Abstract
A series of benzyl, phenyl guanidine, and aminoguandine hydrazone derivatives was designed and in vitro antibacterial activities against two different bacterial strains (Staphylococcus aureus and Escherichia coli) were determined. Several compounds showed potent inhibitory activity against the bacterial strains evaluated, with minimal inhibitory concentration (MIC) values in the low µg/mL range. Of all guanidine derivatives, 3-[2-chloro-3-(trifluoromethyl)]-benzyloxy derivative 9m showed the best potency with MICs of 0.5 µg/mL (S. aureus) and 1 µg/mL (E. coli), respectively. Several aminoguanidine hydrazone derivatives also showed good overall activity. Compounds 10a, 10j, and 10r–s displayed MICs of 4 µg/mL against both S. aureus and E. coli. In the aminoguanidine hydrazone series, 3-(4-trifluoromethyl)-benzyloxy derivative 10d showed the best potency against S. aureus (MIC 1 µg/mL) but was far less active against E. coli (MIC 16 µg/mL). Compound 9m and the para-substituted derivative 9v also showed promising results against two strains of methicillin-resistant Staphylococcus aureus (MRSA). These results provide new and potent structural leads for further antibiotic optimisation strategies.
1. Introduction
Bacterial infections with multidrug-resistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci (VRE), and multidrug-resistant Escherichia coli, pose an increasing threat to the global human population [1,2,3]. These drug-resistant bacteria can cause lethal infections, making the treatment of infected patients increasingly difficult. Therefore, the discovery of novel therapeutic agents that are active against drug-resistant microorganisms remains a fundamental challenge, especially for medicinal chemistry. Most antibiotics currently in clinical use target one of the metabolic pathways of DNA, RNA, protein, or cell wall synthesis [4]. Due to the emergence of pathogens with reduced susceptibility to currently available antibiotic therapies, there is an urgent need to discover new antibiotics with new targets and mechanisms of action.
In recent years, bacterial cell division has attracted considerable attention as a potential antibiotic target [5,6]. Cell division in bacteria is achieved through a highly dynamic macromolecular complex that is characterized by a time-dependent assembly of specific cell division proteins [7], formed in an orchestrated fashion by the essential tubulin homolog FtsZ (filamentous temperature-sensitive protein Z). Most bacteria depend on FtsZ as the main protein for efficient cell division [8,9]. Therefore, FtsZ has been validated as a highly promising target for antibacterial intervention [5].
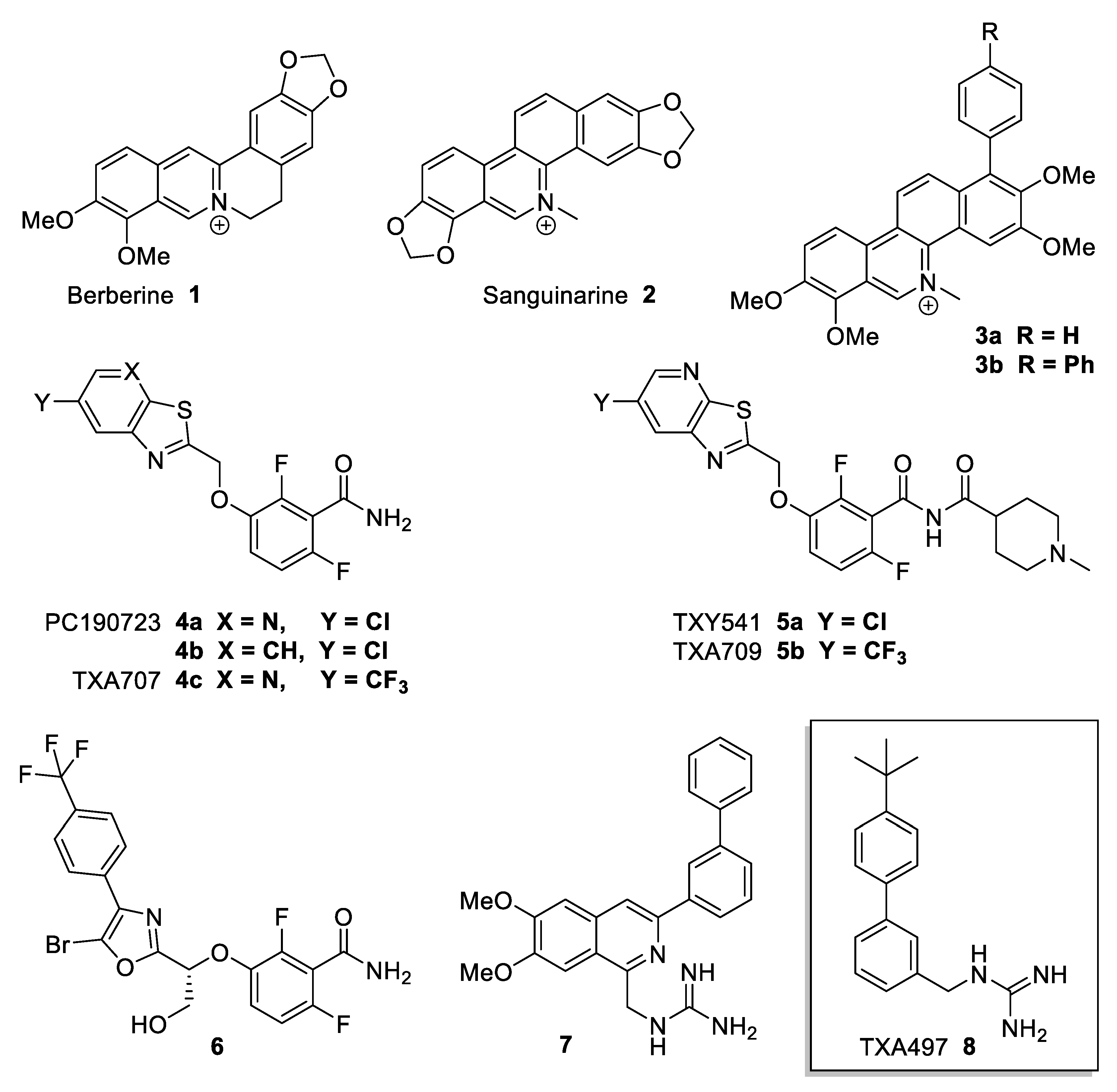
Antibacterial compounds known to target FtsZ are eg Berberine 1 and Sanguinarine 2 (Figure 1). Berberine 1 is a natural plant alkaloid that has been described to target E. coli FtsZ [10,11]. It binds to FtsZ with high affinity in a region that overlaps with the GTP binding site of FtsZ, inhibits FtsZ GTPase activity, and destabilises FtsZ protofilaments [10]. However, it showed only weak antibacterial activity against Gram-positive and Gram-negative species in recent studies [12,13]. Sanguinarine 2 is another, structurally similar, natural plant alkaloid that possesses inhibitory activity against several microorganisms, including MRSA [14]. The optimised derivatives 3a,b showed more enhanced potency [14]. In recent years, a number of compounds have been reported that modulate the assembly/disassembly dynamics of FtsZ, some of which have shown very promising antibacterial activity against important human pathogens and are efficacious even in in vivo models of infection. Benzamide derivative PC190723 4a was identified as an FtsZ inhibitor with antibacterial activity against staphylococci, including multidrug-resistant S. aureus, with minimal inhibitory concentrations (MICs) in the range of 0.5–1.0 µg/mL [15]. PC190723 4a was also effective in a murine septicaemia model of staphylococcal infection and was, thus, the first FtsZ inhibitor with reported in vivo efficacy [15,16]. The closely related analogue 4b showed an improved MIC of 0.25 µg/mL against S. aureus, albeit with an inferior pharmacokinetic profile [17,18]. To improve efficacy, prodrug derivative 5a was developed but showed dechlorination and monooxygenation as a metabolic pathway, a possibility eliminated in 5b by the introduction of CF3 instead of Cl [19,20,21]. Compound 6, a recently reported advanced derivative of PC190723 4a, showed improved antibacterial activity with an average MIC of 0.12 µg/mL against S. aureus and S. epidermidis and high oral bioavailability [22,23].
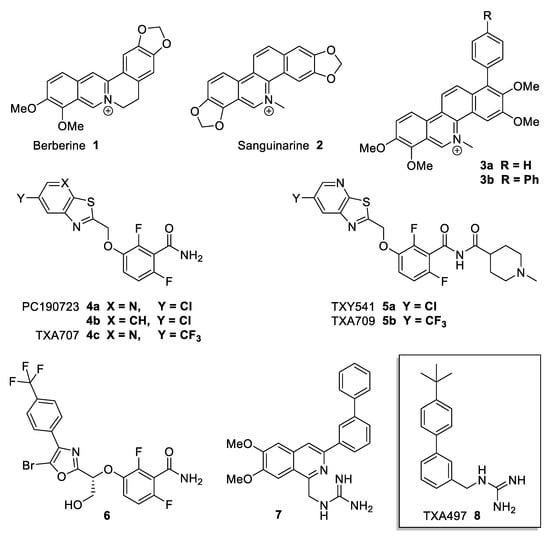
Figure 1.
Antibiotics acting on FtsZ.
A class of compounds regularly reported in the antibiotic context are guanidine derivatives [24]. Guanidine functionalities are commonly found in many biologically relevant molecules that constitute a versatile class of molecules with a wide range of applications. Compounds, either natural or synthetic, containing guanidine as a core unit, either in open or in cyclic form, display an array of pharmacological properties, including antimicrobial, antiviral, antiparasitic, and antifungal activities [24]. The great appeal of the guanidine moiety can be attributed to its hydrogen-bonding capability and protonatability at physiological pH in the context of interaction with biological targets. Bacterial cell envelopes are negatively charged, which may attract the guanidinium cation via electrostatic interaction and favour the binding of these compounds, leading to the disruption of cell membranes and cell walls. Therefore, guanidine derivatives have been exploited as privileged structural motifs in designing novel drugs for the treatment of various infectious and non-infectious diseases. Over many years, a large variety of synthetic small molecules with one or several guanidine units has emerged [24]. There are also synthetic polymeric guanidine derivatives that display very potent antibiotic activities against MRSA in skin infections and against the growth of Aspergillus parasiticus [25,26].
Recent examples of synthetic, small-molecule-type, investigational antibiotics with guanidine motifs are compounds 7 and TXA497 8 (Figure 1) [27,28,29,30]. Biphenyl derivative 8 especially, despite its structural simplicity, displays quite a few remarkable antimicrobial properties [29,30]. First, 8 shows low MICs (1 µg/mL) against several variations of S. aureus including MRSA. Furthermore, 8 also displays a very low MBC value (minimum bactericidal concentration) of 1 µg/mL, leading to a ratio of MBC/MIC = 1 which essentially means that 99.9% of all bacterial cells are killed at the same concentration of minimum inhibition (MIC). The same MBC/MIC ratio for 8 was observed against E. coli, but, at 16 µg/mL, the compound concentrations were much higher [30]. However, more remarkably, it was found that 8 exhibits only a minimal potential for inducing resistance in S. aureus [30]. Initially, 8 was proposed to act on FtsZ dynamics and it was shown to target the GTP binding site of recombinant FtsZ in vitro [30]. However, in bacterial cells, 8 targets the bacterial cell membrane in addition to FtsZ, with some cells predominantly showing the effects of FtsZ inhibition and others predominantly showing the effects of cell membrane disruption [31]. The guanidino/amidino functionality is a key contributor to the antibacterial activity of this class of compounds.
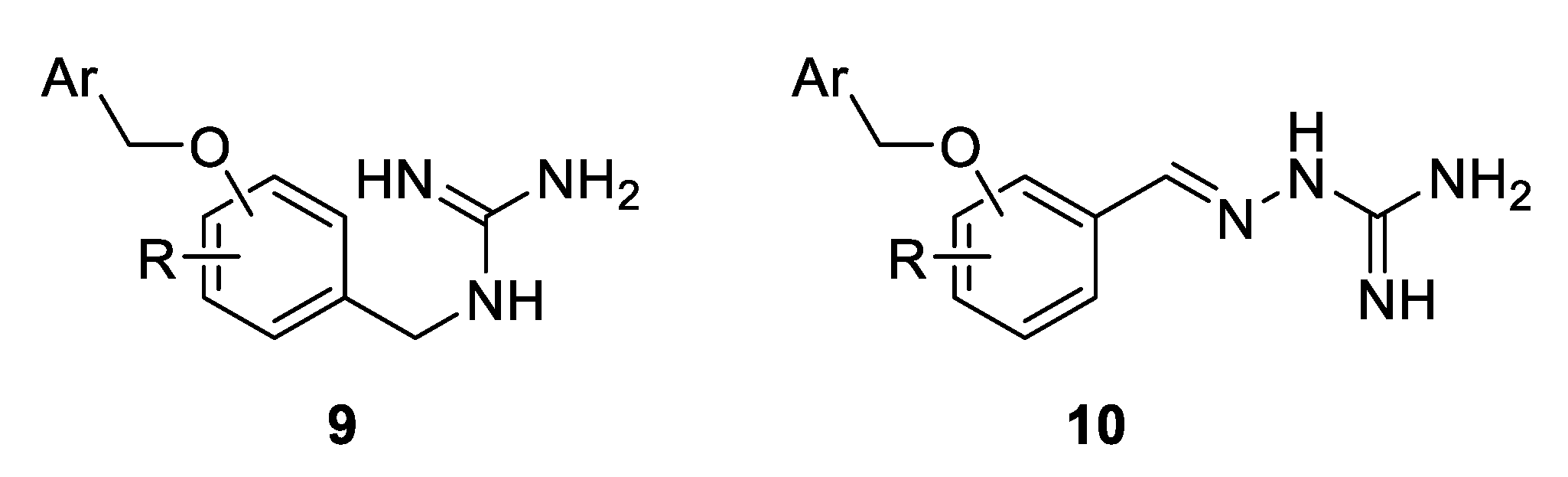
The structural simplicity of 8 was highly attractive from a medicinal chemistry standpoint. As a part of a program to design a library of novel antimicrobial compounds, only a small set of benzyl guanidine 9 and aminoguanidine hydrazone derivative 10 with a variety of benzyloxy groups was initially envisaged (Figure 2). However, the compound series was later expanded, and new broadly-related, but also diverse, guanidine derivatives were additionally synthesised and their antimicrobial activities against S. aureus and E. coli were evaluated [32]. The most potent inhibitors were then tested against the drug-resistant strains MRSA 3 and MRSA 15.
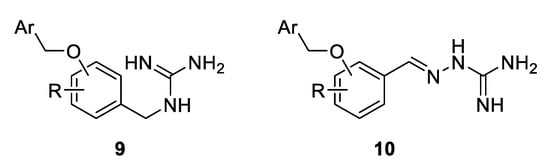
Figure 2.
Design of benzyl guanidine and aminoguanidine hydrazone derivatives with a variety of benzyloxy groups.
2. Results and Discussion
2.1. Chemistry
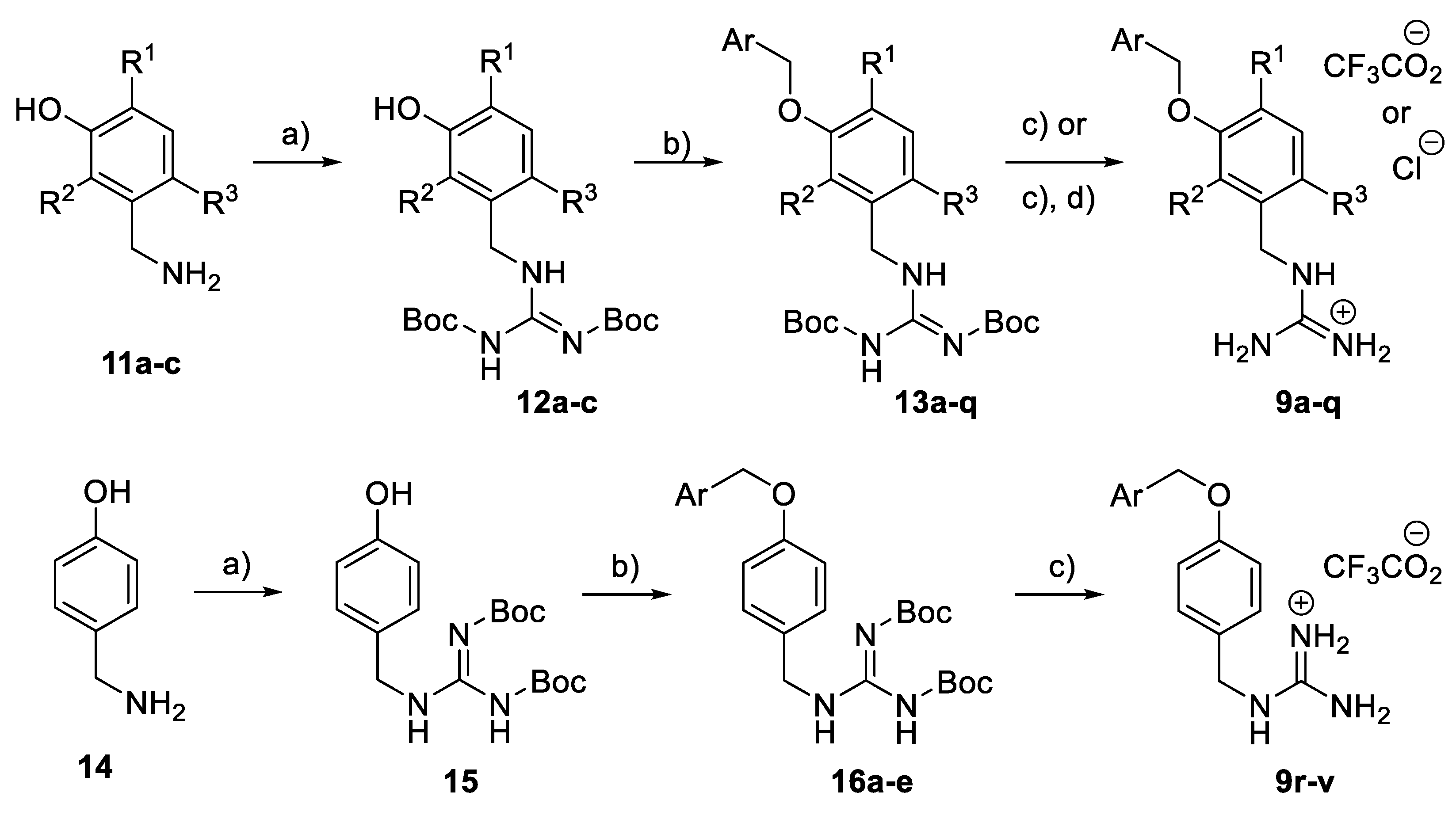
The meta-substituted benzyl guanidine compounds 9a–q were constructed from the corresponding 3-aminomethylphenol derivatives 11a–c via a guanylation reaction using Boc-protected S-methylisothiourea [33], followed by the benzylation of the phenol group under basic conditions to give 13a–q. Finally, treatment with trifluoroacetic acid in dichloromethane led to 9a–q, obtained as their guanidinium trifluoroacetate or chloride salts (Scheme 1). Benzyl guanidine derivatives 9r–v were prepared under the same conditions using 4-aminomethylphenol 14 as the starting material.
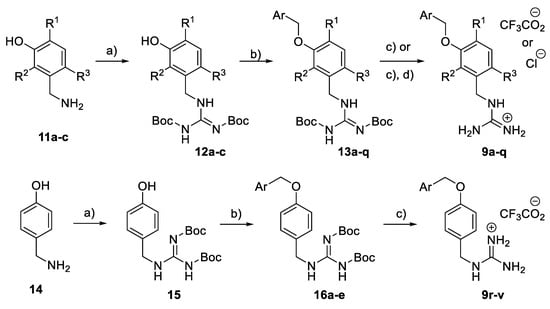
Scheme 1.
Synthesis of benzyl guanidine derivatives 9a–v. Reagents and conditions: (a) BocN=C(SMe)-NHBoc, Et3N, DMF, rt; (b) Benzyl halide, K2CO3, actone; (c) TFA, CH2Cl2, rt; (d) HCl (0.5M in MeOH), rt.
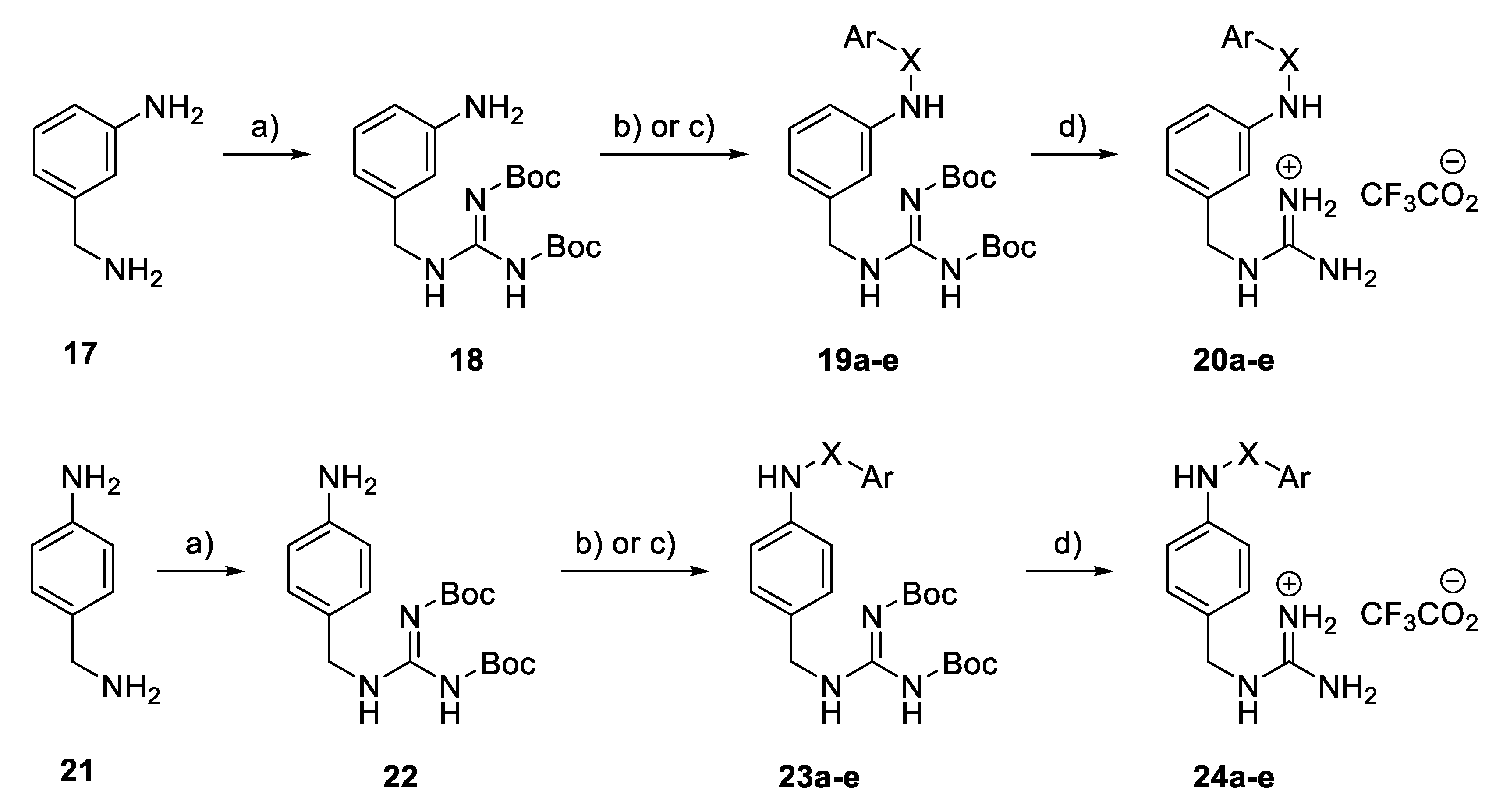
In a benzylguanidine-based structural subset, the meta- and para-substituted compounds 20a–e and 24a–e (Scheme 2) were constructed from 3- and 4-aminomethylaniline 17 and 21 via a guanylation reaction using Boc-protected S-methylisothiourea, followed by the treatment of the resulting 18 and 22, respectively, with the corresponding arylsulfonyl chloride or benzoyl chloride in the presence of a base to achieve Boc-protected derivatives 19a–e and 23a–e. Treatment with trifluoroacetic acid in dichloromethane led to the removal of the Boc groups, and the final compounds 20a–e and 24a–e were obtained as their guanidinium trifluoroacetate salts.
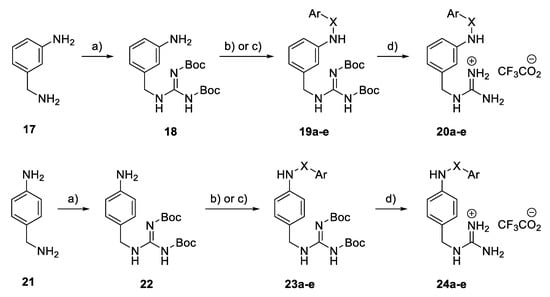
Scheme 2.
Synthesis of benzyl guanidine derivatives 20a–e and 24a–e. Reagents and conditions: (a) BocN=C(SMe) NHBoc, Et3N, DMF, rt; (b) ArSO2Cl, pyridine, CH2Cl2, 0 °C; (c) Benzoyl chloride, K2CO3, actone, 80 °C; (d) TFA, CH2Cl2, rt.
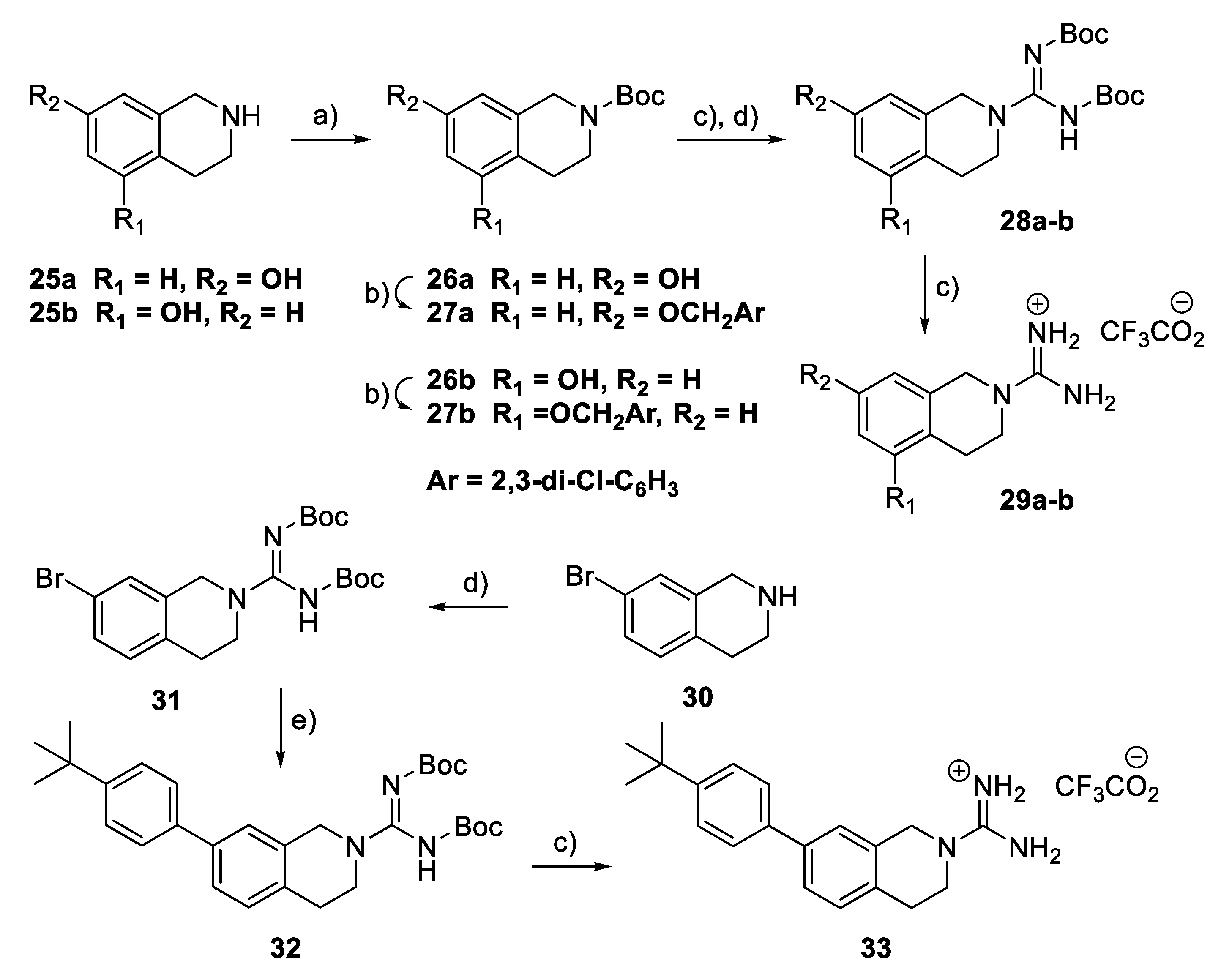
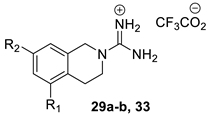
To explore the potential effects of conformational restriction of the guanidine moiety, the tetrahydroisoquinoline-based compounds 29a–b and 33 were prepared via the route shown in Scheme 3. First, compounds 29a–b were prepared from the corresponding hydroxy-substituted 1,2,3,4-tetrahydroisoquinolines 25a–b by N-Boc protection, benzylation, guanylation, and Boc deprotection. Similarly, guanylation of 7-bromo-1,2,3,4-tetrahydroisoquinoline 30 gave the corresponding 2-carboximidamide derivative 31 that was converted to 33 through a route involving a palladium-catalysed Suzuki coupling [30], followed by the removal of the Boc groups with TFA.
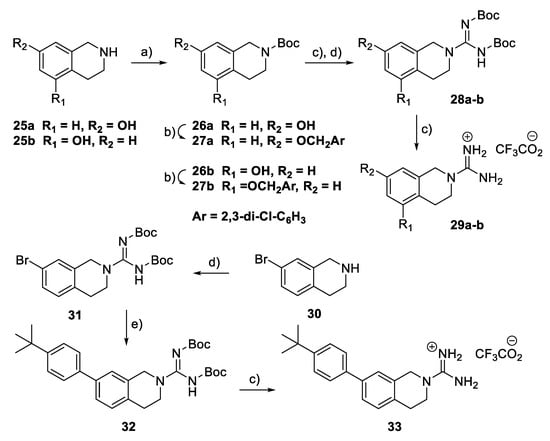
Scheme 3.
Synthesis of tetrahydroisoquinolinylguanidine derivatives 29a–b and 33. Reagents and conditions: (a) Boc2O, THF-H2O, Et3N, rt; (b) 2,3-Dichlorobenzyl bromide, K2CO3, acetone, rt; (c) TFA, CH2Cl2, rt; (d) BocN=C(SMe)-NHBoc, Et3N, DMF, rt; (e) 4-t-Butylphenylboronic acid, Pd(Ph3)4, K2CO3, dioxane, 100 °C.
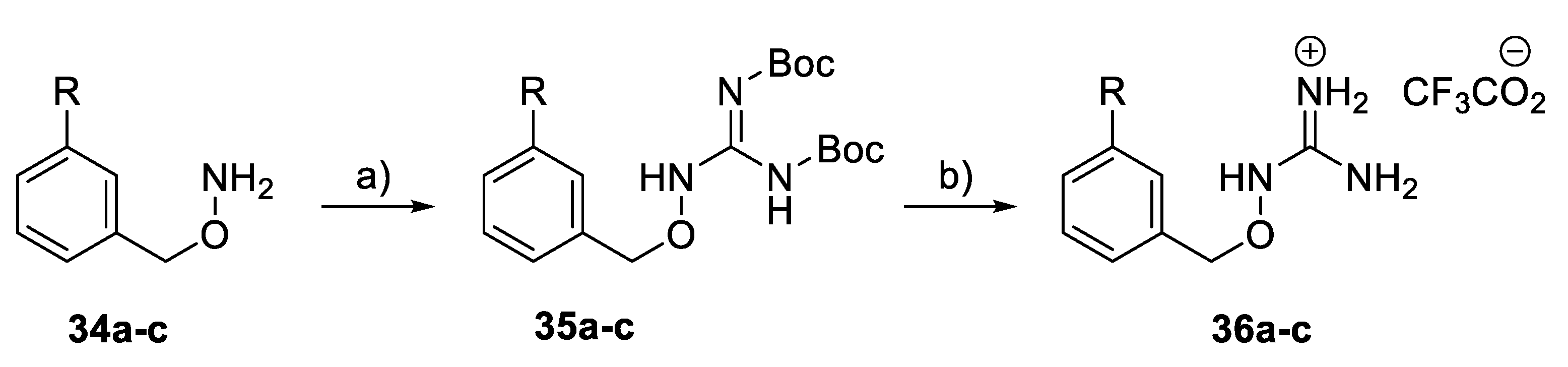
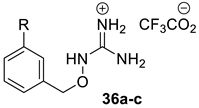
A subset of benzoyloxyguanidine compounds 36a–c (Scheme 4) was synthesised from the corresponding amine via a guanylation reaction, followed by the removal of the Boc protection groups in the presence of TFA.
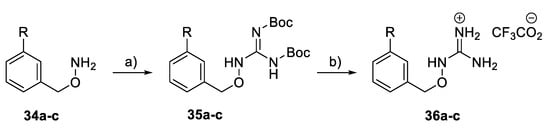
Scheme 4.
Synthesis of benzyloxy guanidine derivatives 36a–c. Reagents and conditions: (a) BocN=C(SMe)-NHBoc, Et3N, DMF, rt; (b) TFA, CH2Cl2, rt.
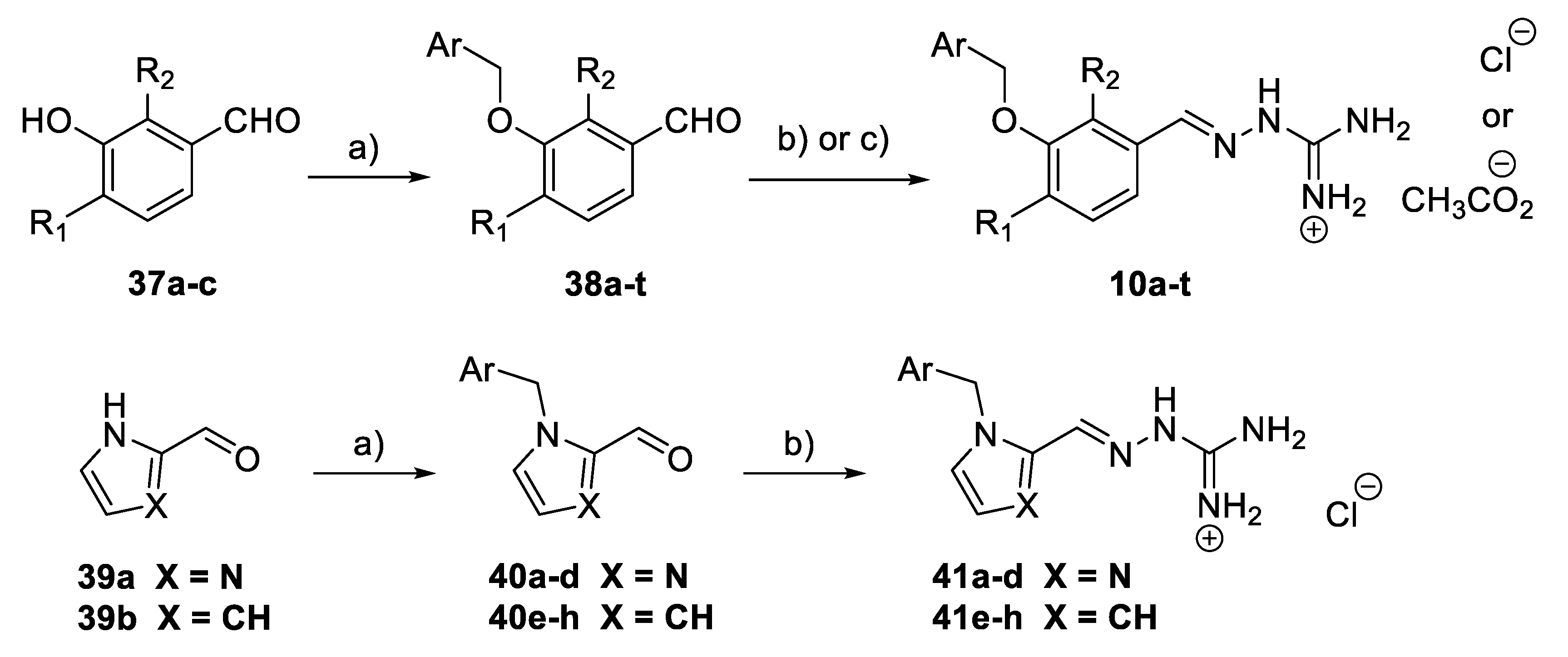
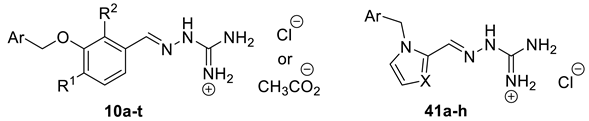
A subset of aminoguanidino hydrazone derivatives 10a–t (Scheme 5) was prepared in two steps from the corresponding 3-hydroxybenzaldehyde derivatives 37a–c, by benzylation of the hydroxyl group and condensation of the corresponding aldehydes 38a–t with N-aminoguanidine bicarbonate [34]. Most of the target compounds were obtained as their chloride salts and a few as acetates. Imidazole aminoguanidine (41a–d) and pyrrole aminoguanidine derivatives (41e–h) were synthesised as chloride salts in the same way using HCl (0.5M in MeOH) at 80 °C.
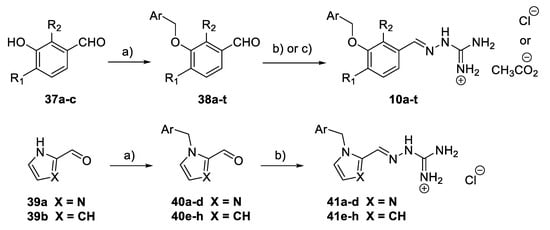
Scheme 5.
Synthesis of aminoguanidino hydrazone derivatives 10a–t and 41a–h. Reagents and conditions: (a) Benzyl halide, K2CO3, DMF, rt; (b) N-aminoguanidine bicarbonate, HCl (0.5 M in MeOH), 80 °C; (c) N-aminoguanidine bicarbonate, AcOH, MeOH, 80 °C.
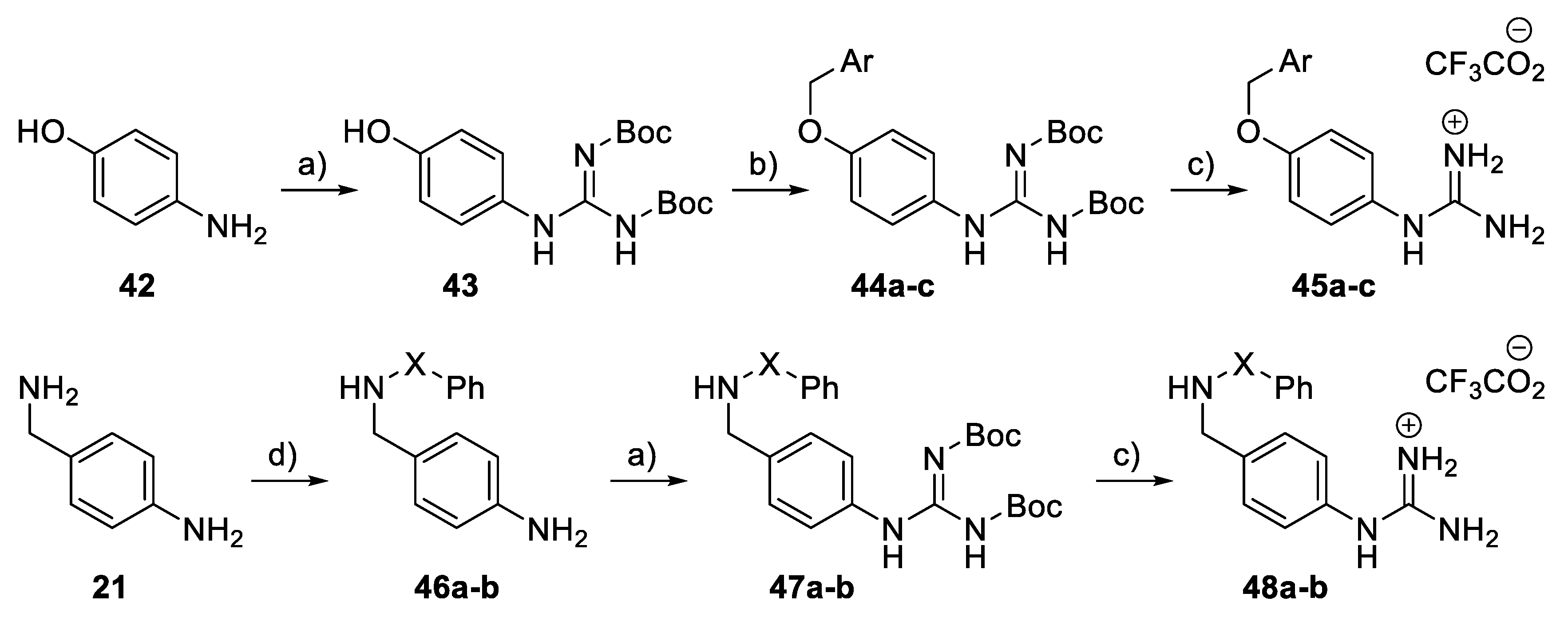
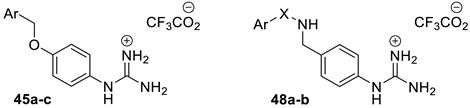
For phenylguanidino derivatives (Scheme 6), a guanylation reaction of para-aminophenol 42 generated the intermediate 43, which was subsequently subjected to benzylation to afford the N,N′-di-Boc protected guanidine derivatives 44a–c. Successive treatment with trifluoroacetic acid in dichloromethane gave 45a–c. Phenyl guanidine derivatives 48a–b were achieved in four steps. In the first step, 4-aminobenzylamine 21 was treated with either benzoyl chloride or benzenesulphonyl chloride and triethylamine in DMF to give 46a–b. Guanylation with Boc-protected S-methylisothiourea in the presence of mercury (II) chloride [35] then achieved 47a–b. Subsequent treatment with TFA removed the Boc groups to give 48a–b.
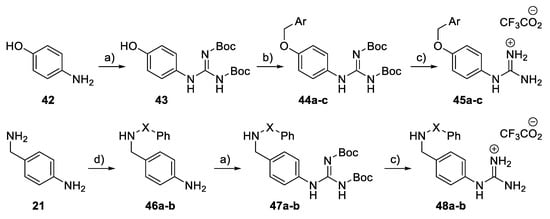
Scheme 6.
Synthesis of phenyl guanidine derivatives 45a–c and 48a–b. Reagents and conditions: (a) BocN=C(SMe)-NHBoc, HgCl2, Et3N, DMF, rt; (b) Benzyl halide, K2CO3, acetone, rt; (c) TFA, CH2Cl2, RT; (d) Benzoyl chloride or benzenesulphonyl chloride, Et3N, DMF, 0 °C.
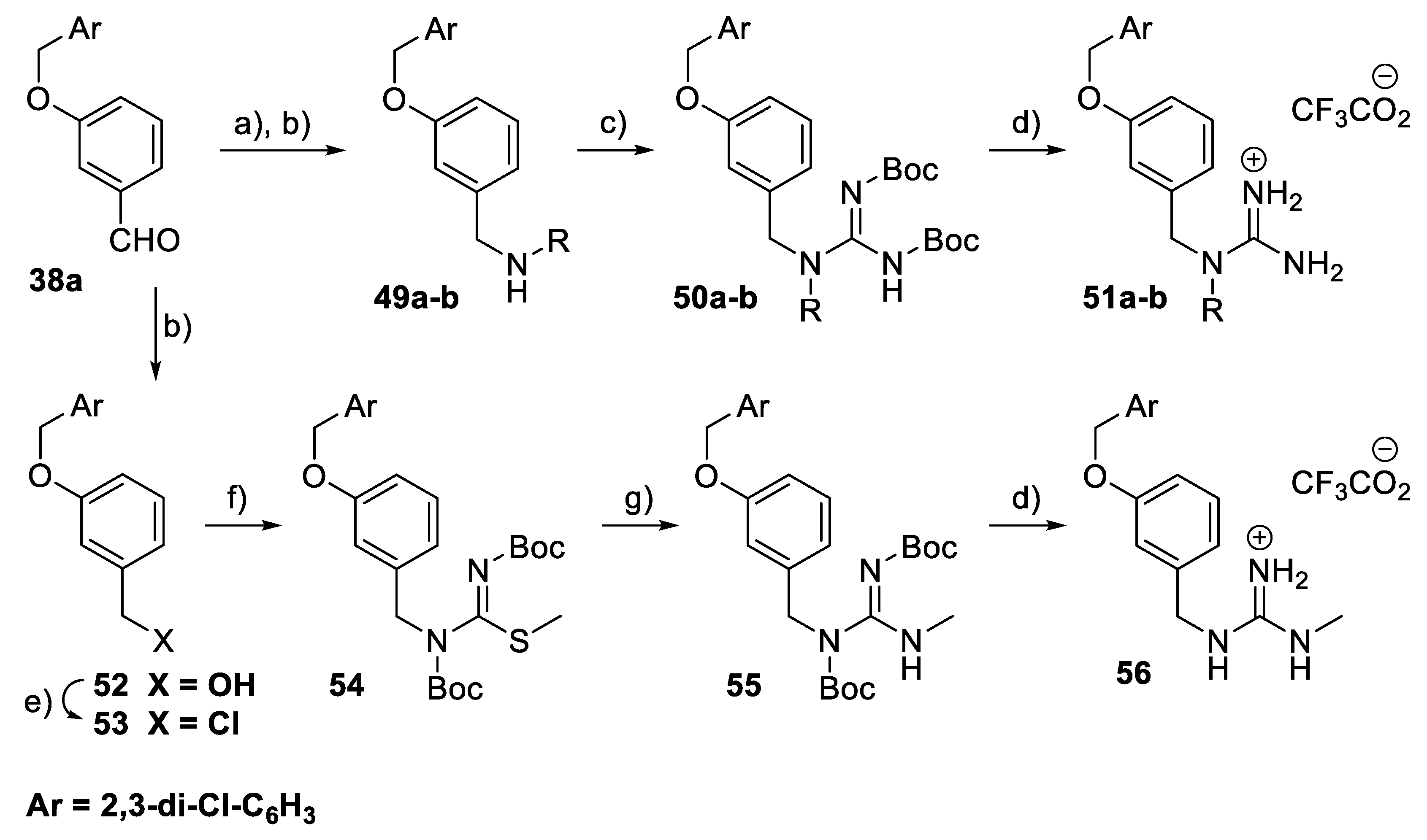
For a subset of benzyl guanidine derivatives 51a–b and 56 (Scheme 7), the reductive amination reaction of the aldehyde 38a (Ar = 2,3-dichlorophenyl) generated the amino intermediates 49a–b, which underwent a guanylation reaction to form the N,N′-di-Boc protected guanidine derivatives 50a–b. Deprotection of the Boc groups generated the guanidinium trifluoroacetate salts 51a–b. The intermediate 53 was obtained through the reduction of the aldehyde 38a, followed by halogenation of the resulting benzyl alcohol 52. Treatment of 53 with S-methyl-N,N′-bis(tert-butoxycarbonyl)isothiourea under basic conditions gave 54. Nucleophilic substitution of 54 with methylamine afforded the N,N′-di-Boc protected guanidine 55, which was then hydrolysed in TFA to give the final compound 56.
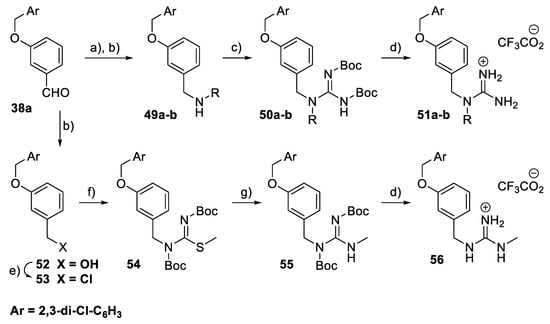
Scheme 7.
Synthesis of benzyl guanidine derivatives 51a–b and 56. Reagents and conditions: (a) R-NH2, MeOH, rt; (b) NaBH4, MeOH, 0 °C; (c) BocN=C(SMe)-NHBoc, Et3N, DMF, rt; (d) TFA, CH2Cl2, rt; (e) CH3SO2Cl, Et3N, CH2Cl2, rt. (f) BocN=C(SMe)-NHBoc, KOH, CH2Cl2, H2O, rt; (g) MeNH2, HgCl2, Et3N, DMF, rt.
2.2. Biology
2.2.1. In Vitro Antimicrobial Activity against S. aureus and E. coli
Table 1 reveals that a significant proportion of the synthesised benzyl guanidine derivatives is more potent against S. aureus than against E. coli. For 9a–m (R1 = R2 = R3 = H), only 9d, 9h, and 9k are more potent against E. coli than against S. aureus, with 9h showing the best activity (MIC = 4 µg/mL) of them. However, the biggest difference in potency against the two strains was found for 9d with MICs of >256 µg/mL and 8 µg/mL, respectively. The most potent compound in this subset was the 2-Cl-3-CF3 derivative 9m with MICs of 0.5 µg/mL and 1 µg/mL, respectively, but 2,3-dichloro derivative 9g showed very similar potency with MICs of 1 µg/mL against both microbial strains. For 9n–o (R1 = MeO, R2 = R3 = H), reduced potency was found. Comparison of 9n with 9c showed a significantly reduced potency for an H to MeO substitution. A MIC of 128 µg/mL for 9n and MICs of 16 µg/mL and 32 µg/mL for 9c were found. 4-Chloro derivatives 9o and 9b showed a similar pattern against E. coli but appeared to be equipotent against S. aureus with MICs of 8 µg/mL for both compounds. Derivatives 9p–q (R1 = H, R2 = R3 = F) proved very potent against S. aureus with MICs of 0.5 µg/mL and 1 µg/mL, respectively. However, in contrast to 9g and 9m, which were two compounds very active against both strains, 9p–q were significantly less potent against E. coli. A few para-substituted benzyl guanidine derivatives 9r–v were also evaluated. The monochlorobenzyl derivatives 9s–t proved both moderately active and did not show any difference in potency between the two microbial strains. The dichlorobenzyl derivatives 9u–v, on the other hand, proved significantly more potent against S. aureus than against E. coli, with 9v showing the best overall antimicrobial activities with MICs of 0.5 µg/mL and 4 µg/mL, respectively. Substitution of one hydrogen at the N atom of the guanidine unit in 9g, where the benzyl unit is attached with a methyl or a methoxyethyl group, led to a significant decrease in antimicrobial potency, from a MIC of 1 µg/mL for 9g to MICs of 32 µg/mL for 51a–b. A slightly better, but still very weak activity, against S. aureus was found for 56, a derivative where the methyl group was introduced at the terminal N atom (MIC of 16 µg/mL).

Table 1.
Antibacterial activities of benzyl guanidine derivatives 9a–v, 51a–b, and 56 against S. aureus and E. coli.
Benzyl guanidines with aminosulfonylaryl or aminobenzoyl motifs as substituents either in the meta- (20a–e) or para-position (24a–e) proved uniquely inactive for both sets of compounds (all MICs > 32 µg/mL, Table 2).

Table 2.
Antibacterial activities of benzyl guanidine derivatives 20a–e and 24a–e against S. aureus and E. coli.
The antimicrobial activities of tetrahydroisoquinoline guanidine derivatives 29a–b and 33 are summarised in Table 3. A comparison of 29a and 29b reveals that substitution in the 5-position seems more favourable than in the 7-positon. However, even 5-substituted derivative 29b proved only moderately active with MICs of 8 µg/mL for both S. aureus and E. coli. Replacing the O-benzyl linkage in the 7-position with a directly attached aromatic ring system seems to further reduce antimicrobial activity. 4-tert-Butylphenyl derivative 33 showed only weak potency with MICs of 64 µg/mL and >128 µg/mL, respectively.

Table 3.
Antibacterial activities of tetrahydroisoquinoline derivatives 29a–b and 33 against S. aureus and E. coli.
The antimicrobial activities of the three benzyloxy guanidine derivatives against S. aureus and E. coli are summarised in Table 4. All compounds of this class that we tested so far displayed no significant potency.

Table 4.
Antibacterial activities of benzyloxy guanidine derivatives 36a–c against S. aureus and E. coli.
The MIC values against S. aureus and E. coli of aminoguanidine hydrazone derivatives 10a–t and 41a–h are summarised in Table 5. Compounds 10a–f (R1 = R2 = H) showed overall moderate to good antimicrobial activities with the majority of MICs between 4 µg/mL and 16 µg/mL. Compound 10d was significantly active against S. aureus (MIC of 1 µg/mL) but less against E. coli (MIC of 16 µg/mL) and was the most potent compound against S. aureus of all aminoguanidine hydrazone derivatives. Methoxy-substituted derivative 10g (R1 = MeO, R2 = H) appeared to be less potent (MICs of 16 µg/mL and 8 µg/mL) when directly compared with 10a (both MICs of 4 µg/mL). Chloro-substituted compounds 10h–t (R1 = H, R2 = Cl) showed MICs between 4 µg/mL and 32 µg/mL. The most potent derivatives here were 10j and 10r–s, all of which are mono-substituted in the benzyloxy motif (4-Cl, 3-CF3, 4-CF3). All three compounds showed MICs of 4 µg/mL against both S. aureus and E. coli. Heterocyclic derivatives, including benzimidazole aminoguanidine hydrazones 41a–d as well as pyrrole aminoguanidine hydrazones 41e–h, displayed only moderate antimicrobial activities, with 41d showing the best potency against S. aureus (MIC of 8 µg/mL).

Table 5.
Antibacterial activities of aminoguanidine hydrazone derivatives 10a–t and 41a–h against S. aureus and E. coli.
Table 6 shows a summary of the MIC values for para-substituted phenyl guanidine derivatives 45a–c and 48a–b against S. aureus and E. coli. All derivatives showed only moderate antimicrobial potency with 45a displaying the best activity against S. aureus (MIC of 8 µg/mL).

Table 6.
Antibacterial activities of phenyl guanidine derivatives 45a–c and 48a–b against S. aureus and E. coli.
2.2.2. Antimicrobial Activity against MRSAs
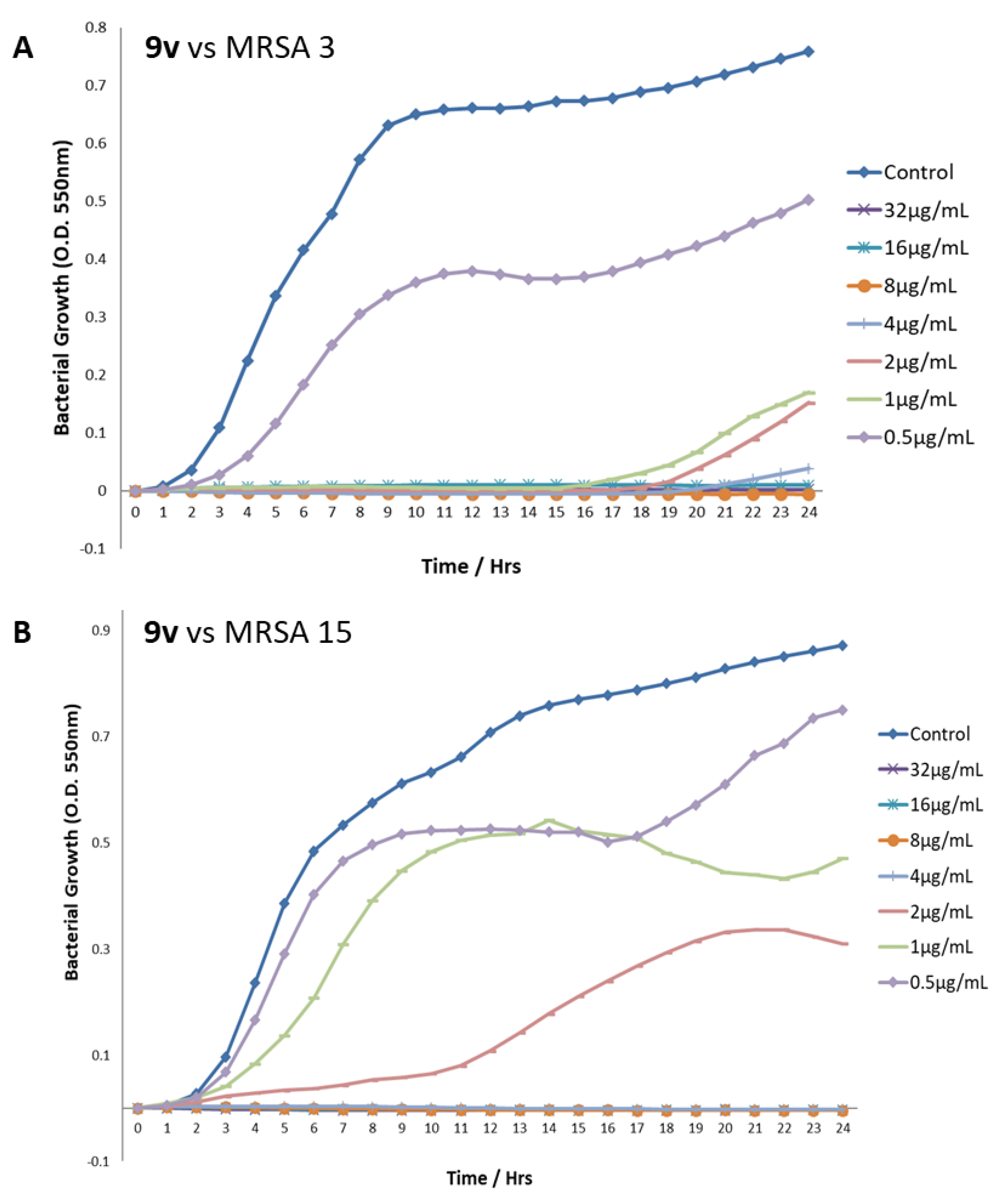
The benzyl guanidine derivatives 9m and 9v were also tested against MRSA 3 and MRSA 15. Bacterial growth was recorded against time at various concentrations of 9m and 9v. The example data for the growth of the MRSAs when treated with differing doses of 9v are shown in Figure 3. The minimum inhibitory concentration (MIC) and the survival index (SI) were established for each experiment (Table 7). In order to determine the SI, the growth of the treated bacteria was compared to the growth (measured as an increase in optical density over time) of the control, untreated bacteria, and the MIC was determined as the concentration that allowed an SI reduction of greater than 50%. As can be seen in Figure 3, as representative plots, although the total optical density change during the control growth varies slightly between the experiments, the growth curve progress and, hence, the overall growth profile of the control bacteria are very consistent, and each experiment’s individual control bacterial growth curve allows for minor variations in growth between the different compound treatments. The majority of the doses above the determined MIC values demonstrate a complete absence of growth of the bacteria over the 24 h of the experiments, with the concentrations around and below the MIC (where present) clearly showing a dose dependent, if only partial, recovery of the usual growth profile of the bacteria. The reported SI values at the MIC concentrations of each compound range between 1.76 and 12.76%, indicating a vastly reduced growth behaviour of the bacteria in all treatments.
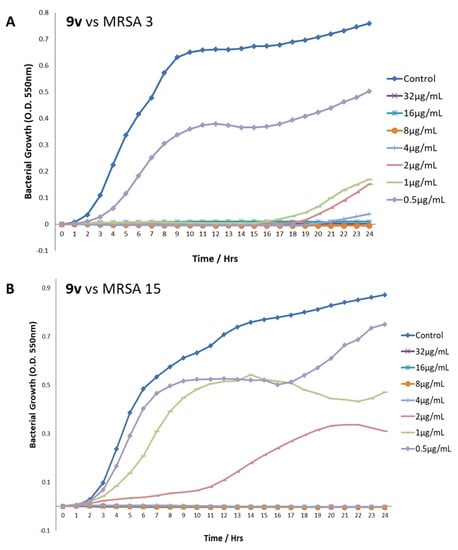
Figure 3.
Growth profiles of MRSA strains 3 and 15 after treatment with different concentrations of compound 9v. (A) 9v vs. MRSA 3. (B) 9v vs. MRSA 15.

Table 7.
Antibacterial activities and survival indices of 9m and 9v against MRSA 3 and MRSA 15.
3. Materials and Methods
3.1. Chemistry
Methods and Materials: All chemicals and anhydrous solvents were purchased from either Sigma-Aldrich (now Merck: Gillingham, UK) or Alfa Aesar (Heysham, UK). All organic solvents of AR grade were supplied by Fisher Scientific (Loughborough, UK). Melting points were determined using a Stanford Research Systems Optimelt MPA100 (Stanford Research Systems, Sunnyvale, CA, USA) and were uncorrected. Thin-layer chromatography (TLC) was performed on pre-coated aluminium plates (Merck, silica gel 60 F254). The products were visualised either by UV irradiation at 254 nm or by staining with 5% w/v phosphomolybdic acid in ethanol, followed by heating. Flash column chromatography was performed on pre-packed silica gel columns (RediSep Rf) and gradient elution (solvents indicated in text) on the Combiflash Rf system (Teledyne Isco). 1H NMR spectra were recorded with a Bruker 400 or 500 MHz spectrometer. The chemical shifts were reported in parts per million (ppm), either relative to the corresponding solvent residual peaks or tetramethylsilane (TMS) as an internal standard. High-resolution mass spectra (HRMS) were recorded on a Bruker MicroTOF with ESI. All compounds were ≥95% pure by 1H NMR spectroscopy.
General Procedure: Guanylation of substituted 3-(aminomethyl)phenols (11a–c): In a solution of the substituted 3-(aminomethyl)phenol hydrochloride 11a–c (6.0 mmol) in DMF (25 mL), S-methyl-N,N′-bis(tert-butoxycarbonyl) isothiourea (1.3 g, 4.5 mmol) was added, followed by Et3N (3.0 mL). The mixture was stirred at r.t. overnight and partitioned between EtOAc (100 mL) and citric acid (50 mL, 5% in water). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give an off-white solid. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 11:9) afforded 12a–c.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}({[(3-hydroxyphenyl)methyl]amino}) methyl]carbamate (12a): A white solid was obtained (75% yield), m.p. 159–161 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 4.55 (2H, d, J = 5.2 Hz, CH2), 6.69–6.80 (3H, m, 3 × ArH), 7.15 (1H, t, J = 7.2 Hz, ArH), 8.69 (1H, t, J = 5.2 Hz, NH), and 11.6 (1H, s, NH). 13C NMR (100 MHz, CDCl3): δ 28.2, 28.4, 44.7, 79.7, 83.5, 114.6, 114.7, 119.6, 129.9, 139.1, 153.3, 156.3, 156.4, and 163.5. HRMS (ESI): Calcd. for C18H28N3O5 (M + H)+ 366.2029 and found 366.2008.
tert-Butyl N-{[(tert-butoxy)carbonyl]imino}({[(3-hydroxy-4-methoxyphenyl) methyl]amino})methyl]carbamate (12b): A white solid was obtained (69% yield), m.p. 127–129 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 3.87 (3H, s, OCH3), 4.51 (2H, d, J = 5.2 Hz, CH2), 6.76–6.79 (2H, m, 2 × ArH), 6.88 (1H, d, J = 1.0 Hz, ArH), 8.49 (1H, br.s, NH), and 11.5 (1H, s, NH). HRMS (ESI): Calcd. for C19H30N3O6 (M + H)+ 396.2135 and found 396.2148.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}({[(2,6-difluoro-3-hydroxyphenyl)methyl]amino})methyl]carbamate (12c): A white solid was obtained (59% yield), m.p. 132–134 °C. 1H NMR (400 MHz, CDCl3) δ 1.47 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.77 (2H, br.s, CH2), 6.79 (1H, m, ArH), 6.99 (1H, m, ArH), 8.70 (1H, br.s, NH), and 11.6 (1H, s, NH). HRMS (ESI): Calcd. for C18H26F2N3O5 (M + H)+ 402.1840 and found 402.1857.
General Procedure: Benzylation of substituted 3-(N,N′-di-Boc-guanydinomethyl)phenols (12a–c): In a solution of the substituted 3-(N,N′-di-Boc-guanydinomethyl)phenol (12a–c) (0.56 mmol) in acetone (8 mL), the substituted benzyl bromide (0.56 mmol) was added, followed by K2CO3 (96 mg). The mixture was stirred at r.t. overnight and partitioned between EtOAc (50 mL) and water (50 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give an off-white solid. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 3:1) afforded 13a–q.
tert-Butyl N-[({[3-(benzyloxy)phenyl]methyl}amino)({[(tert-butoxy) carbonyl] amino})methylidene]carbamate (13a): A white solid was obtained (85% yield), m.p. 90–91 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.60 (2H, d, J = 5.2 Hz, CH2), 5.05 (2H, s, CH2), 6.88–6.92 (2H, m, 2 × ArH), 6.96 (1H, t, J = 2.0 Hz, ArH), 7.25 (1H, t, J = 8.0 Hz, ArH), 7.30–7.45 (5H, m, 5 × ArH), 8.59 (1H, br.s, NH), and 11.52 (1H, s, NH). HRMS (ESI): Calcd. for C25H33N3NaO5+ (M + Na)+ 478.2318 and found 478.2312.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(4-chlorophenyl)methoxy]phenyl}methyl)amino]methylidene]carbamate (13b): A white solid was obtained (64% yield), m.p. 113–115 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 4.59 (2H, d, J = 5.3 Hz, CH2), 5.01 (2H, s, CH2), 6.85–6.95 (3H, m, 3 × ArH), 7.22–7.35 (5H, m, 5 × ArH), 8.58 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C25H33ClN3O5 (M + H)+ 490.2109 and found 490.2122.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(3-chlorophenyl)methoxy]phenyl}methyl)amino]methylidene]carbamate (13c): A clear oil was obtained (85 % yield). 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 4.59 (2H, d, J = 5.5 Hz, CH2), 5.01 (2H, s, CH2), 6.85–6.95 (3H, m, 3 × ArH), 7.22–7.35 (4H, m, 4 × ArH), 7.43 (1H, s, ArH), 8.60 (1H, br.s, NH), and 11.52 (1H, s, NH). HRMS (ESI): Calcd. for C25H33ClN3O5 (M + H)+ 490.2109 and found 490.2135.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(2,4-dichlorophenyl)methoxy]-phenyl}methyl)amino]methylidene]carbamate (13d): A white solid was obtained (55% yield), m.p. 111–112 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.60 (2H, d, J = 5.1 Hz, CH2), 5.11 (2H, s, CH2), 6.88–6.94 (3H, m, 3 × ArH), 7.24–7.28 (2H, m, 2 × ArH), 7.41 (1H, d, J = 1.9 Hz, ArH), 7.49 (1H, d, J = 8.2 Hz, ArH), 8.59 (1H, br.s, NH), and 11.54 (1H, s, NH). HRMS (ESI): Calcd. for C25H31Cl2N3NaO5 (M + Na)+ 546.1538 and found 546.1507.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(3,4-dichlorophenyl) methoxy] phenyl}methyl)amino]methylidene]carbamate (13e): A white solid was obtained (52% yield), m.p. 131–132 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.60 (2H, d, J = 5.2 Hz, CH2), 5.00 (2H, s, CH2), 6.75–6.90 (3H, m, 3 × ArH), 7.22–7.30 (2H, m, 2 × ArH), 7.45 (1H, d, J = 8.2 Hz, ArH), 7.53 (1H, d, J = 2.0 Hz, ArH), 8.59 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C25H31Cl2N3NaO5 (M + Na)+ 546.1538 and found 546.1525.
tert-Butyl N-[{[(tert-Butoxy)carbonyl]amino}[({3-[(2,5-dichlorophenyl) methoxy] phenyl}methyl)amino]methylidene]carbamate (13f): A white solid was obtained (69% yield), m.p. 127–129 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.61 (2H, d, J = 5.1 Hz, CH2), 5.10 (2H, s, CH2), 6.89–6.95 (3H, m, 3 × ArH), 7.22–7.33 (3H, m, 3 × ArH), 7.58 (1H, d, J = 2.5 Hz, ArH), 8.60 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C25H31Cl2N3NaO5 (M + Na)+ 546.1538 and found 546.1506.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(2,3-dichlorophenyl) methoxy] phenyl}methyl)amino]methylidene]carbamate (13g): A white solid was obtained (65% yield), m.p. 98–100 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.59 (2H, d, J = 5.2 Hz, CH2), 5.16 (2H, s, CH2), 6.87–6.94 (3H, m, 3 × ArH), 7.26 (2H, m, 2 × ArH), 7.46 (2H, m, 2 × ArH), 8.59 (1H, br.s, NH), and 11.54 (1H, s, NH). 13C NMR (100 MHz, CDCl3): δ 28.2, 28.4, 45.0, 67.6, 79.6, 83.4, 114.0, 114.6, 120.9, 126.8, 127.6, 129.8, 130.1, 130.7, 133.2, 137.2, 139.2, 153.3, 156.3, 158.7, and 163.7. HRMS (ESI): Calcd. for C25H31Cl2N3NaO5 (M + Na)+ 546.1538 and found 546.1529.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(3-{[4-(trifluoromethyl)phenyl] methoxy}phenyl)methyl]amino})methylidene]carbamate (13h): A white solid was obtained (79% yield), m.p. 108–110 °C. 1H NMR (400 MHz, CDCl3) δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.59 (2H, d, J = 5.2 Hz, CH2), 5.12 (2H, s, CH2), 6.88–6.95 (3H, m, 3 × ArH), 7.25 (1H, t, J = 8.0 Hz, ArH), 7.55 (2H, d, J = 8.2 Hz, 2 × ArH), 7.65 (2H, d, J = 8.2 Hz, 2 × ArH), 8.59 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C26H33F3N3O5 (M + H)+ 524.2372 and found 524.2354.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(3-{[3-(trifluoromethyl)phenyl] methoxy}phenyl)methyl]amino})methylidene]carbamate (13i): A clear oil was obtained (71% yield). 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.60 (2H, d, J = 5.2 Hz, CH2), 5.12 (2H, s, CH2), 6.87–6.97 (3H, m, 3 × ArH), 7.27 (1H, t, J = 7.8 Hz, ArH), 7.50 (1H, t, J = 7.6 Hz, ArH), 7.58 (1H, d, J = 8.5 Hz, ArH), 7.62 (1H, d, J = 8.5 Hz, ArH), 7.70 (1H, s, ArH), 8.59 (1H, br.s, NH), and 11.54 (1H, s, NH). HRMS (ESI): Calcd. for C26H32F3N3NaO5 (M + Na)+ 546.2192 and found 546.2206.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(4-bromophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (13j): A white solid was obtained (59% yield), m.p. 121–122 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 4.59 (2H, d, J = 5.3 Hz, CH2), 5.00 (2H, s, CH2), 6.84–6.94 (3H, m, 3 × ArH), 7.25 (1H, t, J = 7.9 Hz, ArH), 7.29–7.33 (2H, m, 2 × ArH), 7.50 (2H, d, J = 8.5 Hz, 2 × ArH), 8.58 (1H, br.s, NH), and 11.52 (1H, s, NH). HRMS (ESI): Calcd. for C25H32BrN3NaO5 (M + Na)+ 556.1423 and found 556.1405.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(4-fluorophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (13k): A white solid was obtained (57% yield), m.p. 98–99 °C. 1H NMR (400 MHz, CDCl3): δ 1.49 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.59 (2H, d, J = 5.3 Hz, CH2), 5.01 (2H, s, CH2), 6.86–6.94 (3H, m, 3 × ArH), 7.04–7.08 (2H, m, 2 × ArH), 7.25 (1H, t, J = 8.2 Hz, ArH), 7.38–7.42 (2H, m, 2 × ArH), 8.58 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C25H33FN3O5 (M + H)+ 474.2404 and found 474.2425.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(3-{[2-chloro-3-(trifluoromethyl) phenyl]methoxy}phenyl)methyl]amino})methylidene]carbamate (13m): A white solid was obtained (76% yield), m.p. 100–102 °C. 1H NMR (400 MHz, CDCl3): δ 1.49 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.73 (2H, d, J = 5.2 Hz, CH2), 5.21 (2H, s, CH2), 6.91 (1H, d, J = 7.9 Hz, ArH), 6.97 (1H, d, J = 7.9 Hz, ArH), 6.99 (1H, s, ArH), 7.29 (1H, t, J = 7.8 Hz, ArH), 7.40 (1H, t, J = 7.8 Hz, ArH), 7.68 (1H, d, J = 7.8 Hz, ArH), 7.79 (1H, t, J = 7.8 Hz, ArH), 8.65 (1H, br.s, NH), and 11.55 (1H, s, NH). HRMS (ESI): Calcd. for C26H32ClF3N3O5 (M + H)+ 558.1983 and found 558.1971.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(3-chlorophenyl)methoxy]-4-methoxyphenyl}methyl)amino]methylidene]carbamate (13n): A white solid was obtained (82% yield), m.p. 79–81 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 3.87 (3H, s, OCH3), 4.49 (2H, d, J = 5.1 Hz, CH2), 5.09 (2H, s, CH2), 6.75–6.80 (3H, m, 3 × ArH), 7.25–7.35 (3H, m, 3 × ArH), 7.45 (1H, s, ArH), 8.49 (1H, br.s, NH), and 11.52 (1H, s, NH). HRMS (ESI): Calcd. for C26H34ClN3NaO6 (M + Na)+ 542.2034 and found 542.2014.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(4-chlorophenyl)methoxy]-4-methoxyphenyl}methyl)amino]methylidene]carbamate (13o): A white solid was obtained (79% yield), m.p. 126–128 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 3.80 (3H, s, OCH3), 4.49 (2H, d, J = 5.1 Hz, CH2), 5.09 (2H, s, CH2), 6.85–6.90 (3H, m, 3 × ArH), 7.30–7.40 (4H, m, 4 × ArH), 8.49 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C26H34ClN3NaO6 (M + Na)+ 542.2034 and found 542.2050.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({3-[(2,3-dichlorophenyl)methoxy]-2,6-difluorophenyl}methyl)amino]methylidene]carbamate (13p): A white solid was obtained (65% yield), m.p. 140–142 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.74 (2H, d, J = 5.1 Hz, CH2), 5.19 (2H, s, CH2), 6.82–6.89 (2H, m, 2 × ArH), 7.25 (1H, t, J = 8.2 Hz, ArH), 7.42 (1H, d, J = 8.2 Hz, ArH), 7.50 (1H, d, J = 8.1 Hz, ArH), 8.52 (1H, br.s, NH), and 11.54 (1H, s, NH). HRMS (ESI): Calcd. for C25H30Cl2F2N3O5 (M + H)+ 560.1530 and found 560.1511.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(2,6-difluoro-3-{[3-(trifluoromethyl) phenyl]methoxy}phenyl)methyl]amino})methylidene]carbamate (13q): A white solid was obtained (62% yield), m.p. 85–86 °C. 1H NMR (400 MHz, CDCl3): δ 1.46 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 4.72 (2H, d, J = 5.1 Hz, CH2), 5.13 (2H, s, CH2), 6.82–6.90 (2H, m, 2 × ArH), 7.51 (1H, t, J = 8.1 Hz, ArH), 7.59–7.63 (2H, m, 2 × ArH), 7.69 (1H, s, ArH), 8.47 (1H, br.s, NH), and 11.53 (1H, s, NH). HRMS (ESI): Calcd. for C26H31F5N3O5 (M + H)+ 560.2184 and found 560.2172.
General Procedure: Synthesis of benzyl guanidine derivatives (9a–q): In a solution of the substituted N,N′-di-Boc-(guanydinomethyl)benzene (13a–q) (0.3 mmol) in CH2Cl2 (2 mL), TFA (1 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with Et2O, and dried in vacuo to give 9a–q as a white or off-white solid.
1-(3-(Benzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9a): A white solid was obtained (95% yield). 1H NMR (400 MHz, CD3OD): δ 4.26 (2H, s, CH2), 4.98 (2H, s, CH2), 6.80–6.95 (2H, m, 2 × ArH), 7.18–7.29 (2H, m, 2 × ArH), 7.24 (2H, m, 2 × ArH), and 7.33 (2H, m, 2 × ArH). HRMS (ESI): Calcd. for C15H18N3O (M + H)+ 256.1450 and found 256.1454.
1-(3-(4-Chlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9b): An off-white solid was obtained (96% yield). 1H NMR (400 MHz, CD3OD): δ 4.39 (2H, s, CH2), 5.16 (2H, s, CH2), 6.98–7.05 (3H, m, 3 × ArH), 7.37 (1H, t, J = 8.3 Hz, ArH), and 7.43–7.50 (4H, m, 4 × ArH). HRMS (ESI): Calcd. for C15H17ClN3O (M + H)+ 290.1060 and found 290.1066.
1-(3-(3-Chlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9c): An off-white solid was obtained (85% yield). 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, s, CH2), 5.16 (2H, s, CH2), 6.98–7.05 (3H, m, 3 × ArH), 7.32–7.40 (4H, m, 4 × ArH), and 7.51 (1H, s, ArH). HRMS (ESI): Calcd. for C15H17ClN3O (M + H)+ 290.1060 and found 290.1067.
1-(3-(2,4-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9d): A white solid was obtained (98% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (2H, d, J = 5.0 Hz, CH2), 5.20 (2H, s, CH2), 6.95–7.05 (3H, m, 3 × ArH), 7.38 (1H, t, J = 8.0 Hz, ArH), 7.55 (1H, dd, J = 7.9, 1.9 Hz, ArH), 7.67 (1H, d, J = 7.9 Hz, ArH), 7.78 (1H, t, J = 2.1 Hz, ArH), and 8.05 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H16Cl2N3O (M + H)+ 324.0670 and found 324.0665.
1-(3-(3,4-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9e): A white solid was obtained (99% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.39 (2H, d, J = 5.1 Hz, CH2), 5.19 (2H, s, CH2), 6.93–7.05 (3H, m, ArH), 7.38 (1H, td, J = 7.9, 1.9 Hz, ArH), 7.50 (1H, dd, J = 8.0, 1.9 Hz, ArH), 7.72 (1H, d, J = 8.1 Hz, ArH), 7.78 1H, (t, J = 1.9 Hz, ArH), and 8.02 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H16Cl2N3O (M + H)+ 324.0670 and found 324.0667.
1-(3-(2,5-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9f): A white solid was obtained (98% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.42 (2H, d, J = 6.0 Hz, CH2), 5.20 (2H, s, CH2), 6.95–7.08 (3H, m, 3 × ArH), 7.40 (1H, td, J = 8.0, 1.8 Hz, ArH), 7.55 (1H, dd, J = 8.0, 2.1 Hz, ArH), 7.63 (1H, d, J = 8.0 Hz, ArH), 7.72 (1H, t, J = 2.1 Hz, ArH), and 8.10 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H16Cl2N3O (M + H)+ 324.0670 and found 324.0675.
1-(3-(2,3-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9g): A white solid was obtained (92% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (2H, d, J = 5.5 Hz, CH2), 5.25 (2H, s, CH2), 6.95–7.08 (3H, m, 3 × ArH), 7.40 (1H, td, J = 8.0, 1.8 Hz, ArH), 7.48 (1H, t, J = 8.0 Hz, ArH), 7.63 (1H, dd, J = 8.0, 1.8 Hz, ArH), 7.72 (1H, dd, J = 8.0, 1.8 Hz, ArH), and 8.05 (1H, br.s, NH). 13C NMR (100 MHz, DMSO-d6): δ 43.9, 67.3, 113.6, 113.9, 120.0, 128.5, 129.9, 130.3, 130.5, 132.0, 136.9, 139.0, 156.8, and 157.2. (HRMS (ESI): Calcd. for C15H16Cl2N3O (M + H)+ 324.0670 and found 324.0661.
1-(3-(2,3-Dichlorobenzyloxy)benzyl)guanidinium chloride (9g.HCl): Compound 9g (5 mg) was converted to the hydrogen chloride salt by dissolving in a HCl-methanol (0.5M, 2 mL) solution and concentrating in vacuo. A white solid was obtained (4 mg). HRMS (ESI): Calcd. for C15H16Cl2N3O (M + H)+ 324.0670 and found 324.0677.
1-(3-[4-(Trifluoromethyl)benzyloxy]benzyl)guanidinium 2,2,2-trifluoroacetate (9h): A white solid was obtained (97% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (d2H, J = 5.5 Hz, CH2), 5.25 (2H, s, CH2), 6.95–7.12 (2H, m, 2 × ArH), 7.38 (2H, m, 2 × ArH), 7.72 (2H, d, J = 8.2 Hz, 2 × ArH), 7.82 (2H, d, J = 8.2 Hz, 2 × ArH), and 8.05 (1H, br.s, NH). HRMS (ESI): Calcd. for C26H33F3N3O5 (M + H)+ 524.2372 and found 524.2360.
1-(3-[3-(Trifluoromethyl)benzyloxy]benzyl)guanidinium 2,2,2-trifluoroacetate (9i): An off-white solid was obtained (98% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (2H, d, J = 6.1 Hz, CH2), 5.27 (2H, s, CH2), 6.95–7.08 (3H, m, 3 × ArH), 7.13 (1H, td, J = 7.9, 1.7 Hz, ArH), 7.71 (1H, t, J = 7.9 Hz, ArH), 7.78 (1H, d, J = 8.0 Hz, ArH), 7.82 (1H, d, J = 8.0 Hz, ArH), 7.87 (1H, s, ArH), and 8.05 (1H, br.s, NH). HRMS (ESI): Calcd. for C26H33F3N3O5 (M + H)+ 524.2372 and found 524.2397.
1-(3-(4-Bromobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9j): A white solid was obtained (97% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.39 (2H, d, J = 6.2 Hz, CH2), 5.14 (2H, s, CH2), 6.95–7.08 (3H, m, 3 × ArH), 7.37 (1H, t, J = 8.0 Hz, ArH), 7.46 (2H, d, J = 8.0 Hz, 2 × ArH), 7.65 (2H, d, J = 8.1 Hz, 2 × ArH), and 8.10 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H16BrN3NaO (M + Na)+ 356.0374 and found 356.0375.
1-(3-(4-Fluorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9k): A white solid was obtained (90% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (2H, d, J = 6.2 Hz, CH2), 5.11 (2H, s, CH2), 6.97–7.10 (3H, m, 3 × ArH), 7.35 (1H, td, J = 8.0, 1.5 Hz, ArH), 7.45 (2H, d, J = 8.2 Hz, 2 × ArH), 7.60 (2H, d, J = 8.2 Hz, 2 × ArH), and 8.07 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H17FN3O (M + H)+ 274.1356 and found 274.1366.
1-(3-[2-Chloro-3-(trifluoromethyl)benzyloxy]benzyl)guanidinium 2,2,2-trifluoroacetate (9m): A white solid was obtained (99% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.41 (2H, d, J = 6.1 Hz, CH2), 5.31 (2H, s, CH2), 6.96–7.08 (3H, m, 3 × ArH), 7.40 (1H, t, J = 8.0 Hz, ArH), 7.68 (1H, t, J = 8.1 Hz, ArH), 7.95 (1H, d, J = 7.9 Hz, ArH), 7.99 (1H, d, J = 7.9 Hz, ArH), and 8.12 (1H, br.s, NH). HRMS (ESI): Calcd. for C16H16ClF3N3O (M + H)+ 358.0934 and found 358.0979.
1-(3-[2-Chloro-3-(trifluorobenzyloxy)benzyl]guanidinium chloride (9m.HCl): Compound 9m (9 mg) was converted to the hydrogen chloride salt by dissolving in a HCl-methanol (0.5 M, 2 mL) solution and concentrating in vacuo. A white solid was obtained (7 mg). HRMS (ESI): Calcd. for C16H16ClF3N3O (M + H)+ 358.0934 and found 358.0897.
1-(3-[3-Chlorobenzyloxy]-4-methoxybenzyl)guanidinium 2,2,2-trifluoroacetate (9n): A white solid was obtained (97% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.36 (2H, d, J = 6.0 Hz, CH2), 5.27 (2H, s, CH2), 6.90–7.15 (3H, m, 3 × ArH), 7.39–7.45 (3H, m, 3 × ArH), 7.65 (1H, s, ArH), and 8.12 (1H, br.s, NH). HRMS (ESI): Calcd. for C16H18ClN3NaO2 (M + Na)+ 342.0985 and found 342.0977.
1-(3-[4-Chlorobenzyloxy]-4-methoxybenzyl)guanidinium 2,2,2-trifluoroacetate (9o): An off-white solid was obtained (95% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.40 (2H, d, J = 6.0 Hz, CH2), 5.29 (2H, s, CH2), 6.87–7.02 (3H, m, 3 × ArH), 7.30–7.39 (4H, m, 4 × ArH), and 8.07 (1H, br.s, NH). HRMS (ESI): Calcd. for C16H18ClN3NaO2 (M + Na)+ 342.0985 and found 342.0994.
1-(3-(2,3-Dichlorobenzyloxy]-2,6-difluorobenzyl)guanidinium 2,2,2-trifluoroacetate (9p): A white solid was obtained (93% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.50 (2H, s, CH2), 5.35 (2H, s, CH2), 7.30–7.40 (2H, m, 2 × ArH), 7.50 (1H, t, J = 7.9 Hz, ArH), 7.63 (1H, d, J = 7.9 Hz, ArH), 7.75 (1H, d, J = 8.0 Hz, ArH), and 7.96 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H14Cl2F2N3O (M + H)+ 360.0482 and found 360.0493.
1-(2,6-Difluoro-3-[3-(trifluoromethyl)benzyloxy]benzyl)guanidinium 2,2,2-trifluoroacetate (9q): A white solid was obtained (98% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.49 (2H, d, J = 5.3 Hz, CH2), 5.35 (2H, s, CH2), 7.18 (1H, dt, J = 7.9, 1.6 Hz, ArH), 7.32–7.40 (2H, m, 2 × ArH), 7.72 (1H, t, J = 8.1 Hz, ArH), 7.81 (1H, d, J = 7.9 Hz, ArH), 7.88 (1H, s, ArH), and 8.00 (1H, br.s, NH). HRMS (ESI): Calcd. for C16H15F5N3O (M + H)+ 360.1135 and found 360.1248.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}({[(4-hydroxyphenyl)methyl]amino}) methyl]carbamate (15): In a solution of 4-(aminomethyl)phenol hydrochloride 14 (6.0 mmol) in DMF (20 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (1.6 g, 5.6 mmol) was added, followed by Et3N (2.0 mL). The mixture was stirred at r.t. overnight and partitioned between EtOAc (100 mL) and citric acid (50 mL, 5% in water). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give an off-white solid. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 11:9) afforded 15 as a white solid (1.4 g, 68% yield). mp 137–138 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.49 (9H, s, t-Bu), 4.51 (2H, d, J = 5.2 Hz, CH2), 6.91 (2H, d, J = 8.1 Hz, 2 × ArH), 7.10 (2H, d, J = 8.1 Hz, 2 × ArH), 8.50 (1H, br.s, NH), and 11.5 (1H, s, NH). HRMS (ESI): Calcd. for C18H28N3O5 (M + H)+ 366.2029 and found 366.2037.
General Procedure: Benzylation of 4-(N,N′-di-Boc-guanydinomethyl)phenol (15): In a solution of 4-(N,N′-di-Boc-guanydinomethyl)phenol 15 (0.56 mmol) in acetone (8 mL), the substituted benzyl bromide (0.56 mmol) was added, followed by K2CO3 (96 mg, 0.7 mmol). The mixture was stirred at r.t. overnight and then partitioned between EtOAc (50 mL) and water (50 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give an off-white solid. Purification by flash column chromatography eluting with a gradient solvent (petrol ether to petrol ether/EtOAc 3:1) afforded 16a–e.
tert-Butyl N-[({[4-(benzyloxy)phenyl]methyl}amino)({[(tert-butoxy) carbonyl]-amino})methylidene]carbamate (16a): A white solid was obtained (82% yield), m.p. 115–116 °C. 1H NMR (400 MHz, CDCl3): δ 1.34 (9H, s, t-Bu), 1.49 (9H, s, t-Bu), 5.10 (2H, s, CH2), 5.15 (2H, br.s, CH2), 6.88 (2H, dt, J = 8.8, 2.0 Hz, 2 × ArH), 7.19 (2H, dt, J = 8.8, 2.1 Hz, 2 × ArH), 7.28–7.44 (5H, m, 5 × ArH), 9.30 (1H, br.s, NH), and 10.5 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H33N3NaO5 (M + Na)+ 478.2318 and found 478.2329.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({4-[(4-chlorophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (16b): A white solid was obtained (79% yield), m.p. 120–121 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.65 (2H, br.s, CH2), 5.01 (2H, s, CH2), 6.92 (2H, d, J = 8.2 Hz, 2 × ArH), 7.25 (2H, d, J = 8.2 Hz, 2 × ArH), 7.35 (4H, s, 4 × ArH), 8.75 (1H, br.s, NH), and 11.54 (1H, br.s, NH).
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({4-[(3-chlorophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (16c): A white solid was obtained (67% yield), m.p. 120–122 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.60 (2H, br.s, CH2), 5.02 (2H, s, CH2), 6.93 (2H, dd, J = 7.1, 2.0 Hz, 2 × ArH), 7.20–7.30 (5H, m, 5 × ArH), 7.43 (1H, s, ArH), 8.62 (1H, br.s, NH), and 11.5 (1H, br.s, NH).
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({4-[(3,4-dichlorophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (16d): A white solid was obtained (79% yield), m.p. 156–158 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 4.63 (2H, br.s, CH2), 5.00 (2H, s, CH2), 7.22 (1H, d, J = 7.9 Hz, ArH), 7.43 (1H, d, J = 8.1 Hz, ArH), 7.51 (1H, d, J = 1.6 Hz, ArH), 6.91 (2H, d, J = 7.9 Hz, 2 × ArH), 8.80 (1H, br.s, NH), and 11.5 (1H, br.s, NH).
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}[({4-[(2,3-dichlorophenyl)methoxy] phenyl}methyl)amino]methylidene]carbamate (16e): A white solid was obtained (89% yield), m.p. 99–101 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.53 (9H, s, t-Bu), 4.73 (2H, br.s, CH2), 5.16 (2H, s, CH2), 6.95 (2H, d, J = 8.1 Hz, 2 × ArH), 7.25–7.31 (3H, m, 3 × ArH), 7.43 (1H, d, J = 8.2 Hz, ArH), 7.47 (1H, d, J = 8.2 Hz, ArH), 8.90 (1H, br.s, NH), and 11.6 (1H, br.s, NH).
General Procedure: Synthesis of benzyl guanidine derivatives (9r–v): In a solution of the para-substituted N,N′-di-Boc-(4-guanidinomethyl)benzene (100 mg) (16a–e) in CH2Cl2 (2 mL), TFA (1 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with ether, and dried in vacuo to give 9r–v as a white or off-white solid.
1-(4-(Benzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9r): A off-white solid was obtained (96% yield). 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, br.s, CH2), 5.16 (2H, s, CH2), 7.06 (2H, d, J = 8.3 Hz, 2 × ArH), 7.32 (2H, d, J = 8.3 Hz, 2 × ArH), 7.36–7.43 (3H, m, 3 × ArH), and 7.50 (2H, d, J = 8.2 Hz, 2 × ArH). HRMS (ESI): Calcd. for C15H18N3O (M + H)+ 256.1450 and found 256.1539.
1-(4-(4-Chlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9s): A white solid was obtained (87% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.55 (2H, br.s, CH2), 5.17 (2H, s, CH2), 6.94 (2H, d, J = 8.2 Hz, 2 × ArH), 7.27 (2H, d, J = 8.1 Hz, 2 × ArH), 7.37–7.41 (4H, m, 4 × ArH), and 8.15 (br s, 1H, NH). HRMS (ESI): Calcd. for C15H17ClN3O (M + H)+ 290.1060 and found 290.1066.
1-(4-(3-Chlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9t): A white solid was obtained (93% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.33 (2H, br.s, CH2), 5.19 (2H, s, CH2), 7.08 (3H, m, 3 × ArH), 7.30 (2H, m, 2 × ArH), 7.40–7.50 (3H, m, 3 × ArH), and 7.95 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H17ClN3O (M + H)+ 290.1060 and found 290.1071.
1-(4-(3,4-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9u): A white solid was obtained (93% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.33 (2H, d, J = 6.0 Hz, CH2), 5.19 (2H, s, CH2), 7.09 (2H, d, J = 8.6 Hz, 2 × ArH), 7.31 (2H, d, J = 8.5 Hz, 2 × ArH), 7.50 (1H, dd, J = 7.8, 2.1 Hz, 2 × ArH), 7.72 (1H, d, J = 7.7 Hz, ArH), 7.77 (1H, d, J = 2.0 Hz, ArH), and 7.87 (1H, br.s, NH). HRMS (ESI): Calcd. for C15H15Cl2N3O (M + H)+ 324.0670 and found 324.0658.
1-(4-(2,3-Dichlorobenzyloxy)benzyl)guanidinium 2,2,2-trifluoroacetate (9v): A white solid was obtained (98% yield). 1H NMR (400 MHz, DMSO-d6): δ 4.38 (2H, br.s, CH2), 5.35 (2H, s, CH2), 7.14 (2H, d, J = 8.2 Hz, 2 × ArH), 7.32 (2H, d, J = 8.3 Hz, 2 × ArH), 7.46 (1H, t, J = 7.9 Hz, ArH), 7.63 (1H, d, J = 8.1 Hz, ArH), 7.72 (1H, d, J = 7.9 Hz, ArH), and 8.05 (1H, br.s, NH). 13C NMR (DMSO-d6): δ 43.5, 67.3, 114.9, 128.4, 128.4, 128.9, 130.2, 130.5, 132.0, 137.0, 156.8, and 157.5. HRMS (ESI): Calcd. for C15H15Cl2N3O (M + H)+ 324.0670 and found 324.0720.
General procedure: Synthesis of Boc-protected aminobenzyl guanidine derivatives (18, 22): 3-Aminobenzylamine 17 or 4-aminobenzylamine 21 (10 mmol) was dissolved in DMF (8 mL). S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (10.5 mmol) and Et3N (20 mmol) were added successively at 0 °C. The reaction mixture was stirred for 18 h at r.t. and then evaporated at 70 °C. Water (180 mL) and brine (20 mL) were added, and the mixture was extracted with Et2O (2 × 200 mL). The organic layer was dried (Na2SO4), filtered, and concentrated in vacuo. Purification by flash column chromatography (CH2Cl2, 100%) gave 18 and 22.
tert-Butyl N-[(1E)-{[(3-aminophenyl)methyl]amino}({[(tert-butoxy)carbonyl] imino})methyl]carbamate (18): 2.85 g, 78%, white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.44 (9H, s, t-Bu), 1.53 (9H, s, t-Bu), 4.41 (2H, d, J = 5.6 Hz, CH2), 5.14 (2H, br.s, NH2), 6.46 (1H, d, J = 7.2 Hz, ArH), 6.50 (1H, s, ArH), 6.51 (1H, d, J = 7.2 Hz, ArH), 7.02 (1H, t, J = 8.0 Hz, ArH), 8.56 (1H, t, J = 5.6 Hz, NH), and 11.60 (1H, br.s, NH). HRMS (ESI): Calcd. for C18H29N4O4 (M + H)+ 365.2183 and found 365.2189.
tert-Butyl N-[(1E)-{[(4-aminophenyl)methyl]amino}({[(tert-butoxy)carbonyl] imino})methyl]carbamate (22): 2.98 g, 81%, white foam. 1H NMR (400 MHz, DMSO-d6): δ 1.45 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 4.34 (2H, d, J = 5.6 Hz, CH2), 5.10 (2H, br.s, NH2), 6.58 (2H, d, J = 7.6 Hz, 2 × ArH), 7.03 (2H, d, J = 8.0 Hz, 2 × ArH), 8.41 (1H, t, J = 5.6 Hz, NH), and 11.55 (1H, br.s, NH). HRMS (ESI): Calcd. for C18H29N4O4 (M + H)+ 365.2183 and found 365.2187.
General Procedure: Synthesis of Boc-protected sulphonamide derivatives (19a–d, 23a–d): Compound 18 or 22 (0.2 mmol) was dissolved in CH2Cl2 (1.2 mL) and pyridine (0.8 mL) at 0 °C. The corresponding benzene sulphonyl chloride (0.22 mmol) was added, and the reaction mixture was stirred for 2 h at 0 °C. Water (40 mL) and brine (10 mL) were added, and the mixture was extracted with Et2O (2 × 50 mL). The organic layer was dried (Na2SO4), filtered, and concentrated to dryness. The residue was then co-evaporated with toluene (2 × 5 mL) and CH2Cl2 (2 × 5 mL) to give 19a–d or 23a–d.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}({[3-(3-chlorobenzene sulfonamido)phenyl]methyl}amino)methyl]carbamate (19a): 99 mg, 92%, white foam. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.48 (9H, s, t-Bu), 4.51 (2H, s, CH2), 6.98–7.06 (3H, m, 3 × ArH), 7.18 (1H, t, J = 8.0 Hz, ArH), 7.34 (1H, t, J = 7.8 Hz, ArH), 7.46 (1H, ddd, J = 8.0, 2.0, 0.8 Hz, ArH), 7.54 (1H, br.s, NH), 7.64 (1H, ddd, J = 7.8, 1.6, 1.2 Hz, ArH), 7.79 (1H, t, J = 1.8 Hz, ArH), 8.60 (1H, br.s, NH), and 11.52 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H32ClN4O6S (M + H)+ 539.1726 and found 539.1737.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}({[3-(4-chlorobenzene sulfonamido)phenyl]methyl}amino)methyl]carbamate (19b): 98 mg, 91%, foam. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.48 (9H, s, t-Bu), 4.53 (2H, s, CH2), 7.00 (1H, d, J = 2.4 Hz, ArH), 7.02 (1H, d, J = 2.4 Hz, ArH), 7.07 (1H, s, ArH), 7.17 (1H, t, J = 7.8 Hz, ArH), 7.35 (2H, dt, J = 8.8, 2.2 Hz, 2 × ArH), 7.65 (1H, br.s, NH), 7.70 (2H, dt, J = 8.8, 2.2 Hz, 2 × ArH), 8.61 (1H, br.s, NH), and 11.52 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H32ClN4O6S (M + H)+ 539.1726 and found 539.1738.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}({[3-(2,3-dichlorobenzene sulfonamido)phenyl]methyl}amino)methyl]carbamate (19c): 107 mg, 93%, yellow foam. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.49 (9H, s, t-Bu), 4.55 (2H, s, CH2), 6.98–7.04 (2H, m, 2 × ArH), 7.08 (1H, s, ArH), 7.17 (1H, t, J = 7.8 Hz, ArH), 7.27 (1H, t, J = 8.0 Hz, ArH), 7.46 (1H, br.s, NH), 7.60 (1H, d, J = 8.0 Hz, ArH), 7.97 (1H, d, J = 8.0 Hz, ArH), 8.61 (1H, br.s, NH), and 11.52 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H31Cl2N4O6S (M + H)+ 573.1336 and found 573.1349.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}[({3-[3-(trifluoromethyl) benzenesulfonamido]phenyl}methyl)amino]methyl]carbamate (19d): 107 mg, 93%, colourless glass. 1H NMR (400 MHz, CDCl3): δ 1.47 (18H, s, 2 × t-Bu), 4.52 (2H, s, CH2), 6.99–7.07 (3H, m, 3 × ArH), 7.18 (1H, t, J = 7.8 Hz, ArH), 7.55 (1H, t, J = 7.8 Hz, ArH), 7.68 (1H, br.s, NH), 7.75 (1H, d, J = 7.6 Hz, ArH), 7.95 (1H, d, J = 7.6 Hz, ArH), 8.05 (1H, s, ArH), 8.62 (1H, br.s, NH), and 11.51 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H32F3N4O6S (M + H)+ 573.1989 and found 573.1998.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}({[4-(3-chloro benzenesulfonamido)phenyl]methyl}amino)methyl]carbamate (23a): 105 mg, 97%, pale yellow foam. 1H NMR (400 MHz, CDCl3): δ 1.46 (9H, s, t-Bu), 1.47 (9H, s, t-Bu), 4.54 (2H, s, CH2), 7.07 (2H, d, J = 8.0 Hz, 2 × ArH), 7.13 (2H, d, J = 8.4 Hz, 2 × ArH), 7.34 (1H, t, J = 8.0 Hz, ArH), 7.47 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.65 (1H, d, J = 7.6 Hz, ArH), 7.73 (1H, br.s, NH), 7.78 (1H, s, ArH), 8.61 (1H, br.s, NH), and 11.50 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H32ClN4O6S (M + H)+ 539.1726 and found 539.1739.
tert-Butyl N-({[(tert-butoxy)carbonyl]imino}({[4-(4-chlorobenzenesulfonamido) phenyl]methyl}amino)methyl)carbamate (23b): 103 mg, 95%, white foam. 1H NMR (400 MHz, CDCl3): δ 1.46 (9H, s, t-Bu), 1.47 (9H, s, t-Bu), 4.55 (2H, s, CH2), 7.06 (2H, d, J = 8.4 Hz, 2 × ArH), 7.14 (2H, d, J = 8.4 Hz, 2 × ArH), 7.38 (2H, dt, J = 8.8, 2.2 Hz, 2 × ArH), 7.60 (1H, br.s, NH), 7.71 (2H, dd, J = 8.8, 2.2 Hz, 2 × ArH), 8.58 (1H, br.s, NH), and 11.51 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H32ClN4O6S (M + H)+ 539.1726 and found 539.1741.
tert-Butyl N-({[(tert-butoxy)carbonyl]imino}({[4-(2,3-dichlorobenzene sulfonamido)phenyl]methyl}amino)methyl)carbamate (23c): 113 mg, 98%, yellow foam. 1H NMR (400 MHz, CDCl3): δ 1.45 (9H, s, t-Bu), 1.47 (9H, s, t-Bu), 4.51 (2H, s, CH2), 7.08 (2H, d, J = 8.8 Hz, 2 × ArH), 7.13 (2H, d, J = 8.4 Hz, 2 × ArH), 7.27 (1H, t, J = 8.2 Hz, ArH), 7.49 (1H, br.s, NH), 7.61 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.93 (1H, dd, J = 8.0, 1.6 Hz, ArH), 8.51 (1H, br.s, NH), and 11.50 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H31Cl2N4O6S (M + H)+ 573.1336 and found 573.1347.
tert-Butyl N-[(1E)-{[(tert-butoxy)carbonyl]imino}[({4-[3-(trifluoromethyl) benzenesulfonamido]phenyl}methyl)amino]methyl]carbamate (23d): 111 mg, 97%, pale yellow foam. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.48 (9H, s, t-Bu), 4.57 (2H, s, CH2), 7.07 (2H, d, J = 8.4 Hz, 2 × ArH), 7.13 (2H, d, J = 8.4 Hz, 2 × ArH), 7.56 (1H, t, J = 7.8 Hz, ArH), 7.69 (1H, br.s, NH), 7.76 (1H, d, J = 8.0 Hz, ArH), 7.96 (1H, d, J = 8.0 Hz, ArH), 8.04 (1H, s, ArH), 8.66 (1H, br.s, NH), and 11.50 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H32F3N4O6S (M + H)+ 573.1989 and found 573.1996.
General Procedure: Synthesis of Boc-protected amide derivatives (19e, 23e): Compound 18 or 22 (0.5 mmol) and K2CO3 (1.0 mmol) were placed in an oven-dried 50 mL glass tube. Acetone (2.0 mL) and benzoyl chloride (0.75 mmol) were added successively, and the reaction mixture was stirred for 18 h at 80 °C. Water (80 mL) and brine (20 mL) were added, and the mixture was extracted with Et2O (2 × 100 mL). The organic layer was dried (Na2SO4), filtered, and concentrated to dryness. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 4:1) gave 19e or 23e.
tert-Butyl N-[(1E)-{[(3-benzamidophenyl)methyl]amino}({[(tert-butoxy) carbonyl]-imino})methyl]carbamate (19e): 45 mg, 19%, colourless glass. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.48 (9H, s, t-Bu), 4.59 (2H, d, J = 5.2 Hz, CH2), 7.01 (1H, d, J = 7.6 Hz, ArH), 7.30 (1H, t, J = 8.0 Hz, ArH), 7.42–7.49 (3H, m, 3 × ArH), 7.53 (1H, t, J = 7.6 Hz, CH), 7.70 (1H, t, J = 8.0 Hz, ArH), 7.87–7.93 (2H, m, 2 × ArH), 8.19 (1H, s, NH), 8.70 (1H, s, NH), and 11.53 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H33N4O5 (M + H)+ 469.2445 and found 469.2452.
tert-Butyl N-[(1E)-{[(4-benzamidophenyl)methyl]amino}({[(tert-butoxy) carbonyl]-imino})methyl]carbamate (23e): 27 mg, 11%, colourless glass. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.48 (9H, s, t-Bu), 4.58 (2H, d, J = 5.2 Hz, CH2), 7.24 (2H, d, J = 8.4 Hz, 2 × ArH), 7.45 (2H, t, J = 7.4 Hz, 2 × ArH), 7.52 (1H, t, J = 7.2 Hz, ArH), 7.61 (2H, d, J = 8.8 Hz, 2 × ArH), 7.87 (2H, d, J = 8.4 Hz, 2 × ArH), 8.14 (1H, s, NH), 8.63 (1H, s, NH), and 11.53 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H33N4O5 (M + H)+ 469.2445 and found 469.2451.
General procedure: Synthesis of Amino((arylsulfonamido)benzyl) amino) meth-animinium 2,2,2-trifluoroacetate derivatives (20a–d, 24a–d): Compound 19a–d, 23a–d (0.1 mmol) was dissolved in CH2Cl2 (0.8 mL) and then TFA (0.2 mL) was added. The reaction mixture was stirred for 2 h at r.t. and concentrated to dryness to give 20a–d and 24a–d.
Amino((3-((3-chlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (20a): 89 mg, 98%, glass. 1H NMR (400 MHz, CD3OD): δ 4.37 (2H, s, CH2), 7.07 (1H, d, J = 8.0 Hz, ArH), 7.13 (1H, d, J = 7.6 Hz, ArH), 7.23 (1H, s, ArH), 7.33 (1H, t, J = 7.8 Hz, ArH), 7.54 (1H, t, J = 8.0 Hz, ArH), 7.65 (1H, d, J = 8.0 Hz, ArH), 7.75 (1H, d, J = 8.0 Hz, ArH), and 7.81 (1H, s ArH). HRMS (ESI): Calcd. for C14H16ClN4O2S (M + H)+ 339.0677 and found 339.0681.
Amino((3-((4-chlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (20b): 88 mg, 97%, glass. 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, s, CH2), 7.07 (1H, d, J = 8.0 Hz, ArH), 7.11 (1H, d, J = 7.6 Hz, ArH), 7.23 (1H, s, ArH), 7.31 (1H, t, J = 8.0 Hz, ArH), 7.54 (2H, d, J = 8.4 Hz, 2 × ArH), and 7.81 (2H, d, J = 8.4 Hz, 2 × ArH). HRMS (ESI): Calcd. for C14H16ClN4O2S (M + H)+ 339.0677 and found 339.0685.
Amino((3-((2,3-dichlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (20c): 96 mg, 98%, glass. 1H NMR (400 MHz, CD3OD): δ 4.39 (2H, s, CH2), 7.06 (1H, d, J = 7.6 Hz, ArH), 7.13 (1H, d, J = 8.0 Hz, ArH), 7.22 (1H, s, ArH), 7.30 (1H, t, J = 8.0 Hz, ArH), 7.49 (1H, dt, J = 8.2, 0.8 Hz, ArH), 7.81 (1H, d, J = 8.0 Hz, ArH), and 8.11 (1H, d, J = 8.0 Hz, ArH). HRMS (ESI): Calcd. for C14H15Cl2N4O2S (M + H)+ 373.0287 and found 373.0295.
Amino((3-((3-(trifluoromethyl)phenyl)sulfonamido)benzyl)amino) methaniminium 2,2,2-trifluoroacetate (20d): 95 mg, 97%, glass. 1H NMR (400 MHz, CD3OD): δ 4.42 (2H, s, CH2), 7.07 (1H, d, J = 8.0 Hz, ArH), 7.14 (1H, d, J = 7.6 Hz, ArH), 7.24 (1H, s, ArH), 7.32 (1H, t, J = 8.0 Hz, ArH), 7.77 (1H, d, J = 8.2 Hz, ArH), 7.96 (1H, d, J = 7.6 Hz, ArH), and 8.07 (2H, s, 2 × ArH). HRMS (ESI): Calcd. for C15H16F3N4O2S (M + H)+ 373.0941 and found 373.0946.
Amino((4-((3-chlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (24a): 90 mg, 99%, glass. 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, s, CH2), 7.17–7.22 (2H, m, 2 × ArH), 7.24–7.31 (2H, m, 2 × ArH), 7.53 (1H, t, J = 7.4 Hz, ArH), 7.60–7.66 (1H, m, ArH), 7.71–7.77 (1H, m, ArH), and 7.77–7.81 (1H, m, ArH). HRMS (ESI): Calcd. for C14H16ClN4O2S (M + H)+ 339.0677 and found 339.0685.
Amino((4-((4-chlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (24b): 89 mg, 98%, glass, 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, s, CH2), 7.20 (2H, d, J = 8.4 Hz, 2 × ArH), 7.27 (2H, d, J = 8.4 Hz, 2 × ArH), 7.55 (2H, d, J = 8.4 Hz, 2 × ArH), and 7.80 (2H, d, J = 8.4 Hz, 2 × ArH). HRMS (ESI): Calcd. for C14H16ClN4O2S (M + H)+ 339.0677 and found 339.0686.
Amino((4-((2,3-dichlorophenyl)sulfonamido)benzyl)amino)methaniminium 2,2,2-trifluoroacetate (24c): 97 mg, >99%, glass. 1H NMR (400 MHz, CD3OD): δ 4.34 (2H, s, CH2), 7.21–7.28 (4H, m, 4 × ArH), 7.46 (1H, t, J = 8.0 Hz, ArH), 7.81 (1H, dd, J = 8.0, 1.6 Hz, ArH), and 8.10 (1H, dd, J = 8.0, 1.6 Hz, ArH). HRMS (ESI): Calcd. for C14H15Cl2N4O2S (M + H)+ 373.0287 and found 373.0296.
Amino((4-((3-trifluoromethyl)phenyl)sulfonamido)benzyl)amino) methaniminium 2,2,2-trifluoroacetate (24d): 95 mg, 97%, glass. 1H NMR (400 MHz, CD3OD): δ 4.38 (2H, s, CH2), 7.20 (2H, d, J = 8.8 Hz, 2 × ArH), 7.29 (2H, d, J = 8.4 Hz, 2 × ArH), 7.76 (1H, t, J = 8.2 Hz, ArH), 7.95 (1H, d, J = 8.0 Hz, ArH), and 8.05–8.11 (2H, m, 2 × ArH). HRMS (ESI): Calcd. for C15H16F3N4O2S (M + H)+ 373.0941 and found 373.0945.
General procedure: Synthesis of amino((benzamidobenzyl)amino) methaniminium 2,2,2-trifluoroacetate derivatives (20e, 24e): Compound 19e or 23e (0.1 mmol) was dissolved in CH2Cl2 (0.8 mL) and then TFA (0.2 mL) was added. The reaction mixture was stirred for 2 h at 0 °C. The mixture was concentrated to dryness to give 20a or 24e.
Amino((3-benzamidobenzyl)amino)methaniminium 2,2,2-trifluoroacetate (20e): 38 mg, 99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 4.44 (2H, s, CH2), 7.10 (1H, d, J = 8.0 Hz, ArH), 7.42 (1H, t, J = 8.0 Hz, ArH), 7.56–7.62 (3H, m, 3 × ArH), 7.65 (1H, d, J = 7.2 Hz, ArH), 7.90 (1H, s, ArH), 8.00 (2H, d, J = 7.2 Hz, 2 × ArH), and 8.01 (1H, s, NH). HRMS (ESI): Calcd. for C15H17N4O (M + H)+ 269.1397 and found: 269.1401.
Amino((4-benzamidobenzyl)amino)methaniminium 2,2,2-trifluoroacetate (24e): 38 mg, 99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 4.39 (2H, s, CH2), 7.35 (2H, d, J = 8.4 Hz, 2 × ArH), 7.59 (2H, t, J = 7.2 Hz, 2 × ArH), 7.66 (1H, t, J = 7.2 Hz, ArH), 7.84 (2H, d, J = 8.4 Hz, 2 × ArH), 8.00 (2H, d, J = 8.0 Hz, 2 × ArH), and 8.01 (1H, s, NH). HRMS (ESI): Calcd. for C15H17N4O (M + H)+ 269.1397 and found: 269.1402.
tert-Butyl 7-hydroxy-1,2,3,4-dihydroisoquinoline-2-carboxylate (26a): In a solution of 25a (349 mg, 2.34 mmol) in THF/water (5 mL/1 mL), Boc2O (545 mg, 2.5 mmol) and Et3N (0.4 mL, 2.8 mmol) were added. The mixture was stirred at r.t. for 16 h and partitioned between EtOAc (50 mL) and water (50 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography (petrol ether/EtOAc 7:3) gave a white solid (475 mg, 81% yield), m.p. 130–131 °C. 1H NMR (400 MHz, CDCl3): δ 1.49 (9H, s, t-Bu), 2.74 (2H, t, J = 5.9 Hz, CH2), 3.62 (2H, t, J = 6.0 Hz, CH2), 4.51 (2H, s, CH2), 6.62–6.65 (2H, m, 2 × ArH), and 6.98 (1H, d, J = 8.2 Hz, ArH).
tert-Butyl 7-(2,3-dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (27a): In a solution of 26a (370 mg, 1.48 mmol) in acetone (15 mL), 2,3-dichlorobenzyl bromide (437 mg, 1.6 mmol) was added, followed by K2CO3 (262 mg, 1.9 mmol). The mixture was stirred at r.t. overnight and partitioned between EtOAc (30 mL) and water (30 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 3:1) afforded 27a as clear oil (500 mg, 83%). 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 2.76 (2H, t, J = 5.7 Hz, CH2), 3.62 (2H, t, J = 5.7 Hz, CH2), 4.52 (2H, s), 5.15 (2H, s), 6.75–6.79 (2H, m, 2 × ArH), 7.05 (1H, d, J = 8.4 Hz, ArH), 7.23 (1H, t, J = 7.9 Hz, ArH), and 7.45 (2H, m, 2 × ArH). HRMS (ESI): Calcd. for C21H23Cl2NNaO3 (M + Na)+ 430.0953 and found 430.0970.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}({7-(2,3-dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinolin-2-yl})methyl]carbamate (28a): In a solution of 27a (450 mg, 1.1 mmol) in CH2Cl2 (63 mL), TFA (2 mL) was added. The mixture was shaken at r.t. for 10 h and evaporated in vacuo to give to a yellow residue. The crude product was dissolved in DMF (5 mL) and Et3N (0.6 mL). S-Methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (390 mg, 1.32 mmol) was added, followed by HgCl2 (200 mg, 2.37 mmol). The mixture was stirred at r.t. for 16 h, diluted with EtOAc (50 mL), and filtered through Celite. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 3:2) afforded 28a as a foamy powder solid (340 mg, 56% yield), m.p. 59–61 °C. 1H NMR (400 MHz, CDCl3): δ 1.50 (18H, s, 2 × t-Bu), 2.98 (2H, t, J = 5.7 Hz, CH2), 3.80 (2H, t, J = 5.7 Hz, CH2), 4.75 (2H, s), 5.12 (2H, s), 6.70 (1H, br.s, ArH), 6.81 (1H, dd, J = 8.0, 1.9 Hz, ArH), 7.05 (1H, d, J = 8.0 Hz, ArH), 7.22 (1H, d, J = 8.0 Hz, ArH), 7.40–7.46 (2H m, 2 × ArH), and 10.2 (1H, s, NH). HRMS (ESI): Calcd. for C27H34Cl2N3O5 (M + H)+ 550.1876 and found 550.1876.
7-(2,3-Dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinoline-2-carboximidamide 2,2,2-trifluoroacetate (29a): The compound was synthesised as described for 28a. A white solid (30 mg, 82%) was obtained. 1H NMR (400 MHz, DMSO-d6): δ 2.89 (2H, t, J = 5.5 Hz, CH2), 3.50 (2H, t, J = 5.2 Hz, CH2), 4.51 (2H, s), 5.20 (2H, s), 6.85 (1H, d, J = 1.7 Hz, ArH), 6.95 (1H, dd, J = 7.8, 1.5 Hz, ArH), 7.25 (1H, d, J = 7.7 Hz, ArH), 7.45 (1H, t, J = 7.9 Hz, ArH), 7.60 (1H, d, J = 8.0 Hz, ArH), and 7.70 (1H, d, J = 8.0 Hz, ArH). HRMS (ESI): Calcd. for C17H18Cl2N3O (M + H)+ 350.0827 and found 350.0821.
tert-Butyl 5-hydroxy-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (26b): A solution of 25b (1.0 g, 90% purity, 6.2 mmol) in AcOH (20 mL) was reacted over H2 (1 atm) and PtO2 (85 mg) at r.t. for 48 h. The reaction mixture was then filtered through Celite and concentrated in vacuo. The residue was dissolved in acetone (3 mL) and diluted with Et2O (3 mL). The precipitate was collected and dried in vacuo. The crude product (700 mg, 4.7 mmol) was suspended in THF/water (10 mL/2 mL). Boc2O (1.1 g, 5.0 mmol) and Et3N (1.5 mL, 10 mmol) were added. The mixture was stirred at r.t. for 16 h and partitioned between EtOAc (50 mL) and water (50 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography (petrol ether/EtOAc 7:3) gave 26b as a white solid (490 mg, 42% yield), m.p. 156–158 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (s, 9H, t-Bu), 2.75 (2H, t, J = 6.0 Hz, CH2), 3.65 (2H, t, J = 6.1 Hz, CH2), 4.55 (2H, s, CH2), 6.63 (1H, d, J = 7.8 Hz, ArH), 6.68 (1H, d, J = 7.9 Hz, ArH), and 7.03 (1H, d, J = 7.8 Hz, ArH). HRMS (ESI): Calcd. for C14H19NNaO3 (M + Na)+ 272.1263 and found 272.1244.
tert-Butyl 5-(2,3-dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (27b): The compound was synthesised as described for 27a. A white solid (480 mg, 79%) was obtained, m.p. 138–139 °C. 1H NMR (400 MHz, CDCl3): δ 1.49 (9H, s, t-Bu), 2.86 (2H, t, J = 5.8 Hz, CH2), 3.67 (2H, t, J = 5.9 Hz, CH2), 4.58 (2H, s), 5.16 (2H, s), 6.74–6.77 (2H, m, 2 × ArH), 7.14 (1H, t, J = 8.2 Hz, ArH), 7.22–7.26 (2H, m, 2 × ArH), 7.44 (1H, d, J = 8.7 Hz, ArH), and 7.49 (1H, d, J = 9.0 Hz, ArH). HRMS (ESI): Calcd. for C21H23Cl2NNaO3 (M + Na)+ 430.0953 and found 430.0917.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({5-(2,3-dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinolin-2-yl})methylidene]carbamate (28b): The compound was synthesised as described for 28a. A white solid (280 mg, 59%) was obtained, m.p. 129–130 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 2.91 (2H, t, J = 5.5 Hz, CH2), 3.71 (2H, t, J = 5.5 Hz, CH2), 4.62 (2H, s), 5.21 (2H, s), 6.79–6.82 (2H, m, 2 × ArH), 7.18 (1H, t, J = 8.3 Hz, ArH)), 7.31–7.34 (2H, m, 2 × ArH), 7.46 (1H, d, J = 8.5 Hz, ArH), and 7.52 (1H, d, J = 8.3 Hz, ArH). HRMS (ESI): Calcd. for C27H34Cl2N3O5 (M + H)+ 550.1876 and found 550.1882.
5-(2,3-Dichlorobenzyloxy)-1,2,3,4-tetrahydroisoquinoline-2-carboximidamide hydrochloride (29b): The compound in TFA salt form (100 mg) was synthesised as described for 28a. The TFA salt was converted to hydrochloride 29b using HCl (0.5M in MeOH). 1H NMR (400 MHz, DMSO-d6): δ 2.85 (2H, t, J = 5.5 Hz, CH2), 3.52 (2H, t, J = 5.2 Hz, CH2), 4.60 (2H, s), 5.35 (2H, s), 6.82 (1H, d, J = 7.8 Hz, ArH), 7.05 (1H, d, J = 7.9 Hz, ArH), 7.28 (1H, t, J = 8.0 Hz, ArH), 7.47–7.51 (1H, m, ArH), 7.65 (1H, d, J = 8.3 Hz, ArH), and 7.73 (1H, d, J = 8.5 Hz, ArH). HRMS (ESI): Calcd. for C17H18Cl2N3O (M + H)+ 350.0827 and found 350.0899.
tert-Butyl N-[(7-bromo-1,2,3,4-tetrahydroisoquinolin-2-yl)({[(tert-butoxy) carbon yl]amino})methylidene]carbamate (31): In a solution of 7-bromo-1,2,3,4-tetrahydroisoquinoline 30 (318 mg, 1.5 mmol) in DMF (5 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (436 mg, 1.5 mmol) was added, followed by Et3N (0.5 mL). The mixture was stirred at r.t. for 4 h and partitioned between EtOAc (100 mL) and citric acid (50 mL, 5% in water). The organic layer was washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 3:1) afforded 31 as a white solid (500 mg, 73% yield), m.p. 131–132 °C. 1H NMR (400 MHz, CDCl3): δ 1.50 (18H, s, 2 × t-Bu), 2.90 (2H, t, J = 5.5 Hz, CH2), 3.87 (2H, t, J = 5.7 Hz, CH2), 4.70 (2H, s, CH2), 7.01 (1H, d, J = 8.0 Hz, ArH), 7.23 (1H, br.s, ArH), 7.28 (1H, dd, J = 8.2, 1.9 Hz, ArH), and 10.3 (1H, s, NH). HRMS (ESI): Calcd. for C20H29BrN3O4 (M + H)+ 454.1341 and found 454.1356.
tert-Butyl N-[7-(4-tert-butylphenyl)-1,2,3,4-tetrahydroisoquinoline-2-carboximido yl]carbamate (32): In a solution of 31 (400 mg, 0.88 mmol) in dioxane (6 mL) and water (2 mL), 4-tert-butylphenylboronic acid (188 mg, 1.05 mmol) and K2CO3 (242 mg, 1.76 mmol) were added. The mixture was degassed under vacuum for 1 min and Pd(PPh3)4 (20 mg) was added. The reaction mixture was stirred at 100 °C under N2 for 4 h. After cooling to r.t., the mixture was partitioned between EtOAc (50 mL) and water (50 mL). The organic layer was washed with brine, dried (MgSO4), filtered, and concentrated in vacuo. Purification by flash column chromatography (CH2Cl2/MeOH (9:1) gave 32 as a clear oil (190 mg, 53% yield). 1H NMR (400 MHz, CDCl3): δ 1.35 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 2.90 (2H, t, J = 5.3 Hz, CH2), 3.75 (2H, t, J = 5.2 Hz, CH2), 4.73 (2H, s, CH2), 7.18 (1H, d, J = 8.3 Hz, ArH), 7.35 (1H, br.s, ArH), 7.41 (1H, dd, J = 8.1, 1.5 Hz, ArH), 7.46–7.50 (4H, m, 4 × ArH), and 10.1 (1H, s, NH). HRMS (ESI): Calcd. for C25H34N3O2 (M + H)+ 408.2651 and found 408.2687.
N-[7-(4-tert-Butylphenyl)-1,2,3,4-tetrahydroisoquinoline-2-carboximidoyl]cabam-ate 2,2,2-trifluoroacetate (33): The compound was synthesised as described for 28a. A white solid (35 mg, 77%) was obtained, m.p. 218–219 °C. 1H NMR (400 MHz, CD3OD): δ 1.35 (9H, s, t-Bu), 3.00 (2H, t, J = 5.5 Hz, CH2), 3.70 (2H, t, J = 5.5 Hz, CH2), 4.70 (2H, s, CH2), 7.39 (1H, d, J = 8.2 Hz, ArH), 7.46 (1H, d, J = 1.6 Hz, ArH), and 7.50–7.65 (5H, m, 5 × ArH). HRMS (ESI): Calcd. for C20H26N3 (M + H)+ 308.2127 and found 308.2131.
General Procedure: Guanylation of O-benzylhydroxylamines (34a–c): In a solution of the substituted amine (1.5 mmol) in DMF (5 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (285 mg, 0.98 mmol) was added, followed by Et3N (0.6 mL). The mixture was stirred at r.t. overnight and then partitioned between EtOAc (100) ml and brine (50 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 11:9) afforded 35a–c.
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(3-phenylphenyl)methoxy]amino}) methylidene]carbamate (35a): A foamy powder (190 mg, 69%) was obtained. 1H NMR (400 MHz, CDCl3): δ 1.46 (9H, s, t-Bu), 1.49 (9H, s, t-Bu), 5.13 (2H, s, CH2), 7.33–7.46 (5H, m, 5 × ArH), 7.56–7.63 (3H, m, 3 × ArH), 7.64–7.68 (1H, m, ArH), 7.73 (s, 1H, NH), and 9.16 (s, 1H, NH).
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[(3-phenoxyphenyl)methoxy] amino})methylidene]carbamate (35b): A foamy powder (185 mg, 65%) was obtained. 1H NMR (400 MHz, CDCl3): δ 1.36 (18H, s, 2 × t-Bu), 5.28 (2H, s, CH2), 7.41–7.55 (5H, m, 4 × ArH, NH), 7.59 (1H, dt, J = 8.1, 1.5 Hz, ArH), 7.69–7.74 (2H, m, 2 × ArH), 7.76 (1H, s, NH), 7.81 (1H, d, J = 8.1 Hz, ArH), and 7.82 (1H, d, J = 8.2 Hz, ArH).
tert-Butyl N-[{[(tert-butoxy)carbonyl]amino}({[3-(4-tert-butylphenyl)phenyl]meth- oxy}amino)methylidene]carbamate (35c): A foamy powder (200 mg, 80%) was obtained. 1H NMR (400 MHz, CDCl3): δ 1.36 (9H, s, t-Bu), 1.49 (9H, s, t-Bu), 1.50 (9H, s, t-Bu), 5.12 (2H, s, CH2), 7.38–7.45 (4H, m, 4 × ArH), 7.52–7.58 (3H, m, 3 × ArH), 7.62–7.66 (1H, m, ArH), 7.72 (br.s, 1H, NH), and 9.18 (1H, s, NH).
General Procedure: Synthesis of benzyloxy guanidine derivatives (36a–c): In a solution of the substituted N,N′-di-Boc-guanidino derivative (35a–c) (0.3 mmol) in CH2Cl2 (2 mL), TFA (1 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with diethyl ether, and dried in vacuo to give 36a–c as a white or off-white solid.
1-[(3-Phenylphenyl)methoxy]guanidinium 2,2,2-trifluoroacetate (36a): An off-white solid (65 mg, 92%) was obtained. 1H NMR (400 MHz, DMSO-d6): δ 5.00 (2H, s, CH2), 7.43–7.57 (4H, m, 4 × ArH), 7.72 (1H, t, J = 1.9 Hz, ArH), 7.70–7.76 (4H, m, 4 × ArH), 7.83 (1H, br.s, NH), and 11.2 (1H, s, NH). HRMS (ESI): Calcd. for C14H16N3O (M + H)+ 242.1293 and found 242.1282.
1-[(3-Phenoxyphenyl)methoxy]guanidinium 2,2,2-trifluoroacetate (36b): A white solid (95 mg, 97%) was obtained. 1H NMR (400 MHz, CDCl3): δ 4.76 (2H, s, CH2), 6.96–7.05 (4H, m, 4 × ArH), 7.05 (1H, dt, J = 8.5, 1.9 Hz, ArH), 7.12 (1H, td, J = 8.9, 2.1 Hz, ArH), 7.30–7.35 (3H, m, 3 × ArH), and 10.9 (1H, s, NH). HRMS (ESI): Calcd. for C14H15N3NaO2 (M + Na)+ 280.1062 and found 280.1066.
1-{[3-(4-tert-Butylphenyl)phenyl]methoxy}guanidinium 2,2,2-trifluoroacetate (36c): A white solid (75 mg, 77%) was obtained. 1H NMR (400 MHz, CD3OD): δ 1.43 (9H, s, t-Bu), 5.12 (2H, s, CH2), 7.46 (1H, dt, J = 8.9, 1.7 Hz, ArH), 7.52–7.56 (3H, m, 3 × ArH), 7.63–7.66 (2H, m, 2 × ArH), 7.72 (1H, dt, J = 8.8, 2.0 Hz, ArH), and 7.76–7.79 (1H, m, ArH). HRMS (ESI): Calcd. for C18H24N3O (M + H)+ 298.1919 and found 298.1938.
General Procedure: Synthesis of 3-benzyloxybenzaldehyde derivatives (38a–t): The 3-hydroxybenzaldehyde derivative 37a–c (2 mmol) and K2CO3 (4 mmol) were placed in a round-bottom flask. DMF (3 mL) was added. Then, the corresponding benzyl halide (2.2 mmol) was added and the reaction mixture was stirred at r.t. for 18 h. Water (125 mL) and brine (25 mL) were added, and the mixture was extracted with Et2O (2 × 100 mL) or CH2Cl2 (for 38q; 2 × 100 mL). The combined organic layers were dried (NaCl), filtered, and concentrated in vacuo. Crystallisation from Et2O or pentane/Et2O gave the corresponding aldehyde derivatives 38a–c and 38h–t. Aldehyde derivatives 38d–g were purified by flash column chromatography.
3-(2,3-Dichlorobenzyloxy)-benzaldehyde (38a): 445 mg, 79%, white solid. 1H NMR (400 MHz, CDCl3): δ 5.22 (2H, s, CH2), 7.21–7.28 (3H, m, 3 × ArH), 7.42–7.55 (5H, m, 5 × ArH), and 9.99 (1H, s, CH=O). 13C NMR (100 MHz, CDCl3): δ 67.8, 113.5, 122.1, 124.2, 126.8, 127.6, 130.0, 130.4, 130.9, 133.4, 136.6, 138.1, 159.0, and 192.1. HRMS (ESI): Calcd. for C14H11Cl2O2 (M + H)+ 281.0131 and found 281.0142.
3-(2-Chloro-3-(trifluoromethyl)benzyloxy)-benzaldehyde (38b): 474 mg, 75%, white solid. 1H NMR (400 MHz, CDCl3): δ 5.27 (2H, s, CH2), 7.24–7.29 (1H, m, ArH), 7.42 (1H, t, J = 8.0 Hz, ArH), 7.46–7.54 (3H, m, 3 × ArH), 7.70 (1H, d, J = 7.6 Hz, ArH), 7.79 (1H, d, J = 7.6 Hz, ArH), and 9.99 (1H, s, CH=O). HRMS (ESI): Calcd. for C15H11ClF3O2 (M + H)+ 315.0394 and found 315.0403.
3-(4-Chlorobenzyloxy)-benzaldehyde (38c): 430 mg, 87%, white solid, m.p. 52–54 °C. 1H NMR (400 MHz, CDCl3): δ 5.08 (2H, s, CH2), 7.23 (1H, dt, J = 7.6, 2.0 Hz, ArH), 7.34–7.40 (4H, m, 4 × ArH), 7.43–7.51 (3H, m, 3 × ArH), and 9.97 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H12ClO2 (M + H)+ 247.0520 and found 247.0531.
3-(4-(Trifluoromethyl)benzyloxy)-benzaldehyde (38d): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 38d (501 mg, 89%, colourless oil). 1H NMR (400 MHz, CDCl3): δ 5.18 (2H, s, CH2), 7.23–7.28 (1H, m, ArH), 7.44–7.52 (3H, m, 3 × ArH), 7.56 (2H, d, J = 8.0 Hz, 2 × ArH), 7.66 (2H, d, J = 8.0 Hz, 2 × ArH), and 9.97 (1H, d, J = 1.2 Hz, CH=O). HRMS (ESI): Calcd. for C15H12F3O2 (M + H)+ 281.0784 and found 281.0789.
3-(3-Chlorobenzyloxy)-benzaldehyde (38e): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 38e (424 mg, 86%, colourless oil). 1H NMR (400 MHz, CDCl3): δ 5.09 (2H, s, CH2), 7.22–7.26 (1H, m, ArH), 7.29–7.33 (3H, m, 3 × ArH), 7.44–7.51 (4H, m, 4 × ArH), and 9.97 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H12ClO2 (M + H)+ 247.0520 and found 247.0525.
3-(3-(Trifluoromethyl)benzyloxy)-benzaldehyde (38f): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 38f (478 mg, 85%, colourless oil). 1H NMR (400 MHz, CDCl3): δ 5.17 (2H, s, CH2), 7.24–7.28 (1H, m, ArH), 7.45–7.51 (3H, m, 3 × ArH), 7.53 (1H, d, J = 8.0 Hz, ArH), 7.61 (1H, d, J = 8.8 Hz, ArH), 7.63 (1H, d, J = 8.0 Hz, ArH), 7.72 (1H, s, ArH), and 9.98 (1H, s, CH=O). HRMS (ESI) calcd. for C15H12F3O2+ (M + H)+ 281.0784 and found 281.0790.
3-(2,3-Dichlorobenzyloxy)-4-methoxybenzaldehyde (38g): Purification by flash column chromatography (CH2Cl2 to CH2Cl2/EtOAc 9:1) gave 38g (436 mg, 87%). m.p. 117–119 °C. 1H NMR (400 MHz, CDCl3): δ 3.98 (3H, s, OCH3), 5.28 (2H, s, CH2), 7.08 (1H, d, J = 8.3 Hz, ArH), 7.23 (1H, d, J = 8.1 Hz, ArH), 7.43 (2H, d, J = 8.2 Hz, 2 × ArH), 7.52 (2H, dd, J = 8.0, 1.1 Hz, 2 × ArH), and 9.88 (1H, s, CH=O). HRMS (ESI): Calcd. for C15H13Cl2O3 (M + H)+ 311.0242 and found 311.0237.
2-Chloro-3-(2-chloro-3-methoxybenzyloxy)-benzaldehyde (38h): 597 mg, 96%, white solid, m.p. 124–126 °C. 1H NMR (400 MHz, CDCl3): δ 3.92 (3H, s, OCH3), 5.28 (2H, s, CH2), 6.92 (1H, dd, J = 7.0, 2.6 Hz, ArH), 7.19 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.24–7.33 (3H, m, 3 × ArH), 7.54 (1H, dd, J = 7.8, 1.4 Hz, ArH), and 10.53 (1H, d, J = 0.4 Hz, CH=O). HRMS (ESI): Calcd. for C15H13Cl2O3 (M + H)+ 311.0236 and found 311.0232.
2-Chloro-3-benzyloxybenzaldehyde (38i): 425 mg, 86%, white solid, m.p. 103–105 °C. 1H NMR (400 MHz, CDCl3): δ 5.20 (2H, s, CH2), 7.19 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.29 (1H, t, J = 7.8 Hz, ArH), 7.35 (1H, d, J = 6.8 Hz, ArH), 7.41 (2H, t, J = 7.4 Hz, 2 × ArH), 7.47 (2H, d, J = 7.2 Hz, 2 × ArH), 7.53 (1H, dd, J = 7.8, 1.4 Hz, ArH), and 10.54 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H12ClO2 (M + H)+ 247.0520 and found 247.0528.
2-Chloro-3-(4-chlorobenzyloxy)-benzaldehyde (38j): 522 mg, 93%, white solid, m.p. 94–96 °C. 1H NMR (400 MHz, CDCl3): δ 5.15 (2H, s, CH2), 7.16 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.30 (1H, t, J = 8.0 Hz, ArH), 7.35–7.43 (4H, m, 4 × ArH), 7.54 (1H, dd, J = 7.8, 1.4 Hz, ArH), and 10.53 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C14H11Cl2O2 (M + H)+ 281.0131 and found 281.0127.
2-Chloro-3-(2,3-dichlorobenzyloxy)-benzaldehyde (38k): 600 mg, 95%, white solid, m.p. 150–152 °C. 1H NMR (400 MHz, CDCl3): δ 5.27 (2H, s, CH2), 7.20 (1H, d, J = 8.0 Hz, ArH), 7.28 (1H, t, J = 8.0 Hz, ArH), 7.34 (1H, t, J = 8.0 Hz, ArH), 7.46 (1H, d, J = 8.0 Hz, ArH), 7.57 (1H, d, J = 8.0 Hz, ArH), 7.61 (1H, d, J = 8.0 Hz, ArH), and 10.54 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H10Cl3O2 (M + H)+ 314.9741 and found 314.9748.
2-Chloro-3-(2-chloro-3-(trifluoromethyl)benzyloxy)-benzaldehyde (38m): 632 mg, 90%, white solid, m.p. 128–131 °C. 1H NMR (400 MHz, CDCl3): δ 5.31 (2H, s, CH2), 7.23 (1H, dd, J = 8.2, 1.4 Hz, ArH), 7.36 (1H, t, J = 8.0 Hz, ArH), 7.47 (1H, t, J = 7.8 Hz, ArH), 7.59 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.71 (1H, d, J = 8.0 Hz, ArH), 7.94 (1H, d, J = 7.6 Hz, ArH), and 10.55 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C15H10Cl2F3O2 (M + H)+ 349.0004 and found 349.0011.
2-Chloro-3-(2,4-dichlorobenzyloxy)-benzaldehyde (38n): 580 mg, 92%, white solid, m.p. 115–116 °C. 1H NMR (400 MHz, CDCl3): δ 5.22 (2H, s, CH2), 7.20 (1H, dd, J = 8.2, 1.4 Hz, ArH), 7.30–7.36 (2H, m, 2 × ArH), 7.43 (1H, d, J = 2.4 Hz, ArH), 7.57 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.62 (1H, d, J = 8.4 Hz, ArH), and 10.54 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C14H10Cl3O2 (M + H)+ 314.9741 and found 314.9748.
2-Chloro-3-(2,5-dichlorobenzyloxy)-benzaldehyde (38o): 535 mg, 85%, white solid, m.p. 132–134 °C. 1H NMR (400 MHz, CDCl3): δ 5.22 (2H, s, CH2), 7.21 (1H, dd, J = 8.4, 1.2 Hz, ArH), 7.27 (1H, dd, J = 8.4, 2.4 Hz, ArH), 7.34 (1H, d, J = 8.8 Hz, ArH), 7.35 (1H, t, J = 8.0 Hz, ArH), 7.58 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.69 (1H, d, J = 2.4 Hz, ArH), and 10.55 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H10Cl3O2 (M + H)+ 314.9741 and found 314.9745.
2-Chloro-3-(3,4-dichlorobenzyloxy)-benzaldehyde (38p): 610 mg, 97%, white solid, m.p. 140–142 °C. 1H NMR (400 MHz, CDCl3): δ 5.13 (2H, s, CH2), 7.15 (1H, dd, J = 8.2, 1.4 Hz, ArH), 7.29–7.34 (2H, m, 2 × ArH), 7.48 (1H, d, J = 8.4 Hz, ArH), 7.56 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.58 (1H, s, ArH), and 10.53 (1H, s, CH=O). HRMS (ESI): Calcd. for C14H10Cl3O2 (M + H)+ 314.9741 and found 314.9744.
2-Chloro-3-(2,3,5-trichlorobenzyloxy)-benzaldehyde (38q): 610 mg, 87%, white solid, m.p. 158–161 °C. 1H NMR (400 MHz, CDCl3): δ 5.22 (2H, s, CH2), 7.20 (1H, dd, J = 8.2, 1.4 Hz, ArH), 7.36 (1H, t, J = 8.0 Hz, ArH), 7.48 (1H, d, J = 2.4 Hz, ArH), 7.60 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.64 (1H, d, J = 2.4 Hz, ArH), and 10.55 (1H, d, J = 0.4 Hz, CH=O). HRMS (ESI): Calcd. for C14H9Cl4O2 (M + H)+ 348.9351 and found 348.9365.
2-Chloro-3-(3-(trifluoromethyl)benzyloxy)-benzaldehyde (38r): 617 mg, 98%, white solid, m.p. 83–85 °C. 1H NMR (400 MHz, CDCl3): δ 5.23 (2H, s, CH2), 7.19 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.33 (1H, t, J = 8.0 Hz, ArH), 7.54 (1H, t, J = 7.8 Hz, ArH), 7.57 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.62 (1H, d, J = 8.0 Hz, ArH), 7.69 (1H, d, J = 7.6 Hz, ArH), 7.74 (1H, s, ArH), and 10.54 (1H, d, J = 0.4 Hz, CH=O). HRMS (ESI): Calcd. for C15H11ClF3O2 (M + H)+ 315.0394 and found 315.0401.
2-Chloro-3-(4-(trifluoromethyl)benzyloxy)-benzaldehyde (38s): 605 mg, 96%, white solid, m.p. 82–84 °C. 1H NMR (400 MHz, CDCl3): δ 5.25 (2H, s, CH2), 7.18 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.32 (1H, dt, J = 8.0, 0.8 Hz, ArH), 7.56 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.60 (2H, d, J = 8.0 Hz, 2 × ArH), 7.67 (2H, d, J = 7.6 Hz, 2 × ArH), and 10.54 (1H, s, CH=O). HRMS (ESI): Calcd. for C15H11ClF3O2 (M + H)+ 315.0394 and found 315.0398.
2-Chloro-3-(3-chlorobenzyloxy)-benzaldehyde (38t): 418 mg, 74%, white solid. 1H NMR (400 MHz, CDCl3): δ 5.16 (2H, s, CH2), 7.17 (1H, dd, J = 8.2, 1.4 Hz, ArH), 7.31 (1H, t, J = 8.0 Hz, ArH), 7.31–7.37 (3H, m, 3 × ArH), 7.46–7.48 (1H, m, ArH), 7.56 (1H, dd, J = 7.8, 1.4 Hz, ArH), and 10.54 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C14H11Cl2O2 (M + H)+ 281.0131 and found 281.0139.
(E)-Amino(2-(3-(2,3-dichlorobenzyloxy)benzylidene)hydrazineyl)methaniminium acetate (10a): A mixture of 38a (228 mg, 0.81 mmol) and N-aminoguanidine bicarbonate (110 mg, 0.81 mmol) in MeOH-AcOH (3 mL/0.2 mL) was refluxed under N2 for 4 h, cooled to r.t., and concentrated in vacuo. CH2Cl2 (1 mL) was added, and the precipitate was collected, washed with CH2Cl2, and dried in vacuo to give 10a as a white solid (170 mg, 53%, m.p. 159–160 °C). 1H NMR (400 MHz, DMSO-d6): δ 1.87 (3H, s, CH3CO2), 5.29 (2H, s, CH2), 7.03 (1H, d, J = 8.9 Hz, ArH), 7.07 (4H, br.s, 2 × NH2), 7.48 (t, J = 7.9 Hz, 1H, ArH), 7.53–7.55 (2H, m, 2 × ArH), 7.66–7.72 (2H, m, 2 × ArH), 7.58 (1H, s, ArH), and 8.08 (1H, s, CH=N). HRMS (ESI): Calcd. for C15H15Cl2N4O (M + H)+ 337.0623 and found 337.0693.
(E)-Amino(2-(3-(2-chloro-3-(trifluoromethyl)benzyloxy)benzylidene)hydrazine-yl)-methaniminium acetate (10b): A mixture of 38b (320 mg, 1.02 mmol) and N-aminoguanidine bicarbonate (138 mg, 1.02 mmol) in MeOH-AcOH (4 mL/0.2 mL) was refluxed under N2 for 6 h, cooled to r.t., and concentrated in vacuo. EtOAc (3 mL) was added, and the precipitate was collected, washed with EtOAc, and dried in vacuo to give 10b as a white solid (283 mg, 75%, m.p. 190–192 °C). 1H NMR (400 MHz, DMSO-d6): δ 1.82 (3H, s, CH3CO2), 5.25 (2H, s, CH2), 6.95 (4H, br.s, 2 × NH2), 7.02 (1H, dd, J = 7.8, 1.9 Hz, ArH), 7.26–7.33 (2H, m, 2 × ArH), 7.50 (1H, s, ArH), 7.58 (1H, t, J = 7.8 Hz, ArH), 7.86 (1H, d, J = 8.0 Hz, ArH), 7.91 (1H, d, J = 7.8 Hz, ArH), and 7.99 (s, 1H, CH=N). HRMS (ESI): Calcd. for C16H15ClF3N4O (M + H)+ 371.0886 and found 371.0895.
(E)-Amino(2-(3-(2,3-dichlorobenzyloxy)-4-methoxybenzylidene)hydrazineyl)-methaniminium acetate (10g): A white solid was obtained (278 mg, 80%). 1H NMR (400 MHz, DMSO-d6): δ 1.89 (3H, s, CH3CO2), 3.85 (3H, s, OCH3), 5.30 (2H, s, CH2), 6.98 (4H, br.s, 2 × NH2), 7.07 (1H, d, J = 8.1 Hz, ArH), 7.28 (1H, d, J = 8.2 Hz, ArH), 7.50 (1H, t, J = 7.9 Hz, ArH), 7.65–7.75 (3H, m, 3 × ArH), and 8.19 (1H, s, CH=N). HRMS (ESI): Calcd. for C16H17Cl2N4O2 (M + H)+ 367.0729 and found 367.0701.
General procedure: Synthesis of (E)-amino(2-(3-(benzyloxy)benzylidene) hydrazineyl)methaniminium chloride derivatives (10c–f, 10h–t): 3-benzyloxybenzaldehyde derivatives 36c–t (0.2 mmol) and N-aminoguanidine bicarbonate (0.205 mmol) were placed in a 50 mL round-bottom flask. HCl (0.5M in MeOH, 2.0 mL) was added, and the reaction mixture was stirred at 80 °C for 0.5 h and then evaporated to dryness. Crystallisation from Et2O (~5–6 mL) with a very small portion of MeOH (~0.3–0.5 mL) gave the corresponding N-aminoguanidinium chloride or acetate salts 10c–f or 10h–t.
(E)-Amino(2-(3-(4-chlorobenzyloxy)benzylidene)hydrazineyl)methaniminium chloride (10c): 54 mg, 79%, white solid, m.p. 191–193 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.21 (2H, s, CH2), 7.11–7.18 (1H, m, ArH), 7.38–7.45 (2H, m, 2 × ArH), 7.52 (2H, d, J = 8.4 Hz, 2 × ArH), 7.56 (2H, d, J = 8.4 Hz, 2 × ArH), 7.67 (1H, s, ArH), 7.86 (4H, s, br, 2 × NH2), 8.20 (1H, s, CH=N), and 11.97 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H16ClN4O (M + H)+ 303.1007 and found 303.1013.
(E)-Amino(2-(3-(4-(trifluoromethyl)benzyloxy)benzylidene) hydrazineyl) methan-iminium chloride (10d): 58 mg, 77%, white solid, m.p. 176–179 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.33 (2H, s, CH2), 7.14–7.20 (1H, m, ArH), 7.40–7.47 (2H, m, 2 × ArH), 7.67 (1H, s, ArH), 7.75 (2H, d, J = 8.0 Hz, 2 × ArH), 7.83 (2H, d, J = 8.4 Hz, 2 × ArH), 7.85 (4H, s, br, 2 × NH2), 8.20 (1H, s, CH=N), and 11.96 (1H, s, N-NH). HRMS (ESI): Calcd. for C16H16F3N4O (M + H)+ 337.1271 and found 337.1278.
(E)-Amino(2-(3-(3-chlorobenzyloxy)benzylidene)hydrazineyl)methaniminium chloride (10e): 69 mg, >99%, beige glass. 1H NMR (400 MHz, DMSO-d6): δ 5.22 (2H, s, CH2), 7.12–7.18 (1H, m, ArH), 7.42 (2H, d, J = 6.0 Hz, 2 × ArH), 7.45 (1H, dd, J = 5.0, 2.0 Hz, ArH), 7.49 (2H, d, J = 6.0 Hz, 2 × ArH), 7.59 (1H, s, ArH), 7.66 (1H, d, J = 2.8 Hz, ArH), 7.84 (4H, s, br, 2 × NH2), 8.21 (1H, s, CH=N), and 11.97 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H16ClN4O (M + H)+ 303.1007 and found 303.1015.
(E)-Amino(2-(3-(4-(trifluoromethyl)benzyloxy)benzylidene)hydrazineyl)methan-iminium chloride (10f): 68 mg, 92%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.31 (2H, s, CH2), 7.15–7.21 (1H, m, ArH), 7.41–7.45 (2H, m, 2 × ArH), 7.69 (1H, d, J = 2.8 Hz, ArH), 7.71 (1H, d, J = 8.4 Hz, ArH), 7.76 (1H, d, J = 8.0 Hz, ArH), 7.84 (1H, d, J = 7.6 Hz, ArH), 7.86 (4H, s, br, 2 × NH2), 7.89 (1H, s, ArH), 8.22 (1H, s, CH=N), and 11.97 (1H, s, N-NH). HRMS (ESI): Calcd. for C16H16F3N4O (M + H)+ 337.1271 and found 337.1282.
(E)-Amino(2-(2-chloro-3-(2-chloro-3-methoxybenzyloxy)benzylidene) hydrazine-yl)methaniminium chloride (10h): 68 mg, 84%, white solid, m.p. 258–260 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.33 (2H, s, CH2), 7.23 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.28 (1H, dd, J = 7.6 Hz, 1.2, ArH), 7.37 (1H, dd, J = 8.0, 1.6 Hz, ArH), 7.42 (1H, d, J = 8.0 Hz, ArH), 7.43 (1H, t, J = 7.8 Hz, ArH), 7.93 (4H, s, br, 2 × NH2), 7.96 (1H, dd, J = 7.8, 1.4 Hz, ArH), 8.65 (1H, s, CH=N), and 12.26 (1H, s, N-NH). HRMS (ESI): Calcd. for C16H17Cl2N4O2 (M + H)+ 367.0723 and found 367.0734.
(E)-Amino(2-(3-(benzyloxy)-2-chlorobenzylidene)hydrazineyl)methaniminium chloride (10i): 56 mg, 82%, white solid, m.p. 203–205 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.30 (2H, s, CH2), 7.37 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.39–7.44 (2H, m, 2 × ArH), 7.47 (2H, t, J = 7.4 Hz, 2 × ArH), 7.53 (1H, s, ArH), 7.55 (1H, d, J = 1.6 Hz, ArH), 7.92 (4H, s, br, 2 × NH2), 7.94 (1H, dd, J = 7.6, 1.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.27 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H16ClN4O (M + H)+ 303.1007 and found 303.1012.
(E)-Amino(2-(2-chloro-3-(4-chlorobenzyloxy)benzylidene)hydrazineyl)methan-iminium chloride (10j): 63 mg, 84%, white solid, m.p. 237–239 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.30 (2H, s, CH2), 7.35 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.42 (1H, t, J = 8.0 Hz, ArH), 7.51–7.59 (4H, m, 4 × ArH), 7.93 (4H, s, br, 2 × NH2), 7.95 (1H, dd, J = 7.6, 1.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.32 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H15Cl2N4O (M + H)+ 337.0617 and found 337.0625.
(E)-Amino(2-(2-chloro-3-(2,3-dichlorobenzyloxy)benzylidene)hydrazineyl) meth-animinium chloride (10k): 71 mg, 87%, white solid, m.p. 250–253 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.38 (2H, s, CH2), 7.40 (1H, dd, J = 8.4, 1.6 Hz, ArH), 7.46 (1H, t, J = 7.6 Hz, ArH), 7.51 (1H, t, J = 8.0 Hz, ArH), 7.70 (1H, dd, J = 7.8, 1.4 Hz, ArH), 7.73 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.95 (4H, s, br, 2 × NH2), 7.98 (1H, dd, J = 7.6, 1.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.30 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H14Cl3N4O (M + H)+ 371.0228 and found 371.0235.
(E)-Amino(2-(2-chloro-3-(2-chloro-3-(trifluoromethyl)benzyloxy)benzylidene) hydrazineyl)methaniminium chloride (10m): 73 mg, 82%, white solid, m.p. 274–277 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.44 (2H, s, CH2), 7.42–7.48 (2H, m, 2 × ArH), 7.71 (1H, t, J = 7.8 Hz, ArH), 7.95 (1H, d, J = 8.0 Hz, ArH), 7.97 (4H, s, br, 2 × NH2), 7.99 (1H, dd, J = 7.0, 1.4 Hz, ArH), 8.04 (1H, d, J = 7.6 Hz, ArH), 8.66 (1H, s, CH=N), and 12.31 (1H, s, N-NH). 13C NMR (100 MHz, DMSO-d6): δ 67.6 (CH2), 115.5 (CH), 119.8 (CH), 122.3, 127.6 (CH), 127.8 (CH), 127.9 (CH), 132.0, 133.5 (CH), 136.7, 142.9 (CH), 153.5, and 155.2. HRMS (ESI): Calcd. for C16H14Cl2F3N4O (M + H)+ 405.0491 and found 405.0498.
(E)-Amino(2-(2-chloro-3-(2,4-dichlorobenzyloxy)benzylidene)hydrazineyl) meth-animinium chloride (10n): 73 mg, 89%, white solid, m.p. 268–270 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.32 (2H, s, CH2), 7.40 (1H, dd, J = 8.4, 1.6 Hz, ArH), 7.44 (1H, t, J = 8.0 Hz, ArH), 7.58 (1H, dd, J = 8.4, 2.0 Hz, ArH), 7.73 (1H, d, J = 8.4 Hz, ArH), 7.77 (1H, d, J = 2.4 Hz, ArH), 7.95 (4H, s, br, 2 × NH2), 7.98 (1H, dd, J = 7.6, 1.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.34 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H14Cl3N4O (M + H)+ 371.0228 and found 371.0239.
(E)-Amino(2-(2-chloro-3-(2,5-dichlorobenzyloxy)benzylidene)hydrazineyl) meth-animinium chloride (10o): 72 mg, 88%, white solid, m.p. 242–244 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.33 (2H, s, CH2), 7.42 (1H, dd, J = 7.4, 1.6 Hz, ArH), 7.46 (1H, t, J = 7.6 Hz, ArH), 7.56 (1H, dd, J = 8.6, 2.2 Hz, ArH), 7.64 (1H, d, J = 8.8 Hz, ArH), 7.79 (1H, d, J = 2.4 Hz, ArH), 7.93 (4H, s, br, 2 × NH2), 7.99 (1H, dd, J = 7.2, 1.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.25 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H14Cl3N4O (M + H)+ 371.0228 and found 371.0237.
(E)-Amino(2-(2-chloro-3-(3,4-dichlorobenzyloxy)benzylidene)hydrazineyl) meth-animinium chloride (10p): 69 mg, 84%, white solid, m.p. 230–233 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.32 (2H, s, CH2), 7.35 (1H, dd, J = 8.4, 1.2 Hz, ArH), 7.43 (1H, t, J = 7.8 Hz, ArH), 7.53 (1H, dd, J = 8.0, 2.0 Hz, ArH), 7.75 (1H, d, J = 8.4 Hz, ArH), 7.81 (1H, d, J = 2.0 Hz, ArH), 7.92 (4H, s, br, 2 × NH2), 7.96 (1H, dd, J = 8.0, 1.2 Hz, ArH), 8.65 (1H, s, CH=N), and 12.25 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H14Cl3N4O (M + H)+ 371.0228 and found 371.0239.
(E)-Amino(2-(2-chloro-3-(2,3,5-trichlorobenzyloxy)benzylidene)hydrazineyl) methaniminium chloride (10q): 52 mg, 59%, white solid, m.p. 273–276 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.36 (2H, s, CH2), 7.42 (1H, dd, J = 7.4, 2.0 Hz, ArH), 7.46 (1H, t, J = 7.6 Hz, ArH), 7.77 (1H, d, J = 2.4 Hz, ArH), 7.96 (4H, s, br, 2 × NH2), 7.96 (1H, d, J = 2.8 Hz, ArH), 7.99 (1H, dd, J = 7.4, 1.8 Hz, ArH), 8.65 (1H, s, CH=N), and 12.32 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H13Cl4N4O (M + H)+ 404.9838 and found 404.9851.
(E)-Amino(2-(2-chloro-3-(3-(trifluoromethyl)benzyloxy)benzylidene)hydrazineyl) methaniminium chloride (10r): 69 mg, 84%, white powder, m.p. 198–201 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.41 (2H, s, CH2), 7.39 (1H, d, J = 8.4 Hz, ArH), 7.44 (1H, t, J = 7.8 Hz, ArH), 7.73 (1H, t, J = 7.6 Hz, ArH), 7.78 (1H, d, J = 8.0 Hz, ArH), 7.85 (1H, d, J = 7.2 Hz, ArH), 7.91 (1H, s, ArH), 7.94 (4H, s, br, 2 × NH2), 7.97 (1H, d, J = 7.6 Hz, ArH), 8.65 (1H, s, CH=N), and 12.33 (1H, s, N-NH). HRMS (ESI): Calcd. for C16H15ClF3N4O (M + H)+ 371.0881 and found 371.0891.
(E)-Amino(2-(2-chloro-3-(4-(trifluoromethyl)benzyloxy)benzylidene)hydrazineyl) methaniminium chloride (10s): 65 mg, 80%, white solid, m.p. 245–247 °C. 1H NMR (400 MHz, DMSO-d6): δ 5.43 (2H, s, CH2), 7.36 (1H, d, J = 8.0 Hz, ArH), 7.43 (1H, t, J = 8.0 Hz, ArH), 7.76 (2H, d, J = 8.0 Hz, 2 × ArH), 7.85 (2H, d, J = 7.6 Hz, 2 × ArH), 7.95 (4H, s, br, 2 × NH2), 7.96 (1H, d, J = 7.6 Hz, ArH), 8.66 (1H, s, CH=N), and 12.31 (1H, s, N-NH). HRMS (ESI): Calcd. for C16H15ClF3N4O (M + H)+ 371.0881 and found 371.0887.
(E)-Amino(2-(2-chloro-3-(3-chlorobenzyloxy)benzylidene)hydrazineyl) methaniminium chloride (10t): 75 mg, >99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.31 (2H, s, CH2), 7.35 (1H, dd, J = 8.0, 1.2 Hz, ArH), 7.41 (1H, t, J = 8.0 Hz, ArH), 7.43–7.48 (1H, m, ArH), 7.48–7.51 (2H, m, 2 × ArH), 7.59 (1H, s, ArH), 7.92 (4H, s, br, 2 × NH2), 7.94 (1H, dd, J = 8.0, 1.2 Hz, ArH), 8.65 (1H, s, CH=N), and 12.23 (1H, s, N-NH). HRMS (ESI): Calcd. for C15H15Cl2N4O (M + H)+ 337.0617 and found 337.0629.
General Procedure: Synthesis of 1-benzyl-1H-imidazole-2-carbaldehyde derivatives (40a–d) and 1-benzyl-1H-pyrrole-2-carbaldehyde derivatives (40e–h): Imidazole 2-carboxaldehyde 39a (1 mmol) or pyrrole 2-carboxaldehyde 39b (1 mmol) and K2CO3 (4 mmol) were placed in a 25 mL round-bottom flask. DMF (1 mL) was added. Then, the corresponding benzyl halide (1.2 mmol) was added and the reaction mixture was stirred at r.t. for 18 h. Water (80 mL) and brine (20 mL) were added, and the mixture was extracted with Et2O (100 mL). The organic layer was dried (NaCl), filtered, and concentrated in vacuo. All crude compounds 40a–d were purified by flash column chromatography.
1-(3-Chlorobenzyl)-1H-imidazole-2-carbaldehyde (40a): Purification by flash column chromatography (CH2Cl2 to CH2Cl2/EtOAc 9:1) gave 40a (177 mg, 80%, colourless oil). 1H NMR (400 MHz, CDCl3): δ 5.58 (2H, s, CH2), 7.05–7.08 (1H, m, ArH), 7.15 (2H, s, 2 × ArH), 7.25–7.28 (2H, m, 2 × ArH), 7.32 (1H, s, ArH), and 9.84 (1H, d, J = 0.5 Hz, CH=O). HRMS (ESI): Calcd. for C11H10ClN2O (M + H)+ 221.0476 and found 221.0469.
1-(4-Chlorobenzyl)-1H-imidazole-2-carbaldehyde (40b): Purification by flash column chromatography (CH2Cl2 to CH2Cl2/EtOAc 9:1) gave 40b (184 mg, 83%, beige solid). 1H NMR (400 MHz, CDCl3): δ 5.57 (2H, s, CH2), 7.11–7.13 (1H, m, ArH), 7.13–7.15 (2H, m, 2 × ArH), 7.30 (2H, dt, J = 8.4, 2.2 Hz, 2 × ArH), 7.32 (1H, s, ArH), and 9.86 (1H, s, CH=O). HRMS (ESI): Calcd. for C11H10ClN2O (M + H)+ 221.0476 and found 221.0471.
1-(3-(Trifluoromethyl)benzyl)-1H-imidazole-2-carbaldehyde (40c): Purification by flash column chromatography (CH2Cl2 to CH2Cl2/EtOAc 9:1) gave 40c (197 mg, 77%, colourless oil). 1H NMR (400 MHz, DMSO-d6): δ 5.75 (2H, s, CH2), 7.42 (1H, d, J = 0.8 Hz, ArH), 7.52 (1H, d, J = 8.0 Hz, ArH), 7.62–7.67 (2H, m, 2 × ArH), 7.70–7.74 (1H, m, ArH), 7.90 (1H, s, ArH), and 9.75 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C12H10F3N2O (M + H)+ 255.0740 and found 255.0747.
1-(4-(Trifluoromethyl)benzyl)-1H-imidazole-2-carbaldehyde (40d): Purification by flash column chromatography (CH2Cl2 to CH2Cl2/EtOAc 9:1) gave 40d (100 mg, 39%, beige solid). 1H NMR (400 MHz, CDCl3): δ 5.68 (2H, s, CH2), 7.19 (1H, s, ArH), 7.29 (2H, d, J = 8.0 Hz, 2 × ArH), 7.37 (1H, s, ArH), 7.60 (2H, d, J = 8.0 Hz, 2 × ArH), and 9.88 (1H, s, CH=O). HRMS (ESI): Calcd. for C12H10F3N2O (M + H)+ 255.0740 and found 255.0749.
1-(3-Chlorobenzyl)-1H-pyrrole-2-carbaldehyde (40e): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 40e (165 mg, 75%, colourless oil). 1H NMR (400 MHz, CDCl3): δ 5.53 (2H, s, CH2), 6.29 (1H, dd, J = 3.8, 2.6 Hz, ArH), 6.96–6.99 (2H, m, 2 × ArH), 7.07–7.09 (1H, m, ArH), 7.21–7.23 (2H, m, 2 × ArH), 7.25–7.27 (1H, m, ArH), and 9.54 (1H, d, J = 0.8 Hz, CH=O). HRMS (ESI): Calcd. for C12H11ClNO (M + H)+ 220.0524 and found 220.0519.
1-(4-Chlorobenzyl)-1H-pyrrole-2-carbaldehyde (40f): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 40f (185 mg, 84%, beige solid). 1H NMR (400 MHz, CDCl3): δ 5.51 (2H, s, CH2), 6.28 (1H, t, J = 3.2 Hz, ArH), 6.95–6.98 (2H, m, 2 × ArH), 7.07 (2H, dt, J = 8.4, 2.2 Hz, 2 × ArH), 7.26 (2H, dt, J = 8.4, 2.2 Hz, 2 × ArH), and 9.54 (1H, s, CH=O). HRMS (ESI): Calcd. for C12H11ClNO (M + H)+ 220.0524 and found 220.0521.
1-(3-(Trifluoromethyl)benzyl)-1H-pyrrole-2-carbaldehyde (40g): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 40g (216 mg, 85%, beige-brown oil). 1H NMR (400 MHz, CDCl3): δ 5.61 (2H, s, CH2), 6.31 (1H, t, J = 3.2 Hz, ArH), 6.98–7.01 (2H, m, 2 × ArH), 7.30 (1H, d, J = 7.6 Hz, ArH), 7.37 (1H, s, ArH), 7.42 (1H, t, J = 7.8 Hz, ArH), 7.52 (1H, d, J = 8.0 Hz, ArH), and 9.55 (1H, s, CH=O). HRMS (ESI): Calcd. for C13H11F3NO (M + H)+ 254.0787 and found 254.0793.
1-(4-(Trifluoromethyl)benzyl)-1H-pyrrole-2-carbaldehyde (40h): Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 9:1) gave 40h (82 mg, 32%, beige solid). 1H NMR (400 MHz, CDCl3): δ 5.61 (2H, s, CH2), 6.31 (1H, t, J = 3.0 Hz, ArH), 6.98–7.01 (2H, m, 2 × ArH), 7.21 (2H, d, J = 8.0 Hz, 2 × ArH), 7.55 (2H, d, J = 8.0 Hz, 2 × ArH), and 9.54 (1H, s, CH=O). HRMS (ESI): Calcd. for C13H11F3NO (M + H)+ 254.0787 and found 254.0795.
General Procedure: Synthesis of (E)-amino(2-((1-benzyl-1H-imidazol-2-yl)methylene)hydrazineyl)methaniminium chloride derivatives (41a–d) and (E)-Amino(2-((1-benzyl-1H-pyrrol-2-yl)methylene)hydrazineyl)methaniminium chloride derivatives (41e–h): Aldehyde derivatives (40a–h) (0.2 mmol) and N-aminoguanidine bicarbonate (0.24 mmol) were placed in a 50 mL round-bottom flask. HCl (0.5M in MeOH, 2.0 mL) was added, and the reaction mixture was stirred at 80 °C for 2 h and then evaporated to dryness.
(E)-Amino(2-((1-(3-chlorobenzyl)-1H-imidazol-2-yl)methylene)hydrazineyl) methaniminium chloride (41a): 87 mg, >99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.71 (2H, s, CH2), 7.35 (1H, dt, J = 4.4, 1.6 Hz, ArH), 7.46–7.50 (2H, m, 2 × ArH), 7.52 (1H, s, ArH), 7.88 (1H, d, J = 2.2 Hz, ArH), 7.94 (1H, d, J = 2.2 Hz, ArH), 8.35 (4H, s, br, 2 × NH2), 8.56 (1H, s, CH=N), and 12.85 (1H, s, br, N-NH). HRMS (ESI): Calcd. for C12H14ClN6 (M + H)+ 277.0963 and found 277.0956.
(E)-Amino(2-((1-(4-chlorobenzyl)-1H-imidazol-2-yl)methylene)hydrazineyl) methaniminium chloride (41b): 88 mg, >99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.70 (2H, s, CH2), 7.42 (2H, dt, J = 8.4, 2.2 Hz, 2 × ArH), 7.52 (2H, dt, J = 8.4, 2.2 Hz, 2 × ArH), 7.86 (1H, d, J = 1.8 Hz, ArH), 7.91 (1H, d, J = 1.8 Hz, ArH), 8.35 (4H, s, br, 2 × NH2), 8.53 (1H, s, CH=N), and 12.82 (1H, s, br, N-NH). HRMS (ESI): Calcd. for C12H14ClN6 (M + H)+ 277.0963 and found 277.0955.
(E)-Amino(2-((1-(3-(trifluoromethyl)benzyl)-1H-imidazol-2-yl)methylene) hydrazineyl)methaniminium chloride (41c): 94 mg, >99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.80 (2H, s, CH2), 7.65 (1H, d, J = 7.8 Hz, ArH), 7.70 (1H, t, J = 7.8 Hz, ArH), 7.80 (1H, d, J = 7.6 Hz, ArH), 7.85 (1H, s, ArH), 7.90 (1H, d, J = 1.8 Hz, ArH), 7.94 (1H, d, J = 1.8 Hz, ArH), 8.35 (4H, s, br, 2 × NH2), 8.59 (1H, s, CH=N), and 12.85 (1H, s, br, N-NH). HRMS (ESI): Calcd. for C13H14F3N6 (M + H)+ 311.1227 and found 311.1234.
(E)-Amino(2-((1-(4-(trifluoromethyl)benzyl)-1H-imidazol-2-yl)methylene) hydrazineyl)methaniminium chloride (41d): 95 mg, >99%, pale yellow glass. 1H NMR (400 MHz, DMSO-d6): δ 5.83 (2H, s, CH2), 7.58 (2H, d, J = 8.0 Hz, 2 × ArH), 7.82 (2H, d, J = 8.0 Hz, 2 × ArH), 7.88 (1H, d, J = 1.6 Hz, ArH), 7.94 (1H, d, J = 1.6 Hz, ArH), 8.29 (4H, s, br, 2 × NH2), 8.51 (1H, s, CH=N), and 12.73 (1H, s, br, N-NH). HRMS (ESI): Calcd. for C13H14F3N6 (M + H)+ 311.1227 and found 311.1235.
(E)-Amino(2-((1-(3-chlorobenzyl)-1H-pyrrol-2-yl)methylene)hydrazineyl) methan-iminium chloride (41e): 64 mg, >99%, purple-black glass. 1H NMR (400 MHz, DMSO-d6): δ 5.61 (2H, s, CH2), 6.29 (1H, dd, J = 3.8, 2.6 Hz, ArH), 6.77 (1H, dd, J = 3.8, 1.8 Hz, ArH), 7.01 (1H, dt, J = 7.2, 1.6 Hz, ArH), 7.07 (1H, t, J = 1.6 Hz, ArH), 7.26 (1H, dd, J = 2.4, 2.0 Hz, ArH), 7.35 (1H, dt, J = 8.0, 1.8 Hz, ArH), 7.39 (1H, t, J = 7.6 Hz, ArH), 7.59 (4H, s, br, 2 × NH2), 8.09 (1H, s, CH=N), and 11.55 (1H, s, N-NH). HRMS (ESI): Calcd. for C13H15ClN5 (M + H)+ 276.1010 and found 276.1006.
(E)-Amino(2-((1-(4-chlorobenzyl)-1H-pyrrol-2-yl)methylene)hydrazineyl) methan-iminium chloride (41f): 64 mg, >99%, purple-black glass. 1H NMR (400 MHz, DMSO-d6): δ 5.58 (2H, s, CH2), 6.27 (1H, dd, J = 3.8, 1.4 Hz, ArH), 6.76 (1H, dd, J = 3.8, 1.8 Hz, ArH), 7.07 (2H, d, J = 8.0 Hz, 2 × ArH), 7.25 (1H, dd, J = 6.4, 1.6 Hz, ArH), 7.42 (2H, d, J = 8.0 Hz, 2 × ArH), 7.49 (4H, s, br, 2 × NH2), 8.07 (1H, s, CH=N), and 11.54 (1H, s, N-NH). HRMS (ESI): Calcd. for C13H15ClN5 (M + H)+ 276.1010 and found 276.1004.
(E)-Amino(2-((1-(3-(trifluoromethyl)benzyl)-1H-pyrrol-2-yl)methylene) hydrazin-eyl)methaniminium chloride (41g): 81 mg, >99%, purple-black glass. 1H NMR (400 MHz, DMSO-d6): δ 5.72 (2H, s, CH2), 6.30 (1H, dd, J = 3.8, 2.6 Hz, ArH), 6.77 (1H, dd, J = 3.8, 1.8 Hz, ArH), 7.28–7.32 (2H, m, 2 × ArH), 7.44 (1H, s, ArH), 7.50 (4H, s, br, 2 × NH2), 7.60 (1H, t, J = 7.8 Hz, ArH), 7.65 (1H, d, J = 7.6 Hz, ArH), 8.08 (1H, s, CH=N), and 11.57 (1H, s, N-NH). HRMS (ESI): Calcd. for C14H15F3N5 (M + H)+ 310.1274 and found 310.1278.
(E)-Amino(2-((1-(4-(trifluoromethyl)benzyl)-1H-pyrrol-2-yl)methylene) hydrazin-eyl)methaniminium chloride (41h): 80 mg, >99%, purple-black glass. 1H NMR (400 MHz, DMSO-d6): δ 5.71 (2H, s, CH2), 6.31 (1H, t, J = 2.8 Hz, ArH), 6.78–6.79 (1H, m, ArH), 7.23 (2H, d, J = 8.0 Hz, 2 × ArH), 7.27 (1H, t, J = 2.6 Hz, ArH), 7.46 (4H, s, br, 2 × NH2), 7.73 (2H, d, J = 8.0 Hz, 2 × ArH), 8.07 (1H, s, CH=N), and 11.57 (1H, s, N-NH). HRMS (ESI): Calcd. for C14H15F3N5 (M + H)+ 310.1274 and found 310.1281.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}[(4-hydroxyphenyl)amino] methyl]-carbamate (43): In a solution of 4-aminophenol 42 (273 mg, 2.5 mmol) in DMF (6 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (690 mg, 2.37 mmol) was added, followed by Et3N (0.7 mL) and HgCl2 (640 mg, 2.37 mmol). The mixture was stirred at r.t. overnight, and then diluted with EtOAc (30 mL) and filtered through Celite. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo. Purification by flash chromatography (petrol ether to petrol ether/EtOAc 3:2) afforded 43 as a white solid (535 mg, 64% yield), m.p. 182–184 °C. 1H NMR (400 MHz, CDCl3): δ 1.44 (9H, s, t-Bu), 1.47 (9H, s, t-Bu), 6.56 (2H, br.s, 2 × ArH), 7.02 (3H, br.s, 2 × ArH and OH), 9.93 (1H, s, NH), and 11.6 (1H, s, NH). HRMS (ESI): Calcd. for C17H25N3NaO5 (M + Na)+ 374.1692 and found 374.1697.
General Procedure: Benzylation of 4-(N,N′-di-Boc-guanydino)phenol (43): In a solution of 4-(N,N′-di-Boc-guanydino)phenol 43 (110 mg, 0.53 mmol) in acetone (5 mL), the substituted benzyl bromide (0.37 mmol) was added, followed by K2CO3 (72 mg, 0.52 mmol). The mixture was stirred at r.t. overnight and partitioned between EtOAc (30 mL) and water (30 mL). The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give an off-white solid. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 3:1) afforded 44a–c.
tert-Butyl N-[{[4-(benzyloxy)phenyl]amino}({[(tert-butoxy)carbonyl]imino}) meth-yl]-carbamate (44a): A white solid was obtained (79% yield), m.p. 141–143 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 5.05 (2H, s, OCH2), 6.92 (2H, d, J = 8.2 Hz, 2 × ArH), 7.30–7.42 (5H, m, 5 × ArH), 7.47 (2H, d, J = 8.3 Hz, 2 × ArH), 10.2 (1H, br.s, NH), and 11.6 (1H, s, NH). HRMS (ESI): Calcd. for C24H31N3NaO5 (M + Na)+ 464.2161 and found 464.2179.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}({4-[(4-chlorophenyl)methoxy] phenyl}amino)methyl]carbamate (44b): A white solid was obtained (89% yield), m.p. 95–97 °C. 1H NMR (400 MHz, CDCl3): δ 1.48 (9H, s, t-Bu), 1.52 (9H, s, t-Bu), 5.00 (2H, s, OCH2), 6.91 (2H, dd, J = 7.8, 2.4 Hz, 2 × ArH), 7.30 (4H, s, 4 × ArH), 7.51 (2H, dd, J = 7.8, 2.4 Hz, 2 × ArH), 10.2 (1H, br.s, NH), and 11.6 (1H, s, NH).
tert-Butyl N-[{[3-(benzyloxy)phenyl]amino}({[(tert-butoxy)carbonyl]imino}) methyl]carbamate (44c): A white solid was obtained (62% yield), m.p. 79–83 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 5.05 (2H, s, OCH2), 6.92 (2H, d, J = 8.4 Hz, 2 × ArH), 7.25–7.36 (4H, m, 4 × ArH), 7.40 (2H, d, J = 8.3 Hz, 2 × ArH), 10.8 (1H, br.s, NH), and 11.8 (1H, s, NH).
General Procedure: Synthesis of the phenyl guanidine derivatives (45a–c): In a solution of the para-substituted N,N′-di-Boc-(4-guanydino)benzene (100 mg) (44a–c) in CH2Cl2 (2 mL), TFA (1 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with Et2O, and dried in vacuo to give 45a–c as a white or off-white solid.
1-[4-(Benzyloxy)phenyl]guanidinium 2,2,2-trifluoroacetate (45a): A white solid was obtained (95% yield). 1H NMR (400 MHz, CD3OD): δ 5.12 (2H, s, OCH2), 7.12 (2H, d, J = 8.1 Hz, 2 × ArH), 7.22 (2H, d, J = 8.1 Hz, 2 × ArH), 7.32–7.40 (3H, m, 3 × ArH), and 7.46 (2H, d, J = 8.3 Hz, 2 × ArH). HRMS (ESI): Calcd. for C14H16N3O (M + H)+ 242.1293 and found 242.1283.
1-[4-(4-Chlorobenzyloxy)phenyl]guanidinium 2,2,2-trifluoroacetate (45b): A white solid was obtained (88% yield). 1H NMR (400 MHz, CD3OD): δ 5.12 (2H, s, OCH2), 7.21–7.30 (4H, m, 4 × ArH), 7.37 (2H, d, J = 8.2 Hz, 2 × ArH), and 7.55 (2H, d, J = 8.2 Hz, 2 × ArH). HRMS (ESI): Calcd. for C14H15ClN3O (M + H)+ 276.0904 and found 276.0932.
1-[4-(3-Chlorobenzyloxy)phenyl}guanidinium 2,2,2-trifluoroacetate (45c): A white solid was obtained (92% yield). 1H NMR (400 MHz, CD3OD): δ 5.21 (s, 2H, OCH2), 7.32 (2H, d, J = 8.0 Hz, 2 × ArH), 7.38 (1H, s, ArH), 7.47–7.53 (3H, m, 3 × ArH), and 7.59 (2H, d, J = 8.3 Hz, 2 × ArH). HRMS (ESI) calcd. for C14H15ClN3O (M + H)+ 276.0904 and found 276.0911.
General Procedure: Synthesis of Boc-protected amide/sulphonamide derivative (47a-b): 4-Aminobenzylamine 21 (0.5 mmol) was dissolved in DMF (1.0 mL) and Et3N (2.0 mmol). Benzoyl chloride or benzenesulphonyl chloride (0.5 mmol) was added successively, and the reaction mixture was stirred for 2 h at 0 °C. HgCl2 (0.5 mmol) and S-methyl-N,N′-bis(tert-butoxycarbonyl) isothiourea (0.5 mmol) were added, and the reaction mixture was stirred at r.t. for 18 h. Et2O (100 mL) was added, and the mixture was filtered through a paper filter. The organic layer was washed with water (80 mL) and brine (20 mL), dried (NaCl), filtered, and concentrated to dryness. Crystallisation from Et2O gave 47a–b.
tert-Butyl N-[(1Z)-{[(tert-butoxy)carbonyl]imino}({4-[(phenylformamido)methyl] phenyl}amino)methyl]carbamate (47a): 113 mg, 48%, white solid, m.p. 152–156 °C, mixture of conformers as revealed by 1H NMR. Major conformer: 1H NMR (400 MHz, CDCl3): δ 1.45 (9H, s, t-Bu), 1.53 (9H, s, t-Bu), 4.55 (2H, d, J = 5.2 Hz, CH2), 6.64 (1H, br.s, NH), 7.25 (2H, d, J = 8.0 Hz, 2 × ArH), 7.39–7.52 (5H, m, 5 × ArH), 7.81 (2H, d, J = 8.0 Hz, 2 × ArH), 10.40 (1H, br.s, NH), and 11.66 (1H, br.s, NH). HRMS (ESI): Calcd. for C25H33N4O5 (M + H)+ 469.2445 and found 469.2451.
tert-Butyl N-[(1Z)-{[4-(benzenesulfonamidomethyl)phenyl]amino}({[(tert-butoxy)-carbonyl]imino})methyl]carbamate (47b): 128 mg, 50%, white solid, m.p. 154–158 °C. 1H NMR (400 MHz, CDCl3): δ 1.47 (9H, s, t-Bu), 1.53 (9H, s, t-Bu), 4.10 (2H, d, J = 6.0 Hz, CH2), 4.90 (1H, br.s, NH), 7.17 (2H, d, J = 8.0 Hz, 2 × ArH), 7.46 (2H, d, J = 8.4 Hz, 2 × ArH), 7.49–7.55 (2H, m, 2 × ArH), 7.56–7.62 (1H, m, ArH), 7.87 (1H, d, J = 1.6 Hz, ArH), 7.89 (1H, s, ArH), 10.51 (1H, br.s, NH), and 11.65 (1H, br.s, NH). HRMS (ESI): Calcd. for C24H33N4O6S (M + H)+ 505.2115 and found 505.2127.
Amino((4-(benzamidomethyl)phenyl)amino)methaniminium 2,2,2-trifluoroacet-ate (48a): Compound 47a (0.1 mmol) was dissolved in CH2Cl2 (0.8 mL) and TFA (0.2 mL) was added, and the reaction mixture was stirred for 2 h at 0 °C. The mixture was concentrated to dryness to give 48a as a pale yellow glass (38 mg, 99%). The product was a mixture of conformers as revealed by 1H NMR. Major conformer: 1H NMR (400 MHz, DMSO-d6): δ 4.54 (2H, s, CH2), 7.25 (2H, d, J = 8.0 Hz, 2 × ArH), 7.44 (4H, br.s, 2 × NH2), 7.49–7.67 (5H, m, 5 × ArH), 7.94 (2H, d, J = 7.2 Hz, 2 × ArH), 9.17 (1H, t, J = 5.8 Hz, NH), and 9.79 (1H, s, NH). HRMS (ESI): Calcd. for C15H17N4O (M + H)+ 269.1397 and found 269.1405.
Amino((4-(phenylsulfonamidomethyl)phenyl)amino)methaniminium 2,2,2-trifluoroacetate (48b): Compound 47b (0.1 mmol) was dissolved in CH2Cl2 (0.8 mL) and TFA (0.2 mL) was added, and the reaction mixture was stirred for 2 h at 0 °C. The mixture was concentrated to dryness to give 48b as a pale yellow glass (38 mg, 99%). 1H NMR (400 MHz, DMSO-d6): δ 4.03 (2H, d, J = 6.4 Hz, CH2), 7.21 (2H, d, J = 8.0 Hz, 2 × ArH), 7.37 (2H, d, J = 8.0 Hz, 2 × ArH), 7.45 (4H, br.s, 2 × NH2), 7.61–7.72 (3H, m, 3 × ArH), 7.87 (2H, d, J = 7.2 Hz, 2 × ArH), 8.27 (1H, t, J = 6.4 Hz, NH), and 9.78 (1H, br.s, NH). HRMS (ESI): Calcd. for C14H17N4O2S (M + H)+ 305.1067 and found 305.1078.
(3-[2,3-Dichlorobenzyloxy]benzyl)(methyl)amine (49a): In a solution of 38a (330 mg, 1.17 mmol) in MeOH (5 mL), MeNH2 (2.0 M in MeOH, 0.76 mL, 1.5 mmol) was added. The mixture was stirred at r.t. for 10 h and then cooled to 0 °C. NaBH4 (53 mg, 1.4 mmol) was added slowly. The mixture was stirred at r.t. for 4 h and then partitioned between NaOH (1M in water) and EtOAc. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give 49a as a clear oil (340 mg, 98%). 1H NMR (400 MHz, CDCl3): δ 2.45 (3H, s, NCH3), 3.74 (2H, s, CH2), 5.15 (2H, s, OCH2), 6.85 (1H, dd, J = 8.0, 1.5 Hz, ArH), 6.92–7.00 (2H, m, 2 × ArH), 7.18–7.28 (2H, m, 2 × ArH), 7.40 (1H, d, J = 7.9 Hz, ArH), and 7.47 (1H, d, J = 7.9 Hz, ArH). HRMS (ESI): Calcd. for C15H16Cl2NO (M + H)+ 296.0609 and found 296.0680.
(3-[2,3-Dichlorobenzyloxy]benzyl)(2-methoxyethyl)amine (49b): In a solution of 38a (330 mg, 1.17 mmol) in MeOH (5 mL), 2-methoxyethan-1-amine (113 mg, 1.5 mmol) was added. The mixture was stirred at r.t. for 10 h and then cooled to 0°C. NaBH4 (57 mg, 1.5 mmol) was added slowly. The mixture was stirred at r.t. for 4 h and then partitioned between NaOH (1M in water) and EtOAc. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give 49b as a clear oil (350 mg, 88%). 1H NMR (400 MHz, CDCl3): δ 2.80 (2H, t, J = 5.2 Hz, OCH2CH2N), 3.35 (3H, s, OCH3), 3.51 (2H, t, J = 5.3 Hz, OCH2CH2N), 3.80 (2H, s, CH2), 5.16 (2H, s, OCH2), 6.85 (1H, dd, J = 7.9, 2.6 Hz, ArH), 6.96 (1H, d, J = 7.5 Hz, ArH), 6.99 (1H, s, ArH), 7.20–7.26 (2H, m, 2 × ArH), 7.43 (1H, d, J = 8.0 Hz, ArH), and 7.48 (1H, d, J = 7.9 Hz, ArH). HRMS (ESI): Calcd. for C17H20Cl2NO2 (M + H)+ 340.0871 and found 340.0848.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}[(3-(2,3-dichlorobenzyl) benzyl)(methyl)amino]methyl]carbamate (50a): In a solution of 49a (330 mg, 1.11 mmol) in DMF (5 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (389 mg, 1.3 mmol) was added, followed by Et3N (0.3 mL) and HgCl2 (200 mg). The mixture was stirred at r.t. overnight, and then diluted with EtOAc (50 mL) and filtered through Celite. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give a colourless oil. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 4:1) afforded 50a as a white solid (350 mg, 59% yield), m.p. 127–129 °C. 1H NMR (400 MHz, CDCl3): δ 1.50 (18H, s, 2 × t-Bu), 2.90 (3H, s, NCH3), 4.70 (2H, s, CH2), 5.16 (2H, s, OCH2), 6.88–6.97 (2H, m, 2 × ArH), 7.22–7.28 (3H, m, 3 × ArH), 7.41 (1H, d, J = 7.9 Hz, ArH), 7.49 (1H, d, J = 7.9 Hz, ArH), and 10.2 (1H, s, NH). HRMS (ESI): Calcd. for C26H33Cl2N3NaO5 (M + Na)+ 560.1695 and found 560.1697.
tert-Butyl N-[{[(tert-butoxy)carbonyl]imino}[(3-(2,3-dichlorobenzyl)benzyl)(2-methoxyethyl)amino]methyl]carbamate (50b): In a solution of 49b (320 mg, 0.94 mmol) in DMF (3 mL), S-methyl-N,N’-bis(tert-butoxycarbonyl)isothiourea (330 mg, 1.13 mmol) was added, followed by Et3N (0.5 mL) and HgCl2 (306 mg). The mixture was stirred at r.t. overnight, and then diluted with EtOAc (50 mL) and filtered through Celite. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give a colourless oil. Purification by flash column chromatography eluting with a gradient solvent (petrol ether to petrol ether/EtOAc 4:1) afforded 50b as a white solid (340 mg, 62% yield), m.p. 125–126 °C. 1H NMR (400 MHz, CDCl3): δ 1.50 (18H, s, 2 × t-Bu), 3.30 (3H, s, OCH3), 3.45 (2H, s, CH2), 3.65 (2H, br.s, OCH2CH2N), 4.89 (2H, br.s, OCH2CH2N), 5.20 (2H, s, OCH2), 6.86–6.97 (2H, m, 2 × ArH), 7.21–7.28 (3H, m, 3 × ArH), 7.43 (1H, d, J = 8.3 Hz, ArH), 7.49 (1H, d, J = 8.2 Hz, ArH), and 9.56 (1H, s, NH). HRMS (ESI): Calcd. for C28H38Cl2N3O6 (M + H)+ 582.2138 and found 582.2177.
1-(3-(2,3-Dichlorobenzyloxy)benzyl)-1-methylguanidinium chloride (51a): In a solution of 50a (126 mg, 0.23 mmol) in CH2Cl2 (1.5 mL), TFA (0.75 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with Et2O, and dried in vacuo to give a white solid (69 mg, 87%). The solid (18 mg) was dissolved in HCl (0.5M in MeOH, 5 mL), and concentrated in vacuo to give 51a as a white solid (15 mg). 1H NMR (400 MHz, CDCl3): δ 2.96 (3H, s, NCH3), 4.56 (2H, br.s, CH2), 5.18 (2H, s, OCH2), 6.76–6.86 (2H, m, 2 × ArH), 6.98 (1H, d, J = 8.2 Hz, ArH), 7.18–7.36 (2H, m, 2 × ArH), and 7.49 (2H, br.s, 2 × ArH). HRMS (ESI): Calcd. for C16H18Cl2N3O (M + H)+ 338.0827 and found 338.0909.
1-(3-(2,3-Dichlorobenzyl)benzyl)-1-(2-methoxyethyl)guanidinium 2,2,2-trifluoroacetate (51b): In a solution of the intermediate 50b (98 mg, 0.17 mmol) in CH2Cl2 (1.5 mL), TFA (0.75 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. Et2O (1 mL) was added, and the precipitate was collected, washed with Et2O, and dried in vacuo to give 51b as a white solid (70 mg, 86%). 1H NMR (400 MHz, CDCl3): δ 3.31 (3H, s, NCH3), 3.56 (4H, m, OCH2CH2N and CH2), 4.67 (2H, br.s, OCH2CH2N), 5.26 (2H, s, OCH2), 6.85–6.95 (2H, m, 2 × ArH), 7.06 (1H, dd, J = 7.9, 2.1 Hz, ArH), 7.37–7.50 (2H, m, 2 × ArH), 7.62 (1H, dd, J = 8.1, 1.8 Hz, ArH), and 7.73 (1H, dd, J = 8.1, 1.7 Hz, ArH). HRMS (ESI): Calcd. for C18H22Cl2N3O2 (M + H)+ 382.1089 and found 382.1195.
3-(2,3-Dichlorobenzyloxy)phenylmethanol (52): Compound 38a (1.3 g, 4.6 mmol) was dissolved in EtOH (20 mL) and THF (5 mL) and cooled to 0 °C. NaNH4 (176 mg, 4.6 mmol) was added portionwise and the mixture was stirred at r.t. overnight. Acetone (2 mL) was added, and the mixture was partitioned between EtOAc and a saturated NH4Cl solution. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give 52 as a white solid (1.2 g, 92% yield), m.p. 79–80 °C. 1H NMR (400 MHz, CDCl3): δ 4.64 (2H, s, OCH2), 5.24 (2H, s, OCH2), 6.90 (1H, dd, J = 8.0, 1.9 Hz, ArH), 6.96–7.03 (2H, m, 2 × ArH), 7.20–7.31 (2H, m, 2 × ArH), 7.43 (1H, d, J = 8.2 Hz, ArH), and 7.49 (1H, d, J = 8.2 Hz, ArH).
1,2-Dichloro-3-[3-(chloromethyl)phenoxymethyl]benzene (53): In a solution of 52 (1.1 g, 3.9 mmol) in CH2Cl2 (20 mL), Et3N (1.1 mL, 7.8 mmol) was added, followed by methanesulfonyl chloride (0.6 mL, 7.8 mmol). The mixture was stirred at r.t. overnight and partitioned between CH2Cl2 and a saturated NaHCO3 solution. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give 53 as a white solid (0.95 g, 91% yield), m.p. 91–95 °C. 1H NMR (400 MHz, CDCl3): δ 4.53 (2H, s, CH2), 5.17 (2H, s, OCH2), 6.92 (1H, dd, J = 8.1, 1.7 Hz, ArH), 7.00–7.05 (2H, m, 2 × ArH), 7.21–7.32 (2H, m, 2 × ArH), 7.44 (1H, d, J = 8.1 Hz, ArH), and 7.49 (1H, d, J = 8.1 Hz, ArH).
tert-Butyl N-[{[(tert-butoxy)carbonyl](3-(2,3-dichlorobenzyloxy) benzyl)amino}-(methylsulfanyl)methylidene]carbamate (54): In a solution of 53 (330 mg, 1.1 mmol) in CH2Cl2 (5 mL), KOH (112 mg, 2.2 mmol) in water (5 mL) and Bu4NHSO4 (34 mg, 0.1 mmol) were added. The mixture was stirred at r.t. overnight and partitioned between CH2Cl2 and water. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give 54 as a clear oil (300 mg, 49% yield). 1H NMR (400 MHz, CDCl3): δ 1.39 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 2.40 (3H, s, SCH3), 4.75 (2H, s, CH2), 5.15 (2H, s, OCH2), 6.86 (1H, dd, J = 7.9, 1.7 Hz, ArH), 6.95–7.01 (2H, m, 2 × ArH), 7.18–7.26 (2H, m, 2 × ArH), 7.38 (1H, d, J = 8.2 Hz, ArH), and 7.47 (1H, d, J = 8.1 Hz, ArH). HRMS (ESI): Calcd. for C26H32Cl2N2NaO5S (M + Na)+ 577.1306 and found 577.1303.
tert-Butyl N-[{[(tert-butoxy)carbonyl](3-(2,3-dichlorobenzyl) benzyl)amino}(methylamino)methylidene]carbamate (55): In a solution of 54 (200 mg, 0.36 mmol) in DMF (1 mL), MeNH2 (2M in MeOH, 0.27 mL, 0.54 mmol) was added, followed by Et3N (0.2 mL) and HgCl2 (116 mg). The mixture was stirred at r.t. for 1.5 h, and then diluted with EtOAc (30 mL) and filtered through Celite. The organic layer was washed with brine, dried (MgSO4), and concentrated in vacuo to give a colourless oil. Purification by flash column chromatography (petrol ether to petrol ether/EtOAc 4:1) afforded 55 as a white solid (100 mg, 52% yield), m.p. 111–113 °C. 1H NMR (400 MHz, CDCl3): δ 1.46 (9H, s, t-Bu), 1.51 (9H, s, t-Bu), 2.52 (3H, s, NCH3), 4.57 (2H, s, CH2), 5.15 (2H, s, OCH2), 6.87–6.96 (3H, m, 3 × ArH), 7.19–7.26 (2H, m, 2 × ArH), 7.43 (1H, d, J = 7.9 Hz, ArH), 7.49 (1H, d, J = 8.0 Hz, ArH), and 9.87 (1H, s, NH).
3-(3-(2,3-Dichlorobenzyloxy)benzyl)-1-methylguanidinium 2,2,2-trifluoroacetate (56): In a solution of 55 (85 mg, 0.16 mmol) in CH2Cl2 (1 mL), TFA (0.5 mL) was added. The mixture was shaken at r.t. overnight and then evaporated to dryness. The residue was dissolved in MeOH and evaporated to dryness to give 56 as a foamy powder (60 mg, 87%). 1H NMR (400 MHz, CDCl3): δ 2.85 (3H, s, NCH3), 4.39 (2H, s, CH2), 5.19 (2H, s, OCH2), 6.88–6.95 (3H, m, 3 × ArH), 7.24–7.33 (2H, m, 2 × ArH), and 7.52 (2H, d, J = 7.6 Hz, 2 × ArH). HRMS (ESI): Calcd. for C16H18Cl2N3O (M + H)+ 338.0827 and found 338.0945.
3.2. Biology
3.2.1. Materials
Staphylococcus aureus 9518 and Escherichia coli K12 were purchased from the National Collections of Industrial and Marine Bacteria (NCIMB), Aberdeen, UK. MRSA strains were a gift from Dr Llinos Harris, Swansea University.
3.2.2. Methods
Cell Culture
The bacteria were grown for 17 h in 20 mL of tryptic soy broth (TSB) at 30 °C with oscillation (90 oscillations per minute). A total of 1 mL of the overnight culture was taken and centrifuged at 880 g for 7 min. The supernatant was removed, and the pellet resuspended in PBS. The centrifugation step was then repeated, and the resulting pellet redissolved in an appropriate volume to adjust the concentration of the bacterial solution to a density of 1 × 105 cells/mL based on the haemocytometer calculation in a 30 mL universal test tube.
Treatment Protocol
The synthetic compounds were dissolved initially at a concentration of 1 mg/mL in a suitable solvent (ethanol, methanol, or DMSO) before dilution with water in order to generate the relevant concentrations used within the bioassay. Each solvent was tested separately for its own toxicity, and it was ensured that the dilution required to produce the working solutions for the assay was sufficient to remove any toxic effect of the initial solvent used. The bacterial solution (10 µL) was added to 50 µL of either the synthetic compound solution or water as a negative control in a 96-well plate. This was sealed with a polyethylene seal prior to being analysed in a Skanit platereader (Thermoscientific, Cambridge, UK). The optical density at 550 nm was then recorded once every hour for 24 h, with a shaking step immediately prior to each reading being taken.
Quantification Method
The acquired data were then used to determine the growth of each species with or without the addition of each synthetic compound solution. The MIC values were determined as the minimum concentration of compound required to reduce the survival index to less than 50%. The survival indices (Table 7) were determined from the absorbance data as the ratio of the optical density of the control bacteria at the mid-log point of growth to the comparative optical density of the treatment bacteria multiplied by 100, as published previously [36].
4. Conclusions
A range of benzyl, phenyl guanidine, and aminoguanidine hydrazone derivatives were synthesised and evaluated. Some derivatives displayed excellent antimicrobial activity. The best benzyl guanidine derivatives 9g, 9m, and 9v displayed antimicrobial MICs of 0.5–1.0 µg/mL against S. aureus and 1–4 µg/mL against E. coli. The best aminoguanidine hydrazone derivative 10d showed very good potency against S. aureus (MIC 1 µg/mL) but was significantly less potent against E. coli (16 µg/mL), as indeed was benzyl guanidine 9p (0.5 µg/mL and 16 µg/mL, respectively). Overall, 10a, 10j, and 10r–s were less potent against S. aureus (MIC of 4 µg/mL) and more potent against E. coli (MIC of 4 µg/mL) compared to 10d. The most potent benzyl guanidines 9m and 9v were tested against methicillin-resistant Staphylococcus aureus (MRSA 3 and MRSA 15) and showed very promising potencies with MICs of 0.5–2.0 µg/mL and low survival indices (1.76–12.76%). Further work is currently underway to establish the mechanism of action of these new guanidine derivatives, and it seems clear, as expected, that the guanidino/amidino functionality is a key contributor to the antibacterial activity. However, a preliminary further evaluation of a selection of the most potent compounds shows potentially complex profiles that may not include the most expected bacterial cell division machinery, such as FtsZ, as a direct target. Since it is known that the lead agent TXA497 8 targets both the bacterial membrane in addition to FtsZ, with some cells exhibiting the effects of membrane disruption [31], the focus of future studies on the most potent subset of optimised compounds will address both FtsZ and the bacterial cell membrane, and also potential targets elsewhere.
Author Contributions
Conceptualisation, B.V.L.P.; methodology, W.D., X.S., Y.N. and E.D.; validation, W.D., X.S., Y.N., E.D. and B.V.L.P.; formal analysis, W.D., X.S., Y.N., E.D. and B.V.L.P.; investigation, W.D., X.S., Y.N. and E.D.; resources, B.V.L.P.; writing—original draft preparation, W.D., X.S. and B.V.L.P.; writing—review and editing, W.D., Y.N., E.D. and B.V.L.P.; supervision, B.V.L.P.; project administration, B.V.L.P. and Y.N.; funding acquisition, B.V.L.P. and Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge provision of financial support from Research & Innovation, Swansea University, UK.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are available in the article.
Acknowledgments
We thank J.R. Normanton for some useful discussions during this project.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of some compounds may be available from the authors upon request.
References
- Nordmann, P.; Naas, T.; Fortineau, N.; Poirel, L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr. Opin. Microbiol. 2007, 10, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46, S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Sass, P.; Brötz-Oesterhelt, H. Bacterial stress responses to antimicrobial agents. In Stress Responses in Foodborne Microorganisms; Wong, H.-c., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 131–172. [Google Scholar]
- Sass, P.; Brötz-Oesterhelt, H. Bacterial cell division as a target for new antibiotics. Curr. Opin. Microbiol. 2013, 16, 522–530. [Google Scholar] [CrossRef]
- Ma, S.; Ma, S. The Development of FtsZ Inhibitors as Potential Antibacterial Agents. Chem. Med. Chem. 2012, 7, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Gamba, P.; Veening, J.W.; Saunders, N.J.; Hamoen, L.W.; Daniel, R.A. Two-step assembly dynamics of the Bacillus subtilis divisome. J. Bacteriol. 2009, 191, 4186–4194. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.W.; Errington, J. Bacterial cell division: Assembly, maintenance and disassembly of the Z ring. Nat. Rev. Microbiol. 2009, 7, 642–653. [Google Scholar] [CrossRef]
- Erickson, H.P.; Anderson, D.E.; Osawa, M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 2010, 74, 504–528. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Boberek, J.M.; Stach, J.; Good, L. Genetic evidence for inhibition of bacterial division protein FtsZ by berberine. PLoS ONE 2010, 5, e13745. [Google Scholar] [CrossRef]
- Zhang, S.L.; Chang, J.J.; Damu, G.L.; Fang, B.; Zhou, X.D.; Geng, R.X.; Zhou, C.H. Novel berberine triazoles: Synthesis, antimicrobial evaluation and competitive interactions with metal ions to human serum albumin. Bioorg. Med. Chem. Lett. 2013, 23, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.; Lu, S.; Kelley, C.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of substituted dibenzo[a,g]quinolizin-7-ium derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 6962–6966. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.; Kelley, C.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of substituted 5-methylbenzo[c]phenanthridinium derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7080–7083. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.J.; Stokes, N.R.; Ure, R.; Galbraith, G.; Bennett, J.M.; Brown, D.R.; Baker, P.J.; Barynin, V.V.; Rice, D.W.; Sedelnikova, S.E.; et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 2008, 321, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.M.; Therien, A.G.; Lu, J.; Lee, S.H.; Caron, A.; Gill, C.J.; Lebeau- Jacob, C.; Benton-Perdomo, L.; Monteiro, J.M.; Pereira, P.M.; et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to beta-lactam antibiotics. Sci. Transl. Med. 2012, 4, 126ra135. [Google Scholar] [CrossRef]
- Haydon, D.J.; Bennett, J.M.; Brown, D.; Collins, I.; Galbraith, G.; Lancett, P.; Macdonald, R.; Stokes, N.R.; Chauhan, P.K.; Sutariya, J.K.; et al. Creating an antibacterial with in vivo efficacy: Synthesis and characterization of potent inhibitors of the bacterial cell division protein FtsZ with improved pharmaceutical properties. J. Med. Chem. 2010, 53, 3927–3936. [Google Scholar] [CrossRef]
- Andreu, J.M.; Schaffner-Barbero, C.; Huecas, S.; Alonso, D.; Lopez-Rodriguez, M.L.; Ruiz-Avila, L.B.; Núñez-Ramírez, R.; Llorca, O.; Martín-Galiano, A.J. The Antibacterial Cell Division Inhibitor PC190723 Is an FtsZ Polymer-stabilizing Agent That Induces Filament Assembly and Condensation. J. Biol. Chem. 2010, 285, 14239–14246. [Google Scholar] [CrossRef]
- Kaul, M.; Ferrer-Gonzáles, E.; Mark, L.; Parhi, A.K.; LaVoie, E.J.; Pilch, D.S. Combination with a FtsZ inhibitor potentiates the in vivo efficacy of oxacillin against methicillin-resistant Staphylococcus aureus. Med. Chem. Res. 2022, 31, 1705–1715. [Google Scholar] [CrossRef]
- Kaul, M.; Mark, L.; Zhang, Y.; Parhi, A.K.; Lyu, Y.L.; Pawlak, J.; Saravolatz, S.; Saravolatz, L.D.; Weinstein, M.P.; LaVoie, E.J.; et al. TXA709, an FtsZ-Targeting Benzamide Prodrug with Improved Pharmacokinetics and Enhanced In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 4845–4855. [Google Scholar] [CrossRef]
- Fujita, J.; Maeda, Y.; Mizohata, E.; Inoue, T.; Kaul, M.; Parhi, A.K.; LaVoie, E.J.; Pilch, D.S.; Matsumura, H. Structural Flexibility of an Inhibitor Overcomes Drug Resistance Mutation in Staphylococcus aureus FtsZ. ACS Chem. Biol. 2017, 12, 1947–1955. [Google Scholar] [CrossRef]
- Stokes, N.R.; Baker, N.; Bennett, J.M.; Berry, J.; Collins, I.; Czaplewski, L.G.; Logan, A.; Macdonald, R.; Macleod, L.; Peasley, H.; et al. An improved small-molecule inhibitor of FtsZ with superior in vitro potency, drug-like properties, and in vivo efficacy. Antimicrob. Agents Chemother. 2013, 57, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gonzáles, E.; Fujita, J.; Yoshizawa, T.; Nelson, J.M.; Pilch, A.J.; Hillman, E.; Ozawa, M.; Kuroda, N.; Al-Tameemi, H.M.; Boyd, J.M.; et al. Structure-Guided Design of a Fluorescent Probe for the Visualization of FtsZ in Clinically Important Gram-Positive and Gram-Negative Bacterial Pathogens. Sci. Rep. 2019, 9, 20092. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef]
- Kratzer, C.; Tobudic, S.; Assadian, O.; Buxbaum, A.; Graninger, W.; Georgopoulos, A. Validation of AKACID Plus as a Room Disinfectant in the Hospital Setting. Appl. Environ. Microbiol. 2006, 72, 3826–3831. [Google Scholar] [CrossRef][Green Version]
- Kratzer, C.; Tobudic, S.; Macfelda, K.; Graninger, W.; Georgopoulos, A. In Vivo Activity of a Novel Polymeric Guanidine in Experimental Skin Infection with Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3437–3439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelley, C.; Zhang, Y.; Parhi, A.; Kaul, M.; Pilch, D.S.; LaVoie, E.J. 3-Phenyl substituted 6,7-dimethoxyisoquinoline derivatives as FtsZ-targeting antibacterial agents. Bioorg. Med. Chem. 2012, 20, 7012–7029. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 23, 4968–4974. [Google Scholar] [CrossRef]
- Dorrani, M.; Kaul, M.; Parhi, A.; LaVoie, E.J.; Pilch, D.S.; Michniak-Kohn, B. TXA497 as a topical antibacterial agent: Comparative antistaphylococcal, skin deposition, and skin permeation studies with mupirocin. Int. J. Pharm. 2014, 476, 199–204. [Google Scholar] [CrossRef]
- Kaul, M.; Parhi, A.; Zhang, Y.; LaVoie, E.J.; Tuske, S.; Arnold, E.; Kerrigan, J.E.; Pilch, D.S. A Bactericidal Guanidinomethyl Biaryl That Alters the Dynamics of Bacterial FtsZ Polymerization. J. Med. Chem. 2012, 55, 10160–10176. [Google Scholar] [CrossRef]
- Pilch, D.S.; (Department of Pharmacology, Rutgers Robert Wood Johnson Medical School, Rutgers University, Piscataway, NJ, USA). Personal communication, 2022.
- Potter, B.V.L.; Dohle, W.; Su, X.; Normanton, J.; Dudley, E.; Nigam, Y. Antimicrobial Compounds, Compositions and Methods. U.S. Patent WO2016/108045 A2, 7 July 2016. [Google Scholar]
- Yong, Y.F.; Kowalski, J.A.; Thoen, J.C.; Lipton, M.A. A New Reagent for Solid and Solution Phase Synthesis of Protected Guanidines from Amines. Tetrahedron Lett. 1999, 40, 53–56. [Google Scholar] [CrossRef]
- Nishimura, T.; Yamazaki, C.; Toku, H.; Yoshi, S.; Hasegawa, K.; Saito, M.; Nagaki, D. Antiviral Compounds. IV. Synthesis and Anti-influenza Virus Activity of Amidinohydrazones. Chem. Pharm. Bull. 1974, 22, 2444–2447. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.A.; Ramsden, P.D.; Batey, R.A. Phase-Transfer-Catalyzed Alkylation of Guanidines by Alkyl Halides under Biphasic Conditions: A Convenient Protocol for the Synthesis of Highly Functionalized Guanidines. J. Org. Chem. 2003, 68, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, A.; Nigam, Y.; Thomas, S.; Ratcliffe, N.A. Detection and partial purification of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia Sericata and their activity against methicillin resistant Staphylococcus aureus (MRSA). Microbes Infect. 2004, 6, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).