Ionic Liquids Effect on the Stability of 17-Electron Cation Product of the Electrochemical Oxidation of Cymantrene
Abstract
1. Introduction
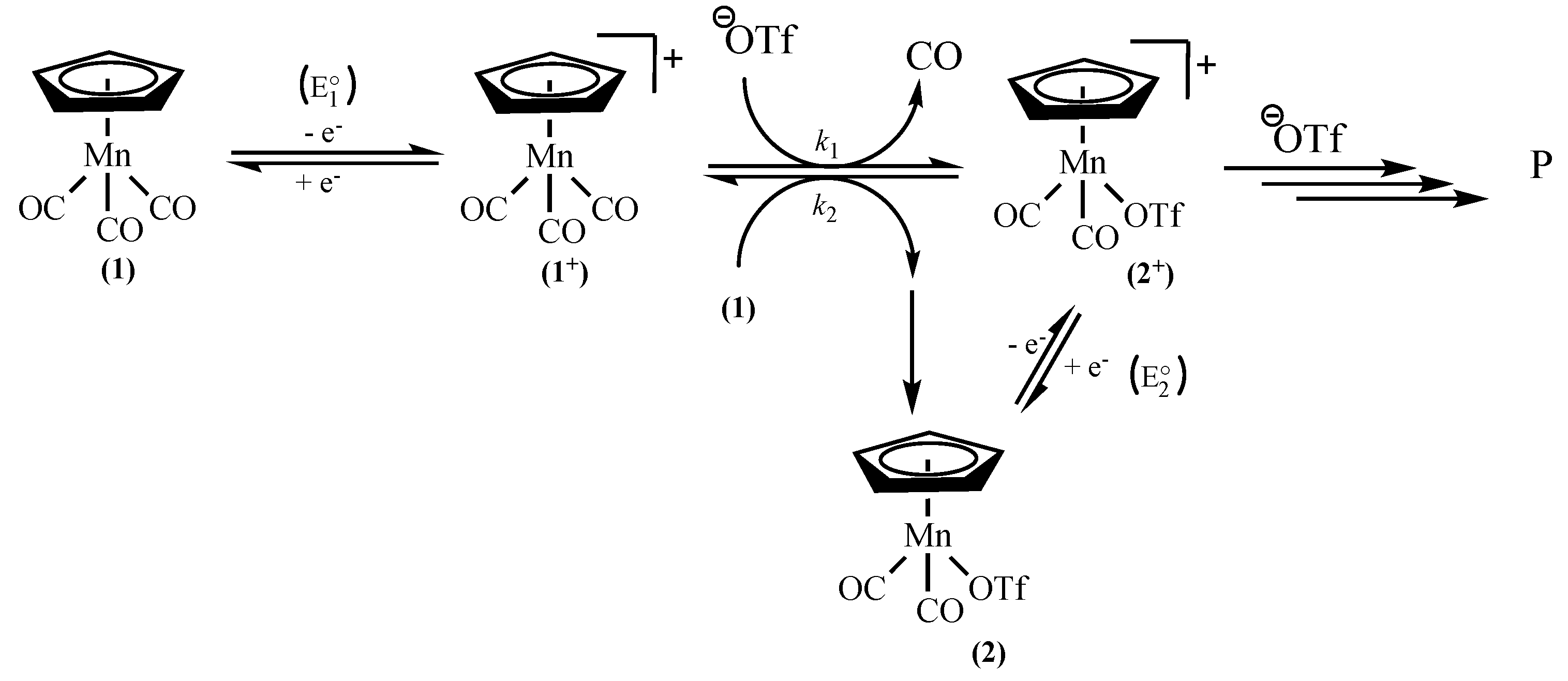
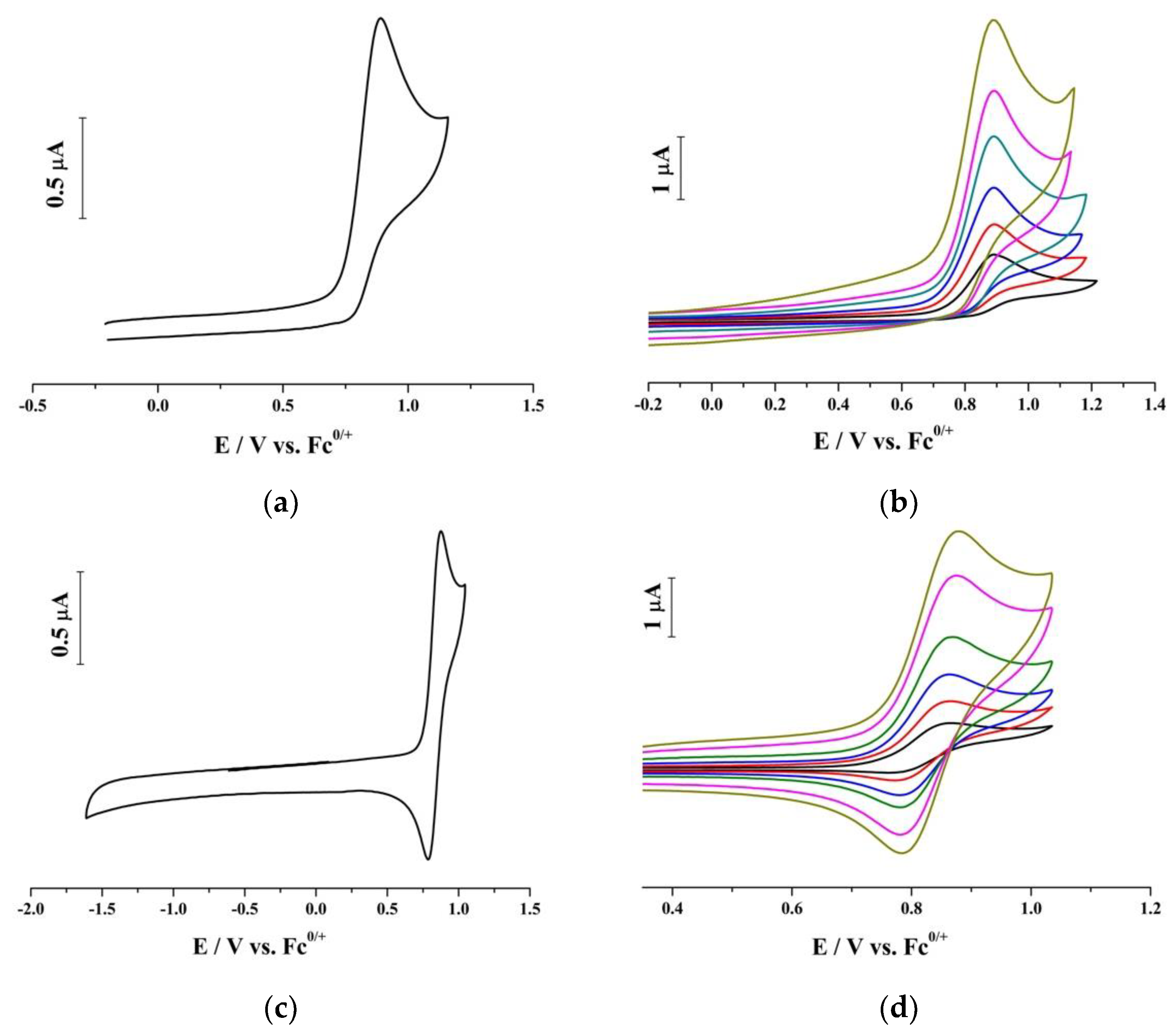
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Barriere, F.; Geiger, W.E. Use of Weakly Coordinating Anions to Develop an Integrated Approach to the Tuning of Delta E1/2 Values by Medium Effects. J. Am. Chem. Soc. 2006, 128, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Barriere, F.; Camire, N.; Geiger, W.E.; Mueller-Westerhoff, U.T.; Sanders, R. Use of Medium Effects to Tune the Delta E1/2 Values of Bimetallic and Oligometallic Compounds. J. Am. Chem. Soc. 2002, 124, 7262–7263. [Google Scholar] [CrossRef] [PubMed]
- Torriero, A.A.J.; Shiddiky, M.J.A.; Bullock, J.P.; Boas, J.F.; MacFarlane, D.R.; Bond, A.M. Electrooxidation of [(η5-C5H5)Fe(CO)2]2 As a Probe of the Nucleophilic Properties of Ionic Liquid Anions. Inorg. Chem. 2010, 49, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Torriero, A.A.J.; Sunarso, J.; Forsyth, M.; Pozo-Gonzalo, C. Assessment of permethylated transition-metal sandwich complexes as internal reference redox systems in ionic liquids. Phys. Chem. Chem. Phys. 2013, 15, 2547–2553. [Google Scholar] [CrossRef]
- Crowhurst, L.; Lancaster, N.L.; Perez Arlandis, J.M.; Welton, T. Manipulating Solute Nucleophilicity with Room Temperature Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 11549–11555. [Google Scholar] [CrossRef]
- Yamagata, M.; Tachikawa, N.; Katayama, Y.; Miura, T. Electrochemical behavior of several iron complexes in hydrophobic room-temperature ionic liquids. Electrochim. Acta 2007, 52, 3317–3322. [Google Scholar] [CrossRef]
- Magna, L.; Chauvin, Y.; Niccolai, G.P.; Basset, J.-M. The Importance of Imidazolium Substituents in the Use of Imidazolium-Based Room-Temperature Ionic Liquids as Solvents for Palladium-Catalyzed Telomerization of Butadiene with Methanol. Organometallics 2003, 22, 4418–4425. [Google Scholar] [CrossRef]
- Earle, M.J.; Hakala, U.; McAuley, B.J.; Nieuwenhuyzen, M.; Ramani, A.; Seddon, K.R. Metal bis[(trifluoromethyl)sulfonyl]amide complexes: Highly efficient Friedel-Crafts acylation catalysts. Chem. Commun. 2004, 12, 1368–1369. [Google Scholar] [CrossRef]
- Williams, D.B.; Stoll, M.E.; Scott, B.L.; Costa, D.A.; Oldham, W.J., Jr. Coordination chemistry of the bis(trifluoromethylsulfonyl)imide anion: Molecular interactions in room temperature ionic liquids. Chem. Commun. 2005, 11, 1438–1440. [Google Scholar]
- Huang, Y.; Carpenter, G.B.; Sweigart, D.A.; Chung, Y.K.; Lee, B.Y. Ligand Substitution at 17-Electron Centers. Electroactivation of Functionalized Cyclopentadienylmanganese Tricarbonyl Complexes to Single- and Double-CO Substitution. Organometallics 1995, 14, 1423–1428. [Google Scholar] [CrossRef]
- Laws, D.R.; Chong, D.S.; Nash, K.; Rheingold, A.L.; Geiger, W.E. Cymantrene radical cation family: Spectral and structural characterization of the half-sandwich analogues of ferrocenium ion. J. Am. Chem. Soc. 2008, 130, 9859–9870. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.; Laws, D.R.; Nafady, A.; Costa, P.J.; Rheingold, A.L.; Calhorda, M.J.; Geiger, W.E. [Re(η5-C5H5)(CO)3]+ Family of 17-Electron Compounds: Monomer/Dimer Equilibria and Other Reactions. J. Am. Chem. Soc. 2008, 130, 2692–2703. [Google Scholar] [CrossRef] [PubMed]
- Zoski, C.G.; Sweigart, D.A.; Stone, N.J.; Rieger, P.H.; Mocellin, E.; Mann, T.F.; Mann, D.R.; Gosser, D.K.; Doeff, M.M.; Bond, A.M. An electrochemical study of the substitution and decomposition reactions of (arene)tricarbonylchromium radical cations. J. Am. Chem. Soc. 1988, 110, 2109–2116. [Google Scholar] [CrossRef]
- Volland, M.A.O.; Kudis, S.; Helmchen, G.; Hyla-Kryspin, I.; Rominger, F.; Gleiter, R. Structure and Bonding Properties of the Complex (h5-Diphenylfulvene)Mn(CO)3+. Organometallics 2001, 20, 227–230. [Google Scholar] [CrossRef]
- Ginzburg, A.G. The chemistry of cymantrene. Russ. Chem. Rev. 1993, 62, 1025–1045. [Google Scholar] [CrossRef]
- Lee, S.; Cooper, N.J. Reductive Activation of the h5-Methylcyclopentadienyl Ligand in [Mn(h5-C5H4Me)(CO)3] and Evidence for a Ring-Slipped Intermediate Containing an h5-Methylcyclopentadienyl Ligand. J. Am. Chem. Soc. 1991, 113, 717–719. [Google Scholar] [CrossRef]
- Sweigart, D.A.; Reingold, J.A. Manganese: Organometallic Chemistry. In Encyclopedia of Inorganic Chemistry; King, R.B., Crabtree, R.H., Lukehart, C.M., Atwood, D.A., Scott, R.A., Eds.; Wiley: New York, NY, USA, 2005; pp. 1–16. [Google Scholar]
- Camire, N.; Nafady, A.; Geiger, W.E. Characterization and Reactions of Previously Elusive 17-Electron Cations: Electrochemical Oxidations of (C6H6)Cr(CO)3 and (C5H5)Co(CO)2 in the Presence of [B(C6F5)4]–. J. Am. Chem. Soc. 2002, 124, 7260–7261. [Google Scholar] [CrossRef]
- Hershberger, J.W.; Klingler, R.J.; Kochi, J.K. Kinetics, thermodynamics, and mechanism of the radical-chain process for ligand substitution of metal carbonyls. J. Am. Chem. Soc. 1983, 105, 61–73. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Pringle, J.M.; Johansson, K.M.; Forsyth, S.A.; Forsyth, M. Lewis base ionic liquids. Chem. Commun. 2006, 1905–1917. [Google Scholar] [CrossRef]
- Beck, W.; Suenkel, K. Metal complexes of weakly coordinating anions. Precursors of strong cationic organometallic Lewis acids. Chem. Rev. 1988, 88, 1405–1421. [Google Scholar] [CrossRef]
- Krossing, I.; Raabe, I. Noncoordinating Anions—Fact or Fiction? A Survey of Likely Candidates. Angew. Chem. Int. Ed. 2004, 43, 2066–2090. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Hydrogen Bonds with BF4− Anion as a Proton Acceptor. Crystals 2020, 10, 460. [Google Scholar] [CrossRef]
- Kim, C.W.; Ahn, J.; Kim, S.M.; Noh, T.H.; Jung, O.-S. Polyatomic anion versus acetonitrile in coordinating ability: Structural properties of AgX-bearing 1,4-bis(2-isonicotinoyloxyethyl)piperazine (X− = NO3−, CF3SO3−, ClO4−, BF4−, and PF6−). Transit. Met. Chem. 2011, 36, 545–551. [Google Scholar] [CrossRef][Green Version]
- Hayashida, T.; Kondo, H.; Terasawa, J.-i.; Kirchner, K.; Sunada, Y.; Nagashima, H. Trifluoromethanesulfonate (triflate) as a moderately coordinating anion: Studies from chemistry of the cationic coordinatively unsaturated mono- and diruthenium amidinates. J. Organomet. Chem. 2007, 692, 382–394. [Google Scholar] [CrossRef]
- Strauss, S.H. The search for larger and more weakly coordinating anions. Chem. Rev. 1993, 93, 927–942. [Google Scholar] [CrossRef]
- Hill, M.G.; Lamanna, W.M.; Mann, K.R. Tetrabutylammonium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate as a noncoordinating electrolyte: Reversible 1e- oxidations of ruthenocene, osmocene, and Rh2(TM4)42+ (TM4 = 2,5-diisocyano-2,5-dimethylhexane). Inorg. Chem. 1991, 30, 4687–4690. [Google Scholar] [CrossRef]
- LeSuer, R.J.; Geiger, W.E. Improved electrochemistry in low-polarity media using tetrakis(pentafluorophenyl)borate salts as supporting electrolytes. Angew. Chem. Int. Ed. 2000, 39, 248–250. [Google Scholar] [CrossRef]
- Cresswell, A.J.; Davies, S.G.; Roberts, P.M.; Thomson, J.E. Beyond the Balz–Schiemann Reaction: The Utility of Tetrafluoroborates and Boron Trifluoride as Nucleophilic Fluoride Sources. Chem. Rev. 2015, 115, 566–611. [Google Scholar] [CrossRef]
- Farooq, O.; Tiers, G.V.D. Alkali metal salts of perfluorinated complex anions. Effective reagents for nucleophilic fluorination. J. Org. Chem. 1994, 59, 2122–2124. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Characterization of decamethylferrocene and ferrocene in ionic liquids: Argon and vacuum effect on their electrochemical properties. Electrochim. Acta 2014, 137, 235–244. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Siriwardana, A.I.; Bond, A.M.; Burgar, I.M.; Dunlop, N.F.; Deacon, G.B.; MacFarlane, D.R. Physical and Electrochemical Properties of Thioether-Functionalized Ionic Liquids. J. Phys. Chem. B 2009, 113, 11222–11231. [Google Scholar] [CrossRef] [PubMed]
- Torriero, A.A.J.; Sunarso, J.; Howlett, P.C. Critical Evaluation of Reference Systems for Voltammetric Measurements in Ionic Liquids. Electrochim. Acta 2012, 82, 60–68. [Google Scholar] [CrossRef]
- Torriero, A.A.J.; Howlett, P.C. Ionic liquid effects on the redox potential of ferrocene. Electrochem. Commun. 2012, 16, 84–87. [Google Scholar] [CrossRef]
- Torriero, A.A.J. Electrochemistry in Ionic Liquids. Volume 1: Fundamentals; Springer: Cham, Switzerland, 2015. [Google Scholar]

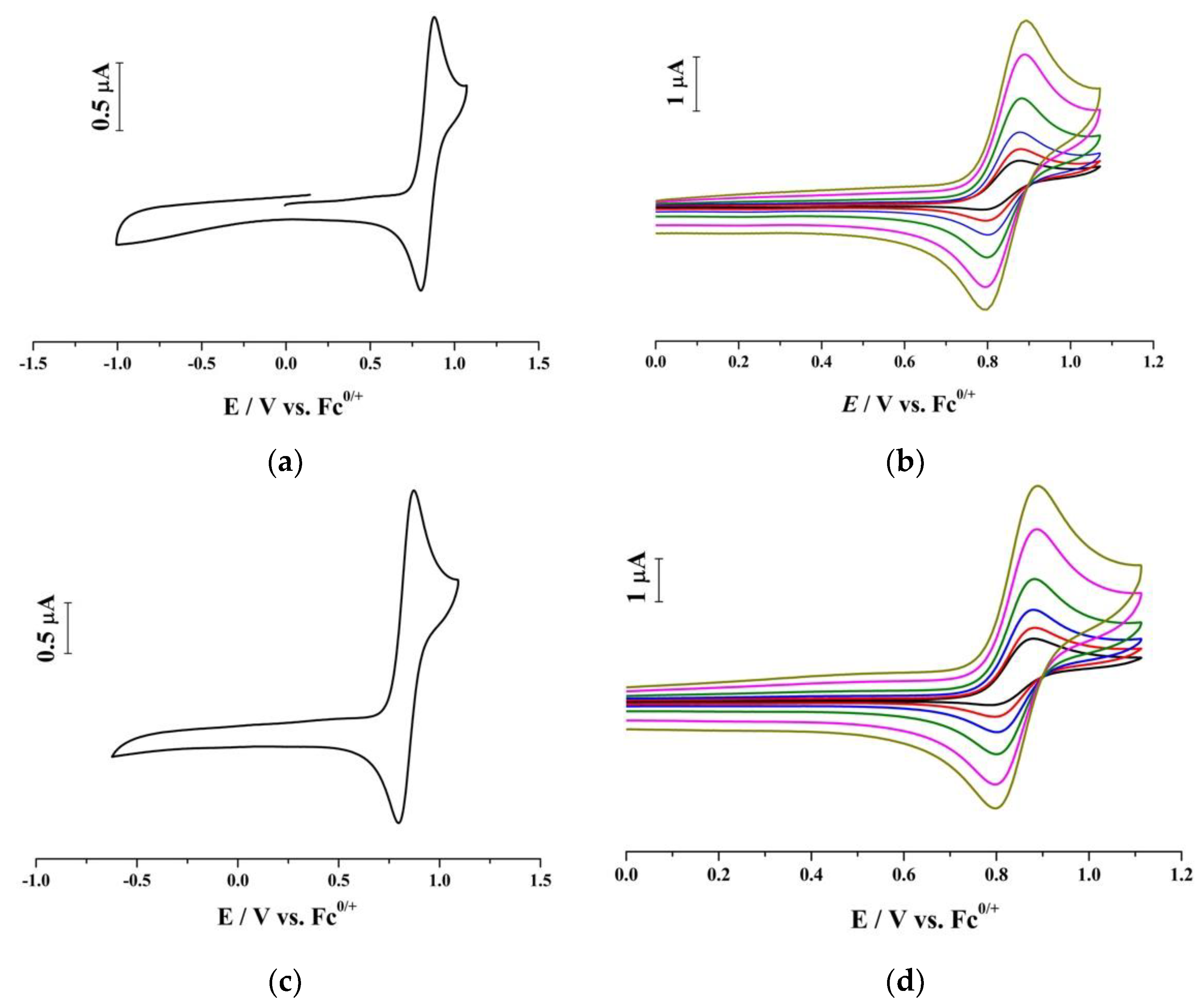

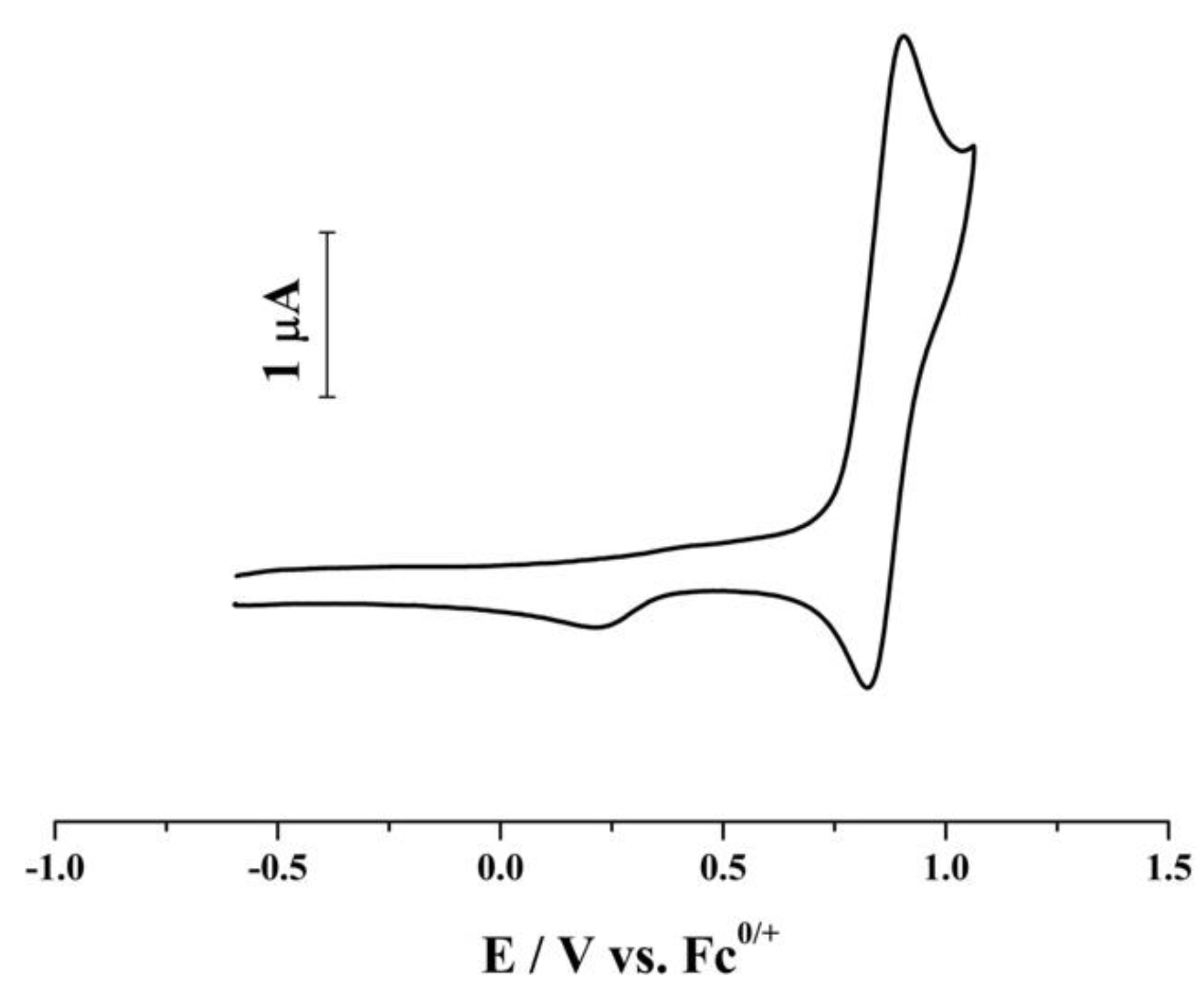
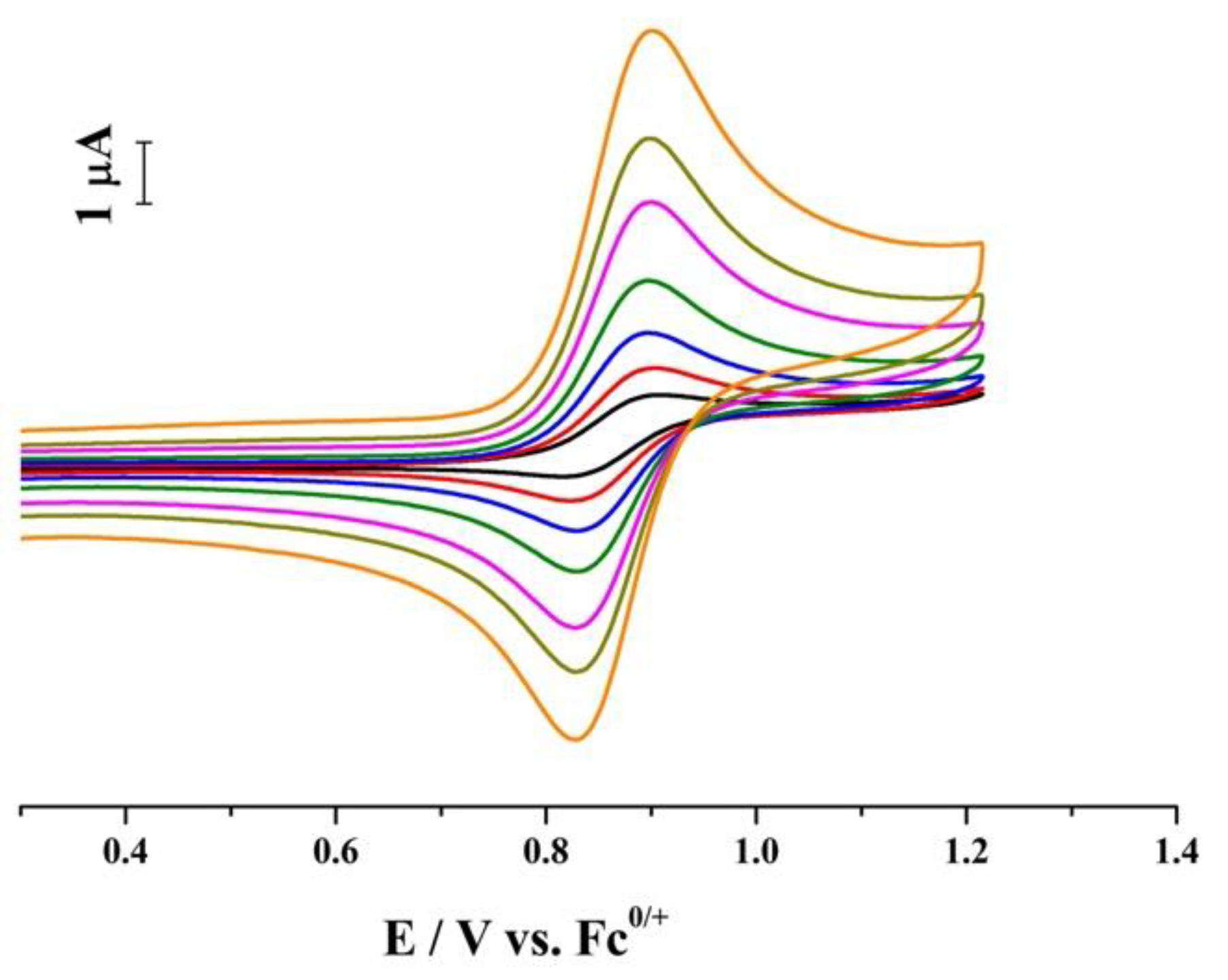
| IL | Scan Rate V s−1 | μA | ∆Ep mV | Em V | Ep−Ep/2 mV | c | 107 D d cm2 s−1 | k1f s−1 |
|---|---|---|---|---|---|---|---|---|
| [bmim][PF6] | 0.1 | 1.79 | - | 0.891 a | 91 | - | - | 1.8 × 104 |
| 0.2 | 2.70 | - | - | |||||
| 0.4 | 3.50 | - | - | |||||
| [bmim][BF4] | 0.1 | 1.42 | 67 | 0.831 b | 68 | 0.76 | 1.2 d | 18 |
| 0.2 | 1.90 | 75 | 0.76 | |||||
| 0.4 | 2.68 | 75 | 0.82 | |||||
| [emim][NTf2] | 0.1 | 2.02 | 72 | 0.838 b | 64 | 0.75 | 3.5 d | 0.45 |
| 0.2 | 2.92 | 75 | 0.83 | |||||
| 0.4 | 4.27 | 84 | 0.87 | |||||
| [bmpyr][NTf2] | 0.1 | 1.26 | 71 | 0.840 | 62 | 0.83 | 1.6 d | 0.45 |
| 0.2 | 1.79 | 76 | 0.89 | |||||
| 0.4 | 2.46 | 88 | 0.92 | |||||
| [emim][OTf] | 0.1 | 2.42 | - | 0.869 a | 77 | - | 3.5 e | 8 × 103 |
| 0.2 | 3.08 | - | 0.57 | |||||
| 0.4 | 4.08 | - | 0.63 |
| Reference Electrode | Em (V) |
|---|---|
| Ag/AgOTf, [emim][OTf] | −0.250 |
| Ag/AgOTf, [bmim][PF6] | 0.043 |
| Ag/AgOTf, [bmim][BF4] | −0.583 |
| Ag/AgOTf, [bmpyr][NTf2] | −0.391 |
| Ag/AgOTf, [emim][ NTf2] | −0.374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torriero, A.A.J. Ionic Liquids Effect on the Stability of 17-Electron Cation Product of the Electrochemical Oxidation of Cymantrene. Molecules 2022, 27, 7428. https://doi.org/10.3390/molecules27217428
Torriero AAJ. Ionic Liquids Effect on the Stability of 17-Electron Cation Product of the Electrochemical Oxidation of Cymantrene. Molecules. 2022; 27(21):7428. https://doi.org/10.3390/molecules27217428
Chicago/Turabian StyleTorriero, Angel A. J. 2022. "Ionic Liquids Effect on the Stability of 17-Electron Cation Product of the Electrochemical Oxidation of Cymantrene" Molecules 27, no. 21: 7428. https://doi.org/10.3390/molecules27217428
APA StyleTorriero, A. A. J. (2022). Ionic Liquids Effect on the Stability of 17-Electron Cation Product of the Electrochemical Oxidation of Cymantrene. Molecules, 27(21), 7428. https://doi.org/10.3390/molecules27217428






