Abstract
Carpesium cernuum L., one of the two Carpesium species occurring in Europe, in the Far East and India, found use as a vegetable and a traditional medicinal remedy for several ailments. In the present study, compositions of essential oils distilled from roots and shoots of C. cernuum plants, cultivated in the open field, have been studied by GC-MS-FID supported by NMR spectroscopy. The analyses led to the identification of 120 compounds in total, of which 115 were found in aerial parts and 37 in roots of the plants. The major constituents found in the oil from shoots were: α-pinene (35%) and 2,5-dimethoxy-p-cymene (thymohydroquinone dimethyl ether, 12%), whereas 2,5-dimethoxy-p-cymene (55%), thymyl isobutyrate (9%) and thymol methyl ether (8%) predominated in the essential oil obtained from the roots. Antibacterial and cytotoxic activities of the essential oils distilled from C. cernuum were also tested. The essential oil from aerial parts of the plant demonstrated good inhibitory activity against Staphylococcus aureus ATCC 29213 and Escherichia coli ATCC 25922 (MIC: 15.6 μL/mL).
1. Introduction
Although plants of the genus Carpesium (Compositae, subtribe: Inuleae-Inulinae), have a long history of use as traditional herbal remedies and seasonal food in the Far East [1,2,3], they have no tradition of medicinal use in Europe. Majority of the species that belong to the genus are native to China, Japan and Korea, and some are endemic to China. Carpesium cernuum L. is a species native to Eurasia and is currently distributed in Europe, Asia and Australia [4,5,6,7]. The plant is a perennial herb, 20–80 cm tall, with an erect branched stem and solitary capitula (15–18 mm), without ray florets, which are subtended by many linear-lanceolate leaves. C. cernuum inhabits waste fields and mountain slopes below 3000 m [4,5]. The whole plant is medicinally utilized [1]. Recently, anti-inflammatory and a tumor migration inhibitory effects of extracts from C. cernuum have been described [8,9].
As a member of the Inuleae-Inulinae subtribe, the genus Carpesium is a close relative to such essential oil-bearing plants as Inula helenium L. and Telekia speciosa (Schreb.) Baumg. [10]. Although many studies on isolation of mono- and sesquiterpenoids from C. cernuum have been published before [1,11,12,13], data on composition of essential oils produced by the plant are not available. Essential oil from the herb of Carpesium abrotanoides L. was shown to induce apoptosis in hepatocellular carcinoma cells in vitro [14]. However, only 16 components of the examined oil were identified, and the established composition was significantly different from that described earlier [15]. A detailed analysis of essential oils from roots and aerial parts of Carpesium divaricatum Sieb. & Zucc. has been published recently [16], revealing the presence of numerous thymol derivatives, especially in the oil from roots of the plant.
The objective of the present study was to examine hitherto unknown chemical composition and biological activity of essential oils from roots and aerial parts of C. cernuum of European origin. Antibacterial activity of the essential oils against both Gram-positive and Gram-negative bacteria has been assessed, as well as cytotoxic activity towards normal and cancer cell lines.
2. Results
Though yields of the essential oil produced by aerial parts of C. cernuum were low (0.015 ± 0.002%; see Table 1), 115 components of the oil, distilled from shoots of the plant collected at the beginning of flowering time, were identified by GC-MS. The identification was supported by NMR spectra analysis of the corresponding components isolated from the C. cernuum root essential oil. The identified compounds constituted 97.7% of the analyzed oil. Three compounds: α-pinene—the predominant constituent (35%, 3), 2,5-dimethoxy-p-cymene (thymohydroquinone dimethyl ether, 12%, 80) and linalool (4%, 30) (see Figure 1) made up about 50% of the essential oil distilled from aerial parts of the plant. Eleven structurally diverse thymol derivatives (55, 80, 88, 89, 114, 115, 120, 121, 126, 127 and 129) composed about 20% of the oil. Structures of six constituents of the essential oil remained unknown. The constituents could not be isolated and spectroscopically analyzed due to their minute amounts in the plant material.

Table 1.
Chemical composition of essential oils from aerial parts and roots of Carpesium cernuum L.
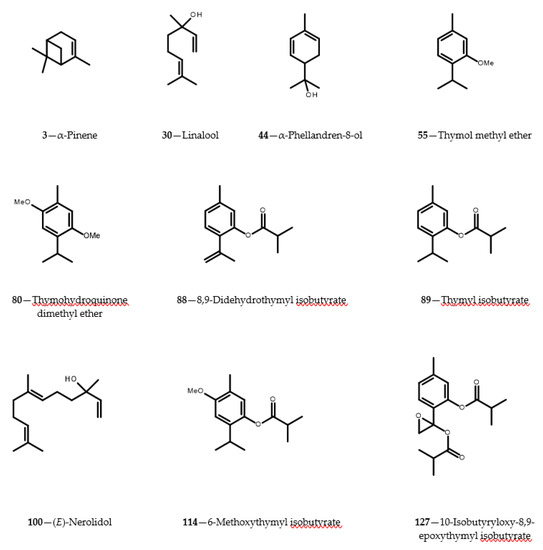
Figure 1.
Structures of selected constituents (contents >3%) identified in essential oils from Carpesium cernuum.
Yield of the essential oil obtained from roots of C. cernuum was much higher than that from the aerial parts of the plant (0.170 ± 0.006%). In contrast to aerial parts, only 37 identified constituents made up nearly 99% of the root essential oil. The main compound found in the oil was 2,5-dimethoxy-p-cymene (80; c. 55%). Together with other thymol derivatives (55, 88, 89, 114, 115, 120, 121, 123, 126, 127 and 129) it accounted for about 88% of the oil. Structure of one component of the root essential oil remained unresolved.
Antibacterial activities of the essential oils from aerial parts and from roots of C. cernuum were tested against the standard bacterial lines, both Gram-positive (Staphylococcus aureus, Enterococcus faecalis) and Gram-negative (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Serratia marcescens, Acinetobacter baumanii). Thymol, a monoterpenoid being found in numerous essential oils and having well-documented antimicrobial activity [17], was used as a reference compound. In general, the essential oil distilled from the aerial parts of C. cernuum demonstrated better bacteriostatic activity than that obtained from roots (see Table 2). A. baumanii ATCC 19606 and S. aureus ATCC 29213 were the most susceptible to both the examined essential oils and the thymol solution. Minimal inhibitory concentrations (MICs) against S. aureus were determined as 15.6 and 62.5 µL/mL for essential oils from C. cernuum aerial parts and roots, respectively. Growth of S. marcescens ATCC 13880 was inhibited only by the highest concentrations of the essential oils tested (MIC ≥ 250 µL/mL).

Table 2.
Antibacterial activities of essential oils from roots (REO) and aerial parts (APEO) of Carpesium cernuum L.
To determine whether the examined essential oils are toxic to human skin, normal skin fibroblasts and keratinocytes were treated with increasing doses (12.5–400 nL/mL) of the C. cernuum essential oils for 48 h. In parallel, the experiments with human melanoma cells lines A375 and C32 were performed, to assess potential anti-cancer activity and selectivity of the cytotoxic effect. Cell viabilities were estimated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [18] (Figure 2), and the half-maximum inhibitory concentration (IC50) values of the essential oils and thymol towards the individual cell lines were calculated (Table 3). Our results indicated that toxicities of both root and aerial parts’ essential oils were almost equal. Moreover, toxic effects of the essential oils were slightly less pronounced than toxicity of thymol used as a reference agent. However, for both essential oils and thymol, no significant difference was found between toxic effect against normal and cancer cells.
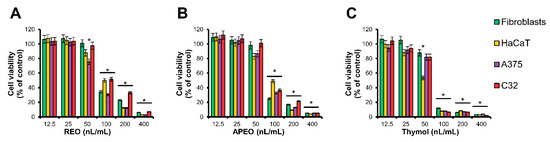
Figure 2.
Cell viabilities of human skin fibroblasts, HaCaT keratinocytes and human melanoma cell lines A375 and C32, treated with different concentrations of C. cernuum essential oils and thymol for 48 h: (A) Essential oil from roots (REO), (B) essential oil from aerial parts (APEO) and (C) thymol. Data are presented as mean ± standard error of the mean (SEM) from three independent experiments. * p < 0.05 compared to control group.

Table 3.
The half-maximum inhibitory concentration (IC50) values (nL/mL) calculated for the essential oils from roots (REO) and aerial parts (APEO) of Carpesium cernuum L. and thymol towards the human skin fibroblasts, keratinocytes (HaCaT) and melanoma cell lines (A375, C32).
3. Discussion
The tribe Inuleae of the Compositae (Asteraceae) comprises 62 genera of flowering plants divided into the two subtribes: Inuleae-Inulinae and Inuleae-Plucheinae [19]. Blumea spp., Pulicaria spp., Dittrichia spp. and Inula spp. have been the most frequently studied taxa of the Inuleae-Inulinae in respect of the production, composition and biological activity of the essential oils. The genera Carpesium and Telekia, though belonging to the same clade as Inula, are much less investigated. Only three papers on essential oils from Carpesium spp. have been published to date [14,15,16]. The two concerning C. abrotanoides [14,15] provided inconsistent data on the composition of the essential oils from the herb. This might be either due to different provenience of the plant material or due to different experimental procedures applied. In total, 16 and 44 components of the oil were identified with, respectively, eudesma-5,11(13)-dien-8,12-olide and β-bisabolene as major constituents. Neither thymol nor its derivatives were mentioned as components of the C. abrotanoides essential oils. As a result of biological activity testing, induction of apoptosis in human hepatocellular carcinoma cells (HepG2) by C. abrotanoides essential oil was experimentally proven [14].
The study on composition of essential oils obtained from roots and aerial parts of C. divaricatum cultivated in Poland [16] revealed marked differences in number and chemical structure of the compounds making up the two kinds of oil. Likewise, in C. cernuum, the main constituent of the essential oil from the aerial parts of C. divaricatum was α-pinene (40%, 3). Thymol and its nine derivatives, including thymohydroqinone dimethyl ether (80, 2%), constituted nearly 5% of the oil. The content of thymol derivatives in the essential oil obtained from aerial parts of C. cernuum (APEO) was much higher (20%) than that found in C. divaricatum. Thymohydroquinone dimethyl ether was the most abundant thymol derivative present in the oil from aerial parts of the plant. Similar to C. divaricatum, roots of C. cernuum were a much better source of the essential oil than aerial parts. Likewise, the essential oils from the aerial parts, also the root essential oils of C. cernuum and root oil from C. divaricatum, differed in respect of the thymol derivatives content. Essential oil from roots of C. cernuum (REO) contained about 88% of the compounds with thymohydroquinone dimethyl ether as a main constituent (80, 55% of the oil), whereas thymol derivatives constituted about 61% of C. divaricatum root essential oil with 10-isobutyryloxy-8,9-epoxythymyl isobutyrate as a major component (127, 29%). Telekia speciosa (Schreb.) Baumg., the closest relative of Carpesium spp., produced essential oils rich in (E,E)-farnesol and (E)-nerolidol (leaves) or in isoalantolactone (flowers, roots). However, 10-isobutyryloxy-8,9-epoxythymyl isobutyrate (127) constituted 20% of the essential oil distilled from the flowers of the plant [20].
Essential oils with a high content of 2,5-dimethoxy-p-cymene are not rare within the Compositae. The compound was identified as a major component of essential oils distilled from plants representing different species of the Inuleae tribe (see Table 4).

Table 4.
Contents of 2,5-dimethoxy-p-cymene in essential oils (EOs) from selected species of the Inuleae.
2,5-Dimethoxy-p-cymene was also the main constituent of some essential oils from plants of the Eupatorieae tribe (Compositae) [31,32,33,34,35,36], especially, fresh leaves of Ayapana triplinervis (Vahl) R.M. King & H. Robinson (syn.: Eupatorium luzoniense Llanos, E. triplinerve Vahl) collected in Reunion Island, from which an essential oil was obtained containing 90% of the compound [35]. Recently, thymohydroquinone dimethyl ether from A. triplinervis has been proven to be active against Zika virus in non-cytotoxic doses [36]. Rhizomes and roots as well as achenes of a popular European medicinal plant Arnica montana L. (tribe Madieae of the Compositae) produced essential oils with a high content of thymohydroquinone dimethyl ether (20–60%), accompanied by smaller amounts of thymol methyl ether (4–27%), that induced apoptosis in cancer cells in vitro [37,38,39]. Roots of Cyathocline purpurea (D. Don) O. Ktze (Compositae), a rare Indian medicinal plant, contained essential oil with 2,5-dimethoxy-p-cymene as a major constituent (57%). The oil demonstrated bactericidal activity against Gram-positive bacteria [40]. Similar composition of essential oils could also be spotted in some species outside of the Compositae family, e.g., in Cyclospermum leptophyllum (Pers.) Sprague ex Britton and P. Wilson (Apiaceae) and Aloe debrana Christian (Xanthorrhoeaceae) [41,42].
The development of new antibiotics remains an ongoing challenge. Plant essential oils, and some individual compounds contained in them, may display good antimicrobial activity themselves or they can act synergistically with the antibiotics currently used in the therapy [43]. The essential oils distilled from dried roots and aerial parts of C. cernuum were active against both Gram-positive and Gram-negative bacteria, though their activity in comparison with that of thymol, used as a reference compound, was low. The determined MICs (see Table 2) were lower in the case of the essential oil obtained from the aerial parts of the plant. Thus, the simple positive correlation between thymol derivatives content or 2,5-dimethoxy-p-cymene content and the antibacterial activity of the oil should be excluded. It is worth to note that the activity of C. cernuum APEO against the standard line of E. coli is equal to that against S. aureus, a Gram-positive bacterium. C. cernuum REO was less active against E. coli which conforms to literature data on antibacterial activity of 2,5-dimethoxy-p-cymene-rich essential oils [29,40]. That activity is usually less pronounced against Gram-negative bacteria. The direct comparison of MICs determined for C. cermuum REO and APEO with the results obtained by the other research teams is difficult due to differences in the laboratory standards applied.
Antiseptic activity of compounds or essential oils should be associated with the lack of toxicity against human cells. Therefore, cytotoxicity of C. cernuum essential oils was tested in vitro using keratinocytes and fibroblasts derived from the human skin. Our data showed higher safety of the examined essential oils over thymol. However, the essential oils from C. cernuum were cytotoxic to the investigated cells in concentrations much lower (IC50: 0.072–0.107 µL/mL; Table 3) than those inhibiting bacterial growth (MIC: 12–250 µL/mL; Table 2). Finally, essential oils were tested in melanoma cell lines to assess anticancer activity. Unlike the essential oil from C. abrotanoides [14] and essential oils from A. montana [38,39], that demonstrated selective cytotoxicity against the cancer cells, C. cernuum REO and APEO did not show selective cytotoxicity towards the cancer cell lines used in this study. Cytotoxic activity of thymol was slightly higher than those of the tested C. cernuum oils. This result agrees with the previous study [44], indicating moderate toxicity of thymol towards a melanoma cell line. Selectivity of the cytotoxic effect in experiments using other types of cells could not be excluded and is worth further studies.
4. Materials and Methods
4.1. General Experimental Procedures
GC-MS-FID analyses of essential oils and their fractions were performed on a Trace GC Ultra Gas Chromatograph coupled with a DSQII mass spectrometer (Thermo Electron, Waltham, MA, USA). Simultaneous GC-FID and GC-MS analyses were performed using a MS-FID splitter (SGE Analytical Science, Ringwood, VIC, Australia). Mass range was 33–550 amu, ion source-heating: 200 °C, ionization energy: 70 eV. One microliter of essential oil solution (80% v/v) diluted in pentane:diethyl ether was injected in split mode at split ratios (50:1). Operating conditions: capillary column Rtx-1 MS (60 m × 0.25 mm, film thickness 0.25 μm), and temperature program: 50 °C (3 min)–300 °C (30 min) at 4 °C/min. Injector and detector temperatures were 280 and 300 °C, respectively. Helium was a carrier gas (constant pressure: 300 kPa). The relative composition of each essential oil sample was calculated from GC peak areas according to total peak normalization. 1H-NMR (700 MHz) and 13C-NMR (175 MHz) spectra for components of essential oils were recorded with a Bruker Avance II Plus 700 MHz spectrometer (Bruker Corp., Billerica, MA, USA) in CDCl3, with tetramethylsilane (TMS) as an internal standard.
4.2. Plant Material
Seeds of Carpesium cernuum L. were delivered by the Anastasie Fătu Botanical Garden of the Alexandru Ioan Cuza University in Iaşi (Romania). The seeds were collected from plants growing in the wild in the natural reserve Poiana cu Cetate (Pădurea Bârnova Natura 2000 site, Iaşi County) and were sown at the end of March 2017. Seedlings and young plantlets were grown in a glasshouse of the Garden of Medicinal Plants, Maj Institute of Pharmacology PAS in Krakow, under controlled conditions (temperatures by day 18–38 °C, by night 12–18 °C), without any chemical treatment. In the third week of May, the plants were transferred into the open field. Aerial parts and roots of the plants were collected at the second year of growth, in the beginning of the flowering period (July 2018), and dried under shade at room temperature. Voucher specimen (5/18) was deposited in the collection kept at the Garden of Medicinal Plants, Maj Institute of Pharmacology, Kraków, Poland. The dry plant material was stored no longer than five months before analysis.
4.3. Isolation of Essential Oil
Essential oils from the dried aerial parts (leaves, stalks, flowers; 4.3 kg) or roots (1.8 kg) of C. cernuum were obtained by hydrodistillation using a Clevenger-type apparatus. The process was conducted for 5 h using 100–550 g of plant material. The yellowish essential oils of specific strong odor were dried over anhydrous magnesium sulphate, and stored at 4 °C in the dark, until tested and analyzed.
4.4. Isolation and NMR Analysis of Major Volatile Components
The relatively high yield of the essential oil (0.17%) obtained from 1.8 kg of the dried roots allowed us to isolate its components that were difficult to identify by the GC-MS method. To isolate the volatiles of interest, the essential oil was flash-chromatographed (FC) on a glass column (500 × 30 mm) filled with silica gel 60 (0.040–0.063 mm, Merck, EM Science, NJ USA), starting the elution with n-hexane and gradually increasing the polarity by addition of diethyl ether. The elution was accelerated by means of pressurized nitrogen (flow rate 100 mL/min). The separation was monitored by TLC and GC-MS analysis. Twenty-two fractions (1–22) of the essential oil distilled from the roots of C. cernuum were obtained and analyzed by GC-MS-FID. Structures of 5 volatiles from the following fractions were confirmed using NMR spectroscopy (1H and/or 13C): fraction 6 (296 mg) thymohydroquinone dimethyl ether (93.3%), fraction 9 (170 mg) 6-methoxythymyl isobutyrate (93.8%), fraction 11 (93 mg) 6-methoxy-8,9-didehydrothymyl isobutyrate (64.2%), fraction 14 (388 mg) 7-isobutyryloxythymyl isobutyrate (85.7%) and fraction 18 (67 mg) 10-isobutyryloxy-8,9-epoxythymyl isobutyrate (69.7%).
4.5. Identification of Essential Oil Constituents
Volatiles from the essential oils were identified based on their MS spectra and their comparison with those from mass spectra libraries: NIST 2012, Wiley Registry of Mass Spectral Data 8th edition and MassFinder 4.1, along with the relative retention indices (RI) on DB-1 column (available from MassFinder 4.1) and on HP-5 column (available from NIST 2012). Isolated compounds were also identified by the comparison of their 1H-NMR and 13C-NMR spectral data with those of the compounds isolated previously in our laboratory or those from the literature.
4.6. Antibacterial Activity of Carpesium Cernuum Essential Oils
4.6.1. Bacterial Lines and Culture Conditions
Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, Enterococcus faecalis ATCC 29212, Klebsiella pneumoniae ATCC 700603, Pseudomonas aeruginosa ATCC 27853, Serratia marcescens ATCC 13880 and Acinetobacter baumanii ATCC 19606 standard bacterial lines, from the collection of the Chair of Microbiology, Immunology and Laboratory Medicine, Pomeranian Medical University in Szczecin, were used to assess antimicrobial activities of the essential oils distilled from roots and aerial parts of C. cernuum. Prior to each experiment, the bacteria were seeded on Columbia agar medium with 5% sheep blood (bioMérieux, Warsaw, Poland) and incubated for 24 h at 37 °C in aerobic conditions. After that, the single bacterial colonies were transferred into the Tryptic Soy Broth (TSB; Merck, Darmstadt, Germany) and incubated for another 18 h at 37 °C in aerobic atmosphere.
4.6.2. Determination of the Minimum Inhibitory Concentrations (MICs)
The MICs of C. cernuum essential oils against the selected Gram-positive and Gram-negative bacteria were determined using a broth microdilution method, according to the recommendations of the Clinical and Laboratory Standards Institute (protocol M07-A9) [45]. Bacterial suspensions in Mueller-Hinton broth (MHB; Merck, Darmstadt, Germany) at the final concentrations of 106 CFU/mL were used in all experiments. Stock solutions of the essential oils (250.0 μL/mL) were prepared with 1% Tween 80 (v/v; Merck, Darmstadt, Germany) and a reference compound—thymol (Ernesto Ventos S.A., Barcelona, Spain)—was solubilized using 2% dimethyl sulfoxide (DMSO; v/v; Loba Chemie, Mumbai, India). Series of dilutions (from 1 to 250 μL/mL) were prepared by diluting the stock solutions with MHB. To each well in a 96-well microplate, containing 50 μL of the essential oil or thymol solution, 50 μL of the bacterial suspension was added. After 18 h of incubation at 37 °C, each well was spiked with 20 µL of 0.02% resazurin (Merck, Darmstadt, Germany) solution. The color change from blue to pink, after 3 h of incubation with resazurin, indicated the presence of viable bacteria. To exclude an inhibitory effect of Tween 80 and DMSO on the bacterial strain’s growth, control assays with MHB and MHB containing 1% (v/v) Tween 80 or 2% (v/v) DMSO were performed, as well as the MHB sterility control. All tests were run in duplicate.
4.7. Cytotoxic Activity of Essential Oils from Carpesium Cernuum
4.7.1. Cell Lines and Culture Conditions
HaCaT keratinocytes were purchased from AddexBio (San Diego, CA, USA). Melanoma cells A375 were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Melanoma cells C32 and normal human skin fibroblasts CCD25Sk were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). The cells were maintained in DMEM (PAN-Biotech GmbH, Aidenbach, Germany) supplemented with 10% fetal bovine serum (Gibco FBS; ThermoFisher Scientific, Waltham, MA, USA) and 1% penicillin/streptomycin (ThermoFisher Scientific, Waltham, MA, USA) at 37 °C in a 5% CO2 incubator.
4.7.2. Cell-Viability Assay
Essential oils and thymol (Sigma-Aldrich Co, St. Louis, MO, USA) were solubilized with dimethyl sulfoxide (DMSO) and stored at −20 °C for up to one month. Final concentration of DMSO in culture medium never exceeded 0.1% and the same concentration of DMSO was used in control.
Viability of cells was determined by the MTT assay [18,46]. Cells were plated in 96-well plates at 1 × 104 cells per well and allowed to adhere for 24 h. Afterwards, the cells were treated with the respective concentration of essential oils or thymol and incubated for 48 h. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich Co., St. Louis, MO, USA) solution was added to each well and the cells were incubated at 37 °C for 4 h. Then, the medium was removed, and formazan crystals were dissolved in 100 μL of DMSO and 12.5 μL of Sorensen’s glycine buffer on a plate shaker. The absorbance was measured at 570 nm on a microplate reader and the results were expressed as a percentage of the absorbance measured in control cells.
Half-maximum inhibitory concentration (IC50) values were calculated by nonlinear regression analysis using GraphPad Prism version 7.04 (GraphPad Software, San Diego, CA, USA). The results were submitted to statistical analysis using one-way analysis of variance (ANOVA) followed by Tukey’s test, accepting * p < 0.05 as significant vs control.
5. Conclusions
This is the first study on composition and biological activity of the essential oils extracted from roots and aerial parts of C. cernuum of European origin. The examined oils differed in composition and antibacterial activity. The essential oil from the plant roots was rich in thymol derivatives, especially thymohydroquinone dimethyl ether (2,5-dimethoxy-p-cymene) of proven antiviral activity (55% of oil). The essential oil from aerial parts, of very complex composition, was rich in α-pinene and demonstrated better antibacterial activity than that determined for the root essential oil. The oils at the concentrations ≥100 nL/mL were cytotoxic in vitro to both cancer and normal cell lines used in the study.
Author Contributions
Conceptualization, J.M. and A.S.; methodology, A.W.-B., Ł.S., P.K. and A.S.; investigation, A.W.-B., Ł.S. and P.K.; resources, A.W.-B., J.M., Ł.S., P.K. and A.S.; data curation, A.W.-B., Ł.S., P.K. and A.S.; writing—original draft preparation, A.W.-B., J.M., Ł.S., P.K. and A.S.; writing—review and editing, A.S.; visualization, A.W.-B., J.M. and Ł.S.; supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available.
References
- Zhang, J.P.; Wang, G.W.; Tian, X.H.; Yang, Y.X.; Liu, Q.X.; Chen, L.P.; Li, H.L.; Zhang, W.D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharm. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 10. [Google Scholar] [CrossRef]
- Hong, L.; Zhuo, J.; Lei, Q.; Zhou, J.; Ahmed, S.; Wang, C.; Long, Y.; Li, F.; Long, C. Ethnobotany of wild plants used for starting fermented beverages in Shui communities of southwest China. J. Ethnobiol. Ethnomed. 2015, 11, 42. [Google Scholar] [CrossRef]
- EFloras. 2008. Available online: http://www.efloras.org (accessed on 14 February 2021). Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA.
- Xu, Z.; Chang, L. Asteraceae. In Identification and Control of Common Weeds: Volume 3, 1st ed.; Zhejiang University Press: Hangzhou, China; Springer Nature: Singapore, 2017; pp. 441–721. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2019. Available online: http://www.plantsoftheworldonline.org (accessed on 15 February 2021).
- Greuter, W. (2006+): Compositae (pro Parte Majore)–In: Greuter, W. & Raab-Straube, E. von (ed.): Compositae. Euro + Med Plant-base—the Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 15 February 2021).
- Park, Y.J.; Cheon, S.Y.; Lee, D.S.; Cominguez, D.C.; Zhang, Z.; Lee, S.; An, H.J. Anti-inflammatory and antioxidant effects of Carpesium cernuum L. methanolic extract in LPS-stimulated RAW 264.7 macrophages. Mediat. Inflamm. 2020, 2020, 3164239. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Li, H.; Ma, C.; Wang, Y.; Tian, J.; Deng, L.; Wang, D.; Jing, X.; Luo, K.; Xing, W.; et al. Identification of Carpesium cernuum extract as a tumor migration inhibitor based on its biological response profiling in breast cancer cells. Phytomedicine 2019, 64, 153072. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Larruscain, D.; Santos-Vicente, M.; Anderberg, A.A.; Rico, E.; Martínez-Ortega, M.M. Phylogeny of the Inula group (Asteraceae: Inuleae): Evidence from nuclear and plastid genomes and a recircumscription of Pentanema. Taxon 2018, 67, 149–164. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, R.; Yang, J.L.; Shi, Y.P. Diversity of sesquiterpenoids from Carpesium cernuum. Helv. Chim. Acta 2010, 93, 595–601. [Google Scholar] [CrossRef]
- Liu, Q.X.; Yang, Y.X.; Zhang, J.P.; Chen, L.P.; Shen, Y.H.; Li, H.L.; Zhang, W.D. Isolation, structure elucidation, and absolute configuration of highly oxygenated germacranolides from Carpesium cernuum. J. Nat. Prod. 2016, 79, 2472–2478. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, W.Q.; Sun, M.; Liang, W.; Wang, T.Y.; Zhang, Y.D.; Ding, X. Carpescernolides A and B, rare oxygen bridge-containing sesquiterpene lactones from Carpesium cernuum. Tetrahedron Lett. 2018, 59, 4063–4066. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, L.; Lin, L.; Zhang, R.; Du, Y.; Chen, H.; Huang, M.; Guo, K.; Yang, X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1055. [Google Scholar] [CrossRef]
- Kameoka, H.; Sagara, K.; Miyazawa, M. Components of essential oils of Kakushitsu (Daucus carota L. and Carpesium abrotanoides L.). Nippon Nōgeikagaku Kaishi 1989, 63, 185–188. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Malarz, J.; Stojakowska, A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland. Molecules 2019, 24, 4418. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Anderberg, A.A. Inuleae. In Systematics, Evolution and Biogeography of Compositae, 1st ed.; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 667–680. [Google Scholar]
- Wajs-Bonikowska, A.; Stojakowska, A.; Kalemba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.S.; Lajide, L.; Villanueva, H.E.; Setzer, W.N. Essential oil composition and insecticidal activity of Blumea perrottetiana growing in Southwestern Nigeria. Nat. Prod. Commun. 2010, 5, 1135–1138. [Google Scholar] [PubMed]
- Joshi, R.K. Chemical composition of Blumea virens roots from India. Chem. Nat. Compd. 2018, 54, 584–585. [Google Scholar] [CrossRef]
- Xu, T.; Gherib, M.; Bekhechi, C.; Atik-Bekkara, F.; Casabianca, H.; Tomi, F.; Casanova, J.; Bighelli, A. Thymyl esters derivatives and a new natural product modhephanone from Pulicaria mauritanica Coss. (Asteraceae) root oil. Flavour Fragr. J. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Onayade, O.A.; Scheffer, J.J.C.; Schripsema, J.; van der Gen, A. 6-Hydroxycarvotanacetone and other constituents of the essential oil of Laggera alata (D. Don) Sch. Bip. ex Oliv. Flavour Fragr. J. 1990, 5, 165–172. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Compositional variation in the essential oils of vegetative and reproductive parts of Laggera crispata (Vahl) Hepper & Wood. Natl. Acad. Sci. Lett. 2013, 36, 447–451. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chanotiya, C.S.; Chauhan, A.; Yadav, A. Chemical investigation of the essential oil of Laggera crispata (Vahl) Hepper & Wood from India. J. Serb. Chem. Soc. 2011, 76, 523–528. [Google Scholar]
- Kambiré, D.A.; Boti, J.B.; Yapi, T.A.; Ouattara, Z.A.; Paoli, M.; Bighelli, A.; Tomi, F.; Casanova, J. Composition and Intraspecific chemical variability of leaf essential oil of Laggera pterodonta from Côte d’Ivoire. Chem. Biodiv. 2020, 17, e1900504. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Ouattara, Z.A.; Tonzibo, Z.F.; Filippi, J.-J. Two new eudesman-4α-ol epoxides from the stem essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2020, 34, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Sharma, V.; Kumar, D.; Gupta, N. Chemical composition, and antibacterial and antioxidant activities of the essential oils from Laggera tomentosa Sch. Bip. ex Oliv. et Hiern (Asteraceae). Turk. J. Chem. 2020, 44, 1539–1548. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shahabi, M.; Shafi, P.M.; Rajeeve, K.R. Medicinal used plants from India: Analysis of the essential oils of Sphaeranthus indicus flowers, roots and stems with leaves. Sci. Pharm. 2003, 71, 251–259. [Google Scholar] [CrossRef]
- Duñg, N.X.; Târn, N.T.; Kruk, C.; Leclercq, P.A. Composition of the oil of Eupatorium stoechadosmum Hance from Vietnam. J. Essent. Oil Res. 1991, 3, 115–116. [Google Scholar] [CrossRef]
- Tabanca, N.; Bernier, U.R.; Tsikolia, M.; Becnel, J.J.; Sampson, B.; Werle, C.; Demirci, B.; Başer, K.H.C.; Blythe, E.K.; Pounders, C.; et al. Eupatorium capillifolium essential oil: Chemical composition, antifungal activity, and insecticidal activity. Nat. Prod. Commun. 2010, 5, 1409–1415. [Google Scholar] [CrossRef]
- Solis-Quispe, L.; Pino, J.A.; Falco, A.S.; Tomaylla-Cruz, C.; Quispe-Tonccochi, E.G.; Solis-Quispe, J.A. Chemical composition and antibacterial activities of essential oil from Ageratina pentlandiana (DC.) R.M. King & H. Rob. leaves grown in the Peruvian Andes. J. Essent. Oil Res. 2019, 31, 409–413. [Google Scholar]
- Mala, J.G.S.; Zoghbi, M.G.B.; da Silva, M.H.L.; Andrade, E.H. A Essential oils of Eupatorium triplinerve Vahl and E. paniculatum Poepp. et Endl. J. Essent. Oil Res. 1999, 11, 541–544. [Google Scholar] [CrossRef]
- Gauvin-Bialecki, A.; Marodon, C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2009, 36, 853–858. [Google Scholar] [CrossRef]
- Haddad, J.G.; Picard, M.; Bénard, S.; Desvignes, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. Ayapana triplinervis essential oil and its main component thymohydroquinone dimethyl ether inhibit Zika virus at doses devoid of toxicity in zebrafish. Molecules 2019, 24, 3447. [Google Scholar] [CrossRef] [PubMed]
- Pljevljakušić, D.; Rančić, D.; Ristić, M.; Vujisić, L.; Radanović, D.; Dajić-Stevanović, Z. Rhizome and root yield of the cultivated Arnica montana L., chemical composition and histochemical localization of essential oil. Ind. Crops Prod. 2012, 39, 177–189. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Winiarczyk, K.; Kowalski, R. Essential oil from Arnica montana achenes: Chemical characteristics and anticancer activity. Molecules 2019, 24, 4158. [Google Scholar] [CrossRef]
- Sugier, P.; Jakubowicz-Gil, J.; Sugier, D.; Kowalski, R.; Gawlik-Dziki, U.; Kołodziej, B.; Dziki, D. Chemical characteristics and anticancer activity of essential oil from Arnica montana L. rhizomes and roots. Molecules 2020, 25, 1284. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical constituents and antibacterial property of the essential oil of the roots of Cyathocline purpurea. J. Ethnopharm. 2013, 145, 621–625. [Google Scholar] [CrossRef]
- Pande, C.; Tewari, G.; Singh, C.; Singh, S. Essential oil composition of aerial parts of Cyclospermum leptophyllum (Pers.) Sprague ex Britton and P. Wilson. Nat. Prod. Res. 2011, 25, 592–595. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. Chemical composition and biological activity of essential oils from Aloe debrana roots. J. Essent. Oil Bear. Pl. 2020, 23, 493–502. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against MRSA strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Satooka, H.; Kubo, I. Effects of thymol on B16-F10 melanoma cells. J. Agric. Food Chem. 2012, 60, 2746–2752. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- Sargent, J.M.; Taylor, C.G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br. J. Cancer 1989, 60, 206–210. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).