Abstract
A N-(2-methoxy-2-oxoethyl)-N-(phenylsulfonyl)glycine monomethyl ester of the respective dicarboxylic acid was involved in a reaction with imines promoted by acetic anhydride at an elevated temperature. Instead of the initially expected δ-lactam products of the Castagnoli–Cushman-type reaction, medicinally important 3-amino-2-azetidinones were obtained as the result of cyclization, involving a methylene group adjacent to an acid moiety. In contrast, replacing alcohol residue with hexafluoroisopropyl in the same substrate made another methylene group (adjacent to the ester moiety) more reactive to furnishing the desired δ-lactam in the Castagnoli–Cushman fashion.
1. Introduction
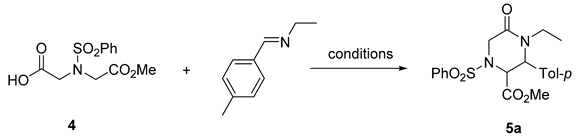
Lactams of various ring sizes (β [1], γ [2], δ [3], ε [4], denoting 4-7-membered rings, respectively, and larger [5]) represent one of the most important heterocyclic moieties employed in medicinal chemistry and drug design [6]. While the size of the lactam ring has a strong bearing on the chemical and physicochemical properties [7] of the lactam-containing compounds as well as their specific biological activity profile [8], the broadly defined lactam chemical class can be confidently defined as privileged [9], i.e., capable of delivering compounds endowed with diverse biological activities (Figure 1). This mandates the development and constant broadening of the current arsenal of synthetic methods to access lactam scaffolds, all with feasible substitution patterns for de novo biological target interrogation and the subsequent medicinal chemistry optimization of the hits emerging from biological screening campaigns.
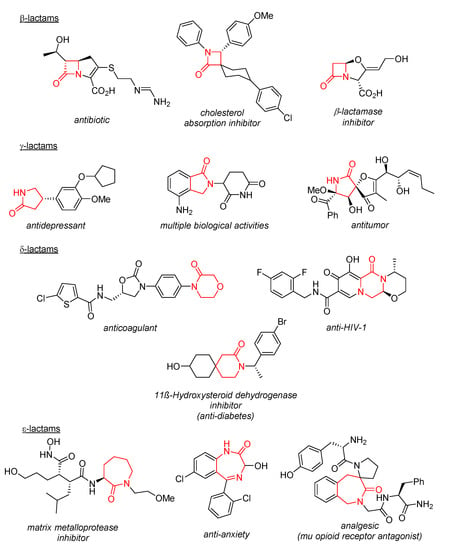
Figure 1.
Selected examples of biologically active compounds comprising lactams rings of various sizes.
Aside from conventional methods of building the lactam core, such as intramolecular amine N-acylation (lactamization) [10], intramolecular amide N-alkylation [11], carbon–carbon-bond-forming reactions [12], various annulation approaches [13,14], the oxidation of cyclic amines [15] and reduction of cyclic imides [16], the reaction of cyclic C-H anhydrides 1 with imines, dubbed the Castagnoli–Cushman reaction [17], represents an efficient way to access 5- to 7-membered [4,18] polysubstituted lactams, often in a diastereoselective fashion [19].
Recently, we demonstrated that, in the well-known synthesis of tetrahydroisoquinolonic acids from imines and homophthalic anhydride (HPA), the latter can be efficiently replaced with 2-(2-methoxy-2-oxoethyl)benzoic acid (2) (HPA monoester) activated by CDI (1,1′-carbonyldiimidazole). This allowed tetrahydroisoquinolonic esters 3 to be obtained and the Castagnoli–Cushman chemistry reaction to be planted onto a new reagent space that did not require the use of cyclic anhydrides [20]. Encouraged by this finding, we proceeded to investigate if the same approach could be extended to dicarboxylic acid monoesters, such as 4, X = NSO2Ph (Scheme 1). Herein, we report our findings obtained during the course of this investigation.
Scheme 1.
(a) The Castagnoli–Cushman reaction; (b) The use of HPA monoester in the Castagnoli–Cushman reaction [20]; (c) Reaction investigated in this work and initially proposed structure of the reaction product.
2. Results and Discussion
Our initial attempt to transfer the reaction conditions optimized for the reaction of HPA monoester 2 with imines (Scheme 1b) was not successful. Therefore, we screened for a suitable carboxylic acid activation regimen that would lead to an appreciable conversion and product yield (Table 1). Among the three other activators tried (oxalyl chloride, trifluoroacetic anhydride and acetic anhydride), only acetic anhydride gave the full conversion of 4 and the best isolated yield for the product (66%, entry 4), whose molecular weight and spectral characteristics appeared to correspond to the desired δ-lactam product 5a and demonstrated the predominant formation of one product diastereomer. Notably, the reaction turned out to be rather sensitive to thermal activation with a sharp decrease in the yield either when raising (entry 5) or reducing (entries 6–7) the temperature. Replacing chlorobenzene with other solvents (entries 8–9) did not improve the yields, while adding a base (entry 10) significantly diminished it.

Table 1.
Condition findings for the reaction of monoester 4 with (E)-N-ethyl-1-(p-tolyl)methanimine.
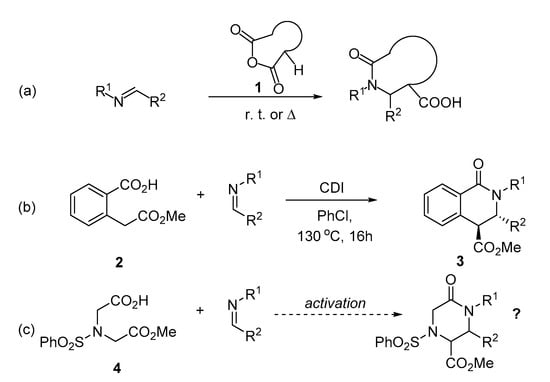
However, a single-crystal X-ray analysis of the reaction product demonstrated, to our surprise, that it was not the expected δ-lactam 5a but rather the trans-diastereomer of β-lactam product 6a (Scheme 2). While the activation of α-C-H carboxylic acids toward such a reaction by acetic anhydride (in the presence of triethylamine) has been described in the literature [21,22], the formation of the β-lactam in the absence of any base (as in our case) is hitherto undescribed. Moreover, the fact that the use of the base is detrimental to the product yield (vide supra) makes this transformation rather unique.
Scheme 2.
The actual outcome of the reaction of monoester 4 with (E)-N-ethyl-1-(p-tolyl)methanimine.
Two possible reaction pathways can be suggested for this observed transformation (Scheme 3). They both begin with a carboxylic acid activation by acetic anhydride and the formation of mixed anhydride I. This intermediate can then either directly acylate imine to form intermediate II or be converted into a ketene III via AcOH elimination (the latter can be base-promoted by imine or traces of amine from imine decomposition). Ketene III also reacts with imine to provide the intermediate II. The last step is a Mannich-type cyclization involving the methylene group adjacent to carbonyl, resulting in formation of a C-C bond and beta-lactam cycle. The selective formation of β-lactams over δ- can be explained by the increased CH acidity of the methylene group closest to the positively charged iminium fragment of intermediate II compared to the second methylene group adjacent to an ester moiety.
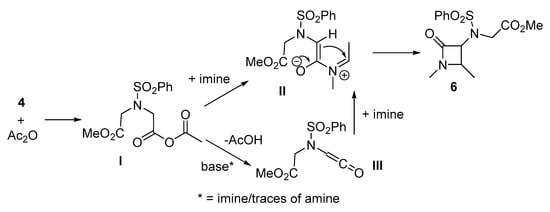
Scheme 3.
Plausible mechanistic pathways for the reaction of mono ester 4, imines and acetic anhydride.
In addition to the novelty of the discovered protocol for β-lactam synthesis, the medicinal importance of the 3-amino-2-azetidinone scaffold comprised by compound 6a is relatively clear. Indeed, it is the core of exemplary antibiotics, such as 7–10 [23] (Figure 2). This motivated us to explore the scope of the new protocol for β-lactams 6 preparation from monoester 4 and various imines using the optimized conditions (Ac2O, PhCl, 130 °C, 16 h).
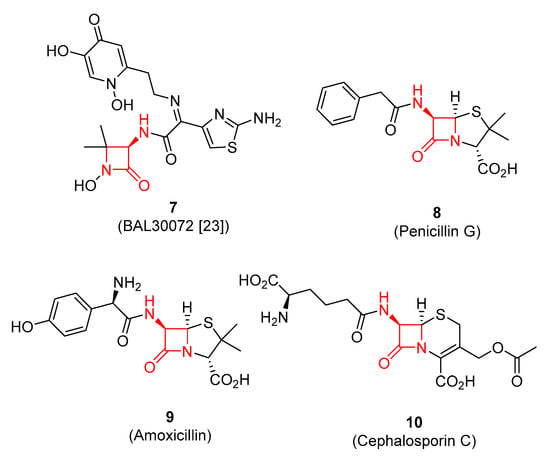
Figure 2.
Examples of antibiotics comprising 3-amino-2-azetidinone scaffold.
Following from the results presented in Scheme 4, polysubstituted 3-amino-2-azetidinones 6a–o can be synthesized in a modest-to-high yield (up to 90%) and high trans-diastereoselectivity (see also Table S1) in case of aldimines (R3 = H), as confirmed by a single-crystal X-ray analysis of two reaction products (6a and 6m). The reaction appeared to work equally well for aldimines derived from both aliphatic and aromatic amines. Symmetrical hydrazones could also be productively involved in the reaction (cf., products 6k–m). In the case of (E)-chalcones, the diastereoselectivity of the reaction deteriorated; however, this was not a detriment to the product yield. The respective diastereomers were separated by HPLC and characterized (compound 6j). The notable products are 6n and 6o, which can be viewed as building blocks for further structural complexity buildup via alkyne-azide click chemistry.
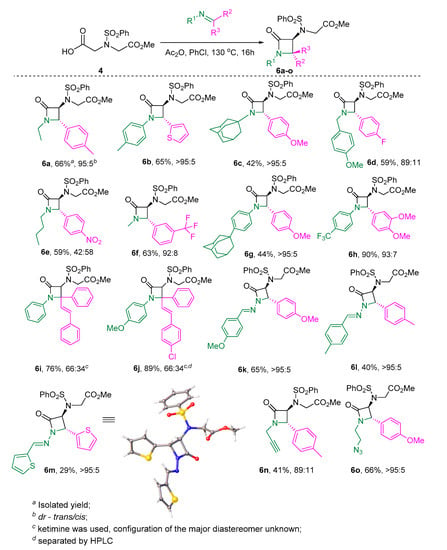
Scheme 4.
The developed novel protocol for synthesis of polysubstituted 3-amino-2-azetidinones 6a–o.
Encouraged by the results obtained with dicarboxylic acid monoester 4, we proceeded to investigate the workability of the new base-free protocol for other carboxylic acids 11a–m in combination with aldimines, aiming to obtain β-lactams 12 (Scheme 5).
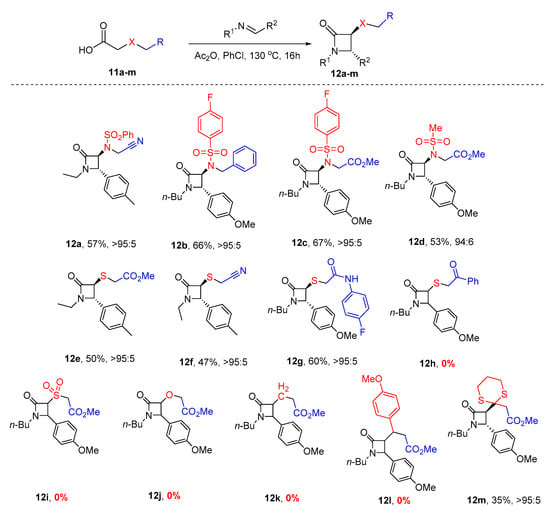
Scheme 5.
The developed novel protocol for synthesis of polysubstituted 3-amino-2-azetidinones 12.
It turned out that the sulfonylamino-substituted acetic acids 11a–d were similarly effective in the β-lactam synthesis, furnishing the respective products 12a–d with good yields and a high diastereoselectivity. When replacing the nitrogen group (X = N) with sulfur, sulfone, oxygen, and carbon linkers had a strong effect on the reaction outcome. The thia-linked carboxylic acids (11e–11g) gave β-lactams in good to fair yields except for, surprisingly, the benzoyl-substituted substrate 11h. Sulfone-, oxygen- and carbon-linked substrates did not deliver the desired products, except for 1,3-dithiane substrate 11m, which furnished the diastereomerically pure product 12m in a modest yield.
Having explored the formation of β-lactams from dicarboxylic monoester 4 and carboxylic acids 11a–m, we continued pondering the possibility of forcing substrates such as 4 to react in the Castagnoli–Cushman fashion and furnish δ-lactam products. One possibility we considered would be to increase the C-H acidity of the ‘ester arm’ of the substrate, thereby making the closure of the six-membered ring more feasible. This could be achieved by placing electron-withdrawing substituents, such as perfluoroalkyl group, in the ester moiety. In our previous work on the surrogate Castagnoli–Cushman reaction of HPA monomethyl ester 2 and its analogs, we observed a notable increase in the reactivity of the 2,2,2-trifluoroethyl (TFE) ester compared to 2 [20]. Similarly, the TFE ester was found to be more reactive towards nucleophilic addition–elimination [24]. Moreover, TFE esters have been used as versatile acylation reagents in various reactions, including transesterification [25], amidation [26], and the kinetic resolution of aliphatic amines [27]. We reasoned that, aside from TFE analog of monoester 4, the hexafluoroisopropyl (HFIP) congener [28] would be even more predisposed to react with the Castagnoli–Cushman (rather than the previously observed) fashion and yield δ-lactams. Hence, we prepared monoesters 13 (TFE) and 14 (HFIP) (see Supplementary Information) and reacted them with N-butyl-1-(4-methoxyphenyl)methanimine (15) in chlorobenzene in the presence of acetic anhydride (Scheme 6). Expectedly, 14 proved more reactive towards imine 15 compared to its TFE counterpart (13) as it required a lower temperature for the reaction to be completed. Both ester products (16 and labile 17) were hydrolyzed to their respective carboxylic acids (18 and 19), and the spectral characteristics of the latter two compounds were compared to each other to reveal that compound 16 was the product of β-lactam synthesis, while compound 17 was the desired δ-lactam formed via the Castagnoli–Cushman reaction (Scheme 5). Indeed, the signals of the vicinal methine protons in 18 resonated differently (doublets at 4.81 and 4.40 ppm) compared to the same signals in 19 (doublets at 5.06 and 4.85 ppm). Small 3J coupling constants (2.0–2.2 Hz) were indicative of the trans-configuration of both carboxylic acids [29].
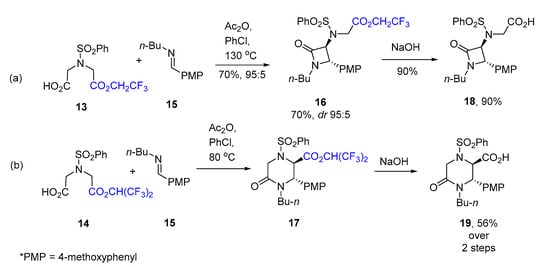
Scheme 6.
TFE (a) and HFIP (b) monoesters 13 and 14 in Ac2O-promoted reactions with imine 15.
Considering the importance of the notable reactivity switch between monoesters 13 and 14 (β-lactam synthesis vs. Castagnoli–Cushman δ-lactam synthesis), we continued scrutinizing the differences in the structures of products 18 and 19 using NMR spectroscopy after our attempts to obtain crystals suitable for X-ray crystallography failed. The unequivocal difference between these compounds was identified in their correlational HMBC spectra. Specifically, compound 18 displayed two key correlations, 3J [CH-CON] and 3J [CH2-CO2H], in contrast to compound 19, where 3J [CH2-CON] and 3J [CH-CO2H] were observable (Figure 3).
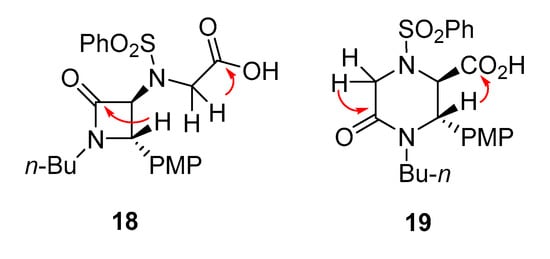
Figure 3.
Key HMBC correlations (red arrows) displayed by compounds 18 and 19.
Finally, we reasoned that β-lactam carboxylic acid 18 could be synthesized via the reaction of monomethyl ester 4 followed by the hydrolysis of ester 6p. Similarly, δ-lactam carboxylic acid 19 could be obtained via the Castagnoli–Cushman reaction of dicarboxylic acid 20 mediated by the in situ generation of the respective cyclic anhydride [18] (Scheme 7). To our delight, this synthetic strategy indeed led to the compounds whose spectral characteristics fully matched those of compounds 18 and 19 synthesized as described in Scheme 6.
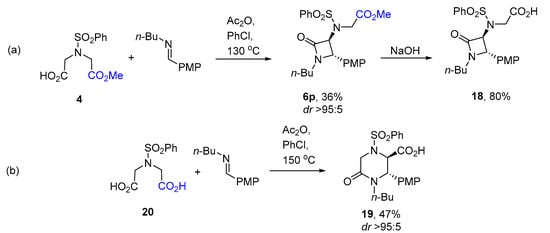
Scheme 7.
Alternative synthesis of compounds (a) 18 and (b) 19.
3. Conclusions
With the aim of involving a dicarboxylic acid monomethyl ester in the recently described Ac2O-promoted Castagnoli–Cushman-type reaction, we identified a novel protocol for β-lactam synthesis instead. A series of 25 novel compounds were prepared in 35–90% yields, mostly as single trans-diastereomers as confirmed by X-ray analysis. The type of substituent in the β-position in the carboxylic acid group of the monoester was found to be crucial for the reaction outcome, while the variation in the imine component was well-tolerated. Additionally, it was discovered that replacing the monomethyl ester with its hexafluoroisopropyl congener not only led to a reduction in the reaction temperature, but also to a marked reactivity switch as the reaction proceeded along the Castagnoli–Cushman-type pathway and furnished the respective δ-lactam. An investigation of the scope of the latter reaction is currently underway in our laboratories and will be reported on in due course.
4. Materials and Methods
NMR spectra were acquired with 400 MHz Bruker Avance III spectrometer (400.13 MHz for 1H, 376.49 MHz for 19F and 100.61 MHz for 13C) or 500 MHz Bruker Avance III (500.03 MHz for 1H and 125.73 MHz for 13C) in CDCl3 or DMSO-d6 and were referenced to residual solvent proton signals (δH = 7.26 and 2.50, respectively) and solvent carbon signals (δC = 77.16 and 39.52, respectively). Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, dd = doublet of doublets, dt = doublet of triplets, ddd = doublet/doublets of doublets; coupling constants, J, are reported in Hz. Mass spectra were acquired with an HRMS-ESI-qTOF spectrometer Nexera LCMS-9030 or MaXis II Bruker Daltonic GmbH (electrospray ionization mode, positive ions detection). IR spectrums were recorded with Fourier transform infrared Shimadzu spectrophotometer IRAffinity-1. Flash column chromatography on silica (Merck, 230–400 mesh) was performed with Biotage Isolera Prime instrument. TLC was performed on aluminium-backed pre-coated plates (0.25 mm) with silica gel 60 F254 with a suitable solvent system and was visualized using UV fluorescence. Preparative HPLC was carried out in a compact preparative system ECOM ECS28P00, equipped with spectrophotometric detector or Shimadzu LC-20AP. Column: YMC-Pack SIL-06, 5 µm, 250 × 20 mm or Agilent Zorbax prepHT XDB-C18, 5 μm, 21.2 × 150 mm. Chlorobenzene was distilled from P2O5 and stored in molecular sieves 4 Å (>24 h). 2-(Benzenesulfonyl-(cyanomethyl)amino)acetic acid (11a) was obtained from commercial sources. Synthesis of other starting materials is reported in ESI. CCDC 2,154,010 (6a) and 2,154,011 (6m) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk (accessed on 22 February 2022).
4.1. General Procedure for Preparation of Beta Lactams 6a–p, 12a–m, 16 and Their Analytical Data
In a screw-cap vial equipped with a magnetic stir bar, imine (1.1 eq) and the corresponding substituted monocarboxylic acid (0.05–1.3 mmol) were mixed in chlorobenzene (0.058 M, 1–6 mL). Then, acetic anhydride (1.1 eq) was added. The resulting mixture was placed in a pre-heated to 130 °C oil bath or metal heating block. After 16h, the mixture was cooled to room temperature, and the solvent was evaporated. The residue was purified by column chromatography in silica gel with a linear gradient (5–75%) of acetone in hexane (total volume of eluent, 400 mL) to provide pure compounds, 6a–p and 12a–m.
4.1.1. Methyl N-((3RS,4RS)-1-Ethyl-2-oxo-4-(p-tolyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6a)
Yield 96 mg, 66%; dr 95:5, yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.65–7.62 (m, 2H), 7.57–7.51 (m, 1H), 7.41 (dd, J = 8.3, 7.4 Hz, 2H), 7.41 (dd, J = 8.3, 7.4 Hz, 2H), 7.21 (d, J = 8.6 Hz, 2H), 4.85 (d, J = 1.8 Hz, 1H), 4.41 (d, J = 1.0 Hz, 1H), 4.26 (d, J = 18.3 Hz, 1H), 4.15 (d, J = 18.3 Hz, 1H), 3.69 (s, 3H), 3.54–3.44 (m, 1H), 2.83 (dqd, J = 14.3, 7.2, 0.9 Hz, 1H), 2.38 (s, 3H), 1.07 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 163.7, 138.7, 138.3, 133.2, 132.7, 129.6, 129.1, 127.5, 126.9, 71.6, 62.5, 52.4, 48.0, 35.4, 21.3, 12.6. HRMS (ESI) m/z: [M + Na]+ Calcd for C21H23N2O5NaS+ 439.1298; found 439.1307.
4.1.2. Methyl N-((3RS,4SR)-2-Oxo-4-(thiophen-2-yl)-1-(p-tolyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6b)
Yield 107 mg, 65%; dr > 95:5, dark orange solid. 1H NMR (500 MHz, CDCl3) δ 7.70 (dd, J = 8.4, 1.3 Hz, 2H), 7.62–7.55 (m, 1H), 7.46 (dd, J = 8.3, 7.4 Hz, 2H), 7.34 (dd, J = 5.0, 3.0 Hz, 1H), 7.29 (dd, J = 3.0, 1.3 Hz, 1H), 7.17 (d, J = 8.4 Hz, 2H), 7.09–7.03 (m, 3H), 5.43 (d, J = 2.1 Hz, 1H), 4.63 (d, J = 2.1 Hz, 1H), 4.31 (d, J = 18.4 Hz, 1H), 4.16 (d, J = 18.4 Hz, 1H), 3.67 (s, 3H), 2.27 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 160.8, 138.3, 137.4, 134.7, 134.4, 133.5, 129.8, 129.3, 127.7, 127.2, 125.7, 123.7, 117.8, 71.5, 59.5, 52.6, 48.2, 21.1. HRMS (ESI) m/z: [M + H]+ Calcd for C23H23N2O5S2+ 471.1043; found 471.1050.
4.1.3. Methyl N-((2RS,3RS)-1-(Adamantan-1-yl)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6c)
Yield 80 mg, 42%; dr > 95:5, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.64–7.55 (m, 2H), 7.53–7.47 (m, 1H), 7.39 (t, J = 7.8 Hz, 2H), 7.26 (d, J = 8.3 Hz, 2H), 6.86 (d, J = 8.8 Hz, 2H), 5.10 (d, J = 4.9 Hz, 1H), 4.88 (d, J = 4.9 Hz, 1H), 3.96 (d, J = 18.5 Hz, 1H), 3.88 (d, J = 18.5 Hz, 1H), 3.82 (s, 3H), 3.48 (s, 3H), 1.98 (br.s, 3H), 1.92 (br.s, 6H), 1.62–1.51 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 168.9, 163.4, 159.8, 139.2, 132.9, 129.5, 128.8, 128.4, 127.9, 113.8, 65.7, 60.6, 56.0, 55.3, 52.2, 49.3, 40.9, 36.1, 29.2. HRMS (ESI) m/z: [M + H]+ Calcd for C29H35N2O6S+ 539.2210; found 539.2219.
4.1.4. Methyl N-((2RS,3RS)-2-(4-Fluorophenyl)-1-(4-methoxybenzyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6d)
Yield 105 mg, 59%; dr 89:11, yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.64–7.58 (m, 2H), 7.54 (t, J = 7.5 Hz, 1H), 7.40 (t, J = 7.9 Hz, 2H), 7.17 (dd, J = 8.7, 5.3 Hz, 2H), 7.06 (t, J = 8.6 Hz, 2H), 7.00 (d, J = 8.6 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 4.72 (d, J = 14.8 Hz, 1H), 4.62 (d, J = 1.9 Hz, 1H), 4.46 (d, J = 1.5 Hz, 1H), 4.22 (d, J = 18.4 Hz, 1H), 4.05 (d, J = 18.4 Hz, 1H), 3.78 (s, 3H), 3.68 (d, J = 14.8 Hz, 1H), 3.60 (s, 3H).13C NMR (126 MHz, CDCl3) δ 169.6, 163.8, 163.0 (d, J = 247.7 Hz), 159.4, 138.4, 133.4, 131.4 (d, J = 3.2 Hz), 130.0, 129.2, 128.9 (d, J = 8.2 Hz), 127.7, 126.8, 116.1 (d, J = 21.6 Hz), 114.3, 72.0, 61.6, 55.4, 52.5, 47.9, 44.3. 19F NMR (376 MHz, CDCl3) δ-112.7. HRMS (ESI) m/z: [M + H]+ Calcd for C26H26FN2O6S+ 513.1490; found 513.1494.
4.1.5. Methyl N-((2RS,3RS)-1-Butyl-2-(4-nitrophenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6e)
Yield 98 mg, 59%; dr 42:58, dark orange oil. 1H NMR (500 MHz, CDCl3) δ 8.28 (d, J = 8.7 Hz, 2H), 8.25 (d, J = 8.7 Hz, 2H), 7.71–7.67 (m, 2H), 7.64 (dd, J = 8.5, 1.3 Hz, 2H), 7.62–7.54 (m, 4H), 7.53–7.50 (m, 2H), 7.49–7.47 (m, 2H), 7.46–7.42 (m, 2H), 5.26 (d, J = 4.7 Hz, 1H), 5.08 (d, J = 1.9 Hz, 1H), 4.97 (d, J = 4.8 Hz, 1H), 4.35 (d, J = 1.8 Hz, 1H), 4.26 (d, J = 18.4 Hz, 1H), 4.16 (d, J = 18.4 Hz, 1H), 3.99 (d, J = 18.3 Hz, 1H), 3.79 (d, J = 18.3 Hz, 1H), 3.72 (s, 3H), 3.61–3.49 (m, 2H), 3.48 (s, 3H), 2.94 (ddd, J = 13.8, 7.4, 6.2 Hz, 1H), 2.81–2.72 (m, 1H), 1.55–1.39 (m, 4H), 1.33–1.26 (m, 6H), 0.88 (q, J = 7.3 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 170.0, 168.4, 163.6, 163.3, 148.3, 148.3, 143.5, 141.8, 138.3, 138.2, 133.6, 133.5, 129.5, 129.4, 129.1, 128.0, 127.5, 127.4, 124.3, 123.8, 72.2, 68.1, 62.8, 62.1, 52.7, 52.3, 49.2, 48.2, 41.3, 40.9, 29.6, 29.3, 20.3, 20.2, 13.6, 13.6. HRMS (ESI) m/z: [M + H]+ Calcd for C22H26N3O7S+ 476.1486; found 476.1487.
4.1.6. Methyl N-((3RS,4RS)-1-Methyl-2-oxo-4-(3-(trifluoromethyl)phenyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6f)
Yield 101 mg, 63%; dr 92:8, light yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.67–7.61 (m, 3H), 7.60–7.47 (m, 4H), 7.45–7.37 (m, 2H), 4.91 (d, J = 1.9 Hz, 1H), 4.43 (d, J = 0.9 Hz, 1H), 4.27 (d, J = 18.4 Hz, 1H), 4.14 (d, J = 18.3 Hz, 1H), 3.71 (s, 3H), 2.77 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.9, 164.0, 138.3, 136.8, 133.5, 131.5 (q, J = 32.5 Hz), 130.5, 129.7, 129.3, 127.6, 125.9 (q, J = 3.6 Hz), 125.0, 124.0 (q, J = 272.6 Hz), 123.7 (q, J = 3.8 Hz), 72.8, 64.5, 52.6, 48.2, 27.4. 19F NMR (376 MHz, CDCl3) δ-62.6. HRMS (ESI) m/z: [M + H]+ Calcd for C20H20F3N2O5S+ 457.1040; found 457.1041.
4.1.7. Methyl N-((2RS,3RS)-1-(4-(Adamantan-1-yl)phenyl)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6g)
Yield 94 mg, 44%; dr > 95:5, yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.70–7.66 (m, 2H), 7.60–7.54 (m, 1H), 7.48–7.40 (m, 2H), 7.28 (d, J = 8.7 Hz, 2H), 7.23–7.17 (m, 4H), 6.90 (d, J = 8.7 Hz, 2H), 5.24 (d, J = 2.2 Hz, 1H), 4.65 (d, J = 2.2 Hz, 1H), 4.31 (d, J = 18.4 Hz, 1H), 4.11 (d, J = 18.4 Hz, 1H), 3.82 (s, 3H), 3.68 (s, 3H), 2.08–2.03 (m, 3H), 1.81 (d, J = 2.9 Hz, 6H), 1.79–1.73 (m, 3H), 1.72–1.66 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 161.4, 160.0, 148.1, 138.4, 134.3, 133.4, 129.2, 128.0, 127.7, 125.6, 119.9, 117.6, 114.5, 72.3, 62.9, 55.4, 52.6, 48.0, 43.2, 36.8, 36.0, 29.0. HRMS (ESI) m/z: [M + H]+ Calcd for C35H39N2O6S+ 615.2523; found 615.2520.
4.1.8. Methyl N-((2RS,3RS)-2-(3,4-Dimethoxyphenyl)-4-oxo-1-(4-(trifluoromethyl)phenyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6h)
Yield 181 mg, 90%; dr 93:7, yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.72–7.69 (m, 2H), 7.62–7.56 (m, 1H), 7.50 (d, J = 8.6 Hz, 2H), 7.48–7.44 (m, 2H), 7.37 (d, J = 8.5 Hz, 2H), 6.92–6.84 (m, 3H), 5.35 (d, J = 2.3 Hz, 1H), 4.62 (d, J = 2.3 Hz, 1H), 4.28 (d, J = 18.4 Hz, 1H), 4.12 (d, J = 18.4 Hz, 1H), 3.90 (s, 3H), 3.86 (s, 3H), 3.67 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 162.2, 149.7 (d, J = 3.9 Hz), 139.6, 138.2, 133.6, 129.3, 127.7, 127.4, 126.6 (d, J = 32.8 Hz), 126.5 (q, J = 3.6 Hz), 124.0 (d, J = 271.7 Hz), 119.5, 118.9, 117.8, 111.6, 109.6, 72.8, 64.0, 56.2, 56.1, 52.7, 48.4. 19F NMR (376 MHz, CDCl3) δ-62.3. HRMS (ESI) m/z: [M + H]+ Calcd for C27H26FN2O7S+ 579.1407; found 579.1411.
4.1.9. Methyl (E)-N-(4-Oxo-1,2-diphenyl-2-styrylazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6i)
Yield 29 mg, 76%; dr 66:34, dark orange oil. 1H NMR (500 MHz, CDCl3) δ 7.90–7.79 (m, 2H), 7.66 (d, J = 7.4 Hz, 2H), 7.60 (td, J = 7.6, 1.6 Hz, 2H), 7.54–7.46 (m, 3H), 7.46–7.27 (m, 26H), 7.20 (t, J = 8.0 Hz, 2H), 7.16–6.99 (m, 7H), 6.91 (d, J = 16.1 Hz, 1H), 6.70 (d, J = 16.2 Hz, 1H), 6.65 (d, J = 16.2 Hz, 1H), 5.28 (d, J = 1.0 Hz, 1H), 4.97 (s, 1H), 4.36 (d, J = 18.5 Hz, 1H), 4.04 (d, J = 18.5 Hz, 1H), 3.73 (d, J = 18.5 Hz, 1H), 3.50 (s, 3H), 3.47 (s, 3H), 3.28 (d, J = 18.5 Hz, 1H), 2.27 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 169.0, 161.2, 161.0, 139.5, 138.2, 137.8, 136.7, 136.7, 136.6, 135.9, 135.9, 135.1, 133.5, 133.3, 129.7, 129.2, 129.2, 129.1, 129.1, 129.0, 129.0, 128.9, 128.8, 128.7, 128.6, 128.6, 128.6, 128.3, 128.2, 128.1, 127.4, 127.1, 127.0, 126.3, 125.9, 124.8, 124.7, 124.0, 119.0, 118.6, 72.8, 70.9, 52.2, 52.2, 49.4, 48.1, 19.9. HRMS (ESI) m/z: [M + Na]+ Calcd for C32H28N2O5S+ 575.1611; found 575.1614.
4.1.10. Methyl (E)-N-(2-(4-Chlorostyryl)-1-(4-methoxyphenyl)-4-oxo-2-phenylazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6j)
Yield 39 mg, 89% (after flash chromatography); dr 66:34, yellow oil. This mixture was further separated by preparative HPLC on silica gel column YMC-Pack SIL-06, 5 µm, 250 × 20 mm (mobile phase hexane-acetone, 5–50% of acetone, total volume 600 mL; flow 20 mL/min, 25 °C) to afford pure diastereomers. Major isomer (16 mg): 1H NMR (500 MHz, CDCl3) δ 7.59–7.55 (m, 2H), 7.54–7.48 (m, 1H), 7.39–7.29 (m, 7H), 7.26 (d, J = 1.5 Hz, 4H), 7.21–7.17 (m, 2H), 7.00 (d, J = 16.3 Hz, 1H), 6.66 (d, J = 9.1 Hz, 2H), 6.52 (d, J = 16.2 Hz, 1H), 4.82 (s, 1H), 4.26 (d, J = 18.6 Hz, 1H), 3.92 (d, J = 18.5 Hz, 1H), 3.66 (s, 3H), 3.43 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.0, 160.2, 156.5, 138.0, 137.7, 134.5, 134.4, 133.5, 132.2, 130.1, 129.2, 129.1, 129.1, 128.7, 128.3, 128.2, 128.1, 126.4, 125.1, 119.9, 114.3, 70.9, 55.5, 52.3, 49.4. Minor isomer (7 mg): 1H NMR (500 MHz, CDCl3) δ 7.83 (dd, J = 8.4, 1.3 Hz, 2H), 7.64–7.57 (m, 1H), 7.53–7.46 (m, 4H), 7.46–7.39 (m, 5H), 7.30 (s, 4H), 6.87 (d, J = 16.2 Hz, 1H), 6.80 (d, J = 9.2 Hz, 2H), 6.62 (d, J = 16.2 Hz, 1H), 5.23 (d, J = 1.0 Hz, 1H), 3.76 (s, 3H), 3.68 (d, J = 18.5 Hz, 1H), 3.44 (s, 3H), 3.27 (d, J = 18.6 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 168.5, 160.4, 156.6, 139.5, 135.1, 134.4, 134.3, 133.4, 132.0, 130.1, 129.1, 129.1, 129.0, 128.8, 128.2, 128.2, 128.2, 128.1, 120.2, 114.4, 72.7, 55.6, 52.2, 48.1. HRMS (ESI) m/z: [M + H]+ Calcd for C33H30ClN2O6S+ 617.1508; found 617.1508.
4.1.11. Methyl N-((2RS,3RS)-1-(((E)-4-Methoxybenzylidene)amino)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6k)
Yield 25 mg, 65%; dr > 95:5, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.66 (dd, J = 8.5, 1.3 Hz, 2H), 7.62 (s, 1H), 7.57 (tt, J = 6.9, 1.2 Hz, 1H), 7.51 (d, J = 8.9 Hz, 2H), 7.44 (t, J = 7.9 Hz, 2H), 7.28 (d, J = 8.7 Hz, 2H), 6.92 (d, J = 8.7 Hz, 2H), 6.83 (d, J = 8.9 Hz, 2H), 5.40 (d, J = 2.0 Hz, 1H), 4.44 (d, J = 2.1 Hz, 1H), 4.33 (d, J = 18.5 Hz, 1H), 4.19 (d, J = 18.5 Hz, 1H), 3.83 (s, 3H), 3.80 (s, 3H), 3.70 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 169.9, 162.1, 160.2, 159.7, 147.7, 138.3, 133.5, 129.6, 129.3, 128.0, 127.7, 126.2, 125.9, 114.7, 114.3, 70.1, 66.6, 55.5, 55.5, 52.7, 48.1. HRMS (ESI) m/z: [M + H]+ Calcd for C27H28N3O7S+ 538.1642; found 538.1647.
4.1.12. Methyl (E)-N-(1-((4-Methylbenzylidene)amino)-2-oxo-4-(p-tolyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6l)
Yield 14 mg, 40%; dr > 95:5, yellow oil. 1H NMR (500 MHz, DMSO-d6 +CDCl3) δ 7.62–7.59 (m, 2H), 7.55 (t, J = 7.5 Hz, 1H), 7.51 (s, 1H), 7.40 (t, J = 7.8 Hz, 2H), 7.35 (d, J = 8.1 Hz, 2H), 7.13 (s, 4H), 7.06 (d, J = 8.1 Hz, 2H), 5.26 (d, J = 2.1 Hz, 1H), 4.47 (d, J = 2.0 Hz, 1H), 4.20 (d, J = 18.4 Hz, 1H), 4.05 (d, J = 18.4 Hz, 1H), 3.61 (s, 3H), 2.29 (s, 3H), 2.25 (s, 3H). 13C NMR (126 MHz, DMSO-d6 +CDCl3) δ 168.8, 159.3, 146.9, 140.7, 138.2, 137.7, 132.9, 130.5, 129.6, 129.3, 128.8, 128.7, 127.1, 126.8, 125.8, 69.6, 65.5, 51.9, 47.3, 20.9, 20.7. HRMS (ESI) m/z: [M + H]+ Calcd for C27H28N3O5S+ 506.1744; found 506.1750.
4.1.13. Methyl N-((3RS,4SR)-2-Oxo-4-(thiophen-2-yl)-1-(((E)-thiophen-2-ylmethylene)amino) azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6m)
Yield 10 mg, 29%; dr > 95:5, brown oil. 1H NMR (500 MHz, CDCl3) δ 8.12 (s, 1H), 7.72 (dd, J = 8.5, 1.2 Hz, 2H), 7.64–7.56 (m, 1H), 7.47 (t, J = 7.9 Hz, 2H), 7.39 (dt, J = 5.1, 1.0 Hz, 1H), 7.37 (dd, J = 5.1, 1.2 Hz, 1H), 7.20 (td, J = 3.7, 1.4 Hz, 2H), 7.06 (dd, J = 5.1, 3.6 Hz, 1H), 7.01 (dd, J = 5.0, 3.7 Hz, 1H), 5.74 (d, J = 2.1 Hz, 1H), 4.57 (d, J = 2.0 Hz, 1H), 4.32 (d, J = 18.4 Hz, 1H), 4.20 (d, J = 18.4 Hz, 1H), 3.70 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 183.1, 169.8, 159.4, 143.1, 138.2, 138.1, 137.6, 133.6, 131.8, 129.9, 129.4, 127.7, 127.7, 127.3, 126.7, 70.8, 63.3, 52.8, 48.3. HRMS (ESI) m/z: [M + H]+ Calcd for C21H20N3O5S3+ 490.0560; found 490.0571.
4.1.14. Methyl N-((3RS,4RS)-2-Oxo-1-(prop-2-yn-1-yl)-4-(p-tolyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (6n)
Yield 61 mg, 41%; dr 89:11, dark brown oil. 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 8.5, 1.3 Hz, 2H), 7.58–7.52 (m, 1H), 7.43–7.36 (m, 2H), 7.19 (q, J = 8.1 Hz, 4H), 4.92 (d, J = 2.1 Hz, 1H), 4.58 (d, J = 2.1 Hz, 1H), 4.33–4.24 (m, 2H), 4.10 (d, J = 18.4 Hz, 1H), 3.70 (s, 3H), 3.53 (ddd, J = 17.8, 2.5, 0.9 Hz, 1H), 2.38 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.5, 163.9, 139.0, 138.4, 133.3, 132.0, 129.8, 129.2, 127.7, 127.0, 76.0, 73.0, 72.2, 62.6, 52.6, 48.0, 30.2, 21.4. HRMS (ESI) m/z: [M + H]+ Calcd for C22H23N2O5S+ 427.1322; found 427.1328.
4.1.15. Methyl N-((2RS,3RS)-1-(2-Azidoethyl)-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6o)
Yield 22 mg, 66%; dr > 95:5, orange oil. 1H NMR (500 MHz, CDCl3) δ 7.66 (dd, J = 8.5, 1.3 Hz, 2H), 7.58–7.53 (m, 1H), 7.42 (td, J = 7.6, 1.7 Hz, 2H), 7.23–7.17 (m, 2H), 6.95–6.91 (m, 2H), 4.90 (d, J = 2.0 Hz, 1H), 4.52 (d, J = 1.9 Hz, 1H), 4.29 (d, J = 18.3 Hz, 1H), 4.14 (d, J = 18.3 Hz, 1H), 3.84 (s, 3H), 3.71 (s, 3H), 3.60 (ddd, J = 14.5, 7.2, 5.4 Hz, 1H), 3.40 (ddd, J = 6.8, 6.0, 5.1 Hz, 2H), 2.92 (dddd, J = 14.6, 6.6, 5.0, 0.7 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 169.9, 164.6, 160.3, 138.5, 133.4, 129.2, 128.4, 127.7, 127.2, 114.6, 72.3, 63.4, 55.5, 52.6, 49.0, 48.1, 39.7. HRMS (ESI) m/z: [M + H]+ Calcd for C21H24N5O6S+ 474.1442; found 474.1442.
4.1.16. Methyl N-((2RS,3RS)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycinate (6p)
Yield 58 mg, 36%, dr > 95:5, orange oil. 1H NMR (400 MHz, CDCl3) δ 7.64–7.59 (m, 2H), 7.53 (q, J = 7.1 Hz, 1H), 7.40 (t, J = 7.8 Hz, 2H), 7.19 (d, J = 8.7 Hz, 2H), 6.92 (d, J = 8.7 Hz, 2H), 4.80 (d, J = 2.0 Hz, 1H), 4.40 (d, J = 2.0 Hz, 1H), 4.28 (d, J = 18.3 Hz, 1H), 4.16 (d, J = 18.3 Hz, 1H), 3.84 (s, 3H), 3.69 (s, 3H), 3.43 (dt, J = 14.6, 7.6 Hz, 1H), 2.72 (dt, J = 14.0, 6.7 Hz, 1H), 1.41 (p, J = 7.5 Hz, 2H), 1.26 (dddd, J = 9.9, 7.3, 5.8, 2.1 Hz, 2H), 0.85 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 169.8, 163.9, 160.1, 138.5, 133.3, 129.1, 128.4, 127.7, 127.6, 114.4, 71.8, 62.8, 55.5, 52.5, 48.1, 40.3, 29.6, 20.2, 13.6. HRMS (ESI) m/z: [M + H]+ Calcd for C23H29N2O6S+ 461.1741; found 461.1746.
4.1.17. N-(Cyanomethyl)-N-((3RS,4RS)-1-ethyl-2-oxo-4-(p-tolyl)azetidin-3-yl)benzenesulfonamide (12a)
Yield 85 mg, 57%; dr > 95:5, dark brown oil. 1H NMR (400 MHz, CDCl3) δ 7.64–7.54 (m, 3H), 7.46–7.39 (m, 2H), 7.22 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 4.79 (d, J = 2.0 Hz, 1H), 4.66 (d, J = 18.3 Hz, 1H), 4.59 (d, J = 2.1 Hz, 1H), 4.29 (d, J = 18.5 Hz, 1H), 3.52 (dq, J = 14.8, 7.4 Hz, 1H), 2.92–2.80 (m, 1H), 2.41 (s, 3H), 1.10 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 162.8, 139.4, 137.1, 134.1, 132.1, 129.9, 129.6, 127.9, 127.1, 114.9, 71.2, 60.6, 35.8, 34.2, 21.4, 12.7. HRMS (ESI) m/z: [M + H]+ Calcd for C20H22N3O3S+ 384.1376; found 384.1374.
4.1.18. N-Benzyl-N-((2RS,3RS)-1-butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-4-fluorobenzenesulfonamide (12b)
Yield 101 mg, 66%; dr > 95:5, yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.89 (ddd, J = 8.9, 5.0, 2.5 Hz, 2H), 7.36 (dd, J = 7.7, 1.9 Hz, 2H), 7.33–7.26 (m, 3H), 7.15 (t, J = 8.6 Hz, 2H), 6.95 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 4.58 (d, J = 15.5 Hz, 1H), 4.53 (d, J = 1.7 Hz, 1H), 4.29 (d, J = 15.5 Hz, 1H), 4.16 (d, J = 2.1 Hz, 1H), 3.80 (s, 3H), 3.30 (dt, J = 14.5, 7.4 Hz, 1H), 2.61 (dt, J = 13.8, 6.7 Hz, 1H), 1.20 (q, J = 7.0 Hz, 2H), 1.13 (dt, J = 13.6, 6.9 Hz, 2H), 0.79 (t, J = 7.2 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 165.3 (d, J = 255.1 Hz), 165.0, 160.0, 136.4, 135.6 (d, J = 3.3 Hz), 130.4 (d, J = 9.4 Hz), 128.8, 128.4, 128.3, 127.8, 127.5, 116.4 (d, J = 22.5 Hz), 114.5, 72.6, 62.5, 55.4, 51.0, 40.2, 29.6, 20.2, 13.7. 19F NMR (376 MHz, CDCl3) δ-104.9. HRMS (ESI) m/z: [M + H]+ Calcd for C27H30FN2O4S+ 497.1905; found 497.1908.
4.1.19. Methyl N-((2RS,3RS)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-((2-nitrophenyl)sulfonyl)glycinate (12c)
Yield 214 mg, 67%; dr > 95:5, yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.72–7.62 (m, 3H), 7.53 (ddd, J = 8.4, 6.9, 1.8 Hz, 1H), 7.24 (d, J = 8.7 Hz, 2H), 6.96 (d, J = 8.7 Hz, 2H), 4.92 (d, J = 1.9 Hz, 1H), 4.57 (d, J = 1.8 Hz, 1H), 4.45 (d, J = 18.7 Hz, 1H), 4.38 (d, J = 18.8 Hz, 1H), 3.87 (s, 3H), 3.76 (s, 3H), 3.47 (dt, J = 14.7, 7.6 Hz, 1H), 2.76 (dt, J = 13.6, 6.6 Hz, 1H), 1.51–1.38 (m, 2H), 1.36–1.23 (m, 2H), 0.89 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.0, 163.4, 160.3, 148.2, 134.1, 132.2, 131.9, 131.4, 128.4, 127.4, 124.6, 114.5, 71.7, 62.5, 55.5, 52.6, 48.6, 40.4, 29.6, 20.2, 13.7. HRMS (ESI) m/z: [M + H]+ Calcd for C23H28N3O8S+ 506.1592; found 506.1593.
4.1.20. Methyl N-((2RS,3RS)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(methylsulfonyl)glycinate (12d)
Yield 94 mg, 53%; dr 94:6, light yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 6.92 (d, J = 8.7 Hz, 2H), 4.75 (d, J = 2.0 Hz, 1H), 4.39 (d, J = 1.9 Hz, 1H), 4.24 (d, J = 18.6 Hz, 1H), 4.09 (d, J = 18.6 Hz, 1H), 3.81 (s, 3H), 3.73 (s, 3H), 3.47 (dt, J = 14.0, 7.6 Hz, 1H), 3.05 (s, 3H), 2.75 (dt, J = 13.7, 6.7 Hz, 1H), 1.47–1.39 (m, 2H), 1.34–1.23 (m, 2H), 0.86 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.2, 164.3, 160.2, 128.0, 127.6, 114.6, 72.7, 62.8, 55.4, 52.6, 49.0, 41.3, 40.2, 29.6, 20.2, 13.7. HRMS (ESI) m/z: [M + H]+ Calcd for C18H27N2O6S+ 399.1584; found 399.1590.
4.1.21. Methyl 2-(((3RS,4RS)-1-Ethyl-2-oxo-4-(p-tolyl)azetidin-3-yl)thio)acetate (12e)
Yield 189 mg, 50%; dr > 95:5, orange oil. 1H NMR (400 MHz, CDCl3) δ 7.20 (s, 4H), 4.46 (d, J = 2.1 Hz, 1H), 3.98 (d, J = 2.3 Hz, 1H), 3.67 (s, 3H), 3.56–3.44 (m, 2H), 3.38 (d, J = 15.3 Hz, 1H), 2.92 (dq, J = 14.3, 7.2 Hz, 1H), 2.35 (s, 3H), 1.08 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.5, 165.5, 139.0, 133.6, 129.8, 126.6, 62.9, 58.6, 52.6, 35.9, 32.6, 21.3, 12.9. HRMS (ESI) m/z: [M + H]+ Calcd for C15H20NO3S+ 294.1158; found 294.1163.
4.1.22. 2-(((3RS,4RS)-1-Ethyl-2-oxo-4-(p-tolyl)azetidin-3-yl)thio)acetonitrile (12f)
Yield 120 mg, 47%; dr > 95:5, light brown oil. 1H NMR (500 MHz, CDCl3) δ 7.29–7.19 (m, 4H), 4.66 (d, J = 2.0 Hz, 1H), 4.06 (d, J = 1.6 Hz, 1H), 3.63–3.45 (m, 3H), 2.99 (dqd, J = 14.5, 7.2, 0.9 Hz, 1H), 2.39 (s, 3H), 1.14 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 164.3, 139.4, 133.0, 130.0, 126.6, 116.8, 62.4, 58.1, 36.2, 21.3, 15.6, 12.8. HRMS (ESI) m/z: [M + Na]+ Calcd for C14H16N2NaOS+ 283.0876; found 283.0877.
4.1.23. 2-(((2RS,3RS)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)thio)-N-(4-fluorophenyl)acetamide (12g)
Yield 102 mg, 60%; dr > 95:5, light brown oil. 1H NMR (500 MHz, CDCl3) δ 9.23 (s, 1H), 7.63–7.54 (m, 2H), 7.23–7.18 (m, 2H), 7.06–6.97 (m, 2H), 6.95–6.87 (m, 2H), 4.37 (d, J = 2.1 Hz, 1H), 3.91 (dd, J = 2.1, 0.8 Hz, 1H), 3.81 (s, 3H), 3.63 (d, J = 15.5 Hz, 1H), 3.51–3.41 (m, 1H), 3.37 (d, J = 15.6 Hz, 1H), 2.85 (dt, J = 13.7, 6.7 Hz, 1H), 1.45 (dt, J = 14.5, 7.3 Hz, 2H), 1.39–1.21 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 167.2, 166.8, 160.5, 160.5, 159.5 (d, J = 243.2 Hz), 134.2 (d, J = 2.6 Hz), 127.9, 127.5, 121.7 (d, J = 8.0 Hz), 115.6 (d, J = 22.4 Hz), 114.7, 62.8, 58.3, 55.5, 41.0, 36.1, 29.6, 20.2, 13.6. 19F NMR (376 MHz, CDCl3) δ-118.1. HRMS (ESI) m/z: [M + Na]+ Calcd for C22H25FN2NaO3S+ 439.1462; found 439.1462.
4.1.24. Methyl 2-(2-((2RS,3SR)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-1,3-dithian-2-yl)acetate (12m)
Yield 18 mg, 35%; light yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.30 (d, J = 8.7 Hz, 2H), 6.90 (d, J = 8.7 Hz, 2H), 4.87 (d, J = 2.2 Hz, 1H), 3.93 (d, J = 2.0 Hz, 1H), 3.81 (s, 3H), 3.60 (s, 3H), 3.46 (d, J = 14.5 Hz, 1H), 3.35 (d, J = 14.5 Hz, 1H), 3.08 (ddd, J = 14.3, 12.1, 2.0 Hz, 2H), 2.88 (dt, J = 14.5, 3.9 Hz, 1H), 2.76–2.62 (m, 2H), 2.12 (dtd, J = 13.5, 5.4, 2.9 Hz, 1H), 1.96–1.84 (m, 1H), 1.47 (ddt, J = 11.7, 9.7, 3.4 Hz, 2H), 1.41–1.30 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 169.2, 165.7, 159.8, 129.5, 128.3, 114.4, 65.8, 57.4, 55.4, 51.9, 49.1, 42.9, 40.1, 29.9, 26.3, 26.3, 24.5, 20.4. HRMS (ESI) m/z: [M + H]+ Calcd for C21H30NNaO4S2+ 424.1611; found 424.1615.
4.1.25. 2,2,2-Trifluoroethyl N-((3RS,4RS)-1-butyl-2-oxo-4-(p-tolyl)azetidin-3-yl)-N-(phenylsulfonyl)glycinate (16)
Yield 109 mg, 70%; dr > 95:5, light yellow oil. 1H NMR (400 MHz, CDCl3) δ 7 7.61–7.51 (m, 3H), 7.45–7.37 (m, 2H), 7.21 (d, J = 8.7 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.77 (d, J = 2.0 Hz, 1H), 4.58–4.41 (m, 2H), 4.38 (d, J = 18.6 Hz, 1H), 4.37 (d, J = 1.8 Hz, 1H), 4.30 (d, J = 18.6 Hz, 1H), 3.85 (s, 3H), 3.43 (dt, J = 14.8, 7.6 Hz, 1H), 2.73 (dt, J = 13.6, 6.8 Hz, 1H), 1.41 (p, J = 7.5 Hz, 2H), 1.32–1.20 (m, 2H), 0.85 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 168.2, 163.6, 160.2, 138.0, 133.5, 129.3, 128.4, 127.6, 127.4, 122.8 (q, J = 277.0 Hz), 114.5, 71.4, 62.9, 61.1 (q, J = 37.0 Hz), 55.5, 47.8, 40.4, 29.6, 20.2, 13.6. 19F NMR (376 MHz, CDCl3) δ-73.6. HRMS (ESI) m/z: [M+H]+ Calcd for C24H28F3N2O6S+ 529.1615; found 529.1619.
4.2. N-((2RS,3RS)-1-Butyl-2-(4-methoxyphenyl)-4-oxoazetidin-3-yl)-N-(phenylsulfonyl)glycine (18)
Compound 16 (37 mg, 0.07 mmol) or 6p (55 mg, 0.12 mmol) was dissolved in a mixture of MeOH and water (3 + 3 mL), followed by addition of NaOH (5 equiv.). After stirring for 24 h at room temperature, MeOH was removed in vacuo, and the residue was acidified with HCl conc to pH 1 and partitioned between water (5 mL) and ethyl acetate (10 mL). The organic layer was separated, dried over sodium sulfate, filtered and concentrated to give pure title compound. Yield 27 mg, 90% (from hydrolysis of compound 16), light yellow oil. Yield 44 mg, 80% (from hydrolysis of compound 6p), light yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 7.5 Hz, 2H), 7.56 (t, J = 7.5 Hz, 1H), 7.42 (t, J = 7.8 Hz, 2H), 7.21 (d, J = 8.5 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.78 (d, J = 2.0 Hz, 1H), 4.39 (d, J = 1.8 Hz, 1H), 4.26 (d, J = 18.5 Hz, 1H), 4.19 (d, J = 18.6 Hz, 1H), 3.85 (s, 3H), 3.44 (dt, J = 14.7, 7.6 Hz, 1H), 2.75 (dt, J = 14.2, 7.0 Hz, 1H), 1.43 (p, J = 7.6 Hz, 2H), 1.28 (dq, J = 15.6, 4.3, 3.7 Hz, 2H), 0.86 (t, J = 7.2 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 172.0, 164.2, 160.3, 138.1, 133.5, 129.3, 128.4, 127.7, 127.4, 114.5, 71.7, 63.0, 55.5, 48.0, 40.5, 29.6, 20.3, 13.7. HRMS (ESI) m/z: [M + Na]+ Calcd for C22H26N2O6NaS+ 469.1404; found 469.1413. IR (KBr): ῦ = 1760 (vs) cm−1 (C=O).
4.3. (2RS,3SR)-4-Butyl-3-(4-methoxyphenyl)-5-oxo-1-(phenylsulfonyl)piperazine-2-carboxylic Acid (19)
Preparation 1 (Scheme 6b). In a screw-cap vial equipped with a magnetic stir bar, N-butyl-1-(4-methoxyphenyl)methanimine (40 mg, 0.21 mmol) and compound 14 (80 mg, 0.19 mmol) were mixed in chlorobenzene (5 mL). Then, acetic anhydride (21 mg, 0.21 mmol) was added. The resulting mixture was placed in a bath pre-heated to 80 °C. After 16 h, the reaction mixture was cooled to RT and concentrated. The residue was dissolved in a mixture of MeOH and water (6 + 6 mL) followed by the addition of NaOH (19 mg, 0.49 mmol, 5 equiv.). After stirring for 24 h at room temperature, MeOH was removed in vacuo and the residue was diluted with water (5 mL) and extracted with ethyl acetate (5 mL). The aqueous layer was separated and acidified with HCl conc to pH 1 and extracted with ethyl acetate (2 × 10 mL). Combined organic layers were dried over sodium sulfate, filtered and concentrated to give pure compound 19. Yield 45 mg, 56%, dr > 95:5, light orange oil.
1H NMR (400 MHz, CDCl3) δ 7.50 (tt, J = 7.0, 1.4 Hz, 1H), 7.41 (dd, J = 8.5, 1.4 Hz, 2H), 7.37–7.29 (m, 2H), 7.00 (d, J = 8.7 Hz, 2H), 6.80 (d, J = 8.7 Hz, 2H), 5.07 (d, J = 2.3 Hz, 1H), 4.84 (d, J = 2.1 Hz, 1H), 4.24 (d, J = 17.1 Hz, 1H), 4.06 (d, J = 17.1 Hz, 1H), 3.90 (dt, J = 13.6, 7.6 Hz, 1H), 3.83 (s, 3H), 2.58 (dt, J = 13.9, 7.1 Hz, 1H), 1.51–1.34 (m, 2H), 1.31–1.12 (m, 2H), 0.85 (t, J = 7.3 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 171.7, 165.0, 160.0, 138.4, 133.0, 129.0, 129.0, 127.5, 127.3, 114.7, 61.2, 61.2, 55.5, 46.2, 45.7, 29.1, 20.0, 13.8. HRMS (ESI) m/z: [M + Na]+ Calcd for C22H26N2O6NaS+ 469.1404; found 469.1411. IR (KBr): ῦ = 1738 (s), 1611 (s) cm−1 (C=O).
Preparation 2 (Scheme 7b). A mixture of N-(phenylsulfonyl)iminodiacetic acid (123 mg, 0.45 mmol), N-butyl-1-(4-methoxyphenyl)methanimine (95 mg, 0.5 mmol) and acetic anhydride (50 mg, 0.5 mmol) in PhCl (1 mL) was placed in a pre-heated bath (150 °C) and stirred for 16h. After cooling to room temperature, the reaction mixture was partitioned between ethyl acetate (5 mL) and saturated sodium bicarbonate solution (5 mL). The aqueous layer was separated, cooled to 0 °C and acidified with HCl concentrated to pH 1, followed by extraction with ethyl acetate (2 × 5 mL). Combined organic layers were dried over sodium sulfate, filtered and concentrated to give pure compound 19. Yield 90 mg, 47%; dr > 95:5, light orange oil.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/molecules27082469/s1. Synthesis of starting materials and their analytical data, crystallographic data, copies of NMR spectra, Table S1 (diastereomeric ratios from crude reaction mixtures and after purification). References [30,31,32,33,34,35,36] are cited in the supplementary materials.
Author Contributions
Conceptualization, O.B., D.D. and M.K.; methodology, O.B.; investigation, A.A., G.K. and O.B.; data curation, O.B. and A.A.; writing—original draft preparation, M.K.; writing—review and editing, D.D. and O.B.; supervision, O.B.; funding acquisition, O.B. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge financial support of this work by the Russian Science Foundation (project grant 20-73-10078).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Research Centre for Magnetic Resonance, the Centre for Chemical Analysis and Materials Research, the Research Centre for X-ray Diffraction Studies and The Center for Study of Extreme States of Materials and Constructions of Saint Petersburg State University Research Park for the analytical data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Llarrull, L.; Testero, S.A.; Fisher, J.F.; Mobashery, S. The future of the β-lactams. Curr. Opin. Microbiol. 2010, 13, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Caruano, J.; Muccioli, G.G.; Robiette, R. Biologically active γ-lactams: Synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. [Google Scholar] [CrossRef] [PubMed]
- Lepikhina, A.; Bakulina, O.; Dar’in, D.; Krasavin, M. The first solvent-free synthesis of privileged γ- and δ-lactams via the Castagnoli–Cushman reaction. RSC Adv. 2016, 6, 83808–83813. [Google Scholar] [CrossRef] [Green Version]
- Bakulina, O.; Chizhova, M.; Dar’in, D.; Krasavin, M. A General Way to Construct Arene-Fused Seven-Membered Nitrogen Heterocycles. Eur. J. Org. Chem. 2018, 2018, 362–371. [Google Scholar] [CrossRef]
- Sapegin, A.; Osipyan, A.; Krasavin, M. Structurally diverse arene-fused ten-membered lactams accessed via hydrolytic imidazoline ring expansion. Org. Biomol. Chem. 2017, 15, 2906–2909. [Google Scholar] [CrossRef] [Green Version]
- Saldivar-Gonzalez, F.I.; Lenci, E.; Trabocchi, A.; Medina-Franco, J.L. Exploring the chemical space and the bioactivity profile of lactams: A chemoinformatic study. RSC Adv. 2019, 9, 27105–27116. [Google Scholar] [CrossRef] [Green Version]
- Imming, P.; Klar, B.; Dix, D.J. Hydrolytic Stability versus Ring Size in Lactams: Implications for the Development of Lactam Antibiotics and Other Serine Protease Inhibitors. Med. Chem. 2000, 43, 4328–4331. [Google Scholar] [CrossRef]
- Wang, D.Y.; Abboud, M.I.; Markoulides, M.S.; Brem, J.; Schofield, C.J. The road to avibactam: The first clinically useful non-β-lactam working somewhat like a β-lactam. Future Med. Chem. 2016, 8, 1063–1084. [Google Scholar] [CrossRef] [Green Version]
- Constantino, L.; Barlocco, D. Privileged Structures as Leads in Medicinal Chemistry. Curr. Med. Chem. 2006, 13, 65–85. [Google Scholar] [CrossRef]
- Krawczyk, H.; Albrecht, Ł.; Wojciechowski, J.; Wolf, W.M.; Krajewska, U.; Różalski, M. Synthesis and antiinflammatory activities of 3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2-ones. Tetrahedron 2008, 64, 6307–6314. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Marepalli, H.R.; Lu, H.-F.; Becker, J.M.; Naider, F. Synthesis, Biological Activity, and Conformational Analysis of Peptidomimetic Analogues of the Saccharomyces cerevisiae α-Factor Tridecapeptide. Biochemistry 1998, 37, 12465–12476. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Gu, Y. Total Synthesis of (−)- and (+)-Dysibetaine. Org. Lett. 2001, 3, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Xi, N.; Arvedson, S.; Eisenberg, S.; Han, N.; Handley, M.; Huang, L.; Huang, Q.; Kiselyov, A.; Liu, Q.; Lu, Y.; et al. N-Aryl-gamma-lactams as integrin alphavbeta3 antagonists. Bioorg. Med. Chem. Lett. 2004, 14, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Elford, T.G.; Hall, D.G. Imine allylation using 2-alkoxycarbonyl allylboronates as an expedient three-component reaction to polysubstituted α-exo-methylene-γ-lactams. Tetrahedron Lett. 2008, 49, 6995–6998. [Google Scholar] [CrossRef]
- Satoh, N.; Yokoshima, S.; Fukuyama, T. Total Synthesis of (−)-Salinosporamide A. Org. Lett. 2011, 13, 3028–3031. [Google Scholar] [CrossRef] [PubMed]
- Bogliotti, N.; Dalko, P.I.; Cossy, J. A Radical Approach for the Construction of the Tricyclic Core of Stemoamide. Synlett 2005, 2005, 349–351. [Google Scholar] [CrossRef]
- Howard, S.Y.; Di Maso, M.J.; Shimabukuro, K.; Burlow, N.P.; Tan, D.Q.; Fettinger, J.C.; Malig, T.C.; Hein, J.E.; Shaw, J.T.J. Mechanistic Investigation of Castagnoli–Cushman Multicomponent Reactions Leading to a Three-Component Synthesis of Dihydroisoquinolones. Org. Chem. 2021, 86, 11599–11607. [Google Scholar] [CrossRef]
- Lepikhina, A.; Dar’in, D.; Bakulina, O.; Chupakhin, E.; Krasavin, M. Skeletal Diversity in Combinatorial Fashion: A New Format for the Castagnoli–Cushman Reaction. ACS Comb. Sci. 2017, 19, 702–707. [Google Scholar] [CrossRef]
- Dar’in, D.; Bakulina, O.; Nikolskaya, S.; Gluzdikov, I.; Krasavin, M. The rare cis-configured trisubstituted lactam products obtained by the Castagnoli–Cushman reaction in N,N-dimethylformamide. RSC Adv. 2016, 6, 49411–49415. [Google Scholar] [CrossRef] [Green Version]
- Guranova, N.; Bakulina, O.; Dar’in, D.; Kantin, G.; Krasavin, M. Homophthalic Esters: A New Type of Reagents for the Castagnoli-Cushman Reaction. Eur. J. Org. Chem. 2022, 2022, e202101281. [Google Scholar] [CrossRef]
- Zarei, M. A straightforward approach to 2-azetidinones from imines and carboxylic acids using dimethyl sulfoxide and acetic anhydride. Tetrahedron Lett. 2014, 55, 5354–5357. [Google Scholar] [CrossRef]
- Zarei, M.J. β-Lactam Preparation via Staudinger Reaction with Activated Dimethylsulfoxide. Heterocycl. Chem. 2016, 54, 1161–1166. [Google Scholar] [CrossRef]
- Page, M.G.P.; Dantier, C.; Desarbe, E. In Vitro Properties of BAL30072, a Novel Siderophore Sulfactam with Activity against Multiresistant Gram-Negative Bacilli. Antimicrob. Agents Chemother. 2010, 54, 2291–2302. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Kirberger, S.E.; Johnson, J.A.; Kimbrough, J.R.; Partridge, D.K.D.; Pomerantz, W.C.K. Efficient Synthesis of 1,4-Thiazepanones and 1,4-Thiazepanes as 3D Fragments for Screening Libraries. Org. Lett. 2020, 22, 3946–3950. [Google Scholar] [CrossRef] [PubMed]
- Duggan, P.J.; Humphrey, D.G.; McCarl, V. Boron compounds. 40. O-Ethylboranediyl derivatives of dulcitol. Aust. J. Chem. 2004, 57, 741–745. [Google Scholar] [CrossRef]
- Caldwell, N.; Jamieson, C.; Simpson, I.; Watson, A.J.B. Catalytic amidation of unactivated ester derivatives mediated by trifluoroethanol. Chem. Commun. 2015, 51, 9495–9498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queyroy, S.; Vanthuyne, N.; Gastaldi, S.; Bertrand, M.P.; Gil, G. Enzymic resolution of racemic amines: Crucial role of the solvent. Adv. Synth. Catal. 2012, 354, 1759–1764. [Google Scholar] [CrossRef]
- Koldobskii, A.B.; Shilova, O.S.; Artyushin, O.I.; Kagramanov, N.D.; Moiseev, S.V.J. Polyfluorinated esters of 4-chloro-2-oxobut-3-ynoic acid. Cycloaddition reactons of hexafluoroisopropyl 4-chloro-2-oxobut-3-ynoate, an incredibly electrophilic alkyne. Fluorine Chem. 2020, 231, 109463. [Google Scholar] [CrossRef]
- Dar’in, D.; Bakulina, O.; Chizhova, M.; Krasavin, M. New Heterocyclic Product Space for the Castagnoli–Cushman Three-Component Reaction. Org. Lett. 2015, 17, 3930–3933. [Google Scholar] [CrossRef]
- Murali Dhar, T.G.; Shen, Z.; Gu, H.H.; Chen, P.; Norris, D.; Watterson, S.H.; Ballentine, S.K.; Fleener, C.A.; Rouleau, K.A.; Barrish, J.C.; et al. 3-Cyanoindole-based inhibitors of inosine monophosphate dehydrogenase: Synthesis and initial structure–Activity relationships. Bioorg. Med. Chem. Lett. 2003, 13, 3557–3560. [Google Scholar] [CrossRef]
- Dorn, C.P. Morpholine and Thiomorpholine Tachykinin Receptor Antagonists. US Patent US-6048859-A, 11 April 2000. [Google Scholar]
- Cherney, R.J.; Duan, J.J.; Voss, M.E.; Chen, L.; Wang, L.; Meyer, D.T.; Wasserman, Z.R.; Hardman, K.D.; Liu, R.Q.; Covington, M.B.; et al. Design, synthesis, and evaluation of benzothiadiazepine hydroxamates as selective tumor necrosis factor-alpha converting enzyme inhibitors. J. Med. Chem. 2003, 46, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Usmanova, L.; Bakulina, O.; Dar’in, D.; Krasavin, M. Spontaneous formation of tricyclic lactones following the Castagnoli–Cushman reaction. Chem. Heterocycl. Compd. 2017, 53, 474–479. [Google Scholar] [CrossRef]
- DeMarinis, R.M.; Boehm, J.C.; Dunn, G.L.; Hoover, J.R.; Uri, J.V.; Guarini, J.R.; Phillips, L.; Actor, P.; Weisbach, J.A. Semisynthetic cephalosporins. Synthesis and structure-activity relationships of analogues with 7-acyl groups derived from 2-(cyanomethylthio)acetic acid or 2-[(2,2,2-trifluoroethyl)thio]acetic acid and their sulfoxides and sulfones. J. Med. Chem. 1977, 20, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Linden, A. Chemistry and structure in Acta Crystallographica Section C. Acta Crystallogr. C Struct. Chem. 2015, 71 Pt 1, 1–2. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).