Abstract
Heterocyclic compounds containing nitrogen and sulfur, especially those in the thiazole family, have generated special interest in terms of their synthetic chemistry, which is attributable to their ubiquitous existence in pharmacologically dynamic natural products and also as overwhelmingly powerful agrochemicals and pharmaceuticals. The thiazolidin-2,4-dione (TZD) moiety plays a central role in the biological functioning of several essential molecules. The availability of substitutions at the third and fifth positions of the Thiazolidin-2,4-dione (TZD) scaffold makes it a highly utilized and versatile moiety that exhibits a wide range of biological activities. TZD analogues exhibit their hypoglycemic activity by improving insulin resistance through PPAR-γ receptor activation, their antimicrobial action by inhibiting cytoplasmic Mur ligases, and their antioxidant action by scavenging reactive oxygen species (ROS). In this manuscript, an effort has been made to review the research on TZD derivatives as potential antimicrobial, antioxidant, and antihyperglycemic agents from the period from 2010 to the present date, along with their molecular mechanisms and the information on patents granted to TZD analogues.
1. Introduction
Small heterocyclic structures containing nitrogen and sulfur have been under investigation for a long time owing to their therapeutic relevance. They offer a wide range of structural varieties and also possess a proven range of diversified therapeutic potentials. Amongst the extensive variety of heterocyclic scaffolds explored in the search for potent bioactive molecules in the process of drug discovery, the thiazolidin-2,4-dione (TZD) (Figure 1) ring system has been acknowledged as a significant scaffold in medicinal chemistry [1].
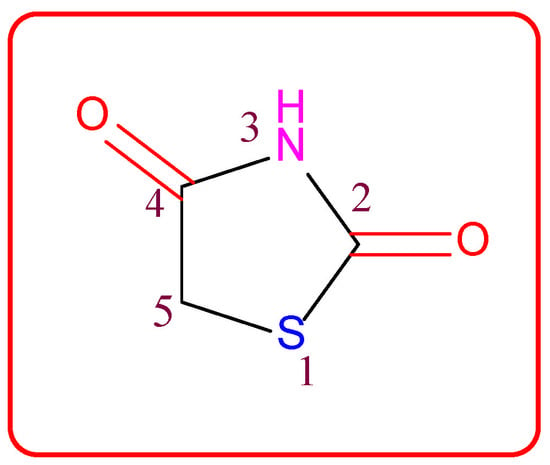
Figure 1.
Structure of the thiazolidine-2,4-dione scaffold.
Thiazolidin-2,4-dione (Figure 1), also called glitazone, is a heterocyclic moiety that consists of a five-membered saturated thiazolidine ring with sulfur at 1 and nitrogen at 3, along with two carbonyl functional groups at the 2 and 4 positions. Substitutions of various moieties are possible only at the third and fifth positions of the Thiazolidin-2,4-dione (TZD) scaffold. The analogues of TZD offer a wide range of structural varieties [2] and also possess a proven range of diversified therapeutic potentials, such as antidiabetic [3,4,5], analgesic, anti-inflammatory [6,7,8], wound healing [9], antiproliferative [10,11,12,13,14], antimalarial [15], antitubercular [16,17], hypolipidemic [18], antiviral [19], antimicrobial, antifungal [20,21,22,23], and antioxidant properties [24,25], etc.
2. The History of Glitazones as Antidiabetics
TZDs are primarily used as hypoglycemic agents over a long time. Ciglitazone is the prototype of the TZD class, which was developed by Takeda Pharmaceuticals (Japan) in the early 1980s but has never been used as a medication due to its hepatotoxicity. In the year 1988, another TZD analog, named troglitazone, was developed by the Sankyo Company (Japan) as an antidiabetic agent, but it was also banned in the year 2000 due to its hepatic toxicity. In 1999, Pfizer (USA) and Takeda (Japan) jointly developed two molecules, pioglitazone (patented in 2002), and englitazone, and subsequently, Pfizer and Smithkline also developed rosiglitazone and darglitazone in the same year. Englitazone and darglitazone were discontinued due to their hepatotoxicity. However, pioglitazone was reported to be safe for use in the hepatic system and is currently in use as an antidiabetic agent. In 2001, rosiglitazone was reported to cause heart failure due to fluid retention and, hence, the Food and Drug Administration (FDA) restricted its use in the year 2010. However, in the year 2013, the restriction was removed by the FDA after a series of trials proved that there was no effect on the heart due to fluid retention. Another drug, lobeglitazone, was developed by Chong Kun Dang Pharmaceuticals (Korea) in 2013, which was approved by the Ministry of Food and Drug Safety of Korea. Some other molecules, such as netaglitazone, rivoglitazone, and balaglitazone, were also developed but then withdrawn during the clinical trial due to their severe toxicities, and these never came to the market. The structures of various TZDs are shown in Figure 2 [26,27].
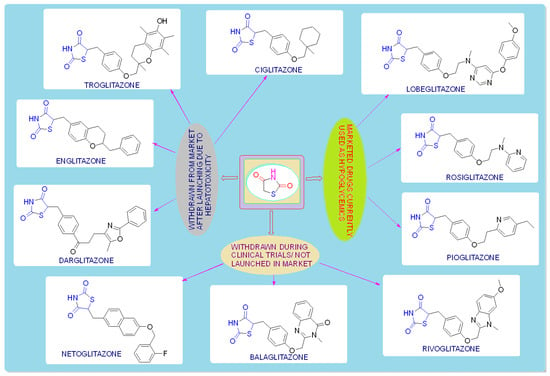
Figure 2.
Antidiabetic molecules developed with the thiazolidin-2,4-dione moiety.
3. Mechanism of Action
TZDs produce their biological response by stimulating the PPARγ receptor (antidiabetic activity) and cytoplasmic Mur ligase enzyme (antimicrobial activity), and by scavenging ROS (antioxidant activity).
- Family of PPARs:
PPARs are a subfamily of transducer proteins that act as transcriptional factors, belonging to the nuclear superfamily of retinoic acid receptors (RARs)/steroid receptors/thyroid hormone receptors (TRs), which are involved in different processes and also help in the regulation and expression of various genes that are vital for glucose and lipid metabolism [28,29]. These nuclear receptors were first identified in rodents in the year 1990s [26].
- Isoforms of PPARs:
To date, three different types of PPARs have been identified, i.e., PPAR-α (NR1C1), PPAR-β/δ (NR1C2), and PPAR-γ (NR1C3), which are encoded by different genes.
- Structure and biological functions of PPARs:
All of the PPAR isoforms have similar functional and structural features. The structure of PPAR has four principal functional domains, known as A/B, C, D, and E/F (Figure 3). The domain A/B, located at the NH2-terminal, comprises ligand-independent activation function 1 (AF-1) and causes the phosphorylation of PPAR. The domain C, also called the DNA-binding domain (DBD), has two zinc atoms that promote the binding of the peroxisome proliferator response element (PPRE) to PPAR in the target gene promoter region. The D site functions as a docking domain for the binding of the coactivators/corepressors to the DNA receptors. The E/F domain near the AF-2 region, also called the ligand-binding domain (LBD), is used to specify the ligand and also activates the binding of PPAR to PPRE, thereby promoting the targeted gene expression. The ligand-dependent activation function 2 (AF-2) recruits the PPAR co-factors that assist in the gene transcription processes. Their biological functions and distribution in the tissues, along with their agonists, are shown in Table 1 [26,30,31,32].

Figure 3.
Principal functional domains of PPARs.

Table 1.
Biological functions of PPARs.
3.1. Mechanism of the TZD as an Antidiabetic
PPAR acts by either transactivation or by trans-repression to enact its antidiabetic activity. In transactivation, PPAR is activated upon binding with the exogenous ligand (TZD) or endogenous ligands (PGs, fatty acids, etc.). PPAR then heterodimerizes with the retinoid X factor (RXR) to form the PPAR–RXR complex. This complex binds with peroxisome proliferator response elements (PPRE) in the target gene with a coactivator that has histone acetylase activity and promotes the transcription of different genes involved in the cellular differentiation and glucose and lipid metabolism (Figure 4) [26,28]. In trans-repression, PPARs interact negatively with other signal transduction pathways, such as the nuclear factor kappa beta (NFκB) pathway, which controls various genes involved in inflammation and also regulates inflammatory mediators, such as leukocytes and cytokines, etc. (Figure 5) [28].
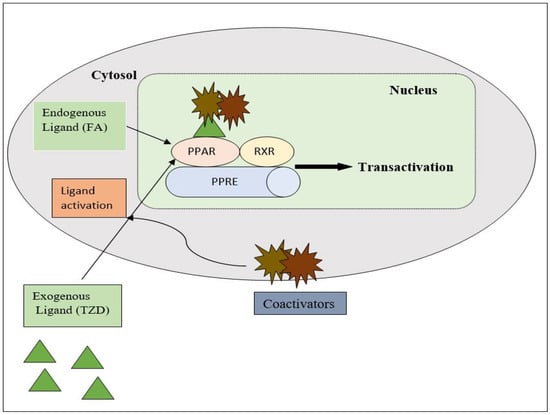
Figure 4.
Gene transcription mechanisms of PPAR.
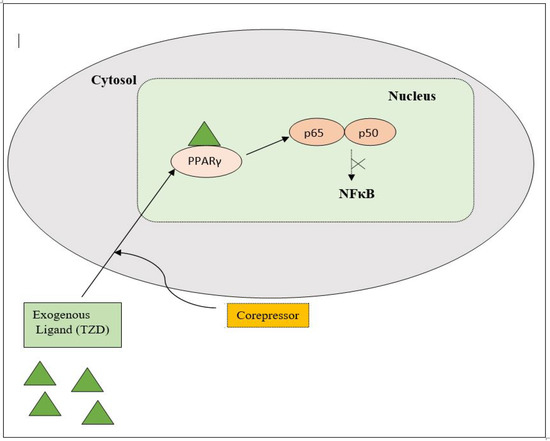
Figure 5.
Gene trans-repression mechanisms of PPAR.
Thiazolidinediones (TZDs) are the most important synthetic moieties with PPAR-γ activation properties, which improve insulin resistance and, hence, lower blood glucose levels in type-II diabetes. In adipose tissues, TZDs activate PPAR-γ, which causes lipid uptake and the storage of triglycerides (TGs). White adipose tissues (WAT) then take up free fatty acids (FFAs) from the tissues (skeletal muscle, liver), where their growth obstructs insulin signaling, known as the lipid steal hypothesis. PPAR-γ also mediates the production of adipocytes. In macrophages, TZDs directly activate PPAR-γ, causing an anti-inflammatory M2 phenotype which leads to a decreased macrophage infiltration in the WAT. TZDs also help to reduce fibrosis and inflammation by acting on PPAR-γ in Kupffer and stellate cells, as well as the parenchymal cells of the steatosis liver, and also mediate atherosclerosis by interacting with PPAR-γ in the macrophages (Figure 6) [26,32,33].
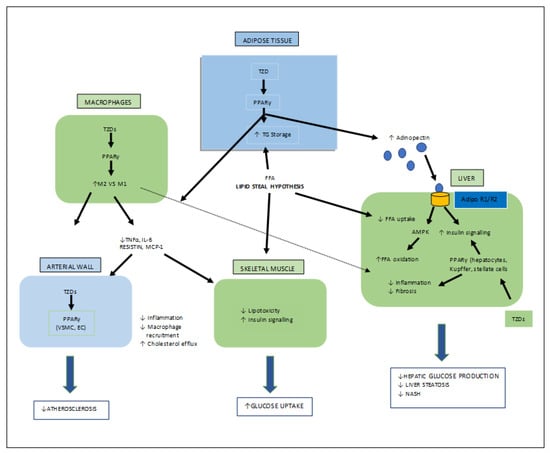
Figure 6.
Various target organs/sites of TZD-PPARγ.
3.2. Mechanism of TZD as Antimicrobial Agent
For bacterial viability, the bacterial cell wall is an important component in maintaining the cell shape and protection. Peptidoglycan is one of the major constituents of the bacterial cell wall and is found on the outer wall of cytoplasmic membrane. Its biosynthetic enzyme inhibition can lead to cell death. The enzymes involved in the synthesis can either be membrane-bound extracellular enzymes (penicillin-binding proteins) or cytoplasmic enzymes (Mur enzymes). Peptidoglycan peptide stem biosynthesis involves four ATP-dependent enzymes, known as the Mur ligases (Mur C-F). They mediate the formation of UDP-MurNAc-pentapeptide through the stepwise additions of MurC (L-alanine), MurD (D-glutamic acid), a diamino acid which is generally a meso-diaminopimelic acid, or MurE (L-lysine) and MurF (dipeptide D-Ala-D-Ala) to the D-lactoyl group of UDP-N-acetylmuramic acid (Figure 7). TZD molecules are supposed to inhibit these cytoplasmic ligases and, hence, lead to bacterial cell death [34,35,36,37].
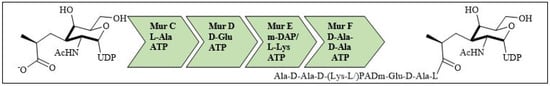
Figure 7.
Peptidoglycan peptide stem formation by the Mur ligases enzymes.
3.3. Mechanism of TZD as an Antioxidant
Oxidative stress occurs due to the production of free radicals. Free radicals are chemically active molecules which are either deficient or have a greater number of electrons. Free radicals containing oxygen are the most significant free radicals, also known as reactive oxygen species (ROS). Oxidants activate various relevant enzymes, such as SOD, catalase, and NADPH oxidase, to convert the oxygen to ROS. ROS scavenge body cells in order to capture or donate protons, thus causing cell, proteins, and DNA damage. TZD derivatives are supposed to work by preventing the cascade effect produced through ROS propagation by donating its proton to the ROS (Figure 8) [38,39,40].
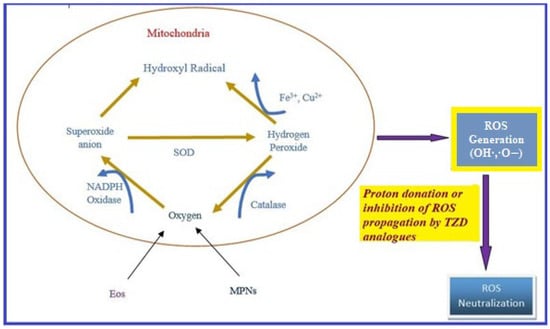
Figure 8.
ROS generation and antioxidant scavenging mechanism of TZDs.
4. Biological Potential of the Thiazolidin-2,4-dione Analogues
4.1. Antimicrobial Activity
In the 1940s, the introduction of antibiotics was believed to have eliminated the curse of all infectious diseases. However, the irrational overuse of antibiotics has led to the development of resistance against antibiotics of several bacterial strains, which has surfaced as one of the serious public health issues. Some of these resistant strains, such as vancomycin-resistant enterococci (VRE) and multidrug-resistant Staphylococcus aureus (MRSA), can survive despite the presence of the majority of the antibiotics currently in use [20]. The appearance of multidrug-resistant microbial pathogens that are resistant to currently available antibiotics/antimicrobials has led to the urgent need for new chemical entities for the treatment of microbial diseases with a unique mechanism of action [41].
In the search of new antimicrobial agents, Shaikh et al. synthesized different series of (E)-5-(substitutedbenzylidene)thiazolidine-2,4-dione and their (6-thiocyanatobenzo- thiazol-2-yl)acetamide derivatives. The in vitro antimicrobial activity of derived molecules against various bacterial and fungal strains was evaluated using a broth microdilution assay measuring the minimum inhibitory concentration (MIC). Among the synthesized derivatives, compounds am1, am2, and am3 were found to be effective against E. coli, S. aureus, and C. albicans, respectively, using ampicillin and griseofulvin as standards (Table 2, Figure 9) [42].

Table 2.
Antimicrobial results of the compounds (am1–am3).
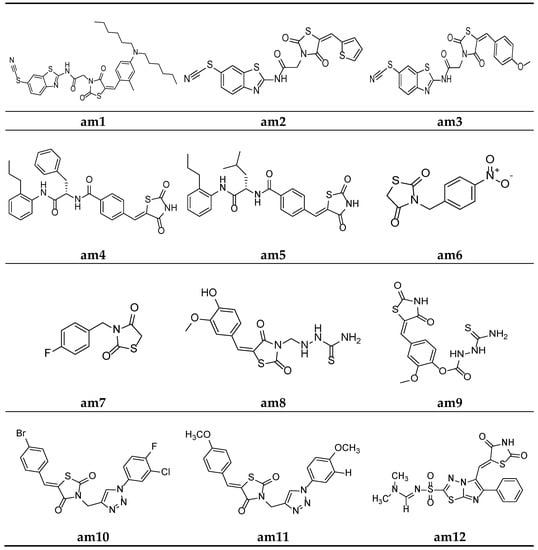
Figure 9.
Molecular structures of the compounds (am1–am12).
To recognize potential new compounds that are active against microbes, 5-benzylidene-2-4-thiazolidinedione-based antibacterial agents were developed by Zvarec et al. The synthesized derivatives were assessed for their in vitro antibacterial activity. Compounds am4 and am5 showed moderate antibacterial activity against B. subtilis (Table 3, Figure 9) [43].

Table 3.
Antimicrobial results of the compounds (am4–am5).
Gaonkar et al. synthesized a new series of N-substituted thiazolidine-2,4-dione derivatives and screened for their in vitro antimicrobial potential against bacterial and fungal strains, using DMF as a solvent in the agar plate disc diffusion method, taking ciprofloxacin and ciclopiroxolamine as standard drugs. The result of the antimicrobial evaluation revealed that compounds am6 and am7 showed a superior antimicrobial potential (Table 4, Figure 9) [44].

Table 4.
Antimicrobial results (inhibitory zone = mm diameter) of the compounds (am6–am7).
Niwale et al. also developed various thiazolidin-2,4-dione analogues and evaluated them for their in vitro antibacterial potential by the serial tube dilution method using streptomycin as a reference drug. The results of the antimicrobial evaluation studies revealed that compounds am8 and am9 were active against all the tested strains of bacteria (Table 5, Figure 9) [45].

Table 5.
Antimicrobial activity (MIC = µg/mL) of the compounds (am8–am9).
A new series of thiazolidine-2,4-diones containing a 1,2,3-triazole scaffold were synthesized by Sindhu et al. and evaluated for their in vitro antimicrobial potential against two bacterial and two fungal strains, using the disc diffusion and poisoned food methods, respectively. The results of the antimicrobial evaluation revealed that compounds am10 and am11 had superior antibacterial and antifungal activities in comparison to ciprofloxacin and fluconazole as the standard drugs (Table 6, Figure 9) [46].

Table 6.
Antimicrobial activity of the compounds (am10–am11).
Alagawadi et al. derived a sequence of TZD analogues containing the 1,3,4-thiadiazole moiety and screened them for their in vitro antimicrobial activity. The results of the antimicrobial evaluation studies showed compounds am12 and am13 to be active against all the tested strains of microbes, using ampicillin and ketoconazole as standards (Table 7, Figure 9 and Figure 10) [47].

Table 7.
Antimicrobial activity (MIC = µg/mL) of the compounds (am12–am13).
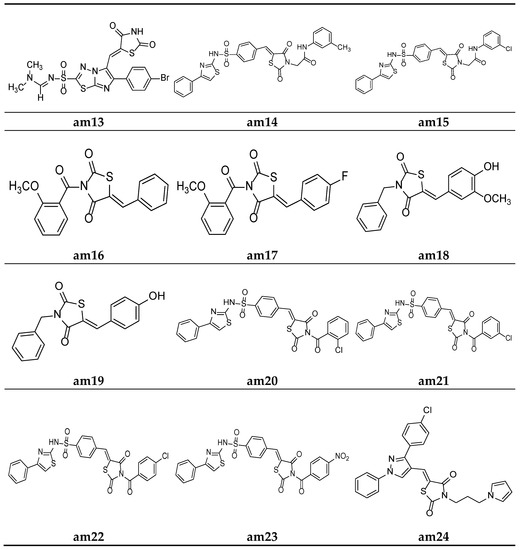
Figure 10.
Molecular structures of the compounds (am16-am24).
In an attempt to identify prospective new TZD derivatives that are active against microbes, a new series of n-phenyl-acetamide derivatives of TZD were developed by Juddhawala et al. The synthesized products were further analyzed for their in vitro antibacterial potential by the disc diffusion method, using ciprofloxacin as the standard. The results of the antimicrobial evaluation revealed that compounds am14 and am15 had a better antimicrobial potential (Table 8, Figure 10) [48].

Table 8.
Antimicrobial activity (Inhibitory Zone = mm diameter) of the compounds (am14–am15).
Patel et al. derived a series of thiazolidine-2,4-diones molecules containing the N-methoxybenzoyl substitution, and they were screened for their in vitro antimicrobial potential by the broth microdilution method, using ampicillin/griseofulvin as the standard, against different bacterial/fungal strains. The results revealed that compounds (am16) and (am17) had better antibacterial and antifungal activities (Table 9, Figure 10) [49].

Table 9.
Results of antimicrobial screening of the synthesized compounds (am16–am17).
Malik et al. derived a series of six new N-substituted 2, 4- thiazolidinedione compounds with active pharmacophores and screened them for their in vitro antibacterial potential against different bacterial strains, using the cup plate method, taking ciprofloxacin as the standard. The compounds (am18) and (am19) were found to be better antibacterial derivatives in the study (Table 10, Figure 10) [50].

Table 10.
Antimicrobial results (inhibitory zone = mm diameter) of the compounds (am18–am19).
Parekh et al. synthesized various TZD molecules containing a benzene sulfonamide scaffold and evaluated them for their in vitro antibacterial activity against different bacterial strains, using the disk diffusion method and taking ciprofloxacin as the standard drug. Among the derivatives tested, compounds am20, am21, am22c, and am23 exhibited promising potential against the microbial strains and showed an activity comparable with that of the standard drug. (Table 11, Figure 10) [51].

Table 11.
Antimicrobial screening results (inhibitory zone = mm diameter) of the compounds (am20–am23).
Desai et al. synthesized a series of thiazolidine-2,4-diones with pyrrole and pyrazole rings. The minimum inhibitory concentration (MIC) of the synthesized derivatives was evaluated through the broth microdilution process and compared with two commercial antibiotics (ampicillin and griseofulvin). Among the derived molecules, compounds, am24, am25, am26, and am27 were found to be the best antimicrobial molecules (Table 12, Figure 10) [52].

Table 12.
Results of antimicrobial screening of the synthesized compounds (am24–am27).
A new series comprising nine novel derivatives of thiazolidine-2,4-diones with a pyrazole ring were developed by Prakash et al. and screened for their in vitro antimicrobial potential against various fungal and bacterial strains, using the agar well diffusion method and poisoned food method, respectively, and they were compared with standard drugs ciprofloxacin and fluconazole. Compounds am28 and am30 were found to be promising antibacterial agents, while compounds am29 and am31 were found to be effective antifungal agents (Table 13, Figure 11) [53].

Table 13.
Antimicrobial activity of the compounds (am28–am31).
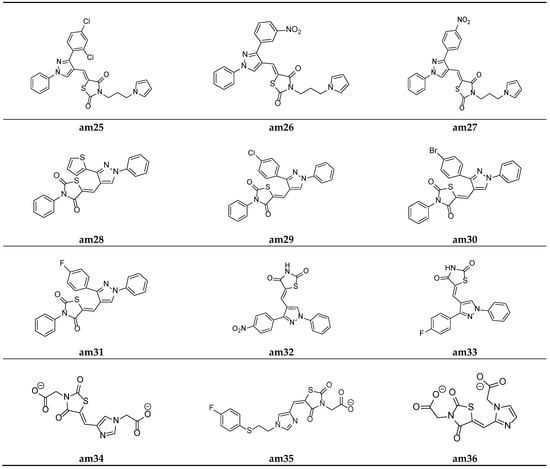
Figure 11.
Molecular structures of the compounds (am25–am36).
Prakash et al. synthesized another series of thiazolidine-2,4-diones molecules and screened them for their in vitro antimicrobial potential against various bacterial and fungal strains. Among the synthesized derivatives, compounds, am32 and am33 were found to be equipotent antibacterial and antifungal agents in comparison to the standard drugs ciprofloxacin and fluconazole as the antibacterial and antifungal agents, respectively (Table 14, Figure 11) [54].

Table 14.
Antimicrobial activity of the compounds (am32–am33).
To identify potential novel agents possessing antimicrobial activity, certain analogues of imidazolyl thiazolidin-2,4-dione and 5-substituted 2,4-thiazolidinedione were derived by Moorthy et al. and evaluated for their in vitro antimicrobial potential by measuring the MIC values using the agar streak dilution method. Compounds am34, am35, and am36 showed moderate antimicrobial activity in comparison to the standard drugs, ciprofloxacin and ketoconazole (Table 15, Figure 11) [20].

Table 15.
Results of antimicrobial screening of the synthesized compounds (am34–am36).
Lobo et al. synthesized a series of 3,5-disubstituted thiazolidine-2,4-diones and screened them for their in vitro antimicrobial potential against different bacterial/fungal strains, using the cup plate method and taking ciprofloxacin and fluconazole as the standards. Among the synthesized analogues, molecules am37 and am38 exhibited a decent activity against all the tested bacterial and fungal strains compared with the standard drug used (Table 16, Figure 12) [55].

Table 16.
Results of antimicrobial activity of the synthesized compounds (am37–am38).
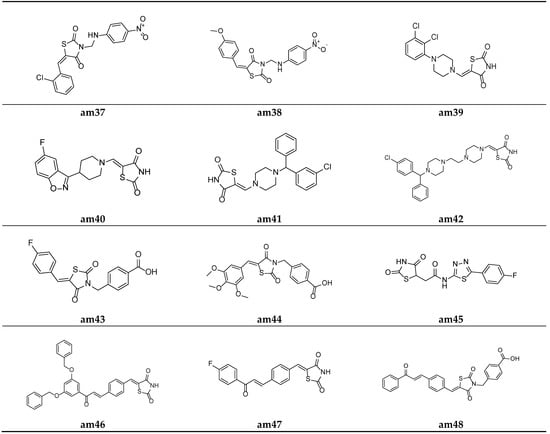
Figure 12.
Molecular structures of the compounds (am37–am48).
A series of substituted 5-(aminomethylene)thiazolidine-2,4-diones derivatives w active pharmacophores were derived by Mohanty et al. and tested for their in vitro antimicrobial potential against different strains of bacteria and fungi using the two-fold serial dilution method and poisoned food method, respectively. Compounds am39 and am40 showed a moderate antibacterial activity, while the compounds am39, am40, am41, and am42 showed a promising antifungal activity compared to the standard drugs, ciprofloxacin and fluconazole respectively (Table 17, Figure 12) [22].

Table 17.
Results of antimicrobial screening of the synthesized compounds (am39–am42).
Alegaon et al. derived various TZD benzoic acid derivatives using a microwave irradiation procedure and evaluated their antimicrobial potential against different bacterial and fungal species. Compounds am43 and am44 were found to be the most active antimicrobial agents in comparison to the standard drugs, ampicillin, ciprofloxacin, and ketoconazole (Table 18, Figure 12) [56].

Table 18.
Antimicrobial activity (MIC = µg/mL) of the compounds (am43–am44).
To improve the previously recognized lead structure, a series of TZD-5-acetic acid amides were developed by Alegaon et al. and screened for their in vitro antimicrobial activity against various fungal and bacterial strains using the twofold sequential dilution procedure, taking commercial drugs, i.e., ciprofloxacin, ampicillin, and ketoconazole, as the standards. The compound am45 was found to possess the best antimicrobial potential amongst all the derived derivatives (Table 19, Figure 12) [57].

Table 19.
Antimicrobial activity (MIC = µg/mL) of the compound (am45).
Avupati et al. synthesized various ((oxoprop-1-enyl)benzylidene)-1,3-thiazolidine-2,4-dione derivatives and tested them for their in vitro antimicrobial potential against many bacterial and fungal strains using the agar well diffusion method. Compounds am46 and am47 showed promising antimicrobial activity when compared with the standard drugs, chloramphenicol and ketoconazole (Table 20 and Table 21, Figure 12) [58].

Table 20.
Antibacterial activity (MIC = µg/mL) of the compounds (am46–am47).

Table 21.
Antifungal activity (MIC = µg/mL) of the compounds (am46–am47).
Liu et al. reported a new series of Thiazolidin-2,4-dione containing methyl benzoic acid molecules and screened them for their in vitro antibacterial potential against many bacterial strains using a serial dilution method with a 96-well microtiter plate. Norfloxacin and ofloxacin were taken as the standards. The results of the antibacterial activity study showed that all the prepared derivatives were inactive against the Gram-negative bacterial strains but active against the Gram-positive bacterial strains. The compounds am48 and am49 exhibited a good antibacterial potential in comparison to the standard drugs amongst all the synthesized molecules (Table 22, Figure 12 and Figure 13) [59].

Table 22.
Inhibitory activity (MIC, µg/mL) of the compounds am48 and am49 against the bacteria and clinical isolates of multidrug-resistant Gram-positive strains.
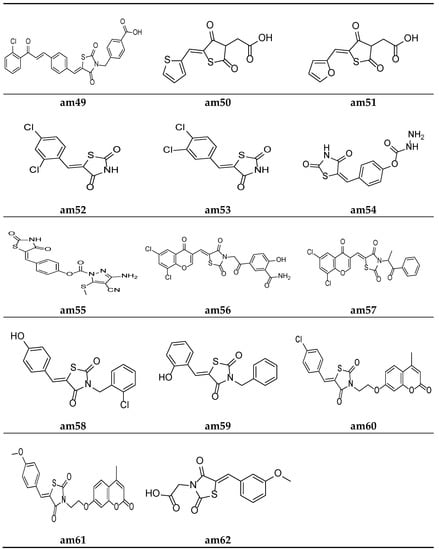
Figure 13.
Molecular structures of the compounds (am49–am62).
Alegaon et al. synthesized another series of TZD acetic acid derivatives and evaluated them for their in vitro antibacterial potential against different microbial strains using a two-fold serial dilution method, taking ciprofloxacin and ketoconazole as the standards. The results of the antimicrobial activity evaluation revealed that the compounds am50 and am51 possessed a moderate antimicrobial activity (Table 23, Figure 13) [60].

Table 23.
Antimicrobial activity (MIC = µg/mL) of the compounds (am50–am51).
Da Silva et al. synthesized 5-arylidene-thiazolidine-2,4-dione derivatives and screened them against different microbial strains to determine their MICs and MBCs (minimum bactericidal concentrations) using the 96-well plate method. The results revealed that compounds am52 and am53 possessed excellent antimicrobial activity against the tested strains in comparison to the standard drug, cefalexin (Table 24, Figure 13) [61].

Table 24.
Results of antimicrobial screening of the synthesized compounds (am52–am53).
Rekha et al. synthesized 5-arylidene-thiazolidine-2,4-dione analogues and tested them against different microbial strains by measuring their zone of inhibition using a slightly modified cup plate method. The results revealed that compound am54 provided a better inhibition of B. subtilis and S. aureus, while am55 possessed excellent potency against P. vulgaris when compared with the standard drug, Amoxycillin (Table 25, Figure 13) [62].

Table 25.
Antimicrobial screening results of the compounds (am54–am55).
Nastasa et al. synthesized a few 2,4-thiazolidinedione derivatives containing a chromene scaffold and tested them for their in vitro antimicrobial potential against certain bacterial and fungal species using the disk diffusion method. The results of the antimicrobial evaluation revealed that compounds am56 and am57 were more active than the commercial drugs gentamicin and fluconazole against the tested species of the microbial strains (Table 26, Figure 13) [21].

Table 26.
Antimicrobial activity of the compounds (am56–am57).
A series of 3-aryl-5-arylidine thiazolidine-2, 4-dione molecules were derived by Purohit et al. and the MICs of the derived compounds were obtained using a twofold serial tube dilution technique, taking ciprofloxacin, norfloxacin, griseofulvin, and fluconazole as the standards. The screening results revealed that compounds am58 and am59 possess a comparable antimicrobial potency to the standard drugs (Table 27, Figure 13) [63].

Table 27.
Antimicrobial activity (MIC = µg/mL) of the compounds (am58–am59).
Mangasuli et al. derived various coumarin-thiazolidinone derivatives and evaluated their antimicrobial potential against different bacterial and fungal species. Compounds am60 and am61 were found to be the most active antimicrobial agents in comparison to the standard drugs, ciprofloxacin and ketoconazole (Table 28, Figure 13) [64].

Table 28.
Antimicrobial activity (MIC = µg/mL) of the compounds (am60–am61).
Alhameed et al. derived various thiazolidine-2,4-dione carboxamide and amino acid derivatives and evaluated their antimicrobial potential against different bacterial and fungal species, taking imipenem and fluconazole as the reference drugs. Compound am62 was found to be the most active analogue against S. aureus (Table 29, Figure 13) [65].

Table 29.
Antimicrobial activity of the compounds (am62).
4.2. Hypoglycemic Activity
Diabetes has been recognized as a metabolic syndrome characterized by raised blood sugar (or blood glucose) levels, affecting a large number of people, both male and female, across the globe. Type-II diabetes mellitus (T2DM) is a multifaceted disease that often affects longevity/the life span due to grave damage to the eyes, kidneys, heart, blood vessels, and nerves [3,66]. According to statistical data, around 200 million people are suffering from diabetes to date, and this figure will be increased to 350 million by 2025 and more than 360 million by 2030 [67,68]. The derivatives of thiazolidine-2,4-done, viz., ciglitazone, troglitazone, rosiglitazone, and many more, were approved as antidiabetic agents but were withdrawn from the market due to their severe hepatotoxicity [69]. Therefore, there is a pressing need to synthesize newer hypoglycemic agents without toxicity.
In the search for new hypoglycemic agents, Jawale et al. synthesized various 1-((2,4-dioxothiazolidin-5-yl)methyl)-3-(substitutedbenzenesulfonyl)urea derivatives and screened them for their in vivo hypoglycemic activity in Sprague–Dawley strain male albino rats using the sucrose-loaded model (SLM) with commercially available metformin as standard. The results of the antihyperglycemic activity test revealed that some of the synthesized compounds in the sucrose-loaded rat model showed substantial antihyperglycemic activity. Furthermore, compounds ad1, ad2, and ad3 showed better activity in the series (Table 30, Figure 14) [70].

Table 30.
Hypoglycemic effect of the compounds (ad1–ad3) on sucrose-loaded hyperglycemic rats.
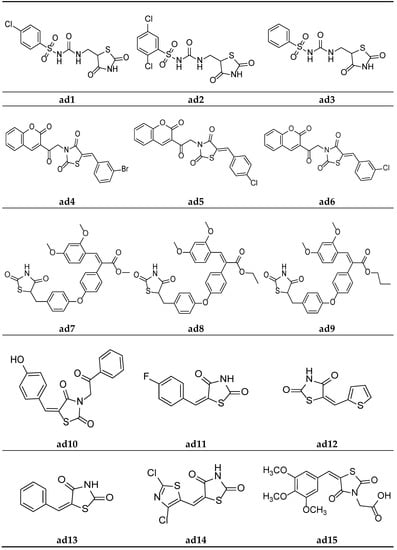
Figure 14.
Molecular structures of the compounds (ad1–ad15).
Mishra et al. synthesized thiazolidine-2,4-dione derivatives with the N-chromen-3-yl ethyl moiety and screened them for their in vivo antidiabetic activity in male Wistar rats weighing 150–250 gm, using the alloxan-induced diabetic model and taking pioglitazone as a standard. The results of the antidiabetic screening demonstrated that all the synthesized derivatives had a promising glucose-reducing potential. Furthermore, compounds ad4, ad5, and ad6 were found to be almost equipotent with the standard (Table 31, Figure 14) [66].

Table 31.
Antidiabetic potential of the compounds (ad4–ad6) in alloxan-induced diabetic rats.
A new series of TZD-acrylic acid alkyl ester molecules were derived by Kumar et al. and screened for their in vivo hypoglycemic potential in neonatal Wistar male rats using the streptozotocin-induced diabetic model. The results of the antihyperglycemic activity test revealed that all the compounds possessed a moderate plasma glucose level (%PGL)-reducing activity in comparison to the marketed drug rosiglitazone. Furthermore, compounds ad7, ad8, and ad9 possessed the maximum activity in the series (Table 32, Figure 14) [71].

Table 32.
Hypoglycemic activity of the compounds (ad7–ad9) using a streptozotocin-induced diabetic rat model.
Gautam et al. designed a series of substituted 5-(arylidene)-thiazolidine-2,4-diones and evaluated them as potential aldose reductase inhibitors by the molecular docking technique (PDB id: 1AH3) using Molegro Virtual Docker (MVD 2012.5.5.0), along with the clinically available drug epalrestat. The affinity of the designed compounds for the aldose reductase enzyme was measured by calculating the Mol-dock score. The results of the docking studies showed that all the compounds had similar or slightly better activity than the clinical drug epalrestat. Compound ad10 in the series possessed the maximum activity and can be used as a lead structure (Table 33, Figure 14) [68].

Table 33.
Hypoglycemic effect of the compound ad10 depicted by docking score.
Swathi et al. synthesized certain 5-substitutedaryl/heteroarylmethylidene-1,3-thiazolidine-2,4-diones and observed their hypoglycemic activity using in silico studies, viz., protein binding energy calculation, drug-likeness, and docking simulation studies, etc. The docking studies were carried out on the PPARγ enzyme (2PRG protein), obtained from a protein data bank, taking rosiglitazone as a standard drug. The results of the docking studies indicated that the synthesized compounds were equipotent with or more potent than standard. Compounds ad11, ad12, and ad13 exhibited the maximum potential in the series (Table 34, Figure 14) [72].

Table 34.
Antidiabetic potential of the compounds (ad11–ad13) using molecular docking studies.
Evcimen et al. synthesized thiazolyl-2,4-thiazolidinediones derivatives and screened them for their in vivo hypoglycemic potential by determining their aldose reductase inhibition using male Wistar albino rats. A Kidney enzyme was isolated and NADPH oxidation was calculated spectrophotometrically using D-L-glyceraldehyde as a substrate. The results of the study revealed that some of the synthesized derivatives possessed significant inhibitory activity. Furthermore, compound ad14 possessed the maximum inhibitory potential in the series (Table 35, Figure 14) [73].

Table 35.
Aldose reductase inhibitory activity of the compound ad14.
Datar et al. synthesized 3,5-disubstituted thiazolidin-2.4-dione molecules and screened them for their in vivo antidiabetic potential using a sucrose-loaded model tested on Wistar rats. The results of the activity test revealed that all the derivatives possessed mild to good activity as compared to the marketed drug, pioglitazone. Compounds ad15 and ad16 were equipotent with standard (Table 36, Figure 14 and Figure 15) [69].

Table 36.
Antidiabetic potential of the compounds (ad15–ad16) using a sucrose-loaded model in rats.
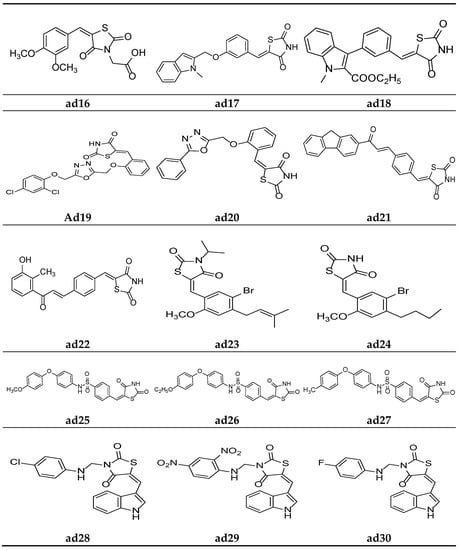
Figure 15.
Molecular structures of the compounds (ad16–ad30).
Verma et al. synthesized compounds ad17 and ad18 (Figure 15) containing indolyl- linked benzylidene molecules with a thiazolidine-2,4-dione scaffold and observed their in silico antidiabetic activity using the Surflex-dock module. The docking was carried out on 1I7I protein complexed with tesaglitazar obtained from the protein data bank using human peroxisome-proliferator-activated receptor gamma (PPARγ), and the compounds were found more potent than standard. The results are depicted in Table 37 [74].

Table 37.
Antidiabetic potential of the compounds (ad17–ad18) based on molecular docking studies.
Nazreen et al. synthesized thiazolidine-2,4-dione derivatives containing the 1,3,4-oxadiazole moiety and screened them for their in vitro PPARγ transactivation assay and in vivo antidiabetic potential using a streptozocin-induced diabetic rat model, comparing them with the commercial drugs rosiglitazone and pioglitazone. Most of the synthesized derivatives possessed mild to moderate activity in PPARγ transactivation and were equipotent with the standards or had a greater potential in lowering the blood glucose levels. Furthermore, compounds ad19 and ad20 were found to be most active derivatives in the series, without hepatotoxicity (Table 38, Figure 15) [75].

Table 38.
Hypoglycemic activity of the compounds (ad19–ad20) in streptozotocin-induced diabetic rats.
Avupati et al. synthesized various (Z)-5-(4-((E)-3-(substituted)-3-oxoprop-1-enyl)benzylidene)-1,3-thiazolidine-2,4-dione derivatives and evaluated them for their in vivo antidiabetic activity using the streptozotocin-induced diabetic model tested on Wistar albino rats, and the results were compared using the marketed drug rosiglitazone. The results of the study revealed that compounds ad21 and ad22 were promising antidiabetic derivatives (Table 39, Figure 15) [58].

Table 39.
Hypoglycemic activity of the compounds (ad21–ad22) using a streptozotocin-induced diabetic model in rats.
Wang et al. synthesized various 5-(benzylidene)thiazolidine-2,4-dione derivatives and evaluated them as competitive inhibitors of protein tyrosine phosphatase 1B (PTP1B), using p-nitrophenyl phosphate (pNPP) as a substrate and ursolic acid as a positive control. The results of activity study revealed that compounds ad23 and ad24 were the most active PTP1B inhibitors amongst all the synthesized molecules (Table 40, Figure 15) [76].

Table 40.
PTP1B inhibition activity of the compounds (ad23–ad24).
Swapna et al. derived different 5-[4′-(S]ubstituted)sulphonylbenzylidene]-2,4-thiazolidinedione molecules and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model tested on Wistar albino rats. All the synthesized derivatives produced comparable results to the standard drug metformin. Compounds ad25, ad26, and ad27 exhibited the maximum activity (Table 41, Figure 15) [77].

Table 41.
Antidiabetic potential of the compounds (ad25–ad27) in alloxan-induced diabetes in rats.
Kumar et al. derived a series of TZD derivatives with indole at the 5th position and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model of Wistar albino rats and compared them with the standard drug glibenclamide. Amongst all the synthesized derivatives, molecules ad28, ad29 and ad30 exhibited significant antidiabetic activity (Table 42, Figure 15) [78].

Table 42.
Antidiabetic potential of the compounds (ad28–ad30) in alloxan-induced diabetes in rats.
Mahapatra et al. derived a series of (Z)-3,5-disubstituted thiazolidine-2,4-dione derivatives containing the thiophen moiety and screened them for their in vitro protein tyrosine phosphatase 1B (PTP1B) inhibition activity colorimetrically, using suramin as a standard inhibitor. The results of the assay revealed that most of the synthesized derivatives were weak PTP1B inhibitors. However, compound ad31 exhibited an almost equipotent inhibition with that of standard inhibitor (Table 43, Figure 16) [79].

Table 43.
Antidiabetic potential of compound ad31 using PTP1B inhibitory studies.
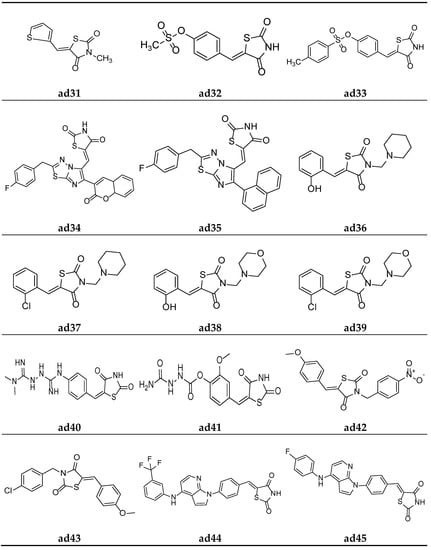
Figure 16.
Molecular structures of the compounds (ad31–ad45).
To further improve the previously recognized lead analogue; a series of (Z)-4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenyl-substitutedbenzenesulfonates were derived by Mahapatrab et al. and screened for their in vitro protein tyrosine phosphatase 1B (PTP1B) inhibition activity, taking the commercial drug suramin as the standard. Compounds ad32 and ad33 were found to be the most potent in the series and possessed greater inhibitory activity than the standard (Table 44, Figure 16) [4].

Table 44.
Antidiabetic potential of the compounds (ad32–ad33) based on PTP1B inhibitory studies.
Badiger et al. derived a series of thiazolidine-2,4-diones derivatives with a 1,3,4-thiadiazole scaffold and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model of Wistar albino rats in comparison to the standard drug pioglitazone. Amongst all the synthesized derivatives, molecules ad34 and ad35 exhibited significant activity (Table 45, Figure 16) [80].

Table 45.
Antidiabetic potential of the compounds (ad34–ad35) in alloxan-induced diabetes in rats.
Alam et al. derived a new series of N-substituted-methyl-1,3-thiazolidine-2,4-dione molecules and evaluated them for their in vivo antidiabetic potential inn Wistar rats using the streptozotocin-induced diabetic model. The results of antidiabetic evaluation revealed that compounds ad36, ad37, ad38, and ad39 were the most active antidiabetic compounds, with equipotent activity to the standard drug glibenclamide (Table 46, Figure 16) [81].

Table 46.
Antidiabetic potential of the compounds (ad36–ad39) in streptozotocin-induced diabetes in rats.
Patil et al. synthesized a new series of TZD molecules and evaluated their in vivo antidiabetic potential in Wistar rats using an alloxan-induced tail tipping diabetic model, taking metformin and pioglitazone as standard drugs. The results of the antidiabetic evaluation revealed that compounds ad40 and ad41 were the most active antidiabetic compounds (Table 47, Figure 16) [82].

Table 47.
Antidiabetic potential of the compounds (ad40–ad41) using the alloxan-induced model in rats.
Garg et al. derived a series of 5-(substituted)arylidene-3-substituted-benzylthiazolidin-2,4-dione analogues and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model tested on Wistar albino rats, and the results were compared with the commercial drug rosiglitazone. Amongst all the synthesized derivatives, ad42 and ad43 exhibited significant activity (Table 48, Figure 16) [83].

Table 48.
Antidiabetic potential of the compounds (ad42–ad43) in alloxan-induced diabetes in rats.
In the search for a lead compound a series of thiazolidine-2,4-dione derivatives clubbed with azole moiety was derived by Senthilkumar et al. and screened for their in vitro α-amylase and α-glucosidase inhibition potential, taking the commercial drug acarbose as the standard. Compounds ad44, ad45, and ad46 were found to be most potent amongst all the derived derivatives and possessed an almost equipotent inhibitory activity with the standard (Table 49, Figure 16 and Figure 17) [84].

Table 49.
Antidiabetic potential of the compounds (ad44–ad46) based on α-amylase and α-glucosidase inhibitory studies.
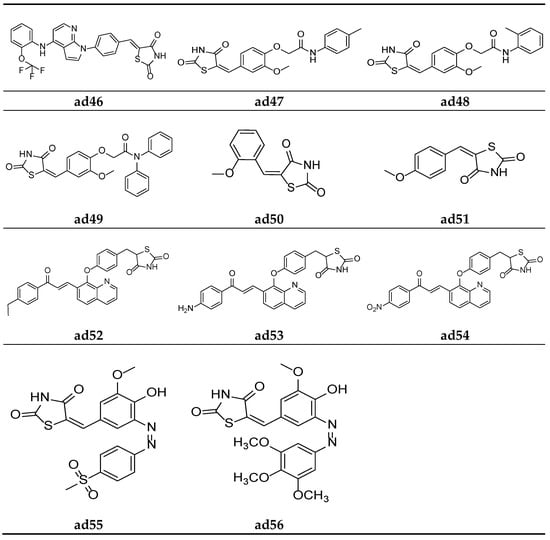
Figure 17.
Molecular structures of the compounds (ad46–ad56).
Nikalje et al. derived a series of thiazolidine-2,4-dione acetamide derivatives and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model of Wistar albino rats, taking pioglitazone as a standard drug. Amongst all the synthesized derivatives, molecules ad47, ad48, and ad49 exhibited a promising activity (Table 50, Figure 17) [85].

Table 50.
Antidiabetic potential of the compounds (ad47–ad49) using an alloxan-induced model in rats.
Sucheta et al. derived a series of 5-(aryl/alkyl)benzylidene-thiazolidine-2,4-dione derivatives and screened them for their in vitro α-amylase inhibitory activity, taking commercial drug acarbose as the standard. Compounds ad50 and ad51 were found to be the most potent of the derived derivatives and exhibited good inhibitory activity as compared to standard (Table 51, Figure 17) [5].

Table 51.
Antidiabetic potential of the compounds (ad50–ad51) according to α-amylase inhibitory activity.
Srikanth et al. derived a series of thiazolidine-2,4-dione derivatives with a quinolin-8-yloxy]benzyl scaffold and screened them for their in vivo antidiabetic activity using an alloxan-induced tail tipping diabetic model tested on Wistar albino rats, taking rosiglitazone as the standard drug. Amongst all the synthesized derivatives, molecules ad52, ad53, and ad54 exhibited moderate activity (Table 52, Figure 17) [86].

Table 52.
Antidiabetic potential of the compounds (ad52–ad54).
Kadium et al. synthesized a series of (z)-5(4-hydroxy-3-methoxybenzylidine)-2,4- thiazolidinedione analogues and tested them for their in vivo antidiabetic potential using a sucrose-loaded model tested on Wistar rats. The results of the activity study revealed that all the derivatives possessed good activity, comparable with the marketed drug pioglitazone. Compounds ad55 and ad56 were found to be more potent than standard (Table 53, Figure 17) [87].

Table 53.
Antidiabetic potential of the compounds (ad55–ad56) using a sucrose-loaded model in rats.
4.3. Antioxidant Activity
Antioxidants are the molecules used to delay or prevent the oxidation of oxidizable substrate when used in low concentrations. The key function of antioxidants is to prevent any cellular damage caused by free radicals by reacting with and stabilizing them. Free radicals are not only produced by smoke, obesity-inducing foods, cigarette, radiation, or exposure to frequent chemical constituents (lead, polycyclic aromatic hydrocarbon, cadmium, etc.), but are also formed by normal cellular processes, during several biochemical reactions, and in diseases such as tumors, atherosclerosis, diabetic and cardiac diseases, etc. Antioxidants work by preventing the cascade effect produced by reactive oxygen species (ROS) propagation by donating their proton to the ROS. Antioxidants neutralize free radicals, hence preventing them from attacking the cell. Natural antioxidants act as natural cleansers and body detoxifiers. They convert toxins of the body into harmless waste products [38,39]. Therefore, the synthesis of highly potent new antioxidant agents is required.
For the development of new antioxidant compounds, Kumar et al. synthesized various thiazolidine-2,4-dione derivatives containing 5-substituted aryl/alkyl moieties, and their in vitro antioxidant potential was evaluated using an 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method, taking ascorbic acid as the reference drug. The results of the antioxidant activity test revealed that all the synthesized compounds exhibited substantial antioxidant activity. Furthermore, compounds ao1 and ao2 were found to be the most potent derivatives in the series (Table 54, Figure 18) [24].

Table 54.
In vitro antioxidant activity of the synthesized compounds (ao1–ao2) using the DPPH scavenging method.
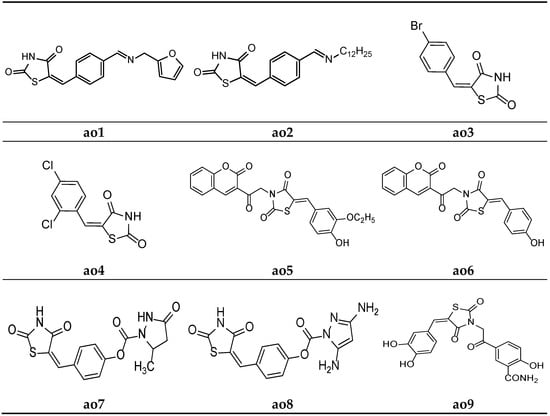
Figure 18.
Molecular structures of the compounds (ao1–ao9).
Sucheta et al. derived a series of 5-(substituted)benzylidene-thiazolidine-2,4-dione derivatives, and in vitro, antioxidant studies were performed, applying a DPPH free radical scavenging method and taking ascorbic acid as a reference molecule. Compounds ao3 and ao4 were found to be the most potent among all the derivatives and possessed a good antioxidant activity (Table 55, Figure 18) [5].

Table 55.
Antioxidant activity of the compounds (ao3–ao4).
Mishra et al. synthesized substituted 5-benzylidene-3-(2-oxo-2-(2-oxo-2H-chromen-3-yl)ethyl)thiazolidine-2,4-dione derivatives and screened them in in vitro antioxidant studies, applying the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and using ascorbic acid as a standard. The results of the assay revealed that compounds ao5 and ao6 possessed a better activity than the other derivatives in the series (Table 56, Figure 18) [66].

Table 56.
In vitro antioxidant activity of the synthesized compounds (ao5–ao6) using the DPPH scavenging method.
Rekha et al. synthesized 5-arylidene-thiazolidine-2,4-dione molecules and tested them for their antioxidant potential applying the DPPH radical method. The results revealed that compounds ao7 and ao8 possessed excellent potency when compared with the standard drug, ascorbic acid (Table 57, Figure 18) [62].

Table 57.
Antioxidant screening results of the synthesized compounds (ao7–ao8) by applying the DPPH scavenging method.
A new series of (E)-5-(2-(5-(substitutedbenzylidene)-2,4-dioxothiazolidin-3-yl)acetyl)-2-hydroxybenzamide were derived by Marc et al. and screened them for their in vitro antioxidant potential using ABTS and DPPH radical scavenging assays. The results of antioxidant activity tests revealed that all the compounds exhibited moderate to potent radical scavenging activity in comparison with Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), ascorbic acid, and butylated hydroxytoluene (BHT) as the standard compounds. Furthermore, compounds ao9 and ao10 exhibited even better activity than the reference compounds (Table 58, Figure 18 and Figure 19) [25].

Table 58.
Antioxidant screening results of the synthesized compounds (ao9-ao10) by applying the DPPH and ABTS+ scavenging methods.
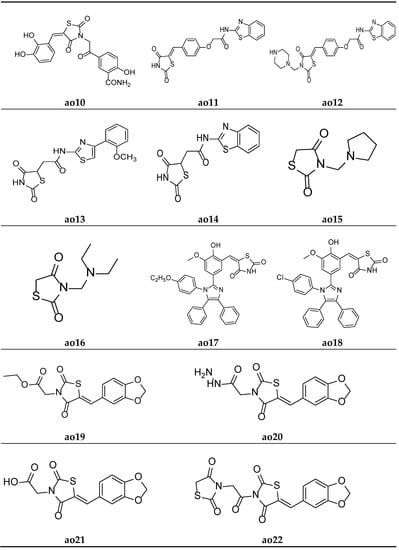
Figure 19.
Molecular structures of the compounds (ao10-ao22).
Khare et al. designed a series of thiazolidine-2,4-dione scaffolds and tested them for their in vitro antioxidant potential using the DPPH radical scavenging method, and the results were compared with standard ascorbic acid. The results of the antioxidant activity test revealed that all the compounds showed moderate radical scavenging activity. Furthermore, compounds ao11 and ao12 possessed better activity than other compounds (Table 59, Figure 19) [38].

Table 59.
In vitro antioxidant activity of the synthesized compounds (ao11–ao12) using the DPPH scavenging method.
Koppireddi et al. synthesized various TZD acetamide analogues with thiazole and benzothiazole moieties and observed their antioxidant potential using superoxide anion scavenging activity, DPPH radical scavenging activity, lipid peroxidation inhibition, and erythrocyte hemolysis inhibition assays. Compounds ao13 and ao14 displayed decent activity when compared with standard ascorbic acid and luteolin (Table 60, Figure 19) [88].

Table 60.
Antioxidant activity of the synthesized compounds (ao13-ao14).
Shahnaz et al. derived N-substituted methyl-2,4-thiazolidinediones molecules and tested them for their in vitro antioxidant potential using a DPPH radical scavenging assay, taking ascorbic acid as the standard. The results of the antioxidant activity tests revealed that all the compounds showed moderate radical scavenging activities. Furthermore, compounds ao15 and ao16 exhibited better activity than the other compounds in the series (Table 61, Figure 19) [89].

Table 61.
In vitro antioxidant activity of the synthesized compounds (ao15–ao16) using the DPPH scavenging method.
Nyaki et al. prepared ((1H-imidazol-2-yl)benzylidene)thiazolidin-2,4-dione molecules and tested them for their in vitro antioxidant potential using a DPPH radical scavenging assay, taking ascorbic acid as the standard. The results of antioxidant activity tests revealed that all the compounds showed moderate to good radical scavenging activities. Furthermore, compounds ao17 and ao18 exhibited better activity than the standard drug (Table 62, Figure 19) [90].

Table 62.
In vitro antioxidant activity of the synthesized compounds (ao17–ao18).
Sameeh et al. derived Benzo[d][1,3]dioxol-5-ylmethylene)-thiazolidin-2,4-dione analogues and screened them for their in vitro antioxidant potential using a DPPH radical scavenging assay, taking ascorbic acid as the reference drug. The results of the assay revealed that molecules ao19 and ao20 exhibited a better antioxidant potential than the standard compound (Table 63, Figure 19) [91].

Table 63.
In vitro antioxidant activity of the synthesized compounds (ao19–ao20).
Sameeh et al. again derived different thiazolidin-2,4-dione analogues and evaluated them for their in vitro antioxidant activity using a DPPH radical scavenging assay, taking ascorbic acid as the reference drug. The results of the assay revealed that molecules ao21 and ao22 exhibited a better antioxidant potential than other drugs in the series (Table 64, Figure 19) [92].

Table 64.
In vitro antioxidant activity of the synthesized compounds (ao21–ao22).
5. Patents Grant Information
Due to the significant potential of the TZD derivatives, many investigators working on thiazolidinediones analogues have contributed a new dimension to the drug design. Several patents have been granted to the TZD analogues, which are shown in Table 65.

Table 65.
Patent grant information of the thiazolidinedione analogues.
6. Conclusions and Future Perspective
The TZD moiety plays a central role in the biological functioning of several essential molecules and possesses significant medicinal potential. The synthetic methodologies are simple and versatile; hence, the discovery of new TZD analogues is easily attainable. The substitution of various moieties at the third and fifth positions of the Thiazolidin-2,4-dione (TZD) scaffold offers the medicinal chemist the potential to derive novel TZD molecules. TZDs are primarily used as antidiabetic agents but also exhibit diverse therapeutic activities, such as antimicrobial, antiviral, anticancer, and antioxidant activities, etc. The present review article attempted to explore the pharmacological potential of TZDs for application as antimicrobial, antioxidant, and hypoglycemic agents, along with their mechanism of action. A brief description of the recent patents granted to TZDs possessing different biological activities was also provided. This review paper will not only be helpful to researchers working on the development of new TZD analogues based on medicinal chemistry, but also for designing new drug molecules in the future. Future investigations of TZD molecules using other heteroatoms may provide us with more encouraging results. Based on the results of this review, TZDs may be considered as an auspicious class of drugs that can be utilized to form new antidiabetic, antimicrobial, or antioxidant agents with a minimal toxic effect.
Author Contributions
Conceptualization, H.K. and R.K.M.; methodology, H.K. and R.K.M.; software, H.K. and R.K.M.; validation, N.A., M.G.M., A.D., H.C., M.M.M., A.R., and T.B.E.; formal analysis, N.A., M.G.M., A.D., H.C., M.M.M., A.R., and T.B.E.; investigation, H.K. and R.K.M.; resources, H.K. and R.K.M.; data curation, H.K. and R.K.M.; writing—original draft preparation, H.K. and R.K.M.; writing—review and editing, N.A., M.G.M., A.D., H.C., M.M.M., A.R., T.B.E., Y.K.M., R.A., T.K.M., M.S., R.K.M., and A.A.-H.; visualization, N.A., M.G.M., A.D., H.C., M.M.M., A.R., T.B.E., Y.K.M., R.A., T.K.M., M.S., R.K.M., and A.A.-H.; supervision, R.K.M., T.B.E., and A.A.-H.; project administration, R.K.M. and T.B.E.; funding acquisition, R.K.M., T.B.E., and A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within the article.
Acknowledgments
The authors are thankful to the Head of the Department of Pharmaceutical Sciences, Maharshi Dayanand University, Rohtak, for providing the facilities necessary to carry out this research.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
List of Abbreviations
| TZD | : | Thiazolidin-2:4-dione |
| SOD | : | Superoxide dismutase |
| E. coli | : | Escherichia coli |
| P. aeruginosa | : | Pseudomonas aeruginosa |
| S. aureus | : | Staphylococcus aureus |
| S. pyrogens | : | Staphylococcus pyrogens |
| C. albicans | : | Candida albicans |
| A. niger | : | Aspergillus niger |
| A. clavatus | : | Aspergillus clavatus |
| S. epidermidis | : | Staphylococcus epidermidis |
| B. subtilis | : | Bacillus subtilis |
| K. pneumoniae | : | Klebsiella pneumoniae |
| A. fumigatus | : | Aspergillus fumigatus |
| A. flavus | : | Aspergillus flavus |
| P. marneffei | : | Penicillium marneffei |
| E.faecalis | : | Enterococcus faecalis |
| C. neoformans | : | Cryptococcus neoformans |
| B. pumilus | : | Bacillus pumilus |
| M. luteus | : | Micrococcus luteus |
| P. vulgaris | : | Proteus vulgaris |
| A. oryzae | : | Aspergillus oryzae |
| P. chrysogenum | : | Penicillium chrysogenum |
| MRSA | : | Methicillin-resistant Staphylococcus aureus |
| QRSA | : | Quinolone-resistant Staphylococcus aureus |
| M. smegmatis | : | Mycobacterium smegmatis |
| L. monocytogenes | : | Listeria monocytogenes |
| S. typhi | : | Salmonella typhi |
| SLM | : | Sucrose-loaded model |
| T2DM | : | Type-II diabetes mellitus |
| PPARγ | : | Peroxisome proliferator-activated receptor Gamma |
| NADPH | : | Nicotinamide Adenine dinucleotide phosphate |
| PTP1B | : | Protein tyrosine phosphatase 1B |
| BHT | : | Butylated hydroxy toluene |
| DPPH | : | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | : | (2,2′-Azino-bis(3-ethylbenzothiazolin-6-sulfonic acid)) |
References
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Deep, A.; Marwaha, R.K. Chemical Synthesis, Mechanism of Action and Anticancer Potential of Medicinally Important Thiazolidin-2,4-dione Derivatives: A Review. Mini. Rev. Med. Chem. 2019, 19, 1476–1516. [Google Scholar] [CrossRef] [PubMed]
- Bansal, G.; Singh, S.; Monga, V.; Thanikachalama, P.V.; Chawla, P. Synthesis and biological evaluation of thiazolidine-2,4-dione-pyrazole conjugates as antidiabetic, anti-inflammatory and antioxidant agents. Bioorg. Chem. 2019, 19, 103271. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, M.K.; Kumar, R.; Kumar, M. Exploring sulfonate esters of 5-arylidene thiazolidine-2,4-diones as PTP1B inhibitors with anti-hyperglycemic activity. Med. Chem. Res. 2018, 27, 476–487. [Google Scholar] [CrossRef]
- Sucheta; Tahlan, S.; Verma, P.K. Synthesis, SAR and in vitro therapeutic potentials of thiazolidine-2,4-diones. Chem. Cent. J. 2018, 12, 129. [Google Scholar]
- Abdellatif, K.R.; Fadaly, W.A.; Kamel, G.M.; Elshaier, Y.A.; El-Magd, M.A. Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γ agonists and anti-inflammatory COX-2 selective inhibitors. Bioorg. Chem. 2019, 82, 86–99. [Google Scholar] [CrossRef]
- Elkamhawy, A.; Kim, N.Y.; Hassan, A.H.E.; Park, J.E.; Paik, S.; Yang, J.E.; Oh, K.S.; Lee, B.H.; Lee, M.Y.; Shin, K.J.; et al. Thiazolidine-2,4-dione-based irreversible allosteric IKK-β kinase inhibitors: Optimization into in vivo active anti-inflammatory agents. Eur. J. Med. Chem. 2020, 188, 111955. [Google Scholar] [CrossRef]
- Asati, V.; Bajaj, S.; Mahapatra, D.K.; Bharti, S.K. Molecular Modeling Studies of Some Thiazolidine-2,4-Dione Derivatives as 15-PGDH Inhibitors. Med. Chem. Res. 2015, 25, 94–108. [Google Scholar] [CrossRef]
- Yu, I.; Choi, D.; Lee, H.-K.; Cho, H. Synthesis and Biological Evaluation of New Benzylidenethiazolidine-2,4-Dione Derivatives as 15-Hydroxyprostaglandin Dehydrogenase Inhibitors to Control the Intracellular Levels of Prostaglandin E2 for Wound Healing. Biotechnol. Bioprocess Eng. 2019, 24, 464–475. [Google Scholar] [CrossRef]
- Upadhyay, N.; Tilekar, K.; Jänsch, N.; Schweipert, M.; Hess, J.D.; Henze Macias, L.; Mrowka, P.; Aguilera, R.J.; Choe, J.; Meyer-Almes, F.-J.; et al. Discovery of novel N-substituted thiazolidinediones (TZDs) as HDAC8 inhibitors: In-silico studies, synthesis, and biological evaluation. Bioorg. Chem. 2020, 100, 103934. [Google Scholar] [CrossRef]
- El-Kashef, H.; Badr, G.; Abo El-Maali, N.; Sayed, D.; Melnyk, P.; Lebegue, N.; Abd El-Khalek, R. Synthesis of a novel series of (Z)-3,5-disubstituted thiazolidine-2,4-diones as promising anti-breast cancer agents. Bioorg. Chem. 2020, 96, 103569. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; El-Adl, K.; El-Hddad, S.S.A.; Elhady, M.M.; Saleh, N.M.; Khalifa, M.M.; Khedr, F.; Alswah, M.; Nayl, A.A.; Ghoneim, M.M.; et al. Design, Molecular Docking, Synthesis, Anticancer and Anti-Hyperglycemic Assessments of Thiazolidine-2,4-diones Bearing Sulfonylthiourea Moieties as Potent VEGFR-2 Inhibitors and PPAR Agonists. Pharmaceuticals 2022, 15, 226. [Google Scholar] [CrossRef]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.M.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, A.A.; Alesawy, M.S.; Metwaly, A.M.; Eissa, I.H. Design and synthesis of thiazolidine-2,4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: In-vitro anticancer evaluation and in-silico studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 1903–1917. [Google Scholar] [CrossRef]
- El-Adl, K.; Sakr, H.; El-Hddad, S.S.; El-Helby, A.G.; Nasser, M.; Abulkhair, H.S. Design, synthesis, docking, ADMET profile, and anticancer evaluations of novel thiazolidine-2,4-dione derivatives as VEGFR-2 inhibitors. Arch. Pharm. 2021, 354, 2000491. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Younis, Y.; Mugumbate, G.; Njoroge, M.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and structure activity-relationship studies of thiazolidinediones as antiplasmodial inhibitors of the Plasmodium falciparum cysteine protease falcipain-2. Eur. J. Med. Chem. 2015, 90, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045. [Google Scholar] [CrossRef]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dionebased thiosemicarbazone derivatives. Bioorg. Chem. 2020, 97, 103676. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Iqbal, A.K.; Khan, A.Y.; Kalashetti, M.B.; Belavagi, N.S.; Gong, Y.-D.; Khazi, I.A.M. Synthesis, hypoglycemic and hypolipidemic activities of novel thiazolidinedione derivatives containing thiazole/triazole/oxadiazole ring. Eur. J. Med. Chem. 2012, 53, 308–315. [Google Scholar] [CrossRef]
- Bahare, R.S.; Ganguly, S.; Choowongkomon, K.; Seetaha, S. Synthesis, HIV-1 RT inhibitory, antibacterial, antifungal and binding mode studies of some novel N-substituted 5-benzylidine-2, 4-thiazolidinediones. DARU J. Pharm. Sci. 2015, 23, 6. [Google Scholar] [CrossRef]
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolyl thiazolidinedione derivatives. Arab. J. Chem. 2019, 12, 413–419. [Google Scholar] [CrossRef]
- Nastasa, C.M.; Duma, M.; Pîrnău, A.; Vlase, L.; Tiperciuc, B.; Oniga, O. Development of new 5-(chromene-3-yl)methylene-2,4-thiazolidinediones as antimicrobial agents. Clujul Med. 2016, 89, 122–127. [Google Scholar]
- Mohanty, S.; Reddy, S.G.; RamaDevi, B.; Karmakar, A.C. An assembly of structurally diverse small and simple 5-aminomethylene derivatives of 2,4-thiazolidinedione and studies of their biological activity. Med. Chem. Res. 2015, 24, 4037–4049. [Google Scholar] [CrossRef]
- Levshin, I.B.; Simonov, A.Y.; Lavrenov, S.N.; Panov, A.A.; Grammatikova, N.E.; Alexandrov, A.A.; Ghazy, E.S.; Savin, N.A.; Gorelkin, P.V.; Erofeev, A.S.; et al. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals 2022, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Deep, A.; Marwaha, R.K. Design, synthesis, in silico studies and biological evaluation of 5-((E)-4-((E)-(substitutedaryl/alkyl)methyl)benzylidene)thiazolidine-2,4-dione derivatives. BMC Chem. 2020, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Marc, G.; Stana, A.; Oniga, S.D.; Pirnau, A.; Vlase, L.; Oniga, O. New phenolic derivatives of thiazolidine-2,4-dione with antioxidant and antiradical properties: Synthesis, characterization, in vitro evaluation, and quantum studies. Molecules 2019, 24, 2060. [Google Scholar] [CrossRef]
- Bansal, G.; Thanikachalam, P.V.; Maurya, R.K.; Chawla, P.; Ramamurthy, S. An overview on medicinal perspective of thiazolidine-2,4-dione: A remarkable scaffold in the treatment of type 2 diabetes. J. Adv. Res. 2020, 23, 163–205. [Google Scholar] [CrossRef]
- Kim, S.G.; Kim, D.M.; Woo, J.-T.; Jang, H.C.; Chung, C.H.; Ko, K.S.; Park, J.H.; Park, Y.S.; Kim, S.J.; Choi, D.S. Efficacy and Safety of Lobeglitazone Monotherapy in Patients with Type 2 Diabetes Mellitus over 24-Weeks: A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo Controlled Trial. PLoS ONE 2014, 9, e92843. [Google Scholar] [CrossRef]
- Kota, B.; Huang, T.; Roufogalis, B. An Overview on Biological Mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94. [Google Scholar] [CrossRef]
- Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118. [Google Scholar] [CrossRef]
- Werman, A.; Hollenberg, A.; Solanes, G.; Bjørbæk, C.; Vidal-Puig, A.J.; Flier, J.S. Ligand-Independent Activation Domain in the N Termsinus of Peroxisome Proliferator-Activated Receptor γ (PPARγ). J. Biol. Chem. 1997, 272, 20230–20235. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Charbonnel, B.; Staels, B. Thiazolidinediones and PPARγ agonists: Time for a reassessment. Trends Endocrinol. Metab. 2012, 23, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Jukic, M.; Gobec, S.; Sova, M. Reaching toward underexplored targets in antibacterial drug design. Drug Dev. Res. 2018, 80, 6–10. [Google Scholar] [CrossRef]
- Tomašić, T.; Zidar, N.; Kovač, A.; Turk, S.; Simčič, M.; Blanot, D.; Müller-Premru, M.; Filipič, M.; Grdadolnik, S.G.; Zega, A.; et al. 5-Benzylidenethiazolidin-4-ones as multitarget inhibitors of bacterial Mur ligases. ChemMedChem 2010, 5, 286–295. [Google Scholar] [CrossRef]
- Barreteau, H.; Kovac, A.; Boniface, A.; Sova, M.; Gobec, S.; Blanot, D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 168–207. [Google Scholar] [CrossRef] [PubMed]
- Sink, R.; Barreteau, H.; Patin, D.; Mengin-Lecreulx, D.; Gobec, S.; Blanot, D. MurD enzymes: Some recent developments. Biomol. Concepts 2013, 4, 539–556. [Google Scholar] [CrossRef]
- Khare, N.; Kapoor, A. Antioxidant evaluation of 2,4-thiazolidinedione and rhodanine derivatives. Der. Pharmacia. Lettre. 2016, 8, 38–46. [Google Scholar]
- Hossain, S.U.; Bhattacharya, S. Synthesis of O-prenylated and O-geranylated derivatives of 5-benzylidene2,4-thiazolidinediones and evaluation of their free radical scavenging activity as well as effect on some phase II antioxidant/detoxifying enzymes. Bioorg. Med. Chem. Lett. 2007, 17, 1149–1154. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Hu, D. Mitochondrial alterations during oxidative stress in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1153–1162. [Google Scholar] [CrossRef]
- Kumar, S.; Narasimhan, B. Therapeutic potential of heterocyclic pyrimidine scaffolds. Chem. Cent. J. 2018, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.M.; Patel, N.B.; Sanna, G.; Busonera, B.; Colla, P.L.; Rajani, D.P. Synthesis of some new 2-amino-6-thiocyanato benzothiazole derivatives bearing 2,4-thiazolidinediones and screening of their in vitro antimicrobial, antitubercular and antiviral activities. Med. Chem. Res. 2015, 24, 3129–3142. [Google Scholar] [CrossRef]
- Zvarec, O.; Polyak, S.W.; Tieu, W.; Kuan, K.; Dai, H.; Pedersen, D.S.; Morona, R.; Zhang, L.; Booker, G.W.; Abell, A.D. 5-Benzylidenerhodanine and 5-benzylidene-2-4-thiazolidinedione based antibacterials. Bioorg. Med. Chem. Lett. 2012, 22, 2720–2722. [Google Scholar] [CrossRef]
- Santosh, L.G.; Namratha, B.; Nitinkumar, S.S.; Hiroki, S. Microwave-assisted synthesis and evaluation of N-substituted thiazolidine-2,4-dione derivatives as antimicrobial agents. Interact. Med. Chem. 2014, 2, 1–5. [Google Scholar]
- Nawale, S.L.; Dhake, A.S. Synthesis and evaluation of novel thiazolidinedione derivatives for antibacterial activity. Der. Pharma. Chemica. 2012, 4, 2270–2277. [Google Scholar]
- Sindhu, J.; Singh, H.; Khurana, J.M.; Sharma, C.; Aneja, K.R. Multicomponent domino process for the synthesis of some novel 5-(arylidene)-3-((1-aryl-1H-1,2,3-triazol-4-yl)methyl)-thiazolidine-2,4-diones using PEG-400 as an efficient reaction medium and their antimicrobial evaluation. Chin. Chem. Lett. 2015, 26, 50–54. [Google Scholar] [CrossRef]
- Alagawadi, K.R.; Alegaon, S.G. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-thiazolidinediones bearing imidazo[2,1-b][1,3,4]thiadiazole moiety. Arab. J. Chem. 2011, 4, 465–472. [Google Scholar] [CrossRef]
- Juddhawala, K.V.; Parekh, N.M.; Rawal, B.M. Synthesis and antibacterial activities of N-chloro aryl acetamide substituted thaizole and 2,4-thazolidinedione derivatives. Arch. Appl. Sci. Res. 2011, 3, 540–548. [Google Scholar]
- Patel, N.B.; Purohit, A.C.; Rajani, D. Newer thiazolopyrimidine-based sulfonamides clubbed with benzothiazole moiety: Synthesis and biological evaluation. Med. Chem. Res. 2014, 23, 4789–4802. [Google Scholar] [CrossRef]
- Malik, N.; Prasad, D.N. Synthesis and antimicrobial evaluation of N-substituted-5-benzylidene-2,4-thiazolidinedione derivatives. Iran. J. Pharm. Res. 2012, 8, 209–214. [Google Scholar]
- Parekh, N.M.; Juddhawala, K.V.; Rawal, B.M. Antimicrobial activity of thiazolyl benzenesulfonamide-condensed 2,4-thiazolidinediones derivatives. Med. Chem. Res. 2013, 22, 2737–2745. [Google Scholar] [CrossRef]
- Desai, N.C.; Satodiya, H.M.; Rajpara, K.M.; Joshi, V.V.; Bhatt, K.; Vaghani, H.V. Synthesis and evaluation of N-substituted thiazolidine-2,4-dione containing pyrazole as potent antimicrobial agents. Anti Infective Agents 2014, 12, 85–94. [Google Scholar] [CrossRef]
- Prakash, O.; Aneja, D.K.; Arora, S.; Sharma, C.; Aneja, K.R. Synthesis and antimicrobial activities of some new 5-((3-(aryl)-1-phenyl-1H-pyrazol-4-yl)methylene)-3-phenylthiazolidine-2,4-diones. Med. Chem. Res. 2012, 21, 10–15. [Google Scholar] [CrossRef]
- Prakash, O.; Aneja, D.K.; Lohan, P.; Hussain, K.; Arora, S.; Sharma, C.; Aneja, K.R. Synthesis and antimicrobial activity of 5-((3-aryl-1-phenyl-1Hpyrazol-4-yl)methylene)thiazolidine-2,4-diones. Med. Chem. Res. 2012, 21, 2961–2968. [Google Scholar] [CrossRef]
- Lobo, P.L.; Poojary, B.; Manjunatha, K.; Prathibha, A.; Kumari, N.S. Novel thiazolidine-2,4-dione mannich bases: Synthesis, characterization and antimicrobial activity. Der. Pharma. Chemica. 2012, 4, 867–871. [Google Scholar]
- Alegaon, S.G.; Alagawadi, K.R. New thiazolidine-2,4-diones as antimicrobial and cytotoxic agent. Med. Chem. Res. 2012, 21, 3214–3223. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R. New thiazolidinedione-5-acetic acid amide derivatives: Synthesis, characterization and investigation of antimicrobial and cytotoxic properties. Med. Chem. Res. 2012, 21, 816–824. [Google Scholar] [CrossRef]
- Avupati, V.R.; Yejella, R.P.; Akula, A.; Guntuku, G.S.; Doddi, B.R.; Vutla, V.R.; Anagani, S.R.; Adimulam, L.S.; Vyricharla, A.K. Synthesis, characterization and biological evaluation of some novel 2,4-thiazolidinediones as potential cytotoxic, antimicrobial and antihyperglycemic agents. Bioorg. Med. Chem.Lett. 2012, 22, 6442–6450. [Google Scholar] [CrossRef]
- Liu, X.F.; Zheng, C.J.; Sun, L.P.; Liu, X.K.; Piao, H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011, 46, 3469–3473. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R.; Pawar, S.M.; Vinod, D.; Rajput, U. Synthesis, characterization, and biological evaluation of thiazolidine-2,4-dione derivatives. Med. Chem. Res. 2014, 23, 987–994. [Google Scholar] [CrossRef]
- da Silva, I.M.; da Silva Filho, J.; Santiago, P.B.; do Egito, M.S.; de Souza, C.A.; Gouveia, F.L.; Ximenes, R.M.; de Sena, K.X.; de Faria, A.R.; Brondani, D.J.; et al. Synthesis and antimicrobial activities of 5-arylidene-thiazolidine-2,4-dione derivatives. Biomed. Res. Int. 2014, 2014, 316082. [Google Scholar] [CrossRef] [PubMed]
- Rekha, S.; Chandrashekhara, S. Antioxidant and antibacterial activities of thiazolidinedione derivatives. Int. J. Pharm. Sci. 2015, 6, 421–428. [Google Scholar]
- Purohit, S.S.; Alman, A.; Shewale, J. Synthesis and antimicrobial activity of a new series of 3,5-disustitutedthiazolidine-2,4-diones. Int. J. Pharm. Pharm. Sci. 2012, 4, 273–276. [Google Scholar]
- Mangasuli, S.N. Synthesis of novel coumarin-thiazolidine-2,4-dione derivatives: An approach to computational studies and biological evaluation. Results Chem. 2021, 3, 100105. [Google Scholar] [CrossRef]
- Alhameed, R.A.; Almarhoon, Z.; Bukhari, S.I.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Synthesis and Antimicrobial Activity of a New Series of Thiazolidine-2,4-diones Carboxamide and Amino Acid Derivatives. Molecules 2020, 15, 105. [Google Scholar] [CrossRef]
- Mishra, G.; Sachana, N.; Chawla, P. Synthesis and evaluation of thiazolidinedione-coumarin adducts as antidiabetic, anti-inflammatory and antioxidant agents. Lett. Org. Chem. 2015, 12, 429–445. [Google Scholar] [CrossRef]
- Dundar, O.B.; Evranos, B.; Evcimen, N.D.; Sarikaya, M.; Ertan, R. Synthesis and aldose reductase inhibitory activity of some new chromonyl- 2,4-thiazolidinediones. Eur. J. Med. Chem. 2008, 43, 2412–2417. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.K.; Thareja, S. Molecular docking studies of novel arylidene-2, 4-thiazolidinediones as potential aldose reductase inhibitors. Lett. Drug Des. Discov. 2013, 10, 604–612. [Google Scholar] [CrossRef]
- Datar, P.A.; Aher, S.B. Design and synthesis of novel thiazolidine-2,4-diones as hypoglycemic agents. J. Saudi Chem. Soc. 2016, 20, S196–S201. [Google Scholar] [CrossRef]
- Jawale, D.V.; Pratap, U.R.; Rahuja, N.; Srivastava, A.K.; Mane, R.A. Synthesis and antihyperglycemic evaluation of new 2,4-thiazolidinediones having biodynamic aryl sulfonylurea moieties. Bioorg. Med. Chem.Lett. 2012, 22, 436–439. [Google Scholar] [CrossRef]
- Kumar, A.; Chawla, A.; Jain, S.; Kumar, P.; Kumar, S. 3-Aryl-2-{4-[4-(2,4-dioxothiazolidin-5-ylmethyl)phenoxy]-phenyl}-acrylic acid alkyl ester: Synthesis and antihyperglycemic evaluation. Med. Chem. Res. 2011, 20, 678–686. [Google Scholar] [CrossRef]
- Swathi, N.; Ramu, Y.; Subrahmanyam, C.V.S.; Satyanarayana, K. Synthesis, quantum mechanical calculation and biological evaluation of 5-(4-substituted aryl/hetero aryl methylidene)-1,3-thiazolidine-2,4-diones. Int. J. Pharm. Pharm. Sci. 2012, 4, 561–566. [Google Scholar]
- Evcimen, N.D.; Sarikaya, M.; Alp, A.S.G.; Dundar, O.B. Aldose reductase inhibitory potential of several thiazolyl-thiazolidine-2,4-diones. Lett. Drug Des. Discov. 2013, 10, 415–419. [Google Scholar] [CrossRef]
- Verma, R.K.; Mall, R.; Singh, A. Indolyl linked meta-substituted benzylidene-based novel PPAR ligands: Synthetic and docking studies. Med. Chem. Res. 2015, 24, 1396–1407. [Google Scholar] [CrossRef]
- Nazreen, S.; Alam, M.S.; Hamid, H.; Yar, M.S.; Shafi, S.; Dhulap, A.; Alam, P.; Pasha, M.A.Q.; Bano, S.; Alam, M.M.; et al. Design, synthesis, in silico molecular docking and biological evaluation of novel oxadiazole based thiazolidine-2,4-diones bis-heterocycles as PPAR-γ agonists. Eur. J. Med. Chem. 2014, 87, 175–185. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Lee, W.; Kim, S.N.; Yoon, G.; Cheon, S.H. Design, synthesis and docking study of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2014, 24, 3337–3340. [Google Scholar] [CrossRef]
- Swapna, D.; Sivagani, B.; Manasa, K.; Rajita, G.; Alagarsamy, V. Synthesis and evaluation of novel thiazolidinedione derivatives for antidiabetic activity. Int. Res. J. Pharma. 2016, 7, 15–19. [Google Scholar]
- Kumar, K.S.; Rao, A.L.; Rao, M.B. Design, synthesis, biological evaluation and molecular docking studies of novel 3-substituted-5-[(indol-3-yl)methylene]-thiazolidine-2,4-dione derivatives. Heliyon 2018, 4, e00807. [Google Scholar]
- Mahapatra, M.K.; Kumar, R.; Kumar, M. N-alkylated thiazolidine-2,4-dione analogues as PTP1B inhibitors: Synthesis, biological activity, and docking studies. Med. Chem. Res. 2017, 26, 1176–1183. [Google Scholar] [CrossRef]
- Badiger, N.P.; Shashidhar, N.; Vaidya, P.N. Synthesis of novel 5-{[2-(4-fluorobenzyl)-6-arylimidazo[2,1-b] [1,3,4]thiadiazol-5-yl]methylene}thiazolidine-2,4-diones as potent antidiabetic agents. Int. J. Sci. Eng. Appl. 2015, 4, 24–29. [Google Scholar] [CrossRef]
- Alam, F.; Dey, K.; Kalita, P. Synthesis, characterization of thiazolidinedione derivatives as oral hypoglycemic agent. Ind. J. Pharm. Sci. Res. 2015, 5, 67–71. [Google Scholar]
- Patil, S.D.; Nawale, S.L.; Balasurbramaniyan, V. Evaluation of thiazolidinedione derivatives for acute toxicity and potential antidiabetic activity. Der. Pharm. Chemica. 2015, 7, 216–223. [Google Scholar]
- Garg, A.; Chawla, P.; Shubhini, S.A. Substituted-arylidene-3-substituted-benzyl-thiazolidine-2,4-dione analogues as anti-hyperglycemic agents. Int. J. Drug Dev. Res. 2012, 4, 141–146. [Google Scholar]
- Senthilkumar, N.; Vijayakumar, V.; Sarveswari, S.; Gayathri, G.A.; Gayathri, M. Synthesis of new thiazolidine-2,-4-dione-azole derivatives and evaluation of their a-amylase and a-glucosidase inhibitory activity. Iran. J. Sci. Technol. Trans. Sci. 2019, 43, 735–745. [Google Scholar] [CrossRef]
- Nikaljea, P.G.A.; Choudharia, S.; Une, H. Design, synthesis and hypoglycemic activity of novel 2-(4-((2, 4-dioxothiazolidin-5-ylidene) methyl)-2-methoxyphenoxy)-N-substituted acetamide derivatives. Euro. J. Exp. Bio. 2012, 2, 1302–1314. [Google Scholar]
- Srikanth, L.; Raghunandan, N.; Srinivas, P.; Reddy, G.A. Synthesis and evaluation of newer quinoline derivatives of thiazolidinedione for their antidiabetic activity. Int. J. Pharm. Bio. Sci. 2010, 1, 120–131. [Google Scholar]
- Kadium, R.T.; Al-Hazam, H.A.; Hameed, B.J. Design, synthesis, and characterization of some novel Thiazolidine-2,4-dione derivatives as antidiabetic agents. Drug Res. 2021, 78, 773–779. [Google Scholar] [CrossRef]
- Koppireddi, S.; Komsani, J.R.; Avula, S.; Pombala, S.; Vasamsetti, S.; Kotamraju, S.; Yadla, R. Novel 2-(2,4-dioxo-1,3-thiazolidin-5-yl)acetamides as antioxidant and/or anti-inflammatory compounds. Eur. J. Med. Chem. 2013, 66, 305–313. [Google Scholar] [CrossRef]
- Shahnaz, M.; Patel, K.B. Synthesis, characterization of 2,4-thiazolidinedione derivatives and evaluation of their antioxidant activity. J. Drug Deliv. Ther. 2013, 3, 96–101. [Google Scholar] [CrossRef]
- Nyaki, H.Y.; Mahmoodi, N.O. Synthesis and characterization of derivatives including thiazolidine-2,4-dione/1-H-imidazole and evaluation of antimicrobial, antioxidant, and cytotoxic properties of new synthetic heterocyclic compounds. Res. Chem. Intermed. 2021, 47, 4129–4155. [Google Scholar] [CrossRef]
- Sameeh, M.Y.; Khowdiary, M.M.; Nassar, H.S.; Abdelall, M.M.; Amer, H.H.; Hamed, A.; Elhenawy, A.A. Thiazolidinedione Derivatives: In Silico, In Vitro, In Vivo, antioxidant and anti-diabetic evaluation. Molecules 2022, 27, 830. [Google Scholar] [CrossRef] [PubMed]
- Sameeh, M.Y.; Khowdiary, M.M.; Nassar, H.S.; Abdelall, M.M.; Alderhami, S.A.; Elhenawy, A.A. Discovery Potent of Thiazolidinedione Derivatives as Antioxidant, α-Amylase Inhibitor, and Antidiabetic Agent. Biomedicines 2022, 10, 24. [Google Scholar]
- Thareja, S.; Verma, S.K.; Jain, A.K.; Bharadwaj, T.R. Novel 5-[4-(2-biphenyl-4-yl-2-oxo-ethoxy)-benzylidene]-thiazolidine-2,4-diones, Their Synthesis and Uses Thereof. World. Intellectual. Property Organisation WO2019/016826A1, 24 January 2019. [Google Scholar]
- Craig, A.S.; Millan, M. Sodium Salts of 5-′4-′2-(n-methyl-n-(2-pyridyl)amino)ethoxy]benzyl]thiazolidine-2,4-dione. World Intellectual Property Organisation WO02/26735A1, 4 April 2002. [Google Scholar]
- Halama, A. Salt of Oxalic Acid with 5-[4-[2-(n-methyl-n-(2-pyridyl)-amino)ethoxy]benzyl]thiazolidin-2,4-dione and A Method of Its Preparation and Its Use. World Intellectual Property Organisation WO2006/010345A1, 2 February 2006. [Google Scholar]
- Epstein, J.W.; Ayral-Kaloustian, S.; Birnberg, G.H.; Salaski, E.J.; Macewan, G.J.; Cheung, K. 5-(arylsulfonyl)-,5-(arylsulfinyl), and 5-(arylsulfanyl)-thiazolidine-2,4-diones Useful for Inhibition of Farnesyl-Protein Transferase. U.S. Patent 10/227,084, 31 August 2004. [Google Scholar]
- Millan, M.J. 5-(4-(2-(n-methyl-n-(2-pyridyl)amino)ethoxy)benzyl)thiazolidine -2,4-dione Malic Acid Salt and Use Against Diabetes Mellitus. World Intellectual Property Organisation WO03/053962A1, 3 July 2003. [Google Scholar]
- Lalanza, M.P.P.; Pedemonte, M.M. 5-[[4-[2-[5-(1-Hydroxyethyl)pyridin-2-yl] ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione for Treating Nonalcoholic Fatty Liver Disease. U.S. Patent US2020/0093812A1, 26 March 2020. [Google Scholar]
- Takashi, S.; Hitoshi, I. Thiazolidinedione Derivatives, Their Production and Their Use. European Patent EP0508740A1, 10 October 1992. [Google Scholar]
- Dolitzky, B.Z. Hydrogenation of Precursors to Thiazolidinedione Antihyperglycemics. World Intellectual Property Organisation WO03/053367A2, 3 July 2003. [Google Scholar]
- Fang, D.; Zhang, J.; Wu, L.; Hu, S.; Hu, X.; Gan, Y. Dimethyldiguanide of the Thiazolidinedione Pharmaceutical, Preparation Method and Use Thereof. Chinese Patent CN101531657A, 16 September 2009. [Google Scholar]
- Fengjiao, G.; Juan, S.; Xinyi, W.; Yang, Z.; Hailiang, Z. Thiazolidinedione-Containing Phenylpiperazine Derivatives as well as Preparation Method and Applications of Thiazolidinedione-Containing Phenylpiperazine Derivatives. Chinese Patent CN104230915A, 24 December 2014. [Google Scholar]
- Hoon, C.; Cheol-Hee, C.; Ying, W. Novel Thiazolidinedione Derivative and Use. Thereof. Patent WO2010/077101A2, World Intellectual Property Organisation, 8 July 2010. [Google Scholar]
- Nara, S.; Kanda, Y.; Asai, A.; Yamashita, N. Thiazolidinedione Derivative. Japanese Patent JP2002/322177A, 8 November 2002. [Google Scholar]
- Colca, G.R.; Kletzein, R.F. Thiazolidinedione Analogues. World Intellectual Property Organisation WO2007/109037A2, 27 September 2007. [Google Scholar]
- Burbridge, M.; Cattan, V.; Jacquet-Bescond, A. Association between 3-[(3-{[4-(4-morpholinylmethyl)-1H-pyrrol-2-yl]methylene}-2-oxo-2,3-dihydro-1h-indol-5-yl)methyl]-1,3-thiazolidine-2,4-dione and a Tyrosine Kinase Inhibitor of the. EGFR. Patent WO2017/021634A1, World Intellectual Property Organisation, 9 February 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).