Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Spectroscopic Analyses
2.2. General Procedure for the Synthesis of Isonicotinic Hydrazid-Based Hydrazone Derivatives
2.2.1. NH1 Tert-butyl (E)-2-(4-((2-Isonicotinoylhydrazineylidene) methyl)-2-methoxyphenoxy) Acetate
2.2.2. NH2 Tert-butyl (E)-2-(4-((2-Isonicotinoylhydrazineylidene) methyl) phenoxy) Acetate
2.2.3. NH3 (E)-N′-(3-Methoxy-4-((3-methylbut-2-en-1-yl)oxy)benzylidene)isonicotinohydrazide
2.2.4. NH4 (E)-N′-(4-((3-Methylbut-2-en-1-yl)oxy)benzylidene)isonicotinohydrazide
2.2.5. NH5 (E)-N′-(3-Methoxy-4-propoxybenzylidene) isonicotinohydrazide
2.2.6. NH6 (E)-N′-(4-Propoxybenzylidene) Isonicotinohydrazide
2.3. Antibacterial Activity
2.3.1. Bacterial Strains Used
2.3.2. Paper Disc Diffusion Method for Zone of Inhibition Assay
2.3.3. Microplate Assay of Minimum Inhibitory Concentration (MIC)
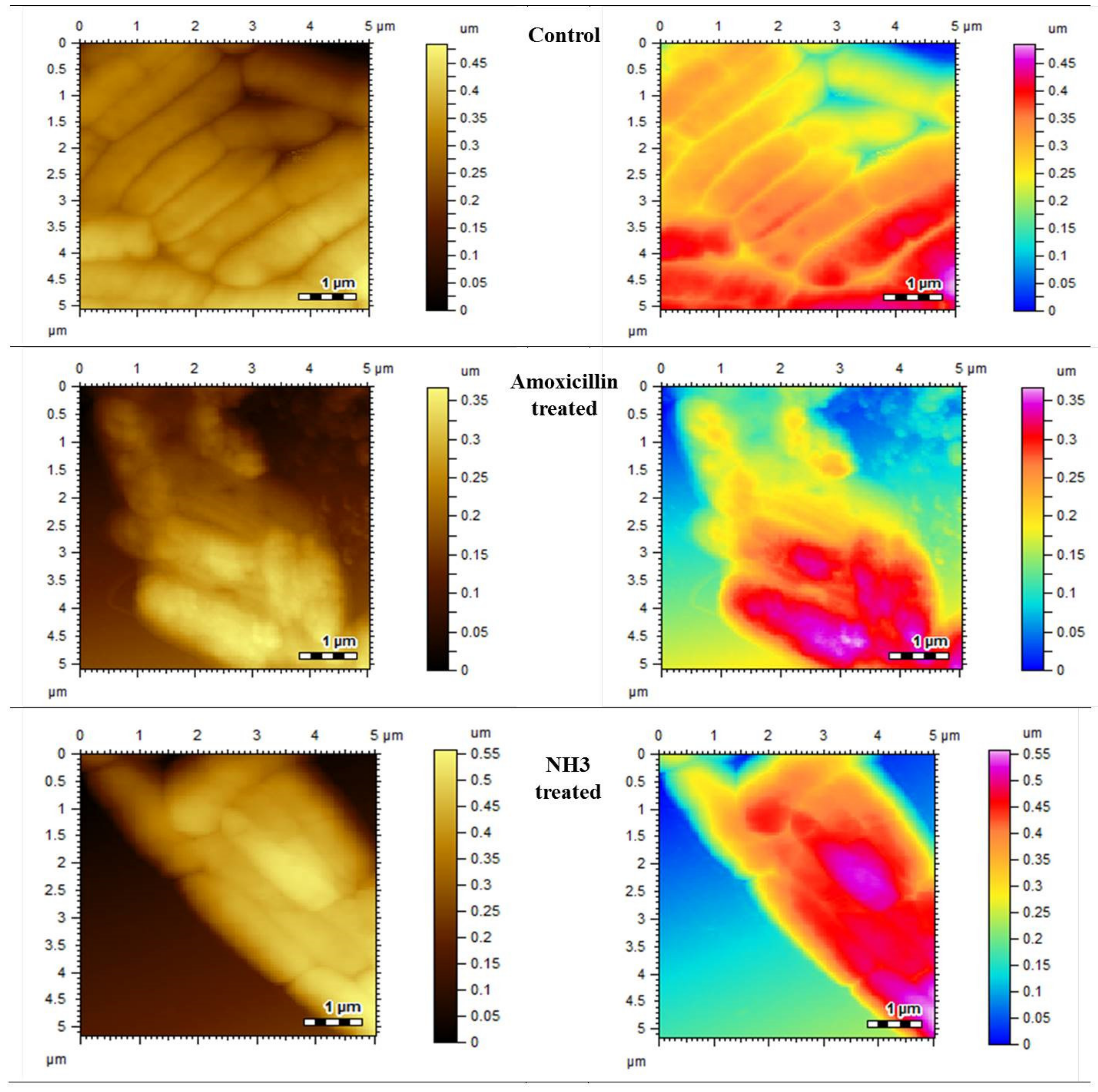
2.3.4. Morphological Changes Studied by Atomic Force Microscopy (AFM)
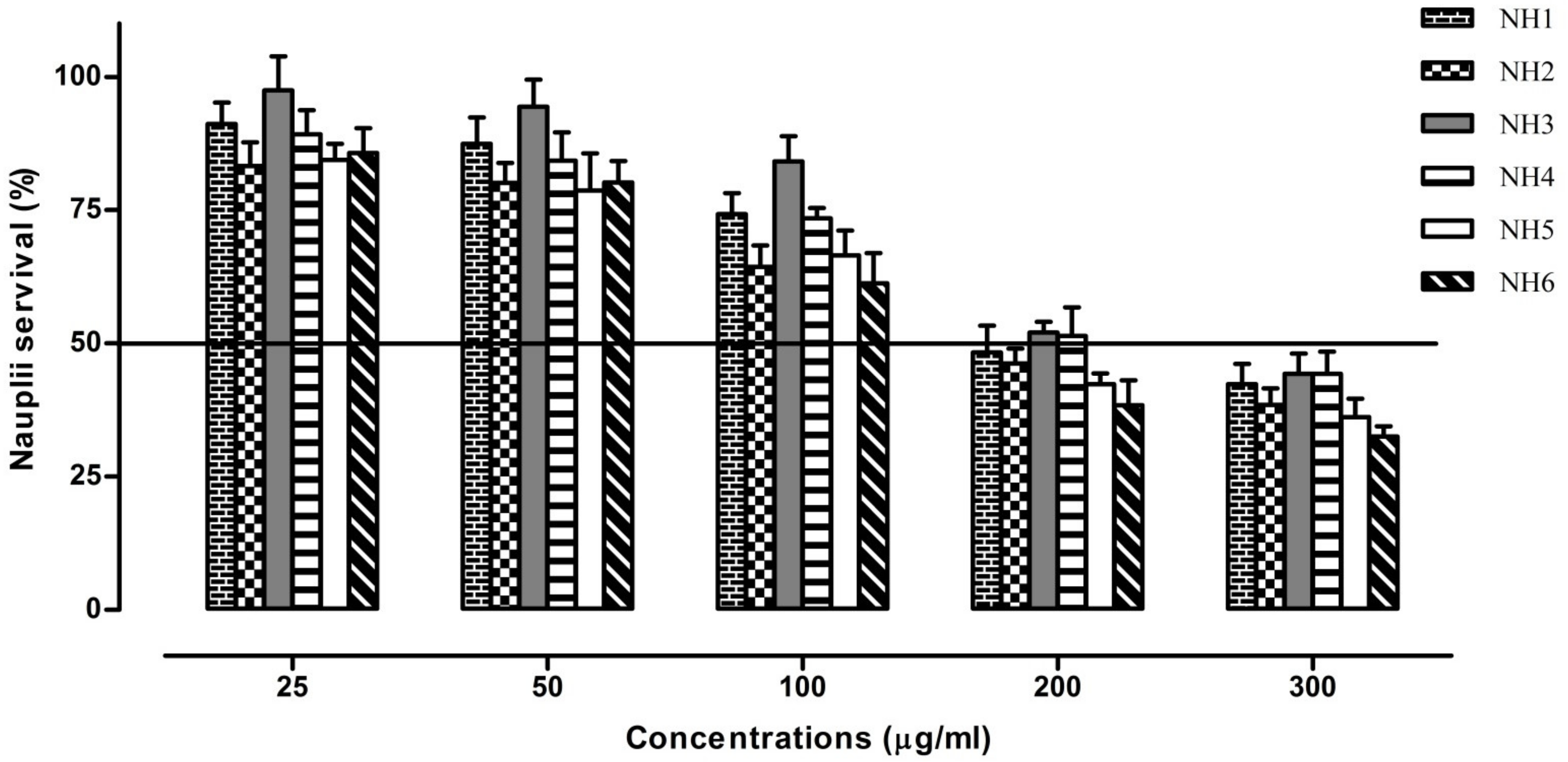
2.4. Cytotoxicity
3. Results and Discussion
3.1. Antibacterial Assay
3.1.1. Determination of Minimum Inhibitory Concentration (MIC)
3.1.2. Morphological Observations Using Atomic Force Microscopy
3.2. Cytotoxic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. Lancet 2006, 367, 859–869. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Easmon, J.; Purstinger, G.; Thies, K.S.; Heinisch, G.; Hofmann, J. Synthesis, structure−activity relationships, and antitumor studies of 2-benzoxazolyl hydrazones derived from alpha-(N)-acyl heteroaromatics. J. Med. Chem. 2006, 49, 6343–6350. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Ghani, U.; Syed, A.S. Thiosemicarbazide binds with the dicopper center in the competitive inhibition of mushroom tyrosinase enzyme: Synthesis and molecular modeling of theophylline analogues. Bioorg. Med. Chem. Lett. 2021, 36, 127826. [Google Scholar] [CrossRef]
- Ahmed, A.; Molvi, I.K.; Patel, M.H.; Ullah, R.; Bari, A. Synthesis of novel 2, 3, 5-tri-substituted thiazoles with anti-inflammatory and antibacterial effect causing clinical pathogens. J. Infect. Public Health 2020, 13, 472–479. [Google Scholar] [CrossRef]
- Sadia, M.; Khan, J.; Naz, R.; Zahoor, M.; Shah, A.S.W.; Ullah, R.; Naz, S.; Bari, A.; Mahmood, M.H.; Ali, S.S.; et al. Schiff base ligand L synthesis and its evaluation as anticancer and antidepressant agent. J. King Saud Univ. Sci. 2021, 33, 101331. [Google Scholar] [CrossRef]
- Khan, A.U.; Hussain, I.; Ullah, R. Synthesis, Characterization, Antimicrobial, Antioxidant Activities of Schiff Base and its Transition Metal Complexes. Chiang Mai J. Sci. 2020, 47, 1241–1254. [Google Scholar]
- De Aguiar Cordeiro, R.; Silva de Melo, C.; De Farias, F.J.; Serpa, R.; de Jesus, A.; Pacheco, E.; Mafezoli, J.; Ferreira de Oliveira, M.; da Silva, M.R.; de Jesus Pinheiro, T.; et al. Synthesis and in vitro antifungal activity of isoniazid-derived hydrazones against Coccidioides posadasii. Microb. Pathogen. 2016, 98, 1–5. [Google Scholar] [CrossRef][Green Version]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef]
- Pahonțu, E.; Ilies, D.-C.; Shova, S.; Oprean, C.; Paunescu, V.; Olaru, O.T.; Radulescu, F.S.; Gulea, A.; Rosu, T.; Draganescu, D. Synthesis, characterization, antimicrobial and antiproliferative activity evaluation of Cu (II), Co (II), Zn (II), Ni (II) and Pt (II) complexes with isoniazid-derived compound. Molecules 2017, 22, 650. [Google Scholar] [CrossRef]
- Naveen Kumar, H.; Jummat, F.; Asmawi, M.Z. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Stefanska, J.; Kielczykowska, M.; Musik, I.; Biernasiuk, A.; Malm, A.; Wujec, M. Synthesis, Dissociation Constants, and Antimicrobial Activity of Novel 2, 3-Disubstituted-1, 3-thiazolidin-4-one Derivatives. J. Heterocycl. Chem. 2016, 53, 393–402. [Google Scholar] [CrossRef]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat, M.A.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar]
- Gao, P.; Wei, Y. Efficient oxidative cyclization of N-acylhydrazones for the synthesis of 2, 5-disubstituted 1, 3, 4-oxadiazoles using t-BuOI under neutral conditions. Heterocycl. Commun. 2013, 19, 113–119. [Google Scholar] [CrossRef]
- Yu, W.; Huang, G.; Zhang, Y.; Liu, H.; Dong, L.; Yu, X.; Li, Y.; Chang, J. I2-mediated oxidative C–O bond formation for the synthesis of 1, 3, 4-oxadiazoles from aldehydes and hydrazides. J. Org. Chem. 2013, 78, 10337–10343. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, X.; Chen, K.; Yan, R.; Shan, Q.; Zhu, H.-L. Design, synthesis, biological evaluation and molecular modeling of 1, 3, 4-oxadiazoline analogs of combretastatin-A4 as novel antitubulin agents. Bioorg. Med. Chem. 2012, 20, 903–909. [Google Scholar] [CrossRef]
- Ali, M.R.; Lu, X.; Chen, K.; Yan, R.; Shan, Q.; Zhu, H.-L. Review of biological activities of hydrazones. Indones. J. Pharm. 2012, 23, 193–202. [Google Scholar]
- Al-Khattaf, F.S.; Mani, A.; Hatamleh, A.A.; Akbar, I. Antimicrobial and cytotoxic activities of isoniazid connected menthone derivatives and their investigation of clinical pathogens causing infectious disease. J. Infect. Public Health 2021, 14, 533–542. [Google Scholar] [CrossRef]
- Maccari, R.; Ottanà, R.; Vigorita, M.G. In vitro advanced antimycobacterial screening of isoniazid-related hydrazones, hydrazides and cyanoboranes: Part 14. Bioorg. Med. Chem. Lett. 2005, 15, 2509–2513. [Google Scholar] [CrossRef]
- Krátký, M.; Bosze, S.; Baranyai, Z.; Stolarikova, J.; Vinsova, J. Synthesis and biological evolution of hydrazones derived from 4-(trifluoromethyl) benzohydrazide. Bioorg. Med. Chem. Lett. 2017, 27, 5185–5189. [Google Scholar] [CrossRef]
- Zarafu, I.; Badea, M.; Ionita, G.; Chifiriuc, M.C.; Bleotu, C.; Popa, M.; Ionita, P.; Tatibouet, A.; Olar, R. Thermal, spectral and biological characterisation of copper (II) complexes with isoniazid-based hydrazones. J. Therm. Anal. Calorim. 2019, 136, 1977–1987. [Google Scholar] [CrossRef]
- Jabeen, M.; Mehmood, K.; Ain Khan, M.; Aslam, N.; Zafar, A.M.; Noreen, S.; Aslam, S.; Ghafoor, A. Microwave and conventional synthesis of Co (II), Cu (II) and Ni (II) metal complexes of some acid hydrazones with their spectral characterization and biological evaluation. Pak. J. Pharm. Sci. 2018, 31, 1003–1011. [Google Scholar] [PubMed]
- Kischner, N. Wolff–Kishner Reduction; J Star Research Inc.: Cranbury, NJ, USA, 2020. [Google Scholar]
- Bakht, J.; Aslam, A.; Ali, H.; Tayyab, M.; Shafi, M. Antimicrobial potentials of Eclipta alba by disc diffusion method. Afr. J. Biotechnol. 2011, 10, 7658–7667. [Google Scholar]
- Kim, Y.H.; Kim, G.H.; Yoon, K.S.; Shankar, S.; Rhim, J.-W. Comparative antibacterial and antifungal activities of sulfur nanoparticles capped with chitosan. Microb. Pathogen. 2020, 144, 104178. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Perumal, S. Determination of antibacterial activity of essential oil of Myristica fragrans Houtt. using tetrazolium microplate assay and its cytotoxic activity against vero cell line. Int. J. Pharmacol. 2012, 8, e6. [Google Scholar]
- Pourali, P.; Badiee, S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Moshi, M.; Innocent, E.; Otieno, J.N.; Magadula, J.J.; Nondo, R.S.; Otieno, D.F.; Wensheit, A.; Mbadazi, P. Antimicrobial and brine shrimp activity of Acanthus pubescens root extracts. Tanzan. J. Health Res. 2010, 12, 155–158. [Google Scholar] [CrossRef][Green Version]
- Niksic, H.; Mikhia, S.; Kapoor, S.; Bhatt, V.; Kumar, R. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
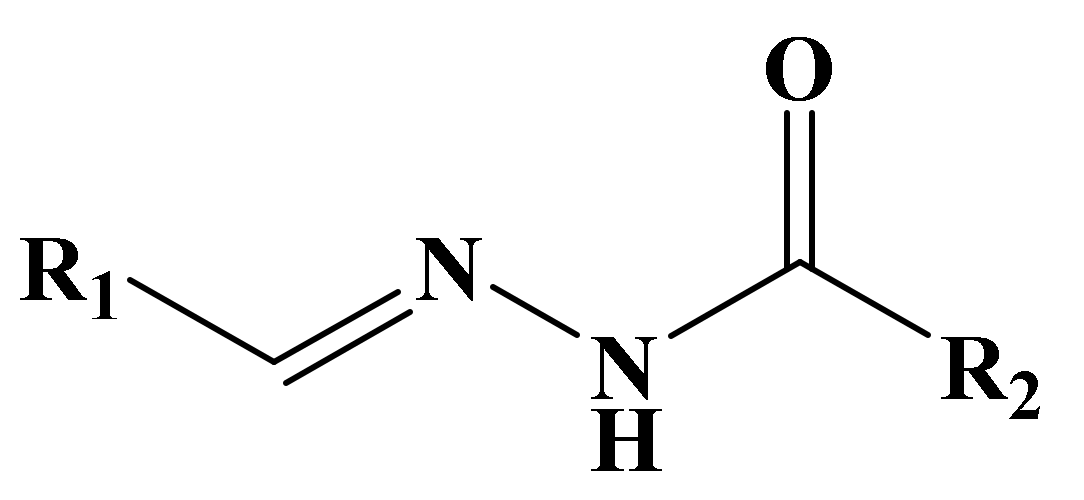



| S. No. | Sample Code | M.F | Molecular Structure | M.P (°C) | RF | %Yield | Color |
|---|---|---|---|---|---|---|---|
| 1 | NH1 | C20H23N3O5 | 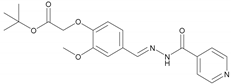 | 169–170 | 0.45 | 68 | Light-yellow |
| 2 | NH2 | C19H21N3O4 | 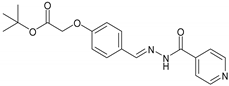 | 173–174 | 0.40 | 69 | Light-yellow |
| 3 | NH3 | C19H21N3O3 | 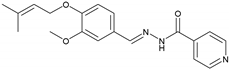 | 178–179 | 0.40 | 70 | Light-yellow |
| 4 | NH4 | C18H19N3O2 | 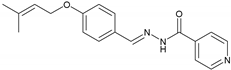 | 179–180 | 0.45 | 66 | Light-yellow |
| 5 | NH5 | C17H19N3O3 | 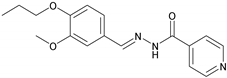 | 152–153 | 0.45 | 71 | Light-yellow |
| 6 | NH6 | C16H17N3O2 | 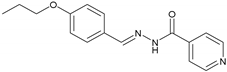 | 143–145 | 0.47 | 63 | Light-yellow |
| Compound | Antibacterial Activity (Paper Disc Diffusion Method) | ||
|---|---|---|---|
| Zone of Inhibition (mm) | |||
| Staphylococcus aureus ATCC 25923 (Gram +ve) | Bacillus subtilis ATCC 11774 (Gram +ve) | Escherichia coli ATCC 10536 (Gram −ve) | |
| NH1 | 20 | 14 | 25 |
| NH2 | 15 | 14 | 14 |
| NH3 | 24 | 22 | 33 |
| NH4 | 20 | 23 | 21 |
| NH5 | 22 | 25 | 24 |
| NH6 | 17 | 23 | 22 |
| Amikacin | 24 | 30 | 38 |
| Conc. µg/mL | % Inhibition of S. aureus ATCC 25923 | ||||||
|---|---|---|---|---|---|---|---|
| Ceftriaxone | NH1 | NH2 | NH3 | NH4 | NH5 | NH6 | |
| 25 | 30 ± 0.8 | - | 11 ± 1.0 | 24 ± 0.7 | 16 ± 0.5 | 5 ± 0.4 | - |
| 50 | 55 ± 0.3 | 10 ± 0.6 | 21 ± 1.2 | 35 ± 1.2 | 24 ± 1.0 | 12 ± 0.4 | 5.5 ± 0.5 |
| 100 | 80 ± 1.2 | 25 ± 0.7 | 42 ± 2 | 44 ± 2.0 | 38 ± 1.5 | 35 ± 0.7 | 25 ± 0.5 |
| 200 | 96 ± 1.0 | 55 ± 1 | 50 ± 2 | 60 ± 1.5 | 55 ± 0.9 | 58 ± 1.1 | 52 ± 1.6 |
| 300 | - | 63 ± 1 | 63 ± 1.5 | 78 ± 1 | 71 ± 2.0 | 77 ± 1.5 | 72 ± 1.2 |
| 400 | - | 69 ± 2 | 74 ± 2 | 89 ± 1.5 | 89 ± 1.5 | - | 80 ± 1.0 |
| 500 | - | 80 ± 1.5 | - | - | - | - | - |
| Conc. µg/mL | % Inhibition of B. subtilis ATCC 11774 | ||||||
|---|---|---|---|---|---|---|---|
| Ceftriaxone | NH1 | NH2 | NH3 | NH4 | NH5 | NH6 | |
| 25 | 40 ± 0.5 | - | 4 ± 0.2 | 12 ± 0.4 | 10 ± 0.7 | - | - |
| 50 | 55 ± 0.7 | 5 ± 0.3 | 12 ± 0.3 | 20 ± 0.7 | 18 ± 0.6 | 10 ± 0.5 | 10 ± 0.6 |
| 100 | 79 ± 1.2 | 26 ± 0.3 | 22 ± 0.3 | 28 ± 0.8 | 29 ± 0.8 | 26 ± 0.5 | 23 ± 0.7 |
| 200 | 92 ± 1.2 | 44 ± 0.4 | 44 ± 0.5 | 53 ± 0.7 | 50 ± 1.1 | 53 ± 0.6 | 52 ± 0.8 |
| 300 | - | 60 ± 0.5 | 60 ± 0.5 | 74 ± 0.9 | 68 ± 1.0 | 73 ± 0.8 | 70 ± 1.1 |
| 400 | - | 75 ± 0.5 | 81 ± 0.6 | 84 ± 0.9 | 77 ± 0.8 | - | - |
| Conc. µg/mL | % Inhibition of E. coli ATCC 10536 | ||||||
|---|---|---|---|---|---|---|---|
| Amoxicillin | NH1 | NH2 | NH3 | NH4 | NH5 | NH6 | |
| 25 | 40 ± 0.5 | 9 ± 0.5 | - | - | - | 10 ± 0.6 | 5 ± 0.2 |
| 50 | 66 ± 0.5 | 18 ± 0.5 | - | 36 ± 0.5 | 5 ± 0.5 | 18 ± 0.6 | 14 ± 0.5 |
| 100 | 85 ± 0.6 | 40 ± 0.4 | 10 ± 0.3 | 50 ± 0.5 | 25 ± 0.5 | 34 ± 0.3 | 28 ± 0.5 |
| 200 | 96 ± 1.0 | 60 ± 0.5 | 25 ± 0.3 | 65 ± 0.2 | 54 ± 0.4 | 58 ± 0.6 | 50 ± 0.7 |
| 300 | - | 79 ± 1.0 | 55 ± 0.5 | 85 ± 1.2 | 75 ± 0.8 | 79 ± 0.8 | 73 ± 0.7 |
| 400 | - | - | 70 ± 0.3 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.A.; Uddin, A.; Shah, M.R.; Ali, I.; Ullah, R.; Hannan, P.A.; Hussain, H. Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential. Molecules 2022, 27, 6770. https://doi.org/10.3390/molecules27196770
Shah MA, Uddin A, Shah MR, Ali I, Ullah R, Hannan PA, Hussain H. Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential. Molecules. 2022; 27(19):6770. https://doi.org/10.3390/molecules27196770
Chicago/Turabian StyleShah, Muhammad Abdullah, Ala Uddin, Muhammad Raza Shah, Imdad Ali, Riaz Ullah, Peer Abdul Hannan, and Hidayat Hussain. 2022. "Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential" Molecules 27, no. 19: 6770. https://doi.org/10.3390/molecules27196770
APA StyleShah, M. A., Uddin, A., Shah, M. R., Ali, I., Ullah, R., Hannan, P. A., & Hussain, H. (2022). Synthesis and Characterization of Novel Hydrazone Derivatives of Isonicotinic Hydrazide and Their Evaluation for Antibacterial and Cytotoxic Potential. Molecules, 27(19), 6770. https://doi.org/10.3390/molecules27196770







