An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives
Abstract
1. Introduction
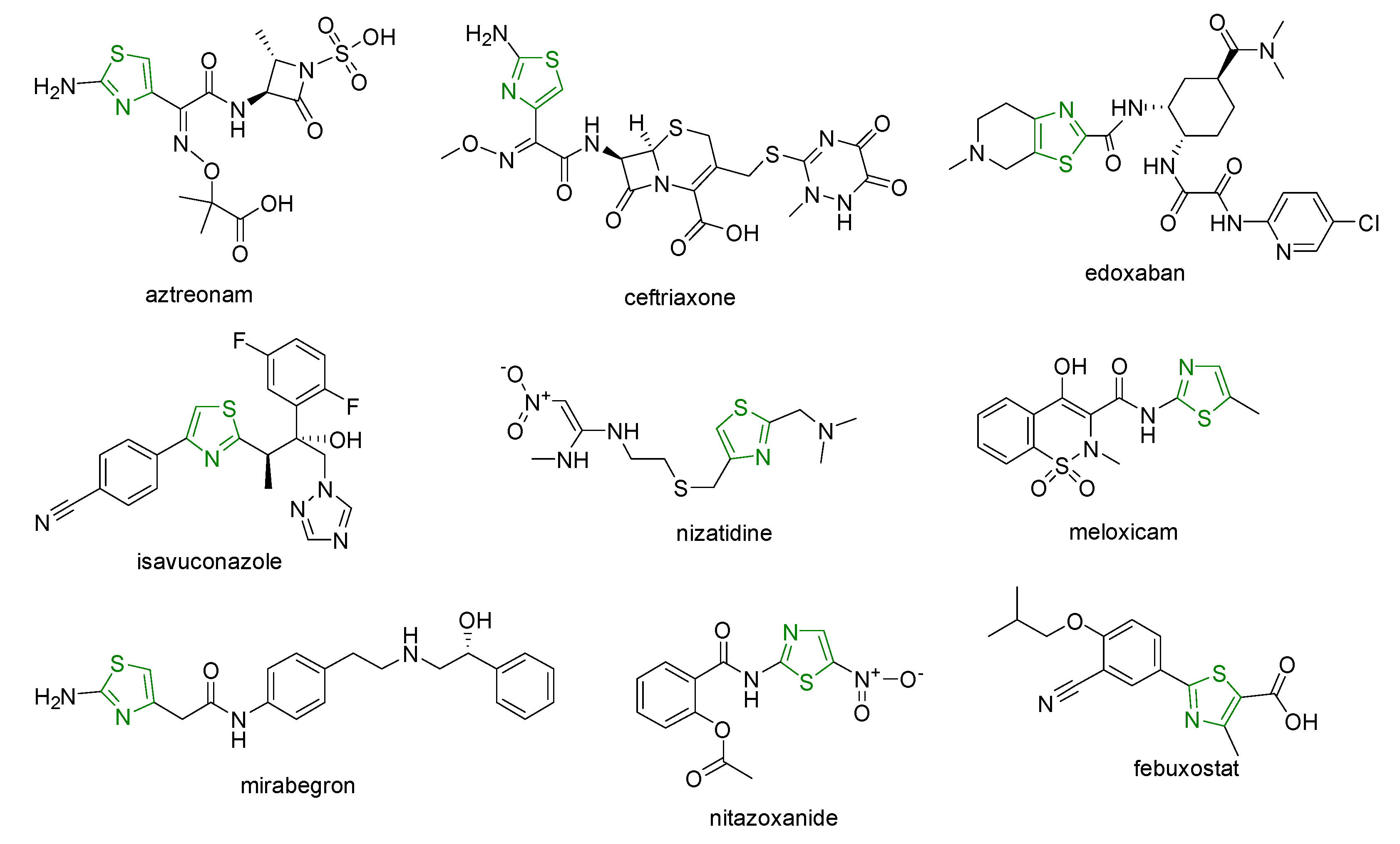
2. Chemistry of Thiazole
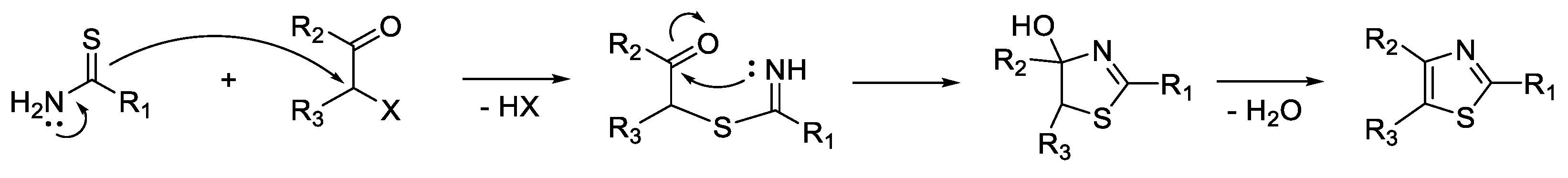
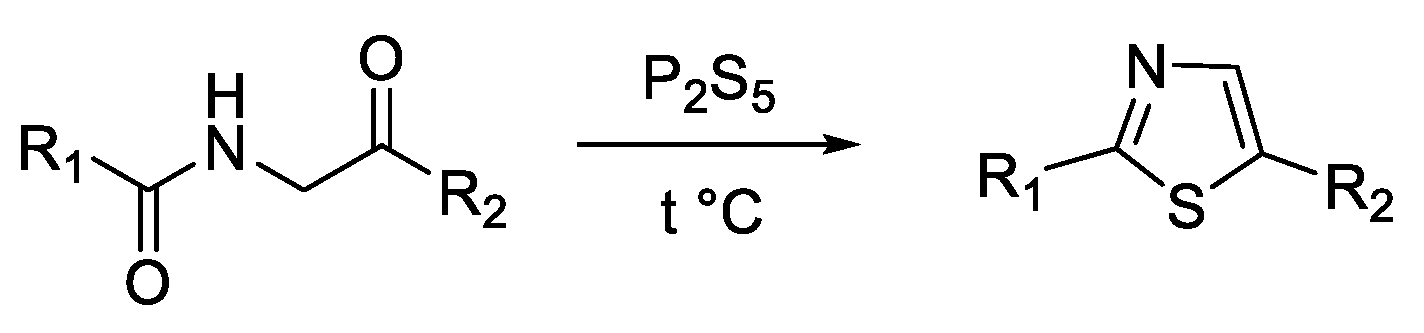
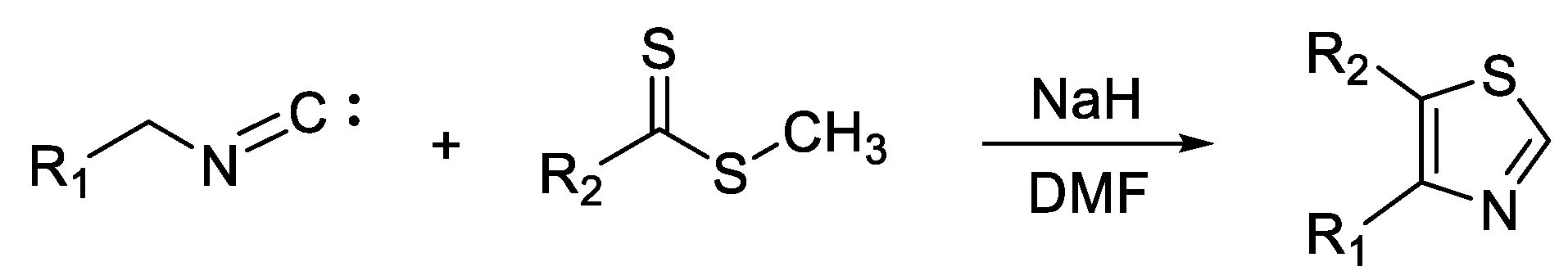
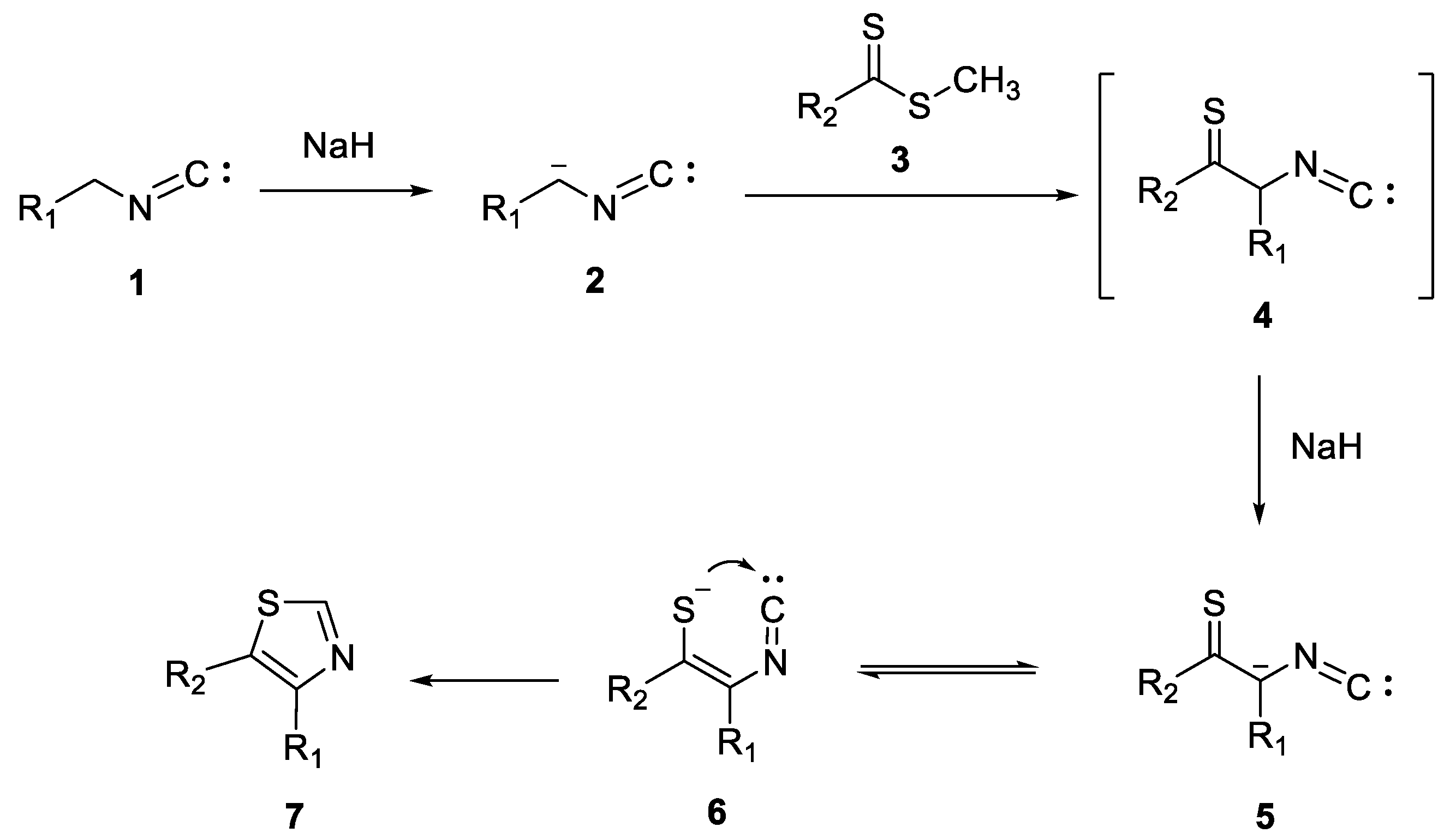
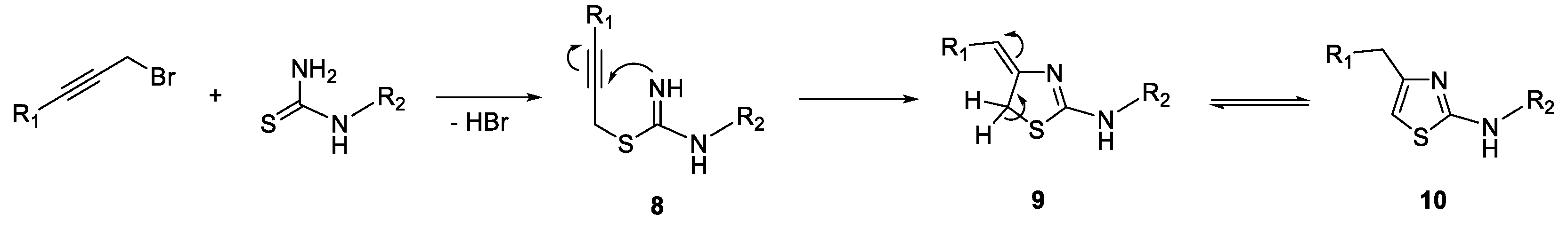
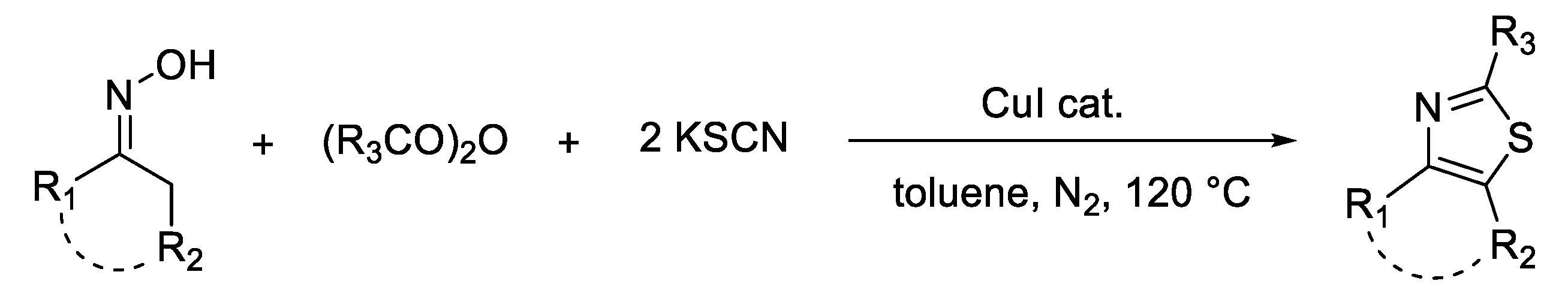
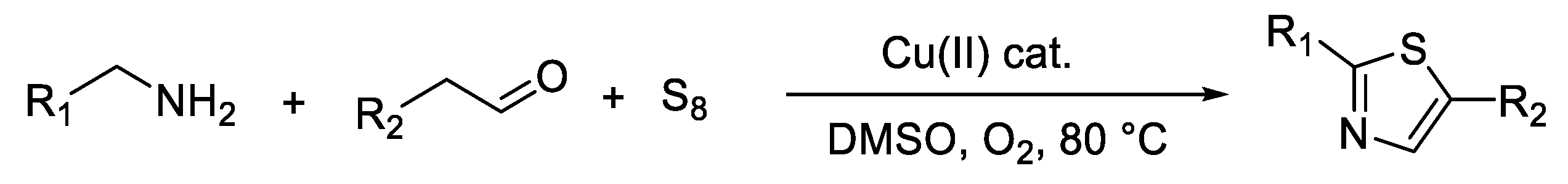
3. Synthesis of Thiazole Derivatives
4. Biological Activity of Thiazole Derivatives
4.1. Antimicrobial Activity
4.2. Antiprotozoal Activity
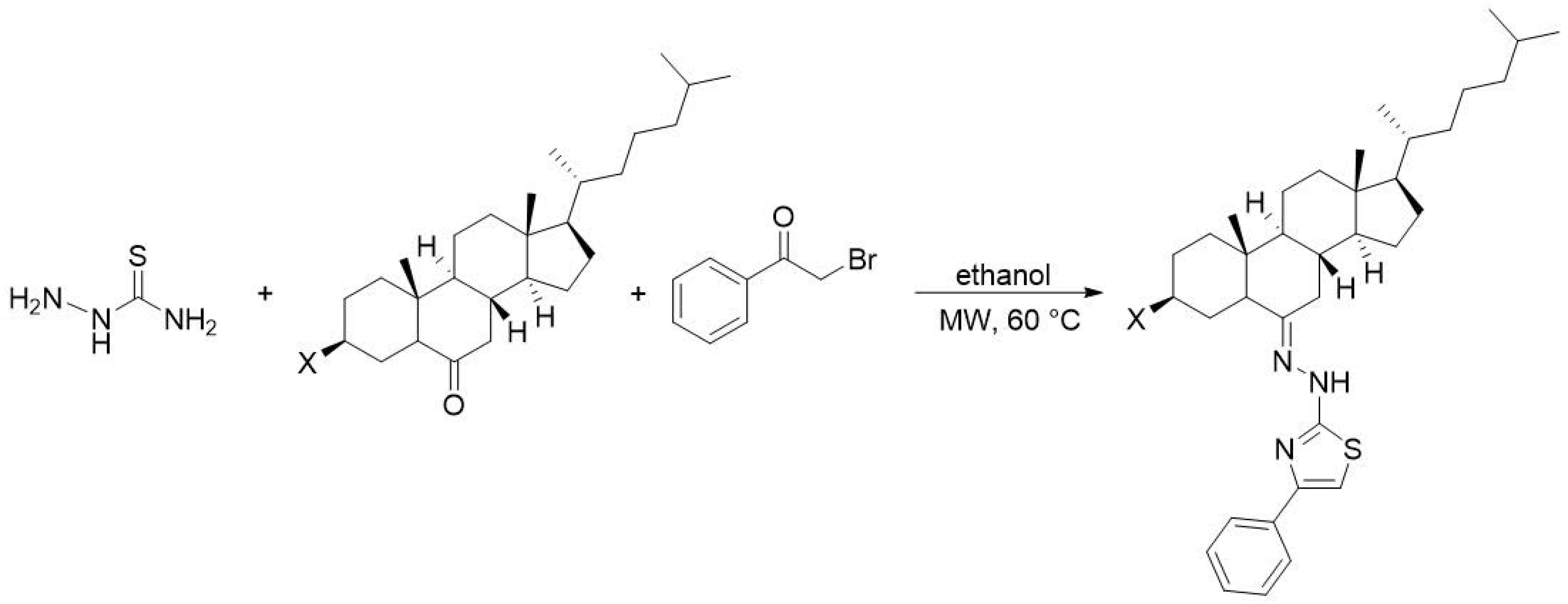
4.3. Antitumor Activity
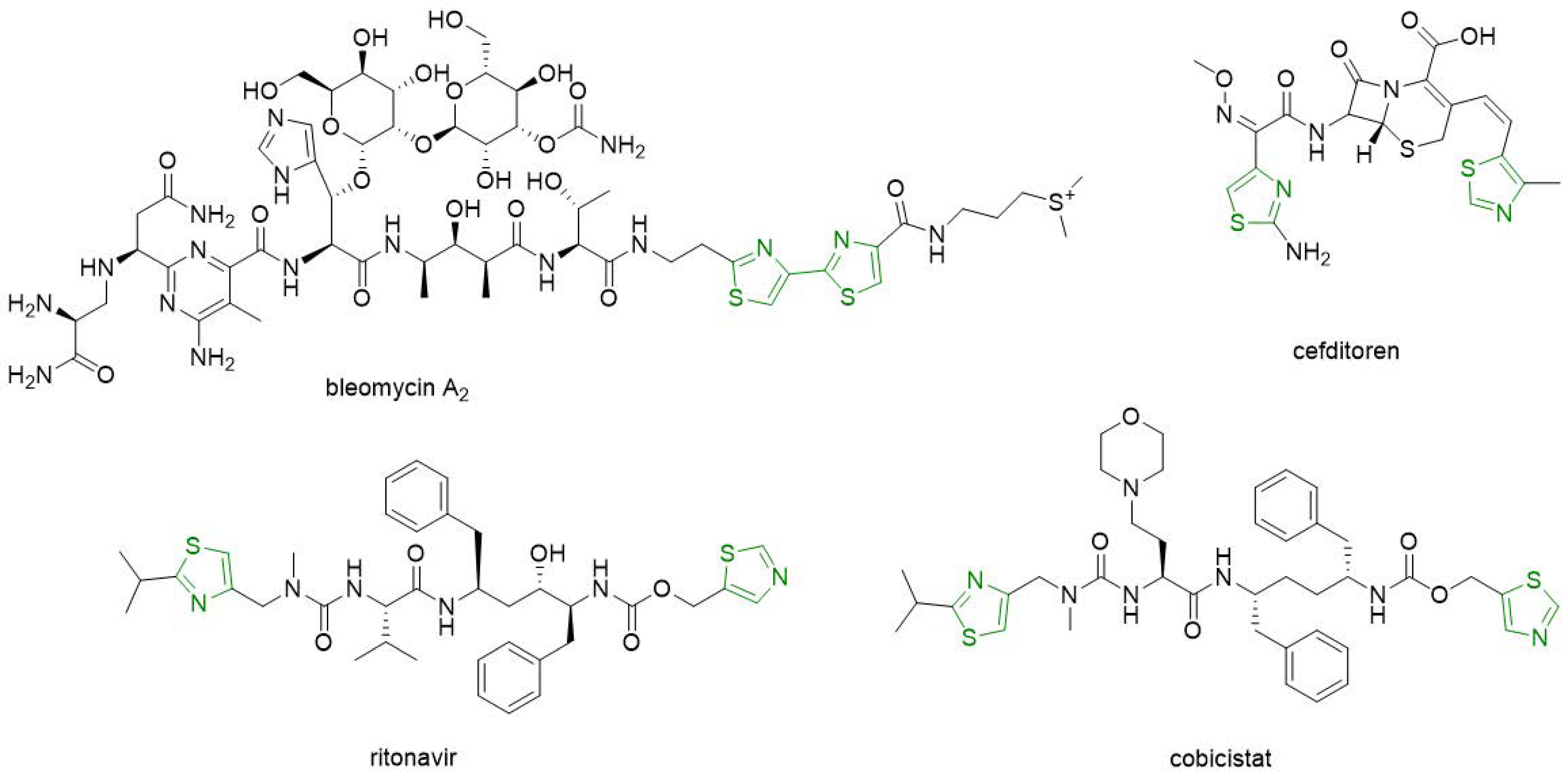
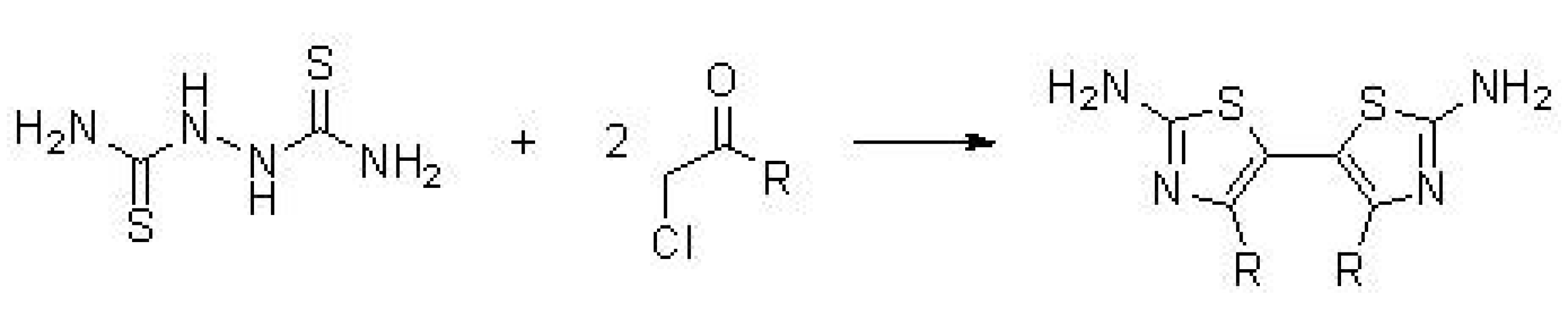
5. Synthesis of Bisthiazole Derivatives (Thiazolyl-Thiazoles and Thiazolyl-Linker-Thiazoles)
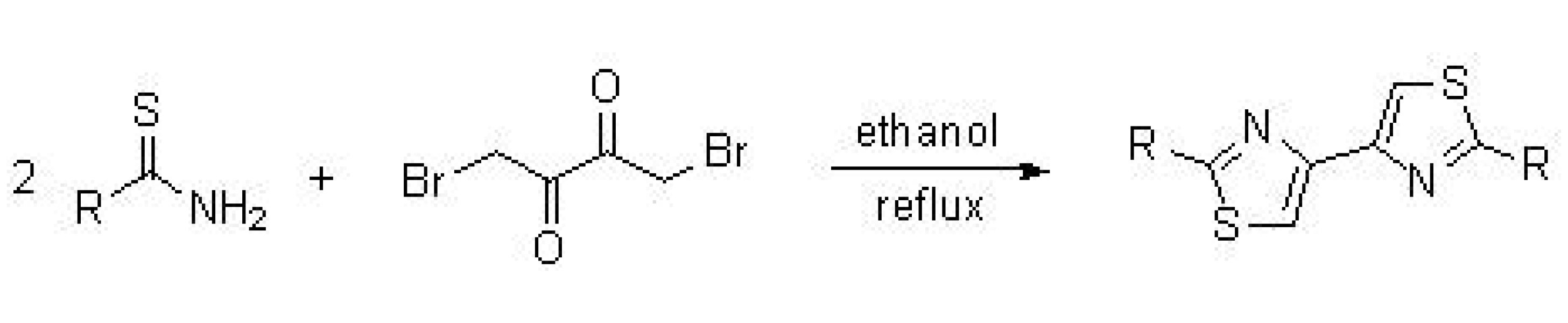
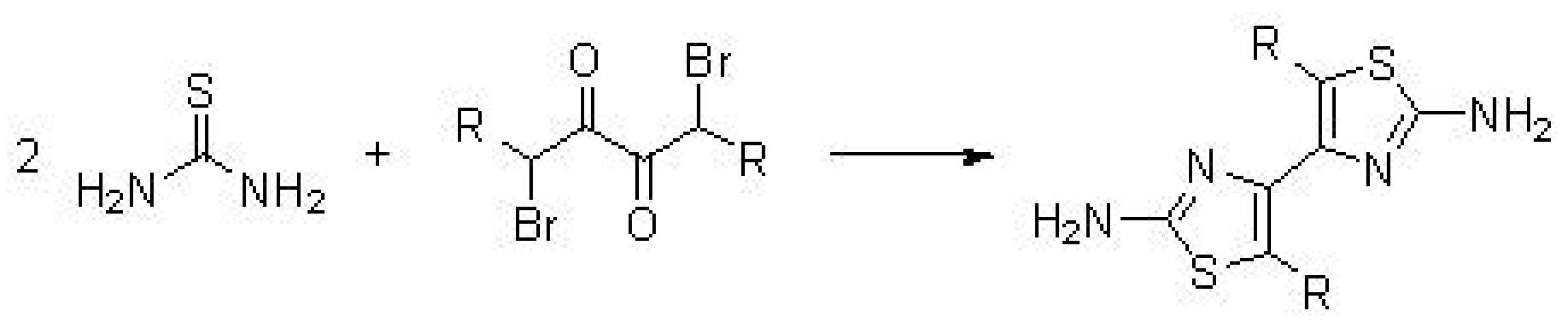
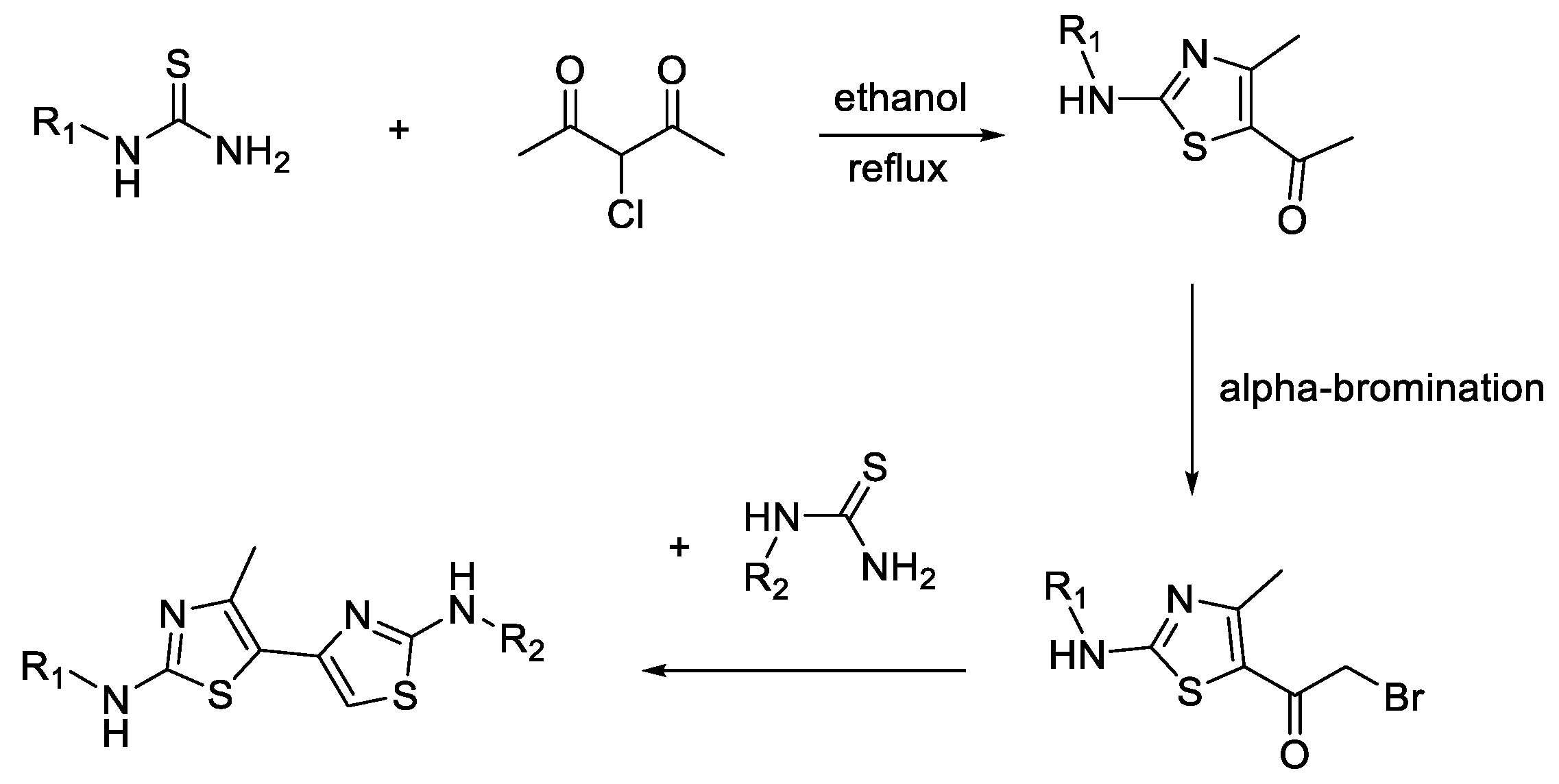
5.1. Synthesis of Thiazolyl-Thiazole Derivatives
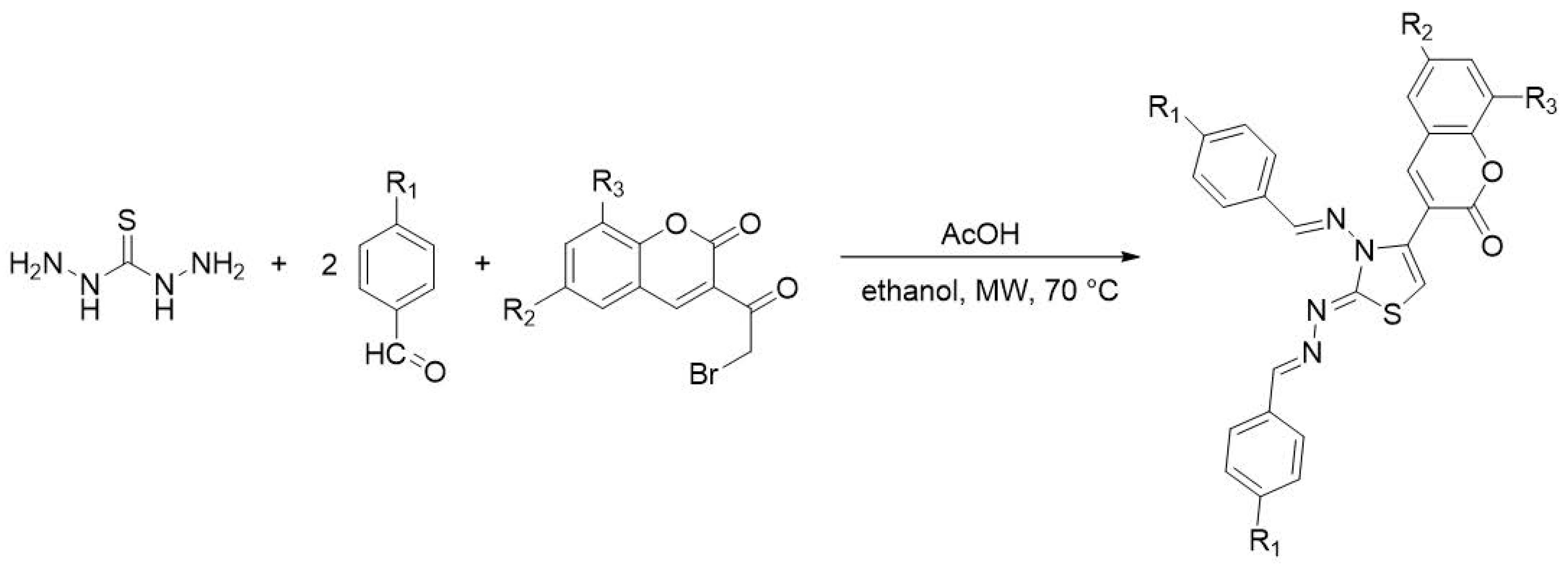
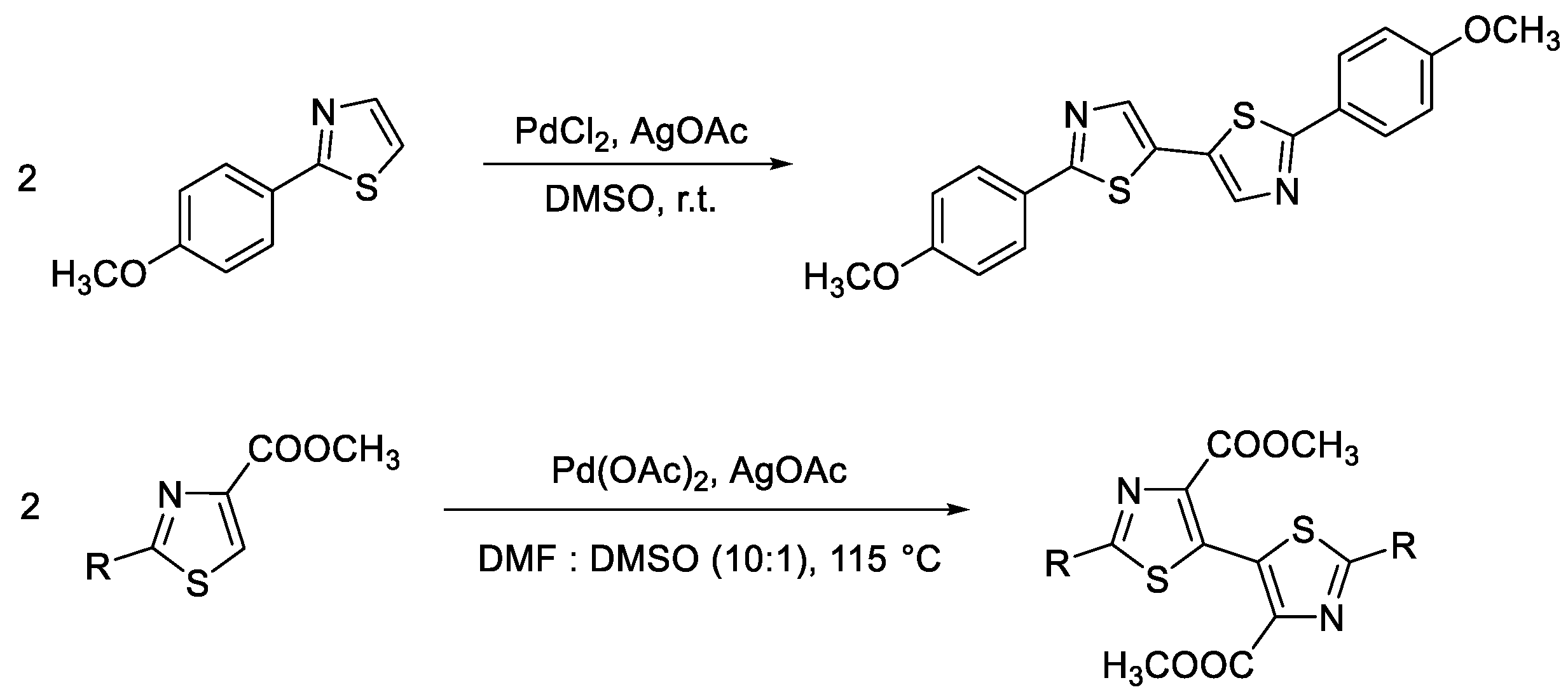
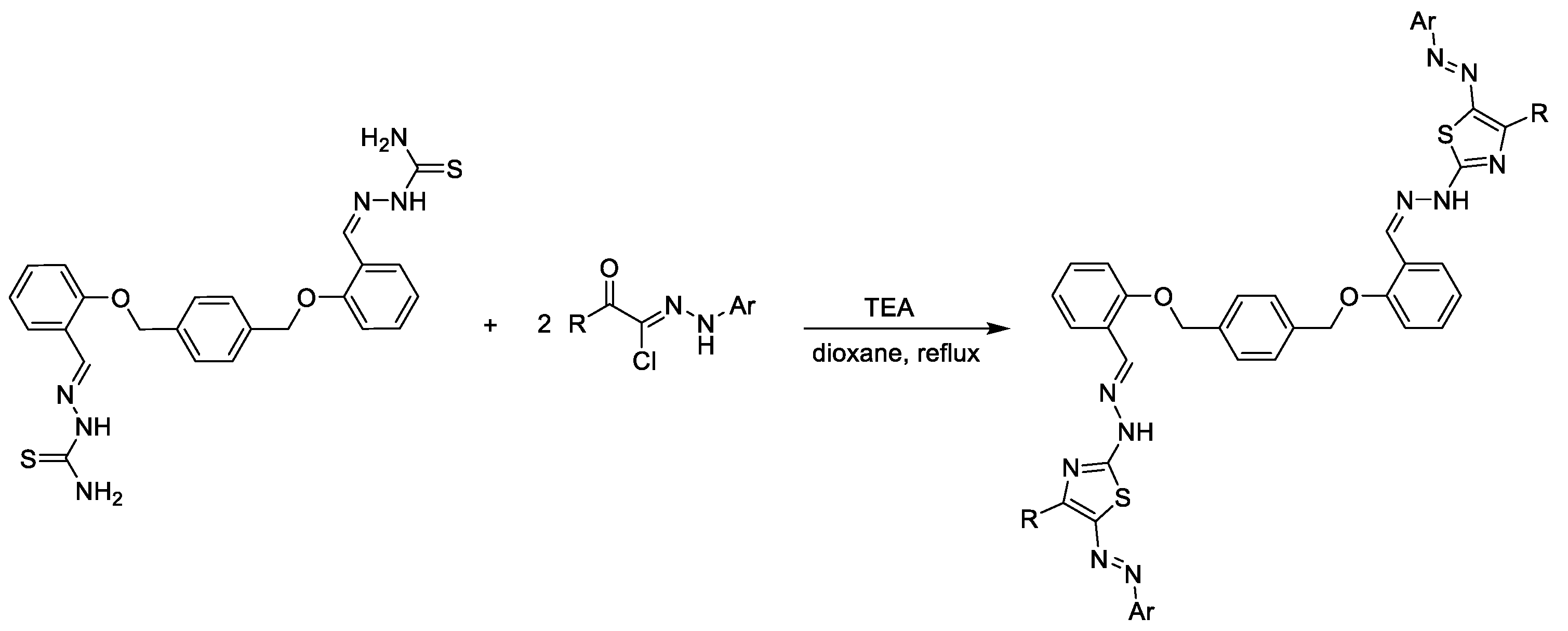
5.2. Synthesis of Thiazolyl-Linker-Thiazole Compounds
6. Biological Activity of Bisthiazole Derivatives
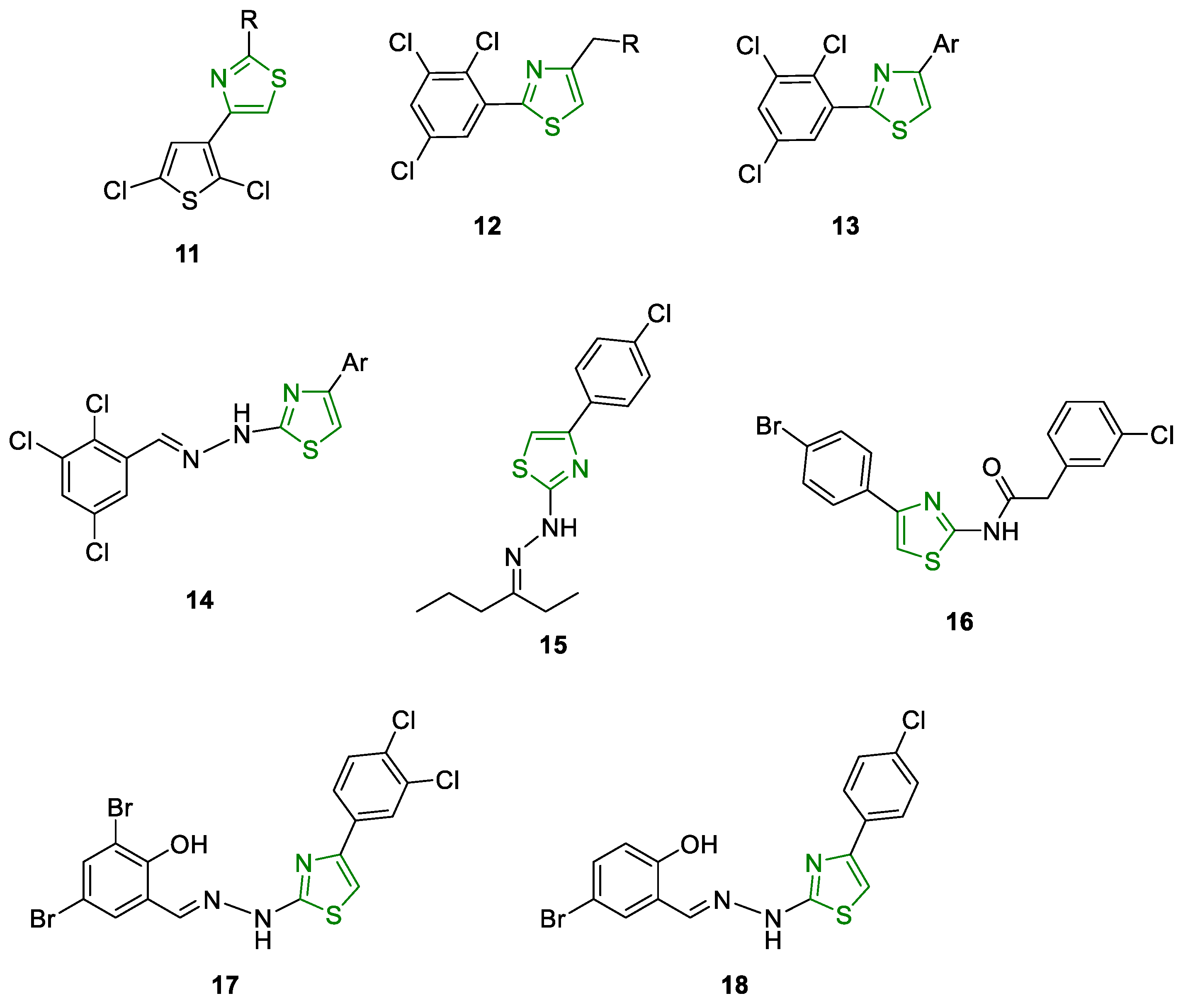
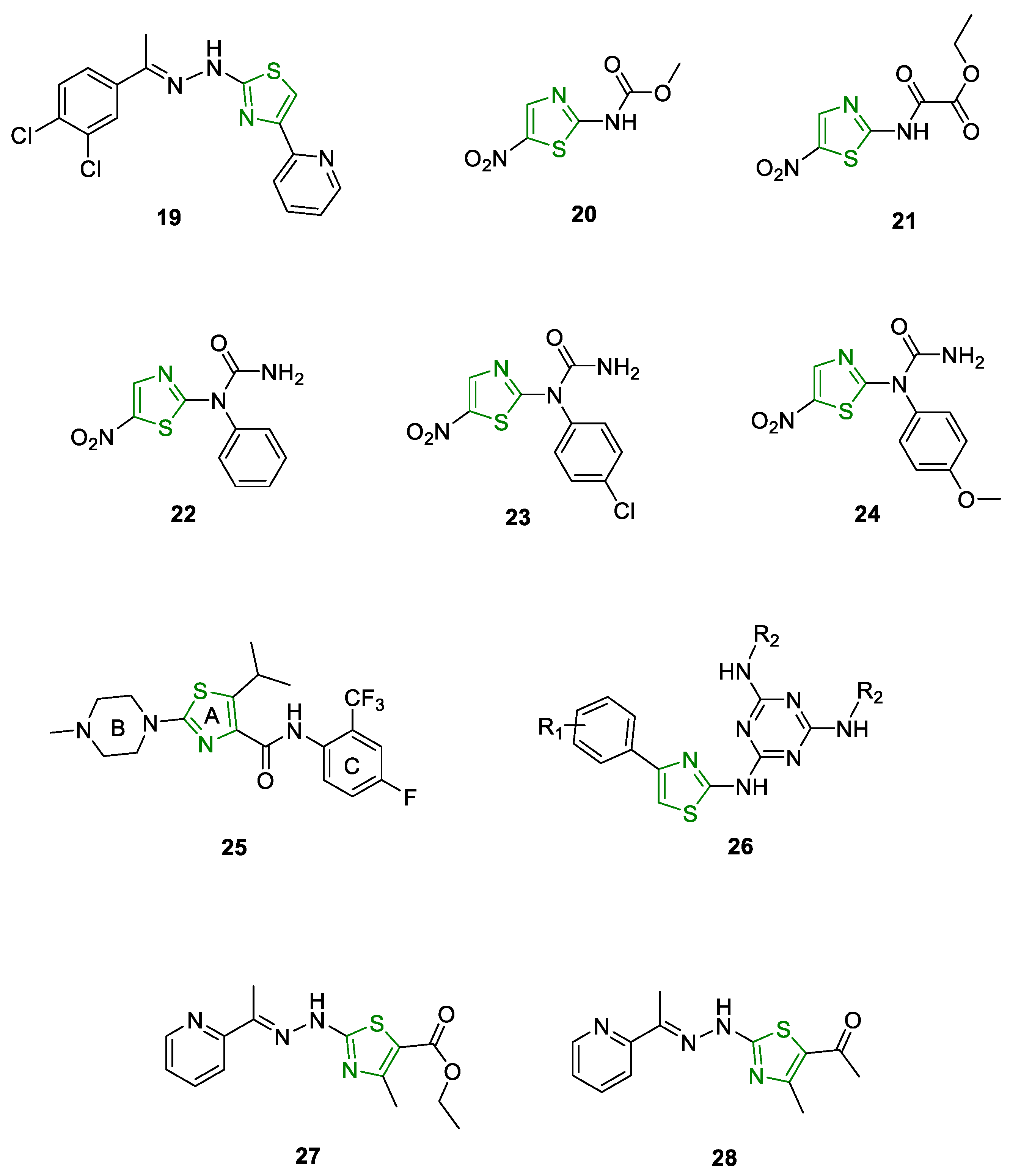
6.1. Antimicrobial Activity
6.2. Antiprotozoal Activity
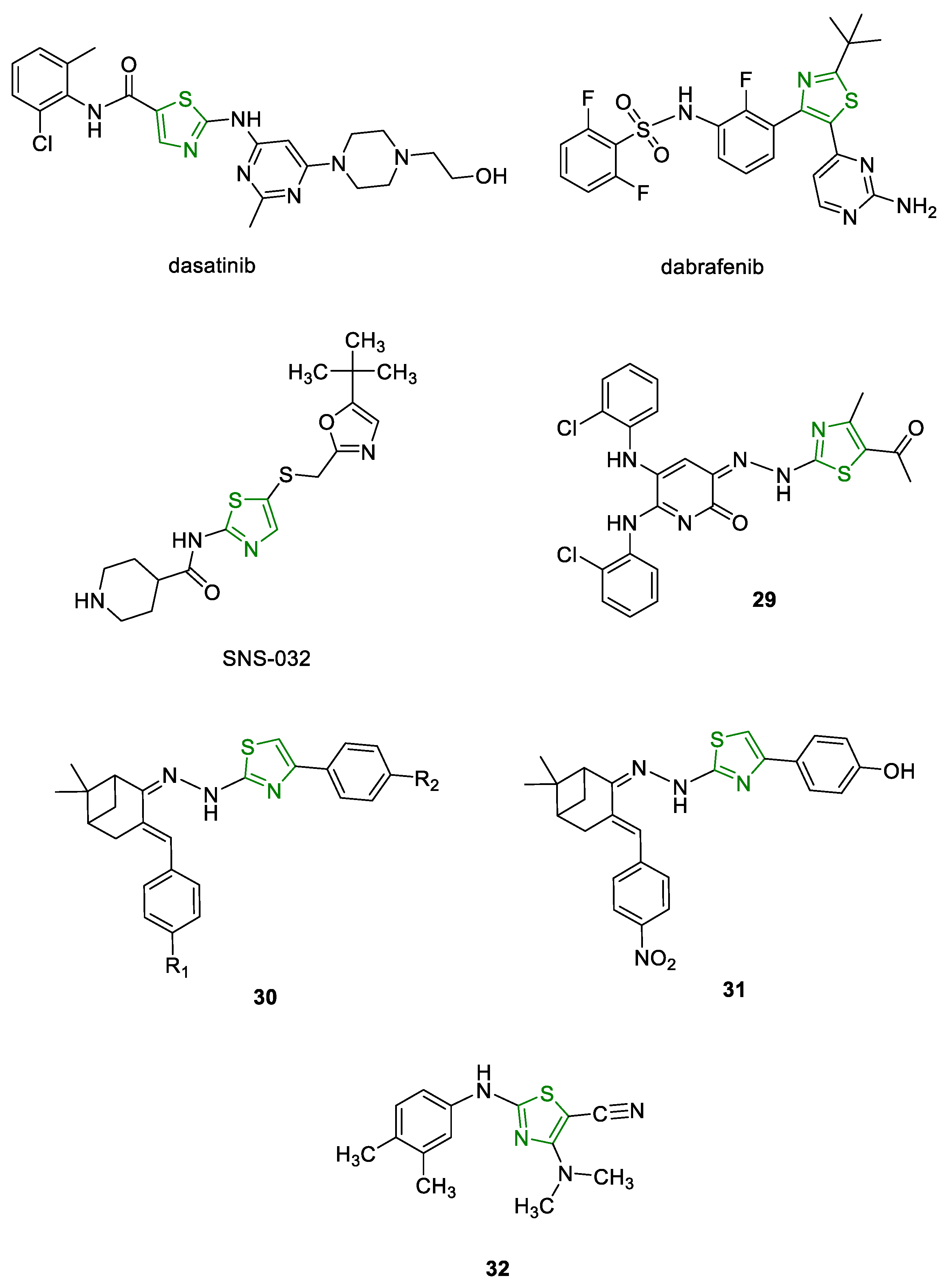
6.3. Antitumor Activity
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Njardarson, J.T. Analysis of US FDA-Approved Drugs Containing Sulfur Atoms. Top. Curr. Chem. 2018, 376, 5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Pola, S. Significance of Thiazole-based Heterocycles for Bioactive Systems. In Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective; Varala, R., Ed.; InTech: Rijeka, Croatia, 2016; pp. 1–47. [Google Scholar]
- Gallant, J.E.; Koenig, E.; Andrade-Villanueva, J.; Chetchotisakd, P.; DeJesus, E.; Antunes, F.; Arastéh, K.; Moyle, G.; Rizzardini, G.; Fehr, J.; et al. Cobicistat Versus Ritonavir as a Pharmacoenhancer of Atazanavir Plus Emtricitabine/Tenofovir Disoproxil Fumarate in Treatment-Naive HIV Type 1–Infected Patients: Week 48 Results. J. Infect. Dis. 2013, 208, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mishra, I.; Mishra, R.; Mujwar, S.; Chandra, P.; Sachan, N. A retrospect on antimicrobial potential of thiazole scaffold. J. Heterocycl. Chem. 2020, 57, 2304–2329. [Google Scholar] [CrossRef]
- Meleddu, R.; Distinto, S.; Corona, A.; Maccioni, E.; Arridu, A.; Melis, C.; Bianco, G.; Matyus, P.; Cottiglia, F.; Sanna, A.; et al. Exploring the thiazole scaffold for the identification of new agents for the treatment of fluconazole resistant Candida. J. Enz. Inhib. Med. Chem. 2016, 31, 1672–1677. [Google Scholar] [CrossRef][Green Version]
- da Silva, E.B.; Oliveira e Silva, D.A.; Oliveira, A.R.; da Silva Mendes, C.H.; dos Santos, T.A.R.; da Silva, A.C.; de Castro, M.C.A.; Ferreira, R.S.; Moreira, D.R.M.; de Oliveira Cardoso, M.V.; et al. Desing and synthesis of potent anti-Trypanosoma cruzi agents new thiazoles derivatives which induce apoptotic parasite death. Eur. J. Med. Chem. 2017, 130, 39–50. [Google Scholar] [CrossRef]
- Singh, I.P.; Gupta, S.; Kumar, S. Thiazole Compounds as Antiviral Agents: An Update. Med. Chem. (Los. Angeles) 2020, 16, 4–23. [Google Scholar] [CrossRef]
- Abu-Melha, S.; Edrees, M.M.; Riyadh, S.M.; Abdelaziz, M.R.; Elfiky, A.A.; Gomha, S.M. Clean grinding technique: A facile synthesis and in silico antiviral activity of hydrazones, pyrazoles, and pyrazines bearing thiazole moiety against SARS-CoV-2 main protease (Mpro). Molecules 2020, 25, 4565. [Google Scholar] [CrossRef]
- Gomha, S.; Edrees, M.; Altalbawy, F. Synthesis and Characterization of Some New Bis-Pyrazolyl-Thiazoles Incorporating the Thiophene Moiety as Potent Anti-Tumor Agents. Int. J. Mol. Sci. 2016, 17, 1499. [Google Scholar] [CrossRef]
- Woods, K.W.; McCroskey, R.W.; Michaelides, M.R.; Wada, C.K.; Hulkower, K.I.; Bell, R.L. Thiazole analogues of the NSAID indomethacin as selective COX-2 Inhibitors. Bioorg. Med. Chem. Lett. 2001, 11, 1325–1328. [Google Scholar] [CrossRef]
- Araniciu, C.; Pârvu, A.; Palage, M.; Oniga, S.; Benedec, D.; Oniga, I.; Oniga, O. The Effect of Some 4,2 and 5,2 Bisthiazole Derivatives on Nitro-Oxidative Stress and Phagocytosis in Acute Experimental Inflammation. Molecules 2014, 19, 9240–9256. [Google Scholar] [CrossRef] [PubMed]
- Liaras, K.; Fesatidou, M.; Geronikaki, A. Thiazoles and Thiazolidinones as COX/LOX Inhibitors. Molecules 2018, 23, 685. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, E.K.A.; Kamel, G.M. Synthesis of new thiazolo-celecoxib analogues as dual cyclooxygenase-2/15-lipoxygenase inhibitors: Determination of regio-specific different pyrazole cyclization by 2D NMR. Eur. J. Med. Chem. 2016, 118, 250–258. [Google Scholar] [CrossRef]
- Rödl, C.B.; Vogt, D.; Kretschmer, S.B.M.; Ihlefeld, K.; Barzen, S.; Brüggerhoff, A.; Achenbach, J.; Proschak, E.; Steinhilber, D.; Stark, H.; et al. Multi-dimensional target profiling of N,4-diaryl-1,3-thiazole-2-amines as potent inhibitors of eicosanoid metabolism. Eur. J. Med. Chem. 2014, 84, 302–311. [Google Scholar] [CrossRef]
- Jaishree, V.; Ramdas, N.; Sachin, J.; Ramesh, B. In vitro antioxidant properties of new thiazole derivatives. J. Saudi Chem. Soc. 2012, 16, 371–376. [Google Scholar] [CrossRef]
- Saravanan, G.; Alagarsamy, V.; Prakash, C.R.; Kumar, P.D.; Selvam, T.P. Synthesis of Novel Thiazole Derivatives as Analgesic Agents. Asian J. Pharm. Sci. 2011, 1, 134–138. [Google Scholar]
- Siddiqui, N.; Ahsan, W. Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur. J. Med. Chem. 2010, 45, 1536–1543. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Gong, Z.; Li, Y. Synthesis, biological evaluation, and docking studies of novel 5,6-diaryl-1,2,4-triazine thiazole derivatives as a new class of α-glucosidase inhibitors. Bioorg. Chem. 2018, 78, 195–200. [Google Scholar] [CrossRef]
- Sowjanya, C.; Seetaram Swamy, S.; Gomathi, S.; Ashok Babu, K. Synthesis, Chemistry and Anti-Hypertensive Activity of Some New Thiazole-Thiadiazole Derivatives. Int. J. Adv. Res. Med. Pharm. Sci. 2016, 1, 6–10. [Google Scholar]
- Sahin, Z.; Ertas, M.; Bender, C.; Bülbül, E.F.; Berk, B.; Biltekin, S.N.; Yurttaş, L.; Demirayak, Ş. Thiazole-substituted benzoylpiperazine derivatives as acetylcholinesterase inhibitors. Drug Dev. Res. 2018, 79, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.R.; Singh, D.P.; Das, R.D.; Panchal, N.B.; Sudarsanam, V.; Nivsarkar, M.; Vasu, K.K. Pharmacological investigation of quinoxaline-bisthiazoles as multitarget-directed ligands for the treatment of Alzheimer’s disease. Bioorg. Chem. 2019, 89, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kadam, K.S.; Jadhav, R.D.; Kandre, S.; Guha, T.; Reddy, M.M.K.; Brahma, M.K.; Deshmukh, N.J.; Dixit, A.; Doshi, L.; Srinivasan, S.; et al. Evaluation of thiazole containing biaryl analogs as diacylglycerol acyltransferase 1 (DGAT1) inhibitors. Eur. J. Med. Chem. 2013, 65, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, P.S. Antibacterial Discovery: 21st Century Challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Jornada, D.H.; Machado, M.G.M.; Ferreira, C.M.R.; dos Santos, J.L.; Chung, M.C. Thiazole, thio and semicarbazone derivatives against tropical infective diseases: Chagas disease, human African trypanosomiasis (HAT), leishmaniasis, and malaria. Eur. J. Med. Chem. 2019, 162, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Hemphill, A. Drug target identification in protozoan parasites. Expert Opin. Drug Discov. 2016, 11, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Sarangi, P.K.N.; Sahoo, J.; Swain, B.D.; Paidesetty, S.K.; Mohanta, G.P. Thiazoles as potent anticancer agents: A review. Indian Drugs 2016, 53, 5–11. [Google Scholar]
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles. Structure, Reactions, Syntheses, and Applications; WILEY-VCH GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3-527-30720-6. [Google Scholar]
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Novel thiazole derivatives: A patent review (2008-2012; Part 1). Expert Opin. Ther. Pat. 2014, 24, 201–216. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef]
- Ali, S.H.; Sayed, A.R. Review of the synthesis and biological activity of thiazoles. Synth. Commun. 2020, 50, 1–31. [Google Scholar] [CrossRef]
- Nayak, S.; Gaonkar, S.L. A Review on Recent Synthetic Strategies and Pharmacological Importance of 1,3-Thiazole Derivatives. Mini-Reviews Med. Chem. 2019, 19, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, G.; Swaroop, T.; Vinayaka, A.; Sharath Kumar, K.; Sadashiva, M.; Rangappa, K. An Easy Access to 4,5-Disubstituted Thiazoles via Base-Induced Click Reaction of Active Methylene Isocyanides with Methyl Dithiocarboxylates. Synthesis (Stuttg) 2012, 44, 1373–1379. [Google Scholar] [CrossRef]
- Castagnolo, D.; Pagano, M.; Bernardini, M.; Botta, M. Domino Alkylation-Cyclization Reaction of Propargyl Bromides with Thioureas/Thiopyrimidinones: A New Facile Synthesis of 2-Aminothiazoles and 5H-Thiazolo[3,2-a]pyrimidin-5-ones. Synlett 2009, 2009, 2093–2096. [Google Scholar] [CrossRef]
- Tang, X.; Yang, J.; Zhu, Z.; Zheng, M.; Wu, W.; Jiang, H. Access to Thiazole via Copper-Catalyzed [3+1+1]-Type Condensation Reaction under Redox-Neutral Conditions. J. Org. Chem. 2016, 81, 11461–11466. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, X.; Wei, J.; Liu, J.; Song, S.; Wang, W.; Jiao, N. Cu-Catalyzed Aerobic Oxidative Sulfuration/Annulation Approach to Thiazoles via Multiple Csp 3 –H Bond Cleavage. Org. Lett. 2018, 20, 2632–2636. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guo, S.; Guo, X.; Zhang, G.; Yu, Y. Selective Access to 4-Substituted 2-Aminothiazoles and 4-Substituted 5-Thiocyano-2-aminothiazoles from Vinyl Azides and Potassium Thiocyanate Switched by Palladium and Iron Catalysts. Org. Lett. 2015, 17, 4698–4701. [Google Scholar] [CrossRef]
- Gomha, S.M.; Edrees, M.M.; Faty, R.A.M.; Muhammad, Z.A.; Mabkhot, Y.N. Microwave-assisted one pot three-component synthesis of some novel pyrazole scaffolds as potent anticancer agents. Chem. Cent. J. 2017, 11, 37. [Google Scholar] [CrossRef]
- Karamthulla, S.; Pal, S.; Khan, M.N.; Choudhury, L.H. “On-water” synthesis of novel trisubstituted 1,3-thiazoles via microwave-assisted catalyst-free domino reactions. RSC Adv. 2014, 4, 37889–37899. [Google Scholar] [CrossRef]
- Prajapati, N.P.; Patel, K.D.; Vekariya, R.H.; Patel, H.D.; Rajani, D.P. Thiazole fused thiosemicarbazones: Microwave-assisted synthesis, biological evaluation and molecular docking study. J. Mol. Struct. 2019, 1179, 401–410. [Google Scholar] [CrossRef]
- Asif, M.; Ali, A.; Zafar, A.; Farhan, M.; Khanam, H.; Hadi, S.M. Shamsuzzaman Microwave-assisted one pot synthesis, characterization, biological evaluation and molecular docking studies of steroidal thiazoles. J. Photochem. Photobiol. B Biol. 2017, 166, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, S.; Peddi, S.R.; Aravilli, R.K.; Jilloju, P.C.; Manga, V.; Vedula, R.R. Microwave irradiated one pot, three component synthesis of a new series of hybrid coumarin based thiazoles: Antibacterial evaluation and molecular docking studies. J. Mol. Struct. 2021, 1225, 129114. [Google Scholar] [CrossRef]
- Chinnaraja, D.; Rajalakshmi, R. A facile, solvent and catalyst free, microwave assisted one pot synthesis of hydrazinyl thiazole derivatives. J. Saudi Chem. Soc. 2015, 19, 200–206. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Berkow, E.; Lockhart, S. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Gümüş, M.; Yakan, M.; Koca, İ. Recent advances of thiazole hybrids in biological applications. Future Med. Chem. 2019, 11, 1979–1998. [Google Scholar] [CrossRef] [PubMed]
- Stoica, C.I.; Ionuț, I.; Vlase, L.; Tiperciuc, B.; Marc, G.; Oniga, S.; Araniciu, C.; Oniga, O. Lipophilicity evaluation of some thiazolyl-1,3,4-oxadiazole derivatives with antifungal activity. Biomed. Chromatogr. 2018, 32, e4221. [Google Scholar] [CrossRef]
- Edrees, M.; Melha, S.; Saad, A.; Kheder, N.; Gomha, S.; Muhammad, Z. Eco-Friendly Synthesis, Characterization and Biological Evaluation of Some Novel Pyrazolines Containing Thiazole Moiety as Potential Anticancer and Antimicrobial Agents. Molecules 2018, 23, 2970. [Google Scholar] [CrossRef]
- Yurttaş, L.; Özkay, Y.; Kaplancıklı, Z.A.; Tunalı, Y.; Karaca, H. Synthesis and antimicrobial activity of some new hydrazone-bridged thiazole-pyrrole derivatives. J. Enz. Inhib. Med. Chem. 2013, 28, 830–835. [Google Scholar] [CrossRef]
- Stana, A.; Enache, A.; Vodnar, D.; Nastasă, C.; Benedec, D.; Ionuț, I.; Login, C.; Marc, G.; Oniga, O.; Tiperciuc, B. New Thiazolyl-triazole Schiff Bases: Synthesis and Evaluation of the Anti-Candida Potential. Molecules 2016, 21, 1595. [Google Scholar] [CrossRef]
- Sarojini, B.K.; Krishna, B.G.; Darshanraj, C.G.; Bharath, B.R.; Manjunatha, H. Synthesis, characterization, in vitro and molecular docking studies of new 2,5-dichloro thienyl substituted thiazole derivatives for antimicrobial properties. Eur. J. Med. Chem. 2010, 45, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Karegoudar, P.; Karthikeyan, M.S.; Prasad, D.J.; Mahalinga, M.; Holla, B.S.; Kumari, N.S. Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.I.; Gonçalves de Souza, I.; Borelli, B.M.; Silvério Matos, T.T.; Santos Teixeira, I.N.; Ramos, J.P.; Maria de Souza Fagundes, E.; de Oliveira Fernandes, P.; Maltarollo, V.G.; Johann, S.; et al. Synthesis, molecular modeling studies and evaluation of antifungal activity of a novel series of thiazole derivatives. Eur. J. Med. Chem. 2018, 151, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Castillo, Y. Bacterial β-Ketoacyl-Acyl Carrier Protein Synthase III (FabH): An Attractive Target for the Design of New Broad-Spectrum Antimicrobial Agents. Mini-Rev. Med. Chem. 2008, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Li, Z.-L.; Zhu, H.-L. Advances in the Research of β-Ketoacyl-ACP Synthase III (FabH) Inhibitors. Curr. Med. Chem. 2012, 19, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Xue, J.-Y.; Zhu, H.-L. Design, synthesis and antibacterial activity studies of thiazole derivatives as potent ecKAS III inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4235–4238. [Google Scholar] [CrossRef]
- Lv, P.-C.; Wang, K.-R.; Yang, Y.; Mao, W.-J.; Chen, J.; Xiong, J.; Zhu, H.-L. Design, synthesis and biological evaluation of novel thiazole derivatives as potent FabH inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6750–6754. [Google Scholar] [CrossRef]
- Kaiser, M.; Mäser, P.; Tadoori, L.P.; Ioset, J.-R.; Brun, R. Antiprotozoal Activity Profiling of Approved Drugs: A Starting Point toward Drug Repositioning. PLoS ONE 2015, 10, e0135556. [Google Scholar] [CrossRef]
- de Oliveira Filho, G.B.; Cardoso, M.V.d.O.; Espíndola, J.W.P.; Oliveira e Silva, D.A.; Ferreira, R.S.; Coelho, P.L.; dos Anjos, P.S.; Santos, E.d.S.; Meira, C.S.; Moreira, D.R.M.; et al. Structural design, synthesis and pharmacological evaluation of thiazoles against Trypanosoma cruzi. Eur. J. Med. Chem. 2017, 141, 346–361. [Google Scholar] [CrossRef]
- Nava-Zuazo, C.; Chávez-Silva, F.; Moo-Puc, R.; Chan-Bacab, M.J.; Ortega-Morales, B.O.; Moreno-Díaz, H.; Díaz-Coutiño, D.; Hernández-Núñez, E.; Navarrete-Vázquez, G. 2-Acylamino-5-nitro-1,3-thiazoles: Preparation and in vitro bioevaluation against four neglected protozoan parasites. Bioorg. Med. Chem. 2014, 22, 1626–1633. [Google Scholar] [CrossRef]
- Bueno, J.M.; Carda, M.; Crespo, B.; Cuñat, A.C.; de Cozar, C.; León, M.L.; Marco, J.A.; Roda, N.; Sanz-Cervera, J.F. Design, synthesis and antimalarial evaluation of novel thiazole derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Ghosh, S.K.; Gahtori, P.; Pratap Singh, U.; Bhattacharyya, D.R.; Bhat, H.R. In silico ADMET study, docking, synthesis and antimalarial evaluation of thiazole-1,3,5-triazine derivatives as Pf-DHFR inhibitor. Pharmacol. Rep. 2019, 71, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Thakur, P.K.; Kannan, T. In vitro and in silico antimalarial activity of 2-(2-hydrazinyl)thiazole derivatives. Eur. J. Pharm. Sci. 2014, 52, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Moghimi, S.; Foroumadi, A. Thiazole in the targeted anticancer drug discovery. Future Med. Chem. 2019, 11, 1929–1952. [Google Scholar] [CrossRef]
- Finiuk, N.S.; Hreniuh, V.P.; Ostapiuk, Y.V.; Matiychuk, V.S.; Frolov, D.A.; Obushak, M.D.; Stoika, R.S.; Babsky, A.M. Antineoplastic activity of novel thiazole derivatives. Biopolym. Cell 2017, 33, 135–146. [Google Scholar] [CrossRef]
- George, R.F.; Samir, E.M.; Abdelhamed, M.N.; Abdel-Aziz, H.A.; Abbas, S.E.S. Synthesis and anti-proliferative activity of some new quinoline based 4,5-dihydropyrazoles and their thiazole hybrids as EGFR inhibitors. Bioorg. Chem. 2019, 83, 186–197. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Lam, W.; Guo, W.; Feng, Y.; Cheng, Y.-C. Targeting tumour microenvironment by tyrosine kinase inhibitor. Mol. Cancer 2018, 17, 43. [Google Scholar] [CrossRef]
- Gagic, Z.; Ruzic, D.; Djokovic, N.; Djikic, T.; Nikolic, K. In silico Methods for Design of Kinase Inhibitors as Anticancer Drugs. Front. Chem. 2020, 7, 1–25. [Google Scholar] [CrossRef]
- de Siqueira, L.R.P.; de Moraes Gomes, P.A.T.; de Lima Ferreira, L.P.; de Melo Rêgo, M.J.B.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef]
- Tridente, G. Dabrafenib. In Adverse Events and Oncotargeted Kinase Inhibitors; Elsevier Inc.: London, UK, 2017; pp. 505–530. ISBN 9780128094006. [Google Scholar]
- Odogwu, L.; Mathieu, L.; Blumenthal, G.; Larkins, E.; Goldberg, K.B.; Griffin, N.; Bijwaard, K.; Lee, E.Y.; Philip, R.; Jiang, X.; et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring BRAF V600E Mutations. Oncologist 2018, 23, 740–745. [Google Scholar] [CrossRef]
- Gomha, S.M.; Kheder, N.A.; Abdelaziz, M.R.; Mabkhot, Y.N.; Alhajoj, A.M. A facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety. Chem. Cent. J. 2017, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wu, Y.; Zhang, J.; Mei, Q.; Zhang, Y.; Zhu, N.; Liu, R.; Zhang, H. Design, synthesis and biological evaluations of novel pyridone-thiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Zhang, Q.; Shan, Y.; Gu, W.; Wang, S. Design, synthesis and biological evaluation of novel β-pinene-based thiazole derivatives as potential anticancer agents via mitochondrial-mediated apoptosis pathway. Bioorg. Chem. 2019, 84, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Anuradha; Patel, S.; Patle, R.; Parameswaran, P.; Jain, A.; Shard, A. Design, computational studies, synthesis and biological evaluation of thiazole-based molecules as anticancer agents. Eur. J. Pharm. Sci. 2019, 134, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.-F.; Ho, C.-L.; Chen, Y.-C.; Wu, W.-J.; Dai, F.-R.; Chui, C.-H.; Huang, S.-P.; Guo, K.-P.; Lin, J.-T.; Tian, H.; et al. New bithiazole-functionalized organic photosensitizers for dye-sensitized solar cells. Dyes Pigments 2013, 96, 516–524. [Google Scholar] [CrossRef]
- Liu, L.; Lam, Y.-W.; Wong, W.-Y. Complexation of 4,4′-di(tert-butyl)-5-ethynyl-2,2′-bithiazole with mercury(II) ion: Synthesis, structures and analytical applications. J. Organomet. Chem. 2006, 691, 1092–1100. [Google Scholar] [CrossRef]
- Cao, J.; Curtis, M.D. Synthesis and characterizations of oligomeric (4,4′-dialkyl-2,2′-bithiazole) carboxylic acids and their LB-film behavior. Synth. Met. 2005, 148, 219–226. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd(OAc)2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Khavasi, H.R.; Abedi, A.; Amani, V.; Notash, B.; Safari, N. Synthesis, characterization and crystal structure determination of zinc (II) and mercury (II) complexes with 2,2′-dimethyl-4,4′-bithiazole. Polyhedron 2008, 27, 1848–1854. [Google Scholar] [CrossRef]
- Sasaki, H. Synthesis of 2,2′-Bis(3,6,9-triazanonyl)-4,4′-bithiazole and Related Compounds as New DNA Cleavage Agents. Chem. Pharm. Bull. (Tokyo) 2007, 55, 1762–1767. [Google Scholar] [CrossRef][Green Version]
- Hosseinian, A.; Mahjoub, A.R. Synthesis, structural characterization and thermal properties of a new Cd(II) complex: As bithiazole precursor for preparation of CdS nanoparticles. J. Mol. Struct. 2011, 985, 270–276. [Google Scholar] [CrossRef]
- Lee, B.W.; Lee, S.D. [5,5] Sigmatropic shift of N-phenyl-N′-(2-thiazolyl)hydrazines and N,N′-bis(2-thiazolyl)hydrazines into 2-amino-5-(p-aminophenyl)thiazoles and 5,5′-bis(2-aminothiazole) derivatives. Tetrahedron Lett. 2000, 41, 3883–3886. [Google Scholar] [CrossRef]
- Turner, G.L.; Morris, J.A.; Greaney, M.F. Direct Arylation of Thiazoles on Water. Angew. Chem. Int. Ed. 2007, 46, 7996–8000. [Google Scholar] [CrossRef]
- Stanetty, P.; Schnürch, M.; Mihovilovic, M.D. Halogenated 2‘-Chlorobithiazoles via Pd-Catalyzed Cross-Coupling Reactions. J. Org. Chem. 2006, 71, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Ikegami, H.; Mori, A. Palladium-Catalyzed C−H Homocoupling of Thiophenes: Facile Construction of Bithiophene Structure. J. Am. Chem. Soc. 2004, 126, 5074–5075. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Huang, Y.; Tang, C.; Xu, J.; Wu, X.; Yao, H. Efficient palladium(II)-catalyzed homocoupling of thiazole-4-carboxylic or oxazole-4-carboxylic derivatives. Tetrahedron 2011, 67, 5550–5555. [Google Scholar] [CrossRef]
- Bach, T.; Heuser, S. Synthesis of 2‘-Substituted 4-Bromo-2,4‘-bithiazoles by Regioselective Cross-Coupling Reactions. J. Org. Chem. 2002, 67, 5789–5795. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.D.; Yoo, C.; Butler, J.D.; Yang, B.; Verkman, A.S.; Kurth, M.J. Design and synthesis of a hybrid potentiator–corrector agonist of the cystic fibrosis mutant protein ΔF508-CFTR. Bioorg. Med. Chem. Lett. 2010, 20, 87–91. [Google Scholar] [CrossRef][Green Version]
- Davison, H.R.; Solano, D.M.; Phuan, P.-W.; Verkman, A.S.; Kurth, M.J. Fluorinated ΔF508-CFTR correctors and potentiators for PET imaging. Bioorg. Med. Chem. Lett. 2012, 22, 1602–1605. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Kuo, Y.-H.; Tien, Y.-W.; Ke, Y.-Y.; Chang, W.-T.; Chang, H.-F.; Ou, L.-C.; Law, P.-Y.; Xi, J.-H.; Tao, P.-L.; et al. The in vivo antinociceptive and μ-opioid receptor activating effects of the combination of N-phenyl-2′,4′-dimethyl-4,5′-bi-1,3-thiazol-2-amines and naloxone. Eur. J. Med. Chem. 2019, 167, 312–323. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Ramakrishna, S.; Rama Murthy, K. Synthesis of novel isoxazolyl bis-thiazolo[3,2-a]pyrimidines. Chin. Chem. Lett. 2012, 23, 899–902. [Google Scholar] [CrossRef]
- Hosny, M.; Salem, M.E.; Darweesh, A.F.; Elwahy, A.H.M. Synthesis of Novel Bis(thiazolylchromen-2-one) Derivatives Linked to Alkyl Spacer via Phenoxy Group. J. Heterocycl. Chem. 2018, 55, 2342–2348. [Google Scholar] [CrossRef]
- Shawali, A.S. A review on bis-hydrazonoyl halides: Recent advances in their synthesis and their diverse synthetic applications leading to bis-heterocycles of biological interest. J. Adv. Res. 2016, 7, 873–907. [Google Scholar] [CrossRef] [PubMed]
- Borcea, A.-M.; Marc, G.; Pîrnău, A.; Vlase, L.; Ionuț, I.; Tiperciuc, B.; Oniga, O. Synthesis and molecular docking study of some new 1,4-phenylene-bisthiazoles as fungal lanosterol 14α-demethylase inhibitors. Farmacia 2017, 65, 683–689. [Google Scholar]
- Hassan, A.A.; El-Sheref, E.M. Chemistry and heterocyclization of dithiobiurea and thioureidoalkylthiourea. J. Heterocycl. Chem. 2010, 47, 764–784. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdelrazek, F.H.; Abdelrahman, A.; Metz, P. Synthesis of Some Novel Thiazole, Thiadiazole and 1,4-Phenylene-bis-thiazole Derivatives as Potent Antitumor Agents. Heterocycles 2016, 92, 954. [Google Scholar] [CrossRef]
- Gomha, S.M.; Farghaly, T.A.; Sayed, A.R. Design, Synthesis, and Characterization of Some New bis -thiazoles. J. Heterocycl. Chem. 2017, 54, 1537–1542. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Kassab, R.M.; Gomha, S.M. Synthesis and characterization of some novel bis-thiazoles. J. Heterocycl. Chem. 2019, 56, 3157–3163. [Google Scholar] [CrossRef]
- Williams, D.R.; Patnaik, S.; Clark, M.P. Total Synthesis of Cystothiazoles A and C. J. Org. Chem. 2001, 66, 8463–8469. [Google Scholar] [CrossRef]
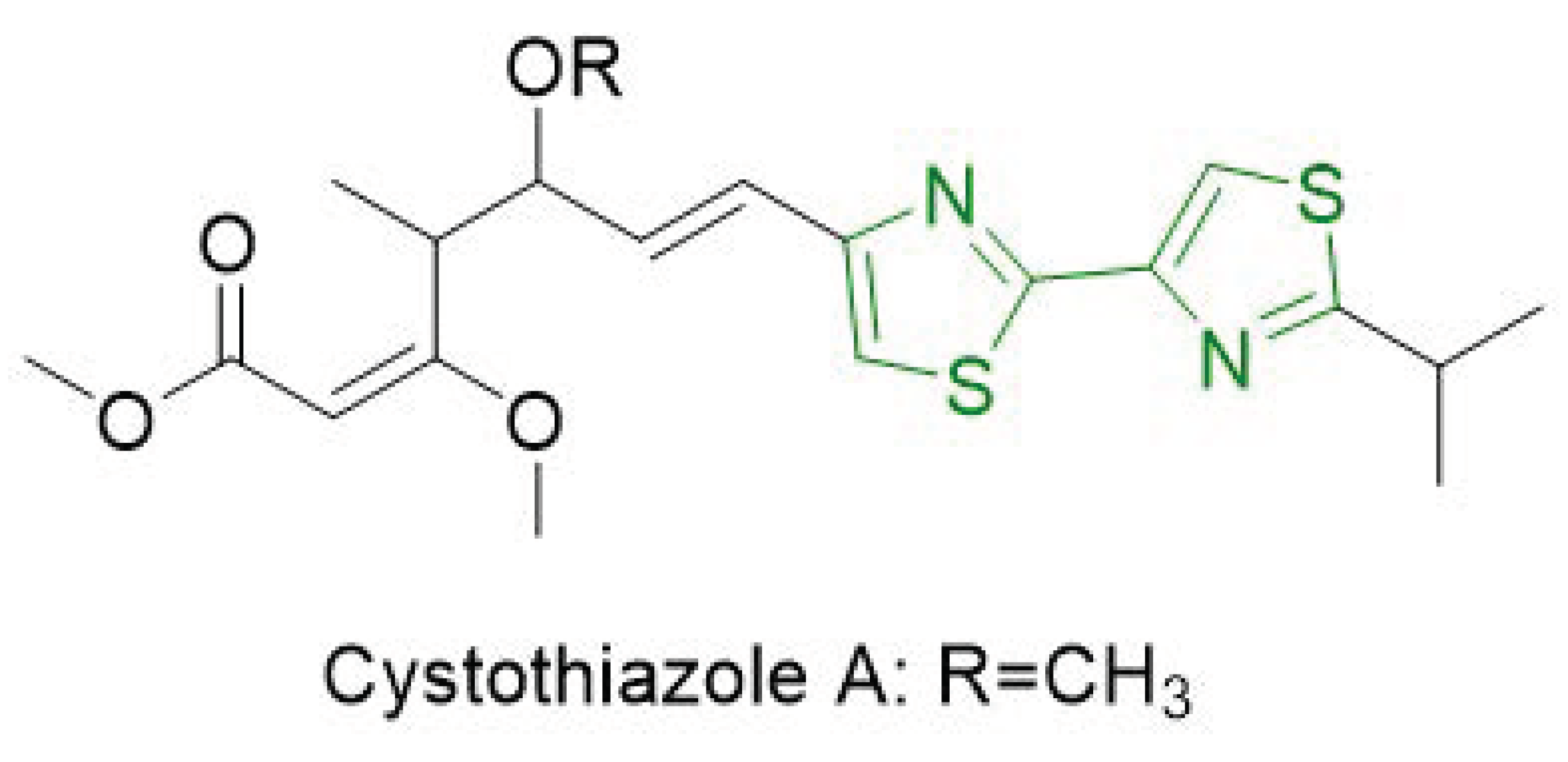
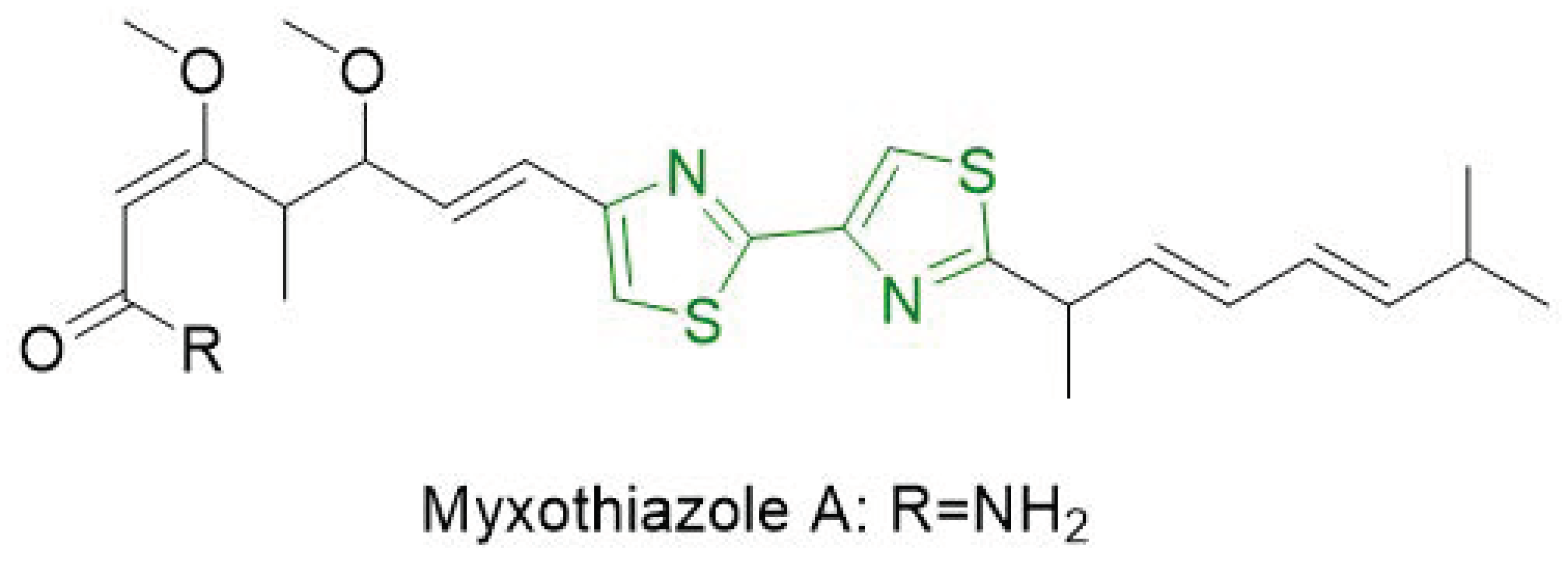
- de Souza, M.V.N. Synthesis and biological activity of natural thiazoles: An important class of heterocyclic compounds. J. Sulfur Chem. 2005, 26, 429–449. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Jang, K.H.; Yang, H.-C.; Oh, K.-B.; Lee, H.-S.; Shin, J. Bithiazole Metabolites from the Myxobacterium Myxococcus fulvus. Chem. Pharm. Bull. (Tokyo) 2007, 55, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.O.; Parvizi, J.; Sharifzadeh, B.; Rassa, M. Facile Regioselective Synthesis of Novel bis -Thiazole Derivatives and Their Antimicrobial Activity. Arch. Pharm. (Weinheim) 2013, 346, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Bikobo, D.S.N.; Vodnar, D.C.; Stana, A.; Tiperciuc, B.; Nastasă, C.; Douchet, M.; Oniga, O. Synthesis of 2-phenylamino-thiazole derivatives as antimicrobial agents. J. Saudi Chem. Soc. 2017, 21, 861–868. [Google Scholar] [CrossRef]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New Series of Thiazole Derivatives: Synthesis, Structural Elucidation, Antimicrobial Activity, Molecular Modeling and MOE Docking. Molecules 2019, 24, 1741. [Google Scholar] [CrossRef] [PubMed]
- Abhale, Y.K.; Sasane, A.V.; Chavan, A.P.; Deshmukh, K.K.; Kotapalli, S.S.; Ummanni, R.; Sayyad, S.F.; Mhaske, P.C. Synthesis and biological screening of 2′-aryl/benzyl-2-aryl-4-methyl-4′,5-bithiazolyls as possible anti-tubercular and antimicrobial agents. Eur. J. Med. Chem. 2015, 94, 340–347. [Google Scholar] [CrossRef]
- Abhale, Y.K.; Shinde, A.D.; Deshmukh, K.K.; Nawale, L.; Sarkar, D.; Choudhari, P.B.; Kumbhar, S.S.; Mhaske, P.C. Synthesis, antimycobacterial screening and molecular docking studies of 4-aryl-4′-methyl-2′-aryl-2,5′-bisthiazole derivatives. Med. Chem. Res. 2017, 26, 2889–2899. [Google Scholar] [CrossRef]
- Bondock, S.; Fouda, A.M. Synthesis and evaluation of some new 5-(hetaryl)thiazoles as potential antimicrobial agents. Synth. Commun. 2018, 48, 561–573. [Google Scholar] [CrossRef]
- Borcea, A.-M.; Marc, G.; Ionuț, I.; Vodnar, D.C.; Vlase, L.; Gligor, F.; Pricopie, A.; Pîrnău, A.; Tiperciuc, B.; Oniga, O. A Novel Series of Acylhydrazones as Potential Anti-Candida Agents: Design, Synthesis, Biological Evaluation and In Silico Studies. Molecules 2019, 24, 184. [Google Scholar] [CrossRef]
- Liu, Z.; Wenzler, T.; Brun, R.; Zhu, X.; Boykin, D.W. Synthesis and antiparasitic activity of new bis-arylimidamides: DB766 analogs modified in the terminal groups. Eur. J. Med. Chem. 2014, 83, 167–173. [Google Scholar] [CrossRef]
- Bansal, K.K.; Bhardwaj, J.K.; Saraf, P.; Thakur, V.K.; Sharma, P.C. Synthesis of thiazole clubbed pyrazole derivatives as apoptosis inducers and anti-infective agents. Mater. Today Chem. 2020, 17, 100335. [Google Scholar] [CrossRef]
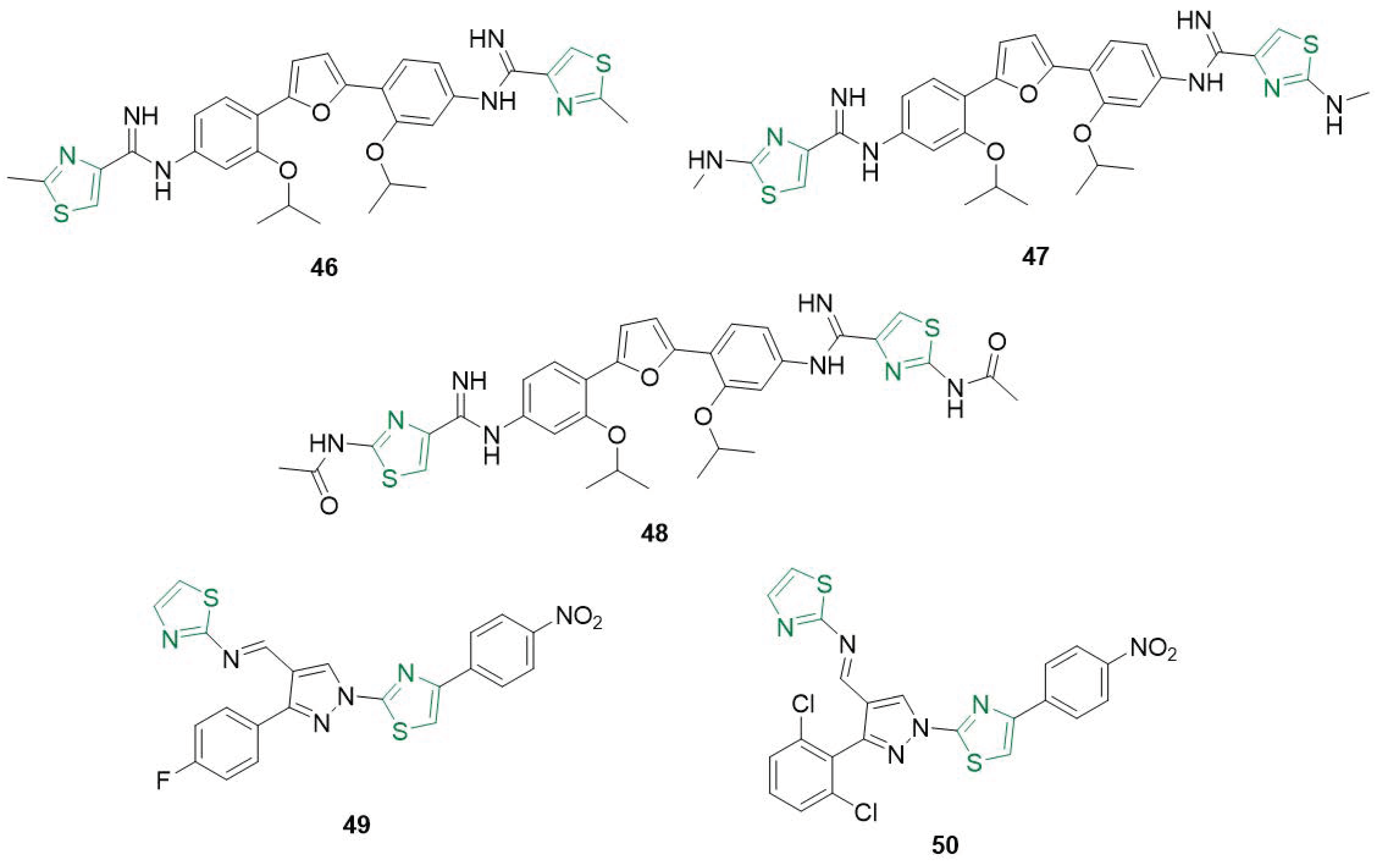
- Turan-Zitouni, G.; Altıntop, M.D.; Özdemir, A.; Kaplancıklı, Z.A.; Çiftçi, G.A.; Temel, H.E. Synthesis and evaluation of bis-thiazole derivatives as new anticancer agents. Eur. J. Med. Chem. 2016, 107, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lucena, D.; Gaboriau, F.; Rivault, F.; Schalk, I.J.; Lescoat, G.; Mislin, G.L.A. Synthesis and biological properties of iron chelators based on a bis-2-(2-hydroxy-phenyl)-thiazole-4-carboxamide or -thiocarboxamide (BHPTC) scaffold. Bioorg. Med. Chem. 2010, 18, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, T.A.; El-Metwaly, N.; Al-Soliemy, A.M.; Katouah, H.A.; Muhammad, Z.A.; Sabour, R. Synthesis, Molecular Docking and Antitumor Activity of New Dithiazoles. Polycycl. Aromat. Compd. 2019, 1–17. [Google Scholar] [CrossRef]
- Sayed, A.R.; Gomha, S.M.; Taher, E.A.; Muhammad, Z.A.; El-Seedi, H.R.; Gaber, H.M.; Ahmed, M.M. One-pot synthesis of novel thiazoles as potential anti-cancer agents. Drug Des. Dev. Ther. 2020, 14, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chai, H.; Su, M.B.; Zhang, Y.M.; Li, J.; Xie, X.; Nan, F.J. Potent and orally efficacious bisthiazole-based histone deacetylase inhibitors. ACS Med. Chem. Lett. 2014, 5, 628–633. [Google Scholar] [CrossRef]
- Gong, C.J.; Gao, A.H.; Zhang, Y.M.; Su, M.B.; Chen, F.; Sheng, L.; Zhou, Y.B.; Li, J.Y.; Li, J.; Nan, F.J. Design, synthesis and biological evaluation of bisthiazole-based trifluoromethyl ketone derivatives as potent HDAC inhibitors with improved cellular efficacy. Eur. J. Med. Chem. 2016, 112, 81–90. [Google Scholar] [CrossRef]
- Fairhurst, R.A.; Imbach-Weese, P.; Gerspacher, M.; Caravatti, G.; Furet, P.; Zoller, T.; Fritsch, C.; Haasen, D.; Trappe, J.; Guthy, D.A.; et al. Identification and optimisation of a 4′,5-bisthiazole series of selective phosphatidylinositol-3 kinase alpha inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3569–3574. [Google Scholar] [CrossRef]
- Fairhurst, R.A.; Gerspacher, M.; Imbach-Weese, P.; Mah, R.; Caravatti, G.; Furet, P.; Fritsch, C.; Schnell, C.; Blanz, J.; Blasco, F.; et al. Identification and optimisation of 4,5-dihydrobenzo[1,2-d:3,4-d]bisthiazole and 4,5-dihydrothiazolo[4,5-h]quinazoline series of selective phosphatidylinositol-3 kinase alpha inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3575–3581. [Google Scholar] [CrossRef]



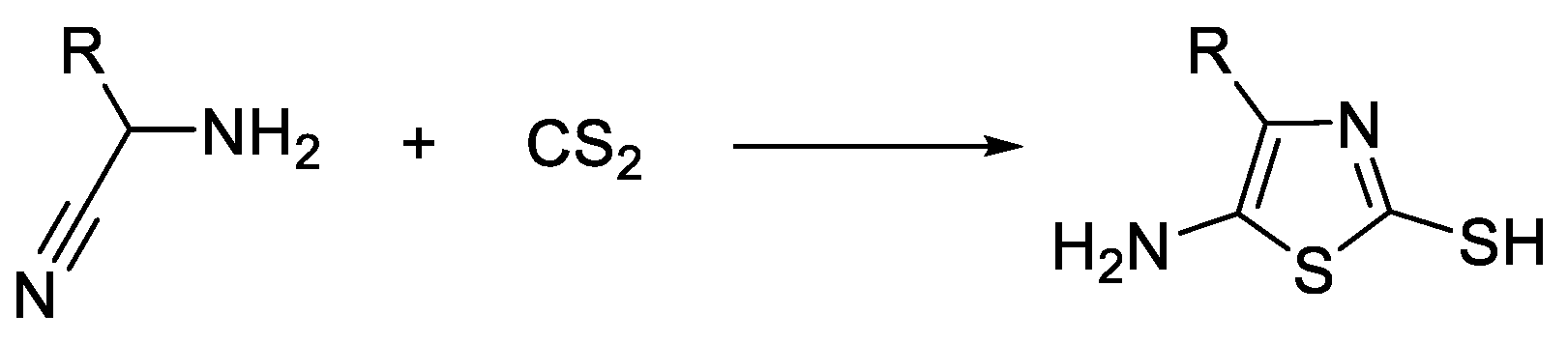
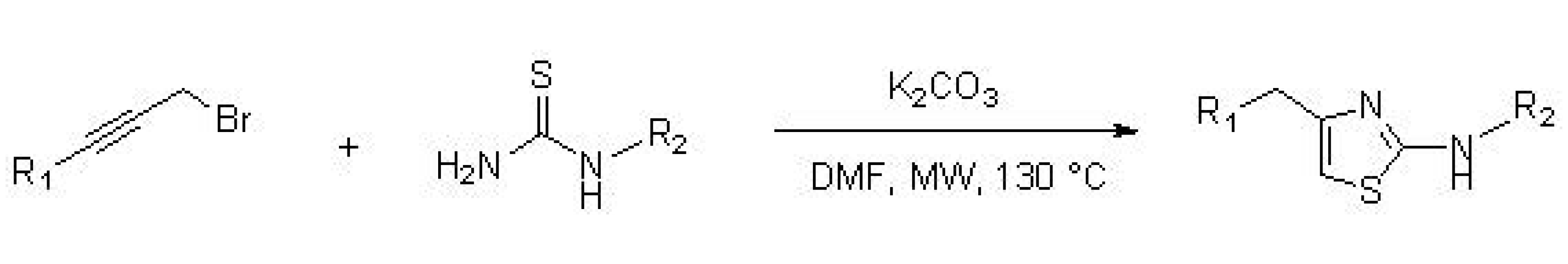



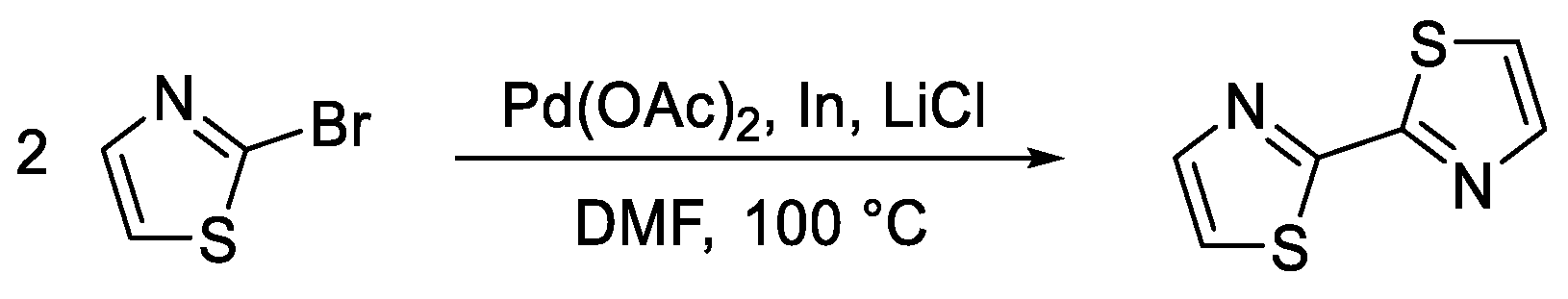

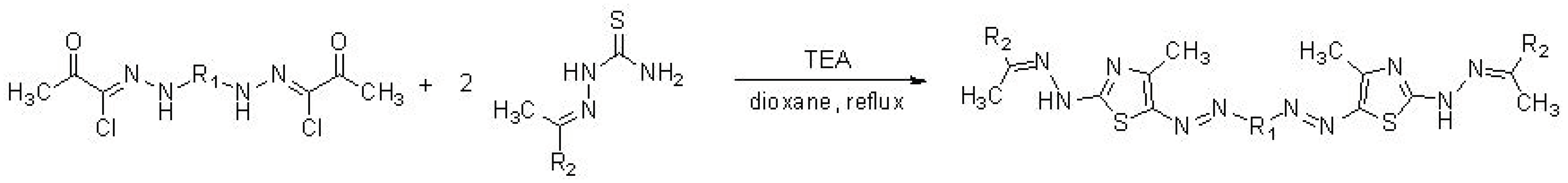


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borcea, A.-M.; Ionuț, I.; Crișan, O.; Oniga, O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules 2021, 26, 624. https://doi.org/10.3390/molecules26030624
Borcea A-M, Ionuț I, Crișan O, Oniga O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules. 2021; 26(3):624. https://doi.org/10.3390/molecules26030624
Chicago/Turabian StyleBorcea, Anca-Maria, Ioana Ionuț, Ovidiu Crișan, and Ovidiu Oniga. 2021. "An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives" Molecules 26, no. 3: 624. https://doi.org/10.3390/molecules26030624
APA StyleBorcea, A.-M., Ionuț, I., Crișan, O., & Oniga, O. (2021). An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules, 26(3), 624. https://doi.org/10.3390/molecules26030624





