Abstract
Prangos ferulacea (L.) Lindl, which belongs to the Apiaceae family, is a species that mainly grows in the eastern Mediterranean region and in western Asia. It has been largely used in traditional medicine in several countries and it has been shown to possess several interesting biological properties. With the aim to provide new insights into the phytochemistry and pharmacology of this species, the essential oils of flowers and leaves from a local accession that grows in Sicily (Italy) and has not yet been previously studied were investigated. The chemical composition of both oils, obtained by hydrodistillation from the leaves and flowers, was evaluated by GC-MS. This analysis allowed us to identify a new chemotype, characterized by a large amount of (Z)-β-ocimene. Furthermore, these essential oils have been tested for their possible antimicrobial and antioxidant activity. P. ferulacea essential oils exhibit moderate antimicrobial activity; in particular, the flower essential oil is harmful at low and wide spectrum concentrations. They also exhibit good antioxidant activity in vitro and in particular, it has been shown that the essential oils of the flowers and leaves of P. ferulacea caused a decrease in ROS and an increase in the activity of superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) in OZ-stimulated PMNs. Therefore, these essential oils could be considered as promising candidates for pharmaceutical and nutraceutical preparations.
1. Introduction
Prangos Lindley. is a genus of the Apiaceae family (subfamily of Apioideae), and according to the Plant of the World Online database [1], it comprises forty-eight accepted species that are widely distributed from Portugal to Tibet, with the Irano-Turanian region being the center of diversity [2]. This genus belongs to the Cachrys group, which also includes the genera Cachrys, Alolacarpum, Bilacunaria, Ferulago, Diplotaenia, Eriocycla, Azilia and Hippomarathrum [3,4].
In Iran, Turkey, Iraq and other Asian countries, the genus Prangos is used as an aromatic and medicinal herb. Both the roots and the aerial parts of the different species are used and, in the literature, scholars have reported the extensive use of essential oils. These have been used to treat gastrointestinal problems, but their uses as an aphrodisiac, coagulant, carminative and tonic have also been reported [5]. Some articles have described the isolation of non-volatile metabolites, such as coumarins, linear and angular furocoumarins, flavonoids, terpenoids [5], and, very recently, a review on the non-volatile metabolites (coumarins and flavonoids) of P. ferulacea (L.) Lindl. (syn. C. ferulacea (L.) Calest.) and their biological properties has been published [6].
Regarding volatile oil compositions, the most studied taxon is P. ferulacea (L.) Lindl. [6,7], although several other investigations that concern other species have been published [5,8,9,10,11,12,13]. Essential oils have always been widely used for different purposes, not only as ingredients in perfumes or cosmetic applications, but also, and most importantly, for medical purposes. They have shown antibacterial, antifungal, virucidal, antiparasitic and insecticidal properties and function as analgesics, sedatives and anti-inflammatories; therefore, they are widely used in the pharmaceutical industry [14].
Prangos ferulacea (L.) Lindl. (synonyms: Cachrys alata Hoffm., C. cylindracea Guss. ex DC., C. ferulacea (L.) Calest., C. goniocarpa Boiss., C. prangoides Boiss., Laserpitium ferulaceum L., Prangos alata Grossh., P. biebersteinii Karjagin, P. foeniculacea C.A. May; Smyrnium laserpitioides Crantz) [1] mainly grows in the eastern Mediterranean region and western Asia on arid, stony, mountain pastures, preferentially on basic soils. It is quite widespread in Sicily where it prefers carbonate mountains, above 1000 m of altitude. In Sicily (Madonie Mountains), grazing cattle and sheep eat the species, giving their milk and derived dairy products, such as cheese and salted ricotta, a characteristic smell and flavor [15].
In Turkey, where P. ferulacea is known as ‘heliz’, this plant provides a characteristic smell and taste to the very famous cheese ‘Otlu’; furthermore, the addition of this plant provides the cheese with antimicrobial properties [16]. In the central, southern, and eastern parts of Turkey, where its vernacular name is ‘çaşir’, the plant is utilized as a vegetable and is boiled, fried, or pickled [17]. Several etnopharmaceutical properties have been reported in Iran, where P. ferulacea (‘jashir’) has been used as a carminative, emollient and tonic for gastrointestinal and liver disorders and has anti-flatulent, sedative, anti-inflammatory, anti-viral, anti-helminthic, antifungal, and antibacterial properties [18]. It is also used in food and yogurt seasoning [19].
In the frame of our ongoing research on the volatiles from the family Apiaceae [20,21,22,23,24], we describe the essential oil composition of an accession of P. ferulacea growing in Sicily that has not yet been reported, together with its antioxidant and antimicrobial activities.
2. Results and Discussion
2.1. Chemical Composition
Fifteen volatile components were identified by GC-MS in the essential oil of flowers from this Sicilian accession of P. ferulacea, and they accounted for 96.58% of the total composition (Table 1). The oil was dominated by monoterpene hydrocarbons (13 components, 94.22%), whereas oxygenated monoterpenes (1 component) were represented only by 4-terpineol (2.08%). The amount of sesquiterpene hydrocarbons (one component, 0.28%) reported was negligible. The major constituent that represented about half of the oil was (Z)-β-ocimene (44.44%). Other monoterpene hydrocarbons that occurred in noteworthy percentages were sabinene (2.80%), 3-thujene (5.79%), and α-pinene (4.28%).

Table 1.
Essential oil composition (%) of the flowers and leaves of Prangos ferulacea.
The composition of the oil from the leaves was quite similar. Nineteen metabolites were identified, accounting for 96.22% of the total composition (Table 1). In addition, the essential oil was characterized by a large amount of monoterpene hydrocarbons (13 components, 88.63%), with the largest amount of (Z)-β-ocimene (61.91%) in comparison to the oil from the flowers. Among the sesquiterpene hydrocarbons (six compounds, 9.59%) the occurrence of caryophyllene should be noted (7.65%), which was completely absent in the oil from the flowers.
In botanical medicine, the presence of β-ocimene in essential oils of several plants has been associated with anticonvulsant activity, antifungal activity, and antitumor activity [25,26,27,28]. Ocimene is also a volatile pheromone that is important for the social regulation of honeybee colonies and its involvement in the plant defensive system against herbivore attacks has been reported [29]. Within the Apiaceae family, this component was found to be particularly abundant in the essential oil from Helosciadium nodiflorum (L.) W.D.J. Koch (Apiaceae), contributing to the plant’s insecticidal capacity [30,31,32].
This analysis showed the occurrence of a new chemotype for this Sicilian accession of P. ferulacea that is characterized by a large amount of (Z)-β-ocimene. A previous investigation into another Sicilian accession showed the presence of two isomers of β-ocimene [7], whereas Seidi Damyeh et al. [33], using an essential oil sample from Iran, reported only the E-isomer of β-ocimene as the most abundant volatile component (28.3%). Considerable variability in the chemical profiles of the essential oils of the populations of P. ferulacea of different geographical origin has been reported and recently reviewed [6]. In fact, for a Turkish accession of P. ferulacea, 2,3,6-trimethyl benzaldehyde was reported as the predominant constituent (66.59%) [34], whereas β-pinene (43.1%), α-pinene (22.1%) and δ-3-carene (16.9%), (E)-caryophyllene (48.2%), α-humulene (10.3%) and spathulenol (9.4%), terpinolene (38.1–56.3%), α-pinene (57%), and α-pinene (36.6%) and β-pinene (31.1%), respectively were detected as volatile markers of different Iranian populations [6]. The chemical diversity between the different oils compared is linked to many factors, such as pedoclimatic conditions, including the climate, amount of rainfall, altitude, distance and effects of proximity to the sea and exposure to wind and sun. Sabinene, the second most abundant component in our oils, was detected among the main constituents only in the essential oil of the fruits of a Sardinian population (15.9%) [35].
2.2. Essential Oil Antimicrobial Activity
Some studies show that plants extracts or components that belong to the genus Prangos exert significant antibacterial, antifungal, antioxidant, anti-inflammatory, hypoglycemic and analgesic activities [5,6]. This study evaluates the antimicrobial and antioxidant activity of P. ferulacea essential oils from its flowers and leaves. The essential oils were tested using vital cell counts to produce data for dose–response curves and the MIC assay. In the first experiment, P. ferulacea oils were used against the following Gram-positive strains: Staphylococcus aureus ATCC6538P, Bacillus subtilis AZ54, Bacillus cereus ATCC10987, and Gram-negative strains, such as Escherichia coli DH5α, Pseudomonas aeruginosa PAOI, and Salmonella tiphymurium ATCC14028. We carried out experiments using the vital count method to create the dose–response curves shown in Figure 1. This figure shows that the oil extract from the flowers (panel A) and leaves (panel B) exhibits dose-dependent antimicrobial activity, the oil extracted from the flowers exhibits good antimicrobial activity against both Gram-positive and negative strains, while the oil extracted from the leaves exhibits greater antimicrobial activity directed against Gram-positive strains. In general, the most sensitive bacteria are the Gram-positive bacilli, which are almost completely killed at a concentration of 200 µg/mL with both essential oils. More details can be found in Supplementary Materials.
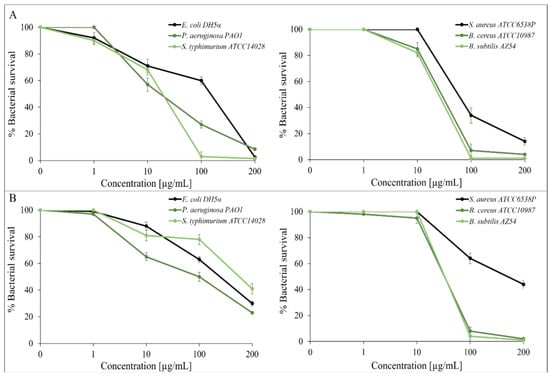
Figure 1.
Antimicrobial activity of P. ferulacea flowers oil (A) and leaves oil (B) at different concentrations (0; 1; 10; 100 and 200 µg/mL) valuated by colony count assay, after 4 h of incubation, against Gram-negative E. coli DH5α, P. aeruginosa PAO1, S. typhimurium ATCC14028 and Gram-positive S. aureus ATCC6538P, B. cereus ATCC10987 and B. subtilis AZ54. The % of bacterial survival is represented by the y axis. The assays were performed in three independent experiments.
The antimicrobial activity of the P. ferulacea oils was also analyzed according to the broth microdilution method. The minimum inhibitory concentration (MIC) values of the oil from P. ferulacea flowers were found to be between 100 and 200 µg/mL for Gram-positive and Gram-negative bacteria, as shown in Table 2. For the P. ferulacea essential oil extract from its leaves, MIC values of 100 and 200 µg/mL were reported against Gram-positive and Gram-negative bacteria. The lowest oil concentrations that inhibited bacterial growth were recorded for the Gram-positive bacilli, according to our previous experiments. Therefore, essential oils that are abundant in terpenes have been shown to possess remarkable antimicrobial activity. However, the weak activity against Gram-negative bacteria could be due to the presence of their outer and inner membranes that protect the Gram-negative bacteria from the effect of the oil components [36].

Table 2.
Minimum inhibitory concentration values (MIC µg/mL) of P. ferulacea oils against Gram-positive and Gram-negative bacteria. Values were obtained from a minimum of three independent experiments.
Different studies have reported that extracts, essential oils and pure compounds of the Prangos species have shown strong antibacterial, antifungal, and antiviral activity. For example, Gram-positive bacteria such as S. aureus and B. cereus were inhibited by various Prangos species, such as P. pabularia and P. platychlaena. In a study performed with different extracts of P. hulusii roots collected in Turkey, the strongest antibacterial activity was associated with the dichloromethane extract of the plant with E. coli with an MIC value of 0.156 mg/mL [37,38].
Regarding the composition of the oils used in our study, monoterpene hydrocarbons accounted for 94.17% and 88.63% for the flowers and leaves, respectively, which could be responsible for most of the antimicrobial activity. However, when the sample under analysis is a complex mixture, it is difficult to attribute the activity to a single molecule or to a group of constituents. Furthermore, in the literature, there is evidence that minor components with synergic action play a critical part in antimicrobial activity [39]. So, for this reason, it is likely that the bioactivity of our oils is due to a synergistic effect between the different compounds present in P. ferulacea essential oil [5].
2.3. Essential Oil Antioxidant Activity
Free radicals produced in all organisms can cause oxidative damage to biological molecules such as DNA, fatty acids, and amino acids. Consequently, oxidative stress plays an important role in the development of some chronic diseases [39], so plants or their extracts with antioxidant activity can play a role in the protection of the health of living organisms. Due to the presence of hydrocarbon monoterpenes in the P. ferulacea essential oils, its radical scavenging ability was evaluated. Figure 2 shows the increasing the % of scavenging activities of ABTS and H2O2 radicals as the concentration of oil (1–1000 µg/mL) increases.
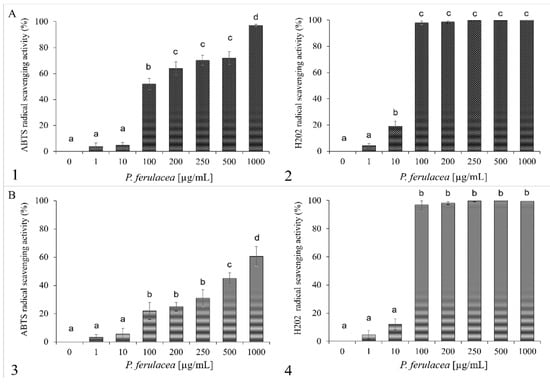
Figure 2.
Antioxidant activity of P. ferulacea flower oil (A) and leaf oil (B). ABTS radical scavenging activity (1, 3) was measured after 10 min of incubation and reported as % of ABTS removed, with respect to the control. Hydrogen peroxide scavenging activity (2, 4) was measured after 30 min of incubation and reported as % of H2O2 removed, with respect to the control. Data were presented as mean and standard error and they were analyzed with a paired t-test. Bars not accompanied by the same letter were significantly different at p < 0.05.
The data shown in Figure 2 are expressed in Table 3 as IC50 values, which demonstrate the oil concentration that causes a 50% reduction in ABTS and H2O2 radicals. The P. ferulacea essential oil extract from flowers shows anti-H2O2 activity with IC50 values of 60 µg/mL and the highest anti-radical effect (IC50 value of 100 µg/mL) for ABTS. P. ferulacea leaf oil shows anti-H2O2 activity with IC50 values of 50 µg/mL and the highest anti-radical effect (IC50 value of 500 µg/mL) for ABTS.

Table 3.
IC50: concentration that inhibited 50% of the free radicals; ABTS: 2,20-azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid); H2O2: hydrogen peroxide. Positive control was represented by ascorbic acid for ABTS; and resveratrol for H2O2.
2.4. Antioxidant Enzymes Measured in PMN Cells
The antioxidant activity of P. ferulacea was investigated by testing the essential oils from flowers and leaves on OZ-stimulated PMNs to induce oxidative stress. Both ROS levels and the activity of SOD, CAT and GST enzymes were evaluated. As can be observed from Figure 3, as a result of the stress induced by OZ, there is a significant increase in ROS, but by treating the PMN with the flowers’ oil, a gradual reduction that is proportional to the increase in concentration is observed. In fact, in the PMNs treated with 25 µg of flower essential oil, a significant reduction in ROS levels can also be observed, and in addition, in the PMNs treated with 100 µg of flower essential oil, ROS levels comparable to the control (non-stressed PMN) can be observed. The leaf oil, on the other hand, induces a significant reduction in ROS levels to 100 µg.
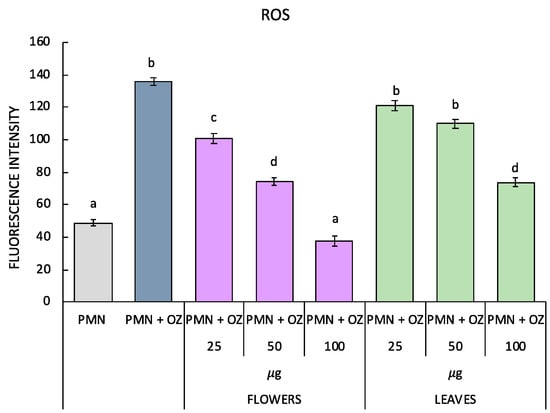
Figure 3.
ROS production in polymorphonuclear cells treated with essential oils of P. ferulacea at concentrations of 0, 25, 50, 100 µg/mL with or without OZ (0.5 mg mL−1). Data were presented as mean and standard error and they were analyzed with a paired t-test. Bars not accompanied by the same letter were significantly different at p < 0.05.
As for the activity of antioxidant enzymes in PMNs treated with essential oils extracted from flowers, they show the same trend. In fact, as can be observed from Figure 4, the activity of CAT SOD and GST statistically increases as the concentration of flower essential oil increases. As for the essential oil of leaves, the activity of SOD and CAT increases in the PMNs treated with 50 and 100 µg compared to the PMNs stimulated with OZ, which were not treated with essential oils. With regard to the activity of GST, it increases in PMNs treated with 50 µg of leaf essential oil compared to PMN that is stimulated with OZ but not treated with essential oils.
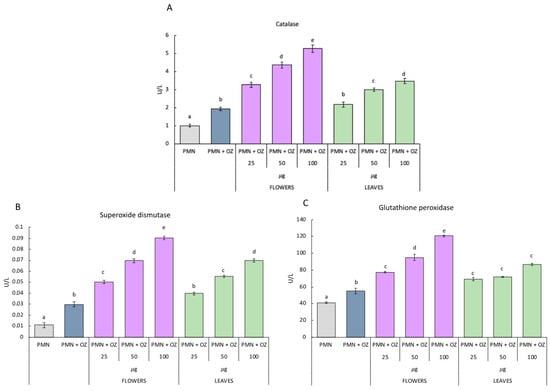
Figure 4.
Activities of antioxidant enzymes: catalase (A); superoxide dismutase (B) and glutathione peroxidase (C) in polymorphonuclear cells treated with essential oils of P. ferulacea at the concentrations of 0, 25, 50, 100 µg/mL with or without OZ (0.5 mg mL−1). Data were presented as mean and standard error and they were analyzed with a paired t-test. Bars not accompanied by the same letter were significantly different at p < 0.05.
Although the main components are expected to be mainly responsible for the antioxidant activity of an essential oil, the key role of some minor components in decreasing or increasing the antioxidant activity cannot be ignored [40].
Ruberto and Baratta [41] have studied the antioxidant efficacy of one hundred pure components of essential oils. The main classes of compounds were analyzed, namely monoterpene hydrocarbons, oxygenated monoterpenes, sesquiterpene hydrocarbons, oxygenated sesquiterpenes, benzene derivatives and nor-isoprenoid components, including alcohols, aldehydes and ketones, which represent the most common constituents of essential oils.
In the work of Çoruh et al. [42], the antioxidant capacities of Heracleum persicum Desf., Prangos ferulacea (L.) Lindl. and Chaerophyllum macropodum Boiss were evaluated. From this comparison, it emerged that P. ferulacea was a better antioxidant than the other two plants in the family. The results of our study showed high antioxidant activity, expressed as the activity of the SOD, CAT, and GST enzymes on the PMNs and, in particular, significant variation in the antioxidant capacity of the different parts of P. ferulacea emerged. The results revealed that the organ can significantly affect their antioxidant abilities. The highest antioxidant activity was recorded for the essential oils of flowers, which can be considered natural antioxidants with higher activity than the essential oils of leaves.
It is assumed that the difference in antioxidant capacity between the two organs of the plant is due to the different compositions of essential oils. The antioxidant efficacy of γ-terpinene, camphene and β-ocimene present in some essential oils has already been demonstrated by Negi et al. in 2012 [43]. α-pinene and limonene may also be responsible for the antioxidant potential [44]. This would explain why greater activity was demonstrated by the extracts of essential oils of flowers compared to those of leaves. In fact, the compounds mentioned are present in greater quantities in the essential oils of the flowers compared to the essential oils of the leaves, with the sole exception of β-ocimene, which is also present in high quantities in the essential oils of the flowers.
3. Materials and Methods
3.1. Plant Material
Flowers (470 g) and leaves (250 g) from twenty individuals of Prangos ferulacea (L.) Lindl., covering about 200 m2, were collected at Piano Zucchi, Palermo, Sicily, Italy, at about 1100 m s/l, 37°53′51″ N; 13°59′50″ E, in June 2022. The samples, identified by Prof. Vincenzo Ilardi (Department STEBICEF, University of Palermo, Italy) by comparisons with the descriptions reported in books and authentic samples, have been stored in the Herbarium of the University of Palermo (Voucher No. PAL 109762).
3.2. Isolation of Volatile Components
Extraction of essential oils was carried out according to the method of Basile et al. [45]. Using a Waring blender, the dried samples, immersed in 0.5 L of distilled water, were extracted by hydrodistillation following the methods reported in [46]. The dried oils were sealed in vials and stored in the freezer (−20 °C) until the time of analysis. The yields of the two oils were 0.62% and 0.28% (w/w) for flowers and leaves, respectively.
3.3. GC-MS Analysis
Analyses of essential oils were performed according to the procedure reported by Badalamenti et al. [47].
3.4. Bacterial Strains
The Gram-negative strains Escherichia coli DH5α, Pseudomonas aeruginosa PAOI and Salmonella tiphymurium ATCC14028 and Gram-positive strains Staphylococcus aureus ATCC6538P, Bacillus subtilis AZ59 and Bacillus cereus ATCC10987 were used to evaluate antimicrobial activity. These strains were grown in LB medium, under agitation at 37 °C.
3.5. Antimicrobial Activity Assay
The method used to evaluate the antimicrobial activity was the cell viability counting method of bacteria [48]. Gram-positive and Gram-negative strains were incubated with both essential oils at different concentrations (1, 10, 100, and 200 µg/mL). Bacterial cells without essential oils represented the positive control and cells with DMSO at 80% concentrations were used as the negative control. Each experiment was carried out in triplicate and the reported result was an average of three independent experiments. (p value was <0.05).
3.6. Determination of Minimal Inhibitory Concentration
The microdilution method established by the Clinical and Laboratory Standards Institute (CLSI) was used to evaluate of minimal inhibitory concentrations (MICs) of P. ferulacea essential oil against the Gram-positive and Gram-negative strains. In addition, ~5 × 105 CFU/mL was added to 95 µL of Mueller–Hinton broth (CAM-HB; Difco) with or without P. ferulacea essential oil at different concentrations (1–200 µg/mL) [49]. After overnight incubation at 37 °C, MIC100 values were determined as the lowest concentration responsible for the absence of visible bacterial growth. Each experiment was conducted in triplicate and the reported result was an average of three independent experiments.
3.7. ABTS Scavenging Capacity Assay
The experiments, according to the reported method [50] with some modifications, are based on ABTS radical cation scavenging. The ABTS solution was prepared to achieve a final absorbance of 0.72 (±0.2) at 734 nm. Then, 1 mL ABTS solution was added to 100 µL of oil (1; 10; 100; 200; 250; 500 and 1000 µg/mL concentrations). Absorbance of ABTS was recorded after 6 min of incubation in the dark, at 734 nm. Finally, the absorbance was measured at 734 nm against a blank, and the percentage inhibition of ABTS radicals was determined from the following equation: ABTS•+ radical scavenging activity (%) = (1 − AS/AC) × 100, where AC represented the absorbance of the ABTS solution and AS was the absorbance of the sample at 734 nm. The concentration required for 50% inhibition was calculated as IC50. Each experiment was carried out in triplicate and the reported result was an average of three independent experiments.
3.8. Hydrogen Peroxide Scavenging Assay
The quantitative determination of H2O2 scavenging activity was recorded by the loss of absorbance at 240 nm, as described by Beers and Sizer [51,52]. Various concentrations of essential oil (1; 10; 100; 200; 250; 500 and 1000 µg/mL) were incubated in 1 mL of hydrogen peroxide solution (50 mM potassium phosphate buffer, pH 7.0; 0.036% (w/w) H2O2). After 30 min, the H2O2 concentration was measured at 240 nm. The percentage of peroxide removed was calculated using the following equation: peroxide removed (%) = (1 − AS/AC) × 100, where AC is the absorbance of 1 mL of H2O2 solution and AS is the absorbance of the sample at 240 nm.
3.9. Antioxidant Enzymes Measured in PMN Cells
The enzymatic antioxidant activity of superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) measured in PMN cells was determined using commercial kit protocols (BioAssay System, San Diego, CA, USA). The activity of the enzymes was expressed as U/L [53]. The essential oils of P. ferulacea were tested at a concentration of 25, 50, 100 µg/mL. The experiments were performed in the presence and absence of OZ (0.5 mg mL−1).
3.10. Reactive Oxygen Species ROS Generation
The dichlorofluorescein (DCF) assay was performed to quantify ROS generation, following the protocol of Manna et al. [54]. The PMNs were treated with P. ferulacea essential oils at a concentration of 25, 50, 100 µg/mL with or without OZ (0.5 mg/mL), following the protocol of Napolitano et al. [55].
3.11. Statistical Analysis
4. Conclusions
Plants of the Prangos genus are considered a great source of phytochemicals with therapeutic and economic applications. Given the growing demand for natural products, many Prangos species have been grown for their use in traditional medicine, the food market, the cosmetic industry and for ornamental purposes. In this work, the chemical and biological properties of the essential oils obtained from the leaves and flowers of P. ferulacea have been analyzed. From the GC-MS analysis, it can be observed that the two essential oils are characterized by the important presence of hydrocarbon monoterpenes, dominated by the high percentage of (Z)-β-ocimene. Although P. ferulacea essential oils showed only moderate antimicrobial activity, both samples exhibited good antioxidant activity in vitro and in particular, they caused a decrease in ROS and an increase in the activity of superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) in OZ-stimulated PMNs. Therefore, these essential oils could be considered as promising candidates for pharmaceutical and nutraceutical preparations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217430/s1, Figure S1: E. coli bacterial cells observed via optical microscopy and fluorescence microscopy. Untreated bacterial cells (A,B); cells treated with P. ferulacea flower oil at a concentration of 200 µg/mL (C,D), cells treated with P. ferulacea leaf oil at a concentration of 200 µg/mL (E,F). References [56,57] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, M.B. and A.B.; methodology, A.Z., V.M. and M.D.N.; formal analysis, N.B.; investigation, V.M. and M.D.N.; data curation, N.B.; writing—original draft preparation, A.B., V.M, A.Z.; writing—review and editing, A.Z., V.M. and N.B.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors of the Department STEBICEF, University of Palermo, Italy.
References
- Plants of the World Online. Available online: https://powo.science.kew.org/ (accessed on 12 September 2022).
- Pimenov, M.G.; Tikhomirov, V.N. The taxonomic problems in the genera Prangos Lindl., Cachrys L., Cryptodiscus Schrenk and Hippomarathrum Hoffmanns. et Link (Umbelliferae–Apioideae). Feddes Reper. 1983, 94, 145–164. [Google Scholar]
- Lyskov, D.F.; Degtjareva, G.V.; Samigullin, T.H.; Pimenov, M.G. The revision of Prangos subsections Koelzella and Fedtschenkoana (Apiaceae) with some notes to phylogeny and biogeography of the genus: Molecular and morphological evidences. Plant Syst. Evol. 2017, 303, 815–826. [Google Scholar] [CrossRef]
- Ajani, Y.; Ajani, A.; Cordes, J.M.; Watson, M.F.; Downie, S.R. Phylogenetic analysis of nrDNA ITS reveals relationships within five groups of Iranian Apiaceae sub family Apioideae. Taxon 2008, 57, 383–401. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Kiss, T.; Tòth, B.; Csupor, D. The Prangos genus: A comprehensive review on traditional use, phytochemistry, and pharmacological activities. Phytochem. Rev. 2020, 19, 1449–1470. [Google Scholar] [CrossRef]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. The nonvolatile and volatile metabolites of Prangos ferulacea and their biological properties. Planta Med. 2019, 85, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Ilardi, V.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Fiorini, D.; Venditti, A.; Maggi, F. Composition and biological activities of the essential oil from a Sicilian accession of Prangos ferulacea (L.) Lindl. Nat. Prod. Res. 2021, 35, 733–743. [Google Scholar] [CrossRef]
- Meshkatalsadat, M.H.; Mirzaei, H.H. Chemical compositions of the essential oils of stems, leaves and flowers of Prangos acaulis (DC) Bornm. Pak. J. Biol. Sci. 2007, 10, 2775–2777. [Google Scholar] [CrossRef][Green Version]
- Meshkatalsadat, M.H.; Bamoniri, A.; Batooli, H. The bioactive and volatile constituents of Prangos acaulis (DC) Bornm extracted using hydrodistillation and nano scale injection techniques. Dig. J. Nanomater. Biostruct. 2010, 5, 263–266. [Google Scholar]
- Mirzaei, H.H.; Meshkatalsadat, M.H.; Soheilivand, S. Determination of essential oil composition of Prangos acaulis (DC) Bornm obtained by hydrodistillation and supercritical fluid extraction methods. J. Appl. Sci. 2007, 7, 2535–2538. [Google Scholar] [CrossRef][Green Version]
- Moharramipour, S.; Taghizadeh, A.; Meshkatalsadat, M.H.; Fathipour, Y.; Talebi, A.A. Repellent activity and persistence of essential oil extracted from Prangos acaulis to three stored-product beetles. Am.-Eurasian. J. Sustain. Agric. 2009, 3, 202–204. [Google Scholar]
- Rustaiyan, A.; Mazloomifar, H.; Masoudi, S.; Aghjani, Z. Volatile oils of Ducrosia assadii Alava. and Prangos acaulis (DC.) Bornm. from Iran. J. Essent. Oil Res. 2006, 18, 682–684. [Google Scholar] [CrossRef]
- Velikorodov, A.V.; Pilipenko, V.N.; Pilipenko, T.A.; Malyi, S.V. Studying the chemical composition of essential oil received from fruits of Prangos odontalgica wild-growing in Astrakhan Region. Khimiya Rastit. Syr’ya 2019, 3, 95–101. [Google Scholar]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Raimondo, F.A. Distribuzione es ecologia di Cachrys ferulacea (L.) Calestani interessante foraggera dei pascoli alto montani della Sicilia. Boll. Stud. Inform. Bot. Giardin. Colon. 1974, 26, 116–129. [Google Scholar]
- Dagdelen, S.; Bilenler, T.; Durmaz, G.; Gokbulut, I.; Hayaloglu, A.A.; Karabulut, I. Volatile composition, antioxidant and antimicrobial activities of herbal plants used in the manufacture of Van Herby (OTLU) cheese. J. Food Process. Pres. 2014, 38, 1716–1725. [Google Scholar] [CrossRef]
- Özcan, M.M.; Dursun, N.; Arslan, D. Some nutritional properties of Prangos ferulacea (L.) Lindl and Rheum ribes L. stems growing wild in Turkey. Int. J. Food Sci. Nutr. 2007, 58, 162–167. [Google Scholar] [CrossRef]
- Kafash-Farkhad, N.; Asadi-Samani, M.; Khaledifar, B. A review on secondary metabolites and pharmacological effects of Prangos ferulacea (L.) Lindl. Life Sci. J. 2013, 10, 360–367. [Google Scholar]
- Sadraei, H.; Shokoohinia, Y.; Sajjadi, S.E.; Mozafari, M. Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction. Res. Pharm. Sci. 2012, 8, 137–144. [Google Scholar]
- Badalamenti, N.; Ilardi, V.; Rosselli, S.; Bruno, M.; Maggi, F.; Leporini, M.; Falco, T.; Loizzo, M.R.; Tundis, R. Ferulago nodosa subsp. geniculata (Guss.) Troia & Raimondo from Sicily (Italy): Isolation of essential oil and evaluation of its bioactivity. Molecules 2020, 25, 3249. [Google Scholar] [CrossRef]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 19, 1–6. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the essential oils of Thymus vulgaris L. and Crithmum maritimum L. as biocidal on two Tholu Bommalu Indian leather puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, V.; Badalamenti, N.; Bruno, M. Chemical composition of the essential oil from different vegetative parts of Foeniculum vulgare subsp piperitum (Ucria) Coutinho (Umbelliferae) growing wild in Sicily. Nat. Prod. Res. 2021, 36, 3587–3597. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Maggio, A.; Badalamenti, N.; Bruno, M.; D’Angelo, G.D.; D’Anneo, A. Essential oil of Foeniculum vulgare subsp. piperitum fruits exerts an anti-tumor effect in triple-negative breast cancer cells. Mol. Med. Rep. 2022, 26, 243. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, L.M.; Menezes, L.R.A.; Rodrigues, A.C.B.C.; Dias, R.B.; Gurgel Rocha, C.A.; Soares, M.P.B.; Neto, A.F.S.; Nascimento, P.M.; Campos, A.F.; Silva, L.C.R.C.; et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 2016, 118, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Nadjafnia, L.; Kamalinejad, M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J. Ethnopharmacol. 2004, 94, 283–287. [Google Scholar] [CrossRef]
- Maggi, F.; Giuliani, C.; Fico, G.; Ricciutelli, M.; Bramucci, M.; Quassinti, L.; Petrelli, D.; Vitali, L.A.; Cianfaglione, K.; Tirillini, B.; et al. Secondary metabolites, secretory structures and biological activity of water celery (Apium nodiflorum (L.) Lag.) growing in central Italy. Plant Biosyst. 2018, 153, 325–335. [Google Scholar] [CrossRef]
- Thakre, A.D.; Mulange, S.V.; Kodgire, S.S.; Zore, G.B.; Karuppayil, S.M. Effects of cinnamaldehyde, ocimene, camphene, curcumin and farnesene on Candida albicans. Adv. Microbiol. 2016, 6, 627–643. [Google Scholar] [CrossRef]
- Cascone, P.; Iodice, L.; Maffei, M.E.; Bossi, S.; Arimura, G.I.; Guerrieri, E. Tobacco overexpressing β-ocimene induces direct and indirect responsesagainst aphids in receiver tomato plants. J. Plant Physiol. 2015, 173, 28–32. [Google Scholar] [CrossRef]
- Afshar, F.H.; Maggi, F.; Iannarelli, R.; Cianfaglione, K.; Isman, M.B. Comparative toxicity of Helosciadium nodiflorum essential oils and combinations of their main constituents against the cabbage looper, Trichoplusia ni (Lepidoptera). Ind. Crops Prod. 2017, 98, 46–52. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Ricciutelli, M.; Lupidi, G.; Maggi, F. Efficacy of the volatile oil from water celery (Helosciadium nodiflorum, Apiaceae) against the filariasis vector Culex quinquefasciatus, the housefly Musca domestica and the African cotton leafworm Spodoptera littoralis. Chem. Biodivers. 2017, 14, e1700376. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Volatile β-ocimene can regulate developmental performance of peach aphid Myzus persicae through activation of defense responses in Chinese cabbage Brassica pekinensis. Front. Plant Sci. 2018, 9, 708. [Google Scholar] [CrossRef] [PubMed]
- Seidi Damyeh, M.; Niakousari, M.; Saharkhiz, M.J. Ultrasound pretreatment impact on Prangos ferulacea Lindl. and Satureja macrosiphonia Bornm. essential oil extraction and comparing their physicochemical and biological properties. Ind. Crops Prod. 2016, 87, 105–115. [Google Scholar] [CrossRef]
- Sumer Ercan, F.; Bas, H.; Koc, M.; Pandir, D.; Oztemiz, S. Insecticidal activity of essential oil of Prangos ferulacea (Umbelliferae) against Ephestia kuehniella (Lepidoptera: Pyralidae) and Trichogramma embryophagum (Hymenoptera: Trichogrammatidae). Turk. J. Agric. For. 2013, 37, 719–725. [Google Scholar] [CrossRef]
- Bertoli, A.; Pistelli, L.; Morelli, I.; Spinelli, G.; Manunta, A. Constituents of Cachrys ferulacea oils. J. Essent. Oil Res. 1998, 10, 533–536. [Google Scholar] [CrossRef]
- Hojjati, M.; Barzegar, H. Chemical composition and biological activities of lemon (Citrus limon) leaf essential oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef]
- Tan, N.; Yazici-Tutunis, S.; Bilgin, M.; Tan, E.; Miski, M. Antibacterial activities of pyrenylated coumarins from the roots of Prangos hulusii. Molecules 2017, 22, 1098. [Google Scholar] [CrossRef]
- Yazici, T.S.; Tan, N.; Mericli, F.; Özsoy, N.; Tan, E. Biological activities of endemic Prangos hulusii. Planta Med. 2013, 79, PN113. [Google Scholar] [CrossRef]
- Mneimne, M.; Baydoun, S.; Nemer, N.; Arnold Apostolides, N. Chemical vomposition and evaluation of antimicrobial activity of essential oils isolated from Achillea kotschyi Boiss. subsp. kotschyi (Asteraceae) of Lebanon. Pharm. Chem. J. 2016, 3, 91–98. [Google Scholar]
- Sarma, A.D.; Mallick, A.R.; Ghosh, A.K. Free radicals and their role in different clinical conditions: An overview. Int. J. Pharm. Sci. Res. 2010, 1, 185–192. [Google Scholar]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Coruh, N.U.R.S.E.N.; Celep, A.S.; Özgökçe, F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007, 100, 1237–1242. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Bisht, R.; Kandari Negi, S. Major constituents, antioxidant and antibacterial activities of Zanthoxylum armatum DC. essential oil. Iran. J. Pharm. Res. 2012, 11, 68–70. [Google Scholar]
- Dhami, A.; Singh, A.; Palariya, D.; Kumar, R.; Prakash, O.; Rawat, D.S.; Pant, A.K. α-Pinene rich bark essential oils of Zanthoxylum armatum DC. from three different altitudes of Uttarakhand, India and their antioxidant, in vitro anti-inflammatory and antibacterial activity. J. Essent. Oil Bear. Plants 2019, 22, 660–674. [Google Scholar] [CrossRef]
- Basile, S.; Badalamenti, N.; Riccobono, O.; Guarino, S.; Ilardi, V.; Bruno, M.; Peri, E. Chemical composition and evaluation of insecticidal activity of Calendula incana subsp. maritima and Laserpitium siler subsp. siculum essential oils against stored products pests. Molecules 2022, 27, 588. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 10.3. 2020. Determination of Essential Oils in Herbal Drugs, 2.8.12., 307. Available online: https://www.edqm.eu/ (accessed on 12 October 2022).
- Badalamenti, N.; Bruno, M.; Schicchi, R.; Geraci, A.; Leporini, M.; Gervasi, L.; Tundis, R.; Loizzo, M.R. Chemical compositions and antioxidant activities of essential oils, and their combinations, obtained from flavedo by-product of seven cultivars of Sicilian Citrus aurantium L. Molecules 2022, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, A.; Pizzo, E.; Di Maro, A.; Varcamonti, M.; D’Alessio, G. The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease. FEBS J. 2010, 277, 1921–1928. [Google Scholar] [CrossRef]
- Prencipe, F.; Zanfardino, A.; Di Napoli, M.; Rossi, F.; D’Errico, S.; Piccialli, G.; Mangiatordi, G.F.; Saviano, M.; Ronga, L.; Varcamonti, M.; et al. Silver (I) n-heterocyclic carbene complexes: A winning and broad spectrum of antimicrobial properties. Int. J. Mol. Sci. 2021, 22, 2497. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cationdecolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Petruk, G.; Donadio, G.; Lanzilli, M.; Isticato, R.; Monti, D.M. Alternative use of Bacillus subtilis spores: Protection against environmental oxidative stress in human normal keratinocytes. Sci. Rep. 2018, 8, 1745. [Google Scholar] [CrossRef]
- Beers, R.; Sizer, I. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Barbosa, P.O.; Pala, D.; Silva, C.T.; de Souza, M.O.; do Amaral, J.F.; Vieira, R.A.L.; de Freitas Folly, G.A.; Volp, A.C.P.; de Freitas, R.N. Açai (Euterpe oleracea Mart.) pulp dietary intake improves cellular antioxidant enzymes and biomarkers of serum in healthy women. Nutrition 2016, 32, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.; Saha, P.; Sarkar, A.; Mukhopadhyay, D.; Bauri, A.K.; Kumar, D.; Das, P.; Chattopadhyay, S.; Chatterjee, M. Malabaricone-A induces a redox imbalance that mediates apoptosis in U937 cell line. PLoS ONE 2012, 7, e36938. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Cicio, A.; Bruno, M.; Zanfardino, A. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira Island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry 2022, 203, 113373. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 30, 3955. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Zanfardino, A.; Taleei, A.; Bushehri, A.A.S.; Hadian, J.; Maresca, V.; Sorbo, S.; Di Napoli, M.; Varcamonti, M.; Basile, A.; et al. Effect of heat stress on yield, monoterpene content and antibacterial activity of essential oils of Mentha piperita var. mitcham and Mentha arvensis var. piperascens. Molecules 2018, 23, 1903. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).