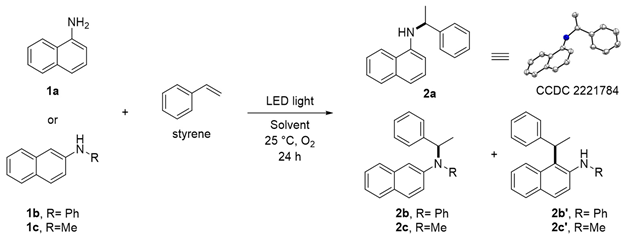
Abstract
This study processes a facile and green approach for the Markovnikov-selective hydroamination of styrene with naphthylamine through irradiation with UV LED light (365 nm) via an electron donor–acceptor complexation between naphthylamines and oxygen in situ. This protocol showcases the synthetic potential for aerobic C–N bond formation without using a metal catalyst and photosensitizer. Three naphthylamines were examined and afforded desired C–N bond formation product in moderate yield.
1. Introduction
Carbon–nitrogen (C–N) bond formation is crucial and has been intensively studied for amine synthesis, comprising important chemical building blocks such as illuminated materials and organocatalysts [,,,]. Hydroamination [] and transition metal-mediated cross-coupling of amines and aryl halides [,] are the most commonly applied strategies for C–N bond-forming reactions. In particular, hydroamination, which can directly connect amines and alkenes with high atom economy and accessibility, is a powerful synthetic approach for C–N bond formation. However, these methods require transition metal catalysts such as palladium, copper, and iron complexes [,,,,]. Owing to the recent rapid progress in green chemistry, more environmentally benign and facile C–N bond formation methods have emerged, such as radical-based photocatalysis [,]. However, most photocatalytic hydroaminations that employ high-oxidative/reductive potential photosensitizers still depend on transition metal catalysis. In addition, no control over the generation of nitrogen-centered radicals can result in various unexpected side reactions []. Therefore, more effective and environmentally friendly C–N bond formation methods, especially those that involve metal-free and photosensitizer-free transformations, are in high demand []. In 2011, Hoffmann et al. found that irradiation with UV light promoted the catalyst-free bond formation of electron-deficient alkenes, but resulted in an excess amount of naphthylamine derivatives (15 equiv.) as coupling partners [].
The discovery of electron donor–acceptor (EDA) complexes that facilitate photocatalyst-free transformation [,] has resulted in the evolution of a wide range of EDA complex-based synthetic C–N bond formation methods. Recently, an EDA complex-initiated annulation reaction of electron-deficient alkenes and alkyl anilines (electron donors) under visible-light irradiation was developed by Sundén et al. [,]. However, the hydroamination of arylamines with styrene is difficult because both substrates are electron donors with relatively low oxidation potentials []. To date, the C–N bond formation between arylamines and styrene has been accomplished mainly via reactions mediated by late-transition metals [,,,] or strong Brønsted acids [,,]. Recently, naphthylamines were also included in the hydroamination reactions. In 2019, Zhang et al. reported visible-light-triggered hydroamination of styrene with arylamines using a copper catalyst and a strong base, which provides facile access to amines with Markovnikov regioselectivity (Figure 1a) []. Thereafter, ortho-alkylation of arylamines with styrene was performed by Patureau [] in 2019 and Gandon in 2020 [] using AgBF4 and the strong Lewis acid Ca(NTf2)2, respectively (Figure 1b,c).
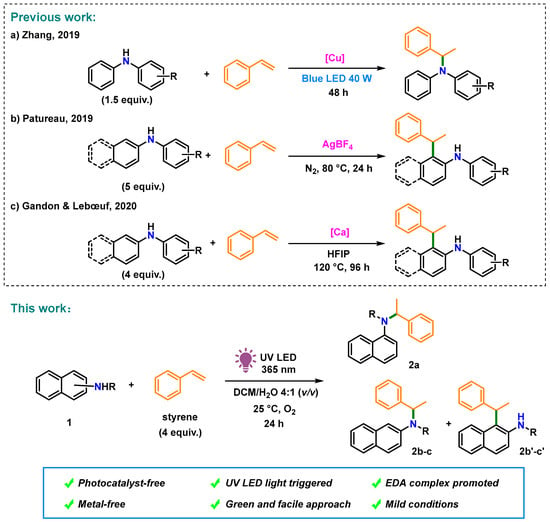
Figure 1.
Photoinduced metal- and photocatalyst-free aerobic C–N bond formation of styrene and naphthylamine derivatives. (a) Zhang, (2019) []; (b) Patureau, 2019 []; (c) Gandon & Lebœuf, 2020 [].
For establishing metal and photosensitizer-free transformations of electron-rich substrates, herein, we report the photocatalytic Markovnikov-selective hydroamination of naphthylamines and styrene via the EDA complexation of naphthylamines and oxygen in situ (Figure 1, This work). This photochemical strategy is eco-friendly, green, and simple to perform, making it a suitable alternative to C–N bond formation under irradiation with 365 nm UV LED light in an O2 atmosphere.
2. Results and Discussions
To investigate the feasibility of the targeted photocatalyst-free reaction triggered by LED light, 1-naphthylamine (1a), and 2-naphthylamine (1b) with styrene were employed (Table 1). Remarkably, when 1a was used in the photocatalytic reaction, N-(1-phenylethyl)-2-naphthylamine (2a, CCDC 2221784) was formed as the major product. In the case of substrate 1b, desired product 2b and 1-alkylated product 2b’ were obtained. The molar ratio of the starting materials was an important factor affecting the yield of desired product 2 (entries 1–3, 5, 12–14); the reaction in a 1:4 ratio of 1a and styrene gave a 61% yield of 2a (entry 5). Among the screened wavelengths from UV to visible light (entries 4–6), the 365 nm LED light was the most suitable for promoting the reaction (entry 5). The solvent system was also a crucial factor that significantly affected the yield of 2 (entries 7–11). After thoroughly evaluating the solvent system (also see Supporting Information), we found that when a binary solvent dichloromethane (DCM) and H2O was used in a ratio of 4:1 as a mixed solvent system, better catalytic performance was observed for both substrates 1a and 1b (entries 5 and 14), respectively. During our screening of reaction conditions, the addition of Cs2CO3 as an inorganic base could slightly improve the total yield of 2b and 2b’ (entries 15–17) []. When photocatalysts were used, no reaction occurred (entries 18–21). Finally, N-methyl-1-naphthylamine 1c was found to be the appropriate substrate. Under the optimized conditions, 30% yield of 2c (C–N product) and 22% yield of 2c’ (C–C product) were obtained, as shown in entry 22.

Table 1.
Optimization of reaction conditions.
To elucidate the reaction mechanism of the proposed UV-light-promoted process, several control experiments were conducted, as shown in Figure 2 and Figure 3. Although the UV–vis spectroscopic measurements of 1a, styrene, and a mixture of 1a and styrene in DCM did not show any peak shifting (Figure 2a), the UV–vis spectroscopic analysis of a mixture of 1a and O2 in DCM showed an increase in the bathochromic displacement and absorption (Figure 2b), which probably supported the formation of an electron donor–acceptor EDA complex between 1a and O2. After mixing oxygen with naphthylamine in DCM, a color change from colorless to orange was immediately observed, which might also support EDA complex generation (also see Supplementary Materials). Next, an “ON-OFF” UV light irradiation (365 nm) experiment was conducted (Figure 3). Under light irradiation, the mixture resulted in a reaction that formed the corresponding product 2a. However, the reaction was completely suppressed under no light irradiation. Finally, continuous 365 nm UV light irradiation resulted in the consumption of 1a to give 2a with 61% yield (see Supplementary Materials for details). In the presence of TEMPO (4 equiv.) as a radical scavenger, the reaction of 1a with styrene was avoided even under the optimized conditions (Figure 4).
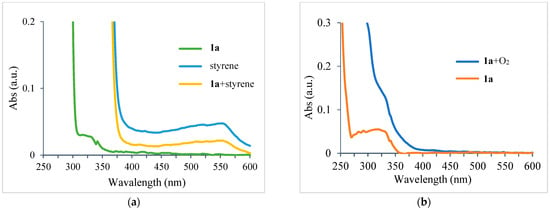
Figure 2.
(a) UV–vis absorbance spectra of 1a, styrene, and mixture of 1a and styrene in DCM; (b) UV–vis absorbance spectra of 1a and mixture of 1a and O2 in DCM.
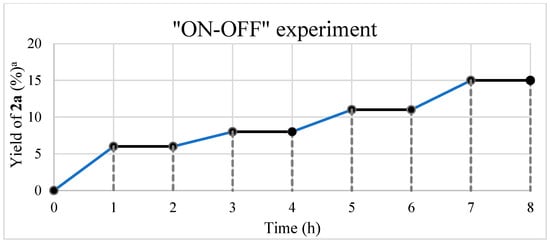
Figure 3.
Result of the ON-OFF experiment using 1a and styrene. Reaction conditions: 1a (28.6 mg, 0.2 mmol) and styrene (0.09 mL, 0.8 mmol) in DCM/H2O (2.0 mL, 4/1) under 365 nm UV irradiation and O2 atmosphere.
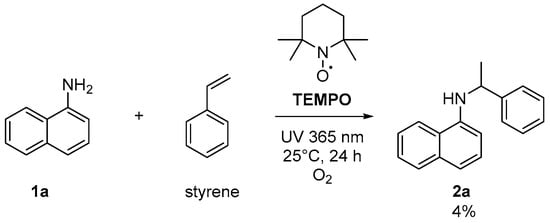
Figure 4.
Reaction in the presence of TEMPO.
A Job plot study to determine the correlation of generating the EDA complex in situ with various ratios of O2 is challenging because of the difficulty in measuring the exact amount of O2 against naphthylamine, although all of these obtained results will probably be in agreement with the proposed mechanism involving the formation of naphthylamine radicals (Figure 5). Triggering of 1b and O2 via UV light induced the formation of EDA complex I*. The generated II species might be in equilibrium with IIA and IIB via electron transfer [], after the bond formation of styrene with IIA and IIB led to the corresponding C–N formation product 2b and C–C formation product 2b’, respectively [] (Figure 5a). When 1a was used as the substrate, only C–N formation occurred, probably owing to the sole generation of N cation radicals (Figure 5b). Although the intramolecular hydroamination to give Markovnikov products via amine radical cation species was reported [], a naphthylamine anion radical generated by a reaction of oxygen anion radical with 1 might be another possible pathway for this C–N bond formation. Currently, the exploration of mechanism is ongoing in our laboratory.
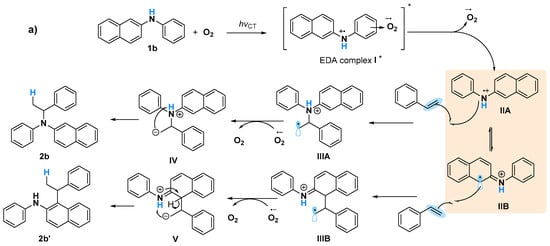
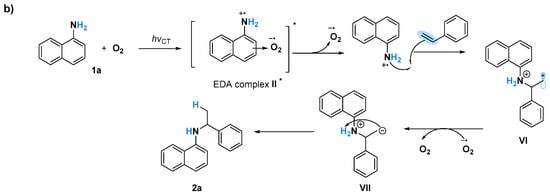
Figure 5.
Proposed mechanism for photoinduced amination reactions using (a) 1b, and (b) 1a.
3. Materials and Methods
3.1. Materials
1-naphthylamine (1a), N-phenyl-2-napthylamine (1b), and styrene were purchased from Tokyo Chemical Industries (TCI). All commercially available organic and inorganic compounds were used directly without further purification.
3.2. Methods
3.2.1. Spectroscopy and Spectrometry
1H- and 13C-NMR spectra were recorded at 25 °C using a JEOL JMN ECS400 FT NMR instrument (1H-NMR 400 MHz; 13C-NMR 100 MHz). The 1H-NMR spectra are reported as follows: chemical shift in ppm downfield of tetramethylsilane and referenced to a residual solvent peak (CHCl3) at 7.26 ppm, integration, multiplicities (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), and coupling constants (Hz). The 13C-NMR spectra are reported in ppm relative to the central line of the triplet for CDCl3 at 77.16 ppm. APCI-MS spectra were obtained using a JMS-T100LC instrument (JEOL). Thin-layer chromatography (TLC) analysis of the reaction mixture was performed on Merck silica gel 60 F254 TLC plates and visualized under UV light. Column chromatography on SiO2 was performed using Kanto Silica Gel 60 (63–210 μm). UV and visible light irradiations were performed with an LED lamp (PER-AMP, Techno Sigma Co., Ltd. Okayama, Japan). Commercial LED lamps (PER-AMP, Techno Sigma Co., Ltd.) were used as a light source to irradiate the Schlenk tube at a distance of 0.5 cm with aluminum foil covering the outside of the tube. A water bath was used for cooling the setup. A thermo-stainless-steel chamber ensured a constant temperature of 25 °C during the reaction. The temperature inside the chamber was also monitored during the experiment to ensure no fluctuations and kept at 25 °C.
3.2.2. Synthetic Procedure of N-methylnaphthalen-2-amine (1c)
Compound 1c was prepared according to the literature procedure []. In a dry 100 mL steel bomb, we added 2-naphthol (500 mg, 3.47 mmol), ammonium chloride (408.5 mg, 7.6 mmol), methylamine (40% in methanol), and ethanol (2.0 M, 1.8 mL). The reaction mixture was heated up to 200 °C, for 12 h. The reaction was quenched by 6 N NaOH after completion. Then, we filtered the residue and extracted it with EtOAc, dried over Na2SO4, and concentrated under reduced pressure. The residue was purified by silica column chromatography (eluent: n-hexane/EtOAc = 20/1) to afford the desired product N-methylnaphthalen-2-amine (1c) in 80% yield as yellow oil. 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 7.6 Hz, 1H), 7.64 (t, J = 8.4 Hz, 2H), 7.40–7.36 (m, 1H), 7.21 (td, J = 7.6, 1.2 Hz, 1H), 6.89 (dd, J = 8.4, 2.3 Hz, 1H), 6.81 (d, J = 2.3 Hz, 1H), 3.88 (s, 1H), 2.95 (s, 3H).
3.2.3. General Protocol for the Photocatalytic Hydroamination of Styrene
An oven-dried Schlenk tube equipped with a magnetic stirring bar was charged with naphthylamines (0.2 mmol) and styrene (0.8 mmol). The tube was evacuated and backfilled with oxygen (three times). Then, 1.6 mL of DCM and 0.4 mL of H2O were added by syringe under an O2 atmosphere. The solution was stirred at 25 °C with the irradiation of a 365 nm UV LED light. After stirring for 24 h, the organic solvent was removed in vacuo, and the remained water phase was extracted with EtOAc. The organic layer was collected and evaporated under vacuum. The residue was purified by column chromatography on silica gel using n-hexane/DCM (20/1) as eluent to obtain the desired products.
N-(1-Phenylethyl)naphthalen-1-amine (2a)
According to the general procedure, a mixture of 1a (28.6 mg, 0.2 mmol) and styrene (0.09 mL, 0.8 mmol) in 2.0 mL DCM/H2O (4:1, v/v) was added under oxygen, then stirred for 24 h under 365 nm UV LED light irradiation to afford 2a as yellow oil in 61% yield. 1H NMR (400 MHz, CDCl3): δ 7.95–7.92 (m, 1H, Ar-CH), 7.80–7.76 (m, 1H, Ar-CH), 7.50–7.42 (m, 4H, Ar-CH), 7.34–7.30 (m, 2H, Ar-CH), 7.25–7.21 (m, 1H, Ar-CH), 7.19–7.16 (m, 2H, Ar-CH), 6.37 (dt, J = 9.3, 4.1 Hz, 1H, Ar-CH), 4.75 (brs, 1H, NH), 4.68 (q, J = 6.7 Hz, 1H, CH), 1.67 (d, J = 6.9 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 144.9, 142.0, 134.2, 128.7, 128.7, 127.0, 126.5, 125.8, 125.6, 124.7, 123.2, 119.8, 117.2, 106.0, 53.6, 25.3; HRMS (APCI): calcd for C18H17N: m/z [M + H]+ 248.1434, found 248.1431.
N-Phenyl-N-(1-phenylethyl)naphthalen-2-amine (2b)
According to the general procedure, a mixture of 1b (43.9 mg, 0.2 mmol), styrene (0.09 mL, 0.8 mmol), and Cs2CO3 (130 mg, 0.4 mmol) in 2.0 mL DCM/H2O (4:1, v/v) was added under oxygen, then stirred for 24 h under 365 nm UV LED light irradiation to afford 2b as yellow oil in 50% yield. 1H NMR (400 MHz, CDCl3): δ 7.71 (d, J = 7.8 Hz, 1H, Ar-CH), 7.64 (d, J = 9.0 Hz, 1H, Ar-CH), 7.60 (d, J = 7.8 Hz, 1H, Ar-CH), 7.42 (d, J = 7.3 Hz, 2H, Ar-CH), 7.39–7.35 (m, 1H, Ar-CH), 7.32–7.28 (m, 3H, Ar-CH), 7.25–7.20 (m, 4H, Ar-CH), 7.05–6.97 (m, 2H, Ar-CH), 6.92 (dd, J = 9.0, 0.9 Hz, 2H, Ar-CH), 5.44 (q, J = 7.0 Hz, 1H, CH), 1.56 (d, J = 7.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 146.9, 144.9, 143.9, 134.5, 129.4, 129.2, 128.7, 128.5, 127.6, 127.2, 127.1, 127.0, 126.2, 124.0, 124.0, 123.9, 122.3, 118.0, 58.1, 19.6; HRMS (APCI): calcd for C24H21N: m/z [M + H]+ 324.1747, found 324.1752.
N-Phenyl-1-(1-phenylethyl)naphthalen-2-amine (2b’)
According to the general procedure, a mixture of 1b (43.9 mg, 0.2 mmol), styrene (0.09 mL, 0.8 mmol), and Cs2CO3 (130 mg, 0.4 mmol) in 2.0 mL DCM/H2O (4:1, v/v) was added under oxygen, then stirred for 24 h under 365 nm UV LED light irradiation to afford 2b’ as yellow oil in 46% yield. 1H NMR (400 MHz, CDCl3): δ 8.11 (d, J = 8.7 Hz, 1H, Ar-CH), 7.82 (d, J = 6.9 Hz, 1H, Ar-CH), 7.70 (d, J = 8.7 Hz, 1H, Ar-CH), 7.48–7.28 (m, 7H, Ar-CH), 7.22 (d, J = 6.9 Hz, 1H, Ar-CH), 7.15 (dd, J = 8.5, 7.6 Hz, 2H, Ar-CH), 6.80 (t, J = 7.6 Hz, 1H, Ar-CH), 6.64 (d, J = 7.6 Hz, 2H, Ar-CH), 5.28 (brs, 1H, NH), 5.24 (t, J = 7.1 Hz, 1H, CH), 1.72 (d, J = 7.6 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ144.5, 144.3, 137.9, 133.2, 131.0, 129.2, 128.8, 127.9, 126.9, 126.5, 126.2, 123.9, 123.3, 119.7, 115.9, 35.6, 16.8; HRMS (APCI): calcd for C24H21N: m/z [M + H]+ 324.1747, found 324.1752.
N-Methyl-N-(1-phenylethyl)naphthalen-2-amine (2c)
According to the general procedure, a mixture of 1c (31.4 mg, 0.2 mmol) and styrene (0.09 mL, 0.8 mmol) in 2.0 mL DCM/H2O (4:1, v/v) was added under oxygen, then stirred for 24 h under 365 nm UV LED light irradiation to afford 2c as yellow oil in 30% yield. 1NMR (400 MHz, CDCl3): δ 7.71 (t, J = 8.5 Hz, 2H, Ar-CH), 7.64 (d, J = 8.5 Hz, 1H, Ar-CH), 7.40–7.28 (m, 7H, Ar-CH), 7.23–7.19 (m, 1H, Ar-CH), 7.01 (d, J = 2.3 Hz, 1H, Ar-CH), 5.29 (q, J = 6.9 Hz, 1H, CH), 2.76 (s, 3H, CH3), 1.60 (d, J = 6.9 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 144.7, 144.6, 133.2, 129.0, 128.8, 128.4, 128.0, 126.9, 126.6, 126.3, 122.0, 121.6, 121.0, 115.0, 35.1, 31.5, 15.7; HRMS (APCI): calcd for C19H19N: m/z [M + H]+ 262.1590, found 262.1591.
N-Methyl-N-(1-phenylethyl)naphthalen-2-amine (2c’)
According to the general procedure, a mixture of 1c (31.4 mg, 0.2 mmol) and styrene (0.09 mL, 0.8 mmol) in 2.0 mL DCM/H2O (4:1, v/v) was added under oxygen, then stirred for 24 h under 365 nm UV LED light irradiation to afford 2c’ as yellow oil in 22% yield. 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 8.2 Hz, 1H, Ar-CH), 7.75 (dd, J = 12.8, 8.0 Hz, 2H, Ar-CH), 7.40 (t, J = 8.0 Hz, 1H, Ar-CH), 7.30 (d, J = 4.6 Hz, 4H, Ar-CH), 7.25–7.10 (m, 3H, Ar-CH), 5.16 (q, J = 7.3 Hz, 1H, NH), 2.70 (s, 3H, CH3), 1.77 (d, J = 7.3 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 148.3, 142.6, 135.2, 128.9, 128.5, 127.5, 127.1, 126.4, 126.3, 122.2, 117.1, 107.3, 57.1, 32.2, 16.4; HRMS (APCI): calcd for C19H19N: m/z [M + H]+ 262.1590, found 262.1591.
3.2.4. Procedure for “ON-OFF” Experiment
The “ON-OFF” experiment was conducted by employing 1a (28.6 mg, 0.2 mmol) and styrene (0.09 mL, 0.8 mmol) in DCM/H2O (2.0 mL, 4/1) under 365 nm UV irradiation in an oxygen atmosphere. Under the UV light irradiation to the mixture, the reaction proceeded to form the corresponding product 2a. However, no light irradiation led to suppressing the reaction completely. Finally, continuous 365 nm UV light resulted in consuming 1a to give 2a with 61% yield.
3.2.5. Procedures for UV–Vis Absorbance Analysis
1a and styrene were employed in the binary solvent system. The color of the reaction mixture immediately changed from colorless to an orange color, and finally to brown in 5 min at the irradiation of 365 nm UV LED under O2 atmosphere (see Supporting Information). In order to understand the generation of EDA complex between substrate 1a and oxygen, a series of UV–vis absorption measurements were carried out. Initially, we took the optical absorption spectra of 1a and styrene in DCM (10 μM) and then measured the mixture of 1a and styrene (1:1 v/v) in DCM (10 μM). The UV–vis absorption spectra indicated that no electron donor–acceptor (EDA) complex formed between the two starting materials. Next, we checked the UV–vis absorption of 1a and oxygen. The sample was prepared by oxygen bubbling for 10 min in the DCM solution of 1a (10 μM). An obvious increasing bathochromic displacement and absorption can be observed in the spectrum, which supported the formation of the EDA complex between 1a and O2.
4. Conclusions
In summary, we have developed a new approach for the Markovnikov-selective hydroamination of styrene with naphthylamines by an irradiation of UV LED light (365 nm) via an in situ electron donor–acceptor (EDA) complexation between naphthylamines and oxygen. This protocol is eco-friendly, green, and facile, showcasing synthetic potential for aerobic C–N bond formation without using any metal catalyst or photosensitizer. The exploration of more C–N bond formation reactions involving naphthylamines and other alkenes is ongoing in our group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28010356/s1, Figure S1: Reaction setup and LED apparatus; Figure S2: “On-off” experiment; Figure S3: Visual characterization of the reaction mixture under UV irradiation; Figure S4: UV-Vis absorption analysis; Figure S5: Proposed mechanism of photoinduced C-N bond formation of 1b and styrene; Figure S6: Compound 2a (1H NMR, 400 MHz, CDCl3); Figure S7: Compound 2a (13C NMR, 100 MHz, CDCl3): Figure S8: Compound 2b (1H NMR, 400 MHz, CDCl3); Figure S9: Compound 2b (13C NMR, 100 MHz, CDCl3); Figure S10: Compound 2b’ (1H NMR, 400 MHz, CDCl3); Figure S11: Compound 2b’ (13C NMR, 100 MHz, CDCl3); Figure S12: Compound 2c (1H NMR, 400 MHz, CDCl3); Figure S13: Compound 2c (13C NMR, 100 MHz, CDCl3); Figure S14: Compound 2c’ (1H NMR, 400 MHz, CDCl3); Figure S15: Compound 2c’ (13C NMR, 100 MHz, CDCl3); Figure S16: Single crystal structure of CCDC 2221784 (2a); Table S1: Optimization of reaction conditions; Table S2: On-off experiment; Table S3: Crystal information of CCDC 2221784 (2a).
Author Contributions
S.T. and H.S.: conceptualization, resources, writing—reviewing and editing. D.F.: investigation, resources, visualization, validation, writing—original draft. A.S.: investigation, resources, validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI, grant number 22K06502 through Grant-in-Aid for Scientific Research (C) and Transformative Research Areas (A), grant number 21A204 through Digitalization-driven Transformative Organic Synthesis (DigiTOS), and grant number 22KK0073 through the Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)), provided by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Japan Society for the Promotion of Science (JSPS), JST CREST (no. JPMJCR20R1), and the Hoansha Foundation.
Informed Consent Statement
The study does not involve humans.
Data Availability Statement
CIF of the crystal of 2a is available as Supplementary. The X-ray crystallographic coordinate for the structure reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC- 2221784 (2a). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 24 December 2022).
Acknowledgments
We acknowledge the technical staff of the Comprehensive Analysis Center of SANKEN, Osaka University (Japan).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müller, T.E.; Beller, M. Metal-Initiated Amination of Alkenes and Alkynes. Chem. Rev. 1998, 98, 675–704. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, J.F. Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res. 2008, 41, 1534–1544. [Google Scholar] [CrossRef]
- Jiao, J.; Murakami, K.; Itami, K. Catalytic Methods for Aromatic C–H Amination: An Ideal Strategy for Nitrogen-Based Functional Molecules. ACS Catal. 2015, 6, 610–633. [Google Scholar] [CrossRef]
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward Renewable Amines: Recent Advances in the Catalytic Amination of Biomass-Derived Oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Müller, T.E.; Hultzsch, K.C.; Yus, M.; Foubelo, F.; Tada, M. Hydroamination: Direct Addition of Amines to Alkenes and Alkynes. Chem. Rev. 2008, 108, 3795–3892. [Google Scholar] [CrossRef]
- Forero-Cortés, P.A.; Haydl, A.M. The 25th Anniversary of the Buchwald–Hartwig Amination: Development, Applications, and Outlook. Org. Process Res. Dev. 2019, 23, 1478–1483. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C-N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Coman, S.M.; Parvulescu, V.I. Nonprecious Metals Catalyzing Hydroamination and C–N Coupling Reactions. Org. Process Res. Dev. 2015, 19, 1327–1355. [Google Scholar] [CrossRef]
- Huang, L.; Arndt, M.; Goossen, K.; Heydt, H.; Goossen, L.J. Late transition metal-catalyzed hydroamination and hydroamidation. Chem. Rev. 2015, 115, 2596–2697. [Google Scholar] [CrossRef] [PubMed]
- Kawatsura, M.; Hartwig, J.F. Palladium-Catalyzed Intermolecular Hydroamination of Vinylarenes Using Arylamines. J. Am. Chem. Soc. 2000, 122, 9546–9547. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Fan, J.; Liu, Y.; Tang, W.; Xue, D.; Li, C.; Xiao, J.; Wang, C. Iron-Catalyzed Anti-Markovnikov Hydroamination and Hydroamidation of Allylic Alcohols. J. Am. Chem. Soc. 2019, 141, 13506–13515. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.K.; Gopalsamuthiram, V.G.; Jawalekar, A.M.; Patre, R.E.; Pal, S. Iron catalyzed C N bond formation. Tetrahedron 2017, 73, 1769–1794. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, W. Recent advances in radical-based C-N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 2018, 47, 2591–2608. [Google Scholar] [CrossRef] [PubMed]
- Pratley, C.; Fenner, S.; Murphy, J.A. Nitrogen-Centered Radicals in Functionalization of sp(2) Systems: Generation, Reactivity, and Applications in Synthesis. Chem. Rev. 2022, 122, 8181–8260. [Google Scholar] [CrossRef] [PubMed]
- Ganley, J.M.; Murray, P.R.D.; Knowles, R.R. Photocatalytic Generation of Aminium Radical Cations for C horizontal line N Bond Formation. ACS Catal. 2020, 10, 11712–11738. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, Q.-Q.; Tan, F.; Lu, L.-Q.; Xiao, W.-J. Visible-Light-Driven Organic Photochemical Reactions in the Absence of External Photocatalysts. Synthesis 2019, 51, 3021–3054. [Google Scholar] [CrossRef]
- Jahjah, R.; Gassama, A.; Dumur, F.; Marinkovic, S.; Richert, S.; Landgraf, S.; Lebrun, A.; Cadiou, C.; Selles, P.; Hoffmann, N. Photochemical electron transfer mediated addition of naphthylamine derivatives to electron-deficient alkenes. J. Org. Chem. 2011, 76, 7104–7118. [Google Scholar] [CrossRef]
- Mulliken, R.S. Molecular Compounds and their Spectra II. J. Am. Chem. Soc. 1952, 74, 811–824. [Google Scholar] [CrossRef]
- Foster, R. Electron donor-acceptor complexes. J. Phys. Chem. 1980, 84, 2135–2141. [Google Scholar] [CrossRef]
- Runemark, A.; Sunden, H. Aerobic Oxidative EDA Catalysis: Synthesis of Tetrahydroquinolines Using an Organocatalytic EDA Active Acceptor. J. Org. Chem. 2022, 87, 1457–1469. [Google Scholar] [CrossRef]
- Runemark, A.; Zacharias, S.C.; Sunden, H. Visible-Light-Driven Stereoselective Annulation of Alkyl Anilines and Dibenzoylethylenes via Electron Donor-Acceptor Complexes. J. Org. Chem. 2021, 86, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.G.S.; Lima, T.M.; Duarte, M.; Jurberg, I.D.; Paixão, M.W. Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6, 1389–1407. [Google Scholar] [CrossRef]
- Li, K.; Horton, P.; Hursthouse, M.; Kuok, K.; Hii, K. Air- and moisture-stable cationic (diphosphine)palladium(II)complexes as hydroamination catalysts: X-ray crystal structures of two[(diphosphine)Pd(NCMe)(OH2)]2+[OTf]–2 complexes. J. Organomet. Chem. 2003, 665, 250–257. [Google Scholar] [CrossRef]
- Johns, A.M.; Utsunomiya, M.; Incarvito, C.D.; Hartwig, J.F. A Highly Active Palladium Catalyst for Intermolecular Hydroamination. Factors that Control Reactivity and Additions of Functionalized Anilines to Dienes and Vinylarenes. J. Am. Chem. Soc. 2006, 128, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Martin, D.; Melaimi, M.; Bertrand, G. Gold-catalyzed hydroarylation of alkenes with dialkylanilines. J. Am. Chem. Soc. 2014, 136, 13594–13597. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, F.; Lerch, S.; Kaliner, M.; Strassner, T. Cobalt-Catalyzed Hydroarylations and Hydroaminations of Alkenes in Tunable Aryl Alkyl Ionic Liquids. Org. Lett. 2018, 20, 6215–6219. [Google Scholar] [CrossRef] [PubMed]
- Beller, M.; Thiel, O.R.; Trauthwein, H. Catalytic Alkylation of Aromatic Amines with Styrene in the Presence of Cationic Rhodium Complexes and Acid. Synlett 1999, 1999, 243–245. [Google Scholar] [CrossRef]
- Anderson, L.L.; Arnold, J.; Bergman, R.G. Proton-Catalyzed Hydroamination and Hydroarylation Reactions of Anilines and Alkenes: A Dramatic Effect of Counteranions on Reaction Efficiency. J. Am. Chem. Soc. 2005, 127, 14542–14543. [Google Scholar] [CrossRef]
- Seshu Babu, N.; Mohan Reddy, K.; Sai Prasad, P.S.; Suryanarayana, I.; Lingaiah, N. Intermolecular hydroamination of vinyl arenes using tungstophosphoric acid as a simple and efficient catalyst. Tetrahedron Lett. 2007, 48, 7642–7645. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, G. Visible-Light-Induced Copper-Catalyzed Intermolecular Markovnikov Hydroamination of Alkenes. Org. Lett. 2019, 21, 7873–7877. [Google Scholar] [CrossRef]
- Rank, C.K.; Ozkaya, B.; Patureau, F.W. HBF4- and AgBF4-Catalyzed ortho-Alkylation of Diarylamines and Phenols. Org. Lett. 2019, 21, 6830–6834. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Force, G.; Guillot, R.; Carpentier, J.-F.; Sarazin, Y.; Bour, C.; Gandon, V.; Lebœuf, D. Lewis Acid/Hexafluoroisopropanol: A Promoter System for Selective ortho-C-Alkylation of Anilines with Deactivated Styrene Derivatives and Unactivated Alkenes. ACS Catal. 2020, 10, 10794–10802. [Google Scholar] [CrossRef]
- Herrero, M.T.; Sarralde, J.D.; SanMartin, R.; Bravo, L.; Domínguez, E. Cesium Carbonate-Promoted Hydroamidation of Alkynes: Enamides, Indoles and the Effect of Iron(III) Chloride. Adv. Synth. Catal. 2012, 354, 3054–3064. [Google Scholar] [CrossRef]
- Bowman, D.F.; Middleton, B.S.; Ingold, K.U. Oxidation of amines with peroxy radicals. I. N-phenyl-2-naphthylamine. J. Org. Chem. 1969, 34, 3456–3461. [Google Scholar] [CrossRef]
- Cortright, S.B.; Huffman, J.C.; Yoder, R.A.; Coalter, J.N.; Johnston, J.N. IAN Amines: Chiral C2-Symmetric Zirconium(IV) Complexes from Readily Modified Axially Chiral C1-Symmetric β-Diketimines. Organometallics 2004, 23, 2238–2250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).