Biosensors Based on Ion-Sensitive Field-Effect Transistors for HLA and MICA Antibody Detection in Kidney Transplantation
Abstract
1. Introduction
2. Results and Discussions
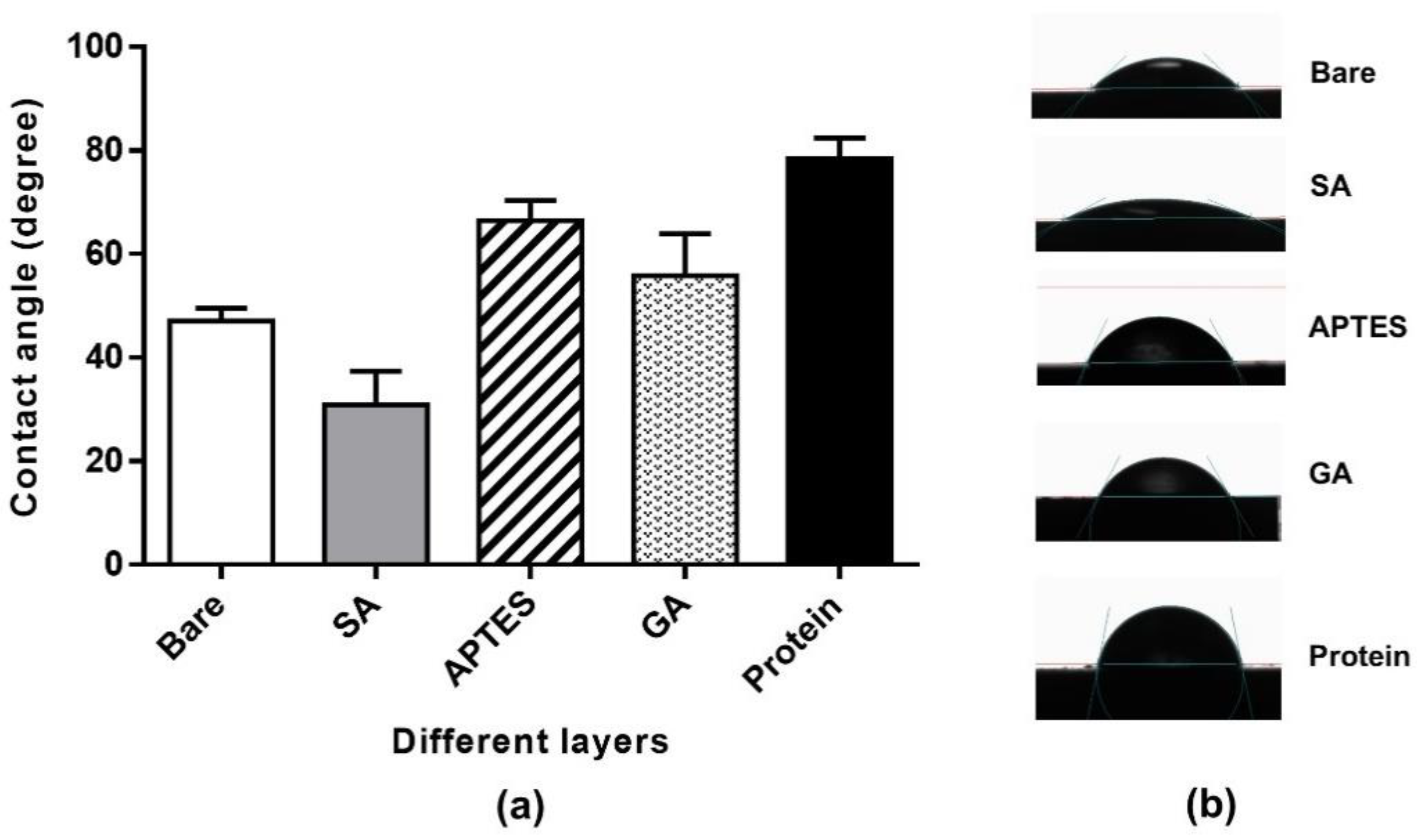
2.1. Contact Angle (CA) Measurement
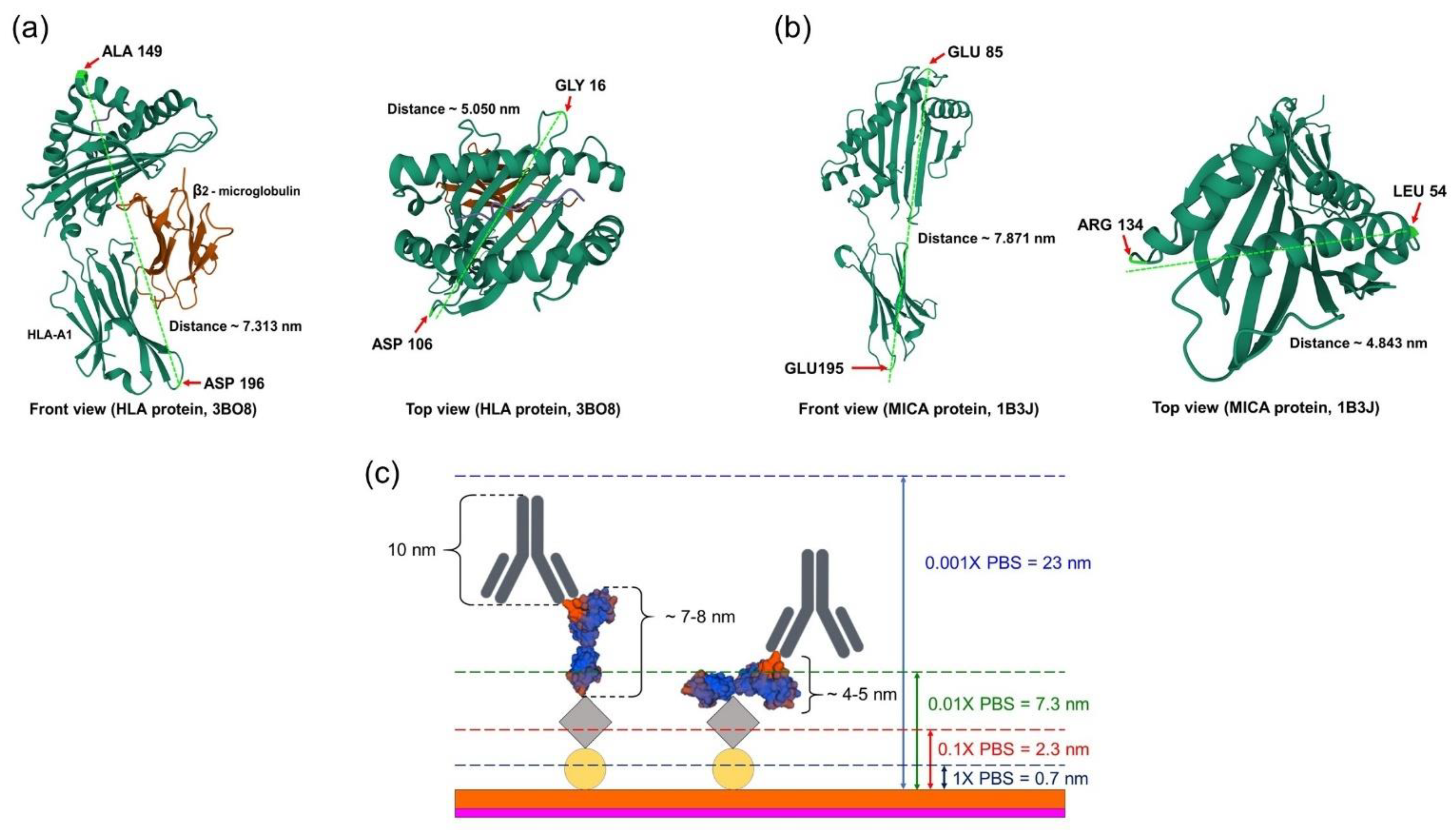
2.2. Surface Thickness
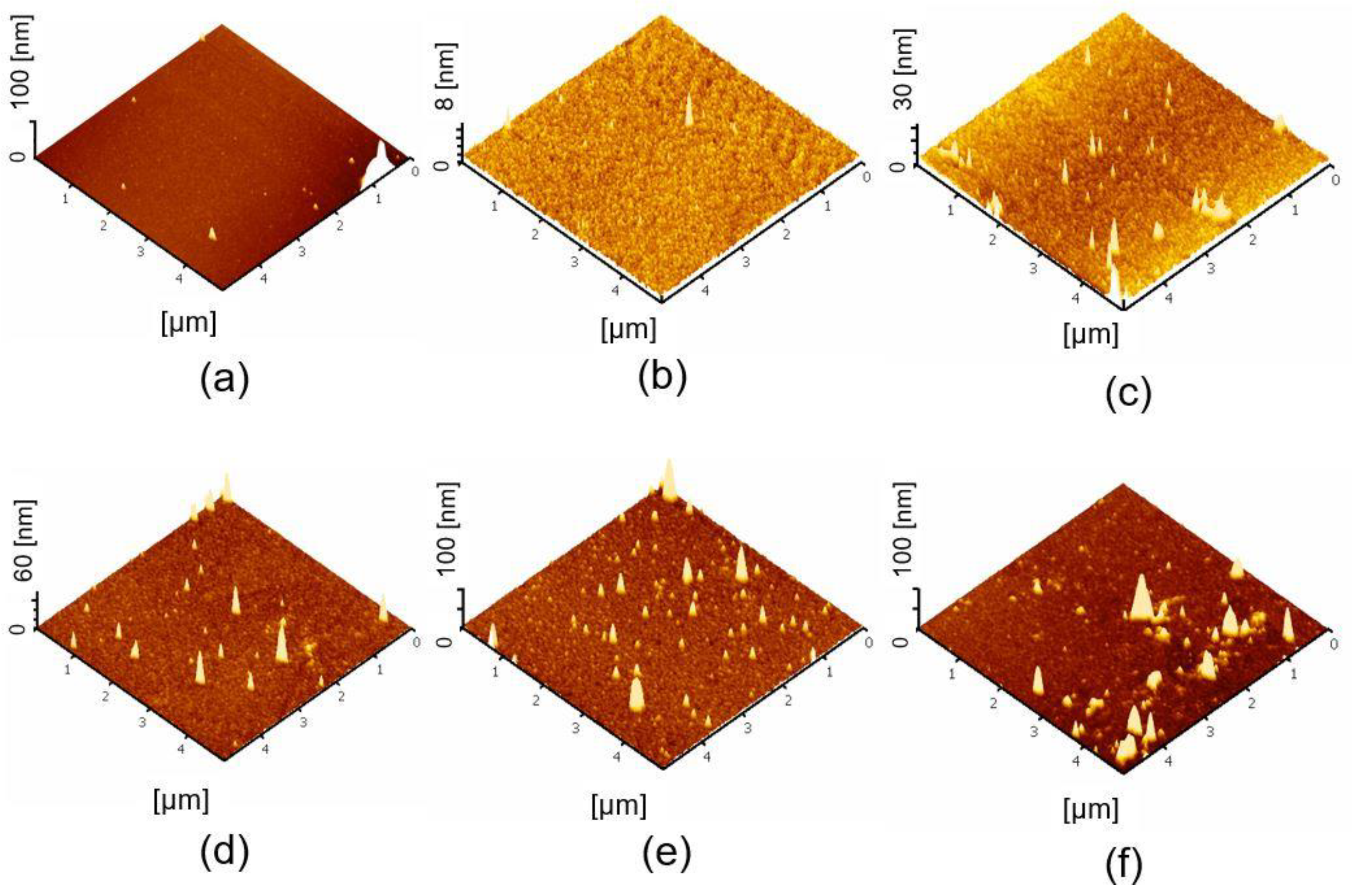
2.3. Surface Topography
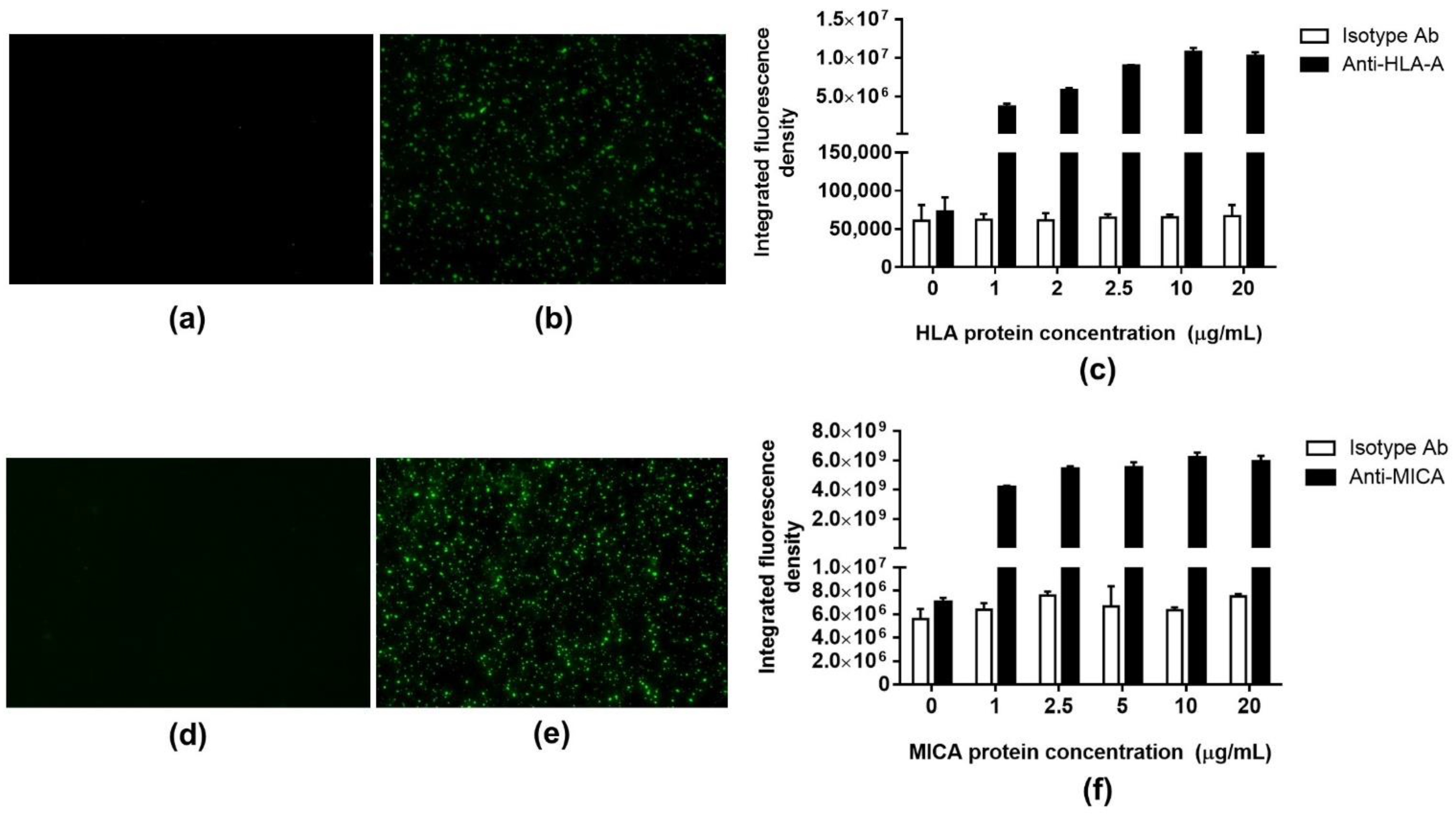
2.4. Fluorescence Detection
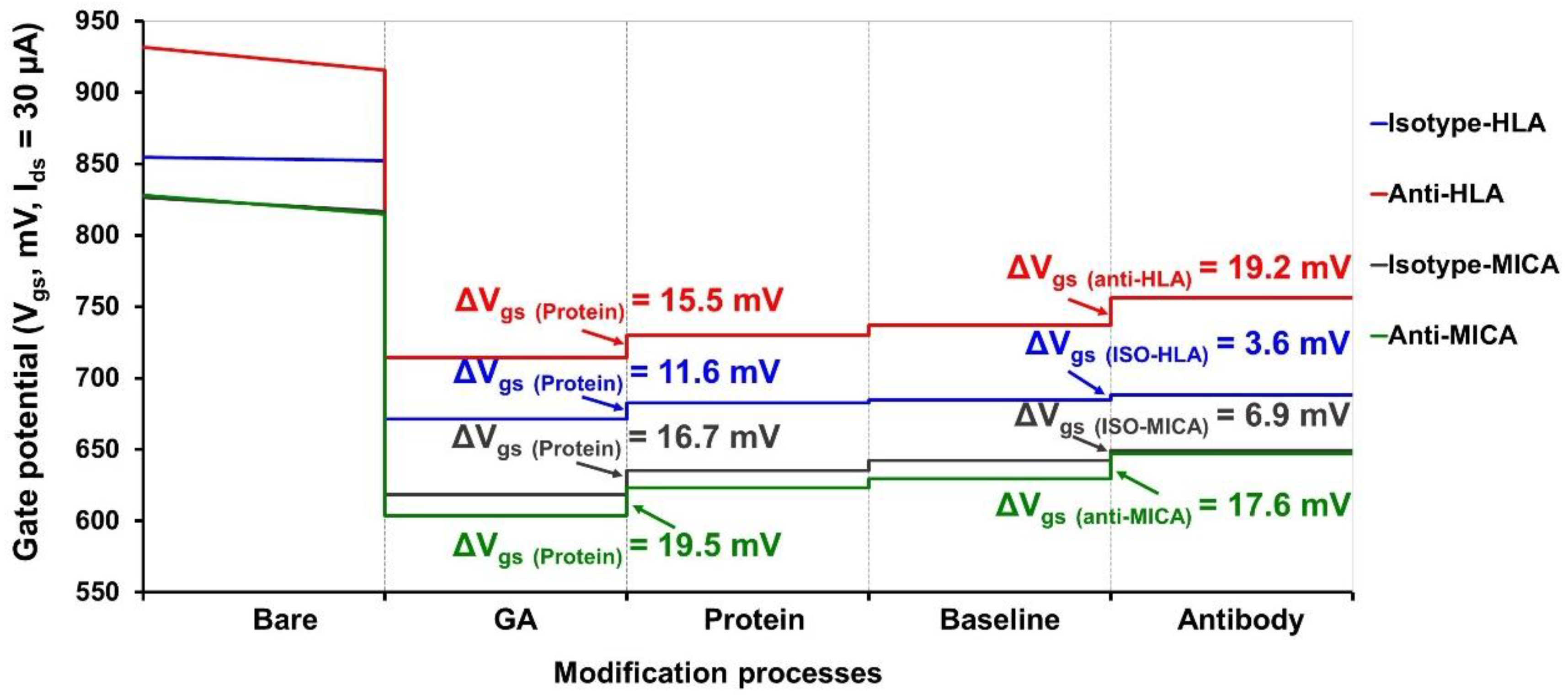
2.5. ISFET Measurement
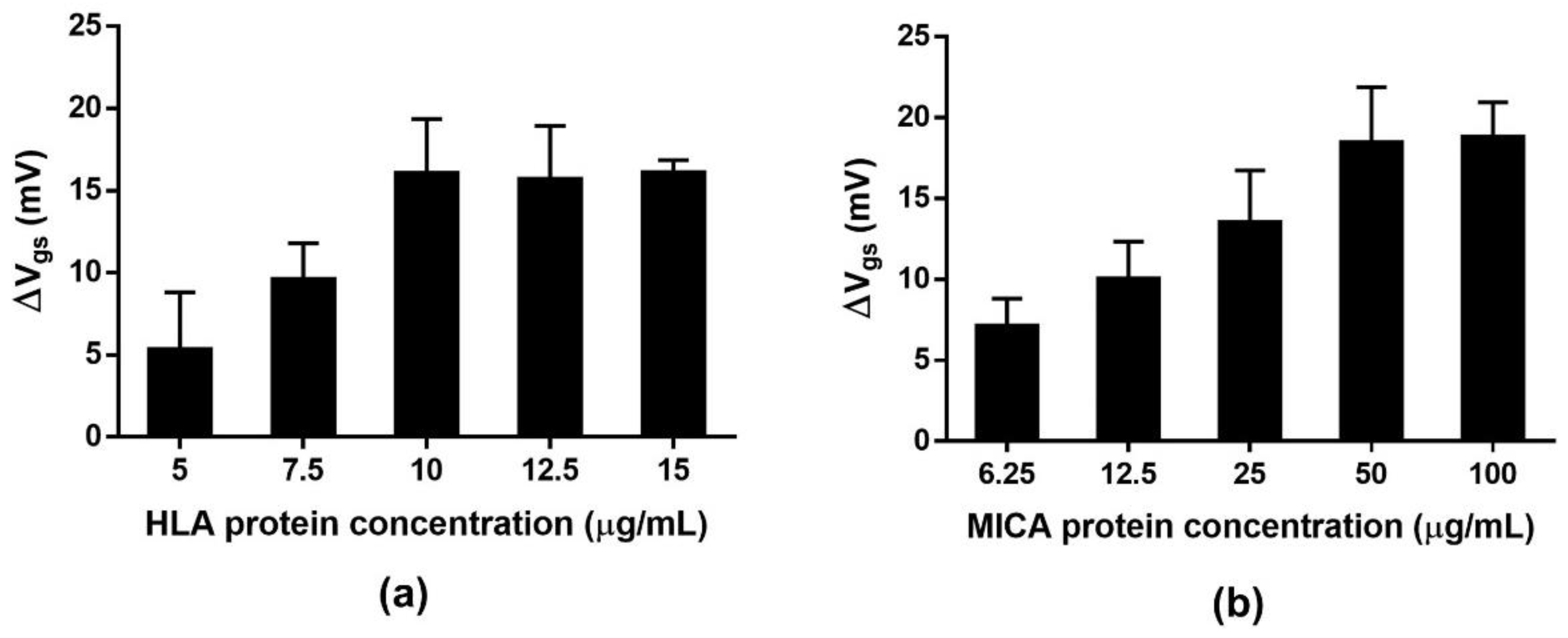
2.6. Protein Concentration Optimization for Electrical Measurement
2.7. Method Validations
2.7.1. Dose–Response Curve
2.7.2. Analytical Precision
2.7.3. Sensitivity and Specificity of the ISFET-Based Immunosensor for Anti-HLA and -MICA Detection in the Experimental Setting
3. Materials and Methods
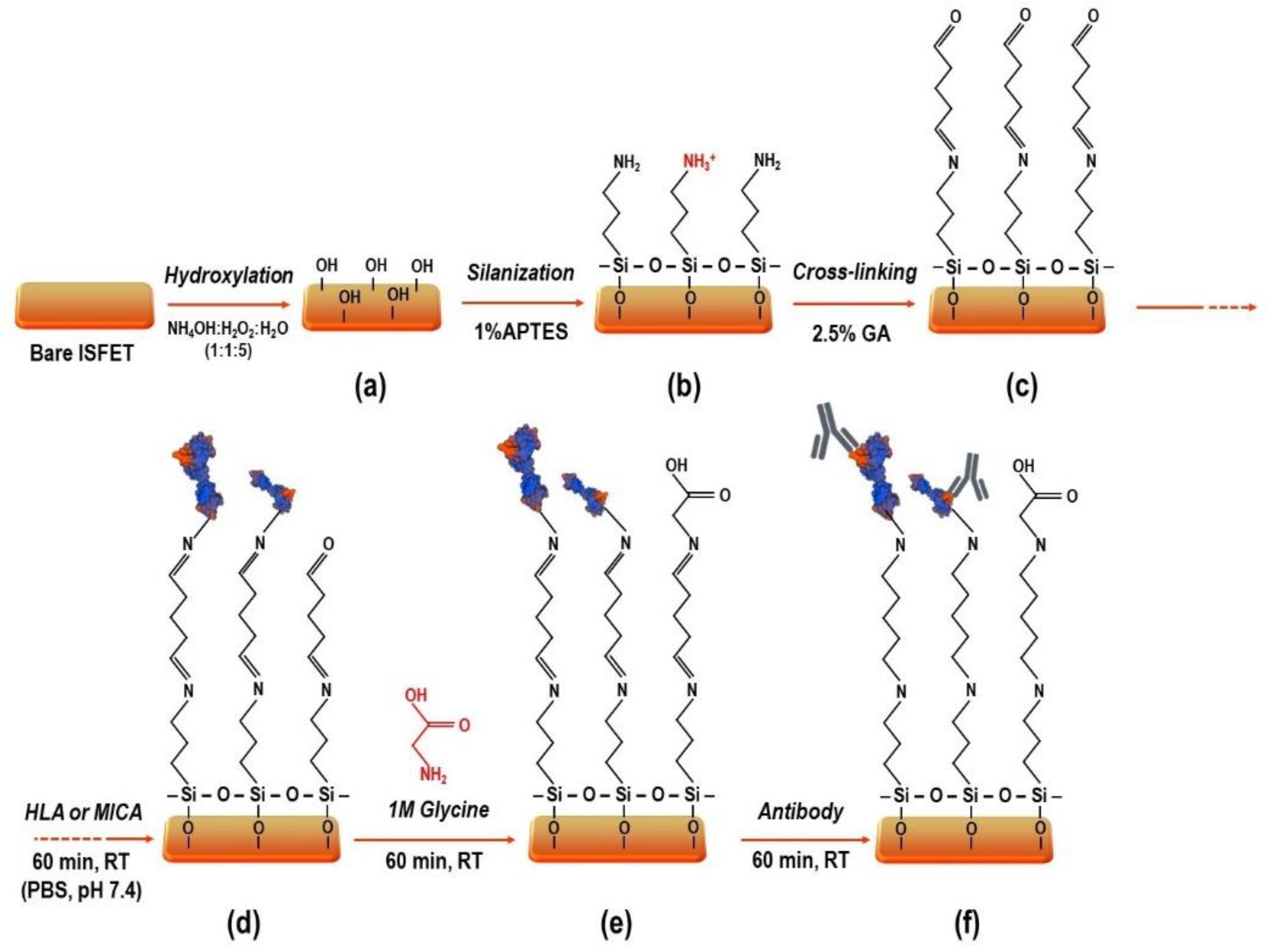
3.1. Modification of Si3N4 Surface
3.2. Characterization of Modified Si3N4 Surface
3.2.1. Contact Angle (CA) Measurement
3.2.2. Ellipsometry
3.2.3. Atomic Force Microscopy (AFM)
3.3. Antigen Immobilization and Antibody Binding
3.3.1. Fluorescence Detection
3.3.2. ISFET Measurement
3.4. Protein Concentration Optimization
3.5. Method Validations
3.5.1. Dose-Response Curve
3.5.2. Limit of Detection (LoD) and Limit of Quantitation (LoQ)
3.5.3. Cut-Off Determination
3.5.4. Analytical Specificity
3.5.5. Analytical Precision
3.5.6. Sensitivity and Specificity for the Experimental Setting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Song, E.Y.; Lee, Y.; Hyun, J.; Kim, Y.S.; Ahn, C.; Ha, J.; Kim, S.J.; Park, M.H. Clinical Relevance of Pretransplant HLA Class II Donor-specific Antibodies in Renal Transplantation Patients with Negative T-cell Cytotoxicity Crossmatches. Ann. Lab. Med. 2012, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, N.W.; Marcos, C.Y.; Mirbaha, F.; Zou, Y.; Stastny, P. Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum. Immunol. 2000, 61, 917–924. [Google Scholar] [CrossRef]
- Buelow, R.; Mercier, I.; Glanville, L.; Regan, J.; Ellingson, L.; Janda, G.; Claas, F.; Colombe, B.; Gelder, F.; Grosse-Wilde, H. Detection of panel-reactive anti-HLA class I antibodies by enzyme-linked immunosorbent assay or lymphocytotoxicity. Results of a blinded, controlled multicenter study. Hum. Immunol. 1995, 44, 1–11. [Google Scholar] [CrossRef]
- Zou, Y.; Heinemann, F.M.; Grosse-Wilde, H.; Sireci, G.; Wang, Z.; Lavingia, B.; Stastny, P. Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum. Immunol. 2006, 67, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tait, B.D. Detection of HLA Antibodies in Organ Transplant Recipients - Triumphs and Challenges of the Solid Phase Bead Assay. Front. Immunol. 2016, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Promptmas, C.; Thanapitak, S.; Srisuwan, A. Optimization of 3-aminopropyltriethoxysilane functionalization on silicon nitride surface for biomolecule immobilization. Talanta 2020, 207, 120305. [Google Scholar] [CrossRef]
- Seo, H.-I.; Kim, C.-S.; Sohn, B.-K.; Yeow, T.; Son, M.-T.; Haskard, M. ISFET glucose sensor based on a new principle using the electrolysis of hydrogen peroxide. Sens. Actuators B Chem. 1997, 40, 1–5. [Google Scholar] [CrossRef]
- Cheng, S.; Hotani, K.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Hashimoto, M.; Mori, Y.; Osaka, T. Field effect transistor biosensor using antigen binding fragment for detecting tumor marker in human serum. Materials 2014, 7, 2490–2500. [Google Scholar] [CrossRef]
- Selvanayagam, Z.E.; Neuzil, P.; Gopalakrishnakone, P.; Sridhar, U.; Singh, M.; Ho, L.C. An ISFET-based immunosensor for the detection of β-Bungarotoxin. Biosens. Bioelectron. 2002, 17, 821–826. [Google Scholar] [CrossRef]
- Saengdee, P.; Chaisriratanakul, W.; Bunjongpru, W.; Sripumkhai, W.; Srisuwan, A.; Hruanun, C.; Poyai, A.; Phunpae, P.; Pata, S.; Jeamsaksiri, W.; et al. A silicon nitride ISFET based immunosensor for Ag85B detection of tuberculosis. Analyst 2016, 141, 5767–5775. [Google Scholar] [CrossRef] [PubMed]
- Jonkheijm, P.; Weinrich, D.; Schroder, H.; Niemeyer, C.M.; Waldmann, H. Chemical strategies for generating protein biochips. Angew. Chem. Int. Ed. 2008, 47, 9618–9647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Jin, G. Covalent immobilization of proteins for the biosensor based on imaging ellipsometry. J. Immunol. Methods 2004, 285, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Saengdee, P.; Chaisriratanakul, W.; Bunjongpru, W.; Sripumkhai, W.; Srisuwan, A.; Jeamsaksiri, W.; Hruanun, C.; Poyai, A.; Promptmas, C. Surface modification of silicon dioxide, silicon nitride and titanium oxynitride for lactate dehydrogenase immobilization. Biosens. Bioelectron. 2015, 67, 134–138. [Google Scholar] [CrossRef]
- Aissaoui, N.; Bergaoui, L.; Landoulsi, J.; Lambert, J.F.; Boujday, S. Silane layers on silicon surfaces: Mechanism of interaction, stability, and influence on protein adsorption. Langmuir 2012, 28, 656–665. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Diao, J.; Ren, D.; Engstrom, J.R.; Lee, K.H. A surface modification strategy on silicon nitride for developing biosensors. Anal. Biochem. 2005, 343, 322–328. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.; Kim, S.; Jang, W.D.; Park, S.; Koh, W.G. Protein-conjugated, glucose-sensitive surface using fluorescent dendrimer porphyrin. J. Mater. Chem. 2009, 19, 5643–5647. [Google Scholar] [CrossRef]
- Lee, C.S.; Kyu Kim, S.; Kim, M. Ion-sensitive field-effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M.; Bergveld, P.; Kooyman, R.P.H.; Greve, J. Possibilities and limitations of direct detection of protein charges by means of an immunological field-effect transistor. Anal. Chim. Acta 1990, 238, 323–329. [Google Scholar] [CrossRef]
- Chen, K.I.; Li, B.R.; Chen, Y.T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Tran, Q.H.; Nguyen, T.H.H.; Mai, A.T.; Nguyen, T.T.; Vu, Q.K.; Phan, T.N. Development of electrochemical immunosensors based on different serum antibody immobilization methods for detection of Japanese encephalitis virus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 015012. [Google Scholar] [CrossRef][Green Version]
- Son, H.W.; Jeun, M.; Choi, J.; Lee, K.H. A strategy to minimize the sensing voltage drift error in a transistor biosensor with a nanoscale sensing gate. Int. J. Nanomed. 2017, 12, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon nanowire field-effect transistors—A versatile class of potentiometric nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Saengdee, P.; Thanapitak, S.; Ongwattanakul, S.; Srisuwan, A.; Pankiew, A.; Thornyanadacha, N.; Chaisriratanakul, W.; Jeamsaksiri, W.; Promptmas, C. A silicon nitride ion sensitive field effect transistor-based immunosensor for determination of urinary albumin. Electrochem. Sci. Adv. 2021, e2100078. [Google Scholar] [CrossRef]
- Rosso, M.; Nguyen, A.T.; De Jong, E.; Baggerman, J.; Paulusse, J.M.J.; Giesbers, M.; Fokkink, R.G.; Norde, W.; Schroën, K.; Van Rijn, C.J.M.; et al. Protein-repellent silicon nitride surfaces: UV-induced formation of oligoethylene oxide monolayers. ACS Appl. Mater. Interfaces 2011, 3, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Gustavo González, A.; Ángeles Herrador, M. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends Anal. Chem. 2007, 26, 227–238. [Google Scholar] [CrossRef]



| Layer | Thickness (nm) | Average | SD |
|---|---|---|---|
| SiO2 | 102.09 | 102.32 | 0.70 |
| 103.11 | |||
| 101.75 | |||
| Si3N4 | 198.72 | 198.59 | 0.89 |
| 197.65 | |||
| 199.41 | |||
| APTES | 2.57 | 2.51 | 0.17 |
| 2.32 | |||
| 2.65 | |||
| GA | 1.15 | 1.52 | 0.46 |
| 1.38 | |||
| 2.03 |
| Antibodies (20 µg/mL) | ΔVgs (antibody) | Protein Concentration from the Inter-Assay | ||
|---|---|---|---|---|
| Mean ± SD (mV) | %CV | Mean ± SD (µg/mL) | %CV | |
| Anti-HLA | 10.03 ± 0.71 | 7.09 | 20.97 ± 2.24 | 10.69 |
| Anti-MICA | 10.31 ± 0.41 | 4.00 | 20.21 ± 1.80 | 8.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, T.Z.M.M.M.; Phanabamrung, S.; Chaisriratanakul, W.; Pankiew, A.; Srisuwan, A.; Chauyrod, K.; Pongskul, C.; Promptmas, C.; Leelayuwat, C. Biosensors Based on Ion-Sensitive Field-Effect Transistors for HLA and MICA Antibody Detection in Kidney Transplantation. Molecules 2022, 27, 6697. https://doi.org/10.3390/molecules27196697
Min TZMMM, Phanabamrung S, Chaisriratanakul W, Pankiew A, Srisuwan A, Chauyrod K, Pongskul C, Promptmas C, Leelayuwat C. Biosensors Based on Ion-Sensitive Field-Effect Transistors for HLA and MICA Antibody Detection in Kidney Transplantation. Molecules. 2022; 27(19):6697. https://doi.org/10.3390/molecules27196697
Chicago/Turabian StyleMin, Thu Zar Ma Ma Moe, Sonwit Phanabamrung, Woraphan Chaisriratanakul, Apirak Pankiew, Awirut Srisuwan, Kondee Chauyrod, Cholatip Pongskul, Chamras Promptmas, and Chanvit Leelayuwat. 2022. "Biosensors Based on Ion-Sensitive Field-Effect Transistors for HLA and MICA Antibody Detection in Kidney Transplantation" Molecules 27, no. 19: 6697. https://doi.org/10.3390/molecules27196697
APA StyleMin, T. Z. M. M. M., Phanabamrung, S., Chaisriratanakul, W., Pankiew, A., Srisuwan, A., Chauyrod, K., Pongskul, C., Promptmas, C., & Leelayuwat, C. (2022). Biosensors Based on Ion-Sensitive Field-Effect Transistors for HLA and MICA Antibody Detection in Kidney Transplantation. Molecules, 27(19), 6697. https://doi.org/10.3390/molecules27196697









