Design, Synthesis and Fungicidal Activity of Ester Derivatives of 4-(3,4-Dichloroisothiazole) 7-Hydroxy Coumarin
Abstract
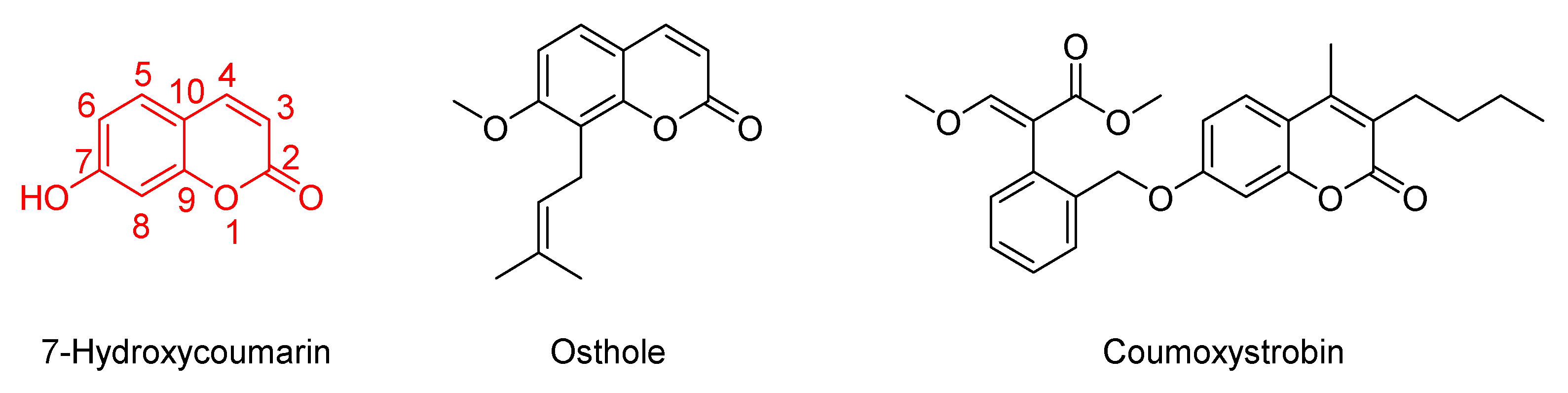
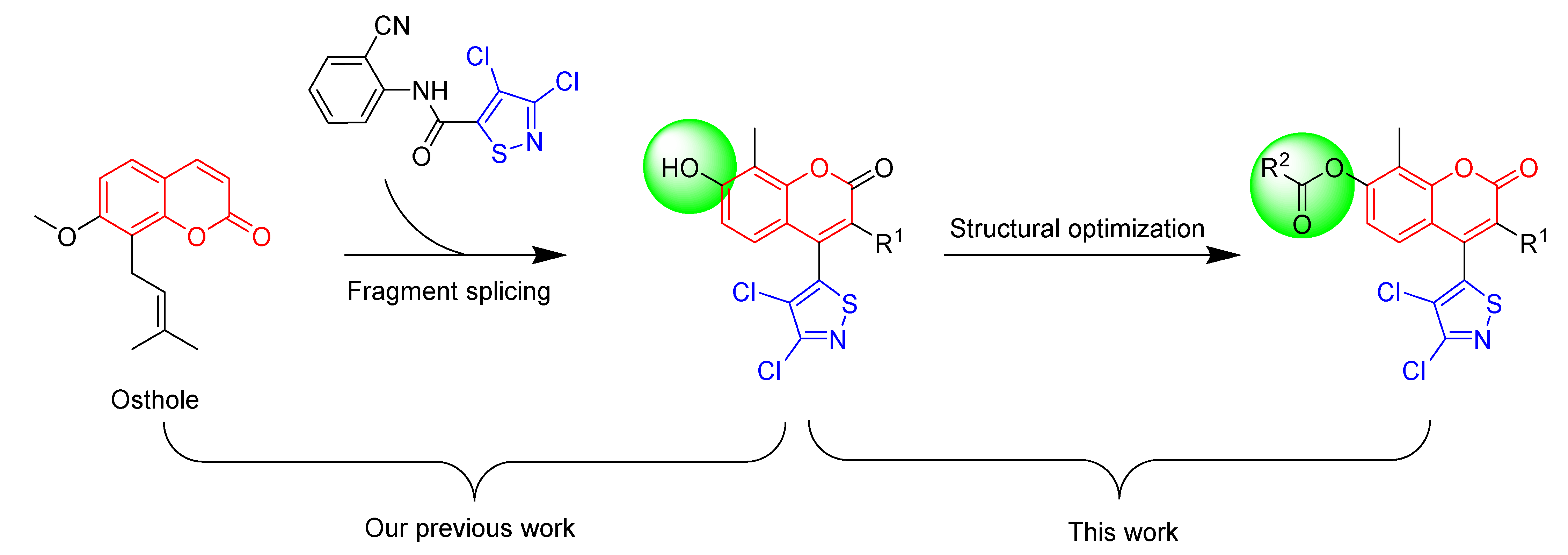
1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
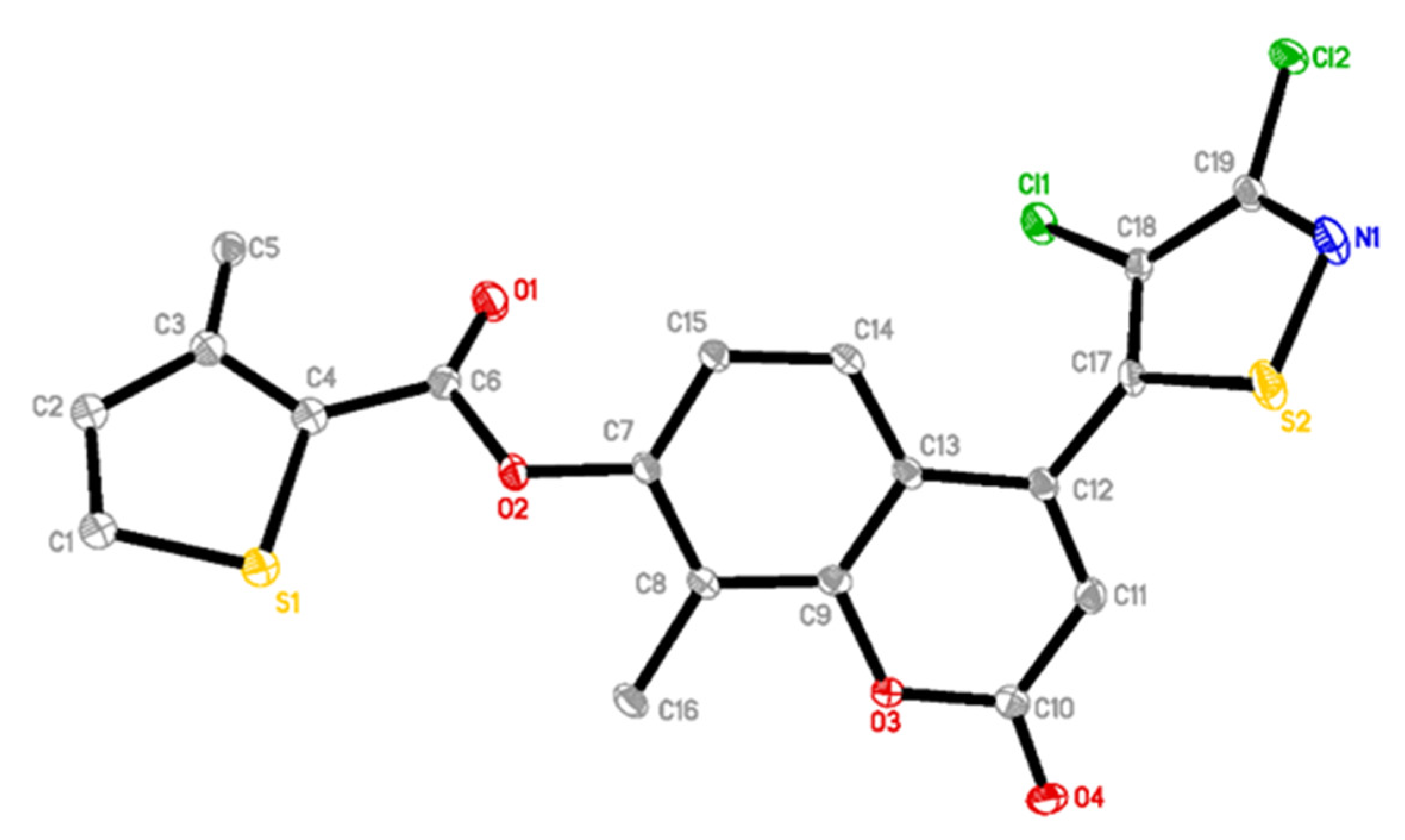
2.2. X-ray Diffraction of Compound 2be
2.3. In Vitro Fungicidal Activity
2.4. In Vivo Fungicidal Activity
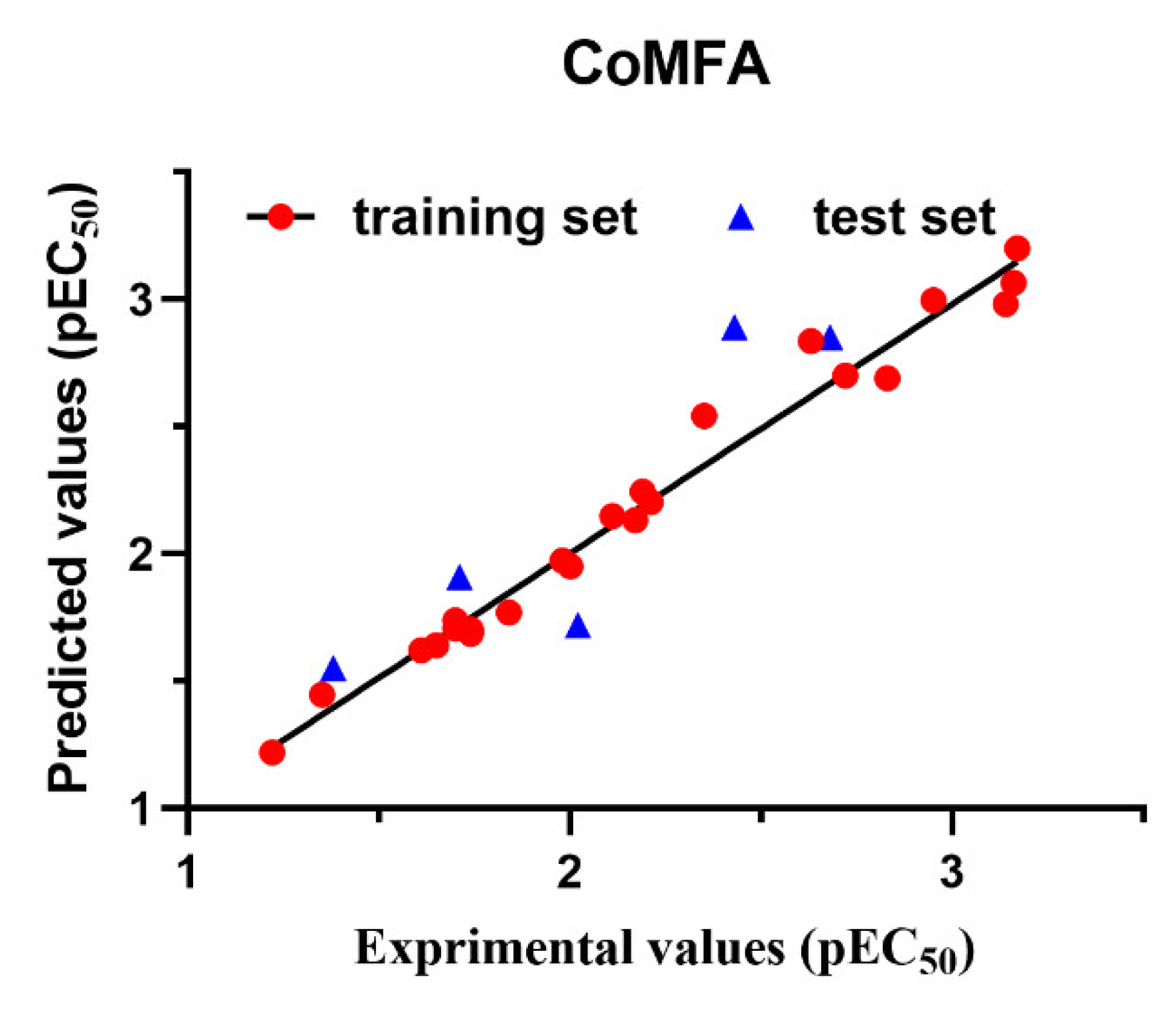
2.5. 3D-QSAR Analysis
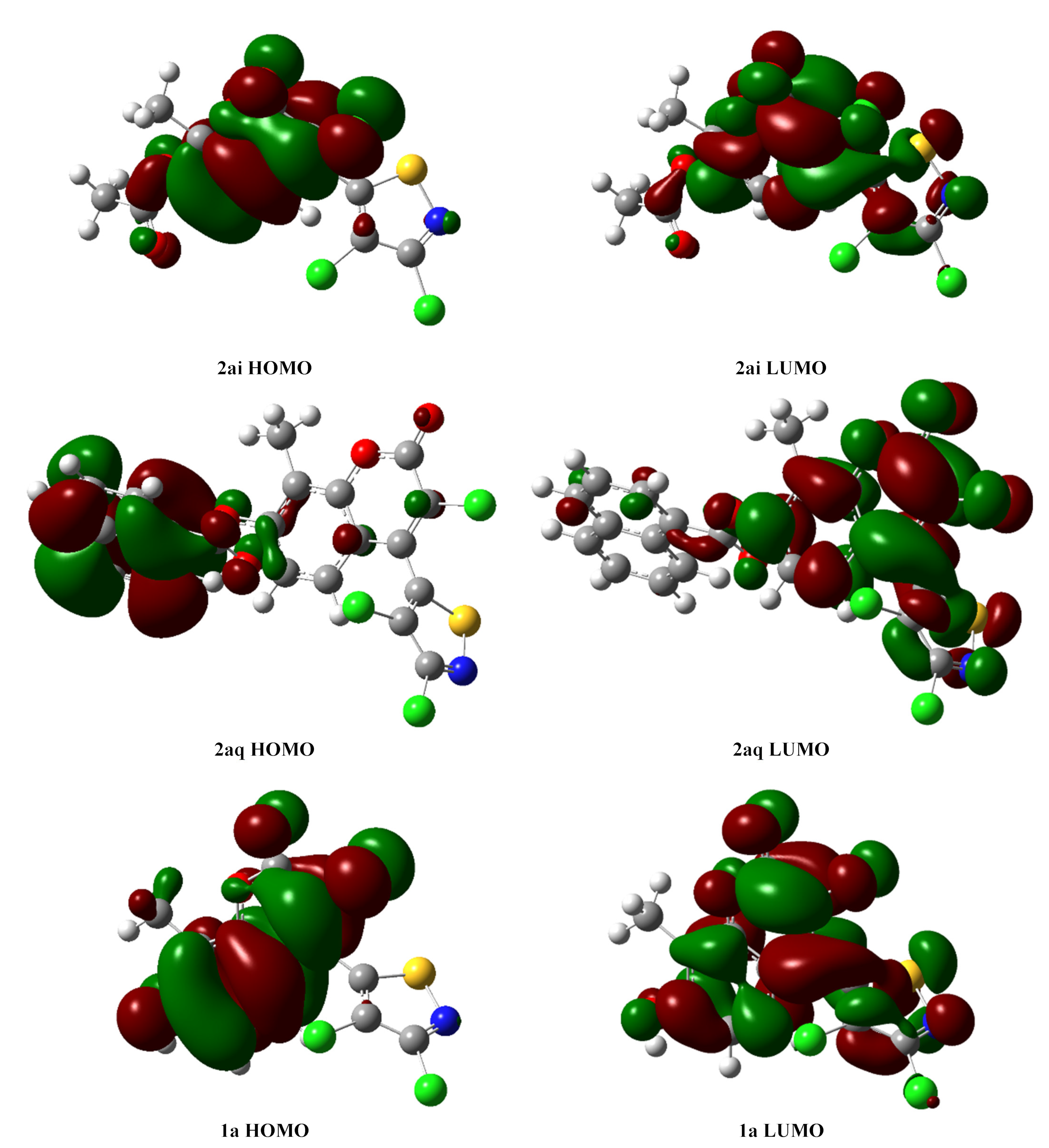
2.6. DFT Calculations of 2ai, 2aq and 1a
3. Materials and Methods
3.1. Instruments and Materials
3.2. General Procedures for Preparation of the Target Compounds 2aa–2bv
3.3. X-ray Diffraction
3.4. In Vitro Fungicidal Activity
3.5. In Vivo Fungicidal Activity
3.6. 3D-QSAR Molecular Modelling
3.7. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lv, Y.; Liu, H.L.; Wang, L.F.; Li, K.; Gao, W.; Liu, X.Y.; Tang, L.F.; Kalinina, T.A.; Glukhareva, T.V.; Fan, Z.J. Discovery of novel 3,4-dichloroisothiazole-containing coumarins as fungicidal leads. J. Agric. Food Chem. 2021, 69, 4253–4262. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, K.; Gao, W.; Hao, Z.; Wang, W.; Liu, X.; Tang, L.; Fan, Z. Design, synthesis and fungicidal activity of 3,4-dichloroisothiazolocoumarin-containing strobilurins. Mol. Divers. 2021, 26, 951–961. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.B.; Yan, L.L.; Chen, L.; Lu, Z.J.; Ge, C.Y.; Zhao, X.Y.; Wang, Z.W.; Lu, A.D.; Wang, Q.M. Marine sesquiterpenes for plant protection: Discovery of laurene sesquiterpenes and their derivatives as novel antiviral and antiphytopathogenic fungal agents. J. Agric. Food Chem. 2022, 70, 6006–6014. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Cao, A.D.; Huang, M.J.; Li, H.J. Effects of haze pollution on pesticide use by rice farmers: Fresh evidence from rural areas of China. Environ. Sci. Pollut. Res. 2021, 28, 62755–62770. [Google Scholar] [CrossRef] [PubMed]
- Bras, A.; Roy, A.; Heckel, D.G.; Anderson, P.; Karlsson Green, K. Pesticide resistance in arthropods: Ecology matters too. Ecol. Lett. 2022, 25, 1746–1759. [Google Scholar] [CrossRef]
- Luderwald, S.; Meyer, F.; Gerstle, V.; Friedrichs, L.; Rolfing, K.; Schreiner, V.C.; Bakanov, N.; Schulz, R.; Bundschuh, M. Reduction of pesticide toxicity under field-relevant conditions? The interaction of titanium dioxide nanoparticles, ultraviolet, and natural organic matter. Environ. Toxicol. Chem. 2020, 39, 2237–2246. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Cammareri, M.; Caretto, S.; Cavallaro, V.; Cominelli, E.; De Palma, M.; Docimo, T.; Giovinazzo, G.; Grandillo, S.; et al. Strategies to modulate specialized metabolism in mediterranean crops: From molecular aspects to field. Int. J. Mol. Sci. 2021, 22, 2887. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest. Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Kim, T.; Ha, M.W.; Kim, J. Recent advances in divergent synthetic strategies for indole-based natural product libraries. Molecules 2022, 27, 2171. [Google Scholar] [CrossRef]
- Ntungwe, E.; Dominguez-Martin, E.M.; Bangay, G.; Garcia, C.; Guerreiro, I.; Colombo, E.; Saraiva, L.; Diaz-Lanza, A.M.; Rosatella, A.; Alves, M.M.; et al. Self-assembly nanoparticles of natural bioactive abietane diterpenes. Int. J. Mol. Sci. 2021, 22, 10210. [Google Scholar] [CrossRef]
- Colone, M.; Calcabrini, A.; Stringaro, A. Drug delivery systems of natural products in oncology. Molecules 2020, 25, 4560. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.G.; Yun, S.; Park, J.R.; Jang, Y.H.; Farooq, M.; Yun, B.J.; Kim, K.M. Bio-efficacy of chrysoeriol7, a natural chemical and repellent, against brown planthopper in rice. Int. J. Mol. Sci. 2022, 23, 1540. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Hu, P.Y.; Yang, M.; Zhang, J.; Liu, Y.L.; Zhu, W.F.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Srivastava, S.; Bimal, D.; Bohra, K.; Singh, B.; Ponnan, P.; Jain, R.; Varma-Basil, M.; Maity, J.; Thirumal, M.; Prasad, A.K. Synthesis and antimycobacterial activity of 1-(beta-d-Ribofuranosyl)-4-coumarinyloxymethyl-/-coumarinyl-1,2,3-triazole. Eur. J. Med. Chem. 2018, 150, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Borges, F. Thiocoumarins: From the synthesis to the biological applications. Molecules 2022, 27, 4901. [Google Scholar] [CrossRef]
- Srivastava, P.; Vyas, V.K.; Variya, B.; Patel, P.; Qureshi, G.; Ghate, M. Synthesis, anti-inflammatory, analgesic, 5-lipoxygenase (5-LOX) inhibition activities, and molecular docking study of 7-substituted coumarin derivatives. Bioorg. Chem. 2016, 67, 130–138. [Google Scholar] [CrossRef]
- Kassem, A.F.; Batran, R.Z.; Abbas, E.M.H.; Elseginy, S.A.; Shaheen, M.N.F.; Elmahdy, E.M. New 4-phenylcoumarin derivatives as potent 3C protease inhibitors: Design, synthesis, anti-HAV effect and molecular modeling. Eur. J. Med. Chem. 2019, 168, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Sharapov, A.D.; Fatykhov, R.F.; Khalymbadzha, I.A.; Zyryanov, G.V.; Chupakhin, O.N.; Tsurkan, M.V. Plant coumarins with anti-HIV activity: Isolation and mechanisms of action. Int. J. Mol. Sci. 2022, 24, 2839. [Google Scholar] [CrossRef]
- Garcia, S.; Mercado-Sanchez, I.; Bahena, L.; Alcaraz, Y.; Garcia-Revilla, M.A.; Robles, J.; Santos-Martinez, N.; Ordaz-Rosado, D.; Garcia-Becerra, R.; Vazquez, M.A. Design of fluorescent coumarin-hydroxamic acid derivatives as inhibitors of hdacs: Synthesis, anti-proliferative evaluation and docking studies. Molecules 2020, 25, 5134. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.T.; Zeng, Y.Y.; Wu, G.J. A review on anti-tumor mechanisms of coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Liu, C.L.; Li, M.; Zhang, H.; Li, Z.N.; Li, Z.M. Design, synthesis and structure-activity relationship of novel coumarin derivatives. Pest. Manag. Sci. 2011, 67, 647–655. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhang, R.R.; Wang, J.Q.; Yu, X.; Zhang, Y.L.; Wang, Q.Q.; Zhang, W.H. Microwave-assisted synthesis and antifungal activity of novel fused Osthole derivatives. Eur. J. Med. Chem. 2016, 124, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Guan, A.Y.; Yang, J.D.; Chai, B.S.; Li, M.; Li, H.C.; Yang, J.C.; Xie, Y. Efficient approach to discover novel agrochemical candidates: Intermediate derivatization method. J. Agric. Food Chem. 2016, 64, 45–51. [Google Scholar] [CrossRef]
- Macías, F.A.; De Siqueira, J.M.; Chinchilla, N.; Marín, D.; Varela, R.M.; Molinillo, J.M.G. New herbicide models from benzoxazinones: Aromatic ring functionalization effects. J. Agric. Food. Chem. 2006, 54, 9843–9851. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Liu, C.L.; Yang, X.P.; Dekeyser, M. Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev. 2014, 114, 7079–7107. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Li, K.M.; Yi, Y.J.; Luo, X.F.; Qiu, L.J.; Zhang, L.J.; Wang, M.; Ye, J.; Ou, X.M.; Li, J.M.; et al. Synthesis, crystal structure, biological evaluation, docking study and DFT calculation of novel strobilurins containing oxime ether phenyl ring or dihydrobenzofuran moiety. J. Mol. Struct. 2023, 1286, 135636–135647. [Google Scholar] [CrossRef]
- Clarkson, G.J.; Roesner, S. Synthesis of benzofuropyridines and dibenzofurans by a metalation/negishi cross-coupling/SNAr reaction sequence. J. Org. Chem. 2023, 88, 684–689. [Google Scholar] [CrossRef]
- Wang, J.G.; Tan, H.Z.; Li, Y.H.; Ma, Y.; Li, Z.M.; Guddat, L.W. Chemical synthesis, in vitro acetohydroxyacid synthase (AHAS) inhibition, herbicidal activity, and computational studies of isatin derivatives. J. Agric. Food Chem. 2011, 59, 9892–9900. [Google Scholar] [CrossRef] [PubMed]


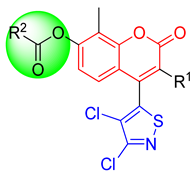
| Compd. | R1 | R2 | Compd. | R1 | R2 | Compd. | R1 | R2 | Compd. | R1 | R2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2aa | Cl |  | 2al | Cl |  | 2ba | H |  | 2bl | H | 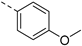 |
| 2ab | Cl | 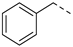 | 2am | Cl | 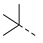 | 2bb | H | 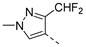 | 2bm | H | 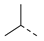 |
| 2ac | Cl | 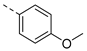 | 2an | Cl | 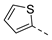 | 2bc | H | 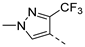 | 2bn | H | 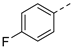 |
| 2ad | Cl | 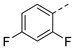 | 2ao | Cl |  | 2bd | H | 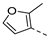 | 2bo | H | 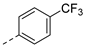 |
| 2ae | Cl | 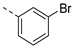 | 2ap | Cl |  | 2be | H | 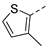 | 2bp | H | 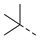 |
| 2af | Cl | 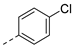 | 2aq | Cl | 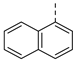 | 2bf | H |  | 2bq | H |  |
| 2ag | Cl | 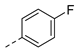 | 2ar | Cl |  | 2bg | H | 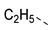 | 2br | H | 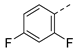 |
| 2ah | Cl |  | 2as | Cl | 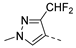 | 2bh | H | 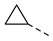 | 2bs | H | 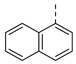 |
| 2ai | Cl | 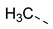 | 2at | Cl | 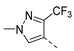 | 2bi | H | 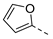 | 2bt | H |  |
| 2aj | Cl | 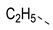 | 2au | Cl |  | 2bj | H |  | 2bu | H | 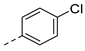 |
| 2ak | Cl | 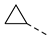 | 2av | Cl | 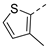 | 2bk | H | 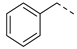 | 2bv | H |  |
| Compd. | A. s | B. c | C. a | F. g | P. p | R. s | S. s | Compd. | A. s | B. c | C. a | F. g | P. p | R. s | S. s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2aa | 4 ± 2 | 8 ± 1 | 0 | 0 | 39 ± 3 | 0 | 7 ± 2 | 2bc | 7 ± 1 | 0 | 14 ± 3 | 0 | 9 ± 2 | 0 | 27 ± 2 |
| 2ab | 12 ± 2 | 16 ± 1 | 4 ± 2 | 6 ± 2 | 18 ± 3 | 0 | 16 ± 2 | 2bd | 7 ± 1 | 7 ± 2 | 13 ± 2 | 0 | 23 ± 2 | 0 | 11 ± 0 |
| 2ac | 6 ± 1 | 4 ± 1 | 0 | 0 | 10 ± 3 | 0 | 6 ± 2 | 2be | 4 ± 1 | 7 ± 3 | 0 | 0 | 10 ± 0 | 0 | 12 ± 2 |
| 2ad | 7 ± 1 | 5 ± 3 | 7 ± 0 | 0 | 62 ± 3 | 0 | 6 ± 2 | 2bf | 7 ± 1 | 8 ± 2 | 5 ± 2 | 0 | 31 ± 2 | 0 | 4 ± 2 |
| 2ae | 6 ± 1 | 0 | 7 ± 3 | 0 | 3 ± 0 | 0 | 4 ± 2 | 2bg | 63 ± 3 | 69 ± 2 | 50 ± 2 | 23 ± 1 | 56 ± 0 | 29 ± 1 | 63 ± 2 |
| 2af | 3 ± 1 | 0 | 4 ± 2 | 0 | 10 ± 0 | 0 | 12 ± 1 | 2bh | 42 ± 3 | 44 ± 2 | 35 ± 3 | 19 ± 3 | 41 ± 3 | 25 ± 3 | 45 ± 3 |
| 2ag | 4 ± 1 | 0 | 9 ± 2 | 0 | 0 | 0 | 11 ± 1 | 2bi | 4 ± 1 | 0 | 0 | 0 | 14 ± 2 | 0 | 5 ± 0 |
| 2ah | 5 ± 2 | 4 ± 2 | 0 | 0 | 4 ± 0 | 0 | 8 ± 1 | 2bj | 0 | 16 ± 2 | 10 ± 3 | 9 ± 3 | 23 ± 2 | 0 | 10 ± 2 |
| 2ai | 77 ± 2 | 76 ± 0 | 86 ± 2 | 20 ± 2 | 69 ± 2 | 46 ± 3 | 76 ± 0 | 2bk | 0 | 15 ± 2 | 13 ± 2 | 9 ± 1 | 24 ± 0 | 0 | 14 ± 3 |
| 2aj | 71 ± 1 | 68 ± 0 | 76 ± 2 | 26 ± 1 | 78 ± 2 | 35 ± 3 | 63 ± 2 | 2bl | 0 | 0 | 11 ± 2 | 0 | 19 ± 2 | 0 | 0 |
| 2ak | 38 ± 2 | 30 ± 0 | 22 ± 2 | 0 | 29 ± 2 | 0 | 24 ± 2 | 2bm | 26 ± 3 | 49 ± 2 | 26 ± 2 | 18 ± 2 | 63 ± 2 | 10 ± 2 | 52 ± 3 |
| 2al | 23 ± 2 | 26 ± 1 | 17 ± 0 | 15 ± 2 | 77 ± 0 | 24 ± 3 | 27 ± 3 | 2bn | 0 | 10 ± 2 | 9 ± 2 | 6 ± 2 | 28 ± 3 | 0 | 10 ± 2 |
| 2am | 0 | 8 ± 1 | 11 ± 2 | 8 ± 2 | 54 ± 3 | 0 | 14 ± 0 | 2bo | 0 | 5 ± 1 | 0 | 0 | 10 ± 0 | 0 | 4 ± 2 |
| 2an | 0 | 7 ± 3 | 13 ± 2 | 11 ± 3 | 10 ± 3 | 0 | 11 ± 2 | 2bp | 6 ± 2 | 13 ± 1 | 14 ± 0 | 14 ± 0 | 35 ± 2 | 0 | 8 ± 3 |
| 2ao | 44 ± 2 | 43 ± 1 | 48 ± 2 | 23 ± 2 | 68 ± 0 | 0 | 35 ± 3 | 2bq | 0 | 0 | 5 ± 2 | 0 | 8 ± 2 | 0 | 4 ± 2 |
| 2ap | 0 | 0 | 0 | 0 | 24 ± 3 | 0 | 0 | 2br | 0 | 0 | 0 | 0 | 8 ± 2 | 0 | 4 ± 2 |
| 2aq | 0 | 0 | 0 | 0 | 16 ± 2 | 0 | 9 ± 2 | 2bs | 0 | 4 ± 1 | 0 | 0 | 18 ± 3 | 0 | 6 ± 2 |
| 2ar | 66 ± 1 | 70 ± 0 | 77 ± 2 | 28 ± 2 | 77 ± 2 | 76 ± 1 | 62 ± 1 | 2bt | 8 ± 3 | 8 ± 1 | 5 ± 2 | 0 | 12 ± 2 | 0 | 8 ± 3 |
| 2as | 25 ± 3 | 23 ± 1 | 19 ± 2 | 0 | 23 ± 2 | 0 | 28 ± 2 | 2bu | 0 | 10 ± 2 | 6 ± 0 | 0 | 5 ± 0 | 0 | 11 ± 0 |
| 2at | 17 ± 2 | 17 ± 1 | 4 ± 2 | 12 ± 3 | 23 ± 3 | 0 | 29 ± 3 | 2bv | 30 ± 2 | 17 ± 0 | 32 ± 3 | 16 ± 1 | 53 ± 0 | 18 ± 3 | 17 ± 0 |
| 2au | 17 ± 0 | 12 ± 1 | 15 ± 2 | 10 ± 3 | 24 ± 0 | 0 | 18 ± 2 | 1a * | 70 ± 2 | 85 ± 2 | 84 ± 3 | 77 ± 1 | 93 ± 2 | 98 ± 1 | 78 ± 2 |
| 2av | 0 | 0 | 5 ± 2 | 0 | 17 ± 3 | 0 | 14 ± 3 | 1b * | 72 ± 3 | 83 ± 1 | 79 ± 3 | 80 ± 1 | 91 ± 2 | 83 ± 2 | 65 ± 1 |
| 2ba | 5 ± 2 | 8 ± 3 | 4 ± 2 | 8 ± 2 | 17 ± 2 | 0 | 6 ± 2 | osthole * | 14 ± 1 | 58 ± 1 | 10 ± 1 | 17 ± 1 | 15 ± 1 | 70 ± 2 | 33 ± 1 |
| 2bb | 7 ± 1 | 16 ± 2 | 15 ± 2 | 6 ± 0 | 18 ± 0 | 0 | 12 ± 2 | isotianil * | 25 ± 2 | 9 ± 2 | 20 ± 1 | 22 ± 1 | 24 ± 1 | 45 ± 1 | 18 ± 2 |
| Compd. | Fungi | Regression Equation | R2 | EC50 (μg/mL) | 95% Confidence Interval |
|---|---|---|---|---|---|
| 2ai | A. s. | y = 4.6354 + 0.7213x | 0.9671 | 3.20 | 2.03~5.06 |
| B. c. | y = 4.7569 + 0.5255x | 0.9738 | 2.90 | 1.90~4.43 | |
| C. a. | y = 4.4115 + 1.0116x | 0.9528 | 3.82 | 2.49~5.85 | |
| P. p. | y = 4.6117 + 0.6906x | 0.9799 | 3.65 | 2.77~4.81 | |
| S. s. | y = 4.2996 + 0.9405x | 0.9704 | 5.56 | 4.15~7.44 | |
| 2aj | A. s. | y = 3.7955 + 0.9777x | 0.9643 | 17.06 | 13.42~21.68 |
| B. c. | y = 3.4733 + 1.1522x | 0.9555 | 21.14 | 14.13~31.63 | |
| C. a. | y = 4.2600 + 1.0479x | 0.9752 | 5.08 | 4.00~6.47 | |
| P. p. | y = 4.1607 + 0.7510x | 0.9502 | 13.11 | 8.86~19.39 | |
| S. s. | y = 4.5100 + 0.7458x | 0.9773 | 4.54 | 3.75~5.49 | |
| 2ar | A. s. | y = 3.6741 + 1.2509x | 0.9886 | 11.48 | 9.54~13.82 |
| B. c. | y = 3.3040 + 1.5643x | 0.9530 | 12.14 | 9.29~15.86 | |
| C. a. | y = 3.9396 + 1.3025x | 0.9580 | 6.52 | 5.07~8.38 | |
| P. p. | y = 3.6776 + 1.2105x | 0.9635 | 12.37 | 9.76~15.68 | |
| R. s. | y = 4.3684 + 1.1211x | 0.9641 | 3.66 | 2.78~4.82 | |
| S. s. | y = 3.8039 + 1.1299x | 0.9740 | 11.44 | 9.42~13.90 | |
| 2bg | A. s. | y = 4.2795 + 0.6054x | 0.9624 | 15.49 | 12.15~19.76 |
| B. c. | y = 4.2014 + 0.7378x | 0.9866 | 12.09 | 10.50~13.92 | |
| S. s. | y = 4.6013 + 0.5269x | 0.9827 | 5.71 | 4.75~6.87 | |
| 1a * | A. s. | y = 4.2794 + 0.9061x | 0.9696 | 6.24 | 4.79~8.12 |
| B. c. | y = 4.7981 + 0.7129x | 0.9716 | 1.92 | 1.39~2.65 | |
| C. a. | y = 4.2191 + 1.0934x | 0.9676 | 5.18 | 3.68~6.95 | |
| P. p. | y = 4.2839 + 0.7369x | 0.9691 | 9.37 | 5.85~15.03 | |
| R. s. | y = 4.9015 + 0.8031x | 0.9718 | 1.33 | 0.93~1.90 | |
| S. s. | y = 4.4350 + 1.0010x | 0.9586 | 3.67 | 2.72~4.95 | |
| 1b * | A. s. | y = 3.3158 + 1.7602x | 0.9841 | 9.05 | 7.78~10.53 |
| B. c. | y = 4.2055 + 1.0298x | 0.9734 | 5.90 | 4.82~7.24 | |
| C. a. | y = 3.5992 + 1.5235x | 0.9555 | 8.31 | 6.46~10.68 | |
| P. p. | y = 3.9657 + 1.3588x | 0.9018 | 5.77 | 3.15~10.58 | |
| R. s. | y = 3.9626 + 1.3553x | 0.9932 | 5.83 | 5.31~6.39 | |
| S. s. | y = 3.8143 + 1.1971x | 0.9499 | 9.78 | 7.00~13.67 | |
| Osthole * | B. c. | y = 3.5371 + 1.6848x | 0.9899 | 7.38 | 6.50~8.39 |
| P. p. | y = 0.5260 + 2.3891x | 0.9727 | 74.59 | 68.27~85.63 | |
| R. s. | y = 3.7903 + 1.3147x | 0.9948 | 8.32 | 7.59~9.12 | |
| S. s. | y = 2.8079 + 1.3590x | 0.9264 | 41.03 | 27.86~64.46 |
| Compd. | 200 μg/mL | 100 μg/mL | 50 μg/mL 2 L | 25 μg/mL 10 μg/mL |
|---|---|---|---|---|
| 2ai | 100 | 100 | 30 40 | 0 nd |
| 2aj | 100 | 100 | 0 75 | 0 nd |
| 2ar | 100 | 100 | 80 0 | 75 nd |
| 2bg | 100 | 100 | 88 20 | 65 nd |
| 1a ** | 100 | 100 | 88 nd | 65 nd |
| 1b ** | 100 | 100 | 75 100 | 20 100 |
| Osthole | 100 | 0 | // 100 | // nd |
| Cyazofamid | 100 | 100 | 100 | 100 |
| Statistical Parameter | CoMFA |
|---|---|
| q2 | 0.797 |
| r2 | 0.979 |
| ONC | 5 |
| SEE | 0.096 |
| F | 166.514 |
| Steric | 0.754 |
| Electrostatic | 0.246 |
| Compd. | EHOMU (Hartree) | ELUMU (Hartree) | ΔE (Hartree/eV) |
|---|---|---|---|
| 2ai | −0.24774 | −0.09462 | 0.15312/4.17 |
| 2aq | −0.23359 | −0.09296 | 0.14063/3.83 |
| 1a | −0.23646 | −0.08930 | 0.14716/4.00 |
| Compd. | Inhibition (%) | D | CoMFA | |
|---|---|---|---|---|
| Pred. * | Res. ** | |||
| 2ac | 10 | 1.74 | 1.70 | 0.04 |
| 2ae | 3 | 1.22 | 1.22 | 0.00 |
| 2af | 10 | 1.74 | 1.70 | 0.04 |
| 2ah | 4 | 1.35 | 1.45 | −0.10 |
| 2ai | 69 | 2.95 | 2.99 | −0.03 |
| 2aj | 78 | 3.17 | 3.20 | −0.03 |
| 2al | 77 | 3.16 | 3.07 | 0.09 |
| 2am | 54 | 2.72 | 2.70 | 0.02 |
| 2ar | 77 | 3.14 | 2.98 | 0.16 |
| 2as | 23 | 2.19 | 2.24 | −0.05 |
| 2at | 23 | 2.21 | 2.20 | 0.01 |
| 2au | 24 | 2.17 | 2.13 | 0.04 |
| 2av | 17 | 2.00 | 1.95 | 0.05 |
| 2ba | 17 | 1.98 | 1.97 | 0.01 |
| 2bc | 9 | 1.70 | 1.71 | −0.01 |
| 2bd | 23 | 2.11 | 2.15 | −0.04 |
| 2be | 10 | 1.70 | 1.74 | −0.04 |
| 2bi | 14 | 1.84 | 1.77 | 0.07 |
| 2bm | 63 | 2.83 | 2.69 | 0.14 |
| 2bo | 10 | 1.74 | 1.69 | 0.05 |
| 2bp | 35 | 2.35 | 2.54 | −0.19 |
| 2bq | 8 | 1.65 | 1.64 | 0.01 |
| 2br | 8 | 1.61 | 1.62 | −0.01 |
| 2bv | 53 | 2.63 | 2.84 | −0.21 |
| 2an *** | 10 | 1.71 | 1.91 | −0.20 |
| 2bg *** | 56 | 2.68 | 2.85 | −0.17 |
| 2bh *** | 41 | 2.43 | 2.89 | −0.46 |
| 2bs *** | 18 | 2.02 | 1.72 | 0.30 |
| 2bu *** | 5 | 1.38 | 1.55 | −0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhang, Y.; Hong, Z.; Yu, Z.; Liu, X.; Duan, Z.; Gao, W.; Tang, L.; Lv, Y.; Fan, Z. Design, Synthesis and Fungicidal Activity of Ester Derivatives of 4-(3,4-Dichloroisothiazole) 7-Hydroxy Coumarin. Molecules 2023, 28, 5205. https://doi.org/10.3390/molecules28135205
Li K, Zhang Y, Hong Z, Yu Z, Liu X, Duan Z, Gao W, Tang L, Lv Y, Fan Z. Design, Synthesis and Fungicidal Activity of Ester Derivatives of 4-(3,4-Dichloroisothiazole) 7-Hydroxy Coumarin. Molecules. 2023; 28(13):5205. https://doi.org/10.3390/molecules28135205
Chicago/Turabian StyleLi, Kun, Yue Zhang, Zeyu Hong, Zhenwu Yu, Xiaoyu Liu, Zhihong Duan, Wei Gao, Liangfu Tang, You Lv, and Zhijin Fan. 2023. "Design, Synthesis and Fungicidal Activity of Ester Derivatives of 4-(3,4-Dichloroisothiazole) 7-Hydroxy Coumarin" Molecules 28, no. 13: 5205. https://doi.org/10.3390/molecules28135205
APA StyleLi, K., Zhang, Y., Hong, Z., Yu, Z., Liu, X., Duan, Z., Gao, W., Tang, L., Lv, Y., & Fan, Z. (2023). Design, Synthesis and Fungicidal Activity of Ester Derivatives of 4-(3,4-Dichloroisothiazole) 7-Hydroxy Coumarin. Molecules, 28(13), 5205. https://doi.org/10.3390/molecules28135205






