Abstract
Three new Pt(II) complexes [(dpp-DAD)PtCl2] (I), [(Mes-DAD(Me)2)PtCl2] (II) and [(dpp-DAD(Me)2)PtCl2] (III) were synthesized by the direct reaction of [(CH3CN)2PtCl2] and corresponding redox-active 1,4-diaza-1,3-butadienes (DAD). The compounds were isolated in a single crystal form and their molecular structures were determined by X-ray diffraction. The purity of the complexes and their stability in solution was confirmed by NMR analysis. The Pt(II) ions in all compounds are in a square planar environment. The electrochemical reduction of complexes I–III proceeds in two successive cathodic stages. The first quasi-reversible reduction leads to the relatively stable monoanionic complexes; the second cathodic stage is irreversible. The coordination of 1,4-diaza-1,3-butadienes ligands with PtCl2 increases the reduction potential and the electron acceptor ability of the DAD ligands. The synthesized compounds were tested in relation to an adenocarcinoma of the ovary (SKOV3).
1. Introduction
Bioinorganic chemistry is one of the most promising areas in coordination chemistry. Based on the amount of accumulated data, it can be confidently stated that the studied metal containing compounds can become promising metallodrugs [1,2,3,4] similarly to cis-diamminedichloroplatinum (cisplatin, CP) [5]—the first FDA-approved platinum compound for treating cancer, which is currently prescribed to almost half of cancer the patients worldwide. However, serious side effects [6,7,8,9], as well as congenital or acquired resistance to CP [10,11], provoked an active search for alternative platinum preparations, but with a different combination of ligands.
Among the promising organic components are red-ox active or non-innocent ligands capable of reversibly accepting electrons [12]. Over the past two decades, it has become clear that redox-active systems based on non-innocent ligands and metals with switchable oxidation states play a crucial role in biological processes [13,14,15], the generation of free radicals can be used for treating of many diseases [16]. One of the most studied redox-active ligands are α-diimines or 1,4-diaza-1,3-butadienes [17,18,19,20]. Compounds of such ligands are mainly investigated for creating efficient catalysts [21,22,23,24,25], small molecules activation [26,27,28] and switchable materials [29,30,31,32,33,34,35]. It has recently been found that platinum and palladium α-diimine complexes reveal cytotoxicity against various cancer cells [36,37].
In searching for new homologues of cisplatin, we prepared three platinum(II) chloride complexes with substituted 1,4-diaza-1,3-butadienes, determined their structures and investigated their electrochemical properties and cytotoxicity compared with cisplatin.
2. Results
2.1. Synthesis and Characterization of [(dpp-DAD)PtCl2] (I), [(Mes-DAD(Me)2)PtCl2] (II) and [(dpp-DAD(Me)2)PtCl2] (III)
The reaction between presynthesized acetonitrile Pt(II) chloride complex with substituted redox-active 1,4-diaza-1,3-butadienes in absolute methanol led to the formation of three new Pt(II) diimine compounds (Scheme 1). The reactions proceed in an inert atmosphere in a sealed thick-wall glass ampoules on heating with color changing from initial yellow to brown-red. The products crystallize with further cooling of the reaction mixture to room temperature without concentration. Decanted and twice washed, the major products were isolated in 64–87% yields. We found that the reaction is best carried out in an inert atmosphere in absolute methanol, whereas the same reactions in air in non-absolute solvents decrease product yields.
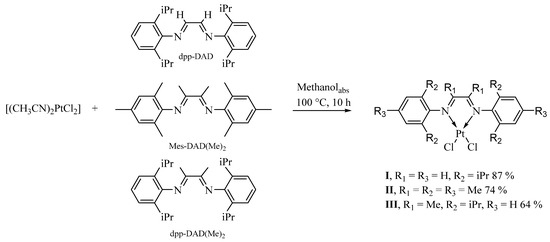
Scheme 1.
Synthesis of complexes I–III.
In the IR spectra (see Supplementary Information) of I–III, the absorption bands at 1588 cm−1 (I), 1601 cm−1 (II), 1588 cm−1 (III) correspond to C=N stretching vibrations of the neutral diimine ligands with a slight decrease in frequencies compared to free 1,4-diaza-1,3-butadienes [17,38]. The band-shift to the lower wavenumbers indicates the coordination of two α-diimine nitrogen atoms [39].
1H and 13C NMR spectra (see Supplementary Information) are in agreement with the structural data. The signal of vinyl protons in 1H NMR spectrum of I is shifted downfield compared to free ligand (8.81 and 8.11 ppm, respectively). Additionally, due to coupling on 195Pt nuclei, satellites are observed with 3J (1H,195Pt) of 91.4 Hz. In 13C NMR spectra of I and III, signals of imine carbon atoms are also shifted downfield compared to free ligands by 3 and 8 ppm, respectively. 13C NMR spectrum of II could not be obtained due to the low solubility of the compound in the common deuterated solvents.
2.2. Molecular Structures of I and II
Complexes I and II crystallize in the monoclinic space groups P21/c and Cc, respectively; experimental and crystal data are presented in Table 1. Compound I is isostructural to known palladium complex [40]. Both Pt(II) compounds are mononuclear, where the central platinum(II) ion coordinates two chloride anions and two nitrogen atoms of α-diimine moiety (Figure 1a,b) forming a square planar environment (selected bond distances and angles for I and II given in Table 2), which is quite typical for Pt2+, e.g., there is the same environment in starting [(CH3CN)2PtCl2] [41] and similar platinum(II) complexes [L2PtCl2], where L is N,N-dimesityl-1,4-diazabutadiene, N,N’-bis(4-hydroxyphenyl)ethylenediamine [37,42].

Table 1.
Crystallographic data, details of data collection, and characteristics of data refinement for I and II.
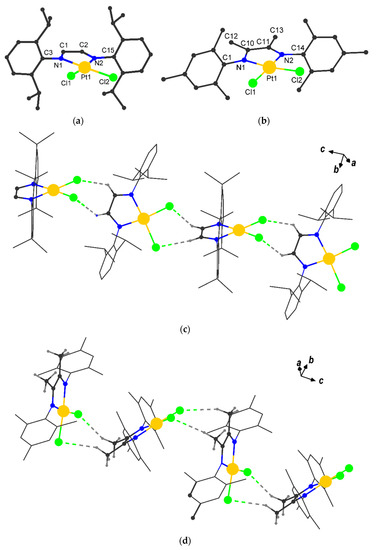
Figure 1.
Molecular structures and crystal packing (along c axis) of I (a,c) and II (b,d). Hydrogen atoms are omitted for clarity, H…Cl interactions are dashed.

Table 2.
Selected bond distances (Å) and angles (deg.) for I and II.
Analysis of the crystal packing revealed intermolecular non-covalent interactions H…Cl (the interatomic distance is shorter than the sum of Bondi’s vdW radii (2.95 Å) [43]) for both compounds (Table S1), which are responsible for the formation of supramolecular chains. In I the chain has a zigzag shape, which is the result of an asymmetric H…Cl contacts of molecules, the angle between the PtN2Cl2 planes is 69.88(7)° (Figure 1c). In the crystal II adjacent molecules form zigzag chain (Figure 1d), the angle between the PtN2Cl2 planes is 62.4(2)°.
2.3. Cyclic Voltammetry of I–III
Complex 1,4-Diaza-1,3-butadienes are non-innocent ligands which can form three different redox states: neutral diimine (L0), radical anion (L−) and dianion (L2−) (Scheme 2). The electrochemical studies allow to observe the existence of the different redox transitions under the conditions of electrochemical experiment. The presence of redox-active ligands in the compounds I–III determines the possibility of their participation in the electrochemical transformations.
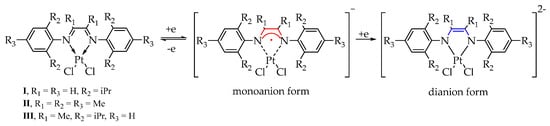
Scheme 2.
Two successive electrochemical oxidation stages for complexes I–III.
The electrochemical behavior of platinum complexes I–III was studied by cyclic voltammetry in dichloromethane solutions using a GC working electrode (Table 3).

Table 3.
The CV data for I–III (CH2Cl2, GC, 0.1 M nBu4ClO4, C = 2·10−3 M, Ar, vs. Ag/AgCl/KCl (sat.)).
The electrochemical reduction of complexes I–III proceeds in two successive cathodic stages (Figure 2, Figure 3 and Figure S9). For all complexes, the first cathodic peak is quasi-reversible and one-electron, indicating the formation of monoanionic species relatively stable over the CV experiment time.
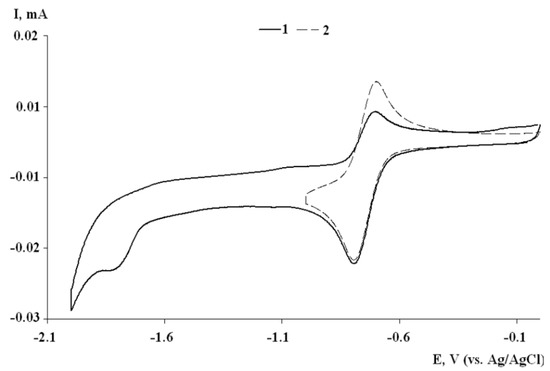
Figure 2.
Cyclic voltammograms of II: the potential switch from 0.0 to −2.0 V (curve 1); the potential switch from 0.0 to −1.0 V (curve 2) (CH2Cl2, C = 2 mmol, 0.1 M TBAP, scan rate 200 mV∙s−1).
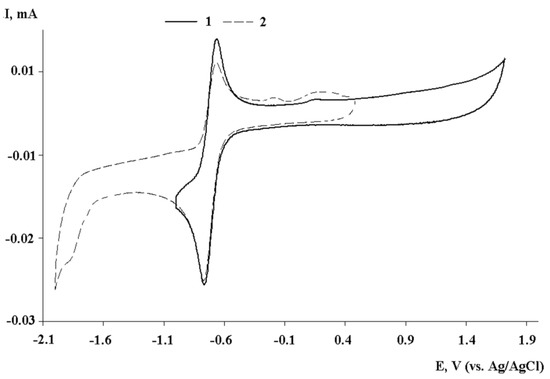
Figure 3.
Cyclic voltammograms of III: the potential switch from 1.7 to −1.0 V (curve 1); the potential switch from 0.5 to −2.0 V (curve 2) (CH2Cl2, C = 2 mmol, 0.1 M TBAP, scan rate 200 mV∙s−1).
Based on the values of current ratios (Ia/Ic), monoanionic complexes formed during the electroreduction of compounds I and III, containing iso-propyl groups in the positions of 2,6-aromatic ring, have the highest stability than complex II with mesityl substituents at nitrogen atoms. The second reduction stage for complexes I–III is irreversible, which suggests the formation of an unstable intermediate (Scheme 2).
The high stability of the electrogenerated monoanionic complexes and the absence of the chloride reoxidation peak (1.40–1.50 V) on the reverse scans of the CVs (Figure 3), which can be formed upon metal reduction, confirmed the ligand participation in the redox processes. An increase in the potential sweep range (up to −2.0 V) with the capture of the second cathodic peak led to a decrease of the anodic current intensity of the first reduction peak (Figure 2, Figure 3 and Figure S9). This fact reveals the occurrence of a chemical stage following the second electron transfer. The electrogenerated dianion forms of complexes are unstable and possessed the basic properties. Additional peaks are observed on the reverse branch of CV in the potential range from −0.1 to 0.1 V (Figure 3).
It is worth noting that the complex I with dpp-DAD ligand undergoes one-electron reduction at the potential (−0.40 V) shifting to the anode potential range compared with other compounds. This behavior is due to the absence of donor methyl groups in the N = CH-CH = N moiety. A similar effect is observed for the reduction peaks of free ligands: −2.07 V (dpp-DAD(Me)2) [44], −1.74 V (Mes-DAD(Me)2) (Figure S10); dpp-DAD [44]. As in the case of the previously discussed complexes of o-(imino)benzoquinones with metal halides [45], the coordination of diazadiene ligands with PtCl2 increased in the reduction potential and, consequently, the electron acceptor ability of the 1,4-diaza-1,3-butadiene ligands. The complex formation favors not only to a shift in the reduction potential of the bound ligand but also hinders the oxidation stage. In the anode potential range, no additional peaks were observed up to 2.0 V (Figure S11).
The first reduction potential of the DAD ligands, being coordinated on the platinum(II), shifts significantly toward the anodic region compared to the corresponding free ligand. This value is 1.3 V for complexes I and III, while for compound II it is equal 0.97 V. The coordination of dpp-DAD with ZnCl2 led to a shift in the reduction potential of the ligand up to −0.54 V [44]. In case of (Mes-DAD(Me)2)AlCl2 complex containing the monoanionic form of the ligand, the potential of redox transition (L−/L0) also becomes more positive (−0.2 V, vs. Ag/AgCl) [46]. The value of the reduction potential shift depends on the Lewis acidity of the metal halides.
Thus, according to CV data, the coordination of DAD ligands to PtCl2 leads to a significant reduction potential shift to the anode range. This trend indicates an increase in oxidizing properties compared with the neutral 1,4-diaza-1,3-butadiene ligands.
2.4. Biological Activity of I–III
Biological tests were performed on ovarian adenocarcinoma SKOV3 for I–III. The cytotoxicity of the complexes was determined against SKOV3 human ovarian adenocarcinoma cells and a primary culture of HDF human fibroblasts as a non-tumor control. Based on the MTT test data, the half-maximal inhibition dose (IC50) for each substance was calculated. Promising drugs are those that cause the death of tumor cells at minimal concentrations, while, to a lesser extent, disrupting the viability of normal cells. The IC50 for SKOV3 and HDF are presented in Table 4 and in Figure 4.

Table 4.
IC50 values of I–III and CP on SKOV3 and HDF.
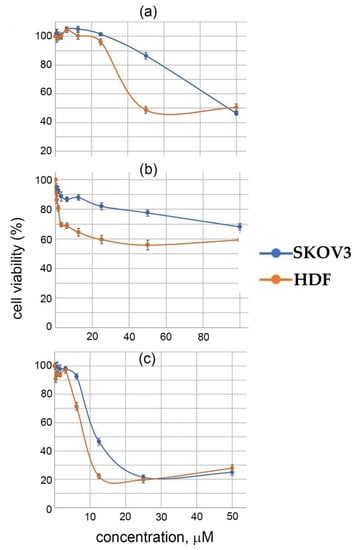
Figure 4.
Survival rates of the SKOV3 (blue) and HDF (orange) cells incubated with various concentrations of complexes I (a), II (b) and III (c) dissolved in DMSO. The mean value of the MTT index ± standard deviation calculated from three independent measurements is given.
3. Materials and Methods
3.1. General Remarks
Synthetic procedures of obtaining new compounds were made in an inert atmosphere using sealed vacuumed glass ampoules or standard Schlenk technique. The synthesized products are stable to the oxygen and moisture. Initial Pt(II) compound [(CH3CN)2PtCl2] was prepared from K2PtCl4 in a water-acetonitrile solution (in a 1:1 ratio). Acetonitrile was used as received. 1,4-diaza-1,3-butadienes Pt(II) complexes were synthesized in a methanol, the latter was dried with sodium metal and stored on the molecular sieves (4 Å), condensed to the reaction mixture prior to use. The 1,4-Bis(2,6-diisopropylphenyl)-1,4-diazabuta-1,3-diene (dpp-DAD) [47], 1,4-Bis(2,4,6-trimethylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene (Mes-DAD(Me)2) [48], and 1,4-Bis(2,6-diisopropylphenyl)-2,3-dimethyl-1,4-diazabuta-1,3-diene (dpp-DAD(Me)2) [23] were synthesized with similar procedures as reported earlier. Product yields were calculated relative to the initial number of ligands.
IR spectra of the compounds were recorded in the range 400–4000 cm−1 on a Perkin Elmer (Waltham, MA, USA) Spectrum 65 spectrophotometer equipped with Quest ATR Accessory (Specac (Orpington, Kent, UK)) using the method of attenuated total reflection (ATR). NMR spectra were recorded on a Bruker Avance III 500 FT spectrometer with operating frequencies of 500.03 and 125.73 MHz for 1H and 13C nuclei, respectively. Chemical shifts are reported in ppm of the δ scale and relate to signals of the solvent.
X-ray diffraction studies of single crystals of complexes I and II were performed on a Bruker D8 Venture (Bruker, Billerica, MA, USA) with a CCD camera and a graphite monochromated MoKα radiation source (λ = 0.71073 Å). Semiempirical absorption corrections were applied for all the experiments using SADABS (University of Gottingen, Gottingen, Germany) [49]. The structures were solved by direct methods and refined in the full-matrix anisotropic approximation for all non-hydrogen atoms. Hydrogen atoms at carbon atoms of organic ligands were generated geometrically and refined in the “riding” model. The calculations were performed in SHELX [50] using Olex2 (OlexSys Ltd., Chemistry Department, Durham University, DH1 3LE, UK) [51]. The main crystallographic parameters and refinement details of compounds I and II are listed in Table 2. The structure parameters were deposited with the Cambridge Structural Database (CCDC Nos. 2216696 (I) and 2216697 (II); deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk/data_request/cif, accessed on 13 October 2022).
Cyclic voltammetry studies were carried out using VERSASTAT-3 potentiostat (PAR) in three-electrode mode. The stationary glassy carbon (d = 2 mm) disk was used as the working electrode; the auxiliary electrode was the platinum-flag electrode. The reference electrode was Ag/AgCl/KCl (sat.) with a watertight diaphragm. All measurements were carried out under argon. The samples were dissolved in the pre-deaerated solvent. The scan rate (ν) was 200 mV∙s−1. The supporting electrolyte 0.1 M Bu4NClO4 was dried under reduced pressure (48 h) at 50 °C. The concentration of compounds was 1–3 mmol.
The cytotoxic effect of various concentrations of the I, II and III complexes on the SKOV3 human ovarian adenocarcinoma cell line and human dermal fibroblast (HDF) primary culture was measured using the MTT assay. This test is based on measuring the activity of the mitochondrial enzyme succinate dehydrogenase and is widely used to evaluate the anticancer activity of potential drugs in vitro. From the MTT test, the half-maximal inhibition dose (IC50) was calculated for both substances. SKOV3 cells were obtained from the ATCC collection, the primary HDF culture was obtained from a healthy donor. SKOV3 and HDF cells were cultured in DMEM (10% FBS, 2 mM glutamine, 1% gentamicin). The cells were cultivated in plastic flasks under sterile conditions, the cells were incubated at 37 °C in 5% CO2.
The stock solutions (50 mM) of compounds I, II and III were prepared in DMSO; before adding to the cells, they were diluted to the required concentrations in a culture medium, and SKOV3 and HDF were seeded in the wells of 96-well plates in the amount of 4 × 103 cells per well and 3.5 × 103 cells per well, respectively. The cells were allowed to fix for 14 h, after which different concentrations of the test compounds or DMSO (as a control sample) were injected in triplicate by the titration method. The final volume of the medium in the wells was 100 μL. At the end of the period of 48 h after the addition of samples, the cell viability was measured using the MTT reagent (Sigma). The 10 μL volume of an MTT working solution (7 mg/mL) was added to each well with cells (100 μL of a medium) and the wells were incubated for 3 h, after which the medium was replaced with a DMSO solution. Using a tablet spectrophotometer (TECAN Infinite M Plex), the absorbance of each well was determined at 570 nm with subsequent subtraction of the background absorbance. The concentration value and the IC50 inhibition dose were determined from the dose–response curves.
3.2. Synthesis of [(dpp-DAD)PtCl2] (I)
Weighed portions of [(CH3CN)2PtCl2] (0.347 g, 1.000 mmol) and dpp-DAD (0.376 g, 1.0 mmol) were placed in a thick-walled glass ampoule (V = 100 mL) and degassed in a dynamic vacuum with a cold trap for 30 min, absolute methanol (70 mL) was condensed into the ampoule, the latter was fire-sealed and heated in an oil bath (100 °C, caution—high pressure!) for 10 h until the all-yellow platinum(II) chloride acetonitrile complex dissolved. The reaction mixture color changed from initial yellow to brown-red. Red prism crystals of the product were grown on the ampoule walls during heating. The product was isolated by further cooling to RT and decantation, twice washed with cold methanol (0.558 g, 87%). Anal. calcd for C26H36Cl2N2Pt (641.19) C, 48.60; H, 5.65; N, 4.36. Found: C, 48.44; H, 5.59; N, 4.32. IR, ν/cm−1: 3071 w, 3027 w, 2958 vs, 2927 s, 2871 m, 1588 w, 1519 m, 1436 s, 1384 w, 1356 w, 1328 m, 1259 m, 1259 m, 1184 m, 1105 m, 1055 m, 939 w, 873 m, 797 vs, 781 s, 701 m, 644 w, 594 m, 544 w, 462 s.
1H NMR (CDCl3): δ 8.81 (s, 2H, HC=N), 7.44 (m, 2H, p-CH), 7.32 (m, 4H, m-CH), 3.24 (sept, 3JH–H = 6.8 Hz, 4H, CH(CH3)2), 1.44 (d, 3JH–H = 6.8 Hz, 12H, CH(CH3)2), 1.21 (d, 3JH–H = 6.8 Hz, 12H, CH(CH3)2). 13C NMR (CDCl3): δ 166.3, 144.0, 141.1, 129.8, 124.6, 28.7, 24.6, 23.2.
3.3. Synthesis of [(Mes-DAD(Me)2)PtCl2] (II)
The complex was obtained by a similar method of I. Weighed portions of [(CH3CN)2PtCl2] (0.347 g, 1.000 mmol) and Mes-DAD(Me)2 (0.320 g, 1.0 mmol) were placed in a thick-walled glass ampoule (V = 100 mL) and degassed in a dynamic vacuum with a cold trap for 30 min, absolute methanol (70 mL) was condensed into the ampoule, the latter was fire-sealed and heated in an oil bath (100 °C, caution—high pressure!) for 10 h until the all-yellow platinum(II) chloride acetonitrile complex dissolved. The solution color changed from initial yellow to brown. The reaction mixture was slowly cooled (10 °C/h) to RT resulted the formation of brown needle-like crystals (0.432 g, 74%). Anal. calcd for C22H28Cl2N2Pt (585.13) C, 45.06; H, 4.81; N, 4.78. Found: C, 45.02; H, 4.76; N, 4.63. IR, ν/cm−1: 3266 w, 3212 w, 3134 w, 3012 w, 2968 w, 2911 w, 2849 w, 1601 m, 1579 m, 1475 s, 1456 s, 1434 s, 1381 s, 1337 m, 1237 m, 1209 w, 1146 m, 990 s, 848 vs, 804 w, 757 w, 716 w, 572 m, 512 w, 465 w. 1H NMR (CDCl3): δ 7.00 (m, 4H, m-CH), 2.34 (s, 6H, p-CH3), 2.24 (s, 12H, o-CH3), 1.26 (s, 6H, N=C–CH3).
3.4. Synthesis of [(dpp-DAD(Me)2)PtCl2] (III)
The complex was obtained by a similar method of I and II. Weighed portions of [(CH3CN)2PtCl2] (0.347 g, 1.000 mmol) and dpp-DAD(Me)2 (0.404 g, 1.0 mmol) were placed in a thick-walled glass ampoule (V = 100 mL) and degassed in a dynamic vacuum with a cold trap for 30 min, absolute methanol (70 mL) was condensed into the ampoule, the latter was fire-sealed and heated in an oil bath (100 °C, caution—high pressure!) for 10 h until the all-yellow platinum(II) chloride acetonitrile complex dissolved. The reaction mixture color changed from initial yellow to red-brown. The solution was slowly cooled (10 °C/h) to RT resulted the formation of brown needle-like crystals (0.428 g, 64%). Anal. calcd for C28H40Cl2N2Pt (669.22) C, 50.15; H, 6.01; N, 4.18. Found: C, 50.07; H, 5.59; N, 4.13. IR, ν/cm−1: 3074 w, 3024 w, 2959 vs, 2924 s, 2867 m, 1589 w, 1522 w, 1450 s, 1383 vs, 1326 s, 1255 m, 1221 m, 1179 m, 1099 w, 1057 m, 996 s, 931 m, 792 vs, 740 s, 704 w, 641 w, 570 w, 538 w, 477 w, 449 m, 404 m. 1H NMR (CDCl3): δ 7.39 (m, 2H, p-CH), 7.30 (m, 4H, m-CH), 3.07 (sept, 3JH–H = 6.8 Hz, 4H, CH(CH3)2), 1.66 (s, 6H, N=C–CH3), 1.45 (d, 3JH–H = 6.8 Hz, 12H, CH(CH3)2), 1.20 (d, 3JH–H = 6.8 Hz, 12H, CH(CH3)2). 13C NMR (CDCl3): δ 168.4, 141.2, 140.2, 129.4, 124.0, 28.8, 23.9, 23.8, 20.7.
4. Conclusions
Thus, here we present the synthesis and characterization of three new platinum(II) compounds with α-diimine ligands. All of them were characterized with 1H and 13C NMR, IR-spectra and elemental analysis. For [(dpp-DAD)PtCl2] (I) and [(Mes-DAD(Me)2)PtCl2] (II) crystal structures were determined reveal square planar geometry of N2PtCl2 moiety. The complexes I–III were characterized by electrochemistry. The cyclic voltammetry of I–III showed two reduction processes. The first cathodic stage was quasi-reversible and one-electron, indicating the formation of monoanionic species. The second reduction step was irreversible. The coordination of DAD ligands to PtCl2 led to the significant reduction potentials shift to anode range compared with the free DAD ligands. Compounds I and II demonstrated low biological activity against SKOV3 human ovarian adenocarcinoma cells, whereas [(dpp-DAD(Me)2)PtCl2] (III) is similar in activity to cisplatin and toxic to healthy fibroblasts. Complexes I and II are safe for healthy cells.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238565/s1, Figures S1–S5: 1H and 13C NMR spectra of I–III; Figures S6–S8: IR-spectra of I–III; Figures S9–S11: cyclic voltammograms of I, III, Mes-DAD(Me)2; Table S1: parameters of hydrogen bonds in the crystal packing in I and II.
Author Contributions
D.S.Y. synthesized tested compounds; design of the study, D.S.Y., I.A.L. and A.A.S.; manuscript writing, review and editing, D.S.Y., S.A.N., M.A.K., I.A.L. and P.A.P.; X-Ray analysis, M.A.K.; funding acquisition, I.L.E. and M.A.K.; electrochemical investigations, I.V.S. and N.T.B.; biological investigation, I.K.M. and V.O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Science Foundation (grant no. 22-13-00175).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The structure parameters of obtained compounds were deposited with the Cambridge Structural Database (CCDC Nos. 2216696 (I) and 2216697 (II); deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk/data_request/cif, accessed on 13 October 2022).
Acknowledgments
X-ray diffraction analysis, elemental and IR-spectra analyzes were performed using the equipment at the Center for Collective Use of the Kurnakov Institute RAS which operates with the support of the state assignment of the IGIC RAS in the field of fundamental scientific research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Sample Availability
Samples of the compounds are available from the authors.
References
- Hogarth, G. Metal-Dithiocarbamate Complexes: Chemistry and Biological Activity. Mini-Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef]
- van Rijt, S.H.; Sadler, P.J. Current Applications and Future Potential for Bioinorganic Chemistry in the Development of Anticancer Drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Viganor, L.; Howe, O.; McCarron, P.; McCann, M.; Devereux, M. The Antibacterial Activity of Metal Complexes Containing 1, 10-Phenanthroline: Potential as Alternative Therapeutics in the Era of Antibiotic Resistance. Curr. Top. Med. Chem. 2017, 17, 1280–1302. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Cooley, M.E.; Davis, L.; Abrahm, J. Cisplatin: A Clinical Review. Part II—Nursing Assessment and Management of Side Effects of Cisplatin. Cancer Nurs. 1994, 17, 283–293. [Google Scholar] [CrossRef]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular Mechanisms of Cisplatin Resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Skatova, A.A.; Chudakova, V.A.; Fukin, G.K. Four-Step Reduction of Dpp-Bian with Sodium Metal: Crystal Structures of the Sodium Salts of the Mono-, Di-, Tri- and Tetraanions of Dpp-Bian. Angew. Chem. Int. Ed. 2003, 42, 3294–3298. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Non-Innocent Ligands in Bioinorganic Chemistry—An Overview. Coord. Chem. Rev. 2010, 254, 1580–1588. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Kaim, W.; Schwederski, B. Cooperation of Metals with Electroactive Ligands of Biochemical Relevance: Beyond Metalloporphyrins. Pure Appl. Chem. 2004, 76, 351–364. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, M.; Zhang, Y.; Fang, F.; Li, M.; An, F.; Zhao, D.; Zhang, J. Free Radical as a Double-Edged Sword in Disease: Deriving Strategic Opportunities for Nanotherapeutics. Coord. Chem. Rev. 2023, 475, 214875. [Google Scholar] [CrossRef]
- Van Koten, G.; Vrieze, K. 1,4-Diaza-1,3-Butadiene (α-Diimine) Ligands: Their Coordination Modes and the Reactivity of Their Metal Complexes. In Advances in Organometallic Chemistry; Academic Press: Cambridge, MA, USA, 1982; Volume 21, pp. 151–239. ISBN 0065-3055. [Google Scholar]
- Nikolaevskaya, E.N.; Druzhkov, N.O.; Syroeshkin, M.A.; Egorov, M.P. Chemistry of Diazadiene Type Ligands with Extra Coordination Groups. Prospects of Reactivity. Coord. Chem. Rev. 2020, 417, 213353. [Google Scholar] [CrossRef]
- Hill, N.J.; Vargas-Baca, I.; Cowley, A.H. Recent Developments in the Coordination Chemistry of Bis(Imino)Acenaphthene (BIAN) Ligands with s- and p-Block Elements. Dalton Trans. 2009, 240–253. [Google Scholar] [CrossRef]
- Mealli, C.; Ienco, A.; Phillips, A.D.; Galindo, A. A Critical Review of Electronic Effects in Enediamido and α-Diimino Complexes of the Group 4 Metals. Eur. J. Inorg. Chem. 2007, 2007, 2556–2568. [Google Scholar] [CrossRef]
- Sandl, S.; Maier, T.M.; van Leest, N.P.; Kröncke, S.; Chakraborty, U.; Demeshko, S.; Koszinowski, K.; de Bruin, B.; Meyer, F.; Bodensteiner, M.; et al. Cobalt-Catalyzed Hydrogenations via Olefin Cobaltate and Hydride Intermediates. ACS Catal. 2019, 9, 7596–7606. [Google Scholar] [CrossRef]
- Bart, S.C.; Hawrelak, E.J.; Lobkovsky, E.; Chirik, P.J. Low-Valent α-Diimine Iron Complexes for Catalytic Olefin Hydrogenation. Organometallics 2005, 24, 5518–5527. [Google Scholar] [CrossRef]
- Crossetti, G.L.; Dias, M.L.; Queiroz, B.T.; Silva, L.P.; Ziglio, C.M.; Bomfim, J.A.S.; Filgueiras, C.A.L. Ethylene Polymerization with Imine and Phosphine Nickel Complexes Containing Isothiocyanate. Appl. Organomet. Chem. 2004, 18, 331–336. [Google Scholar] [CrossRef]
- Lima, G.; Nunes, E.; Dantas, R.; Simone, C.; Meneghetti, M.; Meneghetti, S. Catalytic Behaviors of CoII and MnII Compounds Bearing α-Diimine Ligands for Oxidative Polymerization or Drying Oils. J. Braz. Chem. Soc. 2018, 29, 412–418. [Google Scholar] [CrossRef]
- Palmer, W.N.; Diao, T.; Pappas, I.; Chirik, P.J. High-Activity Cobalt Catalysts for Alkene Hydroboration with Electronically Responsive Terpyridine and α-Diimine Ligands. ACS Catal. 2015, 5, 622–626. [Google Scholar] [CrossRef]
- Moskalev, M.V.; Skatova, A.A.; Razborov, D.A.; Bazanov, A.A.; Bazyakina, N.L.; Sokolov, V.G.; Fedushkin, I.L. Magnesium and Calcium Complexes of ArBIG-Bian and Their Reactivity towards CO2 (ArBIG-Bian=1,2-Bis[(2,6-Dibenzhydryl-4-Methylphenyl)Imino]Acenaphthene). Eur. J. Inorg. Chem. 2021, 2021, 1890–1896. [Google Scholar] [CrossRef]
- Koptseva, T.S.; Sokolov, V.G.; Ketkov, S.Y.; Rychagova, E.A.; Cherkasov, A.V.; Skatova, A.A.; Fedushkin, I.L. Reversible Addition of Carbon Dioxide to Main Group Metal Complexes at Temperatures about 0 °C. Chem.—A Eur. J. 2021, 27, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Skatova, A.A.; Cherkasov, A.V.; Fedushkin, I.L. Addition of Phenylacetylene and Camphor to the Complex [(Dpp-Bian)Eu(Dme)2] (Dpp-Bian Is the 1,2-Bis[(2,6-Diisopropylphenyl)Imino]Acenaphthene Dianion). Russ. Chem. Bull. 2017, 66, 1187–1195. [Google Scholar] [CrossRef]
- Wang, P.; Saber, M.R.; VanNatta, P.E.; Yap, G.P.A.; Popescu, C.V.; Scarborough, C.C.; Kieber-Emmons, M.T.; Dunbar, K.R.; Riordan, C.G. Molecular and Electronic Structures and Single-Molecule Magnet Behavior of Tris(Thioether)–Iron Complexes Containing Redox-Active α-Diimine Ligands. Inorg. Chem. 2021, 60, 6480–6491. [Google Scholar] [CrossRef]
- Khusniyarov, M.M.; Weyhermüller, T.; Bill, E.; Wieghardt, K. Reversible Electron Transfer Coupled to Spin Crossover in an Iron Coordination Salt in the Solid State. Angew. Chem. Int. Ed. 2008, 47, 1228–1231. [Google Scholar] [CrossRef]
- Khusniyarov, M.M.; Weyhermueller, T.; Bill, E.; Wieghardt, K. Tuning the Oxidation Level, the Spin State, and the Degree of Electron Delocalization in Homo- and Heteroleptic Bis(α-Diimine)Iron Complexes. J. Am. Chem. Soc. 2009, 131, 1208–1221. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Yambulatov, D.S.; Cherkasov, A.V.; Demeshko, S.V. Europium Complexes with 1,2-Bis(Arylimino)Acenaphthenes: A Search for Redox Isomers. Russ. Chem. Bull. 2015, 64, 38–43. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Yambulatov, D.S.; Skatova, A.A.; Baranov, E.V.; Demeshko, S.; Bogomyakov, A.S.; Ovcharenko, V.I.; Zueva, E.M. Ytterbium and Europium Complexes of Redox-Active Ligands: Searching for Redox Isomerism. Inorg. Chem. 2017, 56, 9825–9833. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Magdesieva, T.V.; Levitskiy, O.A.; Korchagin, D.V.; Efimov, N.N.; Vasil’ev, P.N.; Goloveshkin, A.S.; Sidorov, A.A.; et al. Complexes of Cobalt(II) Iodide with Pyridine and Redox Active 1,2-Bis(Arylimino)Acenaphthene: Synthesis, Structure, Electrochemical, and Single Ion Magnet Properties. Molecules 2020, 25, 2054. [Google Scholar] [CrossRef] [PubMed]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Kiskin, M.A.; Kholin, K.V.; Khrizanforov, M.N.; Budnikova, Y.G.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Imshennik, V.K.; et al. Generation of a Hetero Spin Complex from Iron(II) Iodide with Redox Active Acenaphthene-1,2-Diimine. Molecules 2021, 26, 2998. [Google Scholar] [CrossRef] [PubMed]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Biancalana, L.; Batchelor, L.K.; Dyson, P.J.; Zacchini, S.; Schoch, S.; Pampaloni, G.; Marchetti, F. α-Diimine Homologues of Cisplatin: Synthesis, Speciation in DMSO/Water and Cytotoxicity. New J. Chem. 2018, 42, 17453–17463. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Nikolaevskii, S.A.; Babeshkin, K.A.; Efimov, N.N.; Kiskin, M.A.; Eremenko, I.L. Synthesis, Structure, and Magnetic Properties of the Cobalt(Ii) Iodide Complex with 1,4-Diazabuta-1,3-Diene Ligand. Russ. Chem. Bull. 2021, 70, 2390–2396. [Google Scholar] [CrossRef]
- Beloglazkina, E.K.; Yudin, I.V.; Majouga, A.G.; Moiseeva, A.A.; Tursina, A.I.; Zyk, N. V Synthesis and Electrochemical Study of 2-(2-Pyridyl)Benzothiazole Complexes with Transition Metals (CoII, NiII, and CuII). Molecular Structure of Aquabis[2-(2-Pyridyl)Benzothiazole]Copper(II) Diperchlorate. Russ. Chem. Bull. 2006, 55, 1803–1809. [Google Scholar] [CrossRef]
- Comerlato, N.M.; Crossetti, G.L.; Howie, R.A.; Tibultino, P.C.D.; Wardell, J.L. Dichloro[N,N’-Bis(2,6-Diisopropylphenyl)-1,2-Ethanediimine-N.,N’]Palladium. Acta Crystallogr. Sect. E 2001, 57, m295–m297. [Google Scholar] [CrossRef]
- Rochon, F.D.; Melanson, R.; Howard-Lock, H.E.; Lock, C.J.L.; Turner, G. The Vibrational Spectra, Crystal and Molecular Structure of Bis(Acetonitrile)Dichloroplatinum(II). Can. J. Chem. 1984, 62, 860–869. [Google Scholar] [CrossRef]
- Tskhovrebov, A.G.; Novikov, A.S.; Odintsova, O.V.; Mikhaylov, V.N.; Sorokoumov, V.N.; Serebryanskaya, T.V.; Starova, G.L. Supramolecular Polymers Derived from the PtII and PdII Schiff Base Complexes via C(Sp2)–H … Hal Hydrogen Bonding: Combined Experimental and Theoretical Study. J. Organomet. Chem. 2019, 886, 71–75. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii of Metals in Covalent Compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Druzhkov, N.O.; Fukin, G.K. Heterometallic Antimony(V)-Zinc and Antimony(V)-Copper Complexes Comprising Catecholate and Diazadiene as Redox Active Centers. J. Organomet. Chem. 2021, 952, 121994. [Google Scholar] [CrossRef]
- Ershova, I.V.; Meshcheryakova, I.N.; Trofimova, O.Y.; Pashanova, K.I.; Arsenyeva, K.V.; Khamaletdinova, N.M.; Smolyaninov, I.V.; Arsenyev, M.V.; Cherkasov, A.V.; Piskunov, A.V. Complexes of Metal Halides with Unreduced O-(Imino)Quinones. Inorg. Chem. 2021, 60, 12309–12322. [Google Scholar] [CrossRef]
- Cole, B.E.; Wolbach, J.P.; Dougherty, W.G.J.; Piro, N.A.; Kassel, W.S.; Graves, C.R. Synthesis and Characterization of Aluminum-α-Diimine Complexes over Multiple Redox States. Inorg. Chem. 2014, 53, 3899–3906. [Google Scholar] [CrossRef] [PubMed]
- Bantreil, X.; Nolan, S.P. Synthesis of N-Heterocyclic Carbene Ligands and Derived Ruthenium Olefin Metathesis Catalysts. Nat. Protoc. 2011, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.A.; Labinger, J.A.; Bercaw, J.E. C−H Bond Activation by Cationic Platinum(II) Complexes: Ligand Electronic and Steric Effects. J. Am. Chem. Soc. 2002, 124, 1378–1399. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).