Structural Evolution and Electronic Properties of Selenium-Doped Boron Clusters SeBn0/− (n = 3–16)
Abstract
1. Introduction
2. Results and Discussion
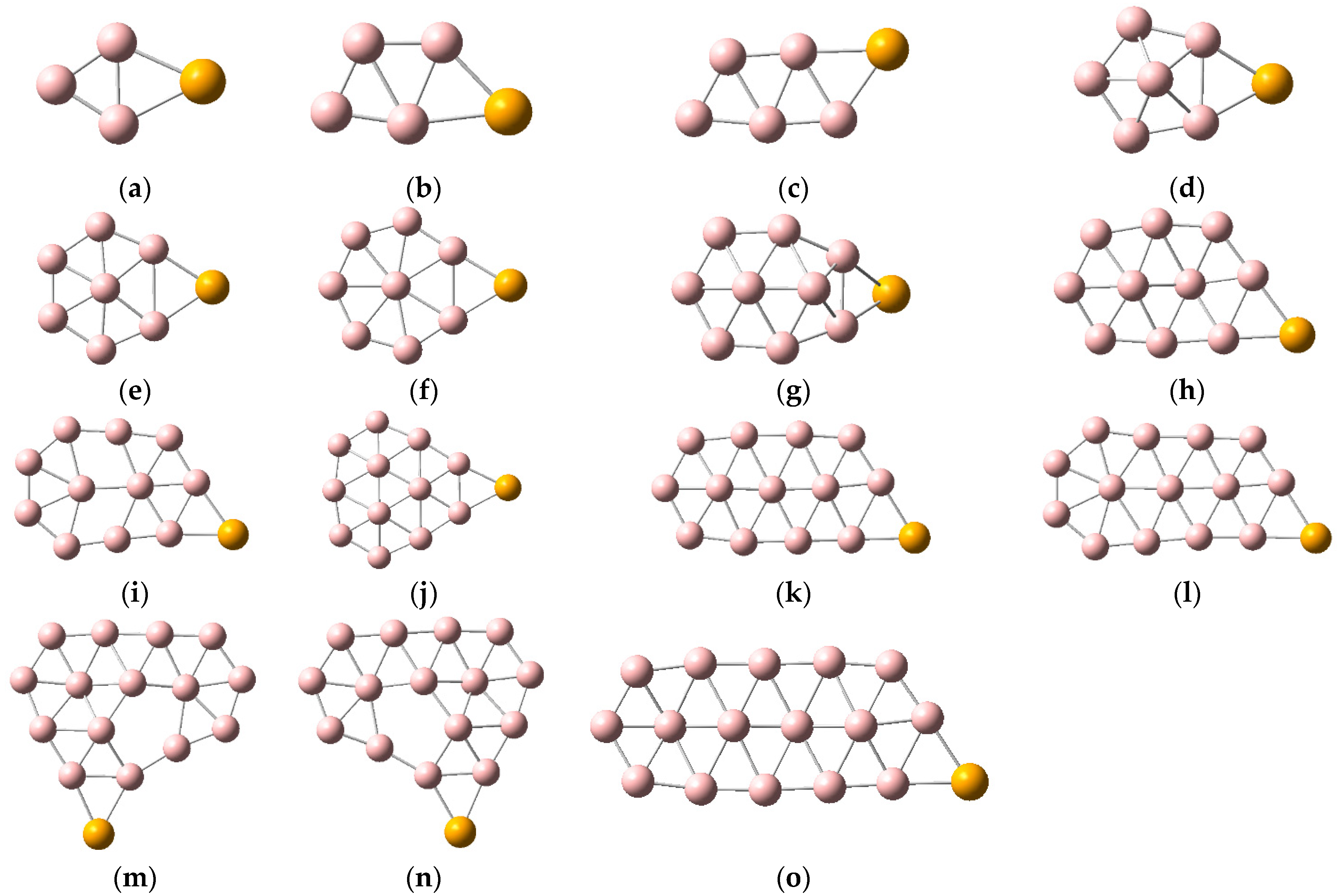
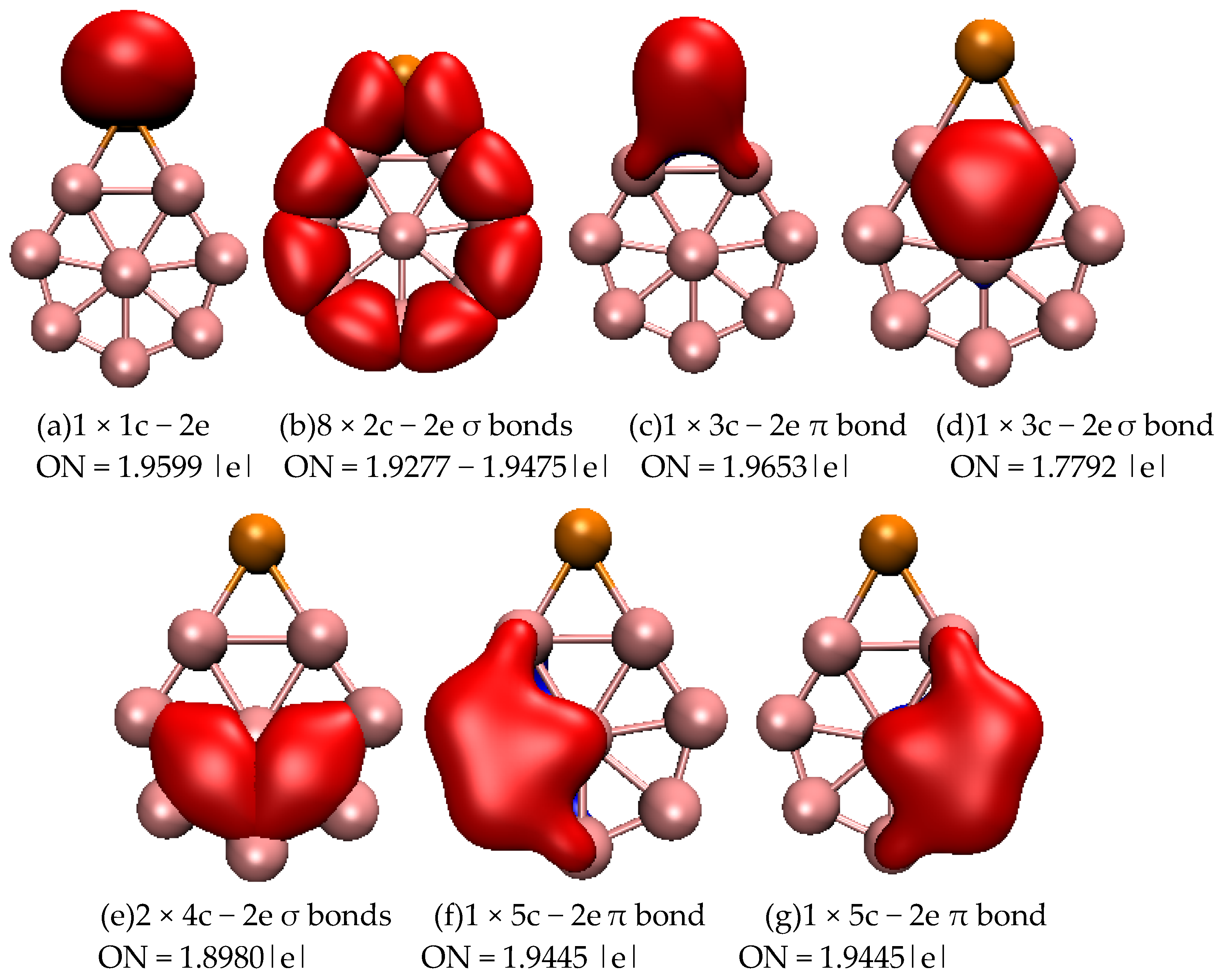
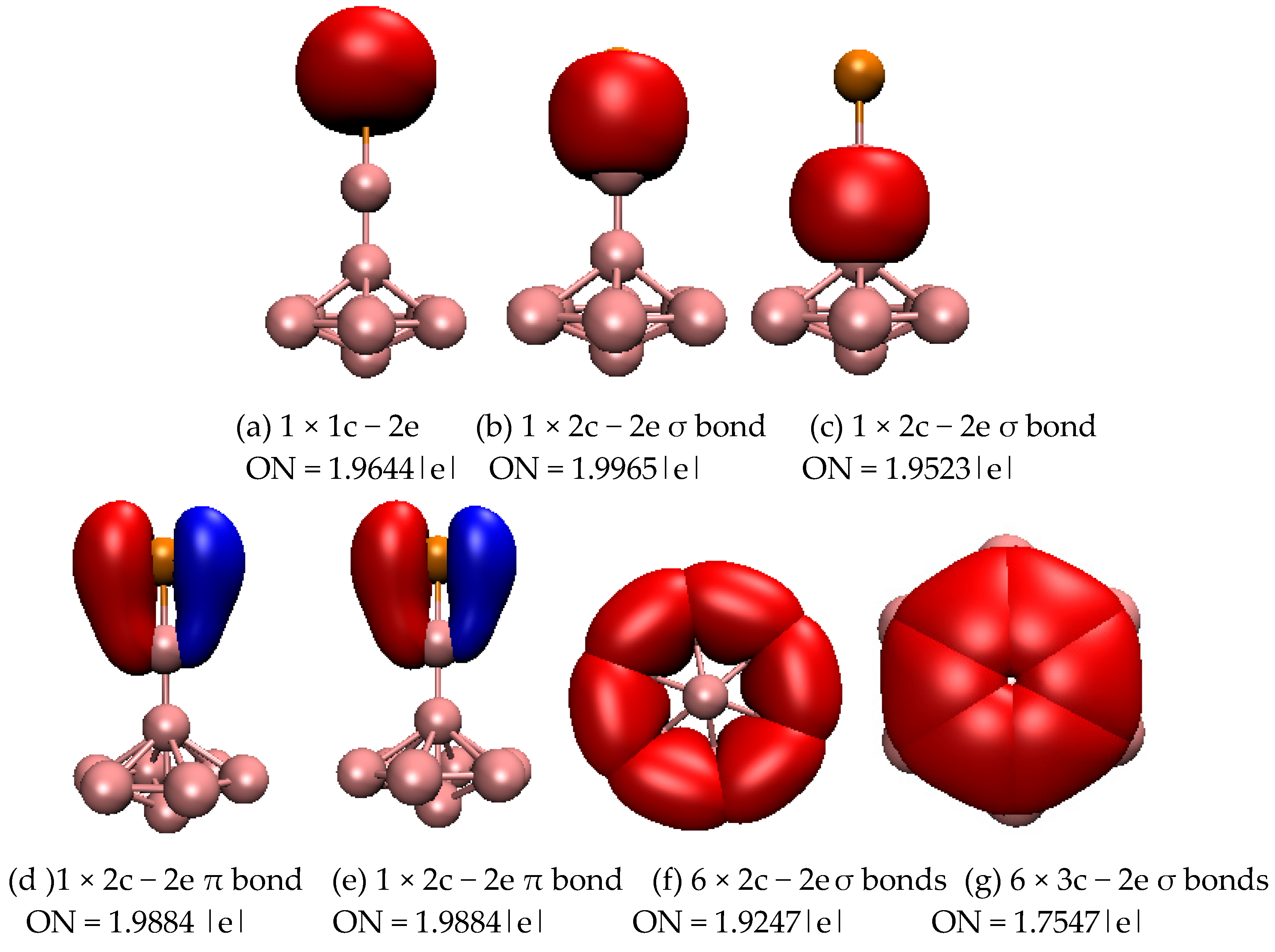
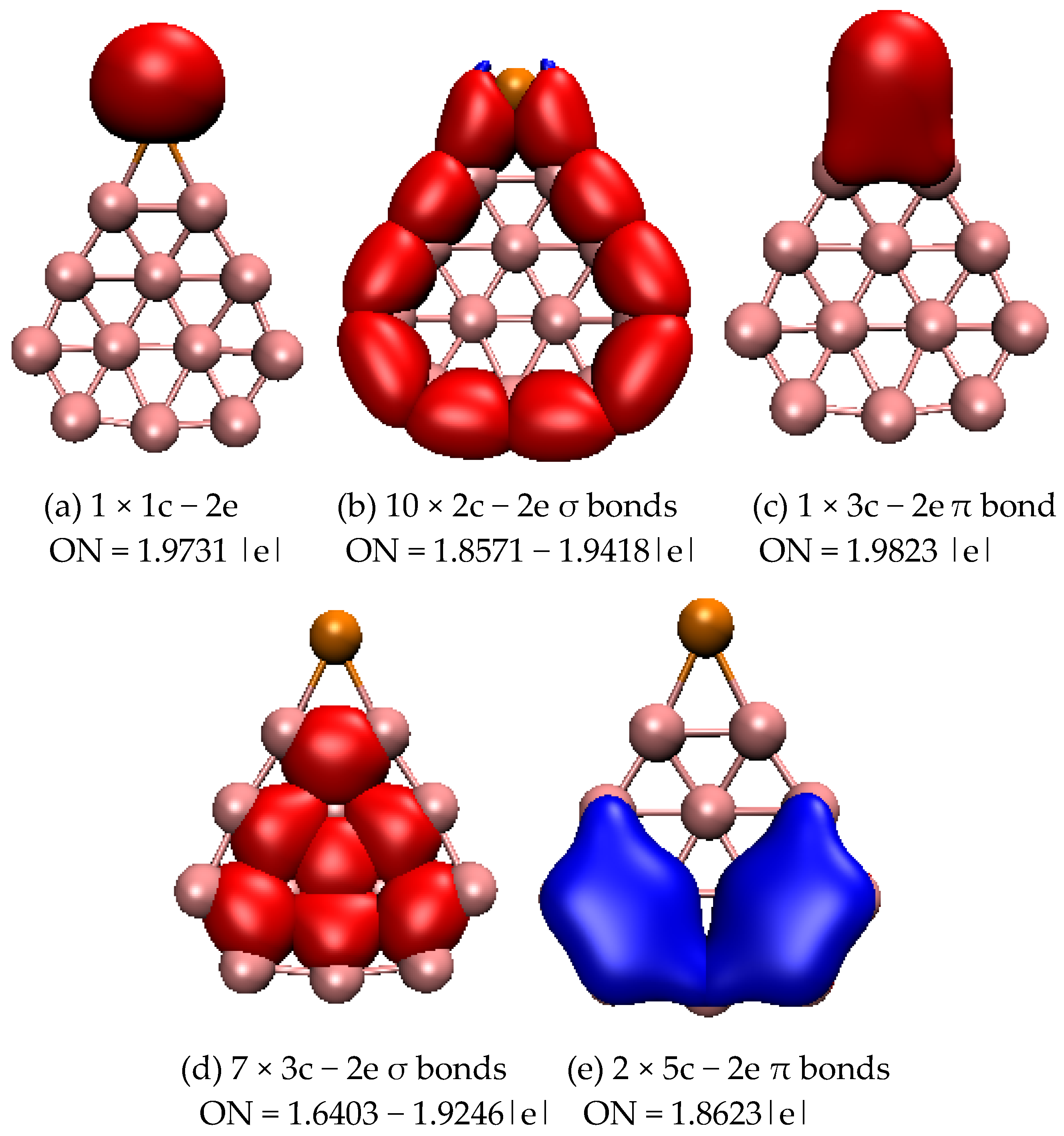
2.1. Structures and Electronic Properties
2.2. Photoelectron Spectra
3. Computation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boustani, I. Systematic ab initio investigation of bare boron clusters: Determination of the geometryand electronic structures of Bn (n = 2 − 14). Phys. Rev. B 1997, 55, 16426–16438. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Wang, L.-S.; Alexandrova, A.N.; Boldyrev, A.I. Electronic structure and chemical bonding of B5− and B5 by photoelectron spectroscopy andab initiocalculations. J. Chem. Phys. 2002, 117, 7917–7924. [Google Scholar] [CrossRef]
- Zhai, H.J.; Kiran, B.; Li, J.; Wang, L.S. Hydrocarbon analogues of boron clusters--planarity, aromaticity and antiaromaticity. Nat. Mater. 2003, 2, 827–833. [Google Scholar] [CrossRef]
- Kiran, B.; Bulusu, S.; Zhai, H.J.; Yoo, S.; Zeng, X.C.; Wang, L.S. Planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc. Natl. Acad. Sci. USA 2005, 102, 961–964. [Google Scholar] [CrossRef]
- Bean, D.E.; Fowler, P.W. Double Aromaticity in “Boron Toroids”. J. Phys. Chem. C 2009, 113, 15569–15575. [Google Scholar] [CrossRef]
- Chen, Q.; Wei, G.F.; Tian, W.J.; Bai, H.; Liu, Z.P.; Zhai, H.J.; Li, S.D. Quasi-planar aromatic B36 and B36- clusters: All-boron analogues of coronene. Phys. Chem. Chem. Phys. 2014, 16, 18282–18287. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Popov, I.A.; Piazza, Z.A.; Li, W.L.; Romanescu, C.; Wang, L.S.; Boldyrev, A.I. Understanding boron through size-selected clusters: Structure, chemical bonding, and fluxionality. Acc. Chem. Res. 2014, 47, 1349–1358. [Google Scholar] [CrossRef]
- Jian, T.; Chen, X.; Li, S.D.; Boldyrev, A.I.; Li, J.; Wang, L.S. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 2019, 48, 3550–3591. [Google Scholar] [CrossRef] [PubMed]
- Casillas, R.; Baruah, T.; Zope, R.R. Geometry and electronic structure of neutral and charged B21 clusters. Chem. Phys. Lett. 2013, 557, 15–18. [Google Scholar] [CrossRef]
- Pham, H.T.; Duong, L.V.; Pham, B.Q.; Nguyen, M.T. The 2D-to-3D geometry hopping in small boron clusters: The charge effect. Chem. Phys. Lett. 2013, 577, 32–37. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhu, L.; Ma, Y. B38: An all-boron fullerene analogue. Nanoscale 2014, 6, 11692–11696. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.J.; Zhao, Y.F.; Li, W.L.; Chen, Q.; Bai, H.; Hu, H.S.; Piazza, Z.A.; Tian, W.J.; Lu, H.G.; Wu, Y.B.; et al. Observation of an all-boron fullerene. Nat. Chem. 2014, 6, 727–731. [Google Scholar] [CrossRef]
- Bai, H.; Chen, Q.; Zhai, H.J.; Li, S.D. Endohedral and exohedral metalloborospherenes: M@B40 (M=Ca, Sr) and M&B40 (M=Be, Mg). Angew. Chem. Int. Ed. 2015, 54, 941–945. [Google Scholar]
- Li, S.-X.; Zhang, Z.-P.; Long, Z.-W.; Qin, S.-J. Structures, stabilities and spectral properties of metalloborospherenes MB400/− (M = Cu, Ag, and Au). RSC Adv. 2017, 7, 38526–38537. [Google Scholar] [CrossRef]
- Dong, H.; Hou, T.; Lee, S.T.; Li, Y. New Ti-decorated B40 fullerene as a promising hydrogen storage material. Sci. Rep. 2015, 5, 9952. [Google Scholar] [CrossRef]
- An, Y.; Zhang, M.; Wu, D.; Fu, Z.; Wang, T.; Xia, C. Electronic transport properties of the first all-boron fullerene B40 and its metallofullerene Sr@B40. Phys. Chem. Chem. Phys. 2016, 18, 12024–12028. [Google Scholar] [CrossRef]
- Bai, H.; Bai, B.; Zhang, L.; Huang, W.; Mu, Y.W.; Zhai, H.J.; Li, S.D. Lithium-Decorated Borospherene B40: A Promising Hydrogen Storage Medium. Sci. Rep. 2016, 6, 35518. [Google Scholar] [CrossRef]
- Shakerzadeh, E.; Biglari, Z.; Tahmasebi, E. M@B40 (M = Li, Na, K) serving as a potential promising novel NLO nanomaterial. Chem. Phys. Lett. 2016, 654, 76–80. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, X. The hydrogen storage capacity of Sc atoms decorated porous boron fullerene B40: A DFT study. Int. J. Hydrogen Energ. 2016, 41, 16992–16999. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Z.; Long, Z.; Chen, D. Structures, Stabilities, and Spectral Properties of Endohedral Borospherenes M@B40 0/- (M = H2, HF, and H2O). ACS Omega 2019, 4, 5705–5713. [Google Scholar] [CrossRef]
- Li, S.X.; Zhang, Z.P.; Long, Z.W.; Chen, D.L. Structures, Electronic, and Spectral Properties of Doped Boron Clusters MB120/− (M = Li, Na, and K). ACS Omega 2020, 5, 20525–20534. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Li, Q.; Kolluru, V.S.C.; Rahn, M.S.; Schwenker, E.; Li, S.; Hennig, R.G.; Darancet, P.; Chan, M.K.Y.; Hersam, M.C. Synthesis of borophane polymorphs through hydrogenation of borophene. Science 2021, 371, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Li, W.L.; Piazza, Z.A.; Boldyrev, A.I.; Wang, L.S. Complexes between planar boron clusters and transition metals: A photoelectron spectroscopy and ab initio study of CoB12− and RhB12−. J. Phys. Chem. A 2014, 118, 8098–8105. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Y.; Das, A.; Dong, X.; Cui, Z.H. Lithium doped tubular structure in LiB20 and LiB20-: A viable global minimum. Phys. Chem. Chem. Phys. 2018, 20, 16202–16208. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Y.-D.; Yan, X.-H. The spin-dependent transport of transition metal encapsulated B40 fullerene. RSC Adv. 2016, 6, 40155–40161. [Google Scholar] [CrossRef]
- Saha, R.; Kar, S.; Pan, S.; Martinez-Guajardo, G.; Merino, G.; Chattaraj, P.K. A Spinning Umbrella: Carbon Monoxide and Dinitrogen Bound MB12- Clusters (M = Co, Rh, Ir). J. Phys. Chem. A 2017, 121, 2971–2979. [Google Scholar] [CrossRef]
- Li, S.-X.; Chen, D.-L.; Zhang, Z.-P.; Long, Z.-W. Ground state structures and properties of Be atom doped boron clusters BeBn (n = 10 − 15). Acta. Physica. Sinica. 2020, 69, 193101. [Google Scholar] [CrossRef]
- Cheung, L.F.; Kocheril, G.S.; Czekner, J.; Wang, L.S. Observation of Mobius Aromatic Planar Metallaborocycles. J. Am. Chem. Soc. 2020, 142, 3356–3360. [Google Scholar] [CrossRef]
- Popov, I.A.; Jian, T.; Lopez, G.V.; Boldyrev, A.I.; Wang, L.S. Cobalt-centred boron molecular drums with the highest coordination number in the CoB16− cluster. Nat. Commun. 2015, 6, 8654. [Google Scholar] [CrossRef]
- Jian, T.; Li, W.L.; Popov, I.A.; Lopez, G.V.; Chen, X.; Boldyrev, A.I.; Li, J.; Wang, L.S. Manganese-centered tubular boron cluster—MnB16 (-): A new class of transition-metal molecules. J. Chem. Phys. 2016, 144, 154310. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Li, W.L.; Bai, H.; Chen, W.J.; Dong, X.R.; Li, J.; Wang, L.S. ReB8- and ReB9-: New Members of the Transition-Metal-Centered Borometallic Molecular Wheel Family. J. Phys. Chem. A 2019, 123, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.F.; Czekner, J.; Kocheril, G.S.; Wang, L.S. ReB6-: A Metallaboron Analog of Metallabenzenes. J. Am. Chem. Soc. 2019, 141, 17854–17860. [Google Scholar] [CrossRef]
- Chen, T.T.; Li, W.L.; Chen, W.J.; Yu, X.H.; Dong, X.R.; Li, J.; Wang, L.S. Spherical trihedral metallo-borospherenes. Nat. Commun. 2020, 11, 2766. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.F.; Kocheril, G.S.; Czekner, J.; Wang, L.S. MnB6-: An Open-Shell Metallaboron Analog of 3d Metallabenzenes. J. Phys. Chem. A 2020, 124, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Kulichenko, M.; Choi, H.W.; Cavanagh, J.; Yuan, D.F.; Boldyrev, A.I.; Wang, L.S. Photoelectron Spectroscopy of Size-Selected Bismuth-Boron Clusters: BiBn− (n = 6 − 8). J. Phys. Chem. A 2021, 125, 6751–6760. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Chen, T.T.; Chen, W.J.; Li, W.L.; Li, J.; Wang, L.S. Expanded Inverse-Sandwich Complexes of Lanthanum Borides: La2B10− and La2B11. J. Phys. Chem. A 2021, 125, 2622–2630. [Google Scholar] [CrossRef]
- Barroso, J.; Pan, S.; Merino, G. Structural transformations in boron clusters induced by metal doping. Chem. Soc. Rev. 2022, 51, 1098–1123. [Google Scholar] [CrossRef] [PubMed]
- Chacko, S.; Kanhere, D.G.; Boustani, I. Ab initiodensity functional investigation of B24 clusters: Rings, tubes, planes, and cages. Phys. Rev. B 2003, 68, 035414. [Google Scholar] [CrossRef]
- Liang, W.; Das, A.; Dong, X.; Wang, M.; Cui, Z. Structural and electronic properties of MB22− (M = Na, K) clusters: Tubular boron versus quasi-planar boron forms. New J. Chem. 2019, 43, 6507–6512. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhang, L.; Lin, H.; Zhao, J.; Ma, Y. Stabilization of fullerene-like boron cages by transition metal encapsulation. Nanoscale 2015, 7, 10482–10489. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, S.X.; Chen, D.L.; Long, Z.W. Structural and Electronic Properties of Single-Atom Transition Metal-Doped Boron Clusters MB24 (M = Sc, V, and Mn). ACS Omega 2021, 6, 30442–30450. [Google Scholar] [CrossRef]
- Li, S.X.; Yang, Y.J.; Chen, D.L.; Long, Z.W. Structures, and electronic and spectral properties of single-atom transition metal-doped boron clusters MB24- (M = Sc, Ti, V, Cr, Mn, Fe, Co, and Ni). RSC Adv. 2022, 12, 16706–16716. [Google Scholar] [CrossRef]
- Dordevic, S.; Radenkovic, S. Electronic structure, stability, and aromaticity of M2B6 (M = Mg, Ca, Sr, and Ba): An interplay between spin pairing and electron delocalization. Phys. Chem. Chem. Phys. 2022, 24, 5833–5841. [Google Scholar] [CrossRef] [PubMed]
- Dordevic, S.; Radenkovic, S. Spatial and Electronic Structures of BeB8 and MgB8: How far Does the Analogy Go? Chemphyschem 2022, 23, e202200070. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Kasuya, A.; Sivamohan, R.; Barnakov, Y.A.; Dmitruk, I.M.; Nirasawa, T.; Romanyuk, V.R.; Kumar, V.; Mamykin, S.V.; Tohji, K.; Jeyadevan, B.; et al. Ultra-stable nanoparticles of CdSe revealed from mass spectrometry. Nat. Mater. 2004, 3, 99–102. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Zhu, L.; Ma, Y. Particle-swarm structure prediction on clusters. J. Chem. Phys. 2012, 137, 084104. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.-J.; Wang, L.-S. All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord. Chem. Rev. 2006, 250, 2811–2866. [Google Scholar] [CrossRef]
- Sergeeva, A.P.; Zubarev, D.Y.; Zhai, H.-J.; Boldyrev, A.I.; Wang, L.-S. A Photoelectron Spectroscopic and Theoretical Study of B16− and B162−: An All-Boron Naphthalene. J. Am. Chem. Soc. 2008, 130, 7244–7246. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Chen, Q.; Li, W.L.; Zhao, Y.F.; Zhang, S.Y.; Hu, H.S.; Bai, H.; Li, H.R.; Tian, W.J.; Lu, H.G.; Zhai, H.J.; et al. Experimental and theoretical evidence of an axially chiral borospherene. ACS Nano 2015, 9, 754–760. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Dong, X.; Jalife, S.; Vasquez-Espinal, A.; Barroso, J.; Orozco-Ic, M.; Ravell, E.; Cabellos, J.L.; Liang, W.Y.; Cui, Z.H.; Merino, G. Li2B24: The simplest combination for a three-ring boron tube. Nanoscale 2019, 11, 2143–2147. [Google Scholar] [CrossRef]
- Dong, X.; Liu, Y.Q.; Liu, X.B.; Pan, S.; Cui, Z.H.; Merino, G. Be4B12+: A Covalently Bonded Archimedean Beryllo-Borospherene. Angew. Chem. Int. Ed. 2022, 61, e202208152. [Google Scholar] [CrossRef]
- Wei, D.; Ren, M.; Lu, C.; Bi, J.; Maroulis, G. A quasi-plane IrB18- cluster with high stability. Phys. Chem. Chem. Phys. 2020, 22, 5942–5948. [Google Scholar] [CrossRef]
- Ren, M.; Jin, S.; Wei, D.; Jin, Y.; Tian, Y.; Lu, C.; Gutsev, G.L. NbB12-: A new member of half-sandwich type doped boron clusters with high stability. Phys. Chem. Chem. Phys. 2019, 21, 21746–21752. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, D.; Jin, Y.; Barroso, J.; Lu, C.; Merino, G. Exhaustive exploration of MgBn (n = 10–20) clusters and their anions. Phys. Chem. Chem. Phys. 2019, 21, 6935–6941. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Le Chen, B.; Sun, W.G.; Kuang, X.Y.; Lu, C.; Xia, X.X.; Shi, H.X.; Maroulis, G. Structural Stability and Evolution of Medium-Sized Tantalum-Doped Boron Clusters: A Half-Sandwich-Structured TaB12- Cluster. Inorg. Chem. 2018, 57, 343–350. [Google Scholar] [CrossRef]
- Li, P.; Du, X.; Wang, J.J.; Lu, C.; Chen, H. Probing the Structural Evolution and Stabilities of Medium-Sized MoBn0/– Clusters. J. Phys. Chem. C 2018, 122, 20000–20005. [Google Scholar] [CrossRef]
- Jin, S.; Chen, B.; Kuang, X.; Lu, C.; Sun, W.; Xia, X.; Gutsev, G.L. Structural and Electronic Properties of Medium-Sized Aluminum-Doped Boron Clusters AlBn and Their Anions. J. Phys. Chem. C 2019, 123, 6276–6283. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-J.; Li, S.-X.; Chen, D.-L.; Long, Z.-W. Structural Evolution and Electronic Properties of Selenium-Doped Boron Clusters SeBn0/− (n = 3–16). Molecules 2023, 28, 357. https://doi.org/10.3390/molecules28010357
Yang Y-J, Li S-X, Chen D-L, Long Z-W. Structural Evolution and Electronic Properties of Selenium-Doped Boron Clusters SeBn0/− (n = 3–16). Molecules. 2023; 28(1):357. https://doi.org/10.3390/molecules28010357
Chicago/Turabian StyleYang, Yue-Ju, Shi-Xiong Li, De-Liang Chen, and Zheng-Wen Long. 2023. "Structural Evolution and Electronic Properties of Selenium-Doped Boron Clusters SeBn0/− (n = 3–16)" Molecules 28, no. 1: 357. https://doi.org/10.3390/molecules28010357
APA StyleYang, Y.-J., Li, S.-X., Chen, D.-L., & Long, Z.-W. (2023). Structural Evolution and Electronic Properties of Selenium-Doped Boron Clusters SeBn0/− (n = 3–16). Molecules, 28(1), 357. https://doi.org/10.3390/molecules28010357






