Abstract
Isocoumarins and dihydroisocoumarins are important skeletons with a wide range of biological activities, such as anti-bacterial, anti-allergy, anti-fungal, anti-tumor, and anti-HIV properties. Herein, we demonstrated divergent syntheses of isocoumarins and 3,4-dihydroisocoumarins by intramolecular dehydrogenative cyclization of 2-(3-oxobutyl) benzoic acids. This transformation undergoes Csp3–H bonds and O–H bonds coupling in air using copper salt. The reactions may undergo free radical process.
1. Introduction
In recent years, oxygen-containing heterocyclic compounds have emerged as the mainstream in drug research and development because of their unique structural characteristics and physiological activities. Compounds with isocoumarins and dihydroisocoumarins, which are important skeletons of many natural products, bioactive substances, and agricultural chemicals, have a wide range of biological activities, such as anti-bacterial and anti-allergy, anti-fungal, anti-tumor, and anti-HIV, and can be used to make herbicides [1,2,3,4,5].
The synthesizing isocoumarins mainly depended on the coupling of carboxyl groups and unsaturated compounds, which involved metal-catalyzed reactions as well as various intramolecular and intermolecular cyclization reactions [6,7,8]. In 1998, Miura’s group [9] firstly employed palladium as a catalyst to synthesize isocoumarins (Figure 1a). In 2009 and 2014, Obushak’s group [10,11] reported the one-pot synthesis of dihydroisocoumarin derivatives and 3-substituted methyl 3,4-dihydroisocoumarin-6-carboxylates under Meerwein’s arylation conditions (Figure 1b). In 2017, Shen’s group [12] discolsed a Fe(NO3)3-catalyzed synthesis of dihydroisocoumarin derivatives (Figure 1c). In 2018, Oh’s group [13] demonstrated A rhodium-catalyzed decarbonylative aerobic oxidation of cyclic α-diketones for the formations of isocoumarins. Among these reactions, transition metal complexes, such as palladium [14,15,16,17,18,19,20], rhodium [21,22,23,24,25,26,27,28,29,30,31], ruthenium [32,33,34], iridium [35], nickel [36], and silver [37], are commonly used catalysts. Moreover, these protocols have the disadvantages of requiring expensive catalysts and halogenated raw materials, and low atomic utilization, etc.
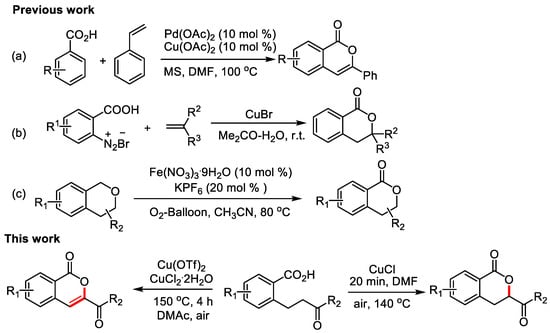
Figure 1.
The works for the synthesis of isocoumarins and dihydroisocoumarins. (a) Miura’s, work; (b) Obushak’s works; (c) Shen’s work.
The synthesis of dihydroisocoumarins is rarely reported. The methods reported in the literature are mainly constructed via the oxidation of methylene groups. Oxygen [12], iridium [35,38], iron [12,39,40], ruthenium [32,33,34,41], manganese [42] can oxidize methylene in isochroman to synthesize dihydroisocoumarins. However, this kind of reaction is not suitable for isocoumarin substrates. Metal-catalyzed intramolecular and intermolecular cyclization reactions, coupling of carbon monoxide with alcohols, and palladium-catalyzed carbonyl insertion have also been used for the synthesis of dihydroisocoumarins.
Our previous study [43] displayed that 2-(3-oxobutyl) benzoic acid can be generated from aromatic carboxylic acids and 1-penten-3-one in one pot via rhodium-catalyzed carboxyl-directed conjugate addition of C–H bonds to α,β-unsaturated ketones in air and water. We envision that whether 2-(3-oxobutyl) benzoic acid can undergo intramolecular cyclization reaction and oxidative dehydrogenation to yield dihydroisocoumarins and isocoumarins in one pot. Herein, we illuminated copper-promoted intramolecular oxidative dehydrogenation of 2-(3-oxoalkyl) benzoic acid for synthesizing dihydroisocoumarins and isocoumarins in a step in air.
2. Results
2.1. General Method for the Synthesis of Dihydroisocoumarins
CuCl (9.9 mg, 0.1 mmol), 0.6 mL of N,N-dimethylformamide, substituted benzoic acid (0.1 mmol) were added in sequence to a microwave reactor. The reaction tube was directly sealed and reacted at 140 °C (oil bath temperature) for 20 min. Then, the mixture was cooled to room temperature and diluted with ethyl acetate, and the salt was removed through a short silica gel column. The crude product was purified using preparative thin-layer chromatography to give the corresponding product.
2.2. General Method for the Synthesis of Isocoumarins
Cu(OTf)2 (217.0 mg, 0.6 mmol), CuCl2·2H2O (17 mg, 0.1 mmol), 0.6 mL of N,N-dimethylacetamide, and substituted benzoic acid (0.2 mmol) were added in the microwave reactor. The mixture reacted at 150 °C (oil bath temperature) for 4 h. After cooling to room temperature, the mixture was diluted with ethyl acetate, and the salt was removed through a short silica gel column. The crude product was purified using preparative thin-layer chromatography to give the corresponding product.
3. Materials and Methods
Experimental Reagents and Instruction
1H NMR and 13C NMR spectra were measured on a Bruker spectrometer, using CDCl3 as the solvent with tetramethylsilane (TMS) as an internal standard at room temperature. High-resolution mass spectrometry was determined using a compass-maxis high-resolution mass spectrometer from Bruker Company, Germany. All solvents used in the experiment were dried using activated molecular sieves, and the other reagents used in the experiment were all analytically pure without any other treatment. Chemical shifts are given in δ relative to TMS, and the coupling constants J are given in Hz. Characterization data of compounds, the conversions of acids and NMR spectra of compounds, See Supplementary Materials.
4. Discussion
2-methyl-6-(3-oxopentyl)benzoic acid was selected as substrate to screen the reaction conditions (Table 1). To our delight, 4% yield of the dihydroisocoumarin product 2a was observed at 150 °C for 24 h with CuI. The 2a were not observed in the atmosphere of nitrogen and oxygen. Using CuCl or CuBr instead of CuI, the yields were increased to 28% and 18% (Table 1, entries 4, 5), only 1% yield was detected using CuF2. Other bivalent coppers, such as CuO, Cu(OAc)2, and CuBr2, failed to generate cyclization product (Table 1, entries 7–9). Lower yields were detected in DMAc, DMSO, THF or tert-pentanol (Table 1, entries 10–13). No 2a were observed in toluene and 1,4-dioxane (Table 1, entries 14, 15). The yield increased to 35% when the amount of CuCl was doubled. It was found that increasing reaction temperature to 140 °C and shortening reaction time to 20 min, the yield of 2a was enhanced to 61% (Table 1, entry 17). Then, the effects of reaction time on the yield were investigated. A reaction time of 20 min was the best among 10 min, 20 min, and 30 min (Table 1, entries 18–20).

Table 1.
Selected results for optimizing reaction conditions a.
When CuO was added, an isocoumarin product 3a in 25% yield was observed (Table 2, entry 1). 16% and 12% yields of 3a were observed in nitrogen and oxygen atmospheres, respectively (Table 2, entries 2, 3). When the solvents were screened (Table 2, entries 4–8), only DMAc gave a slight increase yield (32%, Table 2, entry 4). The yield was 33% when CuCl was replaced by CuCl2·2H2O (Table 2, entry 9). Other copper salts, such as Cu(OH)2, CuCl2, and CuBr, also can deliver 3a (Table 2, entries 10–12). The yields was found to be 50%, 46% and 26% using Cu(OTf)2, AgOTf and AgOAc, respectively (Table 2, entries 13–15). The yield was enhanced to 59% with 3.0 eq. Cu(OTf)2 and 0.5 eq. CuCl2·2H2O at 150 °C for 2.5 h (Table 2, entry 16). It is pleased to find that 65% yield of 3a was observed when the amount of 1a was increased to 0.2 mmol (Table 2, entry 17).

Table 2.
Selected results for optimizing reaction conditions a.
With the optimum reaction conditions in hand, the application of this method was investigated with a series of substituted 2-(3-oxobutyl) benzoic acids. The results are listed in Figure 2 and Figure 3. As can be seen from Figure 2, substituted 2-(3-oxo-amyl) benzoic acids bearing electron-donating groups at ortho-position of carboxyl such as ethyl, phenyl, benzyl, and ethylphenyl delivered moderate yields (2b–2e, 49–57%), and the yield was 74% when there was no substituted group in the benzene ring. Meta- and para-substituted 2-(3-oxamyl) benzoic acids such as 3-methyl, 3-methoxy, 4-methyl, and 4-ethyl, afforded good to excellent yields of cyclization products (2g–2l). 3-Cl-substituted 2-(3-oxamyl) benzoic acid also produced the targeted product (2i) in a 46% yield. Disubstituted 2-(3-oxopentyl) benzoic acids were also compatible, giving rise to moderate to good yields (2m–2u, 44–68%). 3,4,5-Trimethoxy-2-(3-oxamyl) benzoic acid gave 68% yield of 2v. When the benzene ring was substituted by thiophene, the yield was 42% (2w). Employing 3-(3-oxo-amyl)-2-naphthylformic acid and 2-(3-oxo-amyl)-2-naphthylformic acid as substrates, the cyclization products were 72% and 40%, respectively (2x and 2y). 5-Methyl-2-(3-oxobutyl) benzoic acid and 5-methyl-2-(3-oxooctyl) benzoic acid generated 85% and 65% desired products, respectively (2z and 2aa).
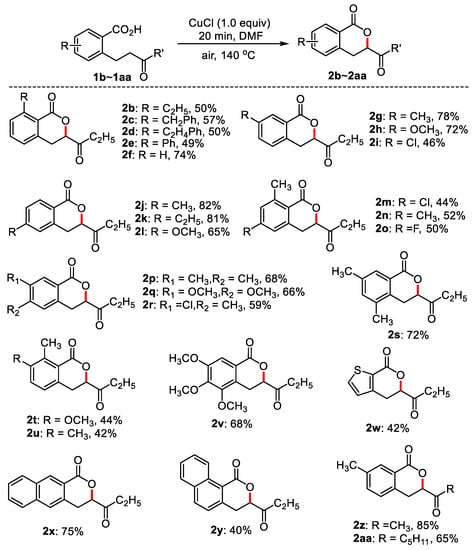
Figure 2.
The synthesis of dihydroisocoumarins. Reaction conditions: substituted benzoic acid (0.1 mmol), CuCl (0.1 mmol), DMF (0.6 mL), 140 °C, 20 min, air, isolated yield.
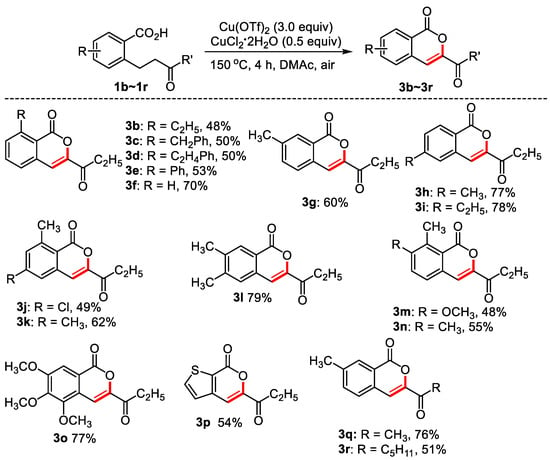
Figure 3.
The synthesis of isocoumarins. Reaction conditions: substituted benzoic acid (0.2 mmol), Cu(OTf)2 (0.6 mmol), CuCl2·2H2O (0.1 mmol), DMAc (0.6 mL), 150 °C, 4 h, air, isolated yield.
As can be seen from Figure 3, the oxidative dehydrogenation of 2-(3-oxopentyl) benzoic acids bearing different substituents produced isocoumarins in moderate to good yields. Similar to the formation of dihydroisocoumarins, donating groups at the ortho, meta, and para-position of carboxyl, 2,3-disubstituted 2-(3-oxo-amyl) benzoic acid, 2,4-disubstituted 2-(3-oxo-amyl) benzoic acid, 3,4-disubstituted 2-(3-oxo-amyl) benzoic acid worked well, and moderate to good yields were obtained (3b–3n). 3,4,5-Trimethoxy-2-(3-oxamyl) benzoic acid delivered 77% yield of targeted product. For heterocyclic 3-(3-oxopentyl) thiophene-2-carboxylic acid, 54% of product was observed. 76% and 51% of the isocoumarins were obtained from 3-methyl-2-(3oxobutyl) benzoic acid and 3-methyl-2-(3oxo-octyl) benzoic acid, respectively.
To make an insight on the mechanism, free radical scavenger TEMPO was added to the reaction mixture. The addition of TEMPO depressed the formation of dihydroisocoumarin, and 21% of isocoumarin was detected, which suggesting that the reaction may undergo a radical process (Figure 4).
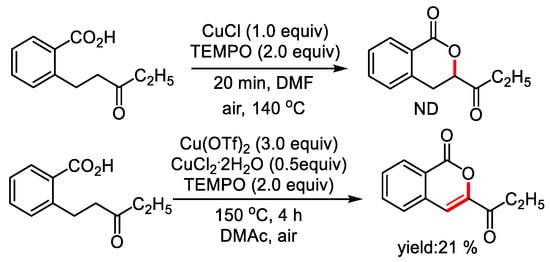
Figure 4.
Exploration of possible free radical reactions.
Based on the above results and radical studies [44,45,46], a plausible mechanism for forming dihydroisocoumarin was given in Figure 5. Firstly, Cu(I) is oxidized by air to afford Cu(II), which reacts with 2-(3-oxopentyl) benzoic acid to form the intermediate (B). Then the free radical intermediate (C) is formed via homolysis of the O–Cu bond. The carbon radical intermediate (D) is obtained via hydrogen transfer from α-H of carbonyl to oxygen free radical. The final product is formed via the copper-catalyzed single-electron intermediate and intramolecular cyclization (F).

Figure 5.
A plausible mechanism.
5. Conclusions
In summary, the divergent syntheses of isocoumarins and 3,4-dihydroisocoumarins were achieved by intramolecular dehydrogenative cyclization of 2-(3-oxobutyl) benzoic acids via Csp3–H bonds and O–H bonds coupling in air using copper salts. The advantages of this protocol include simple operation, air atmosphere, short reaction time, broad substrate scope, and cheap copper salts. The reactions may undergo free radical process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28176319/s1, characterization data of compounds, the conversions of acids and NMR spectra of compounds.
Author Contributions
Conceptualization, Q.Z. and X.-Y.S.; methodology, X.-Y.S. investigation, Q.Z. and L.-Y.Z.; resources, X.-Y.S.; data curation, L.-Y.Z.; writing—original draft preparation, Q.Z.; writing—review and editing, Q.Z. and X.-Y.S.; supervision, X.-Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures were approved by the Committee of Shaanxi University of Technology.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
The authors are grateful to the support of the Shaanxi Normal University experimental testing platform in the process of characterizing compounds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Y.Z.; Rao, X.M.; Xu, J.Y.; Xie, W.J.; Wu, X.M. Research Progress in Agrimonolides with Isocoumarin Skeleton. Prog. Pharm. Sci. 2018, 42, 303–308. [Google Scholar]
- Pal, S.; Chatare, V.; Pal, M. Isocoumarin and Its Derivatives: An Overview on Their Synthesis and Application. Curr. Org. Chem. 2011, 15, 782–800. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Harada, E.; Naitoh, Y.; Inoue, K.; Matsuda, H.; Shimada, H.; Yamahara, J.; Murakami, N. Development of Bioactive Functions in Hydrangeae Dulcis Folium. III. On the Antiallergic and Antimicrobial Principles of Hydrangeae Dulcis Folium. (1). Thunberginols A, B, and F. Chem. Pharm. Bull. 1994, 42, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Meepagala, K.M.; Sturtz, G.; Wedge, D.E. Antifungal Constituents of the Essential Oil Fraction of Artemisia Dracunculus L. var. Dracunculus. J. Agric. Food. Chem. 2002, 50, 6989–6992. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Agata, N.; Kharbanda, S.; Avigan, D.; Kufe, D. A Novel Isocoumarin Derivative Induces Mitotic Phase Arrest and Apoptosis of Human Multiple Myeloma Cells. Cancer Chemother. Pharmacol. 2007, 59, 329–335. [Google Scholar] [CrossRef]
- Saikia, P.; Gogoi, S. Isocoumarins: General Aspects and Recent Advances in their Synthesis. Adv. Synth. Catal. 2018, 360, 2063–2075. [Google Scholar] [CrossRef]
- Drapeau, M.P.; Gooßen, L.J. Carboxylic Acids as Directing Groups for C–H Bond Functionalization. Chem. Eur. J. 2016, 22, 18654–18677. [Google Scholar] [CrossRef]
- Saeed, A.; Larik, F.A. Metal-free Synthesis of Isocoumarins. Chem. Heterocycl. Com. 2016, 52, 450–452. [Google Scholar] [CrossRef]
- Miura, M.; Tsuda, T.; Satoh, T.; Pivsa-Art, S.; Nomura, M. Oxidative Cross-Coupling of N-(1,1'-biphenyl-2-yl) benzenesulfonamides or Benzoic and Naphthoic Acids with Alkenes Using a Palladium-Copper Catalyst System under Air. J. Org. Chem. 1998, 63, 5211–5215. [Google Scholar] [CrossRef]
- Obushak, M.D.; Matiychuk, V.S.; Turytsya, V.V. A New Approach to the Synthesis of 3,4-Dihydroisocoumarin Derivatives. Tetrahedron Lett. 2009, 50, 6112–6115. [Google Scholar] [CrossRef]
- Turytsya, V.V.; Ostapiuk, Y.V.; Matiychuk, V.V.; Obushak, M.D. Synthesis of 3-Aryl/methoxycarbonyl-3,4-dihydroisocoumarin-6-carboxylic Acid Derivatives. J. Heterocycl. Chem. 2014, 51, 1898–1901. [Google Scholar] [CrossRef]
- Hong, C.; Ma, J.; Li, M.; Jin, L.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Shen, Z.L. Ferric Nitrate-Catalyzed Aerobic Oxidation of Benzylic sp3 C–H Bonds of Ethers and Alkylarenes. Tetrahedron 2017, 73, 3002–3009. [Google Scholar] [CrossRef]
- Gangadhararao, G.; Hun, Y.K.; Oh, K. Rhodium(I)-Catalyzed Decarbonylative Aerobic Oxidation of Cyclic α-Diketones: A Regioselective Single Carbon Extrusion Strategy. Org. Lett. 2018, 20, 942–945. [Google Scholar]
- Nandi, D.; Ghosh, D.; Chen, S.J.; Kuo, B.C.; Wang, N.M.; Lee, H.M. One-Step Synthesis of Isocoumarins and 3-Benzylidenephthalides via Ligandless Pd-Catalyzed Oxidative Coupling of Benzoic Acids and Vinylarenes. J. Org. Chem. 2013, 78, 3445–3451. [Google Scholar] [CrossRef]
- Liu, H.X.; Yang, Y.Y.; Wu, J.; Wang, X.L.; Chang, J.B. Regioselective Synthesis of 3,4-Disubstituted Isocoumarins Through the Pd-Catalyzed Annulation of 2-Iodoaromatic Acids with Ynamides. Chem. Commun. 2016, 52, 6801–6804. [Google Scholar] [CrossRef]
- Zhang, J.B.; Han, X.L.; Lu, X.Y. Synthesis of Cyclohexane-Fused Isocoumarins via Cationic Palladium(II)-Catalyzed Cascade Cyclization Reaction of Alkyne-Tethered Carbonyl Compounds Initiated by Intramolecular Oxypalladation of Ester-Substituted Aryl Alkynes. J. Org. Chem. 2016, 81, 3423–3429. [Google Scholar] [CrossRef]
- Jiang, G.S.; Li, J.X.; Zhu, C.L.; Wu, W.Q.; Jiang, H.F. Palladium-Catalyzed Sequential Nucleophilic Addition/Oxidative Annulation of Bromoalkynes with Benzoic Acids to Construct Functionalized Isocoumarins. Org. Lett. 2017, 19, 4440–4443. [Google Scholar] [CrossRef]
- Liu, X.Z.; Chen, G.J.; Zhou, Y.X.; Liu, P.J. Palladium/Copper-catalyzed Tandem Sonogashira Coupling/Lactonization of Methyl 2-(2′,2′-dibromovinyl)benzoate with Terminal Alkynes: Facile Access to 3-Alkynyl Isocoumarins. Tetrahedron Lett. 2018, 59, 3151–3154. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, Z.B.; Zhang, F.L.; Zhu, Y.M. Palladium-catalyzed Carbonylative Synthesis of Isocoumarins and Phthalides by Using Phenyl Formate as a Carbon Monoxide Source. Org. Biomol. Chem. 2017, 15, 1628–1635. [Google Scholar] [CrossRef]
- Nakayama, A.; Hamamoto, K.; Fujiwara, I.; Fukuda, E.; Ozawa, K.; Endo, S.; Yamasaki, R.; Yamanaka, H.; Tamura, Y.; Yamamoto, Y. Concise Synthesis of Isocoumarin-3-carboxylic Acid Esters. Chem. Lett. 2023, 52, 640–643. [Google Scholar] [CrossRef]
- Ueura, K.; Satoh, T.; Miura, M. An Efficient Waste-Free Oxidative Coupling via Regioselective C–H Bond Cleavage: Rh/Cu-Catalyzed Reaction of Benzoic Acids with Alkynes and Acrylates under Air. Org. Lett. 2007, 9, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Ueura, K.; Satoh, T.; Miura, M. Rhodium- and Iridium-Catalyzed Oxidative Coupling of Benzoic Acids with Alkynes via Regioselective C–H Bond Cleavage. J. Org. Chem. 2007, 72, 5362–5367. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y.N.; Wang, X.W.; Gong, B.W.; Tang, X.; Shi, J.J.; Xu, H.E.; Li, W. Water as a Green Solvent for Efficient Synthesis of Isocoumarins Through Microwave-accelerated and Rh/Cu-Catalyzed CH/O–H Bond Functionalization. RSC Adv. 2013, 3, 23402–23408. [Google Scholar] [CrossRef]
- Mo, J.; Wang, L.; Cui, X. Rhodium(III)-Catalyzed C–H Activation/Alkyne Annulation by Weak Coordination of Peresters with O–O Bond as an Internal Oxidant. Org. Lett. 2015, 17, 4960–4963. [Google Scholar] [CrossRef]
- Zhang, M.L.; Zhang, H.J.; Han, T.T.; Ruan, W.Q.; Wen, T.B. Rh(III)-Catalyzed Oxidative Coupling of Benzoic Acids with Geminal-Substituted Vinyl Acetates: Synthesis of 3-Substituted Isocoumarins. J. Org. Chem. 2015, 80, 620–627. [Google Scholar] [CrossRef]
- Hara, Y.; Onodera, S.; Kochi, T.; Kakiuchi, F. Catalytic Formation of α-Aryl Ketones by C–H Functionalization with Cyclic Alkenyl Carbonates and One-Pot Synthesis of Isocoumarins. Org. Lett. 2015, 17, 4850–4853. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Liu, K.; Zou, G.; Liu, P.J. Rhodium(III)-Catalyzed, C–H Activated Annulation to Form Isocoumarins and α-Pyrones using the O N Bond as an Internal Oxidant. Adv. Synth. Catal. 2014, 356, 1496–1500. [Google Scholar] [CrossRef]
- Li, X.G.; Sun, M.; Liu, K.; Jin, Q.; Liu, P.N. Rh(III)-Catalyzed C–H Activation/Cyclization of Benzamides and Diazo Compounds to Form Isocoumarins and α-Pyrones. Chem. Commun. 2015, 51, 2380–2383. [Google Scholar] [CrossRef]
- Yang, C.; He, X.; Zhang, L.; Han, G.; Zuo, Y.P.; Shang, Y.J. Synthesis of Isocoumarins from Cyclic 2-Diazo-1,3-diketones and Benzoic Acids via Rh(III)-Catalyzed C–H Activation and Esterification. J. Org. Chem. 2017, 82, 2081–2088. [Google Scholar] [CrossRef]
- Dalvi, P.B.; Lin, K.L.; Kulkarni, M.V.; Sun, C.M. Rhodium-Catalyzed Regioselective Synthesis of Isocoumarins through Benzothiadiazine-Fused Frameworks. Org. Lett. 2016, 18, 3706–3709. [Google Scholar] [CrossRef]
- Ignacio, F.A.; Feliu, M. Computational Characterization of the Mechanism for the Oxidative Coupling of Benzoic Acid and Alkynes by Rhodium/Copper and Rhodium/Silver Systems. Chem. Eur. J. 2018, 24, 12383–12388. [Google Scholar]
- Warratz, S.; Kornhaa, C. Ruthenium(II)-Catalyzed C-H Activation/Alkyne Annulation by Weak Coordination with O2 as the Sole Oxidant. Angew. Chem. Int. Ed. 2015, 54, 5513–5517. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Shekarrao, K.; Gogoi, S.; Boruah, R.C. Ruthenium-Catalyzed Decarbonylative Addition Reaction of Anhydrides with Alkynes: A Facile Synthesis of Isocoumarins and α-Pyrones. Chem. Commun. 2015, 51, 9972–9974. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.B.; Weix, D.J. Ruthenium-catalyzed C–H arylation of diverse aryl carboxylic acids with aryl and heteroaryl halides. Org. Lett. 2016, 18, 5513–5517. [Google Scholar] [CrossRef] [PubMed]
- Frasco, D.A.; Lilly, C.P.; Boyle, P.D.; Elon, A. Cp*IrIII-Catalyzed Oxidative Coupling of Benzoic Acids with Alkynes. ACS Catal. 2013, 3, 2421–2429. [Google Scholar] [CrossRef]
- Xie, H.; Sun, Q.; Ren, G.; Gao, Z.X. Mechanisms and Reactivity Differences for Cycloaddition of Anhydride to Alkyne Catalyzed by Palladium and Nickel Catalysts: Insight from Density Functional Calculations. J. Org. Chem. 2014, 79, 11911–11921. [Google Scholar] [CrossRef]
- Panda, N.; Mishra, P.; Mattan, I. Synthesis of Isocoumarins via Silver(I)-Mediated Annulation of Enol Esters. J. Org. Chem. 2016, 81, 1047–1056. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, Q.; Yan, C.C.; Dong, J.Y.; Song, H.J.; Li, L.; Liu, Y.X.; Liu, X.M.; Song, H.B. Merging Photoredox with Brønsted Acid Catalysis: The Cross-Dehydrogenative C–O Coupling for sp3 C–H Bond Peroxidation. Chem. Eur. J. 2017, 23, 10871–10877. [Google Scholar] [CrossRef]
- Castro, A.G.; Robertson, C.M.; Xiao, J.L. Dehydrogenative α-Oxygenation of Ethers with an Iron Catalyst. J. Am. Chem. Soc. 2014, 136, 8350–8360. [Google Scholar] [CrossRef]
- Li, R.L.; Zhao, J.; Yang, F.X.; Zhang, Y.C.; Ramella, D.; Peng, Y.; Luan, Y. An Fe3O4@P4VP@FeCl3 Core–Shell Heterogeneous Catalyst for Aerobic Oxidation of Alcohols and Benzylic Oxidation Reaction. RSC Adv. 2017, 7, 51142–51150. [Google Scholar] [CrossRef]
- Maria, Z.; Glenn, M.S. Photoinduced Electron-Transfer-Promoted Redox Fragmentation of N-Alkoxyphthalimides. Org. Lett. 2011, 13, 6264–6267. [Google Scholar]
- Nammalwar, B.; Fortenberry, C.; Bunce, R.A.; Lageshetty, S.K.; Ausman, K.D. Efficient Oxidation of Arylmethylene Compounds Using Nano-MnO2. Tetrahedron Lett. 2013, 54, 2010–2013. [Google Scholar] [CrossRef]
- Han, W.J.; Pu, F.; Li, C.J.; Liu, Z.W.; Fan, J.; Shi, X.Y. Carboxyl-Directed Conjugate Addition of C–H Bonds to α,β-Unsaturated Ketones in Air and Water. Adv. Synth. Catal. 2018, 360, 1358–1363. [Google Scholar] [CrossRef]
- Chen, H.; Sanjaya, S.; Wang, Y.F.; Chiba, S. Copper-Catalyzed Aliphatic C–H Amination with an Amidine Moiety. Org. Lett. 2013, 15, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, P.; Wang, L.D.; Chen, J.P.; Sun, C.L.; Yu, Z.K. Copper-Mediated Intramolecular, Oxidative C–H/C–H Cross-Coupling of α-Oxo Ketene N,S-Acetals for Indole Synthesis. J. Org. Chem. 2014, 79, 10553–10560. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.S.; Xiong, T.; Zhang, Q.; Zhang, X.Y. Copper-Catalyzed Selective Benzylic C–O Cyclization of N-o-Tolylbenzamides: Synthesis of 4H-3,1-benzoxazines. Org. Lett. 2012, 14, 3522–3525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).

