Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy
Abstract
1. Introduction
2. Results
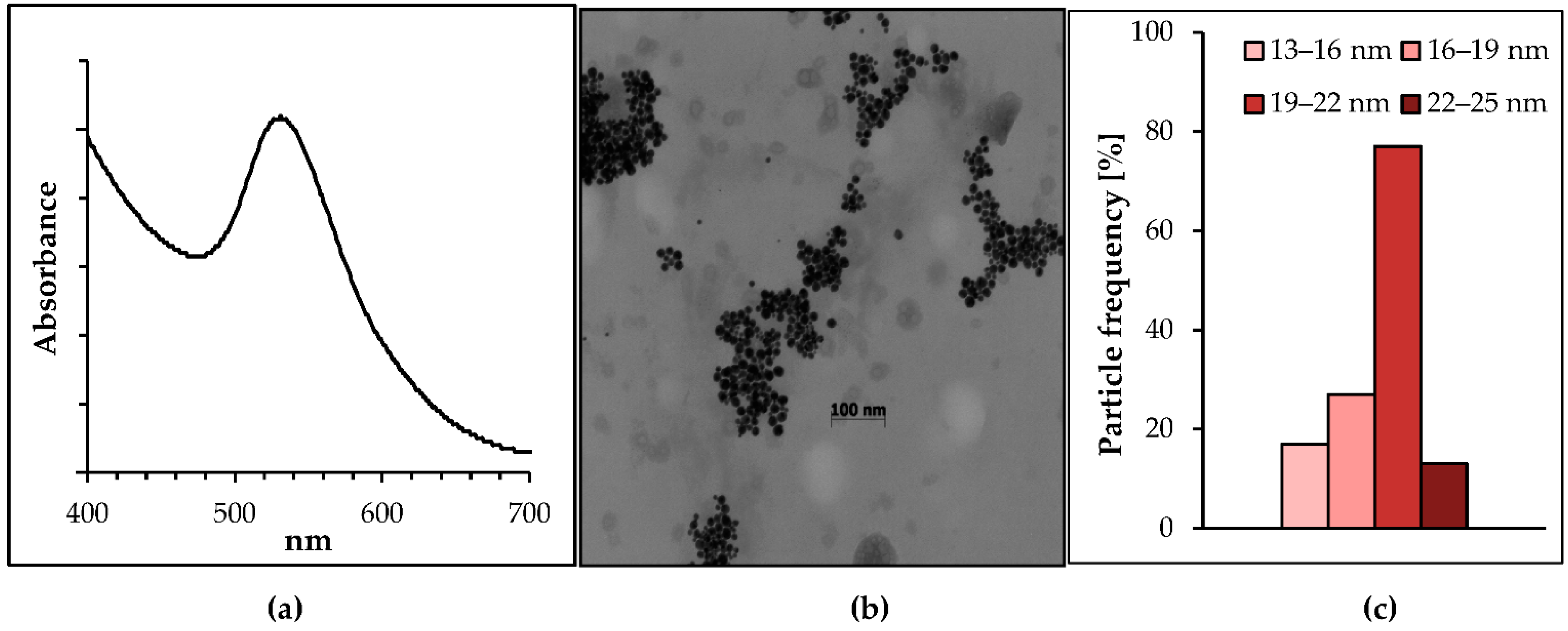
2.1. Synthesis and Characterization of the Biogenic Gold Nanoparticles
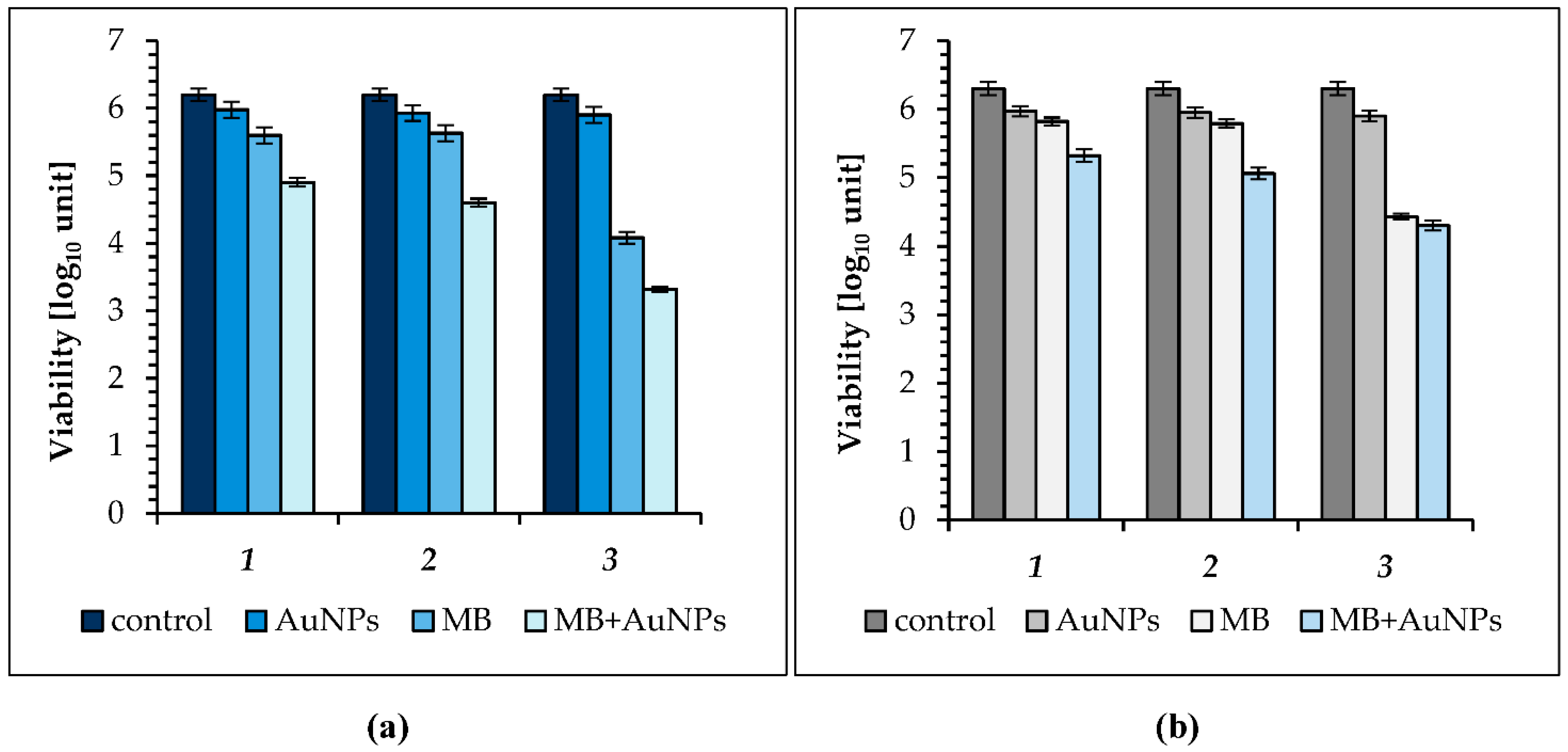
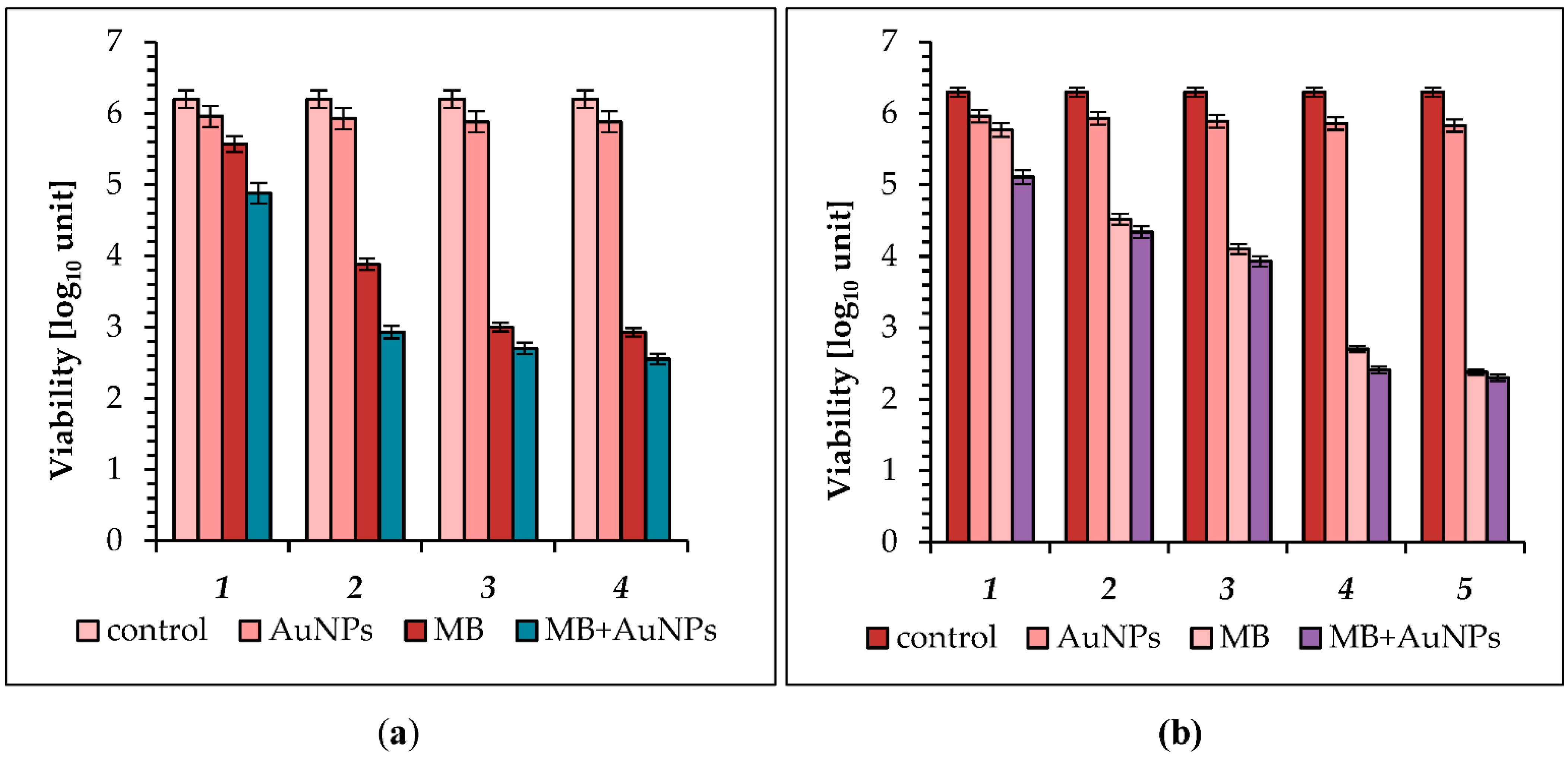
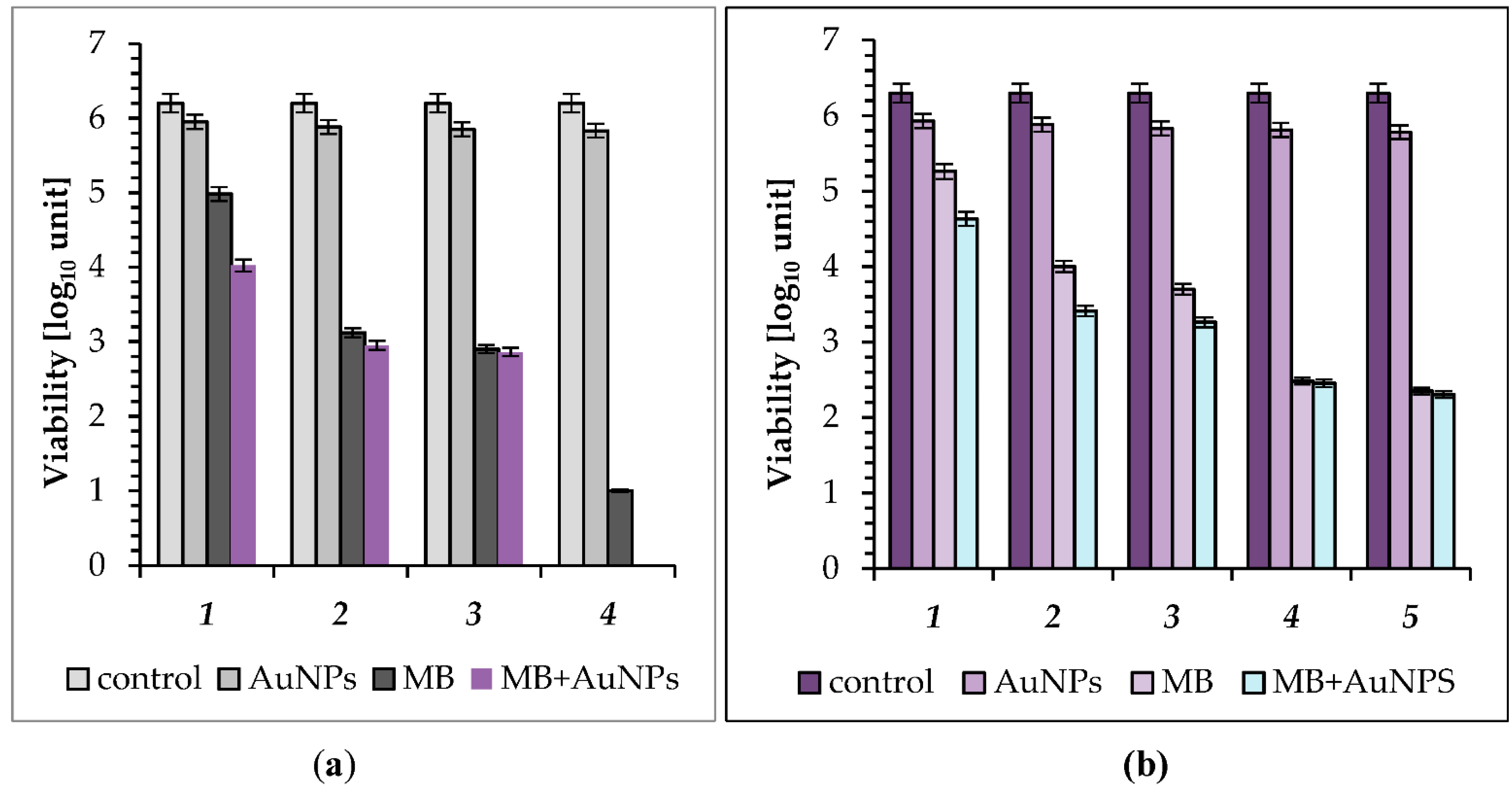
2.2. Photo-Inactivation of Bacteria
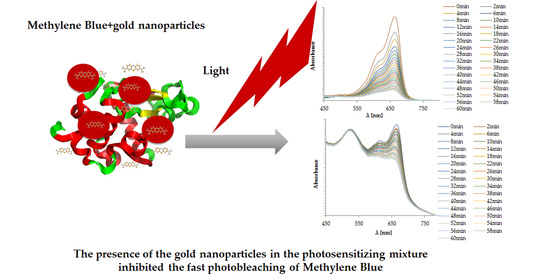
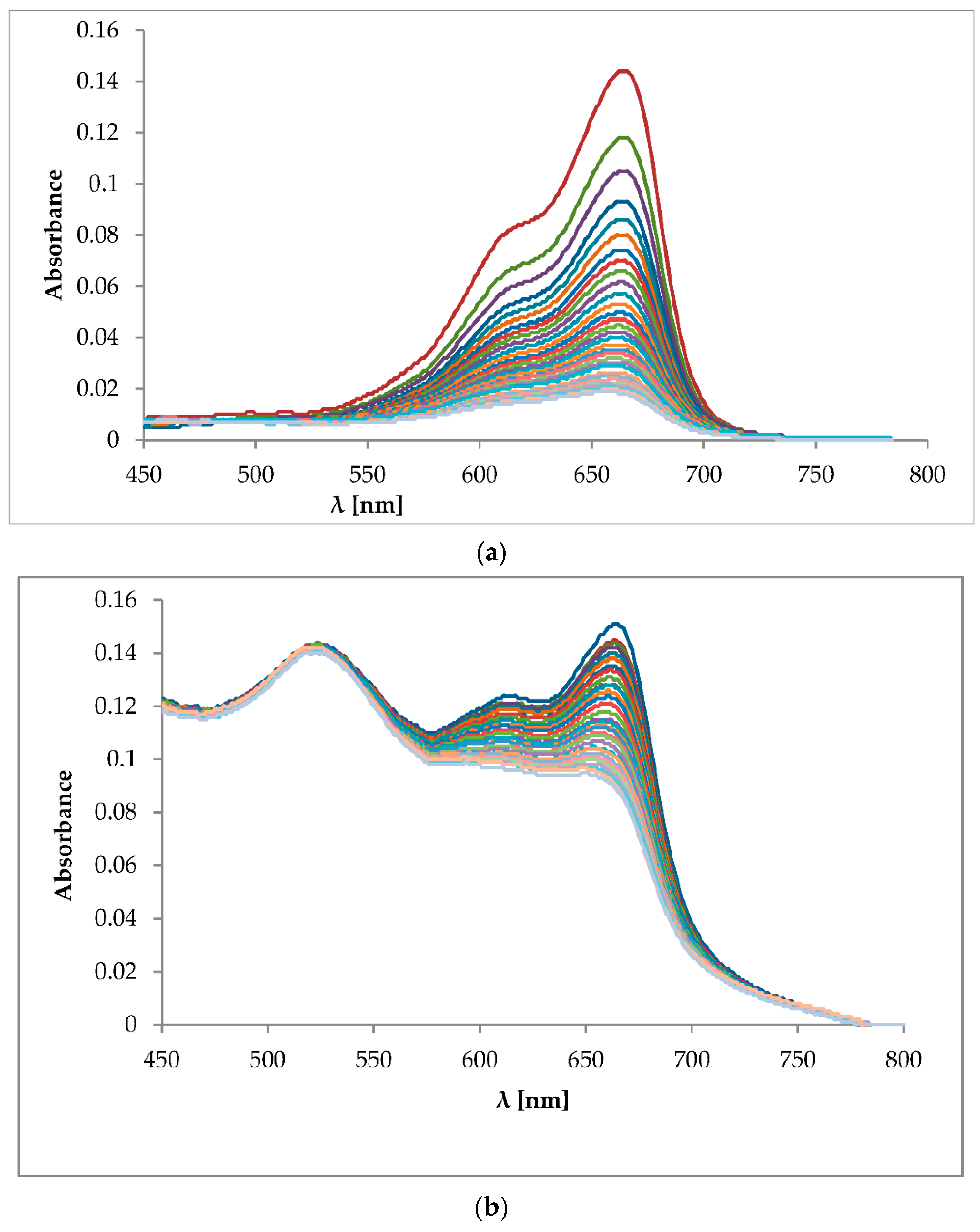
2.3. Photobleaching of Methylene Blue
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Light Source
4.3. Synthesis and Characterization of Gold Nanoparticles
4.4. Dark Toxicity Assays
4.5. In Vitro Photodynamic Inactivation of Bacteria
4.6. Photobleaching of MB
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dancer, S.J. Hospital cleaning in the 21st century. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; Macgregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Coia, J.E.; Hamilton, K.; Taggart, I.; Watson, S.B.; Thakker, B.; Gettinby, G. Environmental decontamination of a hospital isolation room using high-intensity narrow-spectrum light. J. Hosp. Infect. 2011, 76, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Maclean, M.; McKenzie, K.; Anderson, J.G.; Gettinby, G.; MacGregor, S.J. 405 nm light technology for the inactivation of pathogens and its potential role for environmental disinfection and infection control. J. Hosp. Infect. 2014, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef]
- Karrer, S.; Szeimies, R.M.; Hohenleutner, U.; Landthaler, M. Role of lasers and photodynamic therapy in the treatment of cutaneous malignancy. Am. J. Clin. Dermatol. 2001, 2, 229–237. [Google Scholar] [CrossRef]
- Ericson, M.B.; Wennberg, A.M.; Larkö, O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther. Clin. Risk. Manag. 2008, 4, 1–9. [Google Scholar]
- Leman, J.A.; Dick, D.C.; Morton, C.A. Topical 5-ALA photodynamic therapy for the treatment of cutaneous T-cell lymphoma. Clin. Exp. Dermatol. 2002, 27, 516–518. [Google Scholar] [CrossRef]
- Karakullukcu, B.; Oudenaarde, K.; Copper, M.P.; Klop, W.M.C.; Veen, R.; Wildeman, M.; Tan, I.B. Photodynamic therapy of early stage oral cavity and oropharynx neoplasms: An outcome analysis of 170 patients. Eur. Arch. Otorhinolaryngol. 2011, 268, 281–288. [Google Scholar] [CrossRef]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.E.; Takes, R.P.; Poorten, V.V.; Ferlito, A.A. Review of photodynamic therapy for neoplasms of the head and neck. Adv. Ther. 2018, 35, 1–18. [Google Scholar] [CrossRef]
- Huang, Z. A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 2005, 4, 283–293. [Google Scholar] [CrossRef]
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections-state of the art. Photodiagn. Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Derosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill gram-negative bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Camerin, M.M.; Soncin Guidolin, L.; Coppellotti, O. Antimicrobial photodynamic therapy: Basic principles. In Photodynamic Inactivation of Microbial Pathogens, Medical and Environmental Applications; Hamblin, M.R., Jori., G., Eds.; RSC: Cambridge, UK, 2011; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Huang, W.C.; Tsai, P.J.; Chen, Y.C. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine 2007, 2, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of gold and silver nanoparticles with bacterial biofilms: Molecular interactions behind inhibition and resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P.; Pratten, J.; Parkin, I.P.; Wilson, M. Nanoparticles: Their potential use in antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 2011, 10, 712–720. [Google Scholar] [CrossRef]
- Maliszewska, I.; Leśniewska, A.; Olesiak-Bańska, J.; Matczyszyn, K.; Samoć, M. Biogenic gold nanoparticles enhance methylene blue-induced phototoxic effect on Staphylococcus epidermidis. J. Nanopart. Res. 2014, 16, 2457. [Google Scholar] [CrossRef]
- Maliszewska, I.; Lisiak, B.; Popko, K.; Matczyszyn, K. Enhancement of rose bengal-mediated photodynamic fungicidal efficacy against Candida albicans in the presence of biogenic gold nanoparticles. Photochem. Photobiol. 2017, 93, 1081–1091. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Zhang, J.; Aslan, H.; Dong, M. Photoactive antimicrobial nanomaterials. J. Mater. Chem. B 2017, 5, 8631–8652. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, L.; Sun, D.; Chen, H.; Yang, D.; Li, Q. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Kumar, C.G.; Poornachandraa, Y.; Chandrasekhara, C. Green synthesis of bacterial mediated anti-proliferative gold nanoparticles: Inducing mitotic arrest (G2/M phase) and apoptosis (intrinsic pathway). Nanoscale 2015, 7, 18738–18750. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; de Oliveirab, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; Turchiello, R.F.; Mauricio, S.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.H.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Tuite, E.M.; Kelly, J.M. Photochemical interactions of methylene blue and analogues with DNA and other biological substrates. J. Photochem. Photobiol. B 1993, 21, 103–124. [Google Scholar] [CrossRef]
- Fisher, A.M.R.; Murphree, A.L.; Gomer, C.J. Clinical and preclinical photodynamic therapy. Lasers Surg. Med. 1995, 17, 2–31. [Google Scholar] [CrossRef]
- Lambrecht, B.; Mohr, H.; Knüver-Hopf, J.; Schmitt, H. Photoinactivation of viruses in human fresh plasma by phenothiazine dyes in combination with visible light. Vox Sang. 1991, 60, 207–213. [Google Scholar] [CrossRef]
- Bachmann, B.; Knüver-Hopf, J.; Lambrecht, B.; Mohr, H. Target structures for HIV-1 inactivation by methylene blue and light. J. Med. Virol. 1995, 47, 172–178. [Google Scholar] [CrossRef]
- Wainwright, M. Phenothiazinium photosensitisers: V. Photobactericidal activities of chromophore-methylated phenothiazinium salts. Dyes Pigm. 2007, 73, 7–12. [Google Scholar] [CrossRef]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Nejat, A.M.; Kalhori, K.A.M. Antimicrobial photodynamic therapy with nanoparticles versus conventional photosensitizer in oral diseases nanostructures for antimicrobial therapy. In Nanostructures for Antimicrobial Therapy, 1st ed.; Ficai, A., Grumezescu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 10, pp. 237–259. [Google Scholar] [CrossRef]
- Martínez-Torres, A.C.; Zarate-Triviño, D.G.; Lorenzo-Anota, H.Y.; Ávila-Ávila, A.; Rodríguez-Abrego, C.; Rodríguez-Padilla, C. Chitosan gold nanoparticles induce cell death in HeLa and MCF-7 cells through reactive oxygen species production. Int. J. Nanomed. 2018, 13, 3235–3250. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajadram, R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Narband, N.; Uppal, M.; Dunnill, C.W.; Hyett, G.; Wilson, M.; Parkin, I.P. The interaction between gold nanoparticles and cationic and anionic dyes: Enhanced UV-visible absorption. Phys. Chem. Chem. Phys. 2009, 11, 10513–10518. [Google Scholar] [CrossRef]
- Nilsson, R.; Merkel, P.B.; Kearns, D.R. Kinetic properties of the triplet states of methylene blue and other photosensitizing dyes. Photochem. Photobiol. 1972, 16, 109–116. [Google Scholar] [CrossRef]
- Kayser, R.H.; Young, R.H. The photoreduction of methylene blue by amines-I. A flash photolysis study of the reaction between triplet methylene blue and amines. Photochem. Photobiol. 1976, 24, 395–401. [Google Scholar] [CrossRef]
- Lowe, R.D.; Snook, R.D. Photobleaching of Methylene Blue in continuous wave thermal lens spectrometry. Analyst 1993, 118, 613–616. [Google Scholar] [CrossRef]
- Lewis, G.N.; Goldschmid, O.; Magel, T.T.; Bigeleisen, J. Dimeric and other forms of Methylene Blue: Absorption and fluorescence of the pure monomer 1. J. Am. Chem. Soc. 1943, 65, 1150–1154. [Google Scholar] [CrossRef]
- Oster, G.; Wotherspoon, N. Photoreduction of Methylene Blue by ethylenediaminetetraacetic acid1a,b. J. Am. Chem. Soc. 1957, 79, 4836–4838. [Google Scholar] [CrossRef]
- Atherton, S.J.; Harriman, A. Photochemistry of intercalated methylene blue: Photoinduced hydrogen atom abstraction from guanine and adenine. J. Am. Chem. Soc. 1993, 115, 1816–1822. [Google Scholar] [CrossRef]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Sulforhodamine adsorbed Langmuir–Blodgett layers on silver island films: Effect of probe distance on the metal-enhanced fluorescence. J. Phys. Chem. C 2007, 111, 7091–7097. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Krasavin, A.V.; Webster, L.; Segovia, P.; Zayats, A.V.; Richards, D. Spectral variation of fluorescence lifetime near single metal nanoparticles. Sci. Rep. 2016, 6, 21349. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.; Badugu, R.; Lakowicz, J.R. Polyelectrolyte layer-by-layer assembly to control the distance between fluorophores and plasmonic nanostructures. Chem. Mater. 2007, 19, 5902–5909. [Google Scholar] [CrossRef]
- Mishra, H.; Mali, B.L.; Karolin, J.; Dragan, A.I.; Geddes, C.D. Experimental and theoretical study of the distance dependence of metal-enhanced fluorescence, phosphorescence and delayed fluorescence in a single system. Phys. Chem. Chem. Phys. 2013, 15, 19538–19544. [Google Scholar] [CrossRef]
- Hübner, C.G.; Renn, A.; Renge, I.; Wild, U.P. Direct observation of the triplet lifetime quenching of single dye molecules by molecular oxygen. J. Chem. Phys. 2001, 115, 9619–9622. [Google Scholar] [CrossRef]
- Zheng, Q.; Jockusch, S.; Zhou, Z.; Blanchard, S.C. The contribution of reactive oxygen species to the photobleaching of organic fluorophores. Photochem. Photobiol. 2014, 90, 448–454. [Google Scholar] [CrossRef]
- Zaiba, S.; Lerouge, F.; Gabudean, A.M.; Focsan, M.; Lermé, J.; Gallavardin, T.; Maury, O.; Andraud, C.; Parola, S.; Baldeck, P.L. Transparent plasmonic nanocontainers protect organic fluorophores against photobleaching. Nano Lett. 2011, 11, 2043–2047. [Google Scholar] [CrossRef]
- Bucharskaya, A.; Maslyakova., G.; Terentyuk, G.; Yakunin, A.; Avetisyan, Y.; Bibikova, O.; Tuchina, E.; Khlebtsov, B.; Khlebtsov, N.; Tuchin, V. Towards effective photothermal/photodynamic treatment using plasmonic gold nanoparticles. Int. J. Mol. Sci. 2016, 17, 1295. [Google Scholar] [CrossRef]
- Maliszewska, I.; Tylus, W.; Chęcmanowski, J.; Szczygieł, B.; Pawlaczyk-Graja, I.; Pusz, W.; Baturo-Cieśniewska, A. Biomineralization of gold by Mucor plumbeus: The progress in understanding the mechanism of nanoparticles’ formation. Biotechnol. Prog. 2017, 33, 1381–1392. [Google Scholar] [CrossRef]
- Maliszewska, I. Microbial mediated synthesis of gold nanoparticles: Preparation, characterization and cytotoxicity. Dig. J. Nanomater. Biostruct. 2013, 8, 1123–1131. [Google Scholar]


| Concentration of Methylene Blue [mgL−1] | S. aureus | E. coli |
|---|---|---|
| Values of reduction in viability [%] 1 | ||
| 6.25 | 8 ± 2 | 3 ± 1 |
| 12.5 | 11 ± 2 | 5 ± 1 |
| 25.0 | 18 ± 3 | 7 ± 1 |
| 50.0 | 28 ± 4 | 9 ± 2 |
| 100.0 | 45 ± 4 | 15 ± 3 |
| 250.0 | 61 ± 3 | 45 ± 4 |
| Energy Fluence (J cm −2)/Power Density | Mortality Rate (%) | |||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | |||||
| AuNPs | MB | MB + AuNPs | AuNPs | MB | MB + AuNPs | |
| 0.75/2.5 mW cm−2 | 40.3 ± 0.1 | 75.0 ± 0.3 | 95.0 ± 0.2 | 53 ± 1 | 67.25 ± 0.05 | 89.55 ± 0.04 |
| 1.5/5 mW cm−2 | 43 ± 1 | 76.56 ± 0.05 | 95.312 ± 0.008 | 54.4 ± 0.3 | 70.5 ± 0.4 | 93.5 ± 0.2 |
| 2.25/2.5 mW cm−2 | 46.9 ± 0.1 | 73.125 ± 0.005 | 97.5 ± 0.3 | 55 ± 2 | 69.0 ± 0.3 | 94.3 ± 0.1 |
| 3/10 mW cm−2 | 44.4 ± 0.2 | 94.063 ± 0.007 | 99.344 ± 0.007 | 57 ± 3 | 91.0 ± 0.5 | 97.89 ± 0.03 |
| 4.5/2.5 mW cm−2 | 50.4 ± 0.3 | 99.25 ± 0.04 | 99.869 ± 0.008 | 60 ± 3 | 98.65 ± 0.03 | 99.0 ± 0.3 |
| 4.5/5 mW cm−2 | 46.9 ± 0.2 | 99.532 ± 0.007 | 99.947 ± 0.007 | 57.4 ± 0.4 | 98.35 ± 0.04 | 98.9 ± 0.5 |
| 9/5 mW cm−2 | 52.5 ± 0.3 | 99.938 ± 0.006 | 99.969 ± 0.009 | 61.2 ± 0.3 | 99.375 ± 0.005 | 99.575 ± 0.004 |
| 9/10 mW cm−2 | 52.5 ± 0.3 | 99.918 ± 0.007 | 99.94 ± 0.04 | 62 ± 1 | 99.5 ± 0.4 | 99.87 ± 0.03 |
| 13.5/5 mW cm−2 | 54.7 ± 0.2 | 99.947 ± 0.007 | 99.978 ± 0.007 | 63.8 ± 0.5 | 99.975 ± 0.003 | 99.987 ± 0.003 |
| 18/5 mW cm−2 | ND | ND | ND | 66.2 ± 0.5 | 99.988 ± 0.007 | 99.99 ± 0.01 |
| 18/10 mW cm−2 | 55.8 ± 0.1 | 99.95 ± 0.06 | 99.955 ± 0.005 | 66.5 ± 0.4 | 99.75 ± 0.06 | 99.91 ± 0.01 |
| 27/10 mW cm−2 | 57.8 ± 0.1 | 99.999 ± 0.003 | lethal | 68 ± 2 | 99.985 ± 0.004 | 99.986 ± 0.004 |
| 36/10 mW cm−2 | 57.38 ± 0.03 | ND | ND | 70 ± 3 | 99.986 ± 0.004 | 99.99 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maliszewska, I.; Wanarska, E.; Thompson, A.C.; Samuel, I.D.W.; Matczyszyn, K. Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules 2021, 26, 623. https://doi.org/10.3390/molecules26030623
Maliszewska I, Wanarska E, Thompson AC, Samuel IDW, Matczyszyn K. Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules. 2021; 26(3):623. https://doi.org/10.3390/molecules26030623
Chicago/Turabian StyleMaliszewska, Irena, Ewelina Wanarska, Alex C. Thompson, Ifor D. W. Samuel, and Katarzyna Matczyszyn. 2021. "Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy" Molecules 26, no. 3: 623. https://doi.org/10.3390/molecules26030623
APA StyleMaliszewska, I., Wanarska, E., Thompson, A. C., Samuel, I. D. W., & Matczyszyn, K. (2021). Biogenic Gold Nanoparticles Decrease Methylene Blue Photobleaching and Enhance Antimicrobial Photodynamic Therapy. Molecules, 26(3), 623. https://doi.org/10.3390/molecules26030623