A Compiled Update on Nutrition, Phytochemicals, Processing Effects, Analytical Testing and Health Effects of Chenopodium album: A Non-Conventional Edible Plant (NCEP)
Abstract
1. Introduction
2. Characteristics and Cultivation of C. album
2.1. Botanical Description and Vernacular Names
2.2. Cultivation Information
3. Ethnobotanical and Ethnomedicinal Use of C. album
4. Nutritional and Phytochemical Profile of C. album
4.1. Vitamins and Minerals
4.2. Carbohydrates
4.3. Protein and Amino Acids
4.4. Fatty Acids
4.5. Phytochemicals
5. Extraction, Isolation, and Analysis of Bioactive Phytochemicals from C. album
| Plant Part | Sample Processing | Extraction Technique | Solvents Employed in Extraction | Reported Phytochemicals | Analytical Method/Technique (s) Employed for Detetction | Reference |
|---|---|---|---|---|---|---|
| Aerial parts | Shade drying, pulverizing, defatting | Soxhlet extraction, cold maceration | Soxhlation: Ethyl acetate, acetone, and methanol Maceration: 50% Methanol | Flavonoid (7.335 mg/g) (Quercetin) | UV–visible spectroscopy (UV–Vis), Infrared spectroscopy (IR), Nuclear magnetic resonance spectroscopy (NMR), and Mass spectrometry (MS) Aluminium chloride method (for flavonoid) | [86] |
| Washing, drying, grinding | Cold maceration | Maceration: Acetone | Xanthophylls (331 mg/100 g dry wt): neoxanthin, violaxanthin, lutein (11.7 to 185 mg/100 g dry wt), zeaxanthin, Provitamin A: 120 mg/100 g dry wt) | High-performance liquid chromatography—Photodiode Array (HPLC-PDA), LC-MS | [72] | |
| Size reduction | Cold maceration, centrifugation | Maceration: Acetone | Apocarotenoids, chenoalbicin (0.02% yield) | HPLC, UV–Vis, Column chromatography (CC), NMR, High-resolution Electrospray Ionization Mass Spectrometry (HREIMS) | [89] | |
| Crushing, defatting | Solvent extraction | Acetone, water, petroleum ether | β-carotene (0.19–5.91 mg/100 g fresh wt) | Column Chromatography | [90] | |
| Washing, drying, grinding, airtight storage | Solvent extraction | 0.05 M Phosphate buffer, 80% aqueous methanol | Total phenols (304.98 GAE) Saponin (0.027–0.867 g/100 g) Phytic acid (268.33 mg/100 g) Alkaloid (1.27–1.67 mg/100 g) Flavonoid (220.0–406.67 mg/100 g) Oxalate (518.45 mg/100 g) | Folin–Ciocalteu reaction (Phenols), Prothrombin time (saponins), colorimetric method (phytate), UV and MS (flavonoids) Chromatography (alkaloids) | [80] | |
| Washing, shade drying, grinding, airtight storage | Hydro-distillation | Water | Essential oil (0.466% v/w) (mainly containing α-pinene, β-pinene, linalool, α-terpineol, ascaridole, carvacrol, phytane, linolenic acid, diosgenin) | Gas chromatography (GC)-MS | [22] | |
| Pulverization | Hydro-distillation | Water | Essential oil (0.64% v/w) [α-thujene, α-pinene (7%), ascaridole (15.5%), myrcene, sabinene, p-cymene 40.9%, limonene, camphene, carvacrol, elemicin, neral, citronellal, borneol, γ-terpineneol (6.2%)] | Gas chromatography—Flame ionization detector (GC-FID) and GC-MS | [18] | |
| Drying, coarse powdering | Maceration | Methanol, chloroform, n-hexane, petroleum ether, acetone | Alkaloids, amino acids, cardiac glycosides, anthraquinone, flavonoids, steroids, starch | UV–Vis | [17] | |
| Air drying, pulverization | Soxhlation Maceration | Soxhlation: Chloroform, acetone, ethyl acetate, methanol Maceration: 50% methanol | Alkaloids, carbohydrates, amino acids, flavonoids, saponins, tannin, sterol, terpenoids | Thin-layer chromatography (TLC) | [14] | |
| Shade drying, grinding | Soxhlation | Hexane, ethyl acetate Soxhlation: ethanol | Astragalin (50.75% of total extract) | CC, TLC, High-Performance (HP)-TLC, HPLC, UV, Fourier Transform (FT)-IR, NMR | [91] | |
| Shade drying, grinding | Solvent extraction | Ethanol, water | Carbohydrates, protein, alkaloid, tannin, saponin, and flavonoid | HPTLC, Fluorescence spectroscopy | [92] | |
| Cleaning | Microwave-assisted extraction | Petroleum ether, ethyl acetate, methanol, hydroalcoholic, and aqueous solvent | Alkaloids (1.77 to 2.80 mg/g equivalent of atropine), flavonoids (1.72 to 3.81 mg/g equivalent of quercetin), saponins (3.05 to 3.22 mg/g equivalent of diosgenin), total phenols (1.77 to 2.94 mg/g equivalent of gallic acid) | UV, Folin–Ciocalteu reaction (Phenols), Aluminium chloride method (for flavonoid), Vanillin reagent method (saponins), Bromocresol green reaction method (alkaloids) | [93] | |
| Aerial parts and roots | Air drying, grinding | Maceration | n-hexane, acetone, methanol | Total phenolics 64.37 µg PEs/mg of the extract (protocatechuic acid, rutin, hesperidin (9769.13 ± 158.26 μg/g extract), rutin (2935.19 ± 39.92 μg/g extract), apigetrin, quercetin, astragalin, apigenin, and luteolin), Flavonoids (126.67 µg QEs/mg of extract) Fatty acids (mainly with myristic acid 18.26% and cis-10-pentadecanoic acid 15.93%) | Folin–Ciocalteu reaction (Phenols), UV–Vis (flavonoids), LC-MS/MS, GC-MS | [88] |
| Seeds/Grains | Powdering | Solvent extraction | Water | Oleanolic acid, glucose, glucuronic acid, | Ion exchange (IEX) CC, TLC, NMR | [94] |
| Cleaned, dried, milled | Solvent extraction | Sodium hydroxide, water | Carbohydrates, protein, fiber, fat | Colorimetry | [95] | |
| Air drying, grinding | Soxhlation Infusion | Soxhlation: Benzine Infusion: Chloroform, methanol | Lipids (5.8 to 8.9%) (neutral, glycolipids, phospholipids, fatty acids), carotenoids (6.61 mg/100 g) Fatty acids (oleic acid 37.9%, linoleic acid, 26.1%, palmitic acid 17.4%, lignoceric acid 1.1%) | CC, TLC, GC, FTIR | [96] |
6. Impact of Processing Methods on Bioactive Composition and Stability of C. album
6.1. Cooking and Thermal Effects
6.2. Blanching and Drying
6.3. Dehydration
6.4. Ultrasound Treatment
| S. No. | Processing/Storage Method | Time Period | Impact on Nutrient and Mineral Content (and Method Employed for Analysis) | Reference(s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic Acid | β Carotene | Oxalic Acid/ | Phytic Acid | Polyphenols/TPC | Dietary Fiber | PUFA | Calcium | Iron | Zinc | ||||
| 1. | Refrigeration without packaging at 5 °C | 24 h | Decreased by 4.40% (T) | No considerable loss (CM/SM) | Decreased by 3.76% (T) | Decreased by 0.22% (CM/SM) | Decreased by 2.80% (CM/SM) | - | - | - | [111,112] | ||
| 48 h | Decreased by 7.06% (T) | Decreased by 1.75% (CM/SM) | Decreased by 3.76% (T) | Decreased by 0.22% (CM/SM) | Decreased by 4.26% (CM/SM) | - | - | - | [111,112] | ||||
| 2. | Refrigerated in polyethene bags at 5 °C | 24 h | Decreased by 2.03% (T) | No considerable loss (CM/SM) | Decreased by 0.88% (T) | Decreased by 0.22% (CM/SM) | Decreased by 1.19% (CM/SM) | - | - | - | [111,112] | ||
| 48 h | Decreased by 5.65% (T) | Decreased by 0.77% (CM/SM) | Decreased by 4.02% (T) | No considerable loss (CM/SM) | Decreased by 2.28% (CM/SM) | - | - | - | [111,112] | ||||
| 3. | Stored in polyethene bags at 30 °C | 24 h | Decreased by 45.76% (T) | Decreased by 1.87% (CM/SM) | Decreased by 0.97% (T) | Decreased by 0.08% (CM/SM) | Decreased by 2.61% (CM/SM) | - | - | - | [111,112] | ||
| 48 h | Decreased by 66.90% (T) | Decreased by 2.84% (CM/SM) | Decreased by 3.76% (T) | No considerable loss (CM/SM) | Decreased by 3.55% (CM/SM) | - | - | - | [111,112] | ||||
| 4. | Sun Drying | 10 h | Decreased by 88.25% (T) | Decreased by 48.50% (CM/SM) | - * | - * | Decreased by 0.43% (CM/SM) | - | - | - | [111,112] | ||
| Till 6–7% moisture content | Decreased by 29.73% (DRM) | Increased by 758.38% (CC) | Decreased by 29.02% (T) | Decreased by 42.61% (CM/SM) | Decreased by 16.13% | Increased to 459.29% (AAS) | Increased to 536.86% (AAS) | Increased to 322% (AAS) | [113] | ||||
| 5. | Oven drying at 60 to 65 °C | 10 to 12 hr | Decreased by 87.40% (T) | Decreased by 16.03% (CM/SM) | No considerable loss (T) | No considerable loss (CM/SM) | - * | [111,112] | |||||
| Till 6–7% moisture content | Decreased by 43.24% (DRM) | Increased by 842.55% (CC) | Decreased by 47.50% (T) | Decreased by 53.54% (CM/SM) | Decreased by 28.31% | Increased to 523.15% (AAS) | Increased to 639.27% (AAS) | Increased to 368% (AAS) | [113] | ||||
| 6. | Shade drying | Till 6–7% moisture content | Decreased by 8.10% (DRM) | Increased to 822.94% (CC) | Decreased by 19.07% (T) | Decreased by 28.508% (CM/SM) | Decreased by 35.38% | Increased to 484.91% (AAS) | Increased to 589.15% (AAS) | Increased to 396% (AAS) | [113] | ||
| 7. | Solar drying | Till 6–7% moisture content | Decreased by 13.51% (DRM) | Increased by 682.61% (CC) | Decreased by 22.33% (T) | Decreased by 44.69% (CM/SM) | Decreased by 22.54% | Increased to 385.26% (AAS) | Increased to 522.16% (AAS) | Increased to 298% (AAS) | [113] | ||
| 8. | Blanching | 5 min | Decreased by 56.20% (T) | Decreased by 9.82% (CM/SM) | Decreased by 27.69% (T) | Decreased by 1.36% (CM/SM) | Decreased by 3.60% (CM/SM) | - | - | - | [111,112] | ||
| 10 min | Decreased by 71.38% (T) | Decreased by 20.17% (CM/SM) | Decreased by 21.97% (T) | Decreased by 1.89% (CM/SM) | Decreased by 14.63% (CM/SM) | - | - | - | [111,112] | ||||
| 15 min | Decreased by 95.06% (T) | Decreased by 28.49% (CM/SM) | Decreased by 35.38% (T) | Decreased by 2.16% (CM/SM) | Decreased by 23.21% (CM/SM) | - | - | - | [111,112] | ||||
| 9. | Open-pan cooking | 30 min | Decreased by 96.31% (T) | Decreased by 2.48% (CM/SM) | Decreased by 22.71% (T) | Decreased by 0.06% (CM/SM) | Decreased by 1.55% (CM/SM) | - | - | - | [111,112] | ||
| 10 | Pressure cooking | 10 min | Decreased by 89.58% (T) | Decreased by 1.34% (CM/SM) | Decreased by 26.03% (T) | Decreased by 0.08% (CM/SM) | Decreased by 0.76% (CM/SM) | - | - | - | [111,112] | ||
| 10 min | - | Decreased by 19.44% (CC) | - | - | - | Decreased by 0.81 to 9.43% (AAS) | Decreased by 28.79 to 36.34% (AAS) | Decreased by 5.47 to 5.63% (AAS) | [90] | ||||
| 11. | Stir frying | 15 min | - | Decreased by 10.2% (CC) | - | - | - | Decreased by 2.68 to 8.45% (AAS) | Decreased by 13.08 to 13.88% (AAS) | Decreased by 4.93 to 7.81% (AAS) | [90] | ||
| 12. | Germination followed by milling to flour | - | - | - | - | - | Increased to 234.43% (CM/SM) | - | - | - | [114] | ||
| 13. | Germination followed by milling to flour | Increased to 108.76% (DM) | Increased by 1.24% (GC-FID) | Decreased by 15.54% (AAS) | Decreased by 64.7% (AAS) | Increased to 100.82% (AAS) | [25] | ||||||
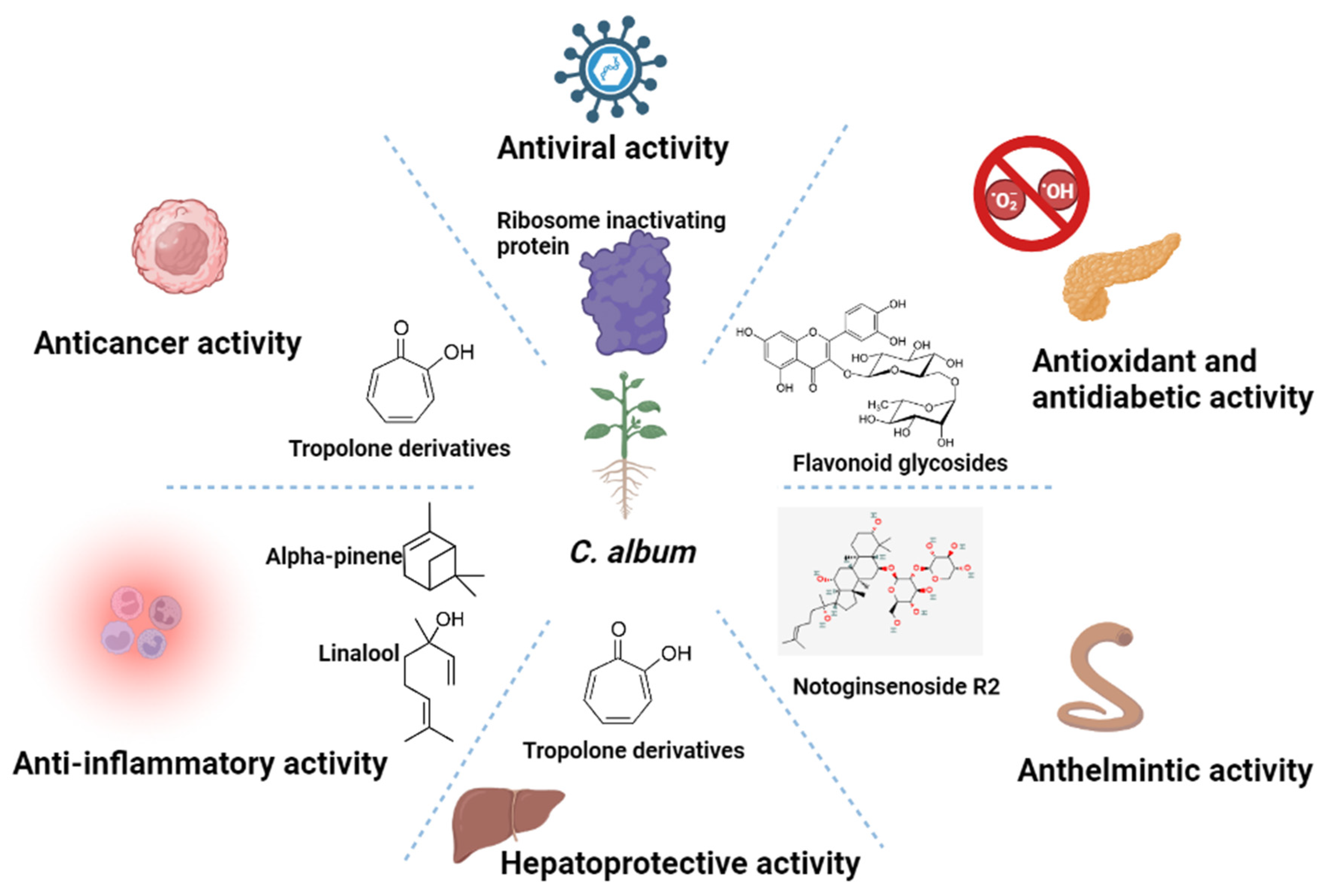
7. Functional Activities of C. album
7.1. Antimicrobial Activities
7.1.1. Antibacterial Activity
7.1.2. Antifungal Activity
7.2. Anthelmintic Activity
7.3. Hepatoprotective Activity
7.4. Antioxidant Activity
| S. No | Antioxidant Assay Method | Plant Part | Type of Extract | Findings | Reference |
|---|---|---|---|---|---|
| 1. | DPPH assay | Whole-plant powder | Methanolic (MT) and aqueous | At 300 µg/mL Aqueous extract inhibited 96%; MT inhibited 73% | [117] |
| Seed | Chloroform (CF), ethyl acetate (EA), acetone (AT) and MT extracts | At 200 µg/mL MT inhibited 87.83%; AT—84.55% EA—86.41% CF—80.44% | [152] | ||
| Aerial parts | Hexane (HE), EA, CF extracts | IC50 (µg/mL): Hexane—>1000, EA—140, CF—435 | [154] | ||
| Seed | MT extracts | At 0.1 mg/mL, MT inhibited 74% | [155] | ||
| 2. | Superoxide anion radical scavenging activity
| Whole-plant powder | MT and aqueous | At 300 µg/mL, aqueous extract inhibited 74% while MT inhibited 85% | [117] |
| Seed | Petroleum Ether (PE), CF, EA, AT, and MT extracts | At 200 µg/mL, MT inhibited 66.79%, AT—60.35%, EA—68.29%, CF—64.04% | [152] | ||
| 3. | Hydroxyl scavenging activity- deoxyribose assay | Whole-plant powder | MT and aqueous | At 300 µg/mL, Aqueous extract inhibited 83% while MT inhibited 94% | [117] |
| 4. | Modified thiobarbituric acid reactive species assay | Whole-plant powder | MT and aqueous | At 300 µg/mL, Aqueous extract inhibited 86% while MT inhibited 78% | [117] |
| 5. | H2O2 scavenging assay | Seed | PE, CF, EA, AT, and MT extracts | At 200 µg/mL, MT inhibited 87.67%, AT—75.85%, EA—78.86%, CF—85.57% | [152] |
| 6. | ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging assay | Seed | PE, CF, EA, AT, and MT extracts | At 200 µg/mL, MT inhibited 85.70%, AT—84.77%, EA—87.17%, CF—88.22% | [152] |
| 7. | β-Carotene bleaching test | Aerial parts | Hexane, EA, CF extracts | IC50 (µg/mL) at 60 min: Hexane—>100, EA—38.03, CF—>100 | [154] |
7.5. Anticancer Activity
7.6. Other Activities
8. Safety and Toxicological Aspects of C. album
9. Potential Food Applications
10. Discussion/Cross-Talk
11. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Dey, A.; De, J.N. Traditional use of medicinal plants as febrifuge by the tribals of Purulia district, West Bengal, India. Asian Pac. J. Trop. Dis. 2012, 2, S800–S803. [Google Scholar] [CrossRef]
- Rana, D.; Bhatt, A.; Lal, B.; Parkash, O.; Kumar, A.; Uniyal, S.K. Use of medicinal plants for treating different ailments by the indigenous people of Churah subdivision of district Chamba, Himachal Pradesh, India. Environ. Dev. Sustain. 2021, 23, 1162–1241. [Google Scholar] [CrossRef]
- Anywar, G. Historical Use of Toxic Plants. In Poisonous Plants and Phytochemicals in Drug Discovery; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 1–17. [Google Scholar]
- Nedelcheva, A.; Dogan, Y.; Obratov-Petkovic, D.; Padure, I.M. The traditional use of plants for handicrafts in southeastern Europe. Hum. Ecol. 2011, 39, 813–828. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Leal, M.L.; Alves, R.P.; Hanazaki, N. Knowledge, use, and disuse of unconventional food plants. J. Ethnobiol. Ethnomedicine 2018, 14, 6. [Google Scholar] [CrossRef]
- Bracale, M.F.; Nóbrega, T.F.; Barreto, R.W. Fungal diseases of non-conventional food plants: First report of Stagonosporopsis caricae causing leaf spots on Vasconcellea monoica. Australas. Plant Dis. Notes 2020, 15, 20. [Google Scholar] [CrossRef]
- Stokes, P.; Rowley-Conwy, P. Iron Age cultigen? Experimental return rates for fat hen (Chenopodium album L.). Environ. Archaeol. 2002, 7, 95–99. [Google Scholar] [CrossRef]
- Khare, C. Indian Medicinal Plants: An Illustrated Dictionary; A Springer Live Reference; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ghosh, A.; Pandey, B.; Agrawal, M.; Agrawal, S.B. Interactive effects and competitive shift between Triticum aestivum L.(wheat) and Chenopodium album L.(fat-hen) under ambient and elevated ozone. Environ. Pollut. 2020, 265, 114764. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, D. Bioactive constituents, phytochemical and pharmacological properties of Chenopodium album: A miracle weed. Int. J. Pharm. 2014, 1, 545–552. [Google Scholar]
- Savage, G.; Vanhanen, L. Oxalate Contents of Raw, Boiled, Wok-Fried and Pesto and Juice Made from Fat Hen (Chenopodium album) Leaves. Foods 2019, 8, 2. [Google Scholar] [CrossRef]
- Joshi, B.K.; Shrestha, R.; Gautam, I.; Poudel, A.; Gotame, T. Neglected and Underutilized Species (NUS), and Future Smart Food (FSF) in Nepal; Taylor & Francis Group: Abingdon, UK, 2019. [Google Scholar]
- Arora, S.K.; Itankar, P.R.; Yende, S.R. Phytochemical screening and TLC studies of different extracts of Chenopodium album. J. Ayurvedic Herb. Med. 2020, 6, 15–20. [Google Scholar] [CrossRef]
- Poonia, A.; Upadhayay, A. Chenopodium album Linn: Review of nutritive value and biological properties. J. Food Sci. Technol. 2015, 52, 3977–3985. [Google Scholar] [CrossRef]
- Chamkhi, I.; Charfi, S.; El Hachlafi, N.; Mechchate, H.; Guaouguaou, F.-E.; El Omari, N.; Bakrim, S.; Balahbib, A.; Zengin, G.; Bouyahya, A. Genetic diversity, antimicrobial, nutritional, and phytochemical properties of Chenopodium album: A comprehensive review. Food Res. Int. 2022, 154, 110979. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, D.; Mittal, A. Antimicrobial and phytochemical potential of Chenopodium album Linn. Int. J. Sci. Technol. Res. 2019, 8, 877–880. [Google Scholar]
- Usman, L.; Hamid, A.; Muhammad, N.; Olawore, N.; Edewor, T.; Saliu, B. Chemical constituents and anti-inflammatory activity of leaf essential oil of Nigerian grown Chenopodium album L. EXCLI J. 2010, 9, 181. [Google Scholar]
- Cumming, B.G.; Seabrook, J.E. Chenopodium. In CRC Handbook of Flowering; CRC Press: Boca Raton, FL, USA, 2019; pp. 196–228. [Google Scholar]
- Singh, R.; Singh, S.; Saxena, D.C. Studies on standardization of alcohol aided starch extraction process from Chenopodium album and its characterization. J. Food Meas. Charact. 2021, 15, 5379–5391. [Google Scholar] [CrossRef]
- Ivanova, T.; Maiorova, O.; Orlova, Y.V.; Kuznetsova, E.; Khalilova, L.; Myasoedov, N.; Balnokin, Y.V.; Tsydendambaev, V. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
- Khomarlou, N.; Aberoomand-Azar, P.; Lashgari, A.P.; Tebyanian, H.; Hakakian, A.; Ranjbar, R.; Ayatollahi, S.A. Essential oil composition and in vitro antibacterial activity of Chenopodium album subsp. Striatum. Acta Biol. Hung. 2018, 69, 144–155. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Physico-chemical, textural, sensory and antioxidant characteristics of gluten—Free cookies made from raw and germinated Chenopodium (Chenopodium album) flour. LWT-Food Sci. Technol. 2016, 71, 281–287. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Effect of storage conditions and packaging materials on the quality attributes of gluten-free extrudates and cookies made from germinated Chenopodium (Chenopodium album) flour. J. Food Meas. Charact. 2017, 11, 1071–1080. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.; Singh, S. Comparative study of raw and germinated Chenopodium (Chenopodium album) flour on the basis of thermal, rheological, minerals, fatty acid profile and phytocomponents. Food Chem. 2018, 269, 173–180. [Google Scholar] [CrossRef] [PubMed]
- CABI. Chenopodium album (Fat Hen); CABI: Wallingford, UK, 2019. [Google Scholar]
- Singh, P.; Shivhare, Y.; Singhai, A.; Sharma, A. Pharmacological and phytochemical profile of Chenopodium album Linn. Res. J. Pharm. Technol. 2010, 3, 960–963. [Google Scholar]
- Pandey, S.; Gupta, R.K. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of Chenopodium album (Bathua). J. Pharmacogn. Phytochem. 2014, 3, 1–9. [Google Scholar]
- Tyagi, K.; Sharma, S.; Rashmi, R.; Kumar, S.; Khair, S. A comparative study of histo-pharmacognosy of Chenopodium album Linn. under the impact of Bicycle Industry Effluent. J. Pharm. Res. 2013, 6, 667–673. [Google Scholar] [CrossRef]
- Arora, C.; Sahua, D.; Bhartib, D.; Tamrakara, V.; Sonia, S.; Sharma, S. Adsorption of hazardous dye crystal violet from industrial waste using low-cost adsorbent Chenopodium album. Desalination Water Treat. 2019, 167, 324–332. [Google Scholar] [CrossRef]
- Ghirardelli, A.; Schiavon, M.; Zanin, G.; Ostapczuk, P.; Masin, R. Short-Term Responses to Salinity of Soybean and Chenopodium album Grown in Single and Mixed-Species Hydroponic Systems. Agronomy 2021, 11, 1481. [Google Scholar] [CrossRef]
- Le, T.H.; Jia, W.; Cho, K.M.; Khaitov, B.; Park, K.W. A Review on the Status of Exotic Weed (Chenopodium album L.) in Korea and Methods to Control. Weed Turfgrass Sci. 2019, 8, 187–197. [Google Scholar]
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2017, 7, 50–53. [Google Scholar] [CrossRef]
- Sharma, S. Realms of Ayurveda: Scientific Excursions by Nineteen Scholars; Arnold-Heinemann: Puram, India, 1979. [Google Scholar]
- Yadav, N.; Vasudeva, N.; Singh, S.; Sharma, S.K. Medicinal properties of genus Chenopodium Linn. Indian J. Nat. Prod. Resour. 2007, 6, 131–134. [Google Scholar]
- Tripathi, D.B. Ashtang Hridyam. Nirmala Hindi Commentary; Chaukhamba Surbharti Prakashan: Delhi, India, 2007; p. 29. [Google Scholar]
- Ballabh, B.; Chaurasia, O.; Ahmed, Z.; Singh, S.B. Traditional medicinal plants of cold desert Ladakh—Used against kidney and urinary disorders. J. Ethnopharmacol. 2008, 118, 331–339. [Google Scholar] [CrossRef]
- Hussain, K.; Shahazad, A.; Zia-ul-Hussnain, S. An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot. Leafl. 2008, 2008, 5. [Google Scholar]
- Shah, A.; Marwat, S.K.; Gohar, F.; Khan, A.; Bhatti, K.H.; Amin, M.; Din, N.U.; Ahmad, M.; Zafar, M. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am. J. Plant Sci. 2013, 4, 98–116. [Google Scholar]
- Abbas, Q.; Hussain, A.; Khan, S.W.; Hussain, A.; Shinwari, S.; Hussain, A.; Ullah, A.; Zafar, M.; Ali, K. Floristic Diversity, Ethnobotany and Traditional Recipes of Medicinal Plants of Maruk Nallah, Haramosh Valley, District Gilgit, Gilgit Baltistan: Traditional recipes of Maruk Nallah, Haramosh Valley, District Gilgit. Proc. Pak. Acad. Sci. B. Life Environ. Sci. 2019, 56, 97–112. [Google Scholar]
- Kumar, G.; Chander, H. Traditional Usage of Ethno-medicinal Plants of Sikandra Hill Range in Mandi District of Himachal Pradesh, India. Asian J. Adv. Basic Sci. 2019, 7, 42–49. [Google Scholar] [CrossRef]
- Sharma, P.; Samant, S. Diversity, distribution and indigenous uses of medicinal plants in Parbati Valley of Kullu district in Himachal Pradesh, Northwestern Himalaya. Asian J. Adv. Basic Sci. 2014, 2, 77–98. [Google Scholar]
- Adak, M.; Kumar, P. Herbal anthelmintic agents: A narrative review. J. Tradit. Chin. Med. 2022, 42, 641–651. [Google Scholar] [CrossRef]
- Sharma, N.; Tanwer, B.S.; Vijayvergia, R. Study of medicinal plants in Aravali regions of Rajasthan for treatment of kidney stone and urinary tract troubles. Int. J. PharmTech Res. 2011, 3, 110–113. [Google Scholar]
- Mahmood, A.; Mahmood, A.; Malik, R.N. Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol. 2012, 143, 338–346. [Google Scholar] [CrossRef]
- Tufail, M.; Hussain, K.; Nawaz, K.; Bhatti, K.H.; Yasin, G.; Ali, S.S. Ethnobotanical Survey of Important Wild Medicinal Plants of Tehsil Gojra, District Toba Tek Singh, Punjab, Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Katewa, S.; Galav, P. Traditional herbal medicines from Shekhawati region of Rajasthan. Indian J. Tradit. Knowl. IJTK 2005, 4, 237–245. [Google Scholar]
- Rehman, K.; Mashwani, Z.-U.-R.; Khan, M.A.; Ullah, Z.; Chaudhary, H.J. An ethno botanical perspective of traditional medicinal plants from the Khattak tribe of Chonthra Karak, Pakistan. J. Ethnopharmacol. 2015, 165, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Pande, M.; Pathak, A. Sexual Function Improving Effect of Chenopodium album (Bathua sag) in Normal Male Mice. Biomed. Pharmacol. J. 2015, 1, 325–332. [Google Scholar]
- Mahmood, A.; Mahmood, A.; Malik, R.N.; Shinwari, Z.K. Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J. Ethnopharmacol. 2013, 148, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Hussain, W.; Ullah, M.; Dastagir, G.; Badshah, L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018, 8, 313–329. [Google Scholar] [PubMed]
- Devi, U.; Seth, M.; Sharma, P.; Rana, J. Study on ethnomedicinal plants of Kibber Wildlife Sanctuary: A cold desert in Trans Himalaya, India. J. Med. Plants Res. 2013, 7, 3400–3419. [Google Scholar]
- Mahmood, A.; Qureshi, R.A.; Mahmood, A.; Sangi, Y.; Shaheen, H.; Ahmad, I.; Nawaz, Z. Ethnobotanical survey of common medicinal plants used by people of district Mirpur, AJK, Pakistan. J. Med. Plants Res. 2011, 5, 4493–4498. [Google Scholar]
- Ahmed, M.M.; Singh, K.P. Traditional knowledge of kidney stones treatment by Muslim Maiba (herbalists) of Manipur, India. Not. Sci. Biol. 2011, 3, 12–15. [Google Scholar] [CrossRef][Green Version]
- Bano, A.; Ahmad, M.; Hadda, T.B.; Saboor, A.; Sultana, S.; Zafar, M.; Khan, M.P.Z.; Arshad, M.; Ashraf, M.A. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J. Ethnobiol. Ethnomedicine 2014, 10, 43. [Google Scholar] [CrossRef]
- Rahman, S.; Husen, A. Potential Role of Medicinal Plants in the Cure of Liver and Kidney Diseases. In Non-Timber Forest Products; Springer: Berlin/Heidelberg, Germany, 2021; pp. 229–254. [Google Scholar]
- Ahmad, M.; Khan, M.P.Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological survey on medicinal plants used in herbal drinks among the traditional communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186. [Google Scholar] [CrossRef]
- Sivasankari, B.; Anandharaj, M.; Gunasekaran, P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014, 153, 408–423. [Google Scholar] [CrossRef]
- Ashfaq, S.; Ahmad, M.; Zafar, M.; Sultana, S.; Bahadur, S.; Abbas, N. Medicinal Plant Biodiversity Used among the Rural Communities of Arid Regions of Northern Punjab, Pakistan; NISCAIR-CSIR: Delhi, India, 2019. [Google Scholar]
- Adhikari, B.S.; Babu, M.; Saklani, P.; Rawat, G. Medicinal plants diversity and their conservation status in Wildlife Institute of India (WII) campus, Dehradun. Ethnobot. Leafl. 2010, 2010, 6. [Google Scholar]
- Shinwari, M.I.; Khan, M.A. Folk use of medicinal herbs of Margalla hills national park, Islamabad. J. Ethnopharmacol. 2000, 69, 45–56. [Google Scholar] [CrossRef]
- Mehra, A.; Bajpai, O.; Joshi, H. Diversity, utilization and sacred values of Ethno-medicinal plants of Kumaun Himalaya. Trop. Plant Res. 2014, 1, 80–86. [Google Scholar]
- Guerrero, J.L.G.; Torija Isasa, M.E. Nutritional composition of leaves of Chenopodium species (C. album L., C. murale L. and C. opulifolium Shraeder). Int. J. Food Sci. Nutr. 1997, 48, 321–327. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Lambsquarters Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169244/nutrients (accessed on 23 April 2023).
- Prasad, R.; Gupta, A.; Parihar, R.; Gangwar, K. In vitro method for predicting the bioavailability of iron from Bathua (Chenopodium album) and Fenugreek (Trigonella foenum graecum) leaves in Indian cookies. J. Appl. Nat. Sci. 2014, 6, 701–706. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A. Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India—An in vitro study. Food Chem. 2015, 170, 430–436. [Google Scholar] [CrossRef]
- Gqaza, B.M.; Njume, C.; Goduka, N.I.; George, G. Nutritional assessment of Chenopodium album L.(Imbikicane) young shoots and mature plant-leaves consumed in the Eastern Cape Province of South Africa. Int. Proc. Chem. Biol. Environ. Eng. 2013, 53, 97–102. [Google Scholar]
- Gesinski, K.; Nowak, K. Comparative analysis of the biological value of protein of Chenopodium quinoa Willd. and Chenopodium album L. Part I. Amino acid composition of the seed protein. Acta Sci. Polonorum. Agric. 2011, 10, 57–65. [Google Scholar]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.; Soomro, H.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3,-6 and-9 fatty acids in animals. Anim. Biotechnol. 2022, 33, 1678–1690. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Rodríguez-García, I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. Eur. Food Res. Technol. 1999, 209, 313–316. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Sangeetha, R.K.; Baskaran, V. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance: Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem. 2010, 119, 1584–1590. [Google Scholar] [CrossRef]
- Jardim, C.M.; Jham, G.N.; Dhingra, O.D.; Freire, M.M. Composition and antifungal activity of the essential oil of the Brazilian Chenopodium ambrosioides L. J. Chem. Ecol. 2008, 34, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Javaid, A.; Shoaib, A. GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha 2017, 35, e017164713. [Google Scholar] [CrossRef]
- Dos Santos Lima, L.A.R.; Johann, S.; Cisalpino, P.S.; Pimenta, L.P.S.; Boaventura, M.A.D. In vitro antifungal activity of fatty acid methyl esters of the seeds of Annona cornifolia A. St.-Hil.(Annonaceae) against pathogenic fungus Paracoccidioides brasiliensis. Rev. Soc. Bras. Med. Trop. 2011, 44, 777–780. [Google Scholar] [CrossRef]
- Poonia, A. Bioactive Compounds of Fat-Hen (Chenopodium album L.). In Bioactive Compounds in Underutilized Vegetables and Legumes. Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 1–11. [Google Scholar]
- Bera, B.; Mukherjee, K.; Ganguly, S. Chemical investigation of the seeds of diploid cytotypes of Chenopodium album. Fitoterapia 1991, 62, 178. [Google Scholar]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Di Marino, C.; Golino, A.; Previtera, L.; Zarrelli, A. Cinnamic acid amides from Chenopodium album: Effects on seeds germination and plant growth. Phytochemistry 2003, 64, 1381–1387. [Google Scholar] [CrossRef]
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Zarrelli, A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem. Biodivers. 2004, 1, 1579–1583. [Google Scholar] [CrossRef]
- Sood, P.; Modgil, R.; Sood, M.; Chuhan, P. Anti-nutrient profile of different Chenopodium cultivars leaves. Ann. Food Sci. Technol. 2012, 13, 68–74. [Google Scholar]
- Anokwuru, C.; Anyasor, G.; Ajibaye, O.; Fakoya, O.; Okebugwu, P. Effect of extraction solvents on phenolic, flavonoid and antioxidant activities of three nigerian medicinal plants. Nat. Sci. 2011, 9, 53–61. [Google Scholar]
- Ahmad Dar, A.; Sangwan, P.; Kumar, A. Chromatography: An important tool for drug discovery. J. Sep. Sci. 2020, 43, 105–119. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Suleman, M.; Hassan, A.U.; Abbas, F.F.I. Antibacterial, Antiparasitic and Phytochemical Activities of Chenopodium album (Bathua) Plant Extract. Bangladesh J. Bot. 2021, 50, 417–421. [Google Scholar] [CrossRef]
- Arora, S.; Itankar, P. Extraction, isolation and identification of flavonoid from Chenopodium album aerial parts. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef]
- Pandey, P.; Tiwari, S. Identification of different phytochemicals in methanolic extract of Chenopodium album (L.) leaf through GC-MS. Pharma Innov. 2020, 9, 175–178. [Google Scholar]
- Yılmaz, P.K.; Ertaş, A.; Akdeniz, M.; Avcı, M.K.; Kolak, U. Chemical compositions by LC-MS/MS and GC-MS and biological activities of Chenopodium album subsp. album var. microphyllum. Ind. Crops Prod. 2019, 141, 111755. [Google Scholar] [CrossRef]
- DellaGreca, M.; Di Marino, C.; Zarrelli, A.; D’Abrosca, B. Isolation and Phytotoxicity of Apocarotenoids from Chenopodium a lbum. J. Nat. Prod. 2004, 67, 1492–1495. [Google Scholar] [CrossRef]
- Sharma, K.D.; Bindal, G.; Rathour, R.; Rana, J. β-Carotene and mineral content of different Chenopodium species and the effect of cooking on micronutrient retention. Int. J. Food Sci. Nutr. 2012, 63, 290–295. [Google Scholar] [CrossRef]
- Mehdi, A.; Al-ani, W.M.; Raoof, A. Isolation of astragalin from IRAQI Chenopodium album. Asian J. Pharm. Clin. Res. 2018, 11, 530–535. [Google Scholar] [CrossRef]
- Pandey, M.K.; Kumar, A.; Singh, R.; Tripathi, M. Scientific standardization of leaves of Chenopodium album L. J. Pharmacogn. Phytochem. 2016, 5, 01–06. [Google Scholar]
- Choudhary, N.; Chatterjee, M.; Kumar, S.; Singh, G.; Suttee, A. Effect of conventional method and microwave assisted extraction on phytoconstituents of Chenopodium album. Mater. Today Proc. 2021, 45, 5362–5367. [Google Scholar] [CrossRef]
- Kumar, S.; Biswas, S.; Mandal, D.; Roy, H.N.; Chakraborty, S.; Kabir, S.N.; Banerjee, S.; Mondal, N.B. Chenopodium album seed extract: A potent sperm-immobilizing agent both in vitro and in vivo. Contraception 2007, 75, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Saxena, D.; Singh, S. Physico-chemical and textural property of starch isolated from Chenopodium (Chenopodium album) grains. Cogent Food Agric. 2015, 1, 1095052. [Google Scholar] [CrossRef]
- Yuldasheva, N.; Ibotov, S.K.; Zakirova, R.; Kurbanova, E.; Gusakova, S. Chemical Characteristics and Biological Activity of Lipids from Chenopodium album Seeds. Chem. Nat. Compd. 2021, 57, 412–415. [Google Scholar] [CrossRef]
- Vimala, B.; Thushara, R.; Nambisan, B.; Sreekumar, J. Effect of processing on the retention of carotenoids in yellow-fleshed cassava (Manihot esculenta Crantz) roots. Int. J. Food Sci. Technol. 2011, 46, 166–169. [Google Scholar] [CrossRef]
- Anjum, F.; Khan, B.A.; Noreen, N.; Masood, T.; Faisal, S. Effect of boiling and storage on beta-carotene content of different vegetables. J. Life Soc. Sci 2008, 6, 63–67. [Google Scholar]
- Singla, N.; Singla, P.; Kaur, N. The Impact of Thermal Processing Methods on The [Beta]-carotene Content of Some Commonly Consumed Vegetables. Int. J. Food Ferment. Technol. 2015, 5, 253. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]
- Modgil, R.; Sood, P. Effect of roasting and germination on carbohydrates and anti-nutritional constituents of indigenous and exotic cultivars of pseudo-cereal (Chenopodium). J. Life Sci. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Meena, S.; Agrawal, M.; Agrawal, K. Effect of blanching and drying on antioxidants and antioxidant activity of selected green leafy vegetables. Int. J. Sci. Res. 2016, 5, 1811–1814. [Google Scholar]
- Singh, A.; Kumari, A.; Chaudhary, H.K. Amaranth, Buckwheat, and Chenopodium: The “ABC” Nutraceuticals of Northwestern Himalayas. In Agricultural Biotechnology: Latest Research and Trends; Springer: Berlin/Heidelberg, Germany, 2021; pp. 587–634. [Google Scholar]
- Yadav, S.K.; Sehgal, S. Effect of domestic processing on total and extractable calcium and zinc content of Bathua (Chenopodium album) and Fenugreek (Trigonella foenum graecum) leaves. Plant Foods Hum. Nutr. 1999, 53, 255–263. [Google Scholar] [CrossRef]
- Kumari, M.; Gupta, S.; Lakshmi, A.J.; Prakash, J. Iron bioavailability in green leafy vegetables cooked in different utensils. Food Chem. 2004, 86, 217–222. [Google Scholar] [CrossRef]
- Gupta, S.; Gowri, B.; Lakshmi, A.J.; Prakash, J. Retention of nutrients in green leafy vegetables on dehydration. J. Food Sci. Technol. 2013, 50, 918–925. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.; Del Toro-Sanchez, C.; Ruiz-Cruz, S.; Márquez-Ríos, E. Effects of high-energy ultrasound on the functional properties of proteins. Ultrason. Sonochemistry 2016, 31, 558–562. [Google Scholar] [CrossRef]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of high intensity ultrasound on structure and foaming properties of pea protein isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Physicochemical, molecular and thermal properties of high-intensity ultrasound (HIUS) treated protein isolates from album (Chenopodium album) seed. Food Hydrocoll. 2019, 96, 433–441. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of lysozyme infusion, high-intensity ultrasound and controlled thermal treatment on the physicochemical and functional characteristics of Chenopodium album protein isolate based active packaging film. Food Packag. Shelf Life 2021, 29, 100686. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sehgal, S. Effect of home processing and storage on ascorbic acid and β-carotene content of bathua (Chenopodium album) and fenugreek (Trigonella foenum graecum) leaves. Plant Foods Hum. Nutr. 1997, 50, 239–247. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sehgal, S. Effect of domestic processing and cooking on selected antinutrient contents of some green leafy vegetables. Plant Foods Hum. Nutr. 2003, 58, 1–11. [Google Scholar] [CrossRef]
- Kaur, N.; Kaur, G. Effect of processing on nutritional and antinutritional composition of bathua (Chenopodium album) leaves. J. Appl. Nat. Sci. 2018, 10, 1149–1155. [Google Scholar] [CrossRef]
- Jan, R.; Saxena, D.C.; Singh, S. Effect of extrusion variables on antioxidant activity, total phenolic content and dietary fibre content of gluten-free extrudate from germinated Chenopodium (Chenopodium album) flour. Int. J. Food Sci. Technol. 2017, 52, 2623–2630. [Google Scholar] [CrossRef]
- Adedapo, A.; Jimoh, F.; Afolayan, A. Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa and Chenopodium album. Acta Pol. Pharm 2011, 68, 83–92. [Google Scholar] [PubMed]
- Korcan, S.E.; Aksoy, O.; Erdoğmuş, S.F.; Ciğerci, İ.H.; Konuk, M. Evaluation of antibacterial, antioxidant and DNA protective capacity of Chenopodium album’s ethanolic leaf extract. Chemosphere 2013, 90, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Lone, B.A.; Chishti, M.; Bhat, F.A.; Tak, H.; Bandh, S.A.; Khan, A. Evaluation of anthelmintic antimicrobial and antioxidant activity of Chenopodium album. Trop. Anim. Health Prod. 2017, 49, 1597–1605. [Google Scholar] [CrossRef]
- Umar, M.F.; Ahmad, F.; Saeed, H.; Usmani, S.A.; Owais, M.; Rafatullah, M. Bio-Mediated Synthesis of Reduced Graphene Oxide Nanoparticles from Chenopodium album: Their Antimicrobial and Anticancer Activities. Nanomaterials 2020, 10, 1096. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Al-khshemawee, H.H.; Al-badri, M.A.K.; Al-srai, M.S.; Daweri, H.H. Antifungal activity and GC-MS detection of leaves and roots parts of Chenopodium album extract against some phytopathogenic fungi. Indian J. Agric. Res. 2020, 54, 117–121. [Google Scholar] [CrossRef]
- Sherazi, A.; Jabeen, K.; Iqbal, S.; Yousaf, Z. Management of Ascochyta rabiei by Chenopodium album extracts. Planta Daninha 2016, 34, 675–680. [Google Scholar] [CrossRef]
- Javaid, A.; Rauf, S. Management of basal rot disease of onion with dry leaf biomass of Chenopodium album as soil amendment. Int. J. Agric. Biol. 2015, 17, 142–148. [Google Scholar]
- Rauf, S.; Javaid, A. Antifungal activity of different extracts of Chenopodium album against Fusarium oxysporum f. sp. cepae, the cause of onion basal rot. Int. J. Agric. Biol. 2013, 15, 367–371. [Google Scholar]
- Peachey, L.; Pinchbeck, G.; Matthews, J.; Burden, F.; Mulugeta, G.; Scantlebury, C.; Hodgkinson, J. An evidence-based approach to the evaluation of ethnoveterinary medicines against strongyle nematodes of equids. Vet. Parasitol. 2015, 210, 40–52. [Google Scholar] [CrossRef]
- Choudhary, N.; Khatik, G.L.; Choudhary, S.; Singh, G.; Suttee, A. In vitro anthelmintic activity of Chenopodium album and in-silico prediction of mechanistic role on Eisenia foetida. Heliyon 2021, 7, e05917. [Google Scholar] [CrossRef]
- Sachan, A.; Shanker, D.; Jaiswal, A.K.; Sudan, V. In vitro ovicidal assessment of methanol, ethyl acetate and chloroform extracts of Annona squamosa and Chenopodium album against caprine gastrointestinal nematodiosis. J. Parasit. Dis. 2015, 39, 62–66. [Google Scholar] [CrossRef][Green Version]
- Sahu, G.; Pradhan, R. Screening of acetylcholinesterase inhibition property in green and leafy vegetables. Biochem. Cell. Arch. 2017, 17, 651–656. [Google Scholar]
- Kant, S. Pharmacological evaluation of antidiabetic and antihyperlipidemic activity of Chenopodium album root extract in male Wistar albino rat models. Int. J. Green Pharm. 2018, 12, 115–122. [Google Scholar]
- Choudhary, N.; Prabhakar, P.K.; Khatik, G.L.; Chamakuri, S.R.; Tewari, D.; Suttee, A. Evaluation of Acute toxicity, In-vitro, In-vivo Antidiabetic Potential of the Flavonoid Fraction of the plant Chenopodium album L. Pharmacogn. J. 2021, 13, 765–779. [Google Scholar] [CrossRef]
- Verma, M.K.; Ahmad, A.; Pant, D.; Kumar, N.; Patwal, P. Chenopodium album ameliorates cyclophosphamide-induced hyperlipidemia in Sprague dawley rats. Pharma Innov. J. 2018, 7, 423–426. [Google Scholar]
- Singh, P.; Shivhare, Y.; Patil, U. Assessment of hypolipidemic potential of Chenopodium album Linn. on triton induced hyperlipidemic rats. Res. J. Pharm. Technol. 2010, 3, 187–192. [Google Scholar]
- Ma, Q.-G.; Wei, R.-R.; Zhang, X.-D.; Sang, Z.-P.; Dong, J.-H.; Lu, Q.-X.; Huang, H.-F.; Guo, D.-M.; Jiang, L. Tropolone derivatives with hepatoprotective and antiproliferative activities from the aerial parts of Chenopodium album Linn. Fitoterapia 2020, 146, 104733. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Ojeswi, B.; Sharma, B.; Srivastava, M.M. Chenopodium album Prevents Progression of Cell Growth and Enhances Cell Toxicity in Human Breast Cancer Cell Lines. Oxidative Med. Cell. Longev. 2009, 2, 160–165. [Google Scholar] [CrossRef]
- Chakraborty, D.; Jain, C.K.; Maity, A.; Ghosh, S.; Choudhury, S.R.; Jha, T.; Majumder, H.K.; Mondal, N.B. Chenopodium album metabolites act as dual topoisomerase inhibitors and induce apoptosis in the MCF7 cell line. Medchemcomm 2016, 7, 837–844. [Google Scholar] [CrossRef]
- Islam, Z.; Amin, A.; Paul, G.K.; Hasan, K.; Rashid, M.; Saleh, M.A.; Islam, N. Anthelmintic, antioxidant, and cytotoxic activities of Chenopodium album against Haemonchus contortus: A combined in vitro and in silico study. Inform. Med. Unlocked 2023, 37, 101194. [Google Scholar] [CrossRef]
- Külcü, D.B.; Gökışık, C.D.; Aydın, S. An investigation of antibacterial and antioxidant activity of nettle (Urtica dioica L.), mint (Mentha piperita), thyme (Thyme serpyllum) and Chenopodium album L. plants from Yaylacık Plateau, Giresun, Turkey. Turk. J. Agric.-Food Sci. Technol. 2019, 7, 73–80. [Google Scholar]
- Javaid, A.; Ali, A.; Khan, I.H.; Ferdosi, M.F. Leaves of Chenopodium album as source of natural fungicides against Sclertium rolfsii. Arab. J. Chem. 2023, 16, 104677. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Vijay, N.; Padmaa, M. Hepatoprotective activity of Chenopodium album Linn. against paracetamol induced liver damage. Pharmacologyonline 2011, 3, 312–328. [Google Scholar]
- Karwani, G.; Sisodia, S.S. Hepatoprotective activity of Chenopodium album Linn. in Ethanol induced Hepatotoxicity in Rats. Res. J. Pharm. Technol. 2015, 8, 669–673. [Google Scholar] [CrossRef]
- Karwani, G.; Sisodia, S.S. Hepatoprotective activity of Chenopodium album Linn. in carbon tetrachloride induced hepatotoxicity rats. Res. J. Pharmacol. Pharmacodyn. 2015, 7, 29–34. [Google Scholar] [CrossRef]
- Nayak, D.P.; Dinda, S.; Swain, P.; Kar, B.; Patro, V. Hepatoprotective activity against CCl4-induced hepatotoxicity in rats of Chenopodium album aerial parts. J. Phytother. Pharmacol. 2012, 1, 33–41. [Google Scholar]
- Parkash, J.; Patel, K.R. Hepatoprotective activity of Chenopodium album leaves extract in CCl4 induced hepatotoxicity in rats. J. Drug Deliv. Ther. 2015, 5, 88–93. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 854–857. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Rezaee, R.; Sahebkar, A. Oxidative stress induces renal failure: A review of possible molecular pathways. J. Cell. Biochem. 2018, 119, 2990–2998. [Google Scholar] [CrossRef]
- Dua, K.; Malyla, V.; Singhvi, G.; Wadhwa, R.; Krishna, R.V.; Shukla, S.D.; Shastri, M.D.; Chellappan, D.K.; Maurya, P.K.; Satija, S. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: An emerging need for novel drug delivery systems. Chem.-Biol. Interact. 2019, 299, 168–178. [Google Scholar] [CrossRef]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Nengroo, Z.; Rauf, A. Fatty acid composition and antioxidant activity of Angelica glauca and Chenopodium album seed extracts from Kashmir. Grasas Y Aceites 2021, 72, e393. [Google Scholar] [CrossRef]
- Verma, M.K.; Ahmad, A.; Pant, D.; Rawat, P.; Kumar, N. Ameliorative effect of Chenopodium album in cyclophosphamide-induced oxidative stress and hematologic toxicity. Pharma Innov. J. 2020, 9, 402–406. [Google Scholar]
- Amodeo, V.; Marrelli, M.; Pontieri, V.; Cassano, R.; Trombino, S.; Conforti, F.; Statti, G. Chenopodium album L. and Sisymbrium officinale (L.) Scop.: Phytochemical Content and In Vitro Antioxidant and Anti-Inflammatory Potential. Plants 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Marwat, M.S.; Chohan, A.M.; Shah, A.H.; Naz, R.; Gul, J.; Bhatti, M.Z.; Saeed, A. Antioxidant Activity in Seeds of Avena fatua and Chenopodium album Weeds Associated with Wheat Crop. Pak. J. Weed Sci. Res. 2018, 24, 203–212. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Rahman, S.; Sana, S.; Biswas, T.K.; Hashem, A.K.M.; Parvin, S.; Mazumder, K. Anticancer potential of Chenopodium album leaf extract against Ehrlich ascites carcinoma cells in Swiss albino mice. Future J. Pharm. Sci. 2020, 6, 65. [Google Scholar] [CrossRef]
- Javed, M.; Bilal, M.; Tabassum, B.; Malik, A.; Adeyinka, O.S.; Tariq, M.; Nasir, I.A. Purification and functional characterization of lectin from Chenopodium album. J. Proteins Proteom. 2022, 13, 55–62. [Google Scholar] [CrossRef]
- Nepal, A.; Chakraborty, M. An overview on medicinal plants of Sikkim Himalayas region with emphasis on antidiabetic: A review. J. Pharmacogn. Phytochem. 2021, 10, 215–217. [Google Scholar] [CrossRef]
- Magama, S.; Asita, A.O. Evaluation of Chenopodium album Linn. crude methanolic leaf extract for central antinociceptive activity in albino mice using the hot plate test. Int. J. Sci. 2017, 6, 36–44. [Google Scholar] [CrossRef][Green Version]
- Mushtaq, A.; Rashid, S.; Jamil, M.; Anwar, R.; Khawaja, N.R. Anti-nociceptive and anti-inflammatory activity of Trapa bispinosa, Chenopodium album and Cuscuta reflexa. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 608–622. [Google Scholar]
- Kumar, S.; Chatterjee, R.; Dolai, S.; Adak, S.; Kabir, S.N.; Banerjee, S.; Mondal, N.B. Chenopodium album seed extract-induced sperm cell death: Exploration of a plausible pathway. Contraception 2008, 77, 456–462. [Google Scholar] [CrossRef]
- Nigam, V.; Paarakh, P.M. Anti-ulcer effect of Chenopodium album Linn. against gastric ulcers in rats. Int. J. Pharm. Sci. Drug Res. 2011, 3, 319–322. [Google Scholar]
- Arora, S.K.; Itankar, P.R.; Verma, P.R.; Bharne, A.P.; Kokare, D.M. Involvement of NFκB in the antirheumatic potential of Chenopodium album L., aerial parts extracts. J. Ethnopharmacol. 2014, 155, 222–229. [Google Scholar] [CrossRef]
- Jabbar, A.; Zaman, M.A.; Iqbal, Z.; Yaseen, M.; Shamim, A. Anthelmintic activity of Chenopodium album (L.) and Caesalpinia crista (L.) against trichostrongylid nematodes of sheep. J. Ethnopharmacol. 2007, 114, 86–91. [Google Scholar] [CrossRef]
- Ibrahim, L.F.; Kawashty, S.A.; Baiuomy, A.R.; Shabana, M.M.; El-Eraky, W.I.; El-Negoumy, S.I. A comparative study of the flavonoids and some biological activities of two Chenopodium species. Chem. Nat. Compd. 2007, 43, 24–28. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Valorization of fruit wastes for circular bioeconomy: Current advances, challenges, and opportunities. Bioresour. Technol. 2022, 359, 127459. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Monga, V.; Bhatia, R. Compendium of naringenin: Potential sources, analytical aspects, chemistry, nutraceutical potentials and pharmacological profile. Crit. Rev. Food Sci. Nutr. 2022, 1–32. [Google Scholar] [CrossRef]
- Singh, L.; Yadav, N.; Kumar, A.; Gupta, A.; Chacko, J.; Parvin, K.; Tripathi, U. Preparation of value added products from dehydrated bathua leaves (Chenopodium album Linn.). Indian J. Tradit. Knowl. IJTK 2007, 6, 6–10. [Google Scholar]
- Fletcher, R.J. Pseudocereals: Overview. In Encyclopedia of Food Grains, 2nd ed.; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 274–279. [Google Scholar] [CrossRef]
- Thejasri, V.; Hymavathi, T.; Roberts, T.P.; Anusha, B.; Devi, S.S. Sensory, physico-chemical and nutritional properties of gluten free biscuits formulated with Quinoa (Chenopodium quinoa Willd.), Foxtail Millet (Setaria italica) and hydrocolloids. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1710–1721. [Google Scholar] [CrossRef]
- Brito, I.L.; de Souza, E.L.; Felex, S.S.S.; Madruga, M.S.; Yamashita, F.; Magnani, M. Nutritional and sensory characteristics of gluten-free quinoa (Chenopodium quinoa Willd)-based cookies development using an experimental mixture design. J. Food Sci. Technol. 2015, 52, 5866–5873. [Google Scholar] [CrossRef]
- Xie, M.; Wang, R.; Wang, Y.; Liu, N.; Qi, J. Effects of dietary supplementation with fermented Chenopodium album L. on growth, nutrient digestibility, immunity, carcase characteristics and meat quality of broilers. Ital. J. Anim. Sci. 2021, 20, 2063–2074. [Google Scholar] [CrossRef]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. Nutraceuticals: Transformation of Conventional Foods into Health Promoters/Disease Preventers and Safety Considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Sharma, M.; Dwivedi, P.; Singh Rawat, A.K.; Dwivedi, A.K. 3—Nutrition nutraceuticals: A proactive approach for healthcare. In Nutraceuticals; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 79–116. [Google Scholar] [CrossRef]
- Mishra, A.; Chaudhari, D.; Patel, H.; Patel, B. Nutraceutical properties of important weeds in India. In Research Anthology on Recent Advancements in Ethnopharmacology and Nutraceuticals; IGI Global: Hershey, PA, USA, 2022; pp. 1245–1263. [Google Scholar]
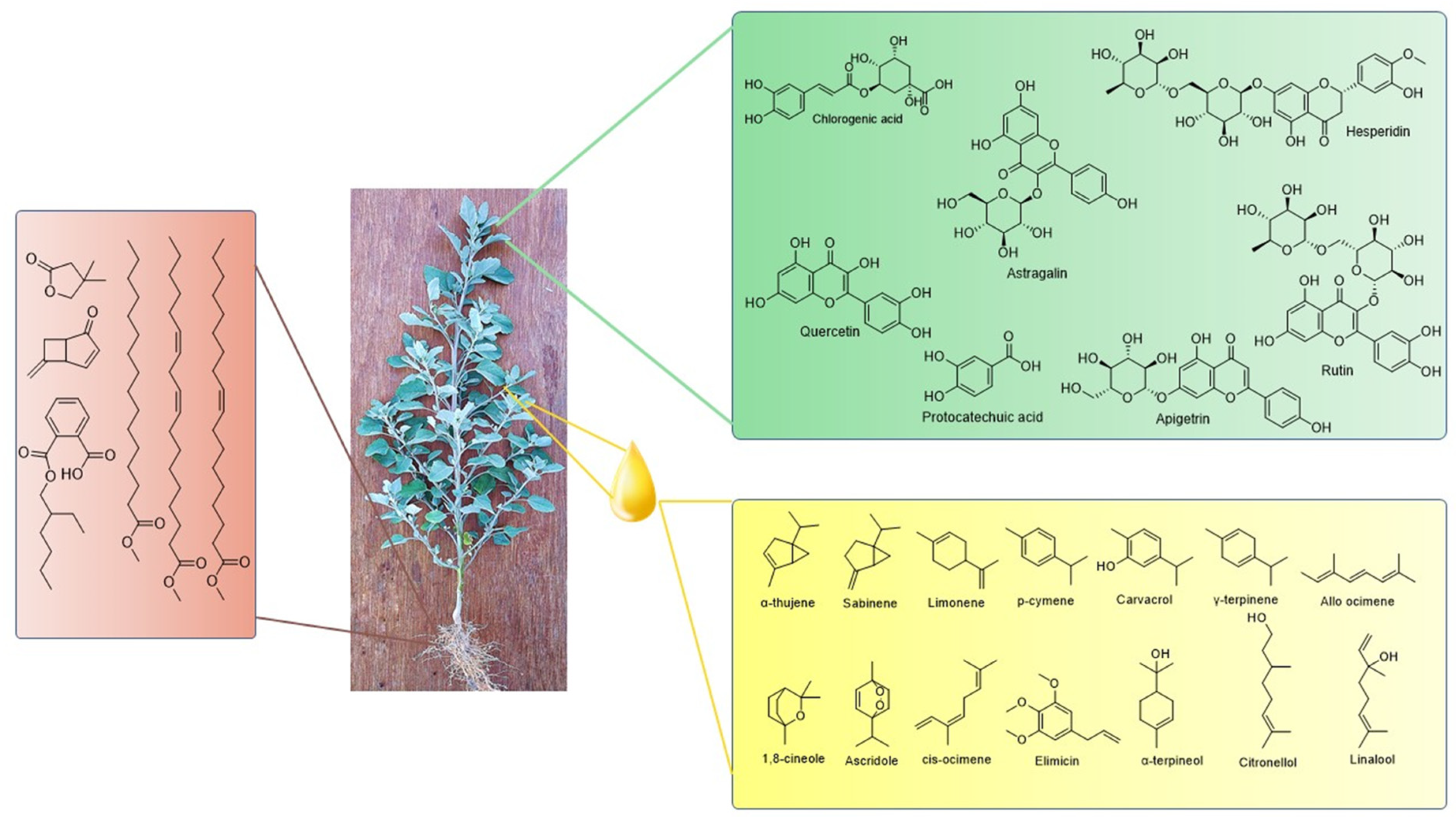
| S. No. | Biological Activity | Plant Part Used | Extraction/Type of Extract | Findings | Reference |
|---|---|---|---|---|---|
| 1. | Anticholinesterase activity | Aerial parts and roots | Methanolic (MT), Acetone (EA), and n-Hexane (HE) extract | Butyrylcholinesterase (BchE) inhibitory activity of MT (52.64 ± 2.78%), AE (65.29 ± 1.56%), and HE (44.31 ± 2.13%) | [88] |
| Fresh leaves | Leaves crushed in pestle mortar were centrifuged and supernatant used for analysis | Acetylcholinesterase inhibitory (AchE) activity (32.13%) | [126] | ||
| 2. | Antidiabetic activity | Roots | MT | Decline in fasting blood glucose after a 12 h treatment with high dose (139.5 ± 4.8 mg/dL), mild dose (144.2 ± 4.1 mg/dL), and low dose (148.3 ±1.5 mg/dL) | [127] |
| Aerial parts | Flavonoid fraction (CAFF), alkaloid fraction (CAAF), saponin fraction (CASF) | Alpha amylase inhibition activity in CAFF (75.66 ± 0.68), CATF (26.97 ± 0.91), CAAF (10.53 ± 1.02), and CASF (6.58 ± 0.71) at concentration of 250 µg/mL | [128] | ||
| 3. | Antihyperlipidemic activity | Roots | MT | High dose MT of C. album normalized plasma lipid status | [127] |
| Aerial parts | Hydroethanolic extract | Rats treated with cyclophosphamide along with extract 440 mg/kg b.w significantly reduced total cholesterol (53.8%), triglycerides (52.42%), and low-density lipoproteins (28.37%) compared to rats treated with cyclophosphamide alone | [129] | ||
| Stems | MT | Rats treated with aqueous insoluble extract dissolved in PVP water mixture were reported to have marked decreased total cholesterol (53.8%), triglycerides (52.42%), and low-density lipoproteins (28.37%) compared to rats treated with cyclophosphamide alone | [130] | ||
| 4. | Antiproliferative activity | Aerial parts | Ethylacetate-soluble extract fraction | IC50 values ranging from 0.5 ± 0.2 to 15.5 ± 2.7 µM | [131] |
| Leaves | Ethylacetate Extract (EA) and MT | % Inhibition of EA and MT (100 mg/mL) against Breast adenocarcinoma estrogen-receptor-positive (MCF-7) and estrogen-receptor-negative (MDA-MB-468) cell lines was 50.40 ± 1.92, 89.09 ± 1.97 (EA), and 28.03 ± 1.97, 49.77 ± 2.01 (MT), respectively | [132] | ||
| Seeds | MT | Desgalactotigonin and oleanolic acid-3-O-β-d-glucuronide found in extract-inhibited MCF-7 cells with IC50 value of 8.27 µM and 11.33 µM, respectively, and inhibited human topoisomerase I and II | [133] | ||
| 5. | Anthelmintic activity | Aerial parts | Petroleum ether extract (PEE), EtOAcE, MTE, hydroalcoholic extract (CAHE), and aqueous extract (CAAE) | EtOAcE (10 mg/mL) was reported to have minimum time for paralysis (10.08 ± 1.11 min) and death (65.28 ± 2.09 min) of Eisenia foetida | [124] |
| Leaves | MTE | MTE treatment for 3 h exhibited 100 ± 0.0% mortality against Haemonchus contortus | [134] | ||
| Leaves and stems | MTE | Treatment with 75% and 100% MTE for 14 h resulted in 100% mortality of Haemonchus contortus | [85] | ||
| 6. | Antimicrobial activity | Aerial parts | Ethanolic extract (EE), Chloroform extract (CE), and HE | MIC value (µg/mL) was reported to be lowest for EE against Enterobacter aerogenes while CE and HE shown equal results against Bacillus subtilis | [135] |
| Leaves and roots | Aqueous extract (AE) | Against Alternaria alternata, Fusarium solani, Rhizoctonia solani, Pythium aphanidermatum, and Sclerotinia sclerotium, the AE (15%) of leaves showed 100%, 83.6%, 100%, 93.33%, and 91.42% mycelial growth inhibition, respectively, while AE (15%) of roots showed complete (100%) inhibition of mycelial growth | [119] | ||
| Leaves | MT | Ethyl acetate fraction (200 mg/mL) of MT was reported to cause maximum decrease (74%) in biomass of Sclerotium rolfsii | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Singh, A.; Hallan, S.S.; Brangule, A.; Kumar, B.; Bhatia, R. A Compiled Update on Nutrition, Phytochemicals, Processing Effects, Analytical Testing and Health Effects of Chenopodium album: A Non-Conventional Edible Plant (NCEP). Molecules 2023, 28, 4902. https://doi.org/10.3390/molecules28134902
Singh S, Singh A, Hallan SS, Brangule A, Kumar B, Bhatia R. A Compiled Update on Nutrition, Phytochemicals, Processing Effects, Analytical Testing and Health Effects of Chenopodium album: A Non-Conventional Edible Plant (NCEP). Molecules. 2023; 28(13):4902. https://doi.org/10.3390/molecules28134902
Chicago/Turabian StyleSingh, Sukhwinder, Amandeep Singh, Supandeep Singh Hallan, Agnese Brangule, Bhupinder Kumar, and Rohit Bhatia. 2023. "A Compiled Update on Nutrition, Phytochemicals, Processing Effects, Analytical Testing and Health Effects of Chenopodium album: A Non-Conventional Edible Plant (NCEP)" Molecules 28, no. 13: 4902. https://doi.org/10.3390/molecules28134902
APA StyleSingh, S., Singh, A., Hallan, S. S., Brangule, A., Kumar, B., & Bhatia, R. (2023). A Compiled Update on Nutrition, Phytochemicals, Processing Effects, Analytical Testing and Health Effects of Chenopodium album: A Non-Conventional Edible Plant (NCEP). Molecules, 28(13), 4902. https://doi.org/10.3390/molecules28134902








