Abstract
Marine invertebrates have been reported to be an excellent resource of many novel bioactive compounds. Studies reported that Indonesia has remarkable yet underexplored marine natural products, with a high chemical diversity and a broad spectrum of biological activities. This review discusses recent updates on the exploration of marine natural products from Indonesian marine invertebrates (i.e., sponges, tunicates, and soft corals) throughout 2007–2020. This paper summarizes the structural diversity and biological function of the bioactive compounds isolated from Indonesian marine invertebrates as antimicrobial, antifungal, anticancer, and antiviral, while also presenting the opportunity for further investigation of novel compounds derived from Indonesian marine invertebrates.
1. Introduction
A wide range of natural products (NPs) has been isolated from various marine organisms, especially marine invertebrates such as sponges, tunicates, soft corals, bryozoans, and nudibranchs. These marine invertebrates are excellent sources of NPs with vast chemical structures and potential biological activities [1,2,3]. During 2012–2017, no less than 550–700 new compounds have been reported from marine invertebrates [4], in which half of these compounds were isolated from marine sponges [5]. Among those, 4% and 22% of the compounds were identified in 2017 and 2016, respectively [4,5]. Between 1998 to 2018, one hundred and fourteen secondary metabolites were isolated from the marine sponges of the genus Suberea [6]. Meanwhile, a hundred and seventy compounds were isolated from soft corals of the genus Dendronephthya alone throughout 1999–2019 [7]. Soft corals belonging to the genus Xenia are rich in terpenoids, with 199 compounds isolated from 1977–2019 [8]. To date, approximately 30,000–40,000 marine natural products (MNPs) have been identified, with the majority of the compounds exhibiting cytotoxic and anticancer properties [4,5,9].
The biological potential of MNPs from marine invertebrates has been proven to be a valuable source for drug discovery and development. Most of the approved commercial marine-based drugs are of marine invertebrate origin. Eight marine drugs have been approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA). The first approved marine drug is Ziconotide (Prialt®) discovered in the marine snail Conus magus. This peptide-derived marine drug is commonly used as an analgesic drug for the management of severe chronic pain through the intrathecal route. The second one is Omega-3-acid-ethyl esters (Lovaza®) derived from fish oil and used as an anti-hypertriglyceridemia drug. The third is Vidarabine (Vira-A®) derived from the sponge Cryptotethya crypta, while the fourth is Iota-carrageenan (Carragelose®), derived from red macroalgae. These two were registered as antivirals. The other four drugs were approved for cancer treatment, i.e., (1) viz. Cytarabine (Cytosar-U® and Depocyt®) discovered in sponge Cryptotethya crypta, (2) Trabectedin (Yondelis®) isolated from the tunicate Ecteinascidia turbinata, (3) Eribulin mesylate (Halaven®) discovered from sponge Halichondria okadai, and (4) Brentuximab vedotin (Adcetris®) derived from the sea hare Dolabella auricularia. Additionally, approximately 30 MNPs were reported in different clinical trial stages, mainly derived from marine invertebrates [9,10,11].
Indonesia is the world’s largest archipelagic country with 17,500 islands and a long coastline of 81,000 km. This extraordinary geographic attribute offers a highly diverse variety of marine organisms, resulting in Indonesia being known as a mega-biodiversity of marine organisms. Like other living organisms, marine species synthesize metabolites, either primary or secondary, to support their lives. Living in an extreme environment often induces these organisms to synthesize multiple secondary metabolites with unique chemical properties. A part of their defense mechanism, these metabolites have also been reported to have diverse biological activities that are important for drug discovery and development [12].
The study of Indonesian MNPs was started in 1972 by Engelbrecht et al., who discovered a compound called 25-hydroxy-24ξ-methylcholesterol derived from a soft coral collected from Nias Island, Indonesia [13]. Following that, Cornery et al. reported two novel cytotoxic compounds from the Indonesian sponge Hyatella sp. called viz. laulimalide and isolaulimalide, which were active against the KB cell line with an IC50 value of 15 ng/mL [14]. Since then, research on Indonesian MNPs has expanded significantly. From the 1970s to the year 2017, about 732 MNPs were isolated from Indonesian sea waters. They were mainly produced by sponges (Porifera), tunicates (Chordata), and soft corals (Cnidaria) [15]. In the past decade, hundreds of novel compounds have been discovered from Indonesian marine organisms, many of which showing potent biological activity [16,17].
This review presents recent updates on Indonesian MNPs isolated from three marine invertebrates (sponges, tunicates, and soft corals), reported from 2007 to 2020, covering the chemical diversity and biological activity.
2. Marine Invertebrates
2.1. Sponges
Among sponges, alkaloids were reported as the most isolated bioactive compounds, followed by terpenoids, peptides, and polyketides (Table 1). Among the isolated alkaloids from sponges, manzamines are mostly reported to exhibit a broad spectrum of biological activities such as cytotoxic, antimicrobial, antimalarial, antiviral, anti-inflammatory, antiatherosclerotic, insecticidal, and proteasome inhibitor [18]. Their structure has a fused tetra- or pentacyclic ring attached to a carboline moiety.

Table 1.
Summary of the marine natural products isolated from the marine sponges from Indonesian oceans.
To date, more than 80 menzamine-derived alkaloids have been isolated from sponges. This report showed 21 alkaloids were isolated from marine sponge A. ingens, five of which were identified as novel manzamine alkaloids, acanthomanzamines A-E (1–5). These five acanthomanzamines were isolated from the marine sponge A. ingens collected in Mantehage, North Sulawesi [18]. The acanthomanzamines A (1) and B (2) were the first compounds to contain the 1,2,3,4-tetrahydroisoquinoline-6,7-diol moiety instead of the β-carboline moiety, while the acanthomanzamine C (3) contains the hexahydrocyclopenta[b]-pyrrol-4(2H)-one ring system, and the acanthomanzamines D (4) and E (5) have the oxazolidine and two methyloxazolidine rings, respectively (Figure 1). In terms of biological activity, the acanthomanzamines 1 and 2 demonstrated more potent cytotoxic activity against cervical cancer HeLa cells (IC50 values 4.2 and 5.7 µM, respectively) than the acanthomanzamines 4 and 5 (IC50 values 15 and >20 µM, respectively). However, the acanthomanzamines 4 and 5 showed better proteasome inhibition against the proteasomal chymotrypsin-like activity (IC50 values of 0.63 and 1.5 µM, respectively). These findings suggested that the presence of 1,2,3,4-tetrahydroisoquinoline-6,7-diol probably enhanced the cytotoxicity. Meanwhile, compounds containing β-carboline perform better on chymotrypsin-like activity. Additionally, the acanthomanzamines 1, 2, 4, and 5 inhibited the accumulation of the cholesterol ester at 20 µM in macrophages with 48%, 73%, 73%, and 61%, respectively [18].
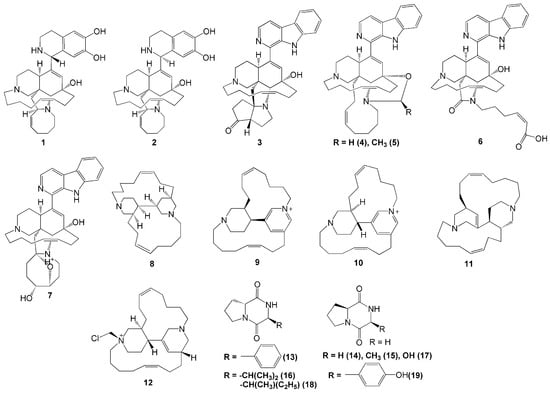
Figure 1.
Chemical structures of 1–19.
The sponge A. ingens also produces other types of nitrogen-containing molecules, i.e., acantholactam (6), pre-neo-kauluamine (7), acathocyclamine A (8), epi-tetradehydrohalicyclamine B (9), tetradehydrohalicyclamine B (10), halicyclamine B (11), chloromethylhalicyclamine B (12), cyclo (d-Pro-l-Phe) (13), cyclo (l-Pro-Gly) (14), cyclo (l-Pro-l-Ala) (15), cyclo (d-Pro-l-Val) (16), cyclo (l-Pro-Ser) (17), cyclo (d-Pro-l-Ile) (18), and cyclo (l-Pro-l-Tyr) (19). Alkaloid compounds 6 and 7 were isolated from A. ingens collected in Bajotalawaan, North Sulawesi [19]. Compound 7 exhibited proteasome inhibitory activity with an IC50 value of 0.34 µM, whereas compound 6 showed little to no activity [19]. These findings indicated that the eight-membered ring in the manzamines plays a key role in their bioactivity.
A novel 3-alkylpiperidine alkaloid (8) was also isolated from A. ingens collected from Wakatobi Marine National Park in Southeast Sulawesi [20]. Recently, the alkaloid compounds 9–19 were also isolated from A. ingens collected from South Sulawesi along with compound 8 [21]. Moreover, compound 8 was reported to have specific antimicrobial activity against E. coli and showed an inhibitory effect on amyloid β-42 production induced by aftin-5 without cytotoxicity at 26 µM. Compounds 8 and 11 exhibited antibacterial activity at 100 µg/disc against E. coli and S. aureus. In addition to that, compound 12 was reported to have selective inhibitory activity against the protein kinase CK1 δ/ε with an IC50 value of 6 µM, while the diketopiperazine compound 13 showed a selective kinase inhibitory activity against CDK2/cyclin A with an IC50 value of 1 µM. However, no bioactivity was reported from compounds 14–19 [21].
A study reported two new pyrimidine-β-carboline alkaloids—namely, ingenines C (20) and D (21), successfully isolated from A. ingens obtained in Sulawesi [22]. The ingenines 20 and 21 exhibited cytotoxic activities towards the human breast MCF-7 and colorectal HCT116 cancer cells (IC values of 4.33 and 6.05 µM, and 2.90 and 3.35 µM, respectively) [22].
Two bromopyrrole alkaloids, dispacamide E (22) and ethyl 3,4-dibromo-1H-pyrrole-2-carboxylate (23), were isolated from methanolic extract of the marine sponge S. massa collected in Papua, Indonesia [23]. Compound 22 had significant protein kinase inhibitory activities against GSK-4, DYRKIA, and CK-1 with IC50 values of 2.1, 6.2, and 4.9 µM, respectively [23]. Another four new alkaloids were isolated from genus Stylissa in Derawan Island, Berau, Northeast Kalimantan—namely, 12-N-methylstevensine (24), 12-N-methyl-2-debromostevensine (25), 3-debromolatonduine B methyl ester (26), and 3-debromolatonduine A (27). Among these four compounds, however, only compound 24 showed significant in-vitro cytotoxic activity against the mouse lymphoma L5187Y cancer cell line with an EC50 value of 8.7 µM [24].
Recently, three new guanidine alkaloids, crambescidins 345 (28), 361 (29), and 373 (30), were isolated from C. bulbotoxa collected in Samalona Island, South Sulawesi [25]. Interestingly, crambescidin 28 was the first crambescidin analogue with a non-alkylated tetrahydropyrane ring (Figure 2). Crambescidin 29 possesses a rare propyl substituent and another tetrahydropyrane ring in place of the left-side unsaturated seven-membered ring. Meanwhile, crambescidin 30 was the first analogue with an ethyl group at the right-side tetrahydropyrane ring, which possesses a methyl group in most reported crambescidin-type alkaloids. These three guanidine alkaloids (28, 29, and 30) exhibited moderate cytotoxicity against the human epidermoid A431 carcinoma cell line with IC50 values of 7.0, 2.5, and 0.94 µM, respectively [25]. They also showed anti-oomycete activity against the oomycete plant pathogen Phytophthora capsici [25].
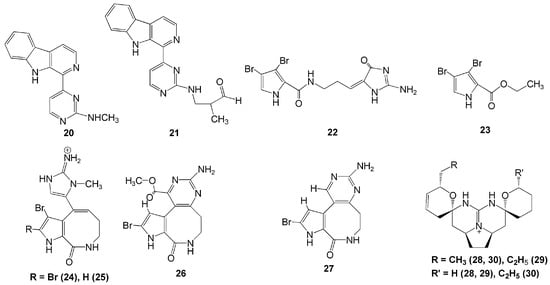
Figure 2.
Chemical structures of 20–30.
Four new imidazole alkaloids were isolated from the Indonesian sponge L. chagosensi (Table 1). The alkaloids methyldorimidazole (31) and preclathridine B (32) were isolated from Kapoposang Island, South Sulawesi [26], whereas the other two alkaloids, naamidines H (33) and I (34), were isolated from L. chagosensi collected in North Sulawesi [27,28]. Compounds 33 and 34 were reported to show cytotoxicity against HeLa cells with IC50 values of 11.3 and 29.6 µM, respectively [27,28]. Another new alkaloid was isolated from L. microraphis, named spironaamidine (35) [28]. The spironaamidine possessed antimicrobial activity against B. cereus. However, the effect was slightly lower than that of naamidine 33 [27,28], probably due to the presence of spiroquinone, which is exceptionally rare in natural sources (Figure 3).
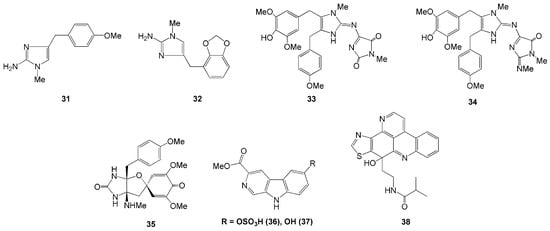
Figure 3.
Chemical Structures of 31–38.
Two new β-carboline alkaloids, variabines A (36) and B (37), were isolated from Indonesian marine sponge Luffariealla variabilis collected in North Sulawesi [29]. Compound 36 was the first sulfated β-carboline alkaloid and the sulfonated derivative of compound 37. A hydroxy group in compound 37 replaces the sulfate group at C-6 in compound 36. Compound 37 was found to inhibit the chymotrypsin-like activity of the proteasome and Ubc13 (E2)-Uev1A interaction with IC50 values of 16.5 and 20.6 µM, respectively. In contrast, compound 36 showed a weak effect on proteasome. This result suggested that the sulfate group in variabines decreases chymotrypsin-like activity [29].
A novel pyridoacridine alkaloid, sagitol C (38), was isolated from the ethyl acetate fraction of Oceaniapia sp. collected in Ambon, Maluku Islands [30]. Alkaloid 38 was found to exhibit cytotoxic activity against the mouse lymphoma L5187Y cancer cell line, HeLa cell, and the rat pheochromocytoma PC12 cell lines with ED50 values of 0.7, 0.9, and 2.3 µM, respectively [30].
Seven novel anti-angiogenic steroidal alkaloids (Figure 4) were isolated from the sponge Corticium simplex collected in Flores Island, East Nusa Tenggara [31,32], namely cortistatins E-H (39–42) and J-L (43–45). These compounds have unique abeo-9(10-19)-stigmastane-type steroidal alkaloids. Compounds 38–42 have oxabicyclo[3.2.1]octene and N-methyl piperidine or 3-methylpyridine units in the side chain, while compounds 43–45 have an isoquinoline unit. Compound 43 showed cytostatic antiproliferative activity against human umbilical vein endothelial cells (HUVECs) at 8 nM [31,32].
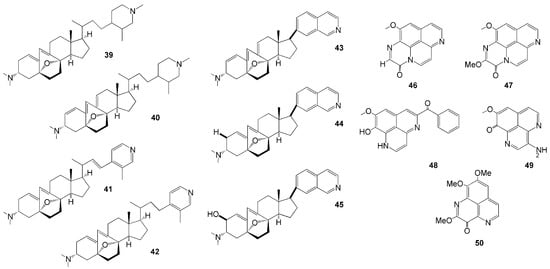
Figure 4.
Chemical structures of 39–50.
The genus Aaptos is also an abundant source of novel aaptamine alkaloids (Table 1). Four new aaptamine derivates were isolated from A. suberitoides collected in Ambon, Maluku Islands [33]—namely, 11-methoxy-3H-[1,6]naphthyridino [6,5,4-def]quinoxalin-3-one (46), 2,11-dimethoxy-3H-[1,6]naphthyridino [6,5,4-def]quinoxalin-3-one (47), 5-benzoyldemethylaaptamine (48), and 3-aminodemethyl(oxy)aaptamine (49). Compound 47 is the 11-methoxy derivative of compound 46 and represents a benzoyl-aaptamine skeleton that has not been previously identified [33]. Concerning the biological activity, compound 48 exhibited cytotoxic activity against the mouse lymphoma L5187Y cancer cell line with an IC50 value of 5.5 µM [33]. Another aaptamine derivate isolated from Aaptos sp. collected from Kupang, East Nusa Tenggara [34],—namely, 2-methoxy-3-oxoaaptamine (50), demonstrated an anti-mycobacterial activity under both aerobic and hypoxic conditions, both with a MIC value of 23 µM [34].
Twenty-one novel psammaplysin derivatives were successfully isolated from A. strongylata collected in Tulamben Bay, Bali [35]. Compounds 19-Hydroxypsammaplysin E (51), psammaplysin K (52), psammaplysin K dimethoxy acetal (53), psammaplysin L (54), and M (55), possess modified aromatic ring substituents. Meanwhile, psammaplysin N-P (56–58), 19-hydroxypsammaplysin P (59), psammaplysin Q (60), 19-hydroxypsammaplysin Q (61), psammaplysin R-S (62–63), 19-hydroxypsammaplysin S (64), psammaplysin T (65), and 19-hydroxypsammaplysin T (66) exhibit various side chains that are saturated fatty acid side chains (Figure 5). On the other hand, psammaplysin U (67), 19-hydroxypsammaplysin U (68), psammaplysin V-W (69–70), and 19-hydroxypsammaplysin W (71) have monoenoic fatty acid side chains. Among these 21 compounds, 19-hydroxypsammaplysin E (51), having the N-substitution of the ethylamino moiety, showed the best antimalarial activity with modest inhibition against P. falciparum (IC50 = 6.4 µM) [35].
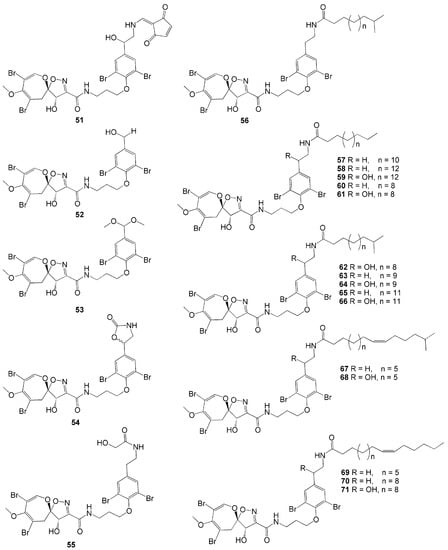
Figure 5.
Chemical structures of 51–71.
Several new terpenes were isolated from Indonesian marine sponges from the Dictyoceratida order (Figure 6), such as L. herbacea [36], Spongia sp. [37], and Carterospongia foliascens [38]. Four new sesquiterpenes were isolated from L. herbacea collected in Manado, North Sulawesi—namely, lamellodysidines A (72) and B (73), O,O-dimethyllingshuiolide A (74), and 11-epi-O,O-dimethyllingshuiolide A (75) [36]. Compound 72 was the first compound identified with a unique bridged polycyclic framework, and compound 73 is a novel nitrogenous sesquiterpene, while compounds 74 and 75 were obtained as an inseparable mixture due to their 11-epimeric nature [36].
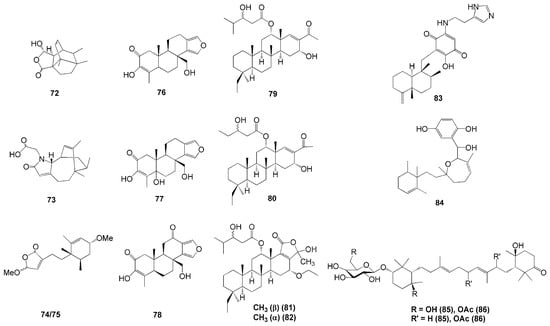
Figure 6.
Chemical structures of 72–86.
Sponges from the genus Spongia are known as a rich source of unique terpenoids (Table 1). This is evident in the successful isolation of three novel terpenoids from Spongia sp. collected in Bunaken Marine Park, North Sulawesi [37]. These three compounds were identified as diterpene—namely, 18-nor-3,17-dihydroxyspongia-3,13(16),14-trien-2-one (76), 18-nor-3,5,17-trihydroxyspongia-3,13(16),14-trien-2-one (77), and spongiapyridine (78). Compound 77 possesses an unusual d-ring substitute, a pyridyl ring system, in the place of δ-lactone (Figure 6). In terms of the biological activity of these four compounds, only compound 76 possessed moderate activity on aromatase inhibition with an IC50 of 34 µM and quinone reductase 1 induction with the concentration needed to double the enzymatic response of 11.2 µM [37].
Four new scalarane-based sesterterpenoids were isolated from C. foliascens associated with the coral reefs of Palau Barang Lompo, near Makasar, South Sulawesi [38]. The closely related compounds were identified as 20,24-bishomo-25-norscalaranes 1 (79) and 2 (80), and 20,24-bishomoscalaranes ketals 3 (81) and 4 (82) (Figure 6). Compounds 81 and 82 were isolated as an inseparable mixture. Compound 80 showed little to no activity toward RCE-protease inhibition compared to 79. Meanwhile, a combination of 80 and 82 (plus 81, as its inseparable mixture) had inhibition activity with IC50 values of 38 and 4.2 µg/mL, respectively [38]. Compound 79 and the mixture of 81 and 82 also showed inhibition against tumour cell lines (human prostate PC3, colorectal LoVo, colorectal adenocarcinoma CACO-2, and breast MDA-468 cancer cell lines) with IC50 values ranging from 2.9–9.5 µg/mL [38].
Other compounds isolated from Sulawesi included nakijiquinone V (83), isolated from Dactylospongia elegans from Tahuna, Sangihe Islands, North Sulawesi [39]. It has three methyl groups attached to a decalin system with an exocyclic double bond [39] (Figure 6). Another was a new meroditerpene—namely, Halioxepine (84), which was isolated from an Indonesian marine sponge from the genus Haliclona collected in Baubau, Southeast Sulawesi [40]. This compound showed moderate cytotoxicity against the rat bladder tumour NBT-T2 cells with IC50 of 11.6 µM and antioxidant activity against 1,1-diphenyl-2-picrylhydrazyl (DPPH) with IC50 of 7.7 µM [40]. Recently, two new terpenoids, melophluosides A (85) and B (86), were successfully isolated from Melophlus sarassinorum collected in Siladen, North Sulawesi. These new compounds belong to the triterpene galactosides of the pouoside class, with compound 85 as the first without an oxygenated group at C-11. Both compounds exhibited moderate cytotoxic activity against HeLa cells with IC50 values of 11.6 and 9.7 mM, respectively [41].
Two new cycoldepsipeptide jaspamide derivatives, jaspamide Q (87) and jaspamide R (88), were identified from the marine sponge J. splendens collected in neighbouring islands near East Kalimantan [42]. They exhibited potent cytotoxic activity against the mouse lymphoma L5187Y cancer cell line with IC50 values of <126.8 µM and <203 µM, respectively [42].
Novel tridecapeptides of the theonellapeptolide family were isolated from T. swinhoei collected from Bunaken Marine Park in Manado, North Sulawesi—namely, sulfinyltheonellapeptolide (89) and theonellapeptolide If (90) [43]. These compounds differ in the abrine moiety. Compound 89 is a theonellapeptolide with a methylsulfinylacetyl group at the N-terminus, while compound 90 was the first theonellapeptolide with four valine residues (Figure 7). Both compounds showed significant antiproliferative activity against human liver HepG2 cancer cells with the same IC50 value of 3 µM [43].
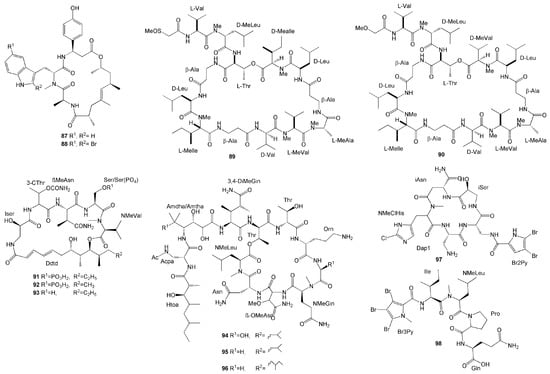
Figure 7.
Chemical structures of 87–98.
S. mirabilis is also one of the rich sources of diverse secondary metabolites, as exemplified by the isolation of six new depsipeptides with two different structural classes named celebesides A-C (91–93) from the species collected in Sulawesi [44]. Compounds 91–93 are cyclic depsipeptides with a polyketide moiety and five amino acid residues (Figure 7). The celebesides A and B (91, 92) possess a 3-carbamoyl threonine and a phosphoserine residue, which is quite uncommon. S. mirabilis also produced the peptides theopapuamides B–D (94–96), which are undecapeptides with an N-terminal fatty acid moiety, with theopapuamide D (96) containing a rare homoisoleucine residue (Figure 7). These compounds exhibited several bioactivities. Theopapuamides B and C (94, 95) were found to have relatively strong antifungal activity against amphotericin B-resistant C. albicans [44]. Additionally, celebeside A (91) and theopapuamide B (94) neutralized HIV-1 in a single-round infectivity assay with an IC50 value of 2.1 ± 0.4 µM and 499.7 ± 0.3 µg/mL [44]. Celebeside 91 and theopapuamides 94 and 95 also demonstrated cytotoxicity against human colon carcinoma cells with IC50 values 9.9 µM, 1.3 µM, and 2.5 µM, respectively [44]. Although they were potent against human colon HCT116 carcinoma cells, celebesides A, B, and C (91-93) were also found to be cytotoxic for healthy cell lines [44].
Haloirciniamide A (97) and seribunamide A (98), new polyhalogenated peptides, have been isolated from Ircinia sp. collected from the coast of Thousand Islands. Compound 97 was the first dibromopyrrole cyclopeptide with a chlorohistidine ring, while compound 98 possess a rare tribromopyrrole ring. Unfortunately, both compounds did not show significant cytotoxicity against four human tumour cell lines [45].
Four new endoperoxyketal polyketides, manadoperoxides A-D (99–102), were isolated from the sponge Plakortis cfr. simplex obtained from the Bunaken Marine Park of Manado, North Sulawesi [46]. In these compounds, the methoxy group at C-6 is replaced by either a methyl or ethyl group instead of a peroxyketal-type (Figure 8), making them slightly different from those previously isolated from the same species in the Caribbean. All compounds isolated from that sponge Plakortis cfr. simplex showed moderate antimalarial activity against D10 and W2 strains of Plasmodium falciparum [46].
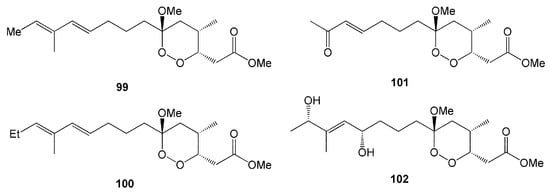
Figure 8.
Chemical structures of 99–102.
A novel cytotoxic macrolide, namely Callyspongiolide (103), was isolated from the Indonesian marine sponge Calyspongia sp [47]. This compound has a notable feature of a conjugated diene-ynic sidechain ending at a benzene ring with bromine, which was not found in the previously isolated marine macrolides (Figure 9). Its cytotoxicity against human acute T cell leukaemia Jurkat J16 T and human Burkitt’s lymphoma Ramos B lymphocytes showed remarkable IC50 values of 70 and 60 nM, respectively, as well as EC50 values of 80 and 50 nM, respectively [47].
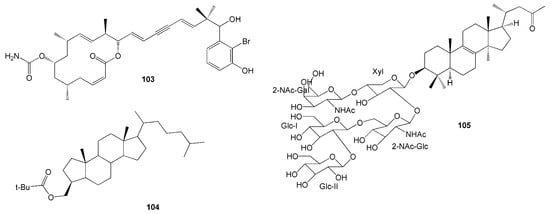
Figure 9.
Chemical structures of 103–105.
A novel A-nor steroid, namely clathruhoate (104), was isolated from a marine sponge Clathria sp., collected from Bintang Samudra Marine Education Park, Southeast Sulawesi [48]. Moreover, a new nortriterpenoid saponin, namely sarasinoside S (105), was identified from the marine sponge Petrosia sp. obtained in North Sulawesi [49]. Compound 105 was the first among the nortriterpenes in the saranoside family with a degraded side chain (Figure 9).
2.2. Tunicates
Tryptamine-derived alkaloids named leptoclinidamide (106) and (-)-leptoclinidamine B (107) were isolated from tunicate L. dubius collected from Lembeh Strait, North Sulawesi [50]. Compound 107 is the enantiomer of the previously isolated compound, (+)-leptoclinidamine B. These compounds have a unique amide moiety with two β-alanine units and d-arginine moiety, respectively [50].
Eleven compounds were identified from tunicate Lissoclinum cf. badium collected in Manado, Indonesia. Among these 11 compounds, there were four new polysulfur aromatic alkaloids named lissoclibadins 4–7 (108–111) (Figure 10), isolated with seven known alkaloids called lissoclibadins 1–3, lissoclinotoxins E and F, 3,4-dimethoxy6-(2′-N,N-dimethylaminoethyl)-5-(methylthio)benzotrithiane, and N,N-dimethyl-5-(methylthio)varacin [51]. Bioactivities of these compounds included inhibition against yeast Saccharomyces cerevisiae demonstrated by compounds 109–111, and antimicrobial activity against S. aureus and E. coli evident from compounds 108–111 [51].
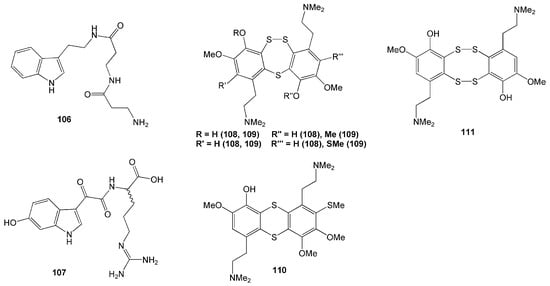
Figure 10.
Chemical structures of 106–111.
Other alkaloids polycarpathiamines A and B (112 and 113), were isolated from tunicate Polycarpa aurata (Table 2). Both alkaloid compounds have a rare 1,2,4-thiadiazole ring (Figure 10). Compound 112 demonstrated potent cytotoxic activity against mouse lymphoma L5187Y cancer cell line with an IC50 value of 0.41 µM [52].

Table 2.
Summary of the marine natural products isolated from the marine tunicates from Indonesian oceans.
Another two novel alkaloids have recently been isolated from another P. aurata from the coast of Siladen, North Sulawesi [53]. Featuring a p-methoxyphenyl group, these compounds were named polyaurines A (114) and B (115). Compound 114 deformed eggs of parasite Schistosoma mansoni, yet it was not toxic to mammalian cells [53]. Two new benzoyl derivatives from this species, ethyl 2-(4-methoxyphenyl)-2-oxoacetate (116) and methyl 2-(4-hydroxyphenyl)-2-oxoacetate (117), were also isolated (Figure 11).
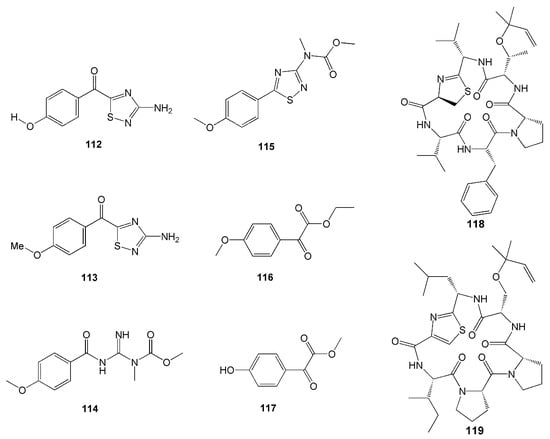
Figure 11.
Chemical structures of 112–119.
Two new cyclic hexapeptides, mollamides B (118) and C (119) (Figure 11), were isolated from D. mole found in Manado Bay, Indonesia. Compound 118 was found to have an antiparasitic activity against Plasmodium falciparum D6 clone (IC50 = 2.9 µM), P. falciparum W2 clone (IC50 = 3 µM), and Leishmania donovani (IC50 = 25.9 µM and IC90 = 50.3 µM) [54]. Compound 118 also displayed antiviral activity against HIV-1 in human PBM cells with an EC50 value of 48.7 µM and anticancer activity against the non-small lung H460, the breast MCF-7, and the human glioblastoma CNS SF268 cancer cell lines [54]. Furthermore, compound 119 showed anticancer activity with a unit zone differential value of 100 against mouse lymphocytic leukaemia L1210, human colon HCT116, and human lung H125 cells. This compound also showed a differential value of 250 against murine colon 38 [54]. However, compounds 118 and 119 did not show anti-inflammatory activity either in vitro in a cell-based assay through a cyclooxygenase enzyme (COX-2) activity assay or in vivo in rat neonatal microglia [54]. Furthermore, compounds 118 and 119 did not show antimicrobial activity against MRSA, Mycobacterium intracellulaire, Candida albicans, C. glabrata, C. krusei, nor Cryptococcus neoformans [54].
2.3. Soft Corals
Terpenoids were often isolated from soft corals, with varying degrees of bioactivities (Table 3). Sarcofuranocembrenolides A (120) and B (121) were isolated from the soft coral Sarcophyton sp. collected in North Sulawesi [55]. Cembranoid 120 is a bisnorcembrenolide featuring a unique carbon skeleton of 8,19-bisnorfuranocembrenolide (Figure 12). On the other hand, cembranoid 121 is a furanocembrenolide with a C1 unit (C-20) attached to C-10. In the ordinary cembrenolides, the C1 unit is attached to C-12 [55].

Table 3.
Summary of the marine natural products isolated from the marine soft corals from Indonesian oceans.
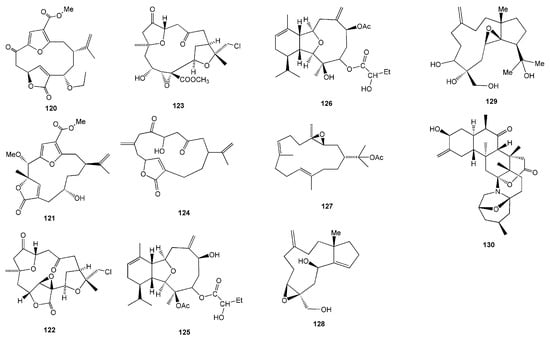
Figure 12.
Chemical structures of 120–130.
Three C-4 norcembranoids-type macrocyclic diterpenoids, namely chloroscabrolide A (122), chloroscabrolide B (123), and prescarbolide (124), were isolated from Sinularia sp. collected from Bunaken Marine Park, Manado, North Sulawesi [56]. Compounds 122 and 123 are two of very few chlorinated compounds from soft coral metabolites and the second example within the class of cembranoids (Figure 12). These two compounds also feature an oxygen bridge connecting C-13 and C-15, which is quite unusual. Meanwhile, the terpenoid prescarbolide (124) is believed to be the precursor of the scabrolide/leptocladolide family of cembranoids [56].
Two new isomeric eunicellin-type diterpenoids were isolated from an Indonesian octocoral Cladiella sp. in the West Pacific Ocean [57]. Cladielloide A (125) and cladielloide B (126) both possess a 2-hydroxybutyroxy group in their structures (Figure 13). Compound 126 exhibited a potent inhibitory effect on superoxide anion generation and elastase release by human neutrophils at 22 µM. It also showed moderate cytotoxicity against the acute lymphocytic leukaemia CCRF-CEM tumour cells with an IC50 value of 10.1 µM [57].
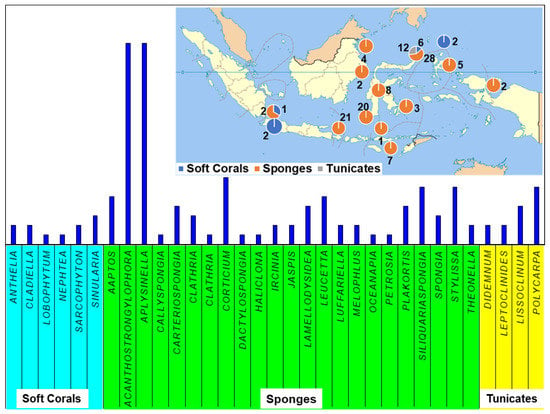
Figure 13.
The distribution of sample origin and the division of compound class by genus.
A terpenoid compound, 3,4-epoxy-nephtenol (127), was isolated from the soft coral Nephthea sp. found in Seribu Islands, DKI Jakarta [58]. Compound 127 showed weak inhibitory growth against three human tumour cell lines, i.e., human glioblastoma SF268, human breast MCF-7, and human non-small cell lung H460 cancer cells [58].
The latest finding on terpenoids from soft coral was discovered from Anthelia sp. collected at Banten, West Java, which successfully isolated two new dolabellane diterpenoids, sangiangol A (128) and sangiangol B (129). These two compounds were found to show weak toxicity against NBT-T2 rat bladder epithelial cells (BRC-1370) at 18 and 28.2 µM, respectively [59]. The first reported zoanthamine-type alkaloid from a marine invertebrate other than zoanthids was named lobozoanthamine (130), isolated from the Indonesian soft coral Lobophytum sp. collected from the Bunaken Marine Park, Manado, North Sulawesi [60]. However, there is no report on the bioactivity of the compounds 120–125 and 130 (Table 3).
3. Conclusions
Indonesian marine biodiversity holds immense potential for drug discovery and bioprospecting, supporting the continuous exploration and investigation of Indonesian MNPs from marine invertebrates such as sponges, tunicates, and soft corals. This is further justified by no less than a hundred and thirty novel compounds reported in this review from 43 publications throughout 2007–2020, the majority of which were isolated from the Indonesian marine invertebrates collected from the North region of Sulawesi, Indonesia (Figure 13).
Indonesian sponges have been known as a major source of many novel MNPs. In this review, sponges were found to contribute to 105 novel compounds reported between 2007–2020, the majority being alkaloids (Figure 14). Most of the sponge alkaloids were isolated from the genera Aplysinella and Acanthostrongulophora. The next large groups of metabolites isolated from Indonesian sponges were terpenes and peptides. Nearly all the sponge terpenoids were isolated from the order Dictyoceratida, in which the dominant genera were Lamellodysidea and Carteriospongia.
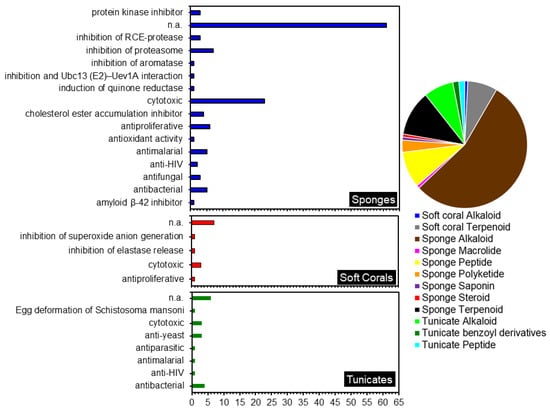
Figure 14.
Biological activities of compounds isolated from Indonesian marine invertebrates (left) and the division of genus by compound class (right).
Meanwhile, all the peptides, with the exception of two peptides from Ircinia sp., were isolated from the order Tetractinellida. On the other hand, macrolides, steroids, and saponins were the least frequently isolated metabolites from Indonesian sponges. The sponge Plakortis simplex was the sole contributor of the polyketides isolated from Indonesia (Table 1).
During 2007–2020, ten alkaloids were reported from Indonesian tunicates, mainly from the family Didemnidae and Styelidae. Soft corals are also a good source of novel metabolites and are well-known as a producer of terpenoids, particularly the group of terpenes and cembranoid diterpenes. Soft coral-derived terpenoids have received significant attention in Indonesian MNPs research (Table 3). Compared to other invertebrates with alkaloids as the most abundant secondary metabolites, only one alkaloid was found in Indonesian soft coral species in this review.
Indonesian soft corals and tunicates yielded far lower novel secondary metabolites than sponges reported throughout 2007–2020. Therefore, this review highlighted the opportunity to explore further the chemical diversity and the biological activity of tunicates from Indonesian waters.
Diverse biological activities were shown by fifty per cent of the compounds isolated from the three Indonesian marine invertebrates discussed (Figure 14). The majority of compounds in this review were reported to possess cytotoxic or antiproliferative activity against various cancer cell lines. Four of these compounds showed a remarkable cytotoxic activity with IC50 values of less than 1 µM, namely compounds 30, 38, 103 and 112. Furthermore, four compounds showed IC50 values between 1–4 µM, and another eight showed moderate cytotoxic with an IC50 value of less than 10 µM. Most of these compounds belong to the alkaloid group, followed by peptides and terpenoids. It is, therefore, evident that the exploration of potential anticancer drugs from Indonesian marine resources warrants further investigation.
The bioactivity of compounds isolated from the Indonesian marine invertebrates (sponges, tunicates, and soft corals) as antimicrobial, antifungal, or antiviral was also described in this review. Unfortunately, little or no such activities were yet to be found from many compounds derived from the three Indonesian marine invertebrates, demonstrating the gap in the knowledge of their beneficial biological activity other than their cytotoxic activity. This knowledge gap opens up the opportunity for further research focusing on exploring and harnessing the potential of Indonesian marine invertebrates as sources of compounds with antimicrobial, antifungal, or antiviral activities, as well as further exploration of Indonesian marine biodiversity for the discovery of novel bioactive compounds.
Author Contributions
Conceptualization, M.Y.P., A.B. and F.I.; resources, F.I., A.B. and M.F.W.; writing—original draft preparation, F.I. and A.B.; writing—review and editing, F.I., A.B., M.F.W., S.I.R., L.S., A.A., A.P., M.Y.P.; visualization, F.I., A.B. and M.F.W.; supervision, M.Y.P. and A.B.; project administration, A.B. and F.I.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Indonesian Institute of Sciences (LIPI) through the DIPA 2021 Research Fund B-10405/IPH/HK.01.03/XI/2020.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Acknowledgments
We would like to thank the Head of Research Center for Biotechnology, Indonesian Institute of Sciences, as well as the research and administration staff members for their support. All authors are supported by the DIPA 2021 Research Fund.
Conflicts of Interest
The authors declare there is no conflict of interest.
Sample Availability
Not Applicable.
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef]
- Jha, R.; Zi-rong, X. Biomedical Compounds from Marine Organisms. Mar. Drugs 2004, 2, 123–146. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Atanasov, A.G.; Horbanczuk, O.K.; Tammam, M.A.; Abdel-Mogib, M.; Hooper, J.N.A.; Sekeroglu, N.; Al-Mourabit, A.; Kijjoa, A. Chemical Diversity and Biological Activities of Marine Sponges of the Genus Suberea: A Systematic Review. Mar. Drugs 2019, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chang, Y.C.; Chen, Y.H.; Zheng, L.G.; Huang, P.C.; Huynh, T.H.; Peng, B.R.; Chen, Y.Y.; Wu, Y.J.; Fang, L.S.; et al. Natural Products from Octocorals of the Genus Dendronephthya (Family Nephtheidae). Molecules 2020, 25, 5957. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Phan, C.S.; Ishii, T.; Kamada, T.; Hamada, T.; Vairappan, C.S. Terpenoids from Marine Soft Coral of the Genus Xenia in 1977 to 2019. Molecules 2020, 25, 5386. [Google Scholar] [CrossRef]
- Jiménez, C. Marine Natural Products in Medicinal Chemistry. ACS Med. Chem. Lett. 2018, 9, 959–961. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed Marine Natural Products in the Pharmaceutical and Cosmeceutical Industries: Tips for Success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Y.-Y.; Shao, C.-L.; Wang, C.-Y. Metabolites from Marine Invertebrates and Their Symbiotic Microorganisms: Molecular Diversity Discovery, Mining, and Application. Mar. Life Sci. Technol. 2019, 1, 60–94. [Google Scholar] [CrossRef]
- Dewi, A.S.; Tarman, K.; Uria, A.R. Marine Natural Products: Prospects and Impacts on the Sustainable Development in Indonesia. In Proceedings of the Indonesian Students’ Scientific Meeting, Delft, The Netherlands, 13–15 May 2008; pp. 54–63. [Google Scholar]
- Engelbrecht, J.; Tursch, B.; Djerassi, C. A New Sterol from an Alcyonarian. Steroid 1972, 53, 1689–1699. [Google Scholar] [CrossRef]
- Corley, D.G.; Herb, R.; Moore, R.E.; Scheuer, P.J. Laulimalides: New Potent Cytotoxic Macrolides from a Marine Sponge and a Nudibranch Predator. J. Org. Chem 1988, 53, 3644–3646. [Google Scholar] [CrossRef]
- Hanif, N.; Murni, A.; Tanaka, C.; Tanaka, J. Marine Natural Products from Indonesian Waters. Mar. Drugs 2019, 17, 364. [Google Scholar] [CrossRef]
- Putra, M.Y.; Jaswir, I. The Alkaloids from Indonesian Marine Sponges. Oceanogr. Open Access 2017, 2. [Google Scholar] [CrossRef]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T. A Review of Chemistry and Biological Activities of the Indonesian Octocorallia. J. Appl. Pharm. Sci. 2017, 7, 219–227. [Google Scholar] [CrossRef]
- Furusato, A.; Kato, H.; Nehira, T.; Eguchi, K.; Kawabata, T.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Takeya, M.; et al. Acanthomanzamines A-E with New Manzamine Frameworks from the Marine Sponge Acanthostrongylophora ingens. Org. Lett. 2014, 16, 3888–3891. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Kato, H.; Eguchi, K.; Kawabata, T.; Fujiwara, Y.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Takeya, M.; Yokosawa, H.; et al. Acantholactam and Pre- Neo -Kauluamine, Manzamine-Related Alkaloids from the Indonesian Marine Sponge Acanthostrongylophora ingens. J. Nat. Prod. 2014, 7, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Dewi, A.S.; Hadi, T.A.; Fajarningsih, N.D.; Blanchfield, J.T.; Bernhardt, P.V.; Garson, M.J. Acanthocyclamine a from the Indonesian Marine Sponge Acanthostrongylophora ingens. Aust. J. Chem. 2014, 67, 1205–1210. [Google Scholar] [CrossRef]
- Esposito, G.; Mai, L.H.; Longeon, A.; Mangoni, A.; Durieu, E.; Meijer, L.; Van Soest, R.; Costantino, V.; Bourguet-Kondracki, M.L. A Collection of Bioactive Nitrogen-Containing Molecules from the Marine Sponge Acanthostrongylophora ingens. Mar. Drugs 2019, 17, 472. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenines C and D, New Cytotoxic Pyrimidine-β-Carboline Alkaloids from the Indonesian Sponge Acanthostrongylophora ingens. Phytochem. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef]
- Ebada, S.S.; Linh, M.H.; Longeon, A.; De Voogd, N.J.; Durieu, E.; Meijer, L.; Bourguet-Kondracki, M.L.; Singab, A.N.B.; Müller, W.E.G.; Proksch, P. Dispacamide e and Other Bioactive Bromopyrrole Alkaloids from Two Indonesian Marine Sponges of the Genus Stylissa. Nat. Prod. Res. 2015, 29, 231–238. [Google Scholar] [CrossRef]
- Fouad, M.A.; Debbab, A.; Wray, V.; Müller, W.E.G.; Proksch, P. New Bioactive Alkaloids from the Marine Sponge Stylissa sp. Tetrahedron 2012, 68, 10176–10179. [Google Scholar] [CrossRef]
- Kasmiati, K.; Yoshioka, Y.; Okamoto, T.; Ojika, M. New Crambescidin-Type Alkaloids from the Indonesian Marine Sponge Clathria bulbotoxa. Mar. Drugs 2018, 16, 84. [Google Scholar] [CrossRef]
- Hassan, W.H.B.; Al-Taweel, A.M.; Proksch, P. Two New Imidazole Alkaloids from Leucetta chagosensis Sponge. Saudi Pharm. J. 2009, 17, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Van Soest, R.W.M.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, Cytotoxic Imidazole Alkaloids from the Indonesian Marine Sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, Y.; Kato, H.; Rotinsulu, H.; Mangindaan, R.E.P.; De Voogd, N.J.; Tsukamoto, S. Spironaamidine, a New Spiroquinone-Containing Alkaloid from the Marine Sponge Leucetta microraphis. Tetrahedron Lett. 2011, 52, 5342–5344. [Google Scholar] [CrossRef]
- Sakai, E.; Kato, H.; Rotinsulu, H.; Losung, F.; Mangindaan, R.E.P.; De Voogd, N.J.; Yokosawa, H.; Tsukamoto, S. Variabines A and B: New β-Carboline Alkaloids from the Marine Sponge Luffariella variabilis. J. Nat. Med. 2014, 68, 215–219. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Elkhayat, E.S.; Fouad, M.A.; Proksch, P. Sagitol C, a New Cytotoxic Pyridoacridine Alkaloid from the Sponge Oceanapia sp. Bull. Fac. Pharmacy, Cairo Univ. 2013, 51, 229–232. [Google Scholar] [CrossRef]
- Watanabe, Y.; Aoki, S.; Tanabe, D.; Setiawan, A.; Kobayashi, M. Cortistatins E, F, G, and H, Four Novel Steroidal Alkaloids from Marine Sponge Corticium simplex. Tetrahedron 2007, 63, 4074–4079. [Google Scholar] [CrossRef]
- Aoki, S.; Watanabe, Y.; Tanabe, D.; Setiawan, A.; Arai, M.; Kobayashi, M. Cortistatins J, K, L, Novel Abeo-9(10-19)-Androstane-Type Steroidal Alkaloids with Isoquinoline Unit, from Marine Sponge Corticium simplex. Tetrahedron Lett. 2007, 48, 4485–4488. [Google Scholar] [CrossRef]
- Pham, C.; Hartmann, R.; Mu, W.E.G.; De Voogd, N.; Lai, D.; Proksch, P. Aaptamine Derivatives from the Indonesian Sponge Aaptos suberitoides. J. Nat. Prod. 2013, 76, 103–106. [Google Scholar] [CrossRef]
- Arai, M.; Han, C.; Yamano, Y.; Setiawan, A.; Kobayashi, M. Aaptamines, Marine Spongean Alkaloids, as Anti-Dormant Mycobacterial Substances. J. Nat. Med. 2014, 68, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Mudianta, I.W.; Skinner-Adams, T.; Andrews, K.T.; Davis, R.A.; Hadi, T.A.; Hayes, P.Y.; Garson, M.J. Psammaplysin Derivatives from the Balinese Marine Sponge Aplysinella strongylata. J. Nat. Prod. 2012, 75, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Torii, M.; Kato, H.; Hitora, Y.; Angkouw, E.D.; Mangindaan, R.E.P.; De Voogd, N.J.; Tsukamoto, S. Lamellodysidines A and B, Sesquiterpenes Isolated from the Marine Sponge Lamellodysidea herbacea. J. Nat. Prod. 2017, 80, 2536–2541. [Google Scholar] [CrossRef] [PubMed]
- Parrish, S.M.; Yoshida, W.Y.; Kondratyuk, T.P.; Park, E.J.; Pezzuto, J.M.; Kelly, M.; Williams, P.G. Spongiapyridine and Related Spongians Isolated from an Indonesian Spongia sp. J. Nat. Prod. 2014, 77, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Hollander, I.; Feldberg, L.; Frommer, E.; Mallon, R.; Tahir, A.; Van Soest, R.; Andersen, R.J. Scalarane-Based Sesterterpenoid RCE-Protease Inhibitors Isolated from the Indonesian Marine Sponge Carteriospongia foliascens. J. Nat. Prod. 2009, 72, 1106–1109. [Google Scholar] [CrossRef]
- Balansa, W.; Mettal, U.; Wuisan, Z.G.; Plubrukarn, A.; Ijong, F.G.; Liu, Y.; Schäberle, T.F. A New Sesquiterpenoid Aminoquinone from an Indonesian Marine Sponge. Mar. Drugs 2019, 17, 158. [Google Scholar] [CrossRef]
- Trianto, A.; Hermawan, I.; De Voogd, N.J.; Tanaka, J. Halioxepine, a New Meroditerpene from an Indonesian Sponge Haliclona sp. Chem. Pharm. Bull. 2011, 59, 1311–1313. [Google Scholar] [CrossRef]
- Sadahiro, Y.; Hitora, Y.; Fukumoto, A.; Ise, Y.; Angkouw, E.D.; Mangindaan, R.E.P.; Tsukamoto, S. Melophluosides A and B, New Triterpene Galactosides from the Marine Sponge Melophlus sarasinorum. Tetrahedron Lett. 2020, 61, 151852. [Google Scholar] [CrossRef]
- Ebada, S.S.; Wray, V.; De Voogd, N.J.; Deng, Z.; Lin, W.; Proksch, P. Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens. Mar. Drugs 2009, 7, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Sinisi, A.; Calcinai, B.; Cerrano, C.; Dien, H.A.; Zampella, A.; D’Amore, C.; Renga, B.; Fiorucci, S.; Taglialatela-Scafati, O. New Tridecapeptides of the Theonellapeptolide Family from the Indonesian Sponge Theonella swinhoei. Beilstein J. Org. Chem. 2013, 9, 1643–1651. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A-C and Theopapuamides B-D, Depsipeptides from an Indonesian Sponge That Inhibit HIV-1 Entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Bayu, A.; Hadi, T.A.; Bueno, S.; Pérez, M.; Cuevas, C.; Putra, M.Y. Unique Polyhalogenated Peptides from the Marine Sponge Ircinia sp. Mar. Drugs 2020, 18, 396. [Google Scholar] [CrossRef]
- Fattorusso, C.; Persico, M.; Calcinai, B.; Cerrano, C.; Parapini, S.; Taramelli, D.; Novellino, E.; Romano, A.; Scala, F.; Fattorusso, E.; et al. Manadoperoxides A-D from the Indonesian Sponge Plakortis Cfr. simplex. Further Insights on the Structure-Activity Relationships of Simple 1,2-Dioxane Antimalarials. J. Nat. Prod. 2010, 73, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.D.; Hartmann, R.; Böhler, P.; Stork, B.; Wesselborg, S.; Lin, W.; Lai, D.; Proksch, P. Callyspongiolide, a Cytotoxic Macrolide from the Marine Sponge Callyspongia sp. Org. Lett. 2014, 16, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Sahidin, I.; Sabandar, C.W.; Wahyuni; Hamsidi, R.; Malaka, M.H.; Sadarun, B.; Aslan, L.O. A-nor Steroids from the Marine Sponge, Clathria Species. Malaysian J. Anal. Sci. 2018, 22, 375–382. [Google Scholar] [CrossRef]
- Maarisit, W.; Yamazaki, H.; Kanno, S.-i.; Tomizawa, A.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Ukai, K.; Kapojos, M.M.; Namikoshi, M. A Tetramic Acid Derivative with Protein Tyrosine Phosphatase 1B Inhibitory Activity and a New Nortriterpene Glycoside from the Indonesian Marine Sponge Petrosia sp. Bioorganic Med. Chem. Lett. 2017, 27, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Wewengkang, D.S.; Nishikawa, T.; Rotinsulu, H.; Mangindaan, R.E.P.; Namikoshi, M. Two New Tryptamine Derivatives, Leptoclinidamide and (-)-Leptoclinidamine B, from an Indonesian Ascidian Leptoclinides dubius. Mar. Drugs 2012, 10, 349–357. [Google Scholar] [CrossRef]
- Nakazawa, T.; Xu, J.; Nishikawa, T.; Oda, T.; Fujita, A.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Kobayashi, H.; Namikoshi, M. Lissoclibadins 4-7, Polysulfur Aromatic Alkaloids from the Indonesian Ascidian Lissoclinum Cf. badium. J. Nat. Prod. 2007, 70, 439–442. [Google Scholar] [CrossRef]
- Pham, C.D.; Weber, H.; Hartmann, R.; Wray, V.; Lin, W.; Lai, D.; Proksch, P. New Cytotoxic 1,2,4-Thiadiazole Alkaloids from the Ascidian Polycarpa Aurata. Org. Lett. 2013, 15, 2230–2233. [Google Scholar] [CrossRef]
- Casertano, M.; Imperatore, C.; Luciano, P.; Aiello, A.; Putra, M.Y.; Gimmelli, R.; Ruberti, G.; Menna, M. Chemical Investigation of the Indonesian Tunicate Polycarpa Aurata and Evaluation of the Effects against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Mar. Drugs 2019, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.S.; Wang, B.; Dunbar, D.C.; Desai, P.V.; Patny, A.; Avery, M.; Hamann, M.T. Mollamides B and C, Cyclic Hexapeptides from the Indonesian Tunicate Didemnum Molle. J. Nat. Prod. 2008, 71, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Kapojos, M.M.; Lee, J.S.; Oda, T.; Nakazawa, T.; Takahashi, O.; Ukai, K.; Mangindaan, R.E.P.; Rotinsulu, H.; Wewengkang, D.S.; Tsukamoto, S.; et al. Two Unprecedented Cembrene-Type Terpenes from an Indonesian Soft Coral Sarcophyton sp. Tetrahedron 2010, 66, 641–645. [Google Scholar] [CrossRef]
- Fattorusso, E.; Luciano, P.; Putra, M.Y.; Taglialatela-Scafati, O.; Ianaro, A.; Panza, E.; Bavestrello, G.; Cerrano, C. Chloroscabrolides, Chlorinated Norcembranoids from the Indonesian Soft Coral Sinularia sp. Tetrahedron 2011, 67, 7983–7988. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tai, C.Y.; Hwang, T.L.; Weng, C.F.; Li, J.J.; Fang, L.S.; Wang, W.H.; Wu, Y.C.; Sung, P.J. Cladielloides A and B: New Eunicellin-Type Diterpenoids from an Indonesian Octocoral Cladiella sp. Mar. Drugs 2010, 8, 2936–2945. [Google Scholar] [CrossRef] [PubMed]
- Januar, H.I.; Chasanah, E.; Motti, C.A.; Tapiolas, D.M.; Liptrot, C.H.; Wright, A.D. Cytotoxic Cembranes from Indonesian Specimens of the Soft Coral Nephthea sp. Mar. Drugs 2010, 8, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Hanif, N.; Murni, A.; Tanaka, J. Sangiangols A and B, Two New Dolabellanes from an Indonesian Marine Soft Coral, Anthelia sp. Molecules 2020, 25, 3803. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Romano, A.; Taglialatela-Scafati, O.; Janib Achmad, M.; Bavestrello, G.; Cerrano, C. Lobozoanthamine, a New Zoanthamine-Type Alkaloid from the Indonesian Soft Coral Lobophytum sp. Tetrahedron Lett. 2008, 49, 2189–2192. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).