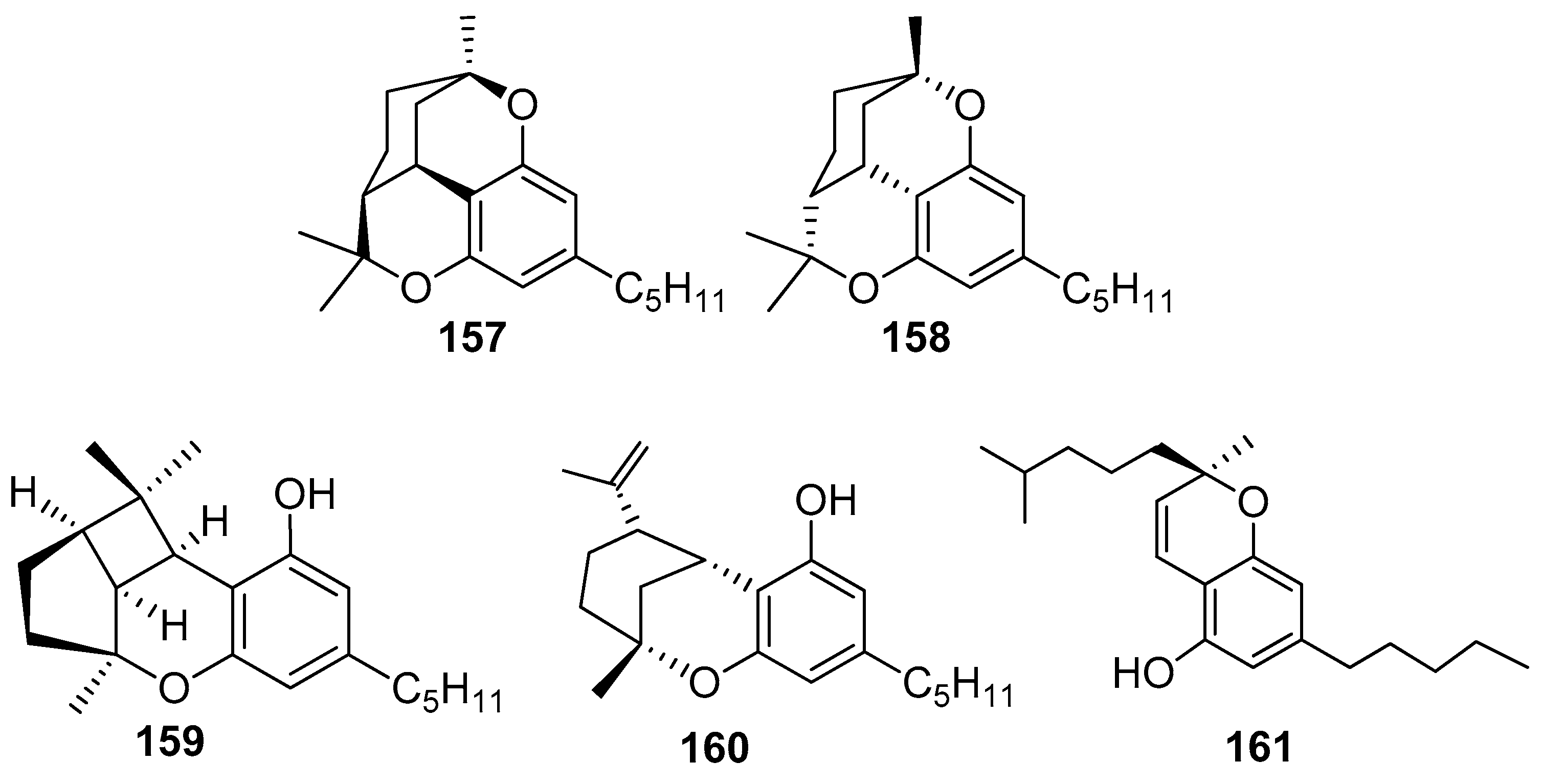
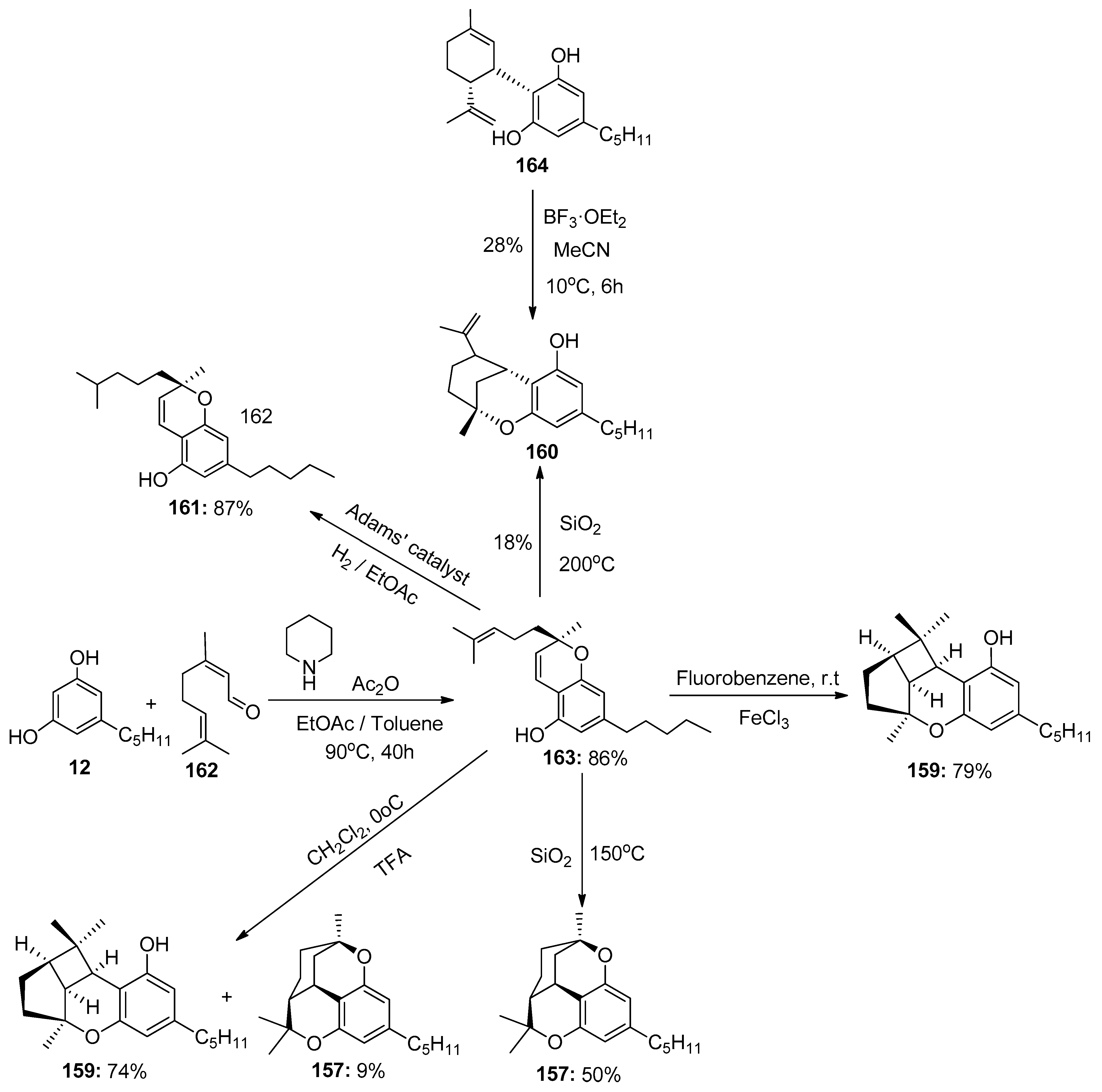
6.2. Cannabicyclol
6.3. Δ8-Iso-Cis-THC
6.4. Tetrahydrocannabichromene
7. Biological Studies of Saturated Cannabinoids
7.1. In Vitro Studies to Determine Affinities of Hydrogenated Cannabinoids for CB1 and CB2 Receptors
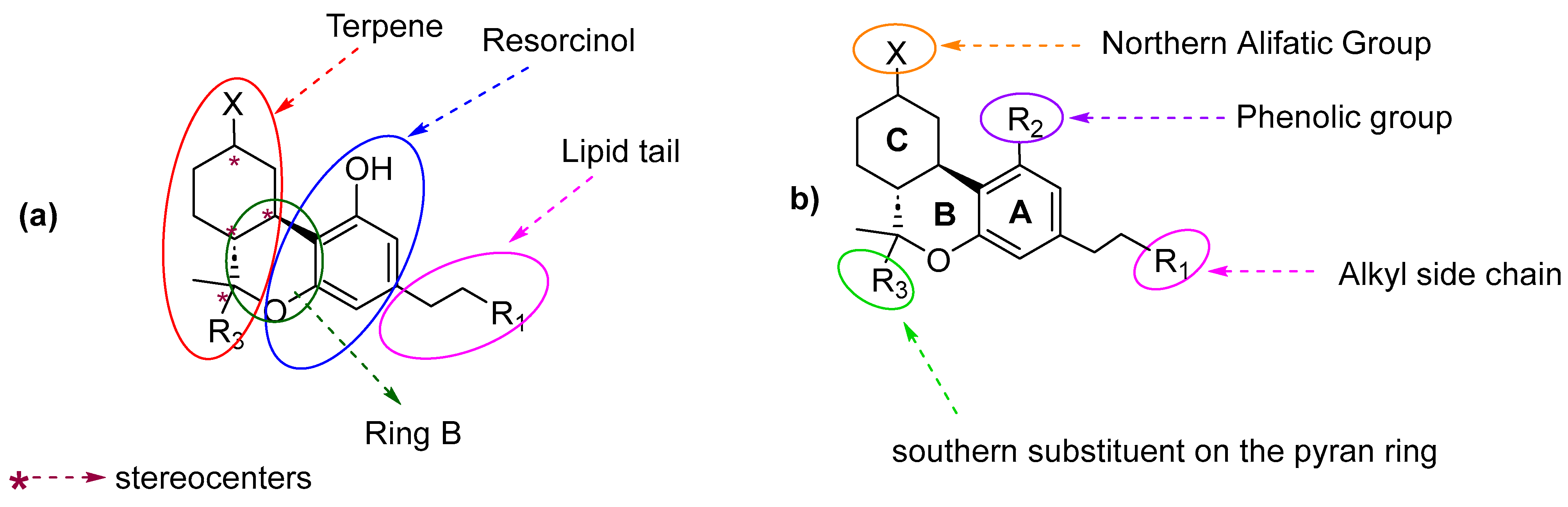
7.2. Southern Aliphatic Hydroxyl Chain (SAH)
7.3. Northern Aliphatic Group (NAG)
7.4. Phenolic Group
7.5. Alkyl Side Chain
 | |||||||
| Compound | Ki (nM) | Function | References | ||||
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
| X1 = H, n = 2 R1 R2 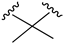 R3 = CH2OH 93 | 3.0 ± 0.8 | - | - | - | 2.1 ± 0.6 | Agonist | [56] |
| X1 = H, n = 2 R1 R2  R3 = OH 89 (Canbisol) | 19.0 ± 0.6 | 13.1 ± 0.2 | - | [56] | |||
| X1 = N3, n = 2 R1 R2 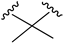 R3 = CH2OH 169 | 0.41 ± 0.05 | - | - | 0.8 ± 0.1 | 1.4 ± 0.06 | Agonist | [56] |
| X1 = N3, n = 2 R1 R2  R3 = CH2OH 170 | 0.40 ± 0.1 | - | - | 0.8 ± 0.1 | 0.8 ± 0.1 | Agonist | [56] |
| X1 = N3, n = 3 R1 R2 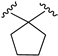 R3 = CH2OH 171 | 0.5 ± 0.2 | - | - | 1.6 ± 0.1 | 1.5 ± 0.3 | Agonist | [56] |
| X1 = NCS, n = 2 R1 R2  R3 = CH2OH 172 | 0.39 ± 0.04 | - | 0.8 ± 0.1 | 3.15 ± 0.04 | Agonist | [56] | |
| X1 = NCS, n = 2 R1 = R2 = H R3 = CH2OH 173 | 5.65 ± 0.1 | 9.0 ± 0.4 | - | 10.50 ± 0.02 | Agonist | [56] | |
| X1 = NCS, n = 2 R1 R2 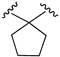 R3 = CH2OH 174 | 1.1 ± 0.1 | - | - | 0.9 ± 0.2 | 1.3 ± 0.05 | Agonist | [56] |
| X1 = NCS, n = 3 R1 R2 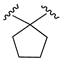 R3 = CH2OH 175 | 0.4 ± 0.1 | - | - | 1.1 ± 0.1 | 1.0 ± 0.2 | Agonist | [56] |
| X1 = CN, n = 2 R1 R2 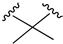 R3 = CH2OH 176 | 0.4 ± 0.05 | - | - | 0.8 ± 0.1 | 0.4 ± 0.2 | Agonist | [56] |
| X1 = CN, n = 2 R1 R2 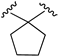 R3 = CH2OH 177 | 0.8 ± 0.2 | - | - | 1.0 ± 0.1 | 1.4 ± 0.2 | Agonist | [56] |
| X1 = CN, n = 3 R1 R2 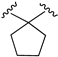 R3 = CH2OH 178 | 0.5 ± 0.1 | - | - | 0.9 ± 0.1 | 0.4 ± 0.05 | Agonist | [56] |
| X1 = N3, n = 2 R1 R2  R3= N3 179 | 0.60 ±0.2 | - | - | - | 2.65 ±0.3 | Agonist | [105] |
| X1 = I, n = 2 R1 R2  R3= N3 180 | 0.67 ± 0.1 | - | - | - | 0.72 ± 0.1 | Agonist | [105] |
 | |||||||
| Compound | Ki (nM) | Function | References | ||||
| rCB1 | hCB1 | rCB2 | hCB2 | mCB2 | |||
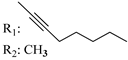 R3: CH2OH 181 | - | 5.8 | - | 61.6 | - | - | [105] |
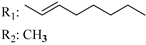 R3: CH2OH 182 | - | 1.2 | - | 5.3 | - | - | [105] |
 R3: CH2OH 183 | - | 0.8 | - | 9.5 | - | - | [105] |
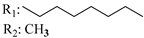 R3: CH2OH 184 | - | 1.7 | - | 14.3 | - | - | [105] |
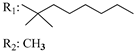 R3: CH2OH 185 | 0.045 | 0.061 | [105] | ||||
 186 | 0.7 | 8.6 | [105] | ||||
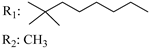 R3: OH 187 | - | 2.3 | - | 2.3 | - | - | [105] |
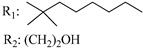 R3: CH2OH 188 | - | 2.8 | - | 2.3 | - | - | [105] |
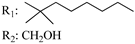 R3: CH2OH 189 | - | 2.9 | - | 2.4 | - | - | [105] |
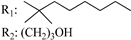 R3: CH2OH 190 | - | 2.2 | - | 3.4 | - | - | [105] |
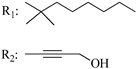 R3: CH2OH 191 | - | 1.21 | - | 0.3 | - | - | [105] |
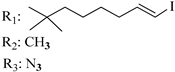 192 | - | 0.80 | - | 0.85 | - | - | [105] |
 193 | - | 0.16 | - | 42.1 | - | CB1 receptor selective antagonist | [106] |
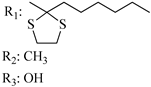 194 | - | 4.51 ± 0.7 | - | 13.9 ± 3.4 | - | - | [56,57] |
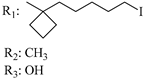 195 | - | 3.16 ± 0.05 | - | 4.21 ± 0.93 | 5.13 ± 1.27 | - | [56,57] |
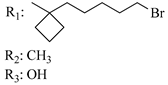 196 | - | 1.37 ± 0.35 | - | 2.76 ± 0.63 | 1.62 ± 0.45 | - | [56,57] |
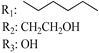 197 | - | 70.5 | - | 1.99 | - | - | [105] |
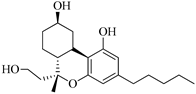 198 | - | 1353.9 | 2476.7 | - | - | [105] | |
 199 | 40.7 | 19.7 | [56,57] | ||||
 | |||||||
| Compound | Ki (nM) | Function | References | ||||
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
 200 | - | 1.82 | - | - | 0.58 | Agonist Mixed CB1/CB2 | [105] |
 201 | - | >20,000 | - | - | 1.94 | CB2 Selective Agonist | [105] |
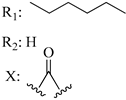 202 | - | 333.0 | - | 265 | - | - | [56,57] |
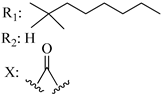 88 (Nabilone) | - | 2.19 | - | 1.84 | Agonist Mixed CB1/CB2 | [56,57] | |
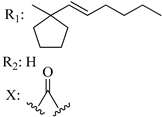 203 | - | 1.23 | - | 5.25 | 7.02 | - | [56,57] |
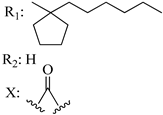 204 | - | 1.76 | - | 0.97 | 3.34 | - | [56,57] |
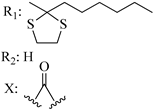 205 | - | 6.57 | - | 42.3 | 32.6 | - | [56,57] |
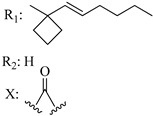 206 | - | 1.13 | - | 12.0 | 15.1 | - | [56,57] |
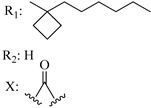 207 | - | 0.84 | - | 13.7 | 11.9 | - | [56,57] |
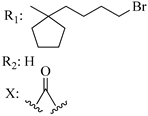 208 | - | 13.1 | - | 13.9 | - | - | [56,57] |
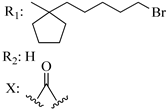 209 | - | 1.03 | - | 2.59 | 1.32 | - | [56,57] |
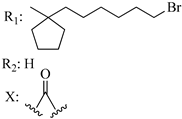 210 | - | 4.96 | - | 1.60 | 3.02 | - | [56,57] |
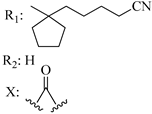 211 | - | 3.14 | 2.78 | - | - | [56,57] | |
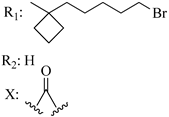 212 | - | 2.33 | 7.56 | - | - | [56,57] | |
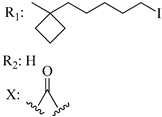 213 | - | 2.11 | 6.18 | - | - | [56,57] | |
| Adamantyl Cannabinoid: | 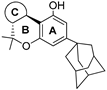 | ||||||
| Compound | Ki (nM) | Function | References | ||||
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
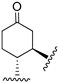 214 | 175.6 | - | - | 249.5 | 338 | - | [107,108] |
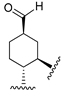 215 | 52.9 | - | - | 25.7 | 5.5 | Agonist | [107,108] |
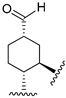 216 | 480.2 | - | - | 200.1 | 90.0 | - | [107,108] |
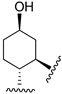 217 | 23.9 | - | - | 39.4 | 40.5 | Agonist | [107,108] |
 218 | 146.3 | - | - | 255.0 | 671.8 | - | [107,108] |
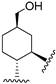 219 | 4.9 | - | - | 12.1 | 11.3 | Agonist | [107,108] |
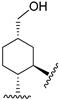 220 | 90.1 | - | - | 95.1 | 121.2 | Agonist | [83,84] |
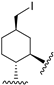 221 | 241.0 | - | - | 345.0 | 261.7 | - | [83,84] |
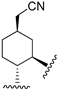 222 | 48.7 | - | - | 87.0 | 100.3 | Agonist | [83,84] |
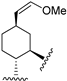 223 | 31.0 | - | - | 90.3 | 67.2 | Agonist | [83,84] |
 224 | 4.6 | - | - | 18.4 | 13.3 | Agonist | [83,84] |
 225 | 40.9 | - | - | 21 | 365.3 | Agonist | [83,84] |
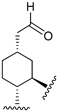 226 | 170.5 | - | - | 80.1 | 70.8 | Agonist | [83,84] |
 227 | 13.2 | - | - | 34.3 | 11.2 | Agonist | [83,84] |
7.6. Seven-Membered Lactone and Quinone in the Terpene Region
7.7. Nonclassical, Bicyclic-Hydrogenated Cannabinoids
7.8. Docking Studies and In Vitro Binding Affinities of HHC
8. Pharmacological and Toxicological Properties of Saturated Cannabinoids
8.1. In Vitro Effects of Saturated Cannabinoid Analogs in Pancreatic Cell Lines
8.2. In Vivo Effects of Saturated Cannabinoid Analogs
9. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Pease, D.C.; Clark, J.H. Isolation of cannabinol, cannabidiol, and quebrachitol from red oil of Minnesota Wild. J. Am. Chem. Soc. 1940, 62, 2194–2196. [Google Scholar] [CrossRef]
- Adams, R.; Wolff, H.; Cain, C.K.; Clark, J.H. Structure of Cannabidiol. V1 Position of the alicyclic double bonds. J. Am. Chem. Soc. 1940, 62, 2215–2219. [Google Scholar] [CrossRef]
- Jacob, A.; Todd, A.R. 119 Cannabis indica Part, I.I. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of cannabinol. J. Chem. Soc. 1940, 649–653. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, and Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Appendino, G. The Early History of Cannabinoid Research. Rend. Lincei Sci. Fis. Nat. 2020, 31, 919–929. [Google Scholar] [CrossRef]
- Adams, R. Marihuana Active Compounds. U.S. Patent 2419937, 6 May 1947. [Google Scholar]
- Ben-Shabat, S.; Hanus, L.O.; Katzavian, G.; Gallily, R. New cannabidiol derivatives: Synthesis, binding to cannabinoid receptor, and evaluation of their antiinflammatory activity. J. Med. Chem. 2006, 49, 1113–1117. [Google Scholar] [CrossRef]
- Collins, A.; Ramirez, G.; Tesfatsion, T.; Ray, K.P.; Caudill, S.; Cruces, W. Synthesis and characterization of the diastereomers of HHC and H4CBD. Nat. Prod. Commun. 2023, 18, 1934578X2311589. [Google Scholar] [CrossRef]
- Collins, A.T.; Tesfastion, G.; Ramirez, K.R.; Cruces, W. Nonclinical In Vitro Safety Assesment Summary of Hemp Derived (R/S)-Hexahydrocannabinol ((R/S)-HHC). Cannabis Sci. Technol. 2022, 5, 23–27. [Google Scholar]
- Geci, M.; Scialdone, M.; Tishler, J. The dark side of cannabidiol: The unanticipated social and clinical implications of synthetic Δ8-THC. Cannabis Cannabinoid Res. 2023, 8, 270–282. [Google Scholar] [CrossRef]
- Ujváry, I. Hexahydrocannabinol and closely related semi-synthetic cannabinoids: A comprehensive review. Drug Test Anal. 2023. [Google Scholar] [CrossRef]
- Busardo, F.P.; Graziano, S.; Varì, M.R.; Pichini, S.; Cassano, T.; Trana, A.D. Hexahydrocannabinol pharmacology, toxicology, and analysis: The first evidence for a recent new psychoactive substance. Curr. Neuropharmacol. 2023, 21, 2424–2430. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Cannabinoids as anticancer drugs: Current status of preclinical research. Br. J. Cancer. 2022, 127, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ross, S.A.; Slade, D.; Radwan, M.M.; Khan, I.A.; ElSohly, M.A. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 2015, 117, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.-F.; von Kiedrowski, G.; Berger, B. Stereo- and Regioselective Synthesis of Enantiomerically Pure (+)- and (−)-Hexahydrocannabinol by Intramolecular Cycloaddition. Angew. Chem. Int. Ed. Engl. 1982, 21, 221–222. [Google Scholar] [CrossRef]
- Bendi, A.; Rao, G.B.D. Strategic One-Pot Synthesis of Glycosyl Annulated Phosphorylated/ Thiophosphorylated 1,2,3-Triazole Derivatives Using CuFe2O4 Nanoparticles as Heterogeneous Catalyst, Their DFT and Molecular Docking Studies as Triazole Fungicides. Lett. Org. Chem. 2023, 20, 568–578. [Google Scholar] [CrossRef]
- Dharma Rao, G.B.; Anjaneyulu, B.; Kaushik, M.P. A Facile One-Pot Five-Component Synthesis of Glycoside Annulated Dihydropyrimidinone Derivatives with 1,2,3-Triazol Linkage via Transesterification/Biginelli/Click Reactions in Aqueous Medium. Tetrahedron Lett. 2014, 55, 19–22. [Google Scholar] [CrossRef]
- Rao, G.B.D.; Anjaneyulu, B.; Kaushik, M.P. Greener and Expeditious One-Pot Synthesis of Dihydropyrimidinone Derivatives Using Non-Commercial β-Ketoesters via the Biginelli Reaction. RSC Adv. 2014, 4, 43321–43325. [Google Scholar] [CrossRef]
- Reddy, K.S.; Rao, B.V. A Facile and Stereoselective Synthesis of the C-2 Epimer of (+)-Deacetylanisomycin. Tetrahedron Asymmetry 2011, 22, 190–194. [Google Scholar] [CrossRef]
- Casiraghi, G.; Cornia, M.; Casnati, G.; Fava, G.G.; Belicchi, M.F. A one-step highly stereocontrolled synthesis of (–)- and (+)-hexahydrocannabinol and related compounds. J. Chem. Soc. Chem. Commun. 1986, 3, 271–273. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.-L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef]
- Lee, Y.R.; Xia, L. Efficient one-pot synthetic approaches for cannabinoid analogues and their application to biologically interesting (−)-hexahydrocannabinol and (+)-hexahydrocannabinol. Tetrahedron Lett. 2008, 49, 3283–3287. [Google Scholar] [CrossRef]
- Scialdone, M.A. Hydrogenation of Cannabis Oil. U.S. Patent 10071127B2, 11 September 2018. [Google Scholar]
- Collins, A.C.; Ray, K.P.; Cruces, W. A Method for Preparing Hexahydrocannabinol. U.S. Patent 63411506, 29 September 2022. [Google Scholar]
- Casati, S.; Rota, P.; Bergamaschi, R.F.; Palmisano, E.; La Rocca, P.; Ravelli, A.; Angeli, I.; Minoli, M.; Roda, G.; Orioli, M. Hexahydrocannabinol on the light cannabis market: The latest “new” entry. Cannabis Cannabinoid Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.K.S.; Rao, B.V.; Raju, S.S. A Common Approach to Pyrrolizidine and Indolizidine Alkaloids; Formal Synthesis of (−)-Isoretronecanol, (−)-Trachelanthamidine and an Approach to the Synthesis of (−)-5-Epitashiromine and (−)-Tashiromine. Tetrahedron Asymmetry 2011, 22, 662–668. [Google Scholar] [CrossRef]
- Nasrallah, D.J.; Garg, N.K. Studies Pertaining to the Emerging Cannabinoid Hexahydrocannabinol (HHC). ACS Chem. Biol. 2023. [Google Scholar] [CrossRef]
- Thapa, D.; Babu, D.; Park, M.-A.; Kwak, M.-K.; Lee, Y.-R.; Kim, J.M.; Kwon, T.K.; Kim, J.-A. Induction of P53-Independent Apoptosis by a Novel Synthetic Hexahydrocannabinol Analog Is Mediated via Sp1-Dependent NSAID-Activated Gene-1 in Colon Cancer Cells. Biochem. Pharmacol. 2010, 80, 62–71. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Harland, E.C.; Benigni, D.A.; Waller, C.W. Cannabinoids in Glaucoma II: The Effect of Different Cannabinoids on Intraocular Pressure of the Rabbit. Curr. Eye Res. 1984, 3, 841–850. [Google Scholar] [CrossRef]
- Skinner, W.A.; Rackur, G.; Uyeno, E. Structure-activity studies on tetrahydro- and hexahydrocannabinol derivatives. J. Pharm. Sci. 1979, 68, 330–332. [Google Scholar] [CrossRef]
- Muhammad, I.; Li, X.C.; Dunbar, D.C.; ElSohly, M.A.; Khan, I.A. Antimalarial (+)-trans-hexahydrodibenzopyran derivatives from Machaerium multiflorum. J. Nat. Prod. 2001, 64, 1322–1325. [Google Scholar] [CrossRef]
- Muhammad, I.; Li, X.-C.; Jacob, M.R.; Tekwani, B.L.; Dunbar, D.C.; Ferreira, D. Antimicrobial and antiparasitic (+)-trans-hexahydrodibenzopyrans and analogues from Machaerium multiflorum. J. Nat. Prod. 2003, 66, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, Q.; Chen, B.; Lu, J.; Wang, H.; She, X.; Pan, X. Total synthesis of (+)-machaeriol D with a key regio- and stereoselective S(N)2′ reaction. Angew. Chem. Int. Ed. Engl. 2006, 45, 3651–3653. [Google Scholar] [CrossRef] [PubMed]
- Dethe, D.H.; Erande, R.D.; Mahapatra, S.; Das, S.; Kumar, B.V. Protecting group free enantiospecific total syntheses of structurally diverse natural products of the tetrahydrocannabinoid family. Chem. Commun. 2015, 51, 2871–2873. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.; Studer, A. Short and protecting-group-free approach to the (−)-Δ8-THC-motif: Synthesis of THC-analogues, (−)-machaeriol B and (−)-machaeriol D. Org. Lett. 2018, 20, 2964–2966. [Google Scholar] [CrossRef] [PubMed]
- Villa, G.; Povie, G.; Renaud, P. Radical chain reduction of alkylboron compounds with catechols. J. Am. Chem. Soc. 2011, 133, 5913–5920. [Google Scholar] [CrossRef]
- Klotter, F.; Studer, A. Short and divergent total synthesis of (+)-machaeriol B, (+)-machaeriol D, (+)-Δ(8)-THC, and analogues. Angew. Chem. Int. Ed. Engl. 2015, 54, 8547–8550. [Google Scholar] [CrossRef]
- Harvey, D.J.; Martin, B.R.; Paton, W.D. Identification of metabolites of delta1- and delta1(6)-tetrahydrocannabinol containing a reduced double bond. J. Pharm. Pharmacol. 1977, 29, 495–497. [Google Scholar] [CrossRef]
- Harvey, D.J.; Brown, N.K. A method based on catalytic hydrogenation for the identification of monohydroxy metabolites of isomeric tetrahydrocannabinols. Rapid Commun. Mass Spectrom. 1990, 4, 67–68. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolomics of Δ9-Tetrahydrocannabinol: Implications in Toxicity. Drug Metab. Rev. 2016, 48, 80–87. [Google Scholar] [CrossRef]
- Kozela, E.; Haj, C.; Hanuš, L.; Chourasia, M.; Shurki, A.; Juknat, A.; Kaushansky, N.; Mechoulam, R.; Vogel, Z. HU-446 and HU-465, derivatives of the non-psychoactive cannabinoid cannabidiol, decrease the activation of encephalitogenic T cells. Chem. Biol. Drug Des. 2016, 87, 143–153. [Google Scholar] [CrossRef]
- Maurya, V.; Appayee, C. Enantioselective total synthesis of potent 9β-11-hydroxyhexahydrocannabinol. J. Org. Chem. 2020, 85, 1291–1297. [Google Scholar] [CrossRef]
- Vidyasagar, M.; Chandrakumar, A. Catalytic Asymmetric Synthesis of 3,4-Disubstituted Cyclohexadiene Carbaldehydes: Formal Total Synthesis of Cyclobakuchiols A and C. Org. Lett. 2018, 20, 4111–4115. [Google Scholar] [CrossRef]
- Archer, R.A.; Blanchard, W.B.; Day, W.A.; Johnson, D.W.; Lavagnino, E.R.; Ryan, C.W.; Baldwin, J.E. Cannabinoids. 3. Synthetic approaches to 9-ketocannabinoids. Total synthesis of nabilone. J. Org. Chem. 1977, 42, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R. Receptors and Channels Targeted by Synthetic Cannabinoid Receptor Agonists and Antagonists. Curr. Med. Chem. 2010, 17, 1360–1381. [Google Scholar] [CrossRef]
- Lemberger, L.; Rowe, H. Clinical Pharmacology of Nabilone, a Cannabinol Derivative. Clin. Pharmacol. Ther. 1975, 18, 720–726. [Google Scholar] [CrossRef]
- Herrmann, N.; Ruthirakuhan, M.; Gallagher, D.; Verhoeff, N.P.L.G.; Kiss, A.; Black, S.E.; Lanctôt, K.L. Randomized Placebo-Controlled Trial of Nabilone for Agitation in Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 2019, 27, 1161–1173. [Google Scholar] [CrossRef]
- Hillen, J.B.; Soulsby, N.; Alderman, C.; Caughey, G.E. Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: A systematic review. Ther. Adv. Drug Saf. 2019, 10, 2042098619846993. [Google Scholar] [CrossRef] [PubMed]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Peball, M.; Krismer, F.; Knaus, H.G.; Djamshidian, A.; Werkmann, M.; Carbone, F.; Ellmerer, P.; Heim, B.; Marini, K.; Valent, D.; et al. Non-Motor Symptoms in Parkinson’s Disease are Reduced by Nabilone. Ann. Neurol. 2020, 88, 712–722. [Google Scholar] [CrossRef]
- Nikas, S.P.; Thakur, G.A.; Parrish, D.; Alapafuja, S.O.; Huestis, M.A.; Makriyannis, A. A concise methodology for the synthesis of (−)-Δ9-tetrahydrocannabinol and (−)-Δ9-tetrahydrocannabivarin metabolites and their regiospecifically deuterated analogs. Tetrahedron 2007, 63, 8112–8123. [Google Scholar] [CrossRef]
- Blaazer, A.R.; Lange, J.H.; van der Neut, M.A.; Mulder, A.; Boon, F.S.D.; Werkman, T.R.; Kruse, C.G.; Wadman, W.J. Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: Design, synthesis, structure-activity relationships, physicochemical properties and biological activity. Eur. J. Med. Chem. 2011, 46, 5086–5098. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Iliopoulos-Tsoutsouvas, C.; Tong, F.; Brust, C.A.; Keenan, C.M.; Raghav, J.G.; Hua, T.; Wu, S.; Ho, J.-H.; Wu, Y.; et al. Novel functionalized cannabinoid receptor probes: Development of exceptionally potent agonists. J. Med. Chem. 2021, 64, 3870–3884. [Google Scholar] [CrossRef] [PubMed]
- Nikas, S.P.; Alapafuja, S.O.; Papanastasiou, I.; Paronis, C.A.; Shukla, V.G.; Papahatjis, D.P.; Bowman, A.L.; Halikhedkar, A.; Han, X.; Makriyannis, A. Novel 1′,1′-chain substituted hexahydrocannabinols: 9β-hydroxy-3-(1-hexyl-cyclobut-1-yl)-hexahydrocannabinol (AM2389) a highly potent cannabinoid receptor 1 (CB1) agonist. J. Med. Chem. 2010, 53, 6996–7010. [Google Scholar] [CrossRef]
- Sharma, R.; Nikas, S.P.; Guo, J.J.; Mallipeddi, S.; Wood, J.T.; Makriyannis, A. C-ring cannabinoid lactones: A novel cannabinergic chemotype. ACS Med. Chem. Lett. 2014, 5, 400–404. [Google Scholar] [CrossRef]
- Itoh, Y.; Yamanaka, M.; Mikami, K. Theoretical Study on the Regioselectivity of Baeyer–Villiger Reaction of α-Me-, -F-, -CF3-Cyclohexanones. J. Org. Chem. 2013, 78, 146–153. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Wieckiewicz, G.; Stokłosa, I.; Stokłosa, M.; Gorczyca, P.; Pudlo, R. Cannabidiol (CBD) in the self-treatment of depression-exploratory study and a new phenomenon of concern for psychiatrists. Front. Psychiatry 2022, 13, 837946. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H.; Jagerovic, N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Waugh, T.M.; Masters, J.; Aliev, A.E.; Marson, C.M. Monocyclic quinone structure-activity patterns: Synthesis of catalytic inhibitors of topoisomerase II with potent antiproliferative activity. ChemMedChem 2020, 15, 114–124. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Zheng, J.; Zhang, J.; Pan, X.; She, X. A general route to 5,6-seco-hexahydrodibenzopyrans and analogues: First total synthesis of (+)-Machaeridiol B and (+)-Machaeriol B. Tetrahedron 2007, 63, 1014–1021. [Google Scholar] [CrossRef]
- Kumarihamy, M.; Tripathi, S.; Balachandran, P.; Avula, B.; Zhao, J.; Wang, M.; Bennett, M.M.; Zhang, J.; Carr, M.A.; Lovell, K.M.; et al. Synthesis and inhibitory activity of machaeridiol-based novel anti-MRSA and anti-VRE compounds and their profiling for cancer-related signaling pathways. Molecules 2022, 27, 6604. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Pagani, A.; Scala, F.; De Petrocellis, L.; Di Marzo, V.; Grassi, G.; Appendino, G. Cannabimovone, a cannabinoid with a rearranged terpenoid skeleton from hemp. European. J. Org. Chem. 2010, 2010, 2067–2072. [Google Scholar] [CrossRef]
- Monroe, A.Z.; Gordon, W.H.; Wood, J.S.; Martin, G.E.; Morgan, J.B.; Williamson, R.T. Structural revision of a Wnt/β-catenin modulator and confirmation of cannabielsoin constitution and configuration. Chem. Commun. 2021, 57, 5658–5661. [Google Scholar] [CrossRef]
- Dennis, D.G.; Anand, S.D.; Lopez, A.J.; Petrovčič, J.; Das, A.; Sarlah, D. Synthesis of the cannabimovone and cannabifuran class of minor phytocannabinoids and their anti-inflammatory activity. J. Org. Chem. 2022, 87, 6075–6086. [Google Scholar] [CrossRef] [PubMed]
- Deora, N.; Carlier, P.R. A computational study of regioselectivity in aluminum hydride ring-opening of cis- and trans-4-t-butyl and 3-methylcyclohexene oxides. Org. Biomol. Chem. 2019, 17, 8628–8635. [Google Scholar] [CrossRef] [PubMed]
- Carreras, J.; Kirillova, M.S.; Echavarren, A.M. Synthesis of (−)-cannabimovone and structural reassignment of anhydrocannabimovone through gold(I)-catalyzed cycloisomerization. Angew. Chem. Int. Ed. Engl. 2016, 55, 7121–7125. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Zvi, Z.; Gaoni, Y. Hashish—XIII. Tetrahedron 1968, 24, 5615–5624. [Google Scholar] [CrossRef] [PubMed]
- Kogan, N.M.; Peters, M.; Mechoulam, R. Cannabinoid Quinones-A review and novel observations. Molecules 2021, 26, 1761. [Google Scholar] [CrossRef]
- Kogan, N.M.; Schlesinger, M.; Priel, E.; Rabinowitz, R.; Berenshtein, E.; Chevion, M.; Mechoulam, R. HU-331, a novel cannabinoid-based anticancer topoisomerase II inhibitor. Mol. Cancer Ther. 2007, 6, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Sturla, S.J.; Boobis, A.R.; FitzGerald, R.E.; Hoeng, J.; Kavlock, R.J.; Schirmer, K.; Whelan, M.; Wilks, M.F.; Peitsch, M.C. Systems toxicology: From basic research to risk assessment. Chem. Res. Toxicol. 2014, 27, 314–329. [Google Scholar] [CrossRef]
- Osman, A.G.; Elokely, K.M.; Yadav, V.K.; Carvalho, P.; Radwan, M.; Slade, D.; Gul, W.; Khan, S.; Dale, O.R.; Husni, A.S.; et al. Bioactive products from singlet oxygen photooxygenation of cannabinoids. Eur. J. Med. Chem. 2018, 143, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Leigh, C.B.; Jin, Z. Cannabinoids and Uses Thereof. U.S. Patent PCT/US2020/063341, 4 December 2020. [Google Scholar]
- Morales, P.; Blasco-Benito, S.; Andradas, C.; Gómez-Cañas, M.; Flores, J.M.; Goya, P.; Fernández-Ruiz, J.; Sánchez, C.; Jagerovic, N. Selective, nontoxic CB(2) cannabinoid o-quinone with in vivo activity against triple-negative breast cancer. J. Med. Chem. 2015, 58, 2256–2264. [Google Scholar] [CrossRef]
- Peña, R.; Martín, P.; Feresin, G.E.; Tapia, A.; Machín, F.; Estévez-Braun, A. Domino synthesis of embelin derivatives with antibacterial activity. J. Nat. Prod. 2016, 79, 970–977. [Google Scholar] [CrossRef]
- Afzal, M.; Gupta, G.; Kazmi, I.; Rahman, M.; Upadhyay, G.; Ahmad, K.; Imam, F.; Pravez, M.; Anwar, F. Evaluation of anxiolytic activity of embelin isolated from Embelia ribes. Biomed. Aging Pathol. 2012, 2, 45–47. [Google Scholar] [CrossRef]
- Sreepriya, M.; Bali, G. Chemopreventive Effects of Embelin and Curcumin against N-Nitrosodiethylamine/Phenobarbital-Induced Hepatocarcinogenesis in Wistar Rats. Fitoterapia 2005, 76, 549–555. [Google Scholar] [CrossRef]
- Xu, M.; Cui, J.; Fu, H.; Proksch, P.; Lin, W.; Li, M. Embelin derivatives and their anticancer activity through microtubule disassembly. Planta Med. 2005, 71, 944–948. [Google Scholar] [CrossRef]
- Schaible, A.M.; Traber, H.; Temml, V.; Noha, S.M.; Filosa, R.; Peduto, A.; Weinigel, C.; Barz, D.; Schuster, D.; Werz, O. Potent Inhibition of Human 5-Lipoxygenase and Microsomal Prostaglandin E2 Synthase-1 by the Anti-Carcinogenic and Anti-Inflammatory Agent Embelin. Biochem. Pharmacol. 2013, 86, 476–486. [Google Scholar] [CrossRef]
- Feresin, G.E.; Tapia, A.; Sortino, M.; Zacchino, S.; de Arias, A.R.; Inchausti, A.; Yaluff, G.; Rodriguez, J.; Theoduloz, C.; Schmeda-Hirschmann, G. Bioactive Alkyl Phenols and Embelin from Oxalis Erythrorhiza. J. Ethnopharmacol. 2003, 88, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Peña, R.; Jiménez-Alonso, S.; Feresin, G.; Tapia, A.; Méndez-Alvarez, S.; Machín, F.; Ravelo, Á.G.; Estévez-Braun, A. Multicomponent Synthesis of Antibacterial Dihydropyridin and Dihydropyran Embelin Derivatives. J. Org. Chem. 2013, 78, 7977–7985. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nikolovska-Coleska, Z.; Wang, G.; Qiu, S.; Wang, S. Design, Synthesis, and Characterization of New Embelin Derivatives as Potent Inhibitors of X-Linked Inhibitor of Apoptosis Protein. Bioorg. Med. Chem. Lett. 2006, 16, 5805–5808. [Google Scholar] [CrossRef]
- Singh, B.; Guru, S.K.; Sharma, R.; Bharate, S.S.; Khan, I.A.; Bhushan, S.; Bharate, S.B.; Vishwakarma, R.A. Synthesis and Anti-Proliferative Activities of New Derivatives of Embelin. Bioorg. Med. Chem. Lett. 2014, 24, 4865–4870. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, S.; Thippeswamy, B.S.; Veerapur, V.P.; Badami, S. Anticonvulsant Activity of Embelin Isolated from Embelia Ribes. Phytomedicine 2011, 18, 186–188. [Google Scholar] [CrossRef]
- Calcaterra, A.; Cianfoni, G.; Tortora, C.; Manetto, S.; Grassi, G.; Botta, B.; Gasparrini, F.; Mazzoccanti, G.; Appendino, G. Natural cannabichromene (CBC) shows distinct scalemicity grades and enantiomeric dominance in Cannabis sativa strains. J. Nat. Prod. 2023, 86, 909–914. [Google Scholar] [CrossRef]
- Wood, J.S.; Gordon, W.H.; Morgan, J.B.; Williamson, R.T. Cannabicitran: Its unexpected racemic nature and potential origins. Chirality 2023, 35, 540–548. [Google Scholar] [CrossRef]
- Caprioglio, D.; Mattoteia, D.; Minassi, A.; Pollastro, F.; Lopatriello, A.; Muňoz, E.; Taglialatela-Scafati, O.; Appendino, G. One-pot total synthesis of cannabinol via iodine-mediated deconstructive annulation. Org. Lett. 2019, 21, 6122–6125. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. The isolation and structure of DELTA-1- tetrahydrocannabinol and other neutral cannabinoids from hashish. J. Am. Chem. Soc. 1971, 93, 217–224. [Google Scholar] [CrossRef]
- Nguyen, G.-N.; Jordan, E.N.; Kayser, O. Synthetic strategies for rare cannabinoids derived from cannabis sativa. J. Nat. Prod. 2022, 85, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Agua, A.R.; Barr, P.J.; Marlowe, C.K.; Pirrung, M.C. Cannabichromene racemization and absolute stereochemistry based on a cannabicyclol analog. J. Org. Chem. 2021, 86, 8036–8040. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.-S.; Li, H.; Tang, Y.; Hsung, R.P. Total syntheses of cannabicyclol, clusiacyclol A and B, iso-eriobrucinol A and B, and eriobrucinol. Org. Lett. 2013, 15, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, Y.R. Efficient and novel one-pot synthesis of polycycles bearing cyclols by FeCl3-promoted [2 + 2] cycloaddition: Application to cannabicyclol, cannabicyclovarin, and ranhuadujuanine A. Org. Biomol. Chem. 2014, 12, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Dysarz, F.A., III; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar] [PubMed]
- Gerard, C.M.; Mollereau, C.; Vassart, G.; Parmentier, M. Molecular Cloning of a Human Cannabinoid Receptor which is also Expressed in Testis. Biochem. J. 1991, 279, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Muhammad, I.; Ibrahim, M.A.; Kumarihamy, M.; Lambert, J.A.; Zhang, J.; Mohammad, M.H.; Khan, S.I.; Pasco, D.S.; Balachandran, P. Cannabinoid and opioid receptor affinity and modulation of cancer-related signaling pathways of machaeriols and machaeridiols from Machaerium Pers. Molecules 2023, 28, 4162. [Google Scholar] [CrossRef]
- Haider, S.; Pandey, P.; Reddy, C.R.; Lambert, J.A.; Chittiboyina, A.G. Novel machaeriol analogues as modulators of cannabinoid receptors: Structure-activity relationships of (+)-hexahydrocannabinoids and their isoform selectivities. ACS Omega 2021, 6, 20408–20421. [Google Scholar] [CrossRef]
- Marzullo, P.; Foschi, F.; Coppini, D.A.; Fanchini, F.; Magnani, L.; Rusconi, S.; Luzzani, M.; Passarella, D. Cannabidiol as the substrate in acid-catalyzed intramolecular cyclization. J. Nat. Prod. 2020, 83, 2894–2901. [Google Scholar] [CrossRef]
- Khanolkar, A.D.; Palmer, S.L.; Makriyannis, A. Molecular probes for the cannabinoid receptors. Chem. Phys. Lipids 2000, 108, 37–52. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Peigneur, S.; Hendrickx, L.A.; Tytgat, J. Targeting cannabinoid receptors: Current status and prospects of natural products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef] [PubMed]
- Thakur, G.A.; Bajaj, S.; Paronis, C.; Peng, Y.; Bowman, A.L.; Barak, L.S.; Caron, M.G.; Parrish, D.; Deschamps, J.R.; Makriyannis, A. Novel adamantyl cannabinoids as CB1 receptor probes. J. Med. Chem. 2013, 56, 3904–3921. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Tius, M.A.; Nikas, S.P.; Tran, N.K.; Tong, F.; Zhou, H.; Zvonok, N.; Makriyannis, A. Oxa-adamantyl cannabinoids. Bioorg. Med. Chem. Lett. 2021, 38, 127882. [Google Scholar] [CrossRef] [PubMed]
- Tius, M.A.; Makriyannis, A.; Long Zoua, X.; Abadji, V. Conformationally restricted hybrids of CP-55,940 and HHC: Stereoselective synthesis and activity. Tetrahedron 1994, 50, 2671–2680. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lu, D.; Kendall, D.A.; Howlett, A.C.; Lichtman, A.H. Molecular mechanism and cannabinoid pharmacology. Handb. Exp. Pharmacol. 2020, 258, 323–353. [Google Scholar] [CrossRef] [PubMed]
- Aviz-Amador, A.; Contreras-Puentes, N.; Mercado-Camargo, J. Virtual screening using docking and molecular dynamics of cannabinoid analogs against CB1 and CB2 receptors. Comput. Biol. Chem. 2021, 95, 107590. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Lee, J.S.; Heo, S.-W.; Lee, Y.R.; Kang, K.W.; Kwak, M.-K.; Choi, H.G.; Kim, J.-A. Novel hexahydrocannabinol analogs as potential anti-cancer agents inhibit cell proliferation and tumor angiogenesis. Eur. J. Pharmacol. 2011, 650, 64–71. [Google Scholar] [CrossRef]
- Thapa, D.; Kang, Y.; Park, P.-H.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Ku, S.K.; Jung, Y.; Kim, J.-A. Anti-tumor activity of the novel hexahydrocannabinol analog LYR-8 in human colorectal tumor xenograft is mediated through the inhibition of akt and hypoxia-inducible factor-1α activation. Biol. Pharm. Bull. 2012, 35, 924–932. [Google Scholar] [CrossRef][Green Version]
- Raïch, I.; Rivas-Santisteban, R.; Lillo, A.; Lillo, J.; Reyes-Resina, I.; Nadal, X.; Ferreiro-Vera, C.; de Medina, V.S.; Majellaro, M.; Sotelo, E.; et al. Similarities and differences upon binding of naturally occurring Δ9-tetrahydrocannabinol-derivatives to cannabinoid CB1 and CB2 receptors. Pharmacol. Res. 2021, 174, 105970. [Google Scholar] [CrossRef]
- Falasca, V.; Falasca, M. Targeting the endocannabinoidome in pancreatic cancer. Biomolecules 2022, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, D.; Collodel, G.; Brizzi, A.; Fiaschi, A.I.; Menchiari, A.; Moretti, E.; Moltoni, L.; Micheli, L. Cytotoxic effects of cannabinoids on human HT-29 colorectal adenocarcinoma cells: Different mechanisms of THC, CBD, and CB83. Int. J. Mol. Sci. 2020, 21, 5533. [Google Scholar] [CrossRef]
- Sheik, A.; Farani, M.R.; Kim, E.; Kim, S.; Gupta, V.K.; Kumar, K.; Huh, Y.S. Therapeutic targeting of the tumor microenvironments with cannabinoids and their analogs: Update on clinical trials. Environ. Res. 2023, 231, 115862. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto Rebelatto, E.R.; Rauber, G.S.; Caon, T. An update of nano-based drug delivery systems for cannabinoids: Biopharmaceutical aspects & therapeutic applications. Int. J. Pharm. 2023, 635, 122727. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Suttithumsatid, W.; Shah, M.A.; Bibi, S.; Panichayupakaranant, P. α-Glucosidase inhibitory activity of cannabidiol, tetrahydrocannabinol and standardized cannabinoid extracts from Cannabis sativa. Curr. Res. Food Sci. 2022, 5, 1091–1097. [Google Scholar] [CrossRef]
- Ramlugon, S.; Levendal, R.-A.; Frost, C.L. Effect of oral cannabis administration on the fat depots of obese and streptozotocin-induced diabetic rats. Phytother. Res. 2023, 37, 1806–1822. [Google Scholar] [CrossRef]
- Alves, V.L.; Gonçalves, J.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. The synthetic cannabinoids phenomenon: From structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020, 50, 359–382. [Google Scholar] [CrossRef]
- Tesfatsion, T.; Collins, A.; Ramirez, G.; Docampo-Palacios, M.L.; Mzannar, Y.; Khan, H.; Aboukameel, O.; Azmi, A.; Jagtap, P.; Ray, K.; et al. Antineoplastic Properties of THCV, HHC and their anti-Proliferative effects on HPAF-II, MIA-paca2, Aspc-1, and PANC-1 PDAC Pancreatic Cell Lines. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Tesfatsion, T.T.; Docampo-Palacios, M.L.; Collins, A.C.; Mzannar, Y.; Khan, H.Y.; Aboukameel, O.; Azmi, A.S.; Jagtap, P.G.; Ray, K.P.; et al. Antitumor Effects of Cannabinoid Analogue CCL104 in Human Pancreatic Ductal Adenocarcinoma MiaPaCa2-Derived Xeno-graft Model. Int. J. Mol. Sci. 2023; submitted. [Google Scholar]
- Ramirez, G.A.; Collins, A.C.; Tesfatsion, T.T.; Mzannar, Y.; Khan, H.Y.; Aboukameel, O.; Azmi, A.S.; Mattos-Pereira, V.; Nair, S.; Jagtap, P.G.; et al. Cytotoxic Cannabinoid Analogs for Prevention of Cancer. In Proceedings of the ACS Spring 2023: Crossroads of Chemistry, Indianapolis, IN, USA, 26–30 March 2023. [Google Scholar] [CrossRef]
- Davis, M.P. Oral Nabilone Capsules in the Treatment of Chemotherapy-Induced Nausea and Vomiting and Pain. Expert Opin. Investig. Drugs 2008, 17, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Abi-Jaoude, E.; Desarkar, P.; Wang, W.; Ameis, S.H.; Lai, M.-C.; Lunsky, Y.; Rajji, T.K. Nabilone Treatment for Severe Behavioral Problems in Adults with Intellectual and Developmental Disabilities: Protocol for a Phase I Open-Label Clinical Trial. PLoS ONE 2023, 18, e0282114. [Google Scholar] [CrossRef] [PubMed]
- Tesfatsion, T.T.; Ramirez, G.A.; Docampo-Palacios, M.L.; Collins, A.C.; Ray, K.P.; Cruces, W. Evaluation of In-Vitro Cytotoxicity, Genotoxicity and Cardiac Safety of Hydrogenated Cannabidiol on Cells Using Metabolic Assay, AMES and hERG Test. Pharmacog. Mag. 2023; accepted. [Google Scholar] [CrossRef]
- Russo, F.; Vandelli, M.A.; Biagini, G.; Schmid, M.; Luongo, L.; Perrone, M.; Ricciardi, F.; Maione, S.; Laganà, A.; Capriotti, A.L.; et al. Synthesis and Pharmacological Activity of the Epimers of Hexahydrocannabinol (HHC). Sci. Rep. 2023, 3, 11061. [Google Scholar] [CrossRef] [PubMed]


























 | ||||
| Entry | Starting Material | Citronellal | Product | Yield (%) |
| 1 |  | 9a |  | 68 |
| 2 |  | 9a | 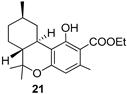 | 87 |
| 3 | 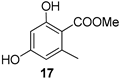 | 9a |  | 75 |
| 3 |  | 9a |  | 92 |
| 4 |  | 9a |  | 72 |
| 5 |  | 9b |  | 70 |
| 6 | 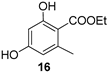 | 9b |  | 87 |
| 7 | 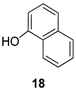 | 9b |  | 90 |
| 8 | 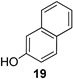 | 9b | 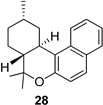 | 75 |
| Compound | Ki (nM) | Function | References | ||||
|---|---|---|---|---|---|---|---|
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
 34b | >10,000 | - | >10,000 | - | - | [63] | |
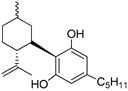 102a | >1000 | - | - | - | - | - | [9,63] |
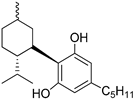 103a | 145 | - | - | - | - | - | [9,63] |
 102b | 124 | - | - | - | - | - | [9,63] |
 103b | 17 | - | - | - | - | - | [9,63] |
| Compound | Ki (μM) | Function | References | ||||
|---|---|---|---|---|---|---|---|
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
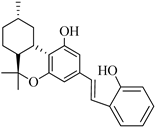 39 (Machaeriol C) | 3.27 | - | 7.76 | - | - | - | [103] |
 43 (Machaeriol D) | 1.75 | - | 1.30 | - | - | - | [103] |
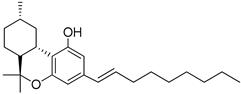 165 | 0.34 | - | 0.57 | - | - | - | [103] |
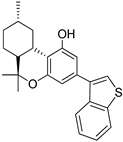 166 | >1000 | - | 0.040 | - | - | CB2 selective agonists | [103] |
 106 (Machaeridiol A) | >1000 | - | 1.77 | - | - | CB2 selective agonists | [103] |
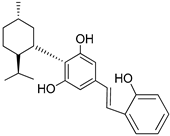 167 (Machaeridiol B) | >1000 | - | 2.18 | - | - | CB2 selective agonists | [103] |
 168 (Machaeridiol C) | >1000 | - | 1.11 | - | - | CB2 selective agonists | [103] |
| Compound | Ki (nM) | Function | References | ||||
|---|---|---|---|---|---|---|---|
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
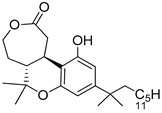 99b | 99.0 ± 11 | - | - | 803.0 ± 87 | 94.1 ± 13 | - | [58] |
 99a | 4.6 ± 2.8 | - | - | 792.3 ± 76 | 54.1 ± 7 | Agonist CB1 | [58] |
| Compound | Ki (nM) | Function | References | ||||
|---|---|---|---|---|---|---|---|
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
 140 | 919.7 | - | - | 2034.1 | - | - | [78] |
 139 | 286.4 | - | - | 464 | - | - | [78] |
| Compound | Ki (nM) | Function | References | ||||
|---|---|---|---|---|---|---|---|
| rCB1 | hCB1 | rCB2 | mCB2 | hCB2 | |||
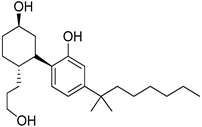 228 (CP-55,940) | - | 0.58 | - | - | 0.69 | Agonist | [109,110] |
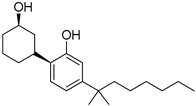 229 | - | 61.6 | - | - | 91.0 | - | [109,110] |
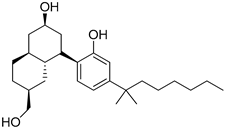 230 | - | 1.0 | - | - | 2.4 | - | [11,110] |
 231 | 7079 | - | - | 7585 | - | [109,110] | |
| Compound | Binding Energy (kcal/mol) | Interaction Type | Ki (nM) | References | ||
|---|---|---|---|---|---|---|
| CB1 | CB2 | hCB1 | hCB2 | |||
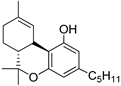 35b | −9.4 | −10.4 | CB1: Alkyl, π-alkyl, π–σ bond, C–H bond, van der Waals. CB2: Alkyl, π-alkyl, π–π-T-shaped, π–σ bond | 15 | 9.1 | [91,111] |
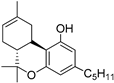 36b | −6.9 | −10.1 | CB2: Alkyl, π-alkyl, π–π-T-shaped, π-Donor-H bond | 440 | 337 | [91,111] |
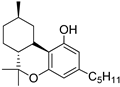 1 | −9.1 | −10.3 | CB2: Alkyl, π-alkyl, π–π-T-shaped, π–σ bond | 15 | 13 | [91,111] |
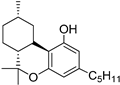 7 | −7.2 | −9.1 | CB2: Alkyl, π-alkyl, π–π-T-shaped, π–π-Stacked | 176 | 105 | [91,111] |
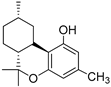 232 | −6.4 | −7.1 | - | >1000 | >100 | [31,112,113] |
 233 | −5.9 | −6.5 | - | >1000 | >100 | [31,112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Docampo-Palacios, M.L.; Ramirez, G.A.; Tesfatsion, T.T.; Okhovat, A.; Pittiglio, M.; Ray, K.P.; Cruces, W. Saturated Cannabinoids: Update on Synthesis Strategies and Biological Studies of These Emerging Cannabinoid Analogs. Molecules 2023, 28, 6434. https://doi.org/10.3390/molecules28176434
Docampo-Palacios ML, Ramirez GA, Tesfatsion TT, Okhovat A, Pittiglio M, Ray KP, Cruces W. Saturated Cannabinoids: Update on Synthesis Strategies and Biological Studies of These Emerging Cannabinoid Analogs. Molecules. 2023; 28(17):6434. https://doi.org/10.3390/molecules28176434
Chicago/Turabian StyleDocampo-Palacios, Maite L., Giovanni A. Ramirez, Tesfay T. Tesfatsion, Alex Okhovat, Monica Pittiglio, Kyle P. Ray, and Westley Cruces. 2023. "Saturated Cannabinoids: Update on Synthesis Strategies and Biological Studies of These Emerging Cannabinoid Analogs" Molecules 28, no. 17: 6434. https://doi.org/10.3390/molecules28176434
APA StyleDocampo-Palacios, M. L., Ramirez, G. A., Tesfatsion, T. T., Okhovat, A., Pittiglio, M., Ray, K. P., & Cruces, W. (2023). Saturated Cannabinoids: Update on Synthesis Strategies and Biological Studies of These Emerging Cannabinoid Analogs. Molecules, 28(17), 6434. https://doi.org/10.3390/molecules28176434







