Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions
Abstract
1. Introduction
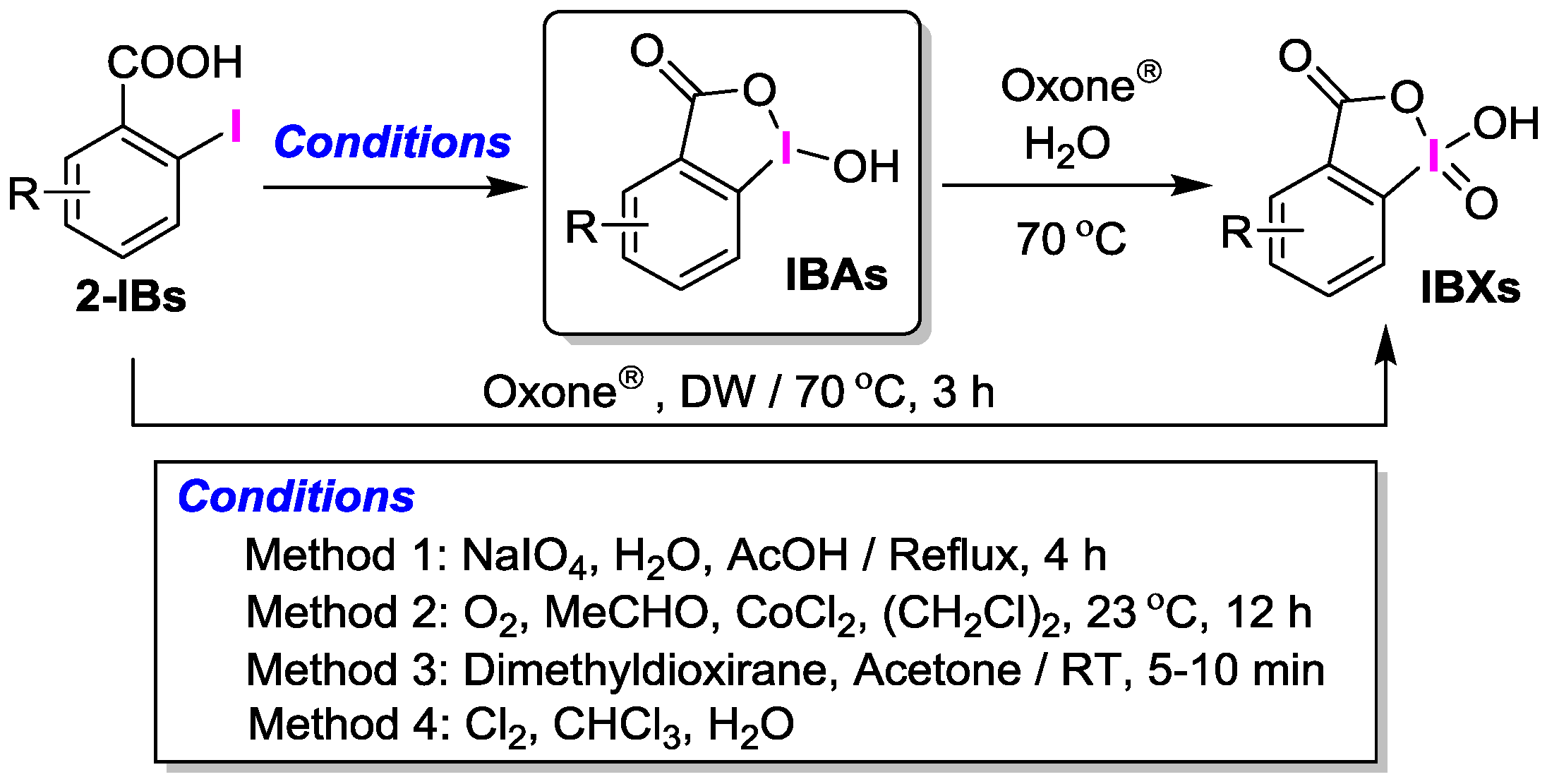
2. Results and Discussion
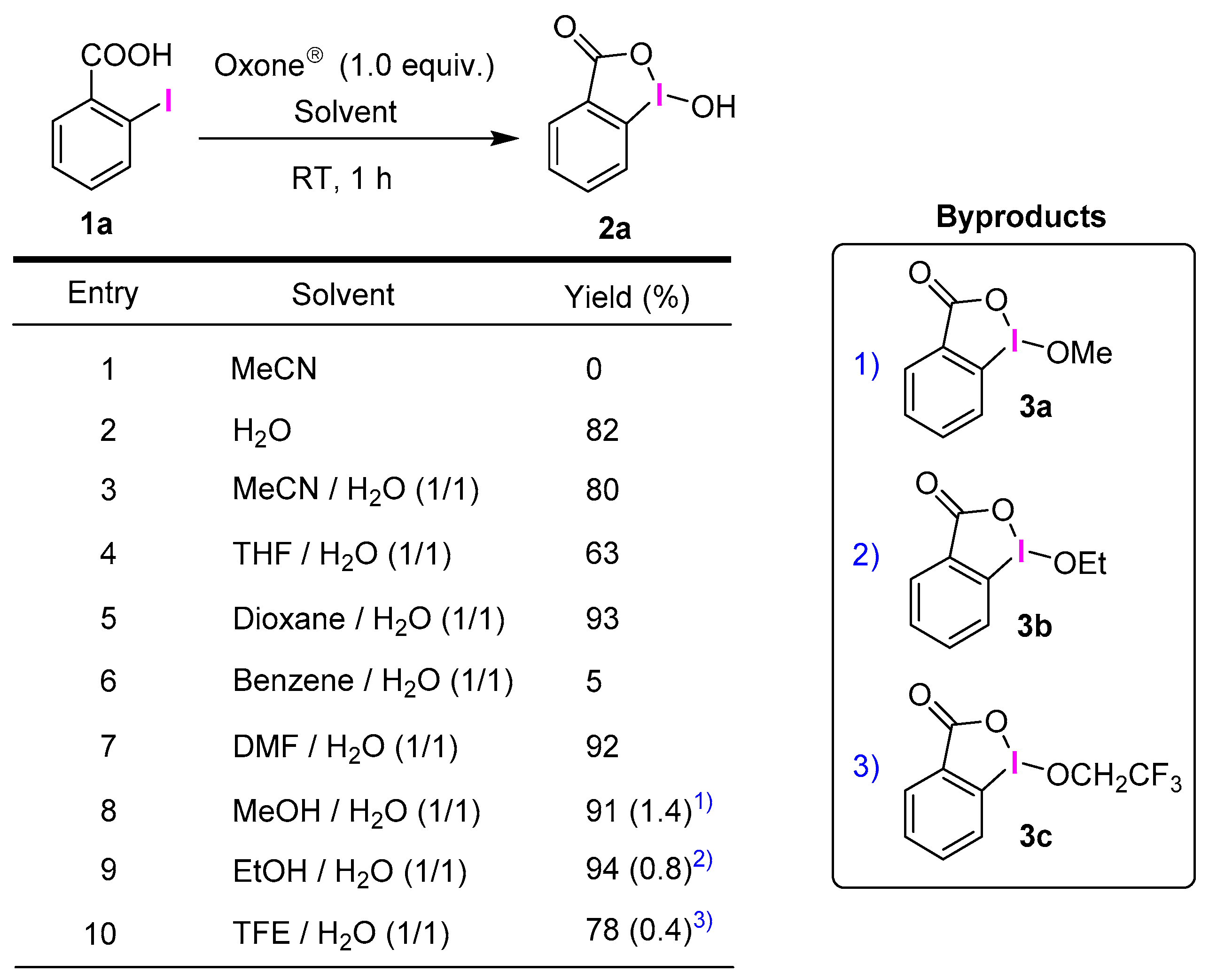
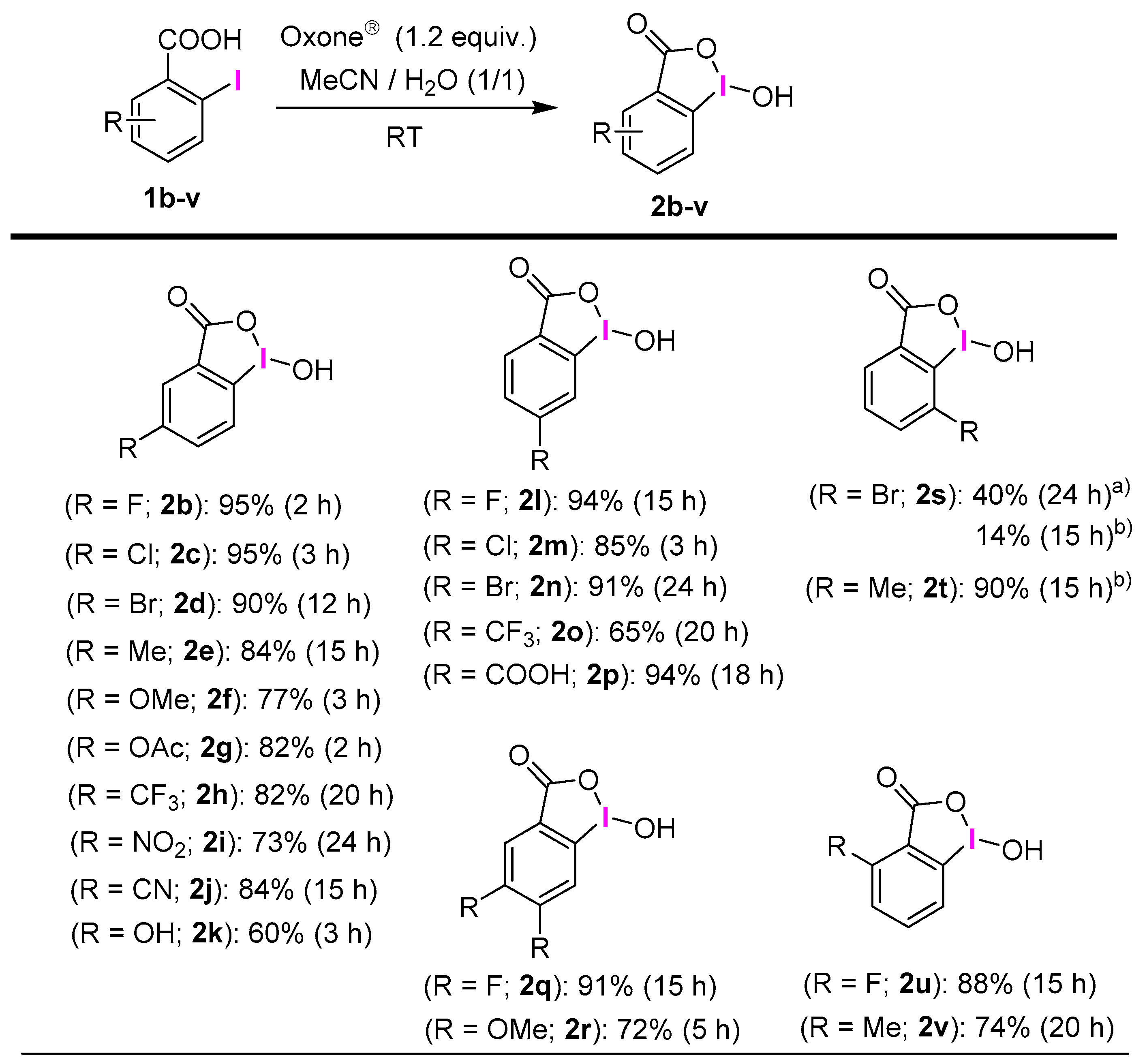
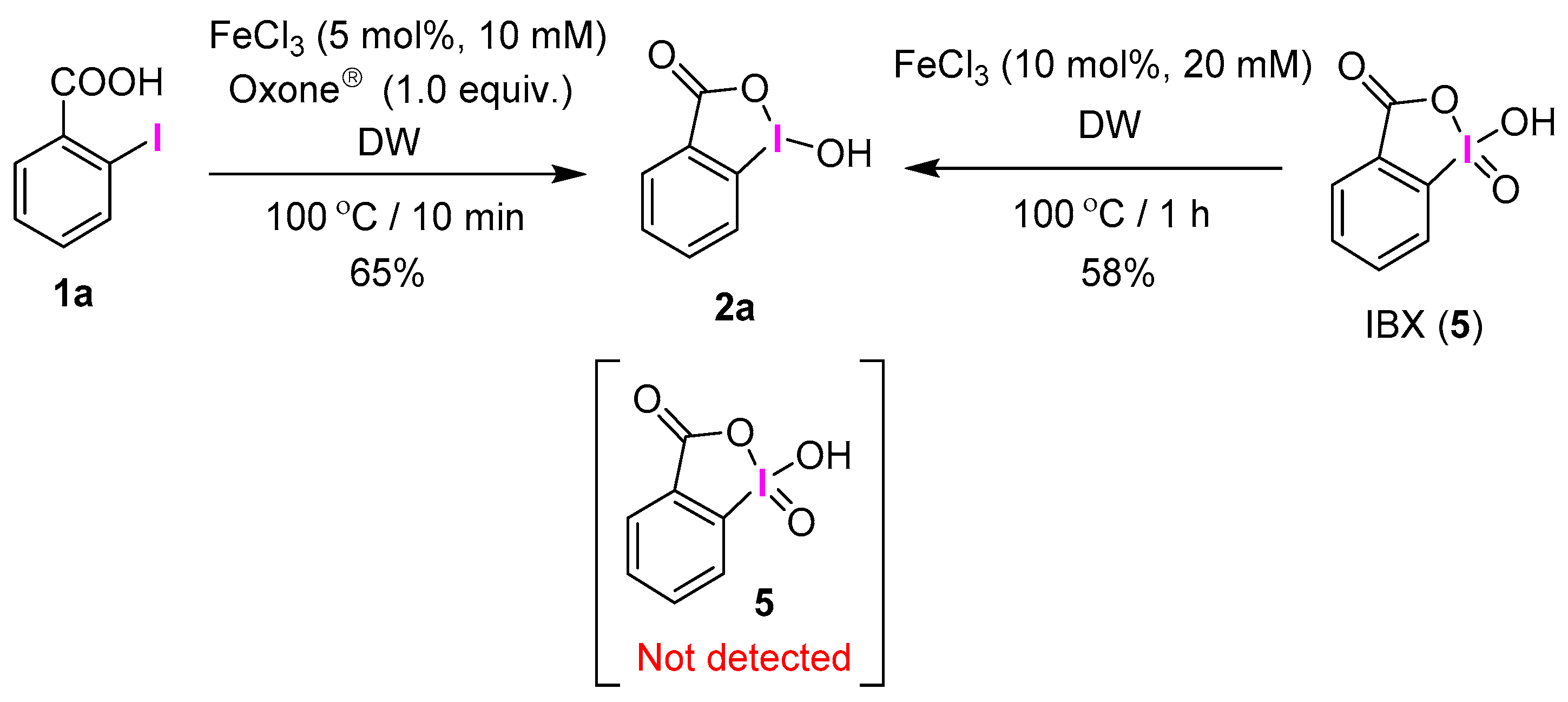
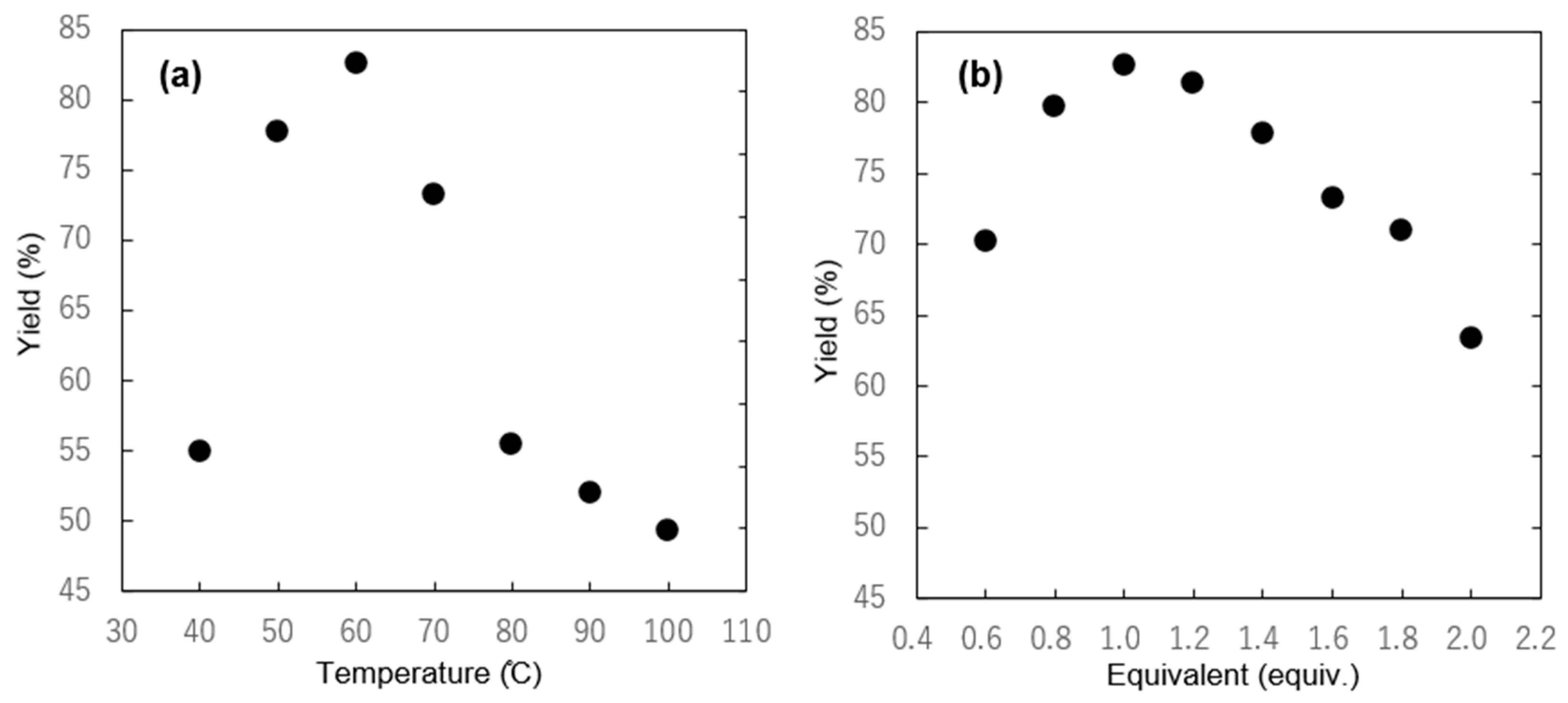
2.1. Selective Synthesis of IBA and Its Analogs
2.2. IBAs Synthesis Using Ferric Effect
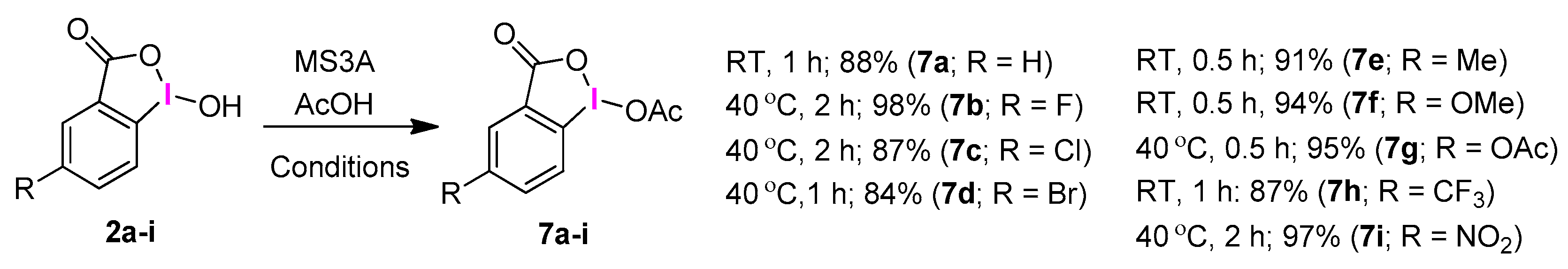
2.3. IBA Derivatives
3. Materials and Methods
3.1. General Information
3.2. Synthesis of IBA Analogues
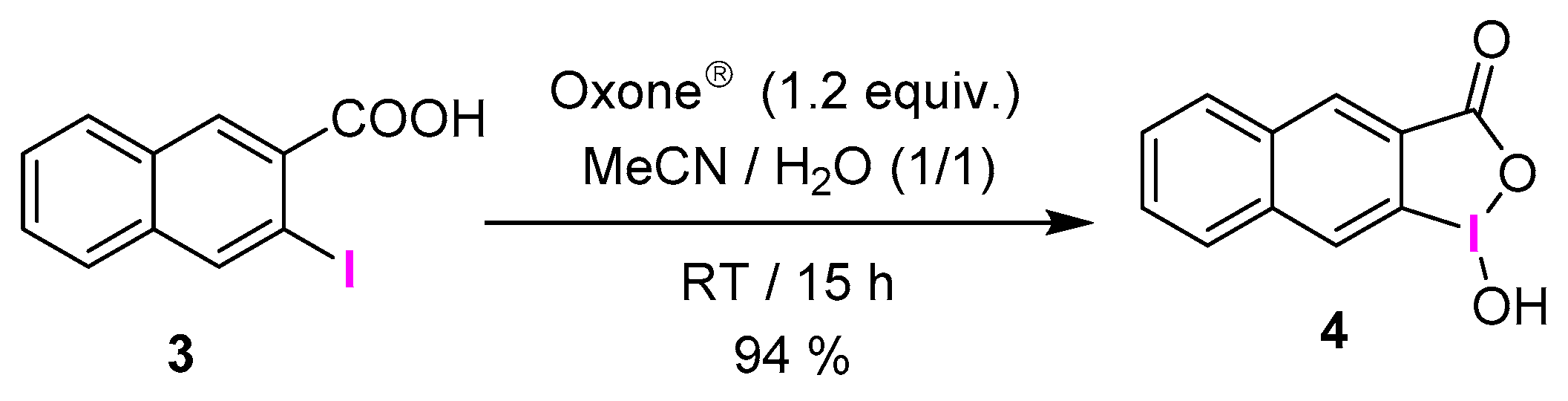
3.2.1. General Procedure for the Synthesis of IBAs 2a–v and 4
3.2.2. 1-Hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2a)
3.2.3. 5-Fluoro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2b)
3.2.4. 5-Chloro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2c)
3.2.5. 5-Bromo-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2d)
3.2.6. 1-Hydroxy-5-methyl-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2e)
3.2.7. 1-Hydroxy-5-methoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2f)
3.2.8. 1-Hydroxy-3-oxo-1,3-dihydro-1λ3-benzo[d][1,2]iodaoxol-5-yl acetate (2g)
3.2.9. 1-Hydroxy-5-(trifluoromethyl)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2h)
3.2.10. 1-Hydroxy-5-nitro-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2i)
3.2.11. 1-Hydroxy-3-oxo-1,3-dihydro-1λ3-benzo[d][1,2]iodaoxole-5-carbonitrile (2j)
3.2.12. 1,5-Dihydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2k)
3.2.13. 6-Fluoro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2l)
3.2.14. 6-Chloro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2m)
3.2.15. 6-Bromo-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2n)
3.2.16. 1-Hydroxy-6-(trifluoromethyl)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2o)
3.2.17. 1-Hydroxy-3-oxo-1,3-dihydro-1λ3-benzo[d][1,2]iodaoxole-6-carboxylic acid (2p)
3.2.18. 5,6-Difluoro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2q)
3.2.19. 1-Hydroxy-5,6-dimethoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2r)
3.2.20. 7-Bromo-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2s)
3.2.21. 1-Hydroxy-7-methyl-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2t)
3.2.22. 4-Fluoro-1-hydroxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2u)
3.2.23. 1-Hydroxy-4-methyl-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (2v)
3.2.24. 1-Hydroxy-1λ3-naphtho[2,3-d][1,2]iodaoxol-3(1H)-one (4)
3.3. Synthesis of Benziodoxole Alkoxides
3.3.1. General Procedure for the Synthesis of Benziodoxole Alkoxides (6)
3.3.2. 1-Methoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6a)
3.3.3. 1-Ethoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6b)
3.3.4. 1-(2,2,2-trifluoroethoxy)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6c)
3.3.5. 1-Propoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6d)
3.3.6. 1-Isopropoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6e)
3.3.7. 1-((1,1,1,3,3,3-hexafluoropropan-2-yl)oxy)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6f)
3.3.8. 1-Butoxy-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6g)
3.3.9. 1-(tert-Butoxy)-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one (6h)
3.4. Synthesis of Benziodoxole Acetates
3.4.1. General Procedure for the Synthesis of Benziodoxole Acetates (7)
3.4.2. 3-Oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7a)
3.4.3. 5-Fluoro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7b)
3.4.4. 5-Chloro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7c)
3.4.5. 5-Bromo-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7d)
3.4.6. 5-Methyl-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7e)
3.4.7. 5-Methoxy-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7f)
3.4.8. 3-Oxo-1λ3-benzo[d][1,2]iodaoxole-1,5(3H)-diyl diacetate (7g)
3.4.9. 3-Oxo-5-(trifluoromethyl)-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7h)
3.4.10. 5-Nitro-3-oxo-1λ3-benzo[d][1,2]iodaoxol-1(3H)-yl acetate (7i)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhdankin, V.V. Organoiodine(V) reagents in organic synthesis. J. Org. Chem. 2011, 76, 1185–1197. [Google Scholar] [CrossRef]
- Singh, F.V.; Wirth, T. Hypervalent iodine-catalyzed oxidative functionalizations including stereoselective reactions. Chem. Asian J. 2014, 9, 950–971. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Advances in synthetic applications of hypervalent iodine compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ji, W.; Wang, L. Sunlight-driven decarboxylative alkynylation of α-keto acids with bromoacetylenes by hypervalent iodine reagent catalysis: A facile approach to ynones. Angew. Chem. Int. Ed. 2015, 54, 8374–8377. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, L.; Luo, S. Enantioselective decarboxylative α-alkynylation of β-ketocarbonyls via a catalytic α-imino radical Intermediate. Org. Lett. 2017, 19, 4924–4927. [Google Scholar] [CrossRef]
- Yang, S.; Tan, H.; Ji, W.; Zhang, X.; Li, P. Visible light-induced decarboxylative acylarylation of phenyl propiolates with α-oxocarboxylic acids to coumarins catalyzed by hypervalent iodine reagents under transition metal-free conditions. Adv. Synth. Catal. 2017, 359, 443–453. [Google Scholar] [CrossRef]
- Sun, X.; Liu, T.; Yang, Y.-T.; Gu, Y.-J.; Liu, Y.W.; Ji, Y.G.; Luo, K.; Zhu, J.; Wu, L. Visible-light-promoted regio- and stereoselective oxyalkenylation of phosphinyl allenes. Adv. Synth. Catal. 2020, 362, 2701–2708. [Google Scholar] [CrossRef]
- Ball, L.T.; Lloyd-Jones, G.C.; Russell, C.A. Gold-catalysed oxyarylation of styrenes and mono- and gem-disubstituted olefins facilitated by an iodine(III) oxidant. Chem. Eur. J. 2012, 18, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Segawa, Y.; Itami, K. Pyridylidene ligand facilitates gold-catalyzed oxidative C–H arylation of heterocycles. Beilstein. J. Org. Chem. 2015, 11, 2737–2746. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lan, T.; Sun, Y.; Chen, H.; Yao, J.; Rao, Y. Unactivated C(sp3)–H hydroxylation through palladium catalysis with H2O as the oxygen source. Chem. Commun. 2015, 51, 14929–14932. [Google Scholar] [CrossRef] [PubMed]
- Bindu, V.H.; Parvathaneni, S.P.; Rao, V.J. Iodosobenzoic acid (IBA) catalysed benzylic and aromatic C–H oxidations. Catal. Lett. 2017, 147, 1434–1440. [Google Scholar] [CrossRef]
- Li, G.-X.; Morales-Rivera, C.A.; Gao, F.; Wang, Y.; He, G.; Liu, P.; Chen, G. A unified photoredox-catalysis strategy for C(sp3)–H hydroxylation and amidation using hypervalent iodine. Chem. Sci. 2017, 8, 7180–7185. [Google Scholar] [CrossRef]
- Wang, D.; Ren, R.; Zhu, C. Manganese-promoted ring-opening hydrazination of cyclobutanols: Synthesis of alkyl hydrazines. J. Org. Chem. 2016, 81, 8043–8049. [Google Scholar] [CrossRef]
- Ye, L.; Gu, Q.-S.; Tian, Y.; Meng, X.; Chen, G.-C.; Liu, X.-Y. Radical asymmetric intramolecular α-cyclopropanation of aldehydes towards bicyclo[3.1.0]hexanes containing vicinal all-carbon quaternary stereocenters. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Amos, S.G.E.; Nicolai, S.; Waser, J. Photocatalytic umpolung of N- and O-substituted alkenes for the synthesis of 1,2-amino alcohols and diols. Chem. Sci. 2020, 11, 11274–11279. [Google Scholar] [CrossRef]
- He, X.-K.; Lu, J.; Zhang, A.-J.; Zhang, Q.-Q.; Xu, G.-Y.; Xuan, J. Bi-OAc-accelerated C3-H alkylation of quinoxaline-2(1H)-ones under visible-light irradiation. Org. Lett. 2020, 22, 5984–5989. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Wu, S.; Chen, Y. Acyl radical smiles rearrangement to construct hydroxybenzophenones by photoredox catalysis. Org. Lett. 2019, 21, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, Y.; Wang, Y. Synthesis of 4-iodoisoquinolin-1(2H)-ones by a dirhodium (II)-catalyzed 1,4-bisfunctionalization of isoquinolinium iodide salts. Org. Lett. 2019, 21, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hao, Y.; Lin, Y.; Wang, Q. Visible-light-induced intramolecular sp3-C-H oxidation of 2-alkyl-substituted benzamides for the synthesis of functionalized iminoisobenzofurans. Chem. Commun. 2019, 55, 13908–13911. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gu, Q.-S.; Li, Z.-L.; Li, Z.; Guo, Y.-L.; Guo, Z.; Liu, X.-Y. Direct photocatalytic synthesis of medium-sized lactams by C−C bond cleavage. Angew. Chem. Int. Ed. 2018, 57, 14225–14229. [Google Scholar] [CrossRef] [PubMed]
- Pawar, G.G.; Robert, F.; Grau, E.; Cramail, H.; Landais, Y. Visible-light photocatalyzed oxidative decarboxylation of oxamic acids: A green route to urethanes and ureas. Chem. Commun. 2018, 54, 9337–9340. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Zhang, F.; Huang, H.; Chen, Y. Visible-light-induced alkoxyl radical generation enables selective C(sp3)–C(sp3) bond cleavage and functionalizations. J. Am. Chem. Soc. 2016, 138, 1514–1517. [Google Scholar] [CrossRef]
- Li, G.-X.; Morales-Rivera, C.A.; Wang, Y.; Gao, F.; He, G.; Liu, P.; Chen, G. Photoredox-mediated Minisci C–H alkylation of N-heteroarenes using boronic acids and hypervalent iodine. Chem. Sci. 2016, 7, 6407–6412. [Google Scholar] [CrossRef]
- Ji, W.; Tan, H.; Wang, M.; Li, P.; Wang, L. Photocatalyst-free hypervalent iodine reagent catalyzed decarboxylative acylarylation of acrylamides with α-oxocarboxylic acids driven by visible-light irradiation. Chem. Commun. 2016, 52, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.-J.; Zhang, W.-M.; Shu, Y.-J.; Sun, Y.-Y.; Xu, J.; Feng, Y.-S.; Xu, H.-J. Deboronative cyanation of potassium alkyltrifluoroborates via photoredox catalysis. Chem. Commun. 2016, 52, 6793–6796. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, G.; Chen, Y. Dual hypervalent iodine (III) reagents and photoredox catalysis enable decarboxylative ynonylation under mild conditions. Angew. Chem. Int. Ed. 2015, 54, 7872–7876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Nian, Y.; Zhou, Y.; Jiang, H.; Liu, H. Palladium-catalyzed picolinamide-directed coupling of C(sp2)–H and C(sp2)–H: A straightforward approach to quinolinone and pyridone scaffolds. Chem. Commun. 2015, 51, 7509–7511. [Google Scholar] [CrossRef]
- Shan, G.; Yang, X.; Zong, Y.; Rao, Y. An efficient palladium-catalyzed C-H alkoxylation of unactivated methylene and methyl groups with cyclic hypervalent iodine (I3+) oxidants. Angew. Chem. Int. Ed. 2013, 52, 13606–13610. [Google Scholar] [CrossRef]
- Zong, Y.; Rao, Y. Developing Pd (II) catalyzed double sp3 C–H alkoxylation for synthesis of symmetric and unsymmetric acetals. Org. Lett. 2014, 16, 5278–5281. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yao, X.; Zhang, C.; Rao, Y. Pd (ii) catalyzed ortho C–H iodination of phenylcarbamates at room temperature using cyclic hypervalent iodine reagents. Chem. Commun. 2015, 51, 10014–10017. [Google Scholar] [CrossRef]
- Yu, Q.-Y.; Zhong, H.-M.; Sun, W.-W.; Zhang, S.-J.; Cao, P.; Dong, X.-P.; Qin, H.-B.; Liu, J.-K.; Wu, B. Palladium-catalyzed C(sp3)−H functionalization at the C3 position of l-pipecolinic acid derivatives. Asian J. Org. Chem. 2016, 5, 608–612. [Google Scholar] [CrossRef]
- Yang, B.; Xu, X.-H.; Qing, F.-L. Synthesis of difluoroalkylated arenes by hydroaryldifluoromethylation of alkenes with α,α-difluoroarylacetic acids under photoredox catalysis. Org. Lett. 2016, 18, 5956–5959. [Google Scholar] [CrossRef]
- Nappi, M.; He, C.; Whitehurst, W.G.; Chappell, B.G.N.; Gaunt, M.J. Selective reductive elimination at alkyl palladium(IV) by dissociative ligand ionization: Catalytic C(sp3)−H amination to azetidines. Angew. Chem. Int. Ed. 2018, 57, 3178–3182. [Google Scholar] [CrossRef] [PubMed]
- Muraki, T.; Togo, H.; Yokoyama, M. Synthetic use of 1-(p-toluenesulfonyloxy)-1,2-benziodoxol-3(1H)-one: Iodination of aromatic rings. Synlett 1998, 3, 286–288. [Google Scholar] [CrossRef]
- Muraki, T.; Togo, H.; Yokoyama, M. Reactivity and synthetic utility of 1-(arenesulfonyloxy)benziodoxolones. J. Org. Chem. 1999, 64, 2883–2889. [Google Scholar] [CrossRef]
- Harper, M.J.; Emmett, E.J.; Bower, J.F.; Russell, C.A. Oxidative 1,2-difunctionalization of ethylene via gold-catalyzed oxyarylation. J. Am. Chem. Soc. 2017, 139, 12386–12389. [Google Scholar] [CrossRef]
- Yoshimura, A.; Nguyen, K.C.; Klasen, S.C.; Postnikov, P.S.; Yusubov, M.S.; Saito, A.; Nemykin, V.N.; Zhdankin, V.V. Hypervalent iodine-catalyzed synthesis of 1,2,4-oxadiazoles from aldoximes and nitriles. Asian J. Org. Chem. 2016, 5, 1128–1133. [Google Scholar] [CrossRef]
- Vinayak, B.; Ravindrakumar, P.V.; Ramana, D.V.; Chandrasekharam, M. Revisiting 1-chloro-1,2-benziodoxol-3-one: Efficient ortho-chlorination of aryls under aqueous conditions. New J. Chem. 2018, 42, 8953–8959. [Google Scholar] [CrossRef]
- Parvathaneni, S.P.; Perumgani, P.C. Regioselective chlorination of aryl C−H bonds with hypervalent iodine(III) reagent 1-chloro-1,2-benziodoxol-3-one. Asian J. Org. Chem. 2018, 7, 324–327. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, L.; Zhang, H.; Zheng, Y.; Jiang, Z.-X.; Yang, Z. Electrophilic chloro(ω-alkoxy)lation of alkenes employing 1-chloro-1,2-benziodoxol-3-one: Facile synthesis of β-chloroethers. Org. Biomol. Chem. 2018, 16, 7203–7213. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Wang, T.; Wang, C.; Xue, D.; Xiao, J. Story of an age-old reagent: An electrophilic chlorination of arenes and heterocycles by 1-chloro-1,2-benziodoxol-3-one. Org. Lett. 2016, 18, 1976–1979. [Google Scholar] [CrossRef] [PubMed]
- Egami, H.; Yoneda, T.; Uku, M.; Ide, T.; Kawato, Y.; Hamashima, Y. Difunctionalization of alkenes using 1-chloro-1,2-benziodoxol-3-(1H)-one. J. Org. Chem. 2016, 81, 4020–4030. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Ni, C.; Hu, J. Hypervalent iodine(III)-catalyzed Balz–Schiemann fluorination under mild conditions. Angew. Chem. Int. Ed. 2018, 57, 9896–9900. [Google Scholar] [CrossRef]
- Jiang, X.; Zheng, C.; Lei, L.; Lin, K.; Yu, C. Synthesis of 2-oxindoles from substituted indoles by hypervalent-iodine oxidation. Eur. J. Org. Chem. 2018, 2018, 1437–1442. [Google Scholar] [CrossRef]
- Jiang, X.; Li, G.; Yu, C. A Diels-Alder reaction/oxa-Michael addition/acyloin rearrangement cascade on tropolonic substrates. Tetrahedron Lett. 2018, 59, 1506–1510. [Google Scholar] [CrossRef]
- Vaillant, F.L.; Wodrich, M.D.; Waser, J. Room temperature decarboxylative cyanation of carboxylic acids using photoredox catalysis and cyanobenziodoxolones: A divergent mechanism compared to alkynylation. Chem. Sci. 2017, 8, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, X.; Hou, C.; Chen, P.; Liu, G. Palladium(II)-catalyzed enantioselective azidation of unactivated alkenes. Angew. Chem. Int. Ed. 2020, 59, 17239–17244. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Zhao, J.; Sun, M.; You, H.; Fang, F.; Li, Z.-Q. Visible-light-promoted site-specific and diverse functionalization of a C(sp3)-C(sp3) bond adjacent to an arene. ACS Catal. 2020, 10, 6603–6612. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Morales-Rivera, C.A.; Li, G.-X.; Huang, X.; He, G.; Liu, P.; Chen, G. Epimerization of tertiary carbon centers via reversible radical cleavage of unactivated C(sp3)-H bonds. J. Am. Chem. Soc. 2018, 140, 9678–9684. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Q. Copper-catalyzed alkene aminoazidation as a rapid entry to 1,2-diamines and installation of an azide reporter onto azaheterocycles. J. Am. Chem. Soc. 2017, 139, 13110–13116. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.-L.; Wang, F.-L.; Guo, Z.; Cheng, Y.-F.; Wang, N.; Dong, X.-W.; Fang, C.; Liu, J.; Hou, C.; et al. Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings. Nat. Commun. 2016, 7, 13852. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.-X.; Yang, G.; He, G.; Chen, G. A visible-light-promoted radical reaction system for azidation and halogenation of tertiary aliphatic C–H bonds. Chem. Sci. 2016, 7, 2679–2683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hartwig, J.F. Metal-catalyzed azidation of tertiary C-H bonds suitable for late-stage functionalization. Nature 2015, 600–604. [Google Scholar] [CrossRef]
- Fumagalli, G.; Rabet, P.T.G.; Boyd, S.; Greaney, M.F. Three-component azidation of styrene-type double bonds: Light-switchable behavior of a copper photoredox catalyst. Angew. Chem. Int. Ed. 2015, 54, 11481–11484. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhu, J.; Sun, X.; Cheng, L.; Wu, L. I2/TBHP mediated divergent C(sp3)-P cleavage of allenylphosphine oxides: Substituent-controlled regioselectivity. Adv. Synth. Catal. 2019, 361, 3532–3537. [Google Scholar] [CrossRef]
- Jiang, H.; He, Y.; Cheng, Y.; Yu, S. Radical alkynyltrifluoromethylation of alkenes initiated by an electron donor–acceptor complex. Org. Lett. 2017, 19, 1240–1243. [Google Scholar] [CrossRef]
- Mangaonkar, S.R.; Kole, P.B.; Singh, F.V. Oxidation of organosulfides to organosulfones with trifluoromethyl 3-oxo-1λ3,2-benziodoxole-1(3H)-carboxylate as an oxidant. Synlett 2018, 29, 199–202. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Q. Copper-catalyzed aminoalkynylation of alkenes with hypervalent iodine reagents. Chem. Sci. 2017, 8, 8265–8270. [Google Scholar] [CrossRef]
- Mukherjee, S.; Garza-Sauchez, R.A.; Tlahuext-Aca, A.; Glorius, F. Alkynylation of Csp2(O)-H bonds enabled by photoredox-mediated hydrogen-atom transfer. Angew. Chem. Int. Ed. 2017, 56, 14723–14726. [Google Scholar] [CrossRef]
- Nicolai, S.; Waser, J. Pd(0)-catalyzed oxy- and aminoalkynylation of olefins for the synthesis of tetrahydrofurans and pyrrolidines. Org. Lett. 2011, 13, 6324–6327. [Google Scholar] [CrossRef]
- Brand, J.P.; Waser, J. Direct alkynylation of thiophenes: Cooperative activation of TIPS–EBX with gold and Brønsted acids. Angew. Chem. Int. Ed. 2010, 49, 7304–7307. [Google Scholar] [CrossRef] [PubMed]
- González, D.F.; Brand, J.P.; Waser, J. Asymmetric synthesis of 4-amino-4H-chromenes by organocatalytic oxa-Michael/aza-Baylis–Hillman tandem reactions. Chem. Eur. J. 2010, 16, 9457–9461. [Google Scholar]
- Baker, G.P.; Mann, G.; Sheppard, N.; Tetlow, A.J. The structure of o-iodosobenzoic acid and of certain derivatives. J. Chem. Soc. 1965, 3721–3728. [Google Scholar] [CrossRef]
- Kraszkiewicz, L.; Skulski, L. Optimized syntheses of iodylarenes from iodoarenes, with sodium periodate as the oxidant. Part II. Arkivoc 2003, 6, 120–125. [Google Scholar] [CrossRef]
- Bosset, C.; Coffinier, R.; Peioto, P.A.; Assal, M.E.; Miqueu, K.; Sotiropoulos, M.; Pouységu, L.; Quideau, S. Asymmetric hydroxylative phenol dearomatization promoted by chiral binaphthylic and biphenylic iodanes. Angew. Chem. Int. Ed. 2014, 53, 9860–9864. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.; Hyun, S.-M.; Powers, D.C. Oxidase catalysis via aerobically generated hypervalent iodine intermediates. Nat. Chem. 2018, 10, 200–204. [Google Scholar] [CrossRef]
- Chandrasekar, S.; Sekar, G. An efficient synthesis of iminoquinones by a chemoselective domino ortho-hydroxylation/oxidation/imidation sequence of 2-aminoarylketones. Org. Biomol. Chem. 2016, 14, 3053–3060. [Google Scholar] [CrossRef]
- Wirth, T. IBX—New reactions with an old reagent. Angew. Chem. Int. Ed. 2001, 40, 2812–2814. [Google Scholar] [CrossRef]
- Ladziata, U.; Zhdankin, V.V. Hypervalent iodine(V) reagents in organic synthesis. Arkivoc 2006, 9, 26–58. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, N.; Pareek, A.; Kishore, D. An insight in to general features of IBX (2-iodoxybenzoic acid). Sci. Revs. Chem. Commun. 2013, 3, 16–42. [Google Scholar]
- Plumb, J.B.; Harper, D.J. IBX is reported to be a shock-sensitive explosive. Chem. Eng. News 1990, 68, 3. [Google Scholar]
- Dess, D.B.; Martin, J.C.J. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Boeckmann, R.K.; Shao, P.; Mullins, J.J. The Dess-Martin periodinane: 1,1,1-triacetoxy-1,1-dihydro-1,2-benziodoxolo-3(1H)-one. Org. Synth. 2000, 77, 141–152. [Google Scholar]
- More, J.D.; Finney, N.S. A simple and advantageous protocol for the oxidation of alcohols with o-iodoxybenzoic acid (IBX). Org. Lett. 2002, 4, 3001–3003. [Google Scholar] [CrossRef]
- Tiffner, M.; Stockhammer, L.; Schörgenhumer, J.; Röser, K.; Waser, M. Towards an asymmetric organocatalytic α-azidation of β-ketoesters. Molecules 2018, 23, 1142. [Google Scholar] [CrossRef]
- Bernini, R.; Barontini, M.; Spatafora, C. New lipophilic piceatannol derivatives exhibiting antioxidant activity prepared by aromatic hydroxylation with 2-iodoxybenzoic acid (IBX). Molecules 2009, 14, 4669–4681. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Ahmed, I. Journey describing applications of oxone in synthetic chemistry. Chem. Rev. 2013, 113, 3329–3371. [Google Scholar] [CrossRef]
- Thottumkara, A.P.; Bowsher, M.S.; Vinod, T.K. In situ generation of o-iodoxybenzoic acid (IBX) and the catalytic use of it in oxidation reactions in the presence of oxone as a co-oxidant. Org. Lett. 2005, 7, 2933–2936. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, M.; Akakura, M.; Ishihara, K. 2-Iodoxybenzenesulfonic acid as an extremely active catalyst for the selective oxidation of alcohols to aldehydes, ketones, carboxylic acids, and enones with oxone. J. Am. Chem. Soc. 2009, 131, 251–262. [Google Scholar] [CrossRef]
- Miura, T.; Nakashima, K.; Tada, N.; Itoh, A. An effective and catalytic oxidation using recyclable fluorous IBX. Chem. Commun. 2011, 47, 1875–1877. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Senapati, K.; Parida, K.N.; Jhulki, S.; Sooraj, K.; Nair, N.N. Twist does a twist to the reactivity: Stoichiometric and catalytic oxidations with twisted tetramethyl-IBX. J. Org. Chem. 2011, 76, 9593–9601. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Uyanik, M.; Crockett, R. 2-Iodoxy-5-methylbenzenesulfonic acid-catalyzed selective oxidation of 4-bromobenzyl alcohol to 4-bromobenzaldehyde or 4-bromobenzoic acid with oxone. Org. Synth. 2012, 89, 105–114. [Google Scholar]
- Bikshapathi, R.; Prathima, P.S.; Rao, V.J. Hypervalent iodine catalysis for selective oxidation of Baylis–Hillman adducts via in situ generation of o-iodoxybenzoic acid (IBX) from 2-iodosobenzoic acid (IBA) in the presence of oxone. New J. Chem. 2016, 40, 10300–10304. [Google Scholar] [CrossRef]
- Yakura, T.; Fujiwara, T.; Yamada, A.; Nambu, H. 2-Iodo-N-isopropyl-5-methoxybenzamide as a highly reactive and environmentally benign catalyst for alcohol oxidation. Beilstein J. Org. Chem. 2018, 14, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Moorthy, J.N. Mechanochemical catalytic oxidations in the solid state with in situ-generated modified IBX from 3,5-di-tert-butyl-2-iodobenzoic acid (DTB-IA)/Oxone. Org. Chem. Front. 2017, 4, 343–349. [Google Scholar] [CrossRef]
- Jhulki, S.; Seth, S.; Mondal, M.; Moorthy, J.N. Facile organocatalytic domino oxidation of diols to lactones by in situ-generated TetMe-IBX. Tetrahedron 2014, 70, 2286–2293. [Google Scholar] [CrossRef]
- Yakura, T.; Horiuchi, Y.; Nishimura, Y.; Yamada, A.; Nambu, H.; Fujiwara, T. Efficient oxidative cleavage of tetrahydrofuran-2-methanols to γ-lactones by a 2-iodobenzamide catalyst in combination with Oxone®. Adv. Synth. Catal. 2016, 358, 869–873. [Google Scholar] [CrossRef]
- Yakura, T.; Yamauchi, Y.; Tian, Y.; Omoto, M. Catalytic hypervalent iodine oxidation of p-dialkoxybenzenes to p-quinones using 4-iodophenoxyacetic acid and Oxone®. Chem. Pharm. Bull. 2008, 56, 1632–1634. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Tian, Y.; Yamauchi, Y.; Omoto, M.; Konishi, T. Catalytic hypervalent iodine oxidation using 4-iodophenoxyacetic acid and Oxone®: Oxidation of p-alkoxyphenols to p-benzoquinones. Chem. Pharm. Bull. 2009, 57, 252–256. [Google Scholar] [CrossRef]
- Zagulyaeva, A.A.; Banek, C.T.; Yusubov, M.S.; Zhdankin, V.V. Hofmann rearrangement of carboxamides mediated by hypervalent iodine species generated in situ from iodobenzene and oxone: Reaction scope and limitations. Org. Lett. 2010, 12, 4644–4647. [Google Scholar] [CrossRef] [PubMed]
- Yakura, T.; Omoto, M.; Yamauchi, Y.; Tian, Y.; Ozono, A. Hypervalent iodine oxidation of phenol derivatives using a catalytic amount of 4-iodophenoxyacetic acid and Oxone® as a co-oxidant. Tetrahedron 2010, 66, 5833–5840. [Google Scholar] [CrossRef]
- Ren, J.; Lu, L.; Xu, J.; Yu, T.; Zeng, B.-B. Selective oxidation of 1-tetralones to 1,2-naphthoquinones with IBX and to 1,4-naphthoquinones with Oxone® and 2-iodobenzoic acid. Synthesis 2015, 47, 2270–2280. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S. A user-friendly entry to 2-iodoxybenzoic acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- China, H.; Tanihara, K.; Sasa, H.; Kikushima, K.; Dohi, T. Regiodivergent oxidation of alkoxyarenes by hypervalent iodine/oxone® system. Catal. Today 2020, 348, 2–8. [Google Scholar] [CrossRef]
- Parida, K.N.; Moorthy, J.N. Synthesis of o-carboxyarylacrylic acids by room temperature oxidative cleavage of hydroxynaphthalenes and higher aromatics with Oxone. J. Org. Chem. 2015, 80, 8354–8360. [Google Scholar] [CrossRef]
- Costello, J.P.; Ferreira, E.M. Regioselectivity influences in platinum-catalyzed intramolecular alkyne O-H and N-H additions. Org. Lett. 2019, 21, 9934–9939. [Google Scholar] [CrossRef]
- Koguchi, S.; Mihoya, A.; Mimura, M. Alcohol oxidation via recyclable hydrophobic ionic liquid-supported IBX. Tetrahedron 2016, 72, 7633–7637. [Google Scholar] [CrossRef]
- Boelke, A.; Kuczmera, T.J.; Caspers, L.D.; Lork, E.; Nachtsheim, B.J. Iodolopyrazolium salts: Synthesis, derivatizations, and applications. Org. Lett. 2020, 22, 7261–7266. [Google Scholar] [CrossRef]
- Kalaj, M.; Mimeni, M.R.; Bentz, K.C.; Barcus, K.S.; Palomba, J.M.; Paesani, F.; Cohen, S.M. Halogen bonding in UiO-66 frameworks promotes superior chemical warfare agent simulant degradation. Chem. Commun. 2019, 55, 3481–3484. [Google Scholar] [CrossRef]
- Sun, W.; Cama, L.D. Preparation of 6H-benzo[c]chromenes as estrogen receptor modulators. PCT Int. Appl. 2004, WO 2004073612, A2. [Google Scholar]
- Caspers, L.D.; Spils, J.; Damrath, M.; Lork, E.; Nachtsheim, B.J. One-pot synthesis and conformational analysis of six-membered cyclic iodonium salts. J. Org. Chem. 2020, 85, 9161–9178. [Google Scholar] [CrossRef] [PubMed]
- Miles, K.C.; Le, C.C.; Stambuli, J.P. Direct carbocyclizations of benzoic acids: Catalyst-controlled synthesis of cyclic ketones and the development of tandem aHH (acyl Heck-Heck) reactions. Chem. Eur. J. 2014, 20, 11336–11339. [Google Scholar] [CrossRef]
- Hamley, P.; Pimm, A.; Tinker, A. Preparation of spiro[pyridoisoindolequinazoline] derivatives and analogs as nitric oxide synthase inhibitors. PCT Int. Appl. 1999, WO 9901455, A1. [Google Scholar]
- Declas, N.; Vaillant, F.L.; Waser, J. Revisiting the Urech synthesis of hydantoins: Direct access to enantiopure 1,5-substituted hydantoins using cyanobenziodoxolone. Org. Lett. 2019, 21, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Bertho, S.; Rey-Rodriguez, R.; Colas, C.; Retailleau, P.; Gillaizeau, I. Regio- and stereoselective iron-catalyzed oxyazidation of enamides using a hypervalent iodine reagent. Chem. Eur. J. 2017, 23, 17674–17677. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wu, J.; Yoshikai, N. Palladium-catalyzed condensation of N-aryl imines and alkynylbenziodoxolones to form multisubstituted furans. J. Am. Chem. Soc. 2014, 136, 11598–11601. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Z.-T.; Zheng, Q.-Y. Organic base-promoted enantioselective electrophilic cyanation of β-keto esters by using chiral phase-transfer catalysts. Org. Biomol. Chem. 2015, 13, 8812–8816. [Google Scholar] [CrossRef]
- Hari, D.P.; Waser, J. Enantioselective copper-catalyzed oxy-alkynylation of diazo compounds. J. Am. Chem. Soc. 2017, 139, 8420–8423. [Google Scholar] [CrossRef]
- Mocci, F.; Uccheddu, G.; Frongia, A.; Cerioni, G. Solution structure of some λ3 iodanes: An 17O NMR and DFT study. J. Org. Chem. 2007, 72, 4163–4168. [Google Scholar] [CrossRef]
- Ito, E.; Fukushima, T.; Kawakami, T.; Murakami, K.; Itami, K. Catalytic dehydrogenative C–H imidation of arenes enabled by photo-generated hole donation to sulfonimide. Chem 2017, 2, 383–392. [Google Scholar] [CrossRef]
- Jia, K.; Pan, Y.; Chen, Y. Selective carbonyl-C (sp3) bond cleavage to construct ynamides, ynoates, and ynones by photoredox catalysis. Angew. Chem. Int. Ed. 2017, 56, 2478–2481. [Google Scholar] [CrossRef]
- Iinuma, M.; Moriyama, K.; Togo, H. Oxidation of alcohols to aldehydes or ketones with 1-acetoxy-1,2-benziodoxole-3(1H)-one derivatives. Eur. J. Org. Chem. 2014, 2014, 772–780. [Google Scholar] [CrossRef]



Sample Availability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
China, H.; Kageyama, N.; Yatabe, H.; Takenaga, N.; Dohi, T. Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions. Molecules 2021, 26, 1897. https://doi.org/10.3390/molecules26071897
China H, Kageyama N, Yatabe H, Takenaga N, Dohi T. Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions. Molecules. 2021; 26(7):1897. https://doi.org/10.3390/molecules26071897
Chicago/Turabian StyleChina, Hideyasu, Nami Kageyama, Hotaka Yatabe, Naoko Takenaga, and Toshifumi Dohi. 2021. "Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions" Molecules 26, no. 7: 1897. https://doi.org/10.3390/molecules26071897
APA StyleChina, H., Kageyama, N., Yatabe, H., Takenaga, N., & Dohi, T. (2021). Practical Synthesis of 2-Iodosobenzoic Acid (IBA) without Contamination by Hazardous 2-Iodoxybenzoic Acid (IBX) under Mild Conditions. Molecules, 26(7), 1897. https://doi.org/10.3390/molecules26071897






