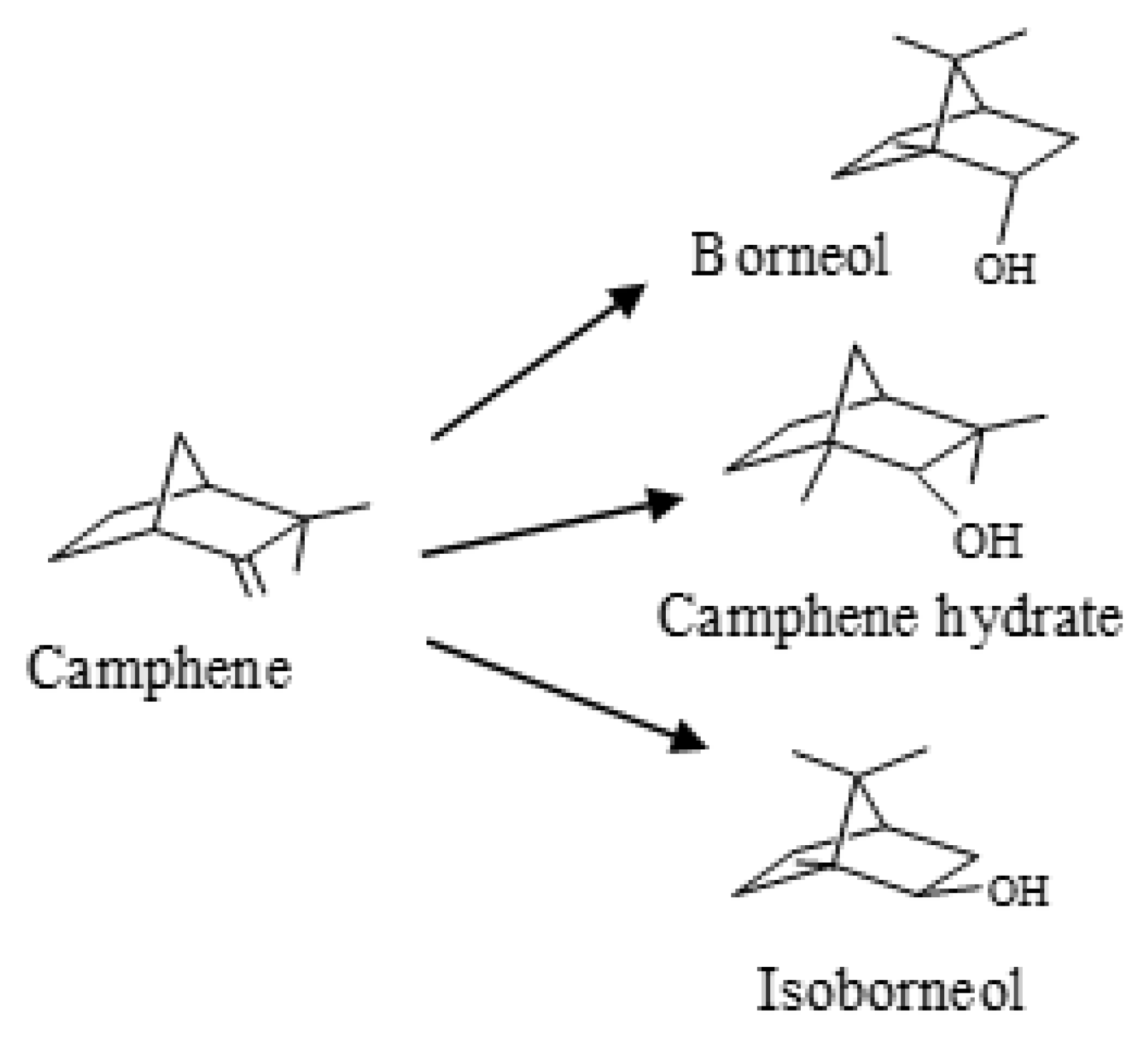
Hydration of Camphene over PW-SBA-15-SO3H
Abstract
1. Introduction
2. Results and Discussion
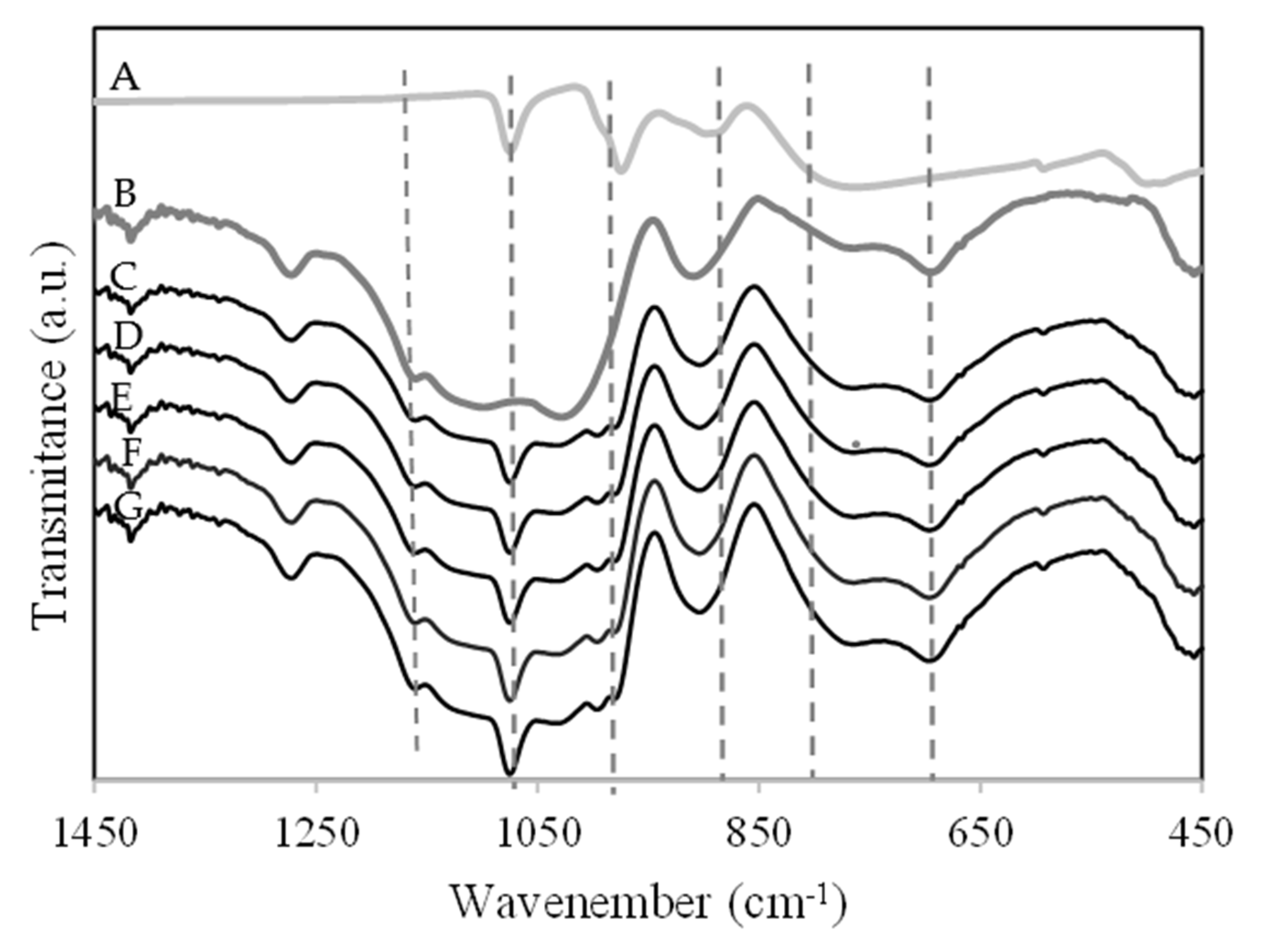
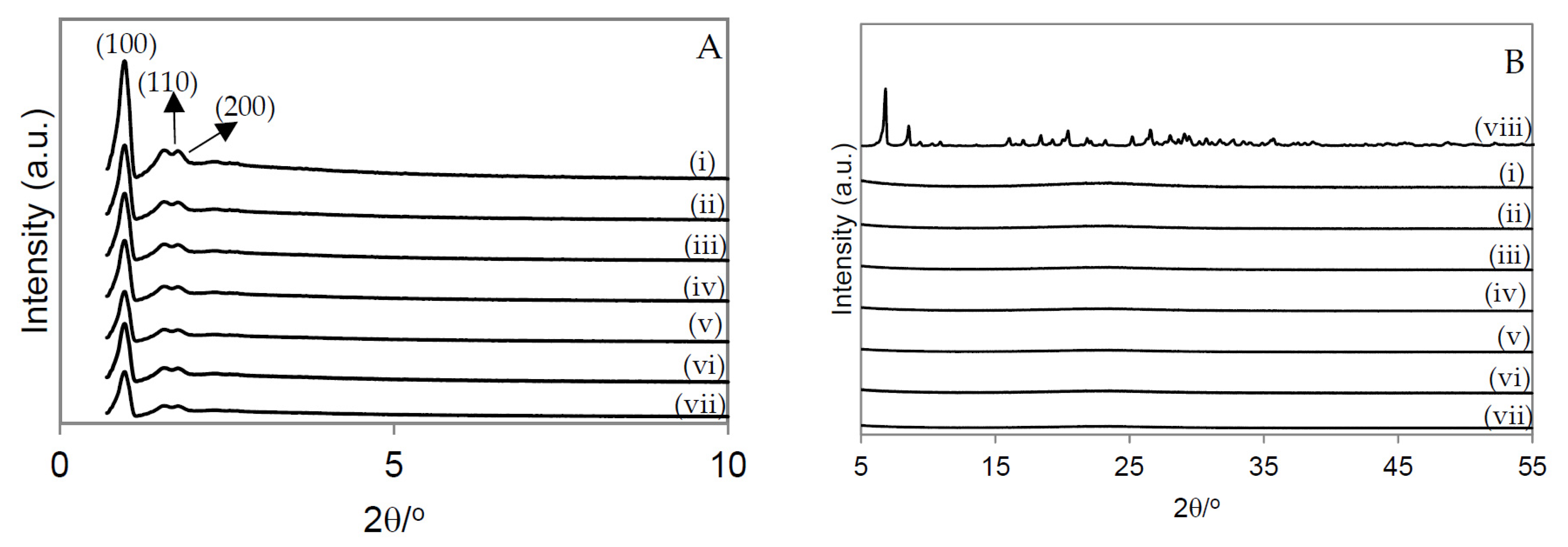
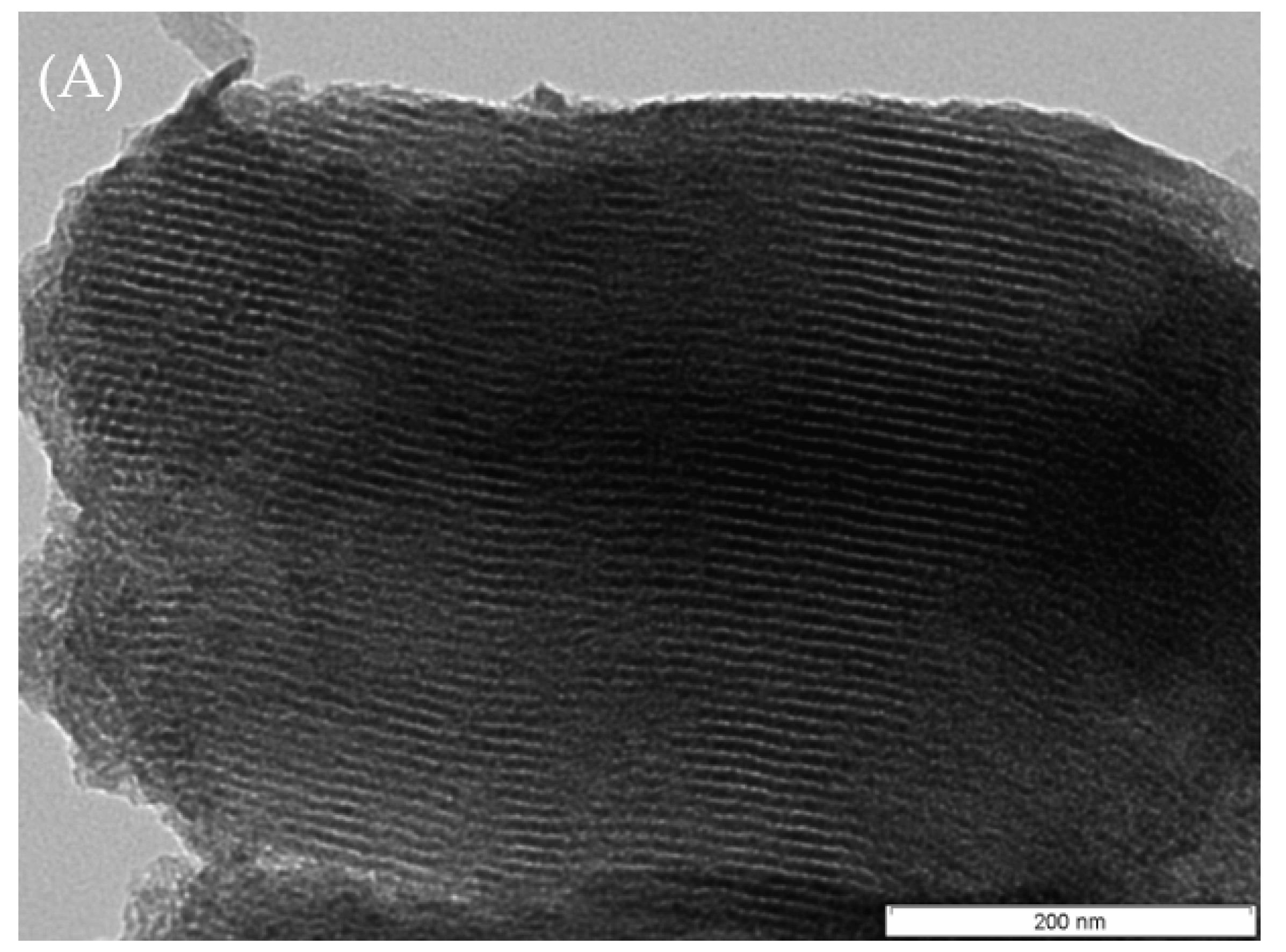
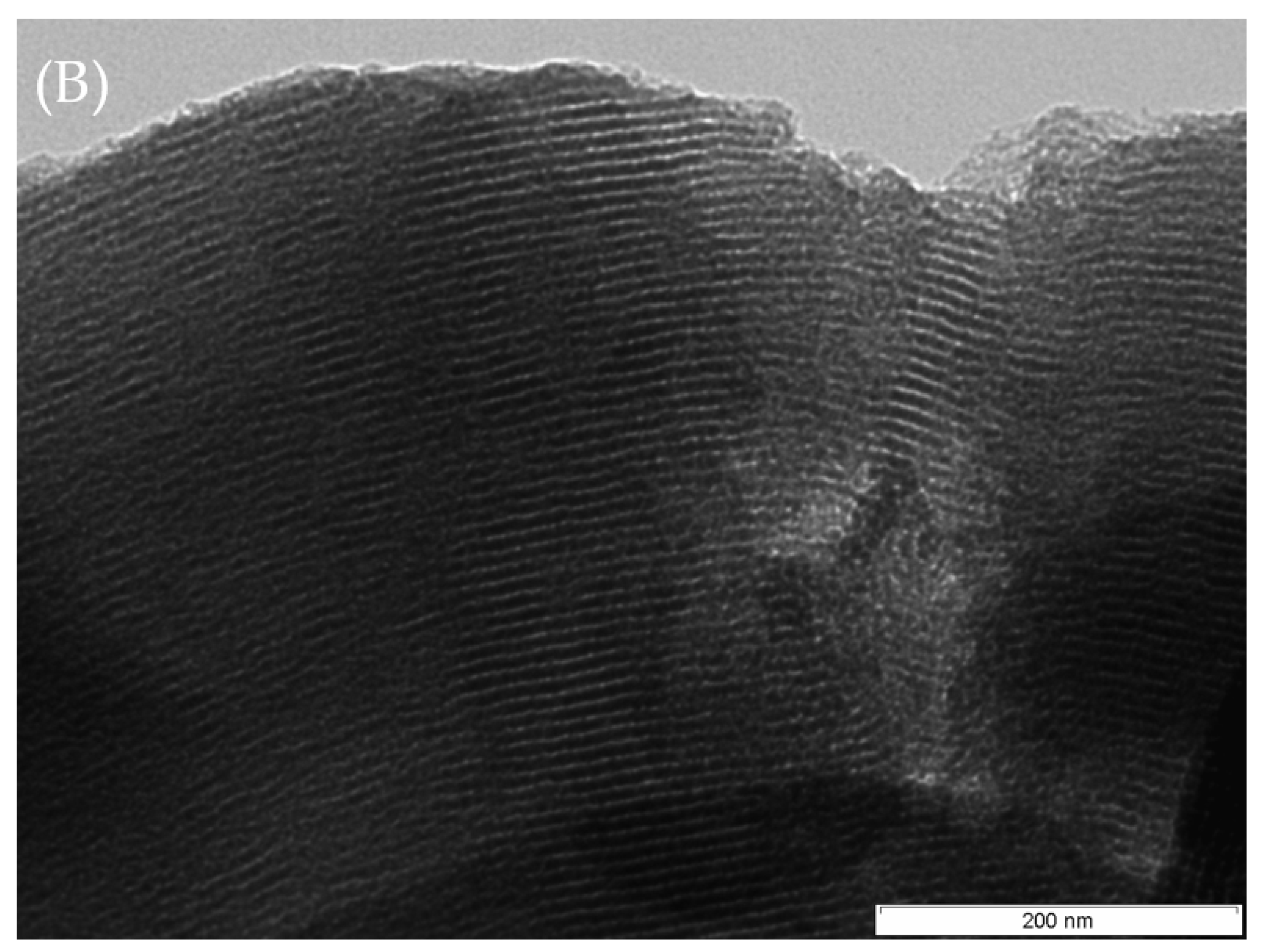
2.1. Catalyst Characterization
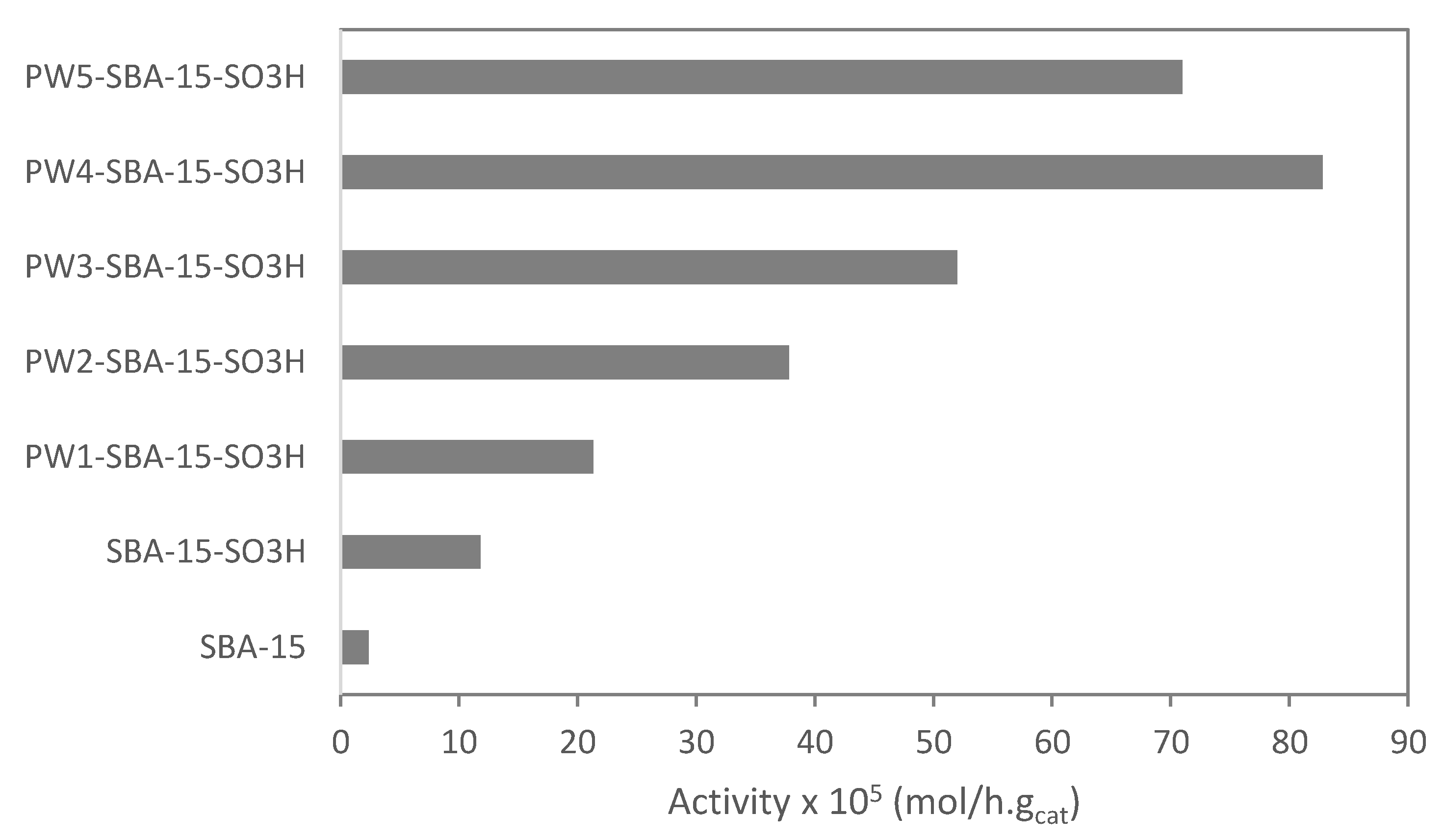
2.2. Catalytic Experiments
- -
- at low water content (high amount of acetone), the catalytic activity increased with increasing water content. This behavior may be due to low amount of water inside the PW4-SBA-15-SO3H surface. When the amount of water increased, the catalytic activity increased as well, until a maximum was reached at 50% of water.
- -
- at high water content (low amount of acetone), it is expected that the solvent inside the PW4-SBA-15-SO3H pore system was richer in water content than the bulk solution. The layer of water molecules surrounding the active sites form a barrier hindering the diffusion of camphene. Consequently, the camphene sorption coefficient as well as the activity, decreased.
2.2.1. Effect of the catalyst amount
2.2.2. Effect of the Initial Concentration of Camphene
2.3. Kinetic Modeling
3. Materials and Methods
3.1. Materials
3.2. Preparation of Materials
3.3. Materials Characterization
3.4. Catalytic Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Newman, A.A. (Ed.) Chemistry of the Terpenes and Terpenoids, 2nd ed.; Academic Press: London, UK, 1972; pp. 1–86. [Google Scholar]
- Gusevskaya, E.V. Reactions of terpenes catalyzed by heteropoly compounds: Valorization of biorenewables. ChemCatChem 2014, 6, 1506–1515. [Google Scholar] [CrossRef]
- Caiado, M.; Machado, A.; Santos, R.N.; Matos, I.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Valente, A.A.; Castanheiro, J.E. Alkoxylation of camphene over silica-occluded tungstophosphoric acid. Appl. Catal. A Gen. 2013, 451, 36–42. [Google Scholar] [CrossRef]
- da Silva Rocha, K.A.; Robles-Dutenhefner, P.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Phosphotungstic heteropoly acid as efficient heterogeneous catalyst for solvent-free isomerization of a-pinene and longifolene. Appl. Catal. A Gen. 2009, 352, 188–192. [Google Scholar] [CrossRef]
- da Silva, K.A.; Kozhevnikov, I.V.; Gusevskaya, E.V. Hydration and acetoxylation of camphene catalyzed by heteropoly acid. J. Mol. Catal. A. Chem. 2003, 192, 129–134. [Google Scholar] [CrossRef]
- Rudakov, G.A. Khimiya i Tekhnologiya Kamfary (Chemistry and Technology of Camphor), 3rd ed.; Lesnaya Prom: Moscow, Russia, 1976. [Google Scholar]
- Radbil’, A.B.; Zolin, B.A.; Radbil’, B.A.; Ryanzanova, T.V.; Klimanskaya, T.V. Direct acid-catalyzed hydration of camphene as a route to isoborneol. Russ. J. Appl. Chem. 2001, 74, 1850–1853. [Google Scholar] [CrossRef]
- Radbil’, A.B.; Kulikov, M.V.; Zolin, B.A. Camphene hydration catalyzed by heteropoly acids. Zh. Prikl. Khim. 2000, 73, 241–245. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [PubMed]
- Maki-Arvela, P.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Recent Progress in Synthesis of Fine and Specialty Chemicals from Wood and Other Biomass by Heterogeneous Catalytic Processes. Catal. Rev. 2007, 49, 197–340. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Thomas, H.J.; Climent, M.J.; Romanelli, G.P.; Iborra, S. Heteropolycompounds as catalysts for biomass product transformations. Catal. Rev. 2016, 58, 497–586. [Google Scholar] [CrossRef]
- Dal Santo, V.; Liguori, F.; Pirovano, C.; Guidotti, M. Design and use of nanostructured single-site heterogeneous catalysts for the selective transformation of fine chemicals. Molecules 2010, 15, 3829–3856. [Google Scholar] [CrossRef]
- Valente, H.; Vital, J. Hydration of a-pinene and camphene over USY zeolites. Stud. Surf. Sci. Catal. 1997, 108, 555–562. [Google Scholar]
- Machado, J.; Castanheiro, J.E.; Matos, I.; Ramos, A.M.; Vital, J.; Fonseca, I.M. SBA-15 with sulfonic acid groups as a Green Catalyst for the acetoxylation of a-pinene. Micropor. Mesopor. Mater. 2012, 163, 237–242. [Google Scholar] [CrossRef]
- Gagea, B.C.; Lorgouilloux, Y.; Altintas, Y.; Jacobs, P.A.; Martens, J.A. Bifunctional conversion of n-decane over HPW heteropoly acid incorporated into SBA-15 during synthesis. J. Catal. 2009, 265, 99–108. [Google Scholar] [CrossRef]
- Chai, S.-H.; Wang, H.-P.; Liang, Y.; Xu, B.-Q. Sustainable production of acrolein: Gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green. Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Castanheiro, J.E.; Fonseca, I.M.; Ramos, A.M.; Vital, J. Tungstophosphoric acid immobilised in SBA-15 as an efficient heterogeneous acid catalyst for the conversion of terpenes and free fatty acids. Micropor. Mesopor. Mat. 2017, 249, 16–24. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Vásquez, P.G.; Cáceres, C.V.; Blanco, M.N. Supported Keggin type heteropolycompounds for ecofriendly reactions. Appl. Catal. A Gen. 2003, 256, 125–139. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Xu, Y.; Wang, Y.; Hu, J.; Shi, F. Sulfonic group functionalized periodic mesoporous ethylenesilica: A highly efficient and reusable catalysts for carbon-carbon coupling reaction, Asian. J. Org. Chem. 2019, 8, 111–114. [Google Scholar] [CrossRef]
- Kozhevnikov, I.V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef]
- Shan, X.; Cheng, Z.; Yuan, P. Reaction kinetics and mechanism for hydration of cyclohexene over ion-exchange resin and H-ZSM-5. Chem. Eng. J. 2011, 175, 423–432. [Google Scholar] [CrossRef]
- Lee, M.; Chou, P.; Lin, H. Kinetic of synthesis and hydrolysis of ethyl benzoate over Amberlyst 39. Ind. Eng. Chem. Res. 2005, 44, 725–732. [Google Scholar] [CrossRef]
- Cruz, V.J.; Izquierdo, J.F.; Cunill, F.; Tejero, J.; Iborra, M.; Fité, C.; Bringué, R. Kinetic modeling of the liquid-phase dimerization of isoamylenes on Amberlyst 35. React. Funct. Polym. 2007, 67, 210–224. [Google Scholar] [CrossRef]
- Marchi, A.J.; Paris, J.F.; Bertero, N.M.; Apesteguía, C.R. Kinetic modeling of the liquid-phase hydrogenation of cinnamaldehyde on copper-based catalysts. Ind. Eng. Chem. Res. 2007, 46, 7657–7666. [Google Scholar] [CrossRef]
- Delgado, P.; Sanz, M.T.; Beltran, S. Kinetic study for esterification of lactic acid with ethanol and hydrolysis of ethyl lactate using an ion-exchange resin catalyst. Chem. Eng. J. 2007, 126, 111–118. [Google Scholar] [CrossRef]
- Igbokwe, P.K.; Ugonabo, V.I.; Iwegbu, N.A.; Akachukwu, P.C.; Olisa, C.J. Kinetics of the catalytic esterification of propanol with ethanoic acid using catalysts obtained from Nigerian clays. J. Univ. Chem. Technol. Metall. 2008, 43, 345–348. [Google Scholar]
- Mao, W.; Wang, X.; Wang, H.; Chang, H.; Zhang, X.; Han, J. Thermodynamic and kinetic study of tert-amyl methyl ether (TAME) synthesis. Chem. Eng. Process. 2008, 47, 761–769. [Google Scholar] [CrossRef]
- Kuusisto, J.; Mikkola, J.P.; Sparv, M.; Wärnå, J.; Karhu, H.; Salmi, T. Kinetics of the catalytic hydrogenation of d-lactose on a carbon supported ruthenium catalyst. Chem. Eng. J. 2008, 139, 69–77. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Zhang, Y.; Tu, H.; Pan, P.; Li, S.; Han, Y.; Piao, M.; Hu, J.; Shi, F.; et al. Phosphotungstic acid and propylsulfonic acid bifunctionalized ordered mesoporous silica: A highly efficient and reusable catalysts for esterification of oleic acid. Chem. Eng. J. 2022, 430, 133059. [Google Scholar] [CrossRef]
 ) PW1-SBA-15-SO3H; (×) PW2-SBA-15-SO3H; (□) PW3-SBA-15-SO3H; (+) PW4-SBA-15-SO3H; (○) PW5-SBA-15-SO3H.
) PW1-SBA-15-SO3H; (×) PW2-SBA-15-SO3H; (□) PW3-SBA-15-SO3H; (+) PW4-SBA-15-SO3H; (○) PW5-SBA-15-SO3H.
 ) PW1-SBA-15-SO3H; (×) PW2-SBA-15-SO3H; (□) PW3-SBA-15-SO3H; (+) PW4-SBA-15-SO3H; (○) PW5-SBA-15-SO3H.
) PW1-SBA-15-SO3H; (×) PW2-SBA-15-SO3H; (□) PW3-SBA-15-SO3H; (+) PW4-SBA-15-SO3H; (○) PW5-SBA-15-SO3H.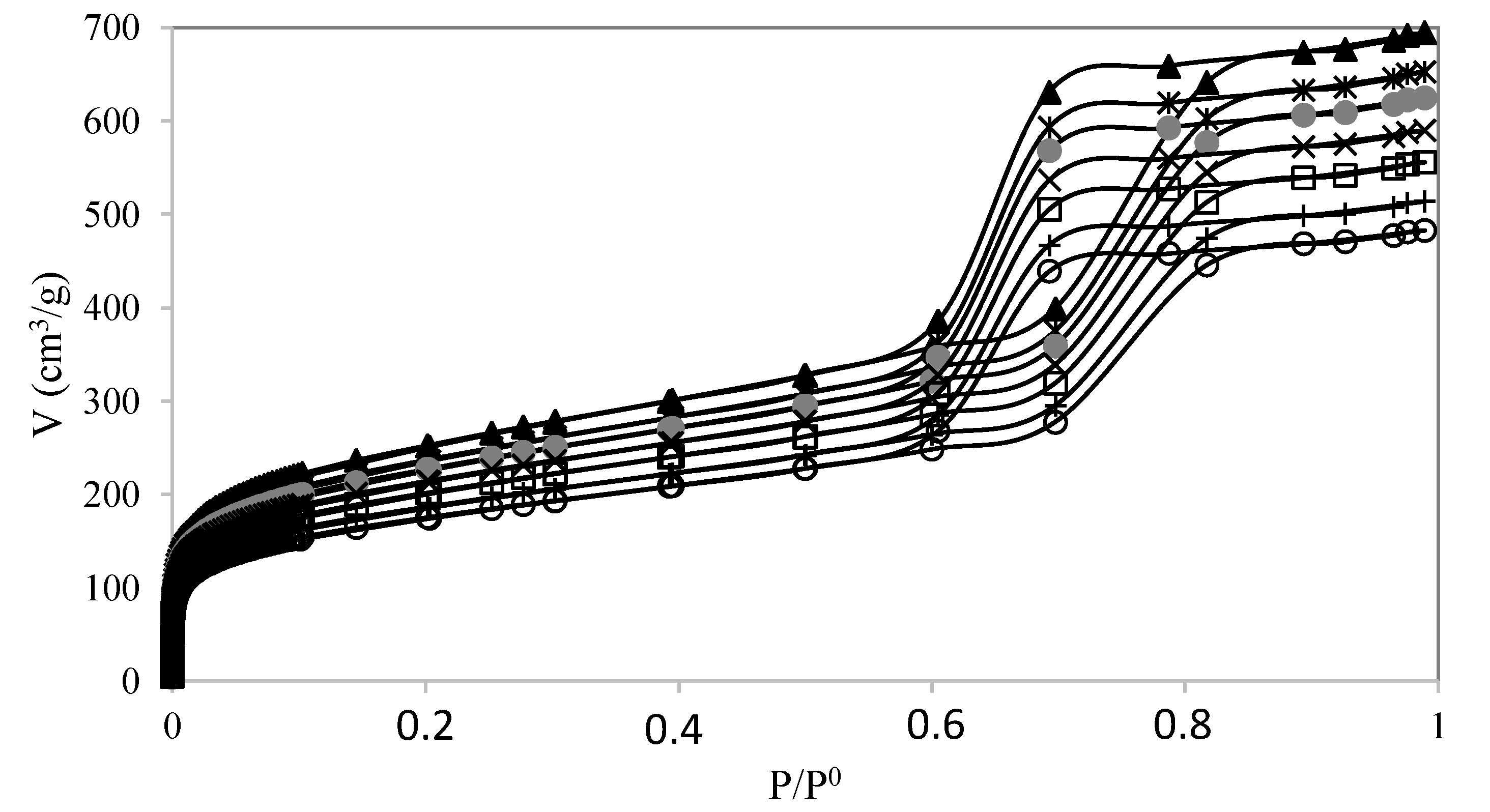
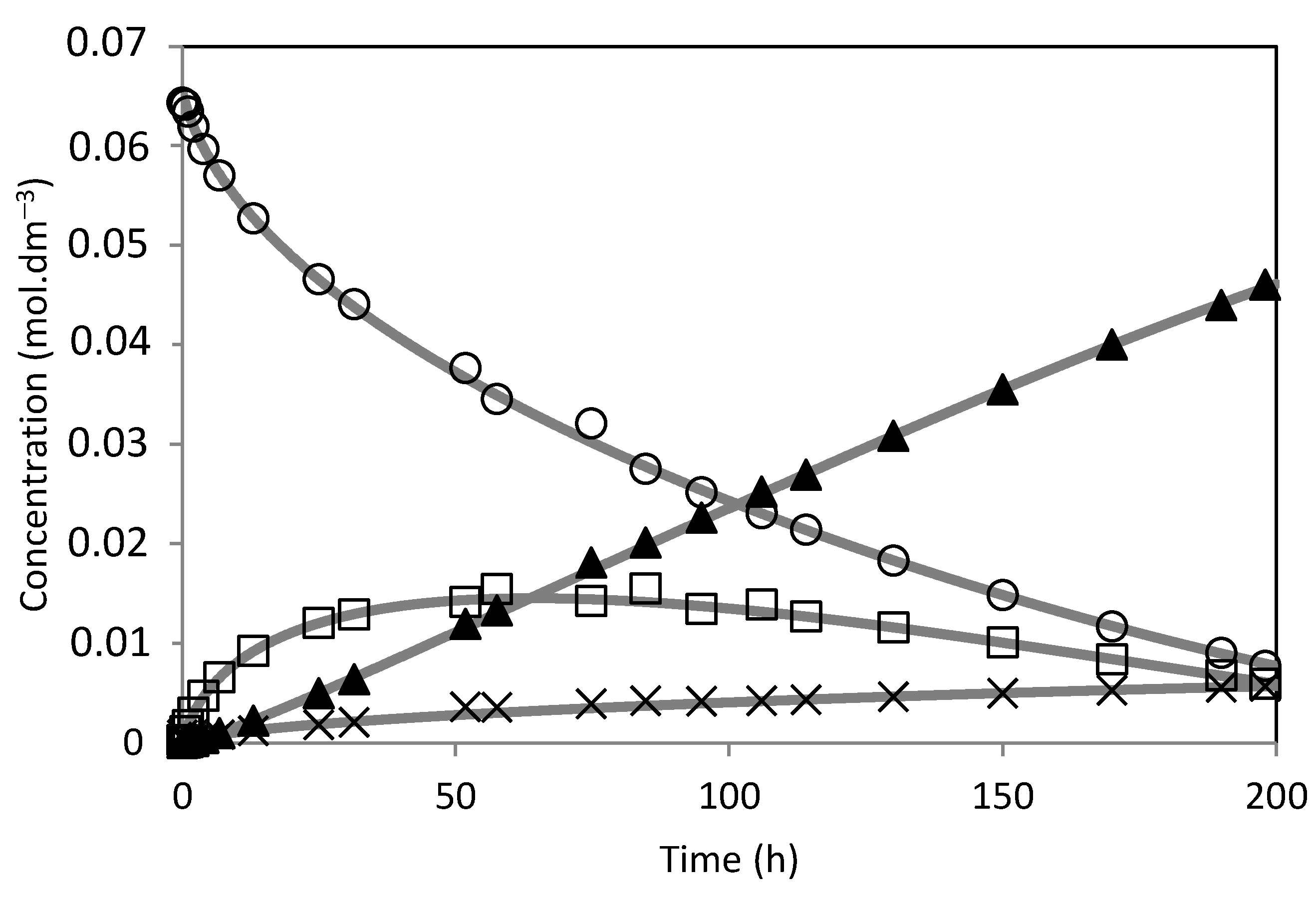
 ) PW2-SBA-15-SO3H; (×) PW3-SBA-15-SO3H; (-) PW4-SBA-15-SO3H; (□) PW5-SBA-15-SO3H. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone (1:1, V/V); ncamphene = 7.5 mmol.
) PW2-SBA-15-SO3H; (×) PW3-SBA-15-SO3H; (-) PW4-SBA-15-SO3H; (□) PW5-SBA-15-SO3H. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone (1:1, V/V); ncamphene = 7.5 mmol.
 ) PW2-SBA-15-SO3H; (×) PW3-SBA-15-SO3H; (-) PW4-SBA-15-SO3H; (□) PW5-SBA-15-SO3H. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone (1:1, V/V); ncamphene = 7.5 mmol.
) PW2-SBA-15-SO3H; (×) PW3-SBA-15-SO3H; (-) PW4-SBA-15-SO3H; (□) PW5-SBA-15-SO3H. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone (1:1, V/V); ncamphene = 7.5 mmol.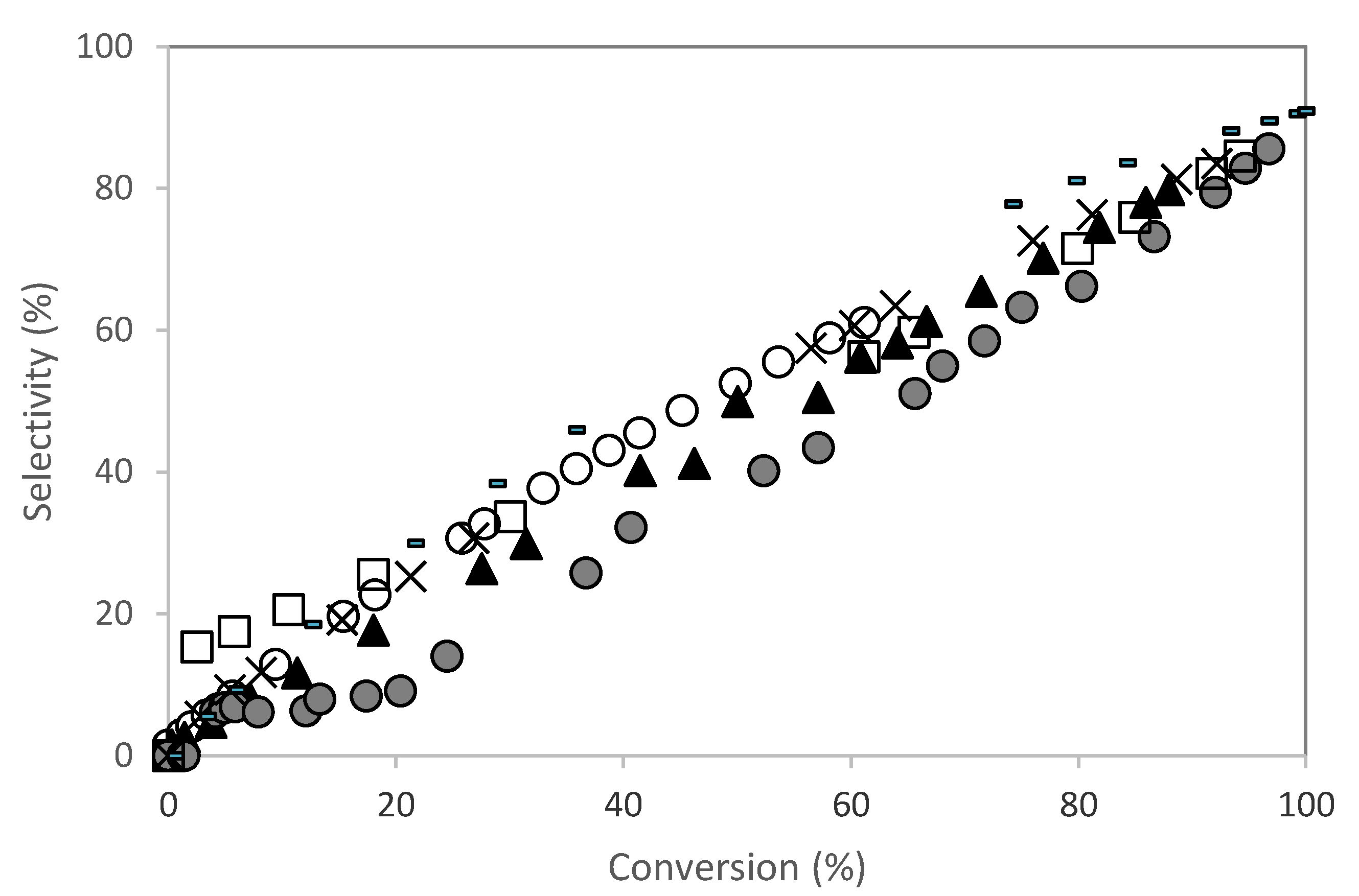
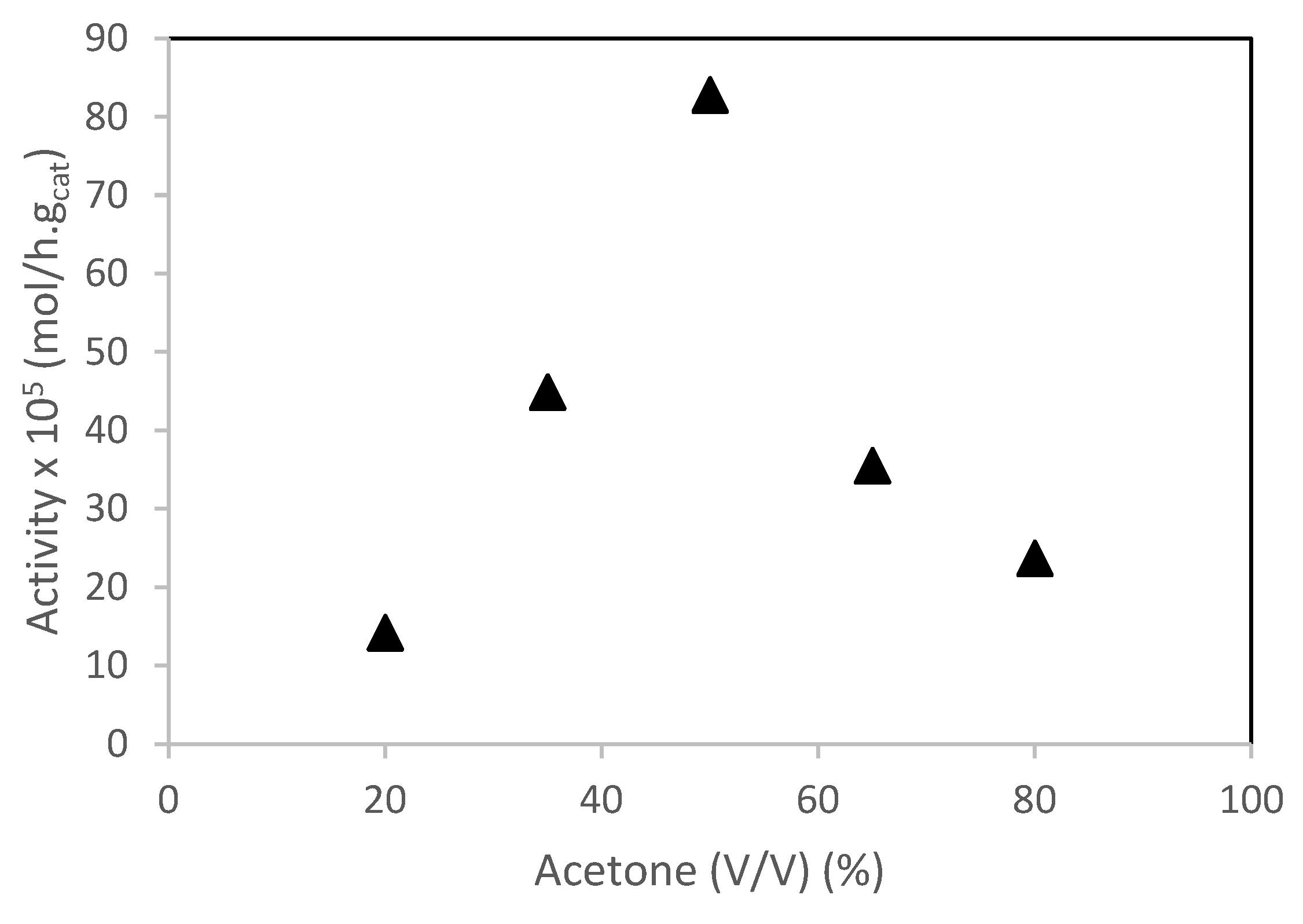
 ) 65% acetone; (□) 80% of acetone; (B) Isoborneol selectivity (%): (○) 20% of acetone; (×) 35% of acetone; (△) 50% of acetone; (□) 65% of acetone; (
) 65% acetone; (□) 80% of acetone; (B) Isoborneol selectivity (%): (○) 20% of acetone; (×) 35% of acetone; (△) 50% of acetone; (□) 65% of acetone; ( ) 80% of acetone. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone; ncamphene = 7.5 mmol.
) 80% of acetone. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone; ncamphene = 7.5 mmol.
 ) 65% acetone; (□) 80% of acetone; (B) Isoborneol selectivity (%): (○) 20% of acetone; (×) 35% of acetone; (△) 50% of acetone; (□) 65% of acetone; (
) 65% acetone; (□) 80% of acetone; (B) Isoborneol selectivity (%): (○) 20% of acetone; (×) 35% of acetone; (△) 50% of acetone; (□) 65% of acetone; ( ) 80% of acetone. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone; ncamphene = 7.5 mmol.
) 80% of acetone. Reaction conditions: T = 50 °C; mcat = 0.482 g; V = 114 mL of aqueous acetone; ncamphene = 7.5 mmol.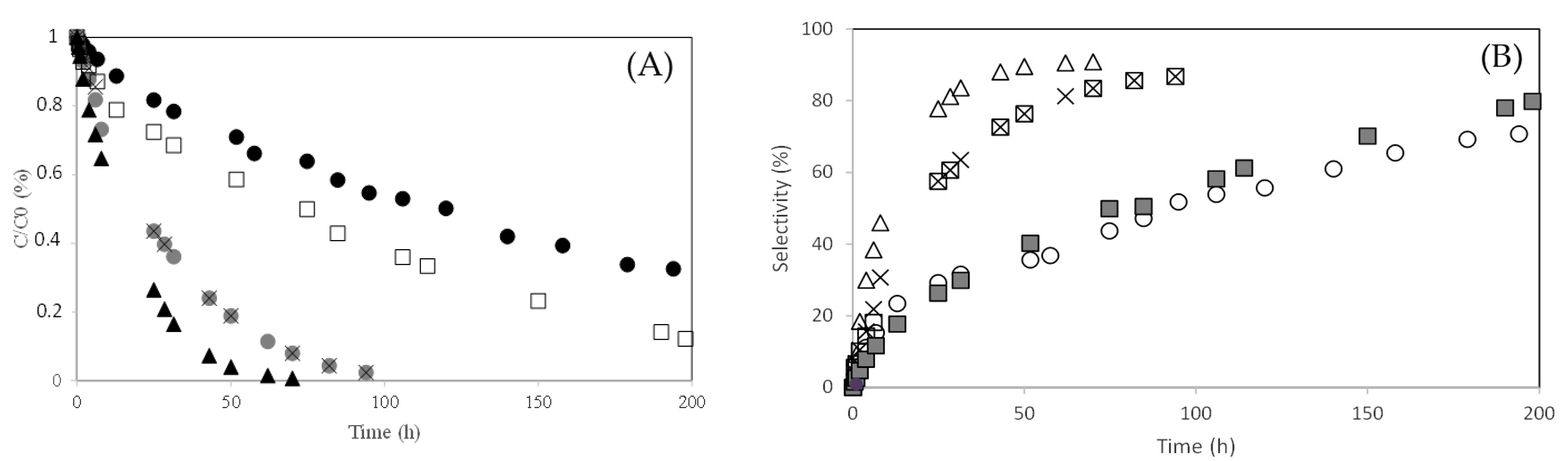
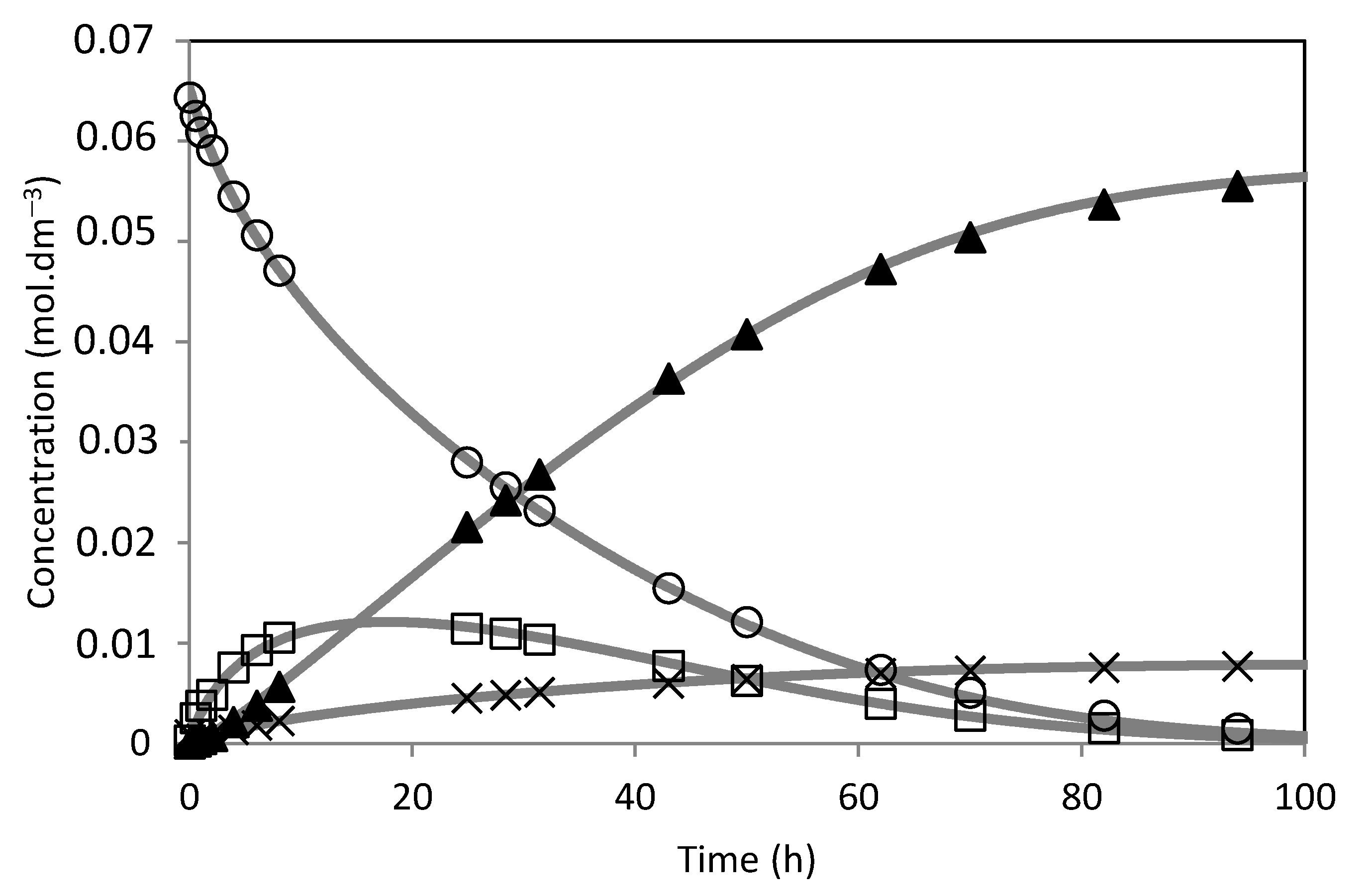
 ) m = 0.48 g; (
) m = 0.48 g; ( ) m = 0.30 g; (
) m = 0.30 g; ( ) m = 0.10 g; Isoborneol selectivity (%): (
) m = 0.10 g; Isoborneol selectivity (%): ( ) m = 0.48 g; (
) m = 0.48 g; ( ) m = 0.30 g; (
) m = 0.30 g; ( ) m = 0.10 g. (B) Camphene conversion (%) and selectivity (%) to the isoborneol at 70 h of reaction.
) m = 0.10 g. (B) Camphene conversion (%) and selectivity (%) to the isoborneol at 70 h of reaction.
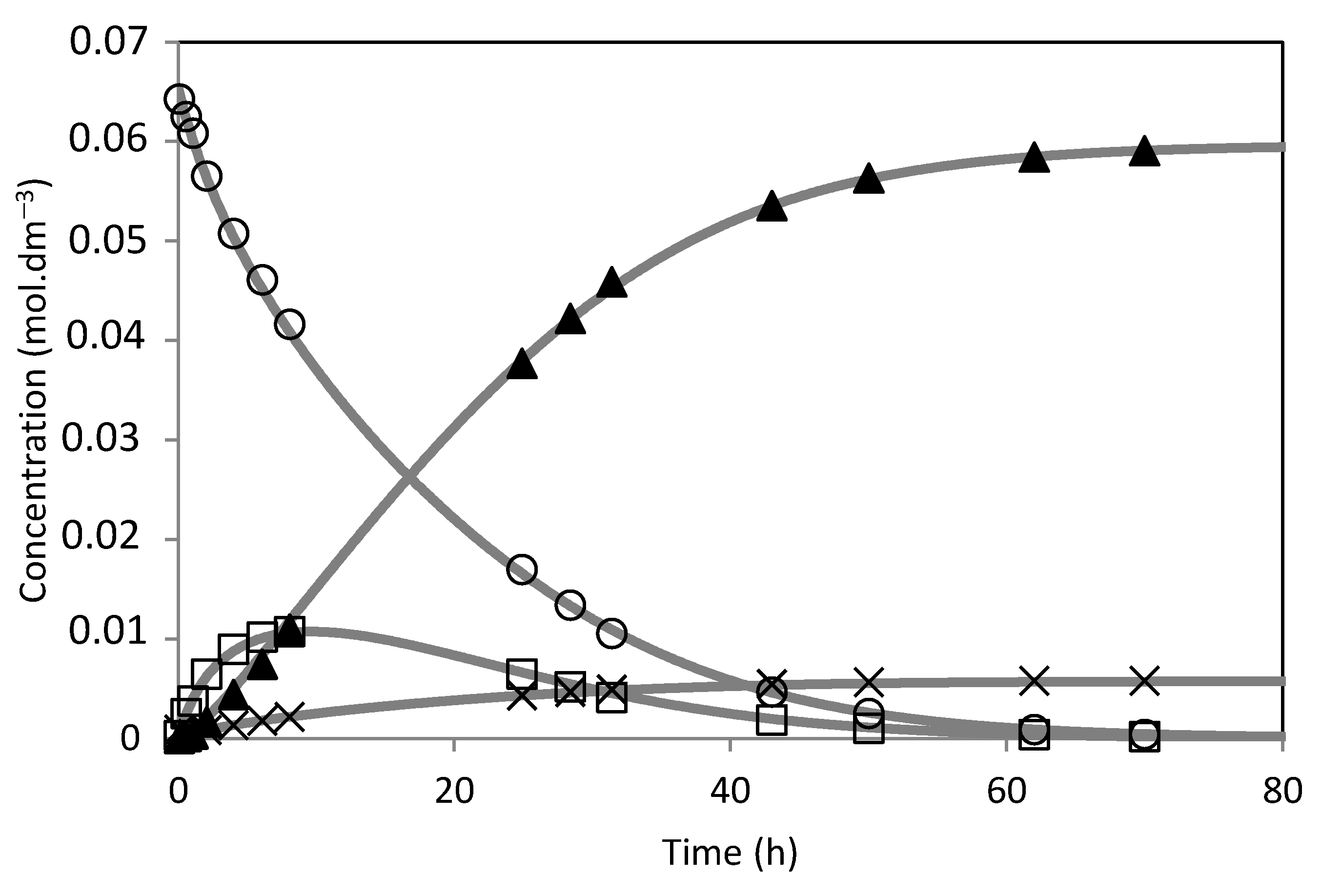
 ) m = 0.48 g; (
) m = 0.48 g; ( ) m = 0.30 g; (
) m = 0.30 g; ( ) m = 0.10 g; Isoborneol selectivity (%): (
) m = 0.10 g; Isoborneol selectivity (%): ( ) m = 0.48 g; (
) m = 0.48 g; ( ) m = 0.30 g; (
) m = 0.30 g; ( ) m = 0.10 g. (B) Camphene conversion (%) and selectivity (%) to the isoborneol at 70 h of reaction.
) m = 0.10 g. (B) Camphene conversion (%) and selectivity (%) to the isoborneol at 70 h of reaction.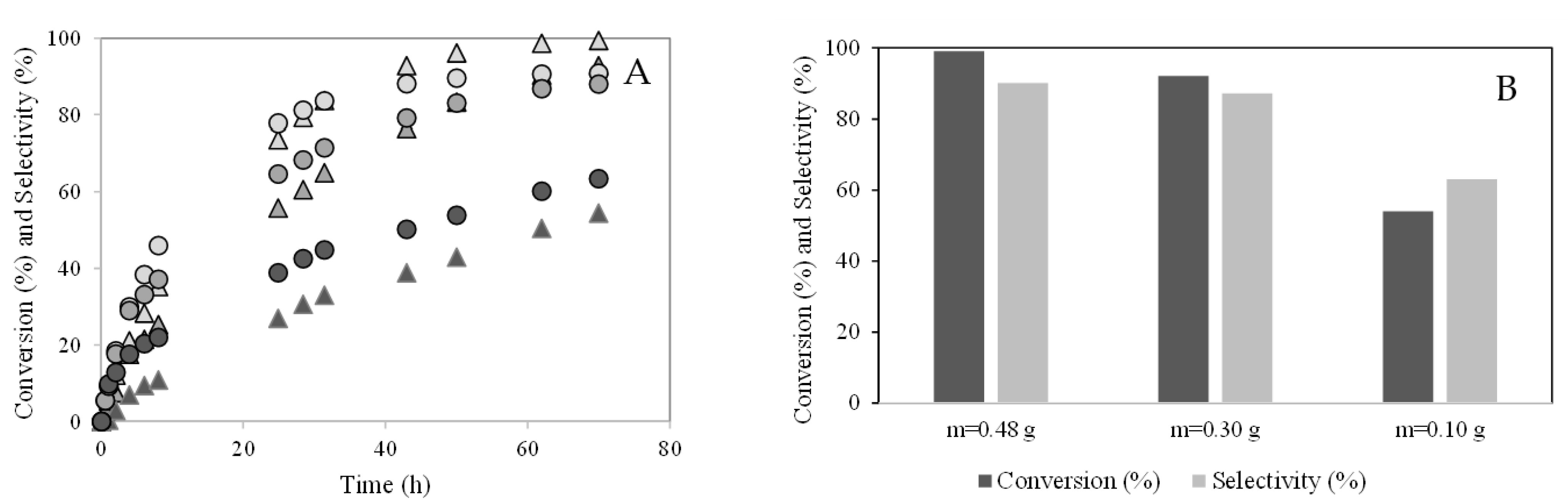

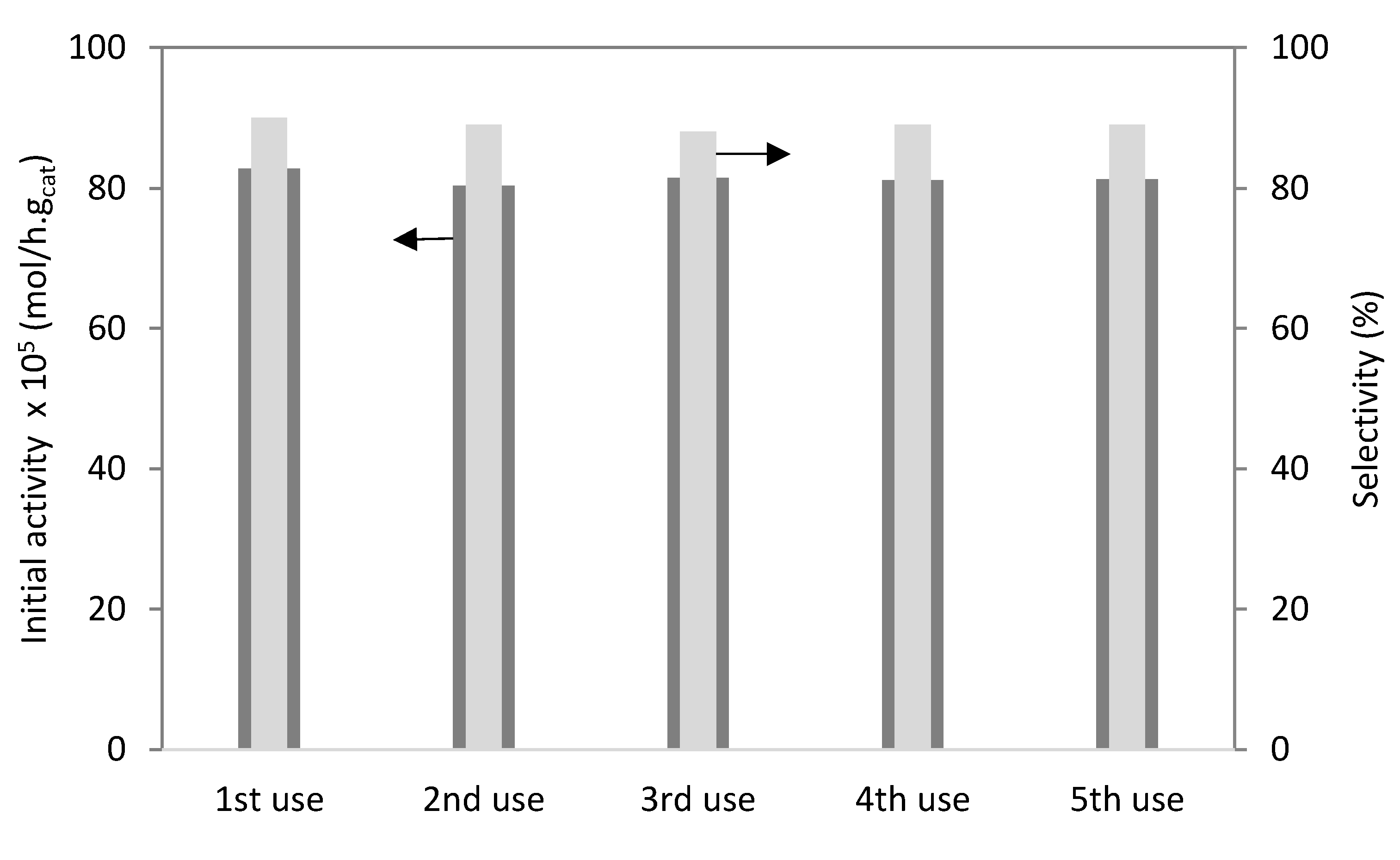




| Materials | Acidity a (mmol H+/g) | HPW Amount (wt%) | ABET (m2/g) | VT b (cm3/g) |
|---|---|---|---|---|
| SBA-15 | - | - | 880 | 0.82 |
| SBA-15-SO3H | 0.40 | - | 772 | 0.74 |
| PW1-SBA-15-SO3H | 0.52 | 1.7 | 723 | 0.71 |
| PW2-SBA-15-SO3H | 0.68 | 4.2 | 707 | 0.68 |
| PW3-SBA-15-SO3H | 0.78 | 8.6 | 687 | 0.65 |
| PW4-SBA-15-SO3H | 1.32 | 16.4 | 654 | 0.61 |
| PW5-SBA-15-SO3H | 1.53 | 22.1 | 583 | 0.56 |
| Catalyst | k1 (mol/g.h) | k2 (mol/g.h) | k3 (mol/g.h) | k4 (mol/g.h) | KC (dm−3/mol) | KHC (dm−3/mol) |
|---|---|---|---|---|---|---|
| SBA-15-SO3H | 0.0003026 | 0.0000510 | 0.0000006 | 0.0000654 | 11.1937638 | 253.3123137 |
| PW1-SBA-15-SO3H | 0.0062009 | 0.0000767 | 0.0000001 | 0.0006799 | 1.3138595 | 241.6666349 |
| PW2-SBA-15-SO3H | 0.0084667 | 0.0000841 | 0.0000002 | 0.0008956 | 2.1688618 | 368.2589676 |
| PW3-SBA-15-SO3H | 0.0150343 | 0.0003588 | 0.0010349 | 0.0022176 | 0.9024512 | 109.2452103 |
| PW4-SBA-15-SO3H | 0.0414648 | 0.0008294 | 0.0034500 | 0.0043307 | 0.4439438 | 78.2818538 |
| PW5-SBA-15-SO3H | 0,0284237 | 0.0003753 | 0.0050847 | 0.0034519 | 0.4343751 | 70.5933381 |
| Catalyst | Time (h) | Conversion (%) | Selectivity (%) Isoborneol | Initial Activity (mol/h.gcat) | Reference |
|---|---|---|---|---|---|
| USY | 50 | 95 | 90 | 4.0 × 10−4 | [5] |
| PW4-SBA-15-SO3H | 70 | 99 | 96 | 8.2 × 10−4 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castanheiro, J. Hydration of Camphene over PW-SBA-15-SO3H. Molecules 2023, 28, 6. https://doi.org/10.3390/molecules28010006
Castanheiro J. Hydration of Camphene over PW-SBA-15-SO3H. Molecules. 2023; 28(1):6. https://doi.org/10.3390/molecules28010006
Chicago/Turabian StyleCastanheiro, José. 2023. "Hydration of Camphene over PW-SBA-15-SO3H" Molecules 28, no. 1: 6. https://doi.org/10.3390/molecules28010006
APA StyleCastanheiro, J. (2023). Hydration of Camphene over PW-SBA-15-SO3H. Molecules, 28(1), 6. https://doi.org/10.3390/molecules28010006






