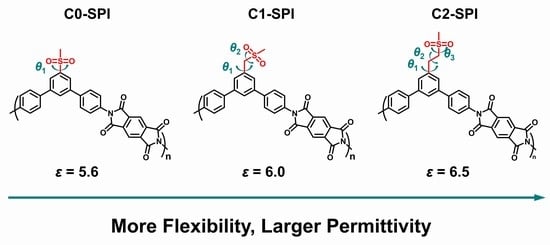
Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Polyimides
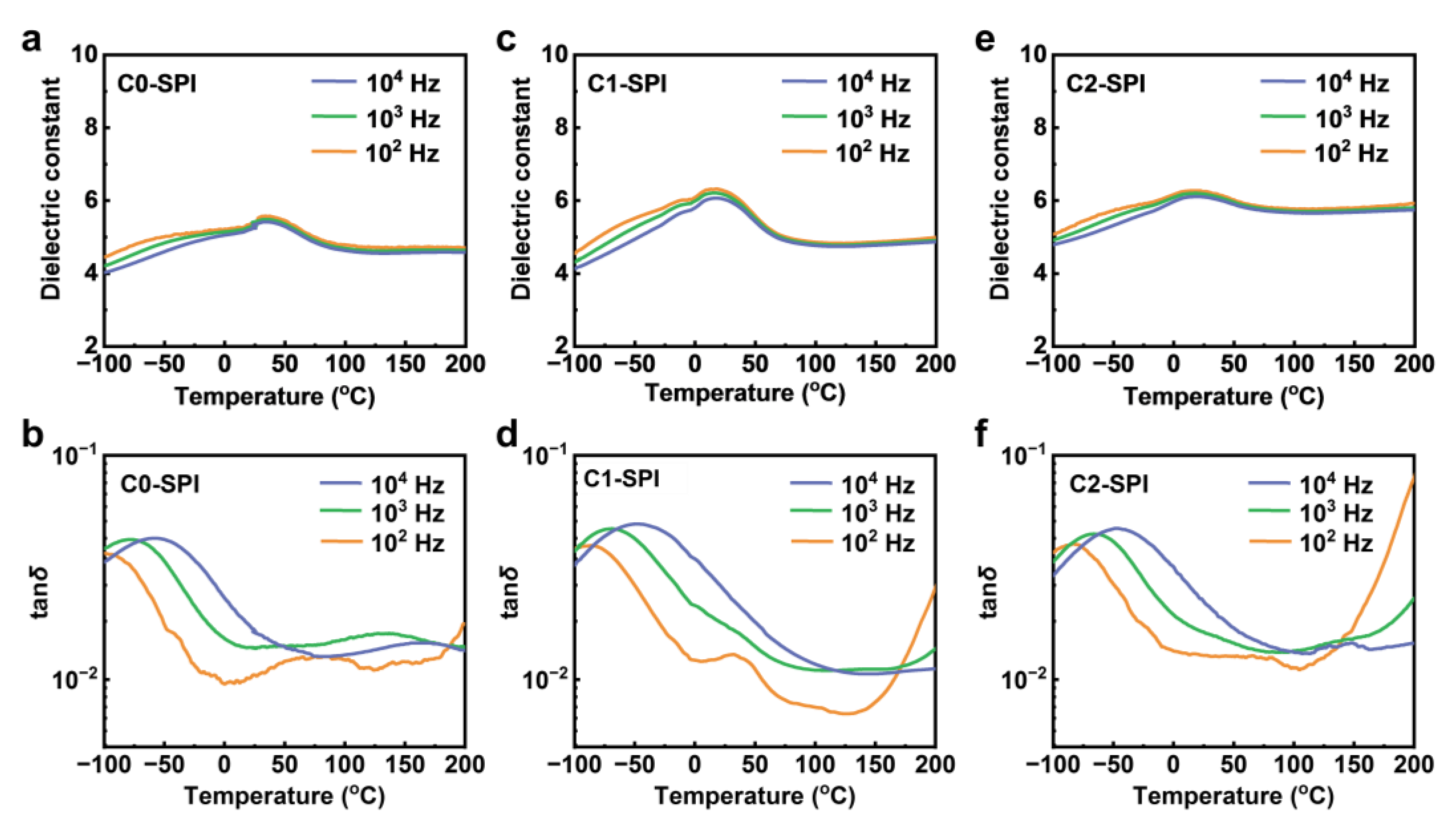
2.2. Dielectric Properties of Polyimides
2.3. Energy Storage Capacity of Polyimides
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Monomers and Polyimides
4.3. Instrumentation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, B.; Yu, T.; Zhang, X.; Li, H.; Shu, H.; Sang, Y.; Jiang, L. Dynamic leader based collective intelligence for maximum power point tracking of PV systems affected by partial shading condition. Energy Convers. Manag. 2019, 179, 286–303. [Google Scholar] [CrossRef]
- Shan, Y.; Hu, J.; Chan, K.W.; Fu, Q.; Guerrero, J.M. Model predictive control of bidirectional DC–DC converters and AC/DC interlinking converters-a new control method for PV-wind-battery microgrids. IEEE Trans. Sustain. Energy 2019, 10, 1823–1833. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Dong, Z.; Ravishankar, J. Three-stage robust inverter-based voltage/var control for distribution networks with high-level PV. IEEE Trans. Smart Grid 2019, 10, 782–793. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, B.; Lu, N. A descriptor system approach for estimation of incipient faults with application to high-speed railway traction devices. IEEE Trans. Syst. Man Cybern.-Syst. 2019, 49, 2108–2118. [Google Scholar] [CrossRef]
- İnci, M.; Büyük, M.; Demir, M.H.; İlbey, G. A review and research on fuel cell electric vehicles: Topologies, power electronic converters, energy management methods, technical challenges, marketing and future aspects. Renew. Sust. Energ. Rev. 2021, 137, 110648. [Google Scholar] [CrossRef]
- Chowdhury, S.; Gurpinar, E.; Ozpineci, B. Capacitor technologies: Characterization, selection, and packaging for next-generation power electronics applications. IEEE Trans. Transp. Electrif. 2022, 8, 2710–2720. [Google Scholar] [CrossRef]
- Huang, L.; Xin, H.; Wang, Z.; Zhang, L.; Wu, K.; Hu, J. Transient stability analysis and control design of droop-controlled voltage source converters considering current limitation. IEEE Trans. Smart Grid 2019, 10, 578–591. [Google Scholar] [CrossRef]
- Samadaei, E.; Kaviani, M.; Bertilsson, K. A 13-levels module (K-type) with two DC sources for multilevel inverters. IEEE Trans. Ind. Electron. 2019, 66, 5186–5196. [Google Scholar] [CrossRef]
- Rui, W.; Qiuye, S.; Dazhong, M.; Xuguang, H. Line impedance cooperative stability region identification method for grid-tied inverters under weak grids. IEEE Trans. Smart Grid 2020, 11, 2856–2866. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, M.; Shen, L.; Lu, L.; Ma, C.; Lu, X.; Lou, X.; Jia, C.L. All-inorganic flexible embedded thin-film capacitors for dielectric energy storage with high performance. ACS Appl. Mater. Interfaces 2019, 11, 5247–5255. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Bai, H.; Ge, G.; Lin, J.; Zhu, K.; Li, G.; Qian, J.; Shen, B.; Zhai, J.; Liu, Z. Boosting energy storage performance of lead-free ceramics via layered structure optimization strategy. Small 2022, 18, 2202575. [Google Scholar] [CrossRef]
- Kotb, H.M.; Ahmad, M.M.; Ansari, S.A.; Kayed, T.S.; Alshoaibi, A. Dielectric properties of colossal-dielectric-constant Na1/2La1/2Cu3Ti4O12 ceramics prepared by spark plasma sintering. Molecules 2022, 27, 779. [Google Scholar] [CrossRef]
- Liu, F.; Li, Q.; Cui, J.; Li, Z.; Yang, G.; Liu, Y.; Dong, L.; Xiong, C.; Wang, H.; Wang, Q. High-Energy-Density Dielectric Polymer Nanocomposites with Trilayered Architecture. Adv. Funct. Mater. 2017, 27, 1606292. [Google Scholar] [CrossRef]
- Dai, Z.; Bao, Z.; Ding, S.; Liu, C.; Sun, H.; Wang, H.; Zhou, X.; Wang, Y.; Yin, Y.; Li, X. Scalable polyimide-poly(amic acid) copolymer based nanocomposites for high-temperature capacitive energy storage. Adv. Mater. 2022, 34, 2101976. [Google Scholar] [CrossRef]
- Zheng, W.; Ren, L.; Zhao, X.; Wang, C.; Yang, L.; Liao, R. Roles of Al2O3@ZrO2 particles in modulating crystalline morphology and electrical properties of P(VDF-HFP) nanocomposites. Molecules 2022, 27, 4289. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, Z.; Chen, W.; Xiao, L.; Yuan, Y.; Li, Z.; Zhuang, Q. In situ synthesis of high dielectric constant GNPs/PBO nanocomposites with enhanced thermostability. IET Nanodielectr. 2019, 2, 97–102. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yu, S.; Zeng, X.; Sun, R.; Wong, C.P. High energy density polymer nanocomposites with Y-doped barium strontium titanate nanoparticles as fillers. IET Nanodielectr. 2018, 1, 137–142. [Google Scholar] [CrossRef]
- Zha, J.W.; Yao, S.C.; Qiu, Y.; Zheng, M.S.; Dang, Z.M. Enhanced dielectric properties and energy storage of the sandwich-structured poly(vinylidene fluoride-co-hexafluoropropylene) composite films with functional BaTiO3@Al2O3 nanofibres. IET Nanodielectr. 2019, 2, 103–108. [Google Scholar] [CrossRef]
- Zhang, G.; Brannum, D.; Dong, D.; Tang, L.; Allahyarov, E.; Tang, S.; Kodweis, K.; Lee, J.-K.; Zhu, L. Interfacial polarization-induced loss mechanisms in polypropylene/BaTiO3 nanocomposite dielectrics. Chem. Mater. 2016, 28, 4646–4660. [Google Scholar] [CrossRef]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, H.U.; Iagodkine, E.; Haque, A.; Chen, L.-Q.; Jackson, T.N.; et al. Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2015, 523, 576–579. [Google Scholar] [PubMed]
- Liu, J.; Li, M.; Zhao, Y.; Zhang, X.; Lu, J.; Zhang, Z. Manipulating H-bonds in glassy dipolar polymers as a new strategy for high energy storage capacitors with high pulse discharge efficiency. J. Mater. Chem. A 2019, 7, 19407–19414. [Google Scholar] [CrossRef]
- Wu, C.; Deshmukh, A.A.; Li, Z.; Chen, L.; Alamri, A.; Wang, Y.; Ramprasad, R.; Sotzing, G.A.; Cao, Y. Flexible Temperature-Invariant Polymer Dielectrics with Large Bandgap. Adv. Mater. 2020, 32, 2000499. [Google Scholar] [CrossRef]
- Hardy, C.G.; Islam, M.S.; Gonzalez-Delozier, D.; Morgan, J.E.; Cash, B.; Benicewicz, B.C.; Ploehn, H.J.; Tang, C. Converting an Electrical Insulator into a Dielectric Capacitor: End-Capping Polystyrene with Oligoaniline. Chem. Mater. 2013, 25, 799–807. [Google Scholar] [CrossRef]
- Qiao, Y.; Islam, M.S.; Han, K.; Leonhardt, E.; Zhang, J.; Wang, Q.; Ploehn, H.J.; Tang, C. Polymers containing highly polarizable conjugated side chains as high-performance all-organic nanodielectric materials. Adv. Funct. Mater. 2013, 23, 5638–5646. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Liao, X.; Han, H.; Li, Y.; Xie, M. Tandem Metathesis Polymerization-Induced Self-Assembly to Nanostructured Block Copolymer and the Controlled Triazolinedione Modification for Enhancing Dielectric Properties. Macromolecules 2018, 51, 10202–10213. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Li, H.; Han, H.; Liao, X.; Sun, R.; Huang, X.; Xie, M. Rational Design and Modification of High-k Bis(double-stranded) Block Copolymer for High Electrical Energy Storage Capability. Chem. Mater. 2018, 30, 1102–1112. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Zhu, M.; Wang, Y.; Li, N.; Zhang, Z.; Chen, Q.; An, L.; Lin, Y.; Nan, C. Controlled functionalization of poly(4-methyl-1-pentene) films for high energy storage applications. J. Mater. Chem. A 2016, 4, 4797–4807. [Google Scholar] [CrossRef]
- Zhang, M.; Li, B.; Wang, J.-J.; Huang, H.-B.; Zhang, L.; Chen, L.-Q. Polymer Dielectrics with Simultaneous Ultrahigh Energy Density and Low Loss. Adv. Mater. 2021, 33, 2008198. [Google Scholar] [CrossRef]
- Peng, X.; Xu, W.; Chen, L.; Ding, Y.; Chen, S.; Wang, X.; Hou, H. Polyimide complexes with high dielectric performance: Toward polymer film capacitor applications. J. Mater. Chem. C 2016, 4, 6452–6456. [Google Scholar] [CrossRef]
- Chen, L.; Ding, Y.; Yang, T.; Wan, C.; Hou, H. Synthesis and properties of a high dielectric constant copolymer of a copper phthalocyanine oligomer grafted to amino-capped polyimide. J. Mater. Chem. C 2017, 5, 8371–8375. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.; Lin, M.; Burlingame, Q.; Chen, Q.; Payzant, A.; Xiao, K.; Zhang, Q.M. Aromatic polythiourea dielectrics with ultrahigh breakdown field strength, low dielectric loss, and high electric energy density. Adv. Mater. 2013, 25, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Kim, D.G.; Ha, T.; Chan Won, J.; Jang, K.S.; Kim, Y.H. Solution-Processable, Thin, and High-kappa Dielectric Polyurea Gate Insulator with Strong Hydrogen Bonding for Low-Voltage Organic Thin-Film Transistors. ACS Appl. Mater. Interfaces 2018, 10, 32462–32470. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, L. Intrinsic polymer dielectrics for high energy density and low loss electric energy storage. Prog. Polym. Sci. 2020, 106, 101254. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.H.; Litt, M.H.; Tan, L.-S.; Zhu, L. High-Temperature and High-Energy-Density Dipolar Glass Polymers Based on Sulfonylated Poly(2,6-dimethyl-1,4-phenylene oxide). Angew. Chem. Int. Ed. 2018, 57, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Thakur, Y.; Dong, R.; Lin, M.; Wu, S.; Cheng, Z.; Hou, Y.; Bernholc, J.; Zhang, Q.M. Optimizing nanostructure to achieve high dielectric response with low loss in strongly dipolar polymers. Nano Energy 2015, 16, 227–234. [Google Scholar] [CrossRef]
- Kussmaul, B.; Risse, S.; Kofod, G.; Waché, R.; Wegener, M.; McCarthy, D.N.; Krüger, H.; Gerhard, R. Enhancement Of Dielectric Permittivity And Electromechanical Response In Silicone Elastomers: Molecular Grafting Of Organic Dipoles To The Macromolecular Network. Adv. Funct. Mater. 2011, 21, 4589–4594. [Google Scholar] [CrossRef]
- Bendler, J.T.; Boyles, D.A.; Edmondson, C.A.; Filipova, T.; Fontanella, J.J.; Westgate, M.A.; Wintersgill, M.C. Dielectric Properties of Bisphenol A Polycarbonate and Its Tethered Nitrile Analogue. Macromolecules 2013, 46, 4024–4033. [Google Scholar] [CrossRef]
- Treufeld, I.; Wang, D.H.; Kurish, B.A.; Tan, L.-S.; Zhu, L. Enhancing electrical energy storage using polar polyimides with nitrile groups directly attached to the main chain. J. Mater. Chem. A 2014, 2, 20683–20696. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, L.; Wang, P.; Li, L.; Chen, J.; Chen, C.; Wu, W.; Wang, G.; Zhang, S. A high-temperature dielectric polymer poly(acrylonitrile butadiene styrene) with enhanced energy density and efficiency due to a cyano group. J. Mater. Chem. A 2020, 8, 15122–15129. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, Q.; Zheng, W.; Bei, R.; Wang, W.; Wu, M.; Liu, S.; Chi, Z.; Zhang, Y.; Xu, J. Intrinsic high-k–low-loss dielectric polyimides containing ortho-position aromatic nitrile moieties: Reconsideration on Clausius–Mossotti equation. Polym. Chem. 2021, 12, 2481–2489. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Z.; Tseng, J.-K.; Treufeld, I.; Liu, X.; Litt, M.H.; Zhu, L. Achieving high dielectric constant and low loss property in a dipolar glass polymer containing strongly dipolar and small-sized sulfone groups. ACS Appl. Mater. Interfaces 2015, 7, 5248–5257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Li, T.; Wang, Z.; Li, L.; Guo, X.; Jiang, P. Novel crosslinkable high-k copolymer dielectrics for high-energy-density capacitors and organic field-effect transistor applications. J. Mater. Chem. A 2017, 5, 20737–20746. [Google Scholar] [CrossRef]
- Zhu, Y.-F.; Zhang, Z.; Litt, M.H.; Zhu, L. High Dielectric Constant Sulfonyl-Containing Dipolar Glass Polymers with Enhanced Orientational Polarization. Macromolecules 2018, 51, 6257–6266. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, J.; Premasiri, K.; Kwok, M.-H.; Li, Q.; Li, R.; Zhang, S.; Litt, M.H.; Gao, X.P.A.; Zhu, L. High-κ polymers of intrinsic microporosity: A new class of high temperature and low loss dielectrics for printed electronics. Mater. Horiz. 2020, 7, 592–597. [Google Scholar] [CrossRef]
- Ma, R.; Baldwin, A.F.; Wang, C.; Offenbach, I.; Cakmak, M.; Ramprasad, R.; Sotzing, G.A. Rationally designed polyimides for high-energy density capacitor applications. ACS Appl. Mater. Interfaces 2014, 6, 10445–10451. [Google Scholar] [CrossRef]
- Li, Z.; Treich, G.M.; Tefferi, M.; Wu, C.; Nasreen, S.; Scheirey, S.K.; Ramprasad, R.; Sotzing, G.A.; Cao, Y. High energy density and high efficiency all-organic polymers with enhanced dipolar polarization. J. Mater. Chem. A 2019, 7, 15026–15030. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, T.; Qu, L.; Liang, X.; Liu, C.; Qian, C.; Zhu, T.; Zhou, Z.; Liu, C.; Liu, S.; et al. Temperature resistant amorphous polyimides with high intrinsic permittivity for electronic applications. Chem. Eng. J. 2022, 436, 135060. [Google Scholar] [CrossRef]
- Liu, X.-J.; Zheng, M.-S.; Chen, G.; Dang, Z.-M.; Zha, J.-W. High-temperature polyimide dielectric materials for energy storage: Theory, design, preparation and properties. Energy Environ. Sci. 2022, 15, 56–81. [Google Scholar] [CrossRef]
- Yelon, A.; Movaghar, B.; Crandall, R.S. Multi-excitation entropy: Its role in thermodynamics and kinetics. Rep. Prog. Phys. 2006, 69, 1145–1194. [Google Scholar] [CrossRef]
- Su, W.; Zhao, K.; Wei, J.; Ngai, T. Dielectric relaxations of poly(N-isopropylacrylamide) microgels near the volume phase transition temperature: Impact of cross-linking density distribution on the volume phase transition. Soft Matter 2014, 10, 8711–8723. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T. Sobtop, Version [1.0(dev2)]. Available online: http://sobereva.com/soft/Sobtop (accessed on 14 March 2022).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T. Molclus Program, Version 1.9.9.5. Available online: http://www.keinsci.com/research/molclus.html (accessed on 3 October 2021).
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Zhu, L. Exploring Strategies for High Dielectric Constant and Low Loss Polymer Dielectrics. J. Phys. Chem. Lett. 2014, 5, 3677–3687. [Google Scholar] [CrossRef]
- Prateek; Thakur, V.K.; Gupta, R.K. Recent Progress on Ferroelectric Polymer-Based Nanocomposites for High Energy Density Capacitors: Synthesis, Dielectric Properties, and Future Aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef]
- Wei, X.K.; Dunin-Borkowski, R.E.; Mayer, J. Structural Phase Transition and In-Situ Energy Storage Pathway in Nonpolar Materials: A Review. Materials 2021, 14, 7854. [Google Scholar] [CrossRef]
- Wei, X.K.; Domingo, N.; Sun, Y.; Balke, N.; Dunin-Borkowski, R.E.; Mayer, J. Progress on Emerging Ferroelectric Materials for Energy Harvesting, Storage and Conversion. Adv. Energy Mater. 2022, 12, 2201199. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Li, Z.; Chen, K.; Liu, S.; Chi, Z.; Xu, J.; Zhang, Y. Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials. Molecules 2022, 27, 6337. https://doi.org/10.3390/molecules27196337
Zheng W, Li Z, Chen K, Liu S, Chi Z, Xu J, Zhang Y. Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials. Molecules. 2022; 27(19):6337. https://doi.org/10.3390/molecules27196337
Chicago/Turabian StyleZheng, Weiwen, Zuhao Li, Kaijin Chen, Siwei Liu, Zhenguo Chi, Jiarui Xu, and Yi Zhang. 2022. "Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials" Molecules 27, no. 19: 6337. https://doi.org/10.3390/molecules27196337
APA StyleZheng, W., Li, Z., Chen, K., Liu, S., Chi, Z., Xu, J., & Zhang, Y. (2022). Temperature-Resistant Intrinsic High Dielectric Constant Polyimides: More Flexibility of the Dipoles, Larger Permittivity of the Materials. Molecules, 27(19), 6337. https://doi.org/10.3390/molecules27196337