Antiviral Evaluation of New Synthetic Bioconjugates Based on GA-Hecate: A New Class of Antivirals Targeting Different Steps of Zika Virus Replication
Abstract
1. Introduction
2. Results
2.1. Assessment of Hecate Stability in Human Serum
2.2. Peptide Synthesis
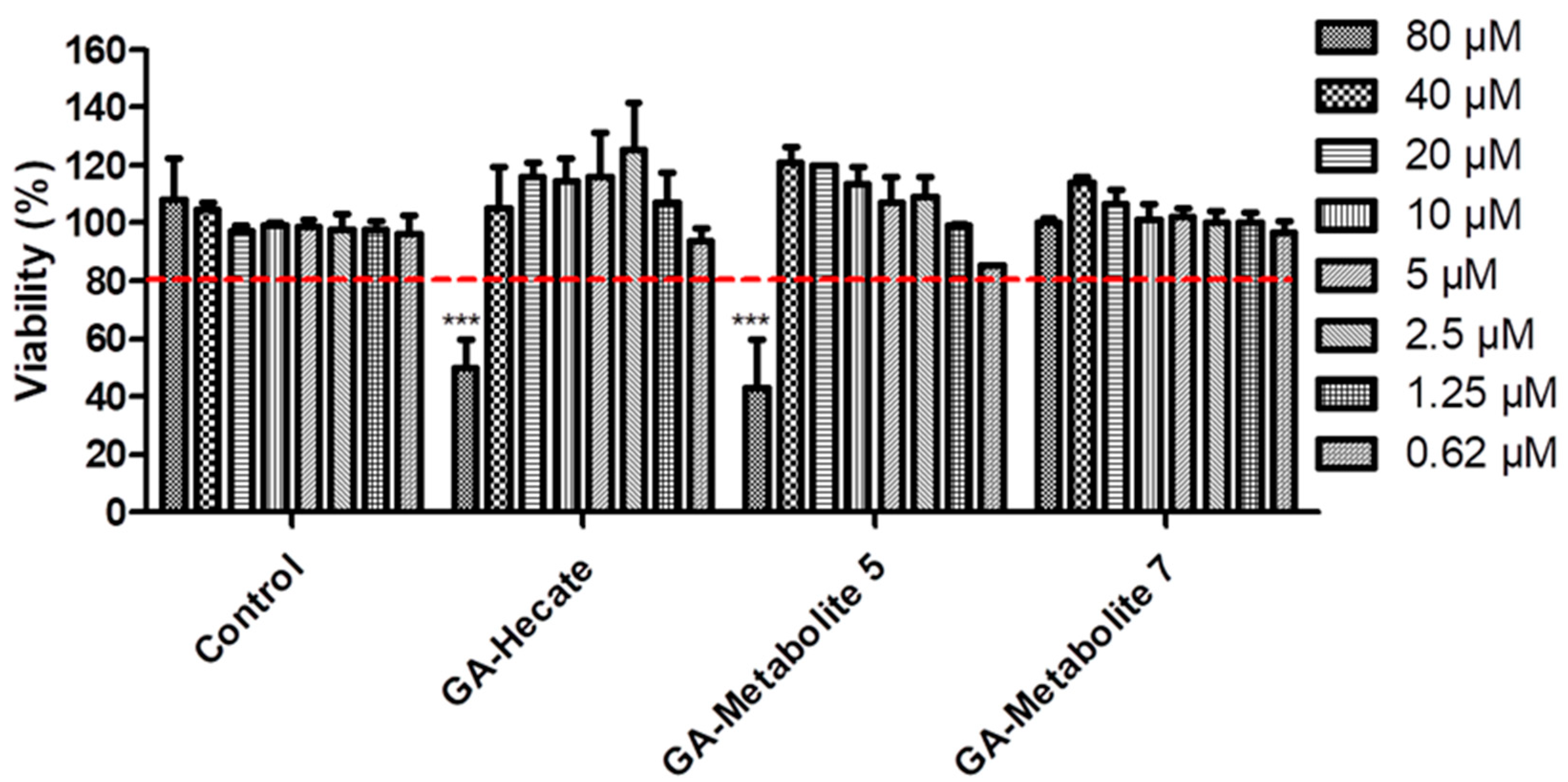
2.3. The Cytotoxicity Profile of GA-Peptides
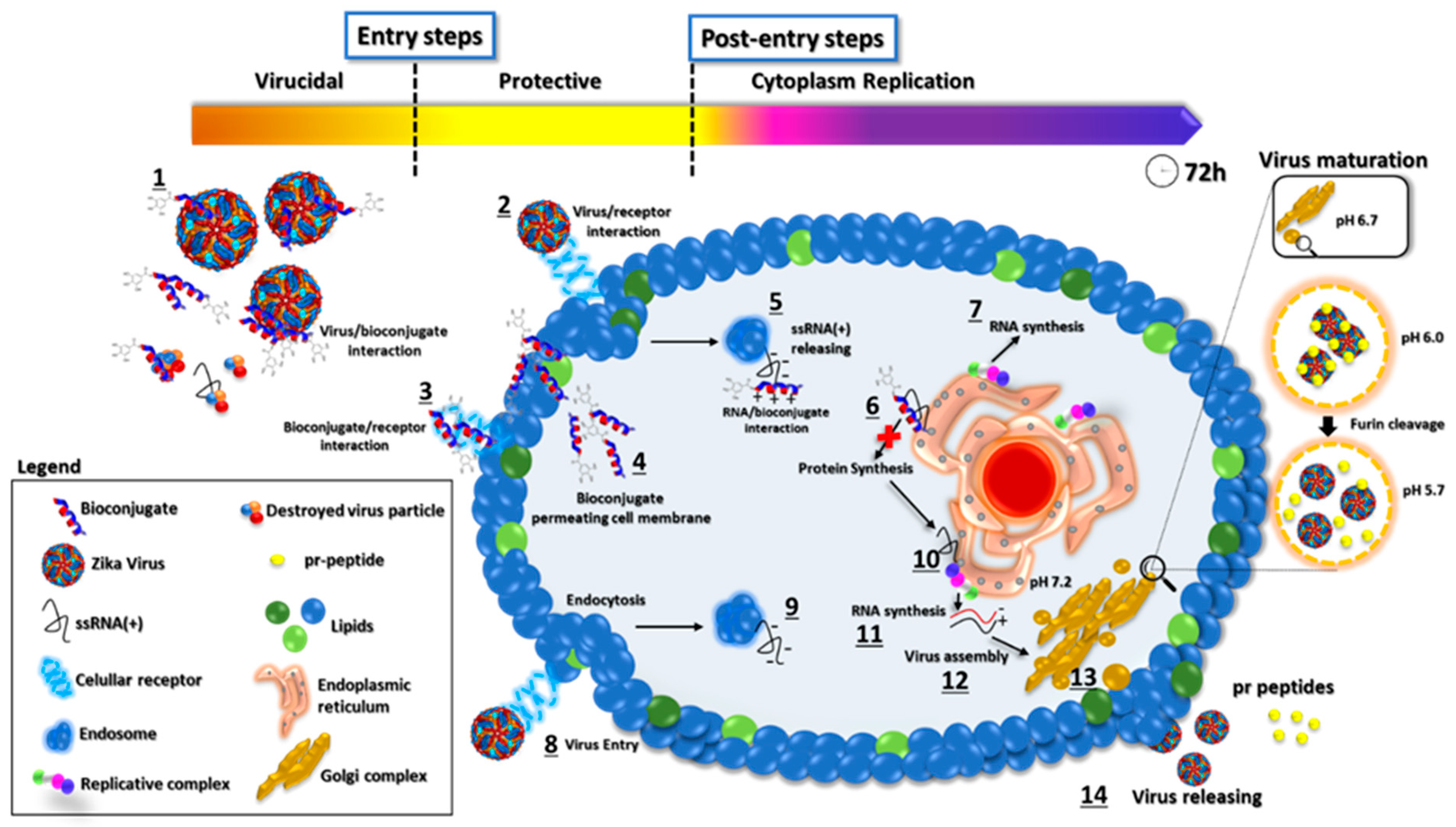
2.4. Antiviral Activity
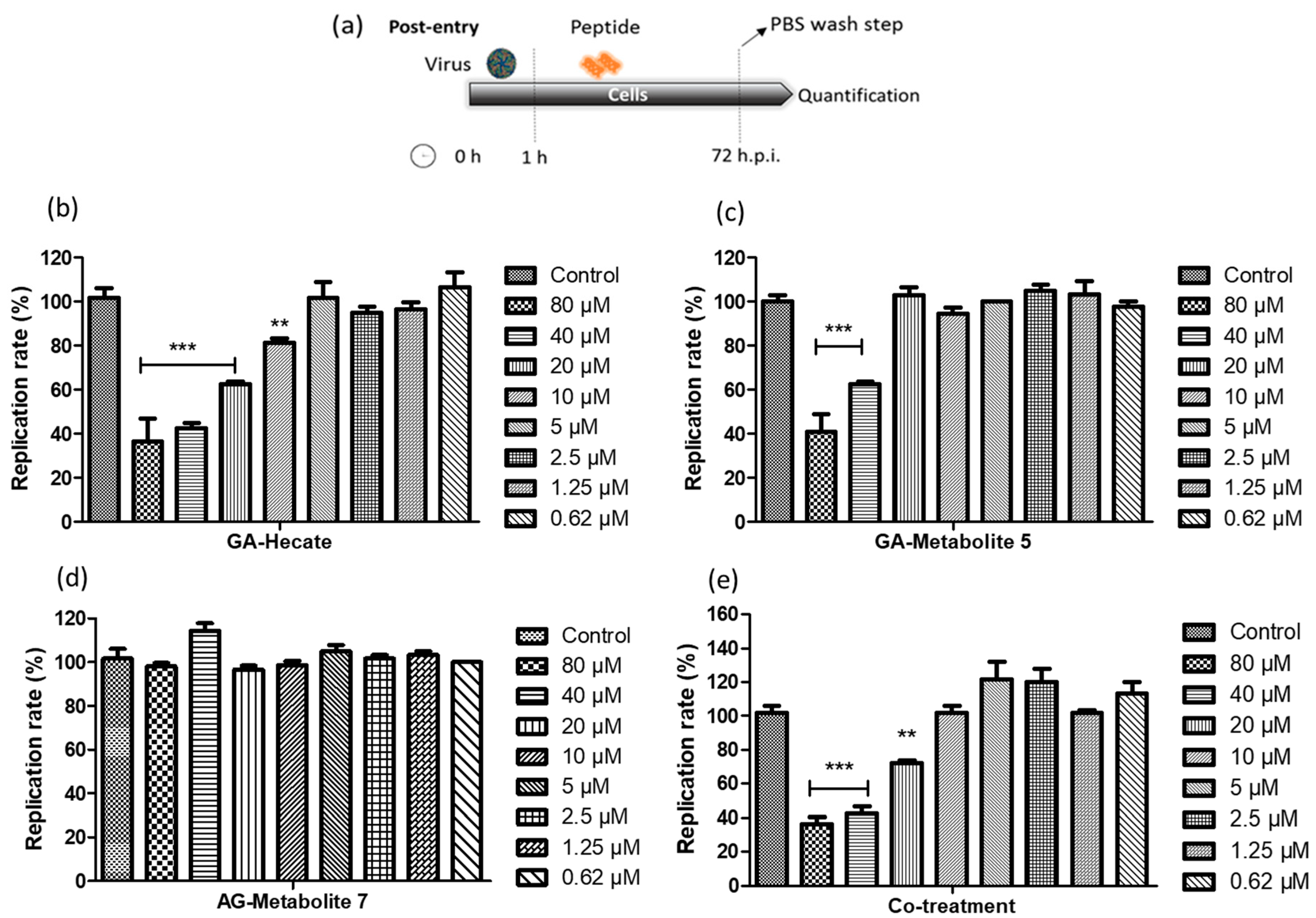
2.4.1. Post-Entry Assessment of GA-Hecate and GA-Metabolites
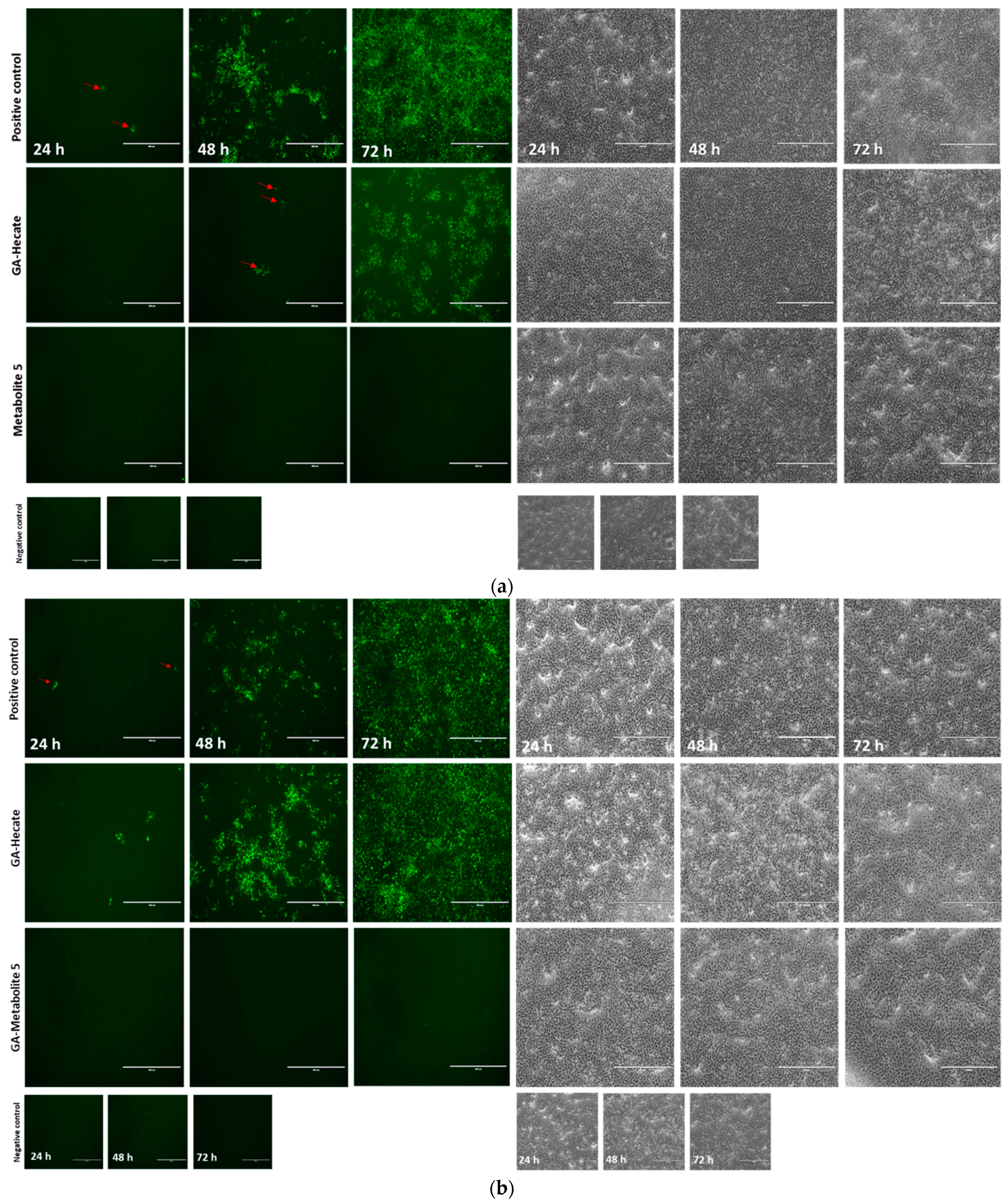
2.4.2. Virucidal Effects of GA-Hecate and GA-Metabolite 5
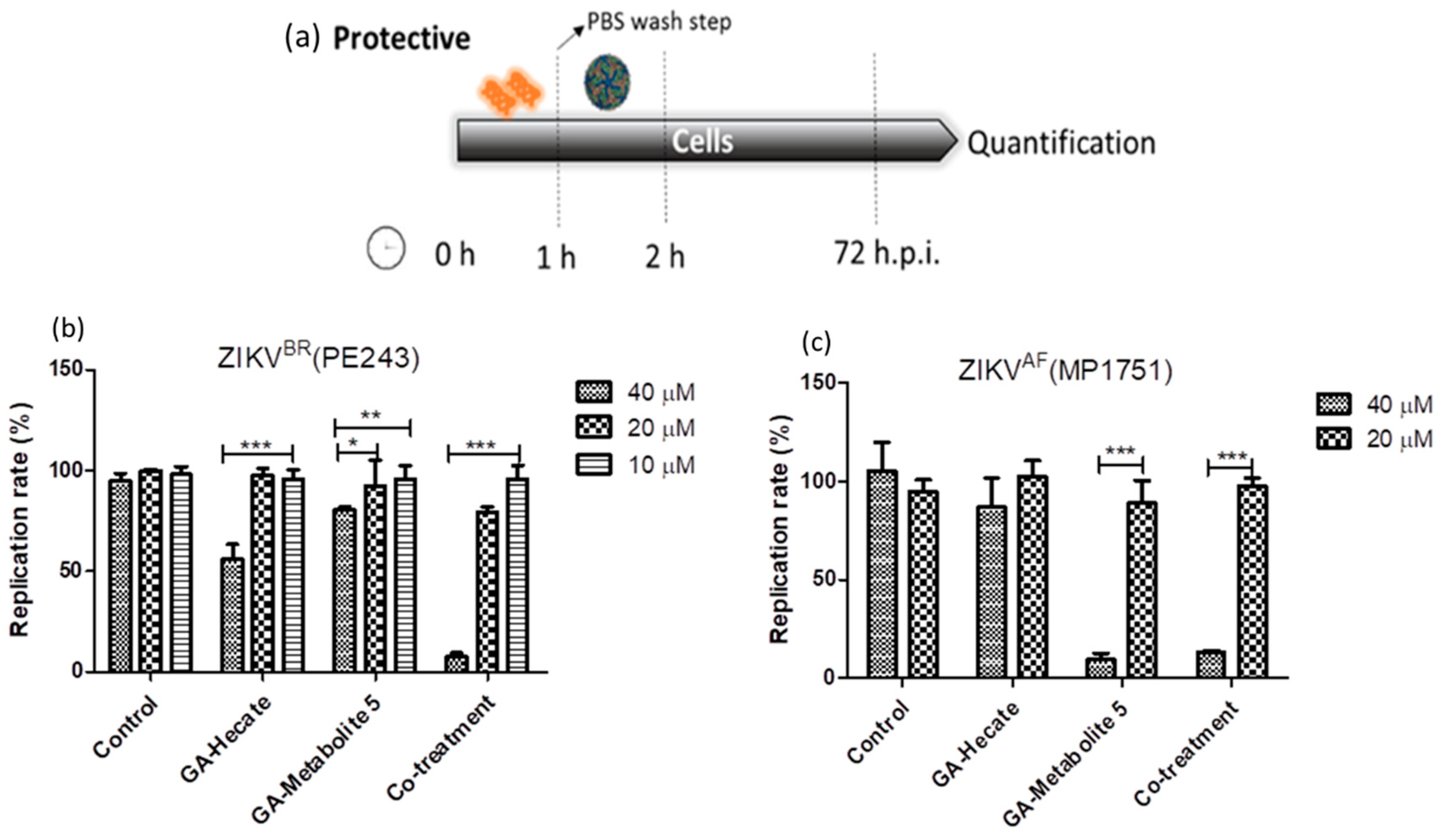
2.4.3. Protective Effect of GA-Hecate and GA-Metabolite 5
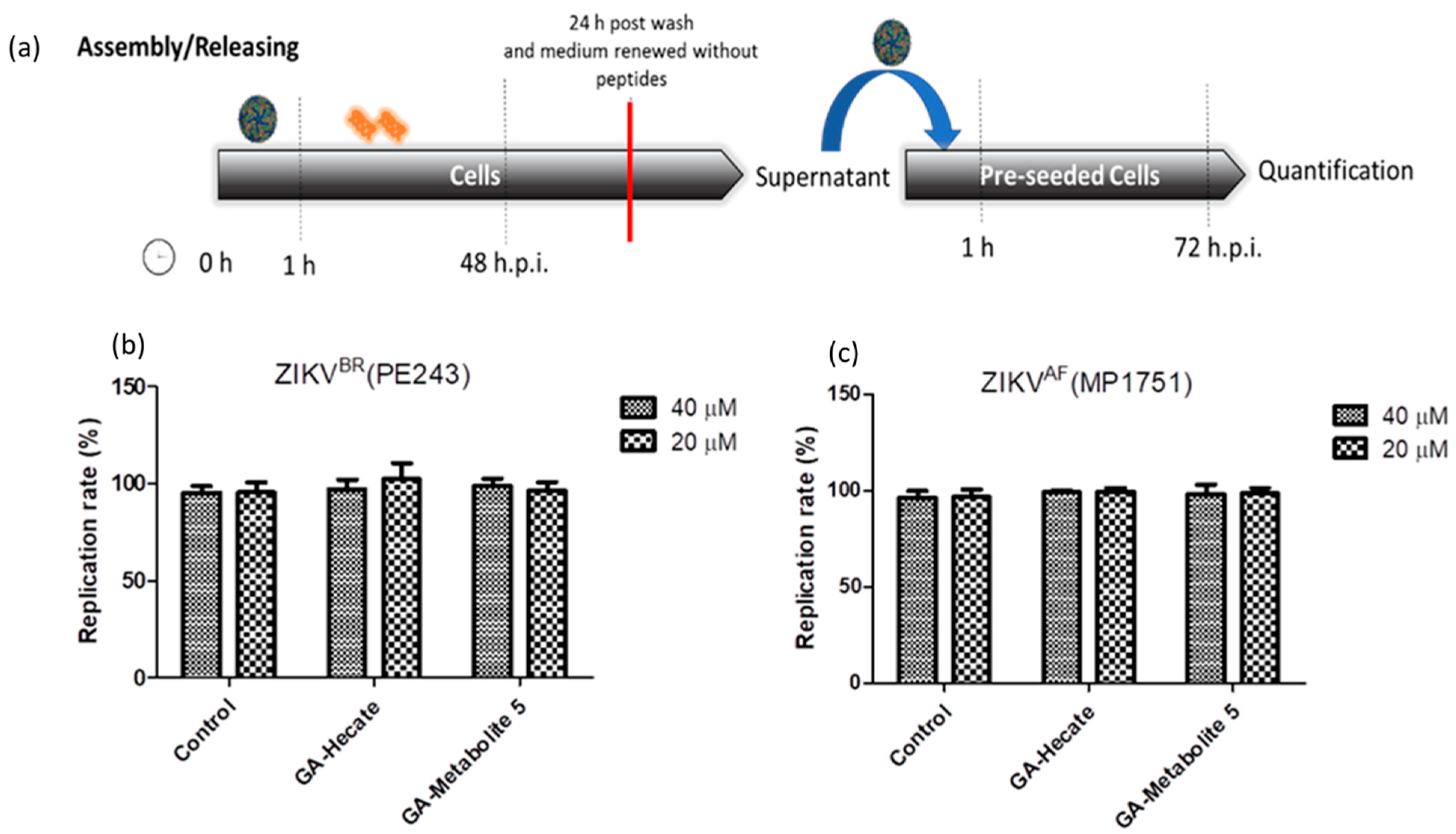
2.4.4. Effect of GA-Hecate and GA-Metabolite 5 on Virus Assembly and Release
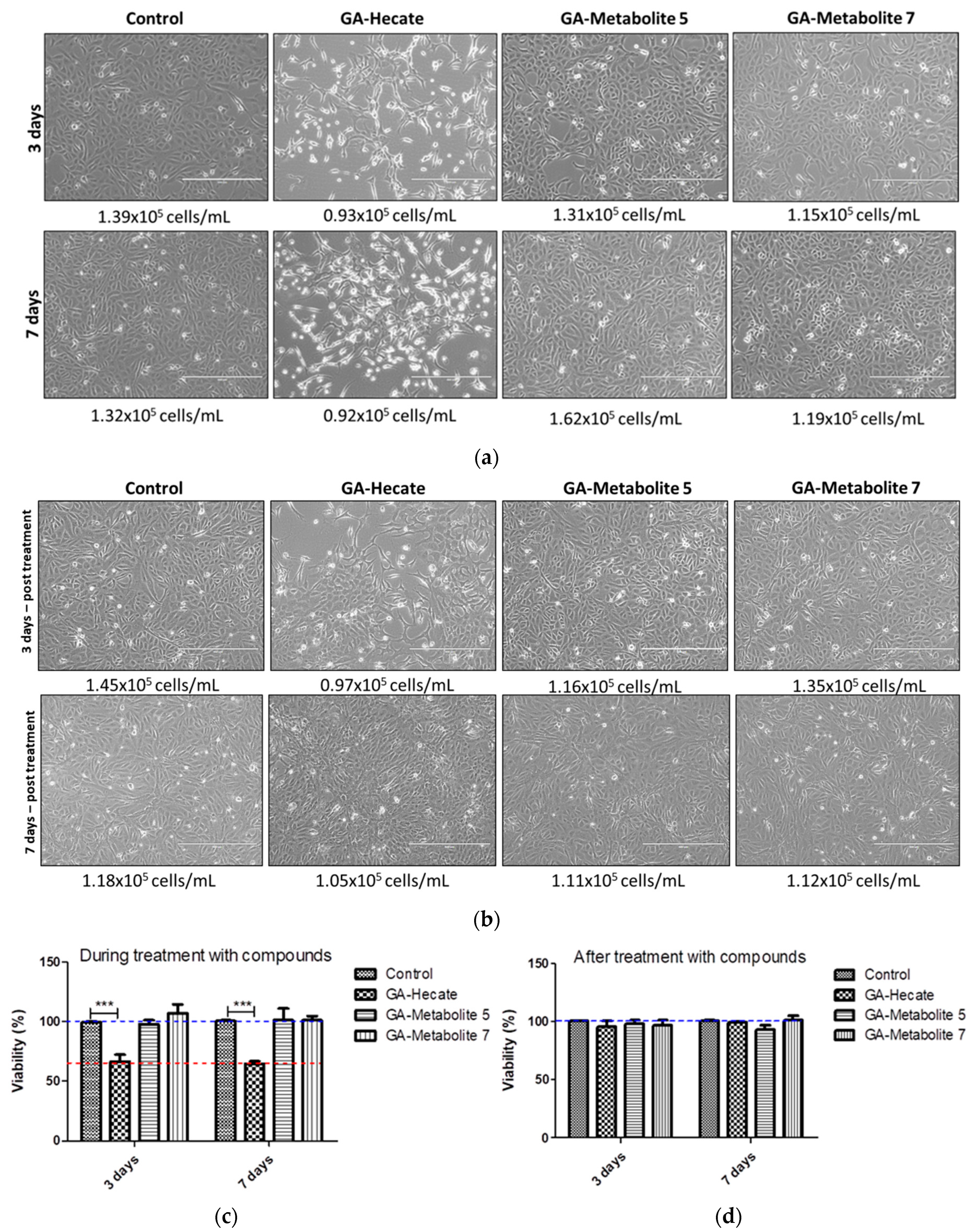
2.5. GA-Hecate and GA-Metabolite Cell Toxicity during Constant Treatment
3. Materials and Methods
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shah, P.S.; Link, N.; Jang, G.M.; Sharp, P.P.; Zhu, T.; Swaney, D.L.; Johnson, J.R.; Von Dollen, J.; Ramage, H.R.; Satkamp, L.; et al. Comparative Flavivirus-Host Protein Interaction Mapping Reveals Mechanisms of Dengue and Zika Virus Pathogenesis. Cell 2018, 175, 1931–1945.e18. [Google Scholar] [CrossRef] [PubMed]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring Cellular Networks by Members of the Flaviviridae Family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Chong, H.Y.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Flavivirus Infection—A Review of Immunopathogenesis, Immunological Response, and Immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Simonin, Y.; Loustalot, F.; Desmetz, C.; Foulongne, V.; Constant, O.; Fournier-Wirth, C.; Leon, F.; Molès, J.P.; Goubaud, A.; Lemaitre, J.M.; et al. Zika Virus Strains Potentially Display Different Infectious Profiles in Human Neural Cells. EBioMedicine 2016, 12, 161–169. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Shi, W.F.; Qin, C.F. The Evolution of Zika Virus from Asia to the Americas. Nat. Rev. Microbiol. 2019, 17, 131–139. [Google Scholar] [CrossRef]
- Beaver, J.T.; Lelutiu, N.; Habib, R.; Skountzou, I. Evolution of Two Major Zika Virus Lineages: Implications for Pathology, Immune Response, and Vaccine Development. Front. Immunol. 2018, 9, 1640. [Google Scholar] [CrossRef]
- Pielnaa, P.; Al-Saadawe, M.; Saro, A.; Dama, M.F.; Zhou, M.; Huang, Y.; Huang, J.; Xia, Z. Zika Virus-Spread, Epidemiology, Genome, Transmission Cycle, Clinical Manifestation, Associated Challenges, Vaccine and Antiviral Drug Development. Virology 2020, 543, 34–42. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Oliva, S.M.; Zhu, H. Modeling the Transmission and Control of Zika in Brazil. Sci. Rep. 2017, 7, 7721. [Google Scholar] [CrossRef]
- Dénes, A.; Ibrahim, M.A.; Oluoch, L.; Tekeli, M.; Tekeli, T. Impact of Weather Seasonality and Sexual Transmission on the Spread of Zika Fever. Sci. Rep. 2019, 9, 17055. [Google Scholar] [CrossRef]
- Metsky, H.C.; Matranga, C.B.; Wohl, S.; Schaffner, S.F.; Freije, C.A.; Winnicki, S.M.; West, K.; Qu, J.; Baniecki, M.L.; Gladden-Young, A.; et al. Zika Virus Evolution and Spread in the Americas. Nature 2017, 546, 411–415. [Google Scholar] [CrossRef]
- Joguet, G.; Mansuy, J.M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of Acute Zika Virus Infection on Sperm and Virus Clearance in Body Fluids: A Prospective Observational Study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef]
- Althaus, C.L.; Low, N. How Relevant Is Sexual Transmission of Zika Virus? PLoS Med. 2016, 13, e1002157. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Giannakopoulos, X.; Sofikitis, N.; Papadopoulou, C. An Update on Sexual Transmission of Zika Virus. Pathogens 2018, 7, 66. [Google Scholar] [CrossRef]
- Aguiar, R.S.; Pohl, F.; Morais, G.L.; Nogueira, F.C.S.; Carvalho, J.B.; Guida, L.; Arge, L.W.P.; Melo, A.; Moreira, M.E.L.; Cunha, D.P.; et al. Molecular Alterations in the Extracellular Matrix in the Brains of Newborns with Congenital Zika Syndrome. Sci. Signal. 2020, 13, eaay6736. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Oliveira, A.L.R.; Wang, H.; Zukor, K.; de Castro, M.V.; Siddharthan, V. Zika Virus Infection Causes Temporary Paralysis in Adult Mice with Motor Neuron Synaptic Retraction and Evidence for Proximal Peripheral Neuropathy. Sci. Rep. 2019, 9, 19531. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Y.; Rojas, M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramírez-Santana, C.; Monsalve, D.M.; Gershwin, M.E.; Anaya, J.M. Guillain–Barré Syndrome, Transverse Myelitis and Infectious Diseases. Cell Mol. Immunol. 2018, 15, 547–562. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika Virus Fact Sheet. WPRO Fact Sheets 2016, 29, 487–524. [Google Scholar] [CrossRef]
- De Carvalho, N.S.; De Carvalho, B.F.; Fugaça, C.A.; Dóris, B.; Biscaia, E.S. Zika Virus Infection during Pregnancy and Microcephaly Occurrence: A Review of Literature and Brazilian Data. Braz. J. Infect. Dis. 2016, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Wilson, M.E.; Touch, S.; McCloskey, B.; Mwaba, P.; Bates, M.; Dar, O.; Mattes, F.; Kidd, M.; Ippolito, G.; et al. Rapid Spread of Zika Virus in The Americas-Implications for Public Health Preparedness for Mass Gatherings at the 2016 Brazil Olympic Games. Int. J. Infect. Dis. 2016, 44, 11–15. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, K.; Chinazzi, M.; Piontti, A.P.Y.; Dean, N.E.; Rojas, D.P.; Merler, S.; Mistry, D.; Poletti, P.; Rossi, L.; et al. Spread of Zika Virus in the Americas. Proc. Natl. Acad. Sci. USA 2017, 114, E4334–E4343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, R.; Shen, H.; Wang, M.; Yin, Z.; Cheng, A. Structures and Functions of the Envelope Glycoprotein in Flavivirus Infections. Viruses 2017, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, M.; Meyniel-Schicklin, L.; Deloire, A.; Coutard, B.; Canard, B.; De Lamballerie, X.; Andre, P.; Rabourdin-Combe, C.; Lotteau, V.; Davoust, N. Flavivirus NS3 and NS5 Proteins Interaction Network: A High-Throughput Yeast Two-Hybrid Screen. BMC Microbiol. 2011, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Mazeaud, C.; Freppel, W.; Chatel-Chaix, L. The Multiples Fates of the Flavivirus RNA Genome During Pathogenesis. Front. Genet. 2018, 9, 595. [Google Scholar] [CrossRef]
- Plevka, P.; Battisti, A.J.; Junjhon, J.; Winkler, D.C.; Holdaway, H.A.; Keelapang, P.; Sittisombut, N.; Kuhn, R.J.; Steven, A.C.; Rossmann, M.G. Maturation of Flaviviruses Starts from One or More Icosahedrally Independent Nucleation Centres. EMBO Rep. 2011, 12, 602–606. [Google Scholar] [CrossRef]
- Rothan, H.A.; Kumar, M. Role of Endoplasmic Reticulum-Associated Proteins in Flavivirus Replication and Assembly Complexes. Pathogens 2019, 8, 148. [Google Scholar] [CrossRef]
- Rastogi, M.; Sharma, N.; Singh, S.K. Flavivirus NS1: A Multifaceted Enigmatic Viral Protein. Virol. J. 2016, 13, 131. [Google Scholar] [CrossRef]
- Perera-Lecoin, M.; Meertens, L.; Carnec, X.; Amara, A. Flavivirus Entry Receptors: An Update. Viruses 2013, 6, 69–88. [Google Scholar] [CrossRef]
- Kaufmann, B.; Rossmann, M.G. Molecular Mechanisms Involved in the Early Steps of Flavivirus Cell Entry. Microbes Infect. 2011, 13, 1–9. [Google Scholar] [CrossRef]
- Smit, J.M.; Moesker, B.; Rodenhuis-Zybert, I.; Wilschut, J. Flavivirus Cell Entry and Membrane Fusion. Viruses 2011, 3, 160–171. [Google Scholar] [CrossRef]
- Jones, C.T.; Ma, L.; Burgner, J.W.; Groesch, T.D.; Post, C.B.; Kuhn, R.J. Flavivirus Capsid Is a Dimeric Alpha-Helical Protein. J. Virol. 2003, 77, 7143–7149. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A Structural Perspective of the Flavivirus Life Cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.N.; Sanches, P.R.d.S.; Carneiro, B.M.; Braga, A.C.S.; Campos, G.R.F.; Cilli, E.M.; Rahal, P. GA-Hecate Antiviral Properties on HCV Whole Cycle Represent a New Antiviral Class and Open the Door for the Development of Broad Spectrum Antivirals. Sci. Rep. 2018, 8, 14329. [Google Scholar] [CrossRef]
- Batista, M.N.; Braga, A.C.S.; Fernandes Campos, G.R.; Michel Souza, M.; de Matos, R.P.A.; Zara Lopes, T.; Maria Candido, N.; Duarte Lima, M.L.; Cristina Machado, F.; de Andrade, S.T.Q.; et al. Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus. Viruses 2019, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Jardim, A.C.G.; Shimizu, J.F.; Rahal, P.; Harris, M. Plant-Derived Antivirals against Hepatitis c Virus Infection. Virol. J. 2018, 15, 34. [Google Scholar] [CrossRef]
- Fink, S.L.; Vojtech, L.; Wagoner, J.; Slivinski, N.S.J.; Jackson, K.J.; Wang, R.; Khadka, S.; Luthra, P.; Basler, C.F.; Polyak, S.J. The Antiviral Drug Arbidol Inhibits Zika Virus. Sci. Rep. 2018, 8, 2–10. [Google Scholar] [CrossRef]
- Fox, J.L. Antivirals Become a Broader Enterprise. Nat. Biotechnol. 2007, 25, 1395–1402. [Google Scholar] [CrossRef]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The Emergence of Peptides in the Pharmaceutical Business: From Exploration to Exploitation. EuPA Open Proteom. 2014, 4, 58–69. [Google Scholar] [CrossRef]
- Tesauro, D.; Accardo, A.; Diaferia, C.; Milano, V.; Guillon, J.; Ronga, L.; Rossi, F. Peptide-Based Drug-Delivery Systems in Biotechnological Applications: Recent Advances and Perspectives. Molecules 2019, 24, 351. [Google Scholar] [CrossRef]
- He, R.; Finan, B.; Mayer, J.P.; DiMarchi, R.D. Peptide Conjugates with Small Molecules Designed to Enhance Efficacy and Safety. Molecules 2019, 24, 1855. [Google Scholar] [CrossRef]
- Lee, A.C.L.; Harris, J.L.; Khanna, K.K.; Hong, J.H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Montero, A.; Gastaminza, P.; Whitten-Bauer, C.; Wieland, S.F.; Isogawa, M.; Fredericksen, B.; Selvarajah, S.; Gallay, P.A.; Ghadiri, M.R.; et al. A Virocidal Amphipathic α-Helical Peptide That Inhibits Hepatitis C Virus Infection in Vitro. Proc. Natl. Acad. Sci. USA 2008, 105, 3088–3093. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.; Wang, S.; Sun, J.; Wang, P.; Xin, Q.; Zhang, L.; Xiao, G.; Wang, W. Antiviral Activity of Peptide Inhibitors Derived from the Protein E Stem against Japanese Encephalitis and Zika Viruses. Antivir. Res. 2017, 141, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Baghian, A.; Jaynes, J.; Enright, F.; Kousoulas, K.G. An Amphipathic α-Helical Synthetic Peptide Analogue of Melittin Inhibits Herpes Simplex Virus-1 (HSV-1)-Induced Cell Fusion and Virus Spread. Peptides 1997, 18, 177–183. [Google Scholar] [CrossRef]
- Freire, M.C.L.C.; Noske, G.D.; Bitencourt, N.V.; Sanches, P.R.S.; Santos-Filho, N.A.; Gawriljuk, V.O.; de Souza, E.P.; Nogueira, V.H.R.; de Godoy, M.O.; Nakamura, A.M.; et al. Non-Toxic Dimeric Peptides Derived from the Bothropstoxin-I Are Potent SARS-CoV-2 and Papain-like Protease Inhibitors. Molecules 2021, 26, 4896. [Google Scholar] [CrossRef]
- Sanches, P.R.S.; Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Lorenzón, E.N.; Rahal, P.; Cilli, E.M. A Conjugate of the Lytic Peptide Hecate and Gallic Acid: Structure, Activity against Cervical Cancer, and Toxicity. Amino Acids 2015, 47, 1433–1443. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef]
- Donald, C.L.; Brennan, B.; Cumberworth, S.L.; Rezelj, V.; Clark, J.J.; Cordeiro, M.T.; Freitas, R.; Franc, D.O.; Pena, L.J.; Wilkie, G.S.; et al. Full Genome Sequence and SfRNA Interferon Antagonist Activity of Zika Virus from Recife, Brazil. PLoS Negl. Trop. Dis. 2016, 10, e0005048. [Google Scholar] [CrossRef]
- Manavalan, B.; Patra, M.C. MLCPP 2.0: An Updated Cell-Penetrating Peptides and Their Uptake Efficiency Predictor. J. Mol. Biol. 2022, 434, 167604. [Google Scholar] [CrossRef]
- Simonin, Y.; van Riel, D.; Van de Perre, P.; Rockx, B.; Salinas, S. Differential Virulence between Asian and African Lineages of Zika Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005821. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in Oral Peptide Therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef]
- Spearman, C. The Method of “Right and Wrong Cases” (Constant Stimuli) without Gauss’s Formula. Br. J. Psychol. 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Hilpert, K.; Fjell, C.D.; Cherkasov, A. Peptide-Based Drug Design; Humana Press: Totowa, NJ, USA, 2008; Volume 494, ISBN 978-1-58829-990-1. [Google Scholar]
- Boöttger, R.; Hoffmann, R.; Knappe, D. Differential Stability of Therapeutic Peptides with Different Proteolytic Cleavage Sites in Blood, Plasma and Serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef] [PubMed]
- López-Camacho, C.; Abbink, P.; Larocca, R.A.; Dejnirattisai, W.; Boyd, M.; Badamchi-Zadeh, A.; Wallace, Z.R.; Doig, J.; Velazquez, R.S.; Neto, R.D.L.; et al. Rational Zika Vaccine Design via the Modulation of Antigen Membrane Anchors in Chimpanzee Adenoviral Vectors. Nat. Commun. 2018, 9, 2441. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.P. Biotransformation and in Vivo Stability of Protein Biotherapeutics: Impact on Candidate Selection and Pharmacokinetic Profiling. Drug Metab. Dispos. 2014, 42, 1873–1880. [Google Scholar] [CrossRef]
- Davenport, A.P.; Scully, C.C.G.; de Graaf, C.; Brown, A.J.H.; Maguire, J.J. Advances in Therapeutic Peptides Targeting G Protein-Coupled Receptors. Nat. Rev. Drug Discov. 2020, 19, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.; Fibriansah, G.; Kostyuchenko, V.A.; Ng, T.S.; Lim, X.X.; Zhang, S.; Lim, X.N.; Wang, J.; Shi, J.; Morais, M.C.; et al. Capsid Protein Structure in Zika Virus Reveals the Flavivirus Assembly Process. Nat. Commun. 2020, 11, 895. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural Biology of Zika Virus and Other Flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Interim Practical Manual: Supporting National Implementation of the WHO Guidelines on Core Components of Infection Prevention and Control Programmes; World Health Organization: Geneva, Switzerland, 2007; 77p. [Google Scholar]
- Andersen, D.O.; Weber, N.D.; Steven, G.W.; Hughes, B.G.; Murray, B.K.; North, J.A. In Vitro Virucidal Activity of Selected Anthraquinones and Anthraquinone Derivatives. Antivir. Res. 1991, 16, 185–196. [Google Scholar] [CrossRef]
- Schinazi, R.F.; Sijbesma, R.; Srdanov, G.; Hill, C.L.; Wudl, F. Synthesis and Virucidal Activity of a Water-Soluble, Configurationally Stable, Derivatized C60 Fullerene. Antimicrob. Agents Chemother. 1993, 37, 1707–1710. [Google Scholar] [CrossRef]
- Beer, B.E.; Doncel, G.F.; Krebs, F.C.; Shattock, R.J.; Fletcher, P.S.; Buckheit, R.W.; Watson, K.; Dezzutti, C.S.; Cummins, J.E.; Bromley, E.; et al. In Vitro Preclinical Testing of Nonoxynol-9 as Potential Anti-Human Immunodeficiency Virus Microbicide: A Retrospective Analysis of Results from Five Laboratories. Antimicrob. Agents Chemother. 2006, 50, 713–723. [Google Scholar] [CrossRef]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.A.; Blomster, R.N. Inactivation of Enveloped Viruses by Anthraquinones Extracted from Plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef]
- Akberova, S.I. New biological properties of p-aminobenzoic acid. Izv. Akad. Nauk. Ser. Biol. 2002, 4, 477–481. (In Russian) [Google Scholar]
- Sample, C.J.; Hudak, K.E.; Barefoot, B.E.; Koci, M.D.; Wanyonyi, M.S.; Abraham, S.; Staats, H.F.; Ramsburg, E. A mastoparan-derived peptide has broad-spectrum antiviral activity against enveloped viruses. Peptides 2013, 48, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lorenzón, E.N.; Sanches, P.R.S.; Nogueira, L.G.; Bauab, T.M.; Cilli, E.M. Dimerization of Aurein 1.2: Effects in Structure, Antimicrobial Activity and Aggregation of Cândida Albicans Cells. Amino Acids 2013, 44, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Vignoli Muniz, G.S.; Duarte, E.L.; Lorenzón, E.N.; Cilli, E.M.; Lamy, M.T. What Different Physical Techniques Can Disclose about Disruptions on Membrane Structure Caused by the Antimicrobial Peptide Hylin A1 and a More Positively Charged Analogue. Chem. Phys. Lipids 2022, 243, 105173. [Google Scholar] [CrossRef]
- Seyfi, R.; Kahaki, F.A.; Ebrahimi, T.; Montazersaheb, S.; Eyvazi, S.; Babaeipour, V.; Tarhriz, V. Antimicrobial Peptides (AMPs): Roles, Functions and Mechanism of Action. Int. J. Pept. Res. Ther. 2020, 26, 1451–1463. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-andres, J.; Fazakerley, J.K.; Hirsch, A.J. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Li, B.; Linhardt, R.J. Pathogenesis and Inhibition of Flaviviruses from a Carbohydrate Perspective. Pharmaceuticals 2017, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Sirohi, D.; Kuhn, R.J. Zika Virus Structure, Maturation, and Receptors. J. Infect. Dis. 2017, 216, S935–S944. [Google Scholar] [CrossRef] [PubMed]
- Jemielity, S.; Wang, J.J.; Chan, Y.K.; Ahmed, A.A.; Li, W.; Monahan, S.; Bu, X.; Farzan, M.; Freeman, G.J.; Umetsu, D.T.; et al. TIM-Family Proteins Promote Infection of Multiple Enveloped Viruses through Virion-Associated Phosphatidylserine. PLoS Pathog. 2013, 9, e1003232. [Google Scholar] [CrossRef] [PubMed]
- Sittampalam, G.S.; Coussens, N.P.; Brimacombe, K. Assay Guidance Manual [Internet]. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences. 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 4 June 2023).
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An Automated Protein Homology-Modeling Server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chan, H.C.S.; Hu, Z. Using PyMOL as a Platform for Computational Drug Design. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7. [Google Scholar] [CrossRef]
- Kasturi, L.; Chen, H.; Shakin-Eshleman, S.H. Regulation of N-Linked Core Glycosylation: Use of a Site-Directed Mutagenesis Approach to Identify Asn-Xaa-Ser/Thr Sequons That Are Poor Oligosaccharide Acceptors. Biochem. J. 1997, 323, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Routhu, N.K.; Lehoux, S.D.; Rouse, E.A.; Bidokhti, M.R.M.; Giron, L.B.; Anzurez, A.; Reid, S.P.; Abdel-Mohsen, M.; Cummings, R.D.; Byrareddy, S.N. Glycosylation of Zika Virus Is Important in Host–Virus Interaction and Pathogenic Potential. Int. J. Mol. Sci. 2019, 20, 5206. [Google Scholar] [CrossRef]
- Nilsson, I.M.; Von Heijne, G. Glycosylation Efficiency of Asn-Xaa-Thr Sequons Depends Both on the Distance from the C Terminus and on the Presence of a Downstream Transmembrane Segment. J. Biol. Chem. 2000, 275, 17338–17343. [Google Scholar] [CrossRef]
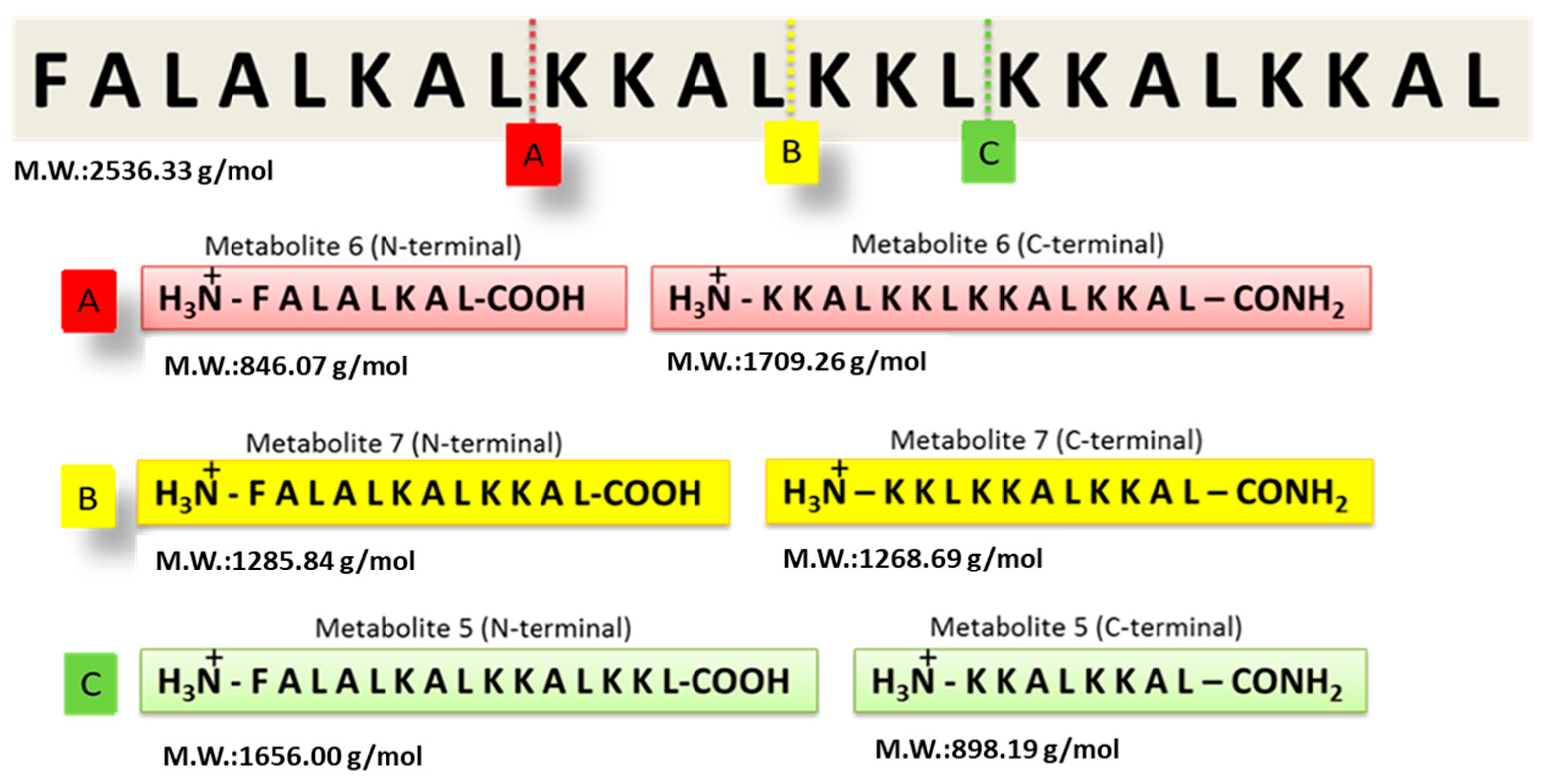


| Peptide | Hecate | Metabolite 5 | Metabolite 6 | Metabolite 7 | |||
|---|---|---|---|---|---|---|---|
| Region | N-terminal | C-Terminal | N-terminal | C-terminal | N-terminal | C-terminal | |
| Theoretical M.W. (g/mol) | 2536.3 | 1656.0 | 898.2 | 846.1 | 1709.3 | 1285.8 | 1268.7 |
| Experimental Results (m/z) | 508.3 (+5) 635.0 (+4) 846.2 (+3) | 1656.6 (+1) | 301.2 (+3) | 846.3 * | 1285.5 (+1) 645.7 (+2) | 1268.7 (+1) | |
| Name | Amino Acid Sequence | Molecular Weight (g/mol) | Net Charge 1 (pH 7.0) | Water 2 Solubility |
|---|---|---|---|---|
| GA-Hecate | GA-FALALKALKKALKKLKKALKKAL-CONH2 | 2688.4 | +9 | Soluble |
| GA-Metabolite 5 | GA-FALALKALKKALKKL-COOH | 1808.3 | +4 | Soluble |
| GA-Metabolite 6 | GA-FALALKAL-COOH | 998.2 | 0 | Not soluble |
| GA-Metabolite 7 | GA-FALALKALKKAL-COOH | 1438.9 | +2 | Soluble |
| Peptide | IC50 (µM) a | CC50 (µM) b | Uptake Efficiency c |
|---|---|---|---|
| GA-Hecate | 19.5 | >70.0 | Low |
| GA-Metabolite 5 | >40.0 | >70.0 | High |
| GA-Metabolite 7 | >80.0 | >80.0 | Low |
| Co-treatment | >40.0 | >70.0 | n.d. |
| FFU/mL | |||
|---|---|---|---|
| Concentration/Time | Control | GA-Hecate | GA-Metabolite 5 |
| 40 µM | |||
| 48 hpi | 9.84 × 105 ± 0.52 | 9.84 ± 0.23 | - |
| 72 hpi | 7.38 × 106 ± 0.08 | 3.11 × 104 ± 0.6 | 7.38 ± 0.82 |
| 20 µM | |||
| 48 hpi | 9.31 × 105 ± 0.65 | 9.63 × 105 ± 0.5 | - |
| 72 hpi | 7.42 × 106 ± 0.45 | 9.38 × 106 ± 0.5 | 9.63 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Sanches, P.R.; Velazquez, R.S.; Batista, M.N.; Carneiro, B.M.; Bittar, C.; De Lorenzo, G.; Rahal, P.; Patel, A.H.; Cilli, E.M. Antiviral Evaluation of New Synthetic Bioconjugates Based on GA-Hecate: A New Class of Antivirals Targeting Different Steps of Zika Virus Replication. Molecules 2023, 28, 4884. https://doi.org/10.3390/molecules28134884
da Silva Sanches PR, Velazquez RS, Batista MN, Carneiro BM, Bittar C, De Lorenzo G, Rahal P, Patel AH, Cilli EM. Antiviral Evaluation of New Synthetic Bioconjugates Based on GA-Hecate: A New Class of Antivirals Targeting Different Steps of Zika Virus Replication. Molecules. 2023; 28(13):4884. https://doi.org/10.3390/molecules28134884
Chicago/Turabian Styleda Silva Sanches, Paulo Ricardo, Ricardo Sanchez Velazquez, Mariana Nogueira Batista, Bruno Moreira Carneiro, Cintia Bittar, Giuditta De Lorenzo, Paula Rahal, Arvind H. Patel, and Eduardo Maffud Cilli. 2023. "Antiviral Evaluation of New Synthetic Bioconjugates Based on GA-Hecate: A New Class of Antivirals Targeting Different Steps of Zika Virus Replication" Molecules 28, no. 13: 4884. https://doi.org/10.3390/molecules28134884
APA Styleda Silva Sanches, P. R., Velazquez, R. S., Batista, M. N., Carneiro, B. M., Bittar, C., De Lorenzo, G., Rahal, P., Patel, A. H., & Cilli, E. M. (2023). Antiviral Evaluation of New Synthetic Bioconjugates Based on GA-Hecate: A New Class of Antivirals Targeting Different Steps of Zika Virus Replication. Molecules, 28(13), 4884. https://doi.org/10.3390/molecules28134884





